The Long Way of Oxytocin from the Uterus to the Heart in 70 Years from Its Discovery
Abstract
1. Introduction
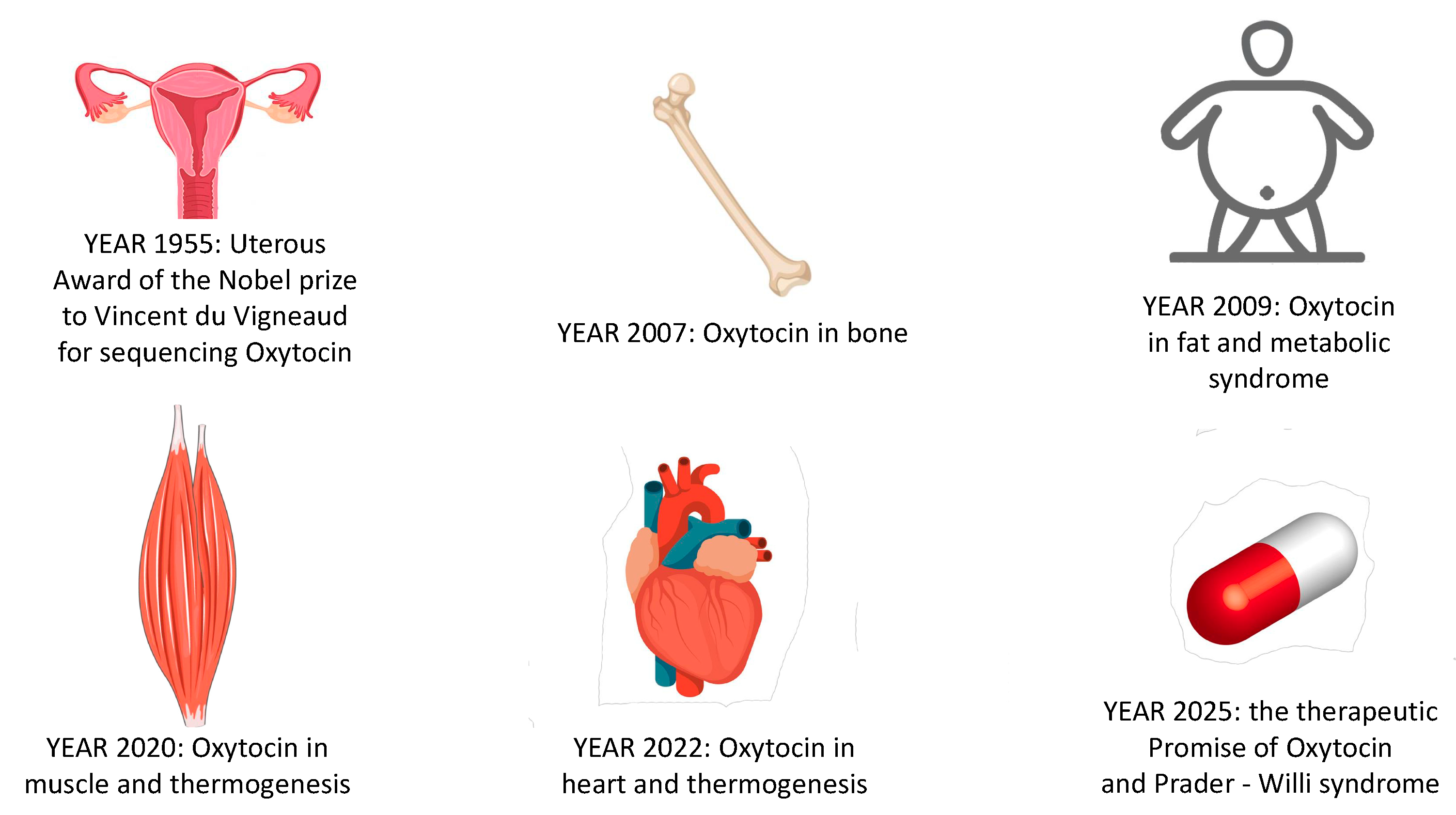
2. Year—1955: Oxytocin Is First Discovered for Its Effects on Uterine Contractility but Expresses Sexual Dimorphism
2.1. Oxytocin in Women Health
2.2. Oxytocin in Men and Women
3. Year—2007: Oxytocin Is in Bone
3.1. In Vitro Studies
3.2. Ex Vivo Studies
4. Year—2009: Oxytocin Is in Fat and Is Involved in the Onset of Metabolic Syndrome
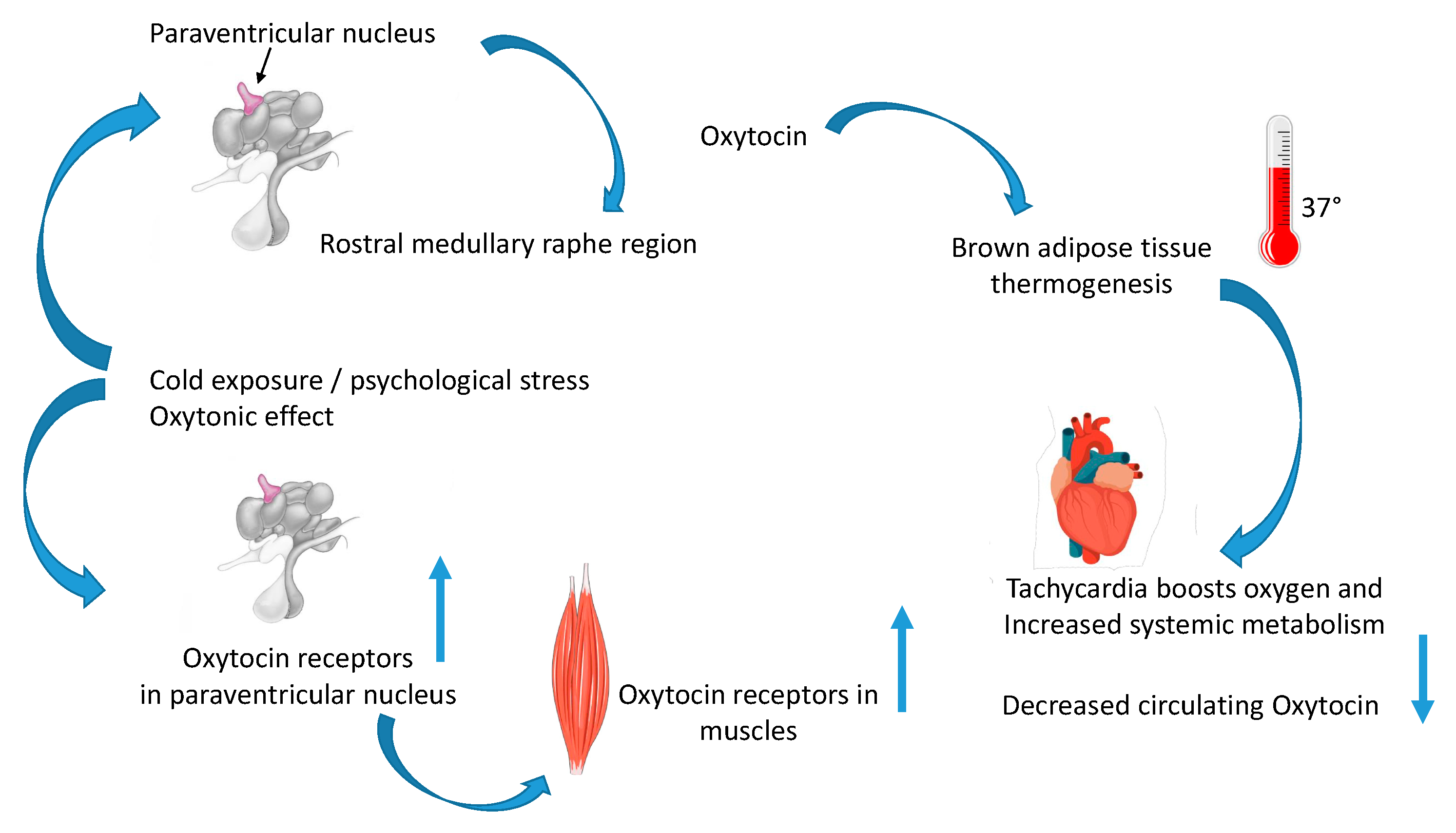
5. Year—2016: Oxytocin Regulates Thermogenesis and “The Oxytonic Effect”
5.1. Oxytocin in Muscle Adaptation after Cold Stress Challenge
5.2. The Oxytonic Effect
6. Year—2022: Oxytocin Is in the Heart
6.1. Beneficial Effect of Oxytocin in Coronary Artery Disease and Atherosclerosis
6.2. Oxytocin in the Myocardium during Thermogenesis
7. Year—2025: The Therapeutic Promise of Oxytocin and Prader–Willi Syndrome
7.1. Oxytocin as an Anti-Obesity Medication
7.2. Oxytocin in Prader–Willi Syndrome
8. Strengths and Limitations of Oxytocin as Anti-Obesity Treatment
8.1. Limitations in the Use of Oxytocin as an Anti-Obesity Medication and the Blood Brain Barrier
8.2. Oxytocin in Clinical Trials
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BBB | Blood–brain barrier |
| BAT | Brown adipose tissue |
| WAT | White adipose tissue |
| iWAT | Inguinal adipose tissue |
| CS | Cold stress |
| CSF | Cerebrospinal fluid |
| BBB | Blood brain barrier |
| HIPP | Hippocampus |
| MyHC | Myosin heavy chain |
| Oxt | Oxytocin |
| Oxtr | Oxytocin receptor |
| PVN | Paraventricular nucleus |
| PWS | Prader–Willi syndrome |
| SON | Supraoptical nucleus |
| Sol | Soleus muscle |
| TA | Tibialis anterioris |
| TRPV1 | Transient receptor potential vanilloid 1 |
References
- Eisenberg, Y.; Dugas, L.R.; Akbar, A.; Reddivari, B.; Layden, B.T.; Barengolts, E. Oxytocin is lower in African American men with diabetes and associates with psycho-social and metabolic health factors. PLoS ONE 2018, 13, e0190301. [Google Scholar] [CrossRef] [PubMed]
- Camerino, C.; Conte, E.; Carratù, M.R.; Fonzino, A.; Lograno, M.D.; Tricarico, D. Oxytocin/Osteocalcin/IL-6 and NGF/BDNF mRNA levels in response to cold stress challenge in mice: Possible oxytonic brain-bone-muscle-interaction. Front. Physiol. 2019, 10, 1437. [Google Scholar] [CrossRef] [PubMed]
- Conte, E.; Romano, A.; De Bellis, M.; De Ceglia, M.L.; Carratù, M.R.; Gaetani, S.; Maqoud, F.; Tricarico, D.; Camerino, C. Oxtr/TRPV1 expression and acclimation of skeletal muscle to cold-stress in male mice. J. Endocrinol. 2021, 249, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, A.; Kataoka, N.; Nakamura, K. An oxytocinergic neural pathway that stimulates thermogenic and cardiac sympathetic outflow. Cell Rep. 2022, 40, 111380. [Google Scholar] [CrossRef] [PubMed]
- Brownstein, M.J.; Russell, J.T.; Gainer, H. Synthesis, transport, and release of posterior pituitary hormones. Science 1980, 207, 373–378. [Google Scholar] [CrossRef]
- Gimpl, G.; Fahrenholz, F. The oxytocin receptor system: Structure, function, and regulation. Physiol. Rev. 2001, 81, 629–683. [Google Scholar] [CrossRef]
- Odekunle, E.A.; Elphick, M.R. Comparative and evolutionary physiology of vasopressin/oxytocin-type neuropeptide signaling in invertebrates. Front. Endocrinol. 2020, 11, 225. [Google Scholar] [CrossRef]
- Oliver, G.; Schäfer, E.A. On the physiological action of extracts of pituitary body and certain other glandular organs: Preliminary communication. J. Physiol. 1895, 18, 277. [Google Scholar] [CrossRef]
- Howell, W.H.; Duke, W.W. Experiments on the isolated mammalian heart to show the relation of the inorganic salts to the action of the accelerator and inhibitory nerves. J. Physiol. 1906, 35, 131. [Google Scholar] [CrossRef]
- Liu, N.; Yang, H.; Han, L.; Ma, M. Oxytocin in women’s health and disease. Front. Endocrinol. 2022, 13, 786271. [Google Scholar] [CrossRef]
- Richter, O.N.; Kübler, K.; Schmolling, J.; Kupka, M.; Reinsberg, J.; Ulrich, U.; Van derVen, H.; Wardelmann, E.; Van derVen, K. Oxytocin receptor gene expression of estrogen-stimulated human myometrium in extracorporeally perfused non-pregnant uteri. Mol. Hum. Reprod. 2004, 10, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Camerino, C. The new frontier in oxytocin physiology: The oxytonic contraction. Int. J. Mol. Sci. 2020, 21, 5144. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.L.; Tasker, J.G.; Lucion, A.B.; Fiedler, J.; Munhoz, C.D.; Wu, T.Y.J.; Deak, T. Factors promoting vulnerability to dysregulated stress reactivity and stress-related disease. J. Neuroendocrinol. 2018, 30, e12641. [Google Scholar] [CrossRef] [PubMed]
- Oladosu, F.A.; Tu, F.F.; Garfield, L.B.; Garrison, E.F.; Steiner, N.D.; Roth, G.E.; Hellman, K.M. Low serum oxytocin concentrations are associated with painful menstruation. Reprod. Sci. 2020, 27, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Mooney, S.J.; Coen, C.W.; Holmes, M.M.; Beery, A.K. Region-specific associations between sex, social status, and oxytocin receptor density in the brains of eusocial rodents. Neuroscience 2015, 303, 261–269. [Google Scholar] [CrossRef]
- Horrell, N.D.; Hickmott, P.W.; Saltzman, W. Neural regulation of paternal behavior in mammals: Sensory, neuroendocrine, and experiential influences on the paternal brain. In Neuroendocrine Regulation of Behavior; Coolen, L., Grattan, D., Eds.; Springer: Cham, Switzerland, 2018; CTBN; Volume 43, pp. 111–160. [Google Scholar] [CrossRef]
- Olazábal, D.E. Role of oxytocin in parental behaviour. J. Neuroendocrinol. 2018, 30, e12594. [Google Scholar] [CrossRef]
- Breuil, V.; Trojani, M.C.; Ez-Zoubir, A. Oxytocin and bone: Review and perspectives. Int. J. Mol. Sci. 2021, 22, 8551. [Google Scholar] [CrossRef]
- Magon, N.; Kalra, S. The orgasmic history of oxytocin: Love, lust, and labor. Indian J. Endocrinol. Metab. 2011, 15 (Suppl. 3), S156. [Google Scholar] [CrossRef]
- De Melo, V.U.; Saldanha, R.R.; Dos Santos, C.R.; Cruz, J.D.C.; Lira, V.A.; Santana-Filho, V.J.; Michelini, L.C. Ovarian hormone deprivation reduces oxytocin expression in paraventricular nucleus preautonomic neurons and correlates with baroreflex impairment in rats. Front. Physiol. 2016, 7, 461. [Google Scholar] [CrossRef]
- Stone, G.; Choi, A.; Meritxell, O.; Gorham, J.; Heydarpour, M.; Seidman, C.E.; Seidman, J.G.; Aranki, S.F.; Body, S.C.; Carey, V.J.; et al. Sex differences in gene expression in response to ischemia in the human left ventricular myocardium. Hum. Mol. Genet. 2019, 28, 1682–1693. [Google Scholar] [CrossRef]
- Elabd, C.; Basillais, A.; Beaupied, H.; Breuil, V.; Wagner, N.; Scheideler, M.; Zaragosi, L.E.; Massiéra, F.; Lemichez, E.; Trajanoski, Z. Oxytocin controls differentiation of human mesenchymal stem cells and reverses osteoporosis. Stem Cells 2008, 26, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Camerino, C. Estrogen-BDNF signalling in neuronal cells: Toward a brain-centric approach to the cure of aging and osteoporosis. IBMS BoneKEy 2012, 202, 1–3. [Google Scholar] [CrossRef]
- Camerino, C. Oxytocin inhibits bone formation through the activation of the sympathetic tone, A new candidate in the central regulation of bone formation. J. Bone Miner. Res. 2008, 23 (Suppl. 1), S56. [Google Scholar]
- Takeda, S.; Elefteriou, F.; Levasseur, R.; Liu, X.; Zhao, L.; Parker, K.L.; Armstrong, D.; Ducy, P.; Karsenty, G. Leptin regulates bone formation via the sympathetic nervous system. Cell 2002, 111, 305–317. [Google Scholar] [CrossRef]
- Moghazy, H.; Mahmoud, A.A.; Elbadre, H.; Aziz, H.O.A. Protective effect of oxytocin against bone loss in a female rat model of osteoporosis. Rep. Biochem. Mol. Biol. 2020, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.N.E.; Bogdanowicz, D.R.; Mitroo, S.; Shan, J.; Kala, S.; Lu, H.H. Cellular interactions regulate stem cell differentiation in tri-culture. Connect. Tissue Res. 2016, 57, 476–487. [Google Scholar] [CrossRef] [PubMed]
- During, A. Osteoporosis: A role for lipids. Biochimie 2020, 178, 49–55. [Google Scholar] [CrossRef] [PubMed]
- De Paula, F.J.; ìRosen, C.J. Marrow adipocytes: Origin, structure, and function. Annu. Rev. Physiol. 2020, 82, 461–484. [Google Scholar] [CrossRef] [PubMed]
- Breuil, V.; Panaia-Ferrari, P.; Fontas, E.; Roux, C.; Kolta, S.; Eastell, R.; Ben Yahia, H.; Faure, S.; Gossiel, F.; Benhamou, C.L.; et al. Oxytocin, a new determinant of bone mineral density in post-menopausal women: Analysis of the OPUS cohort. J. Clin. Endocrinol. Metab. 2014, 99, E634–E641. [Google Scholar] [CrossRef]
- Breuil, V.; Fontas, E.; Chapurlat, R.; Panaia-Ferrari, P.; Yahia, H.B.; Faure, S.; Euller-Ziegler, L.; Amri, E.Z.; Szulc, P. Oxytocin and bone status in men: Analysis of the MINOS cohort. Osteoporos. Int. 2015, 26, 2877–2882. [Google Scholar] [CrossRef]
- Carter, C.S.; Kenkel, W.M.; MacLean, E.L.; Wilson, S.R.; Perkeybile, A.M.; Yee, J.R.; Ferris, C.F.; Nazarloo, H.P.; Porges, S.W.; Davis, J.M. Is oxytocin “nature’s medicine”? Pharmacol. Rev. 2020, 72, 829–861. [Google Scholar] [CrossRef] [PubMed]
- Amri, E.Z.; Pisani, D.F. Control of bone and fat mass by oxytocin. Horm. Mol. Biol. Clin. Investig. 2016, 28, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Abramova, O.; Zorkina, Y.; Ushakova, V.; Zubkov, E.; Morozova, A.; Chekhonin, V. The role of oxytocin and vasopressin dysfunction in cognitive impairment and mental disorders. Neuropeptides 2020, 83, 102079. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, Y.; Kasahara, Y.; Onaka, T.; Takahashi, N.; Kawada, T.; Nishimori, K. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport 2008, 19, 951–955. [Google Scholar] [CrossRef]
- Camerino, C. Low sympathetic tone and obese phenotype in oxytocin-deficient mice. Obesity 2009, 17, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Camerino, C.; Zayzafoon, M.; Rymaszewski, M.; Heiny, J.; Rios, M.; Hauschka, P. Central depletion of brain-derived neurotrophic factor in mice results in high bone mass and metabolic phenotype. Endocrinology 2012, 153, 5394–5405. [Google Scholar] [CrossRef]
- Kublaoui, B.M.; Gemelli, T.; Tolson, K.P.; Wang, Y.; Zinn, A.R. Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Mol. Endocrinol. 2008, 22, 1723–1734. [Google Scholar] [CrossRef]
- Lipschitz, D.L.; Crowley, W.R.; Bealer, S.L. Differential sensitivity of intranuclear and systemic oxytocin release to central noradrenergic receptor stimulation during mid-and late gestation in rats. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E523–E528. [Google Scholar] [CrossRef] [PubMed]
- Camerino, C. Oxytocin thinks globally and acts locally: The oxytocinergic regulation of bone mass. IBMS BoneKEy 2009, 6, 295. [Google Scholar] [CrossRef]
- McCormack, S.E.; Blevins, J.E.; Lawson, E.A. Metabolic effects of oxytocin. Endocr. Rev. 2020, 41, 121–145. [Google Scholar] [CrossRef]
- Elabd, C.; Cousin, W.; Upadhyayula, P.; Chen, R.Y.; Chooljian, M.S.; Li, J.; Kung, S.; Jiang, K.P.; Conboy, I.M. Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nat. Commun. 2014, 5, 4082. [Google Scholar] [CrossRef] [PubMed]
- Dombret, C.; Nguyen, T.; Schakman, O.; Michaud, J.L.; Hardin-Pouzet, H.; Bertrand, M.J.; De Backer, O. Loss of Maged1 results in obesity, deficits of social interactions, impaired sexual behavior and severe alteration of mature oxytocin production in the hypothalamus. Hum. Mol. Genet. 2012, 21, 4703–4717. [Google Scholar] [CrossRef] [PubMed]
- Camerino, C.; Conte, E.; Cannone, M.; Caloiero, R.; Fonzino, A.; Tricarico, D. Nerve growth factor, brain-derived neurotrophic factor and osteocalcin gene relationship in energy regulation, bone homeostasis and reproductive organs analyzed by mRNA quantitative evaluation and linear correlation analysis. Front. Physiol. 2016, 7, 456. [Google Scholar] [CrossRef] [PubMed]
- Camerino, C.; Conte, E.; Caloiero, R.; Fonzino, A.; Carratù, M.; Lograno, M.D.; Tricarico, D. Evaluation of short and long term cold stress challenge of nerve grow factor, brain-derived neurotrophic factor, osteocalcin and oxytocin mRNA expression in BAT, brain, bone and reproductive tissue of male mice using real-time PCR and linear correlation analysis. Front. Physiol. 2018, 8, 1101. [Google Scholar] [PubMed]
- Mizunoya, W.; Okamoto, S.; Miyahara, H.; Akahoshi, M.; Suzuki, T.; Do, M.K.Q.; Ohtsubo, H.; Komiya, Y.; Qahar, M.; Waga, T. Fast-to-slow shift of muscle fiber-type composition by dietary apple polyphenols in rats: Impact of the low-dose supplementation. Anim. Sci. J. 2017, 88, 489–499. [Google Scholar] [CrossRef]
- Scala, R.; Maqoud, F.; Angelelli, M.; Latorre, R.; Perrone, M.G.; Scilimati, A.; Tricarico, D. Zoledronic acid modulation of TRPV1 channel currents in osteoblast cell line and native rat and mouse bone marrow-derived osteoblasts: Cell proliferation and mineralization effect. Cancers 2019, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- Beets, I.; Janssen, T.; Meelkop, E.; Temmerman, L.; Suetens, N.; Rademakers, S.; Jansen, G.; Schoofs, L. Vasopressin/oxytocin-related signaling regulates gustatory associative learning in C. elegans. Science 2012, 338, 543–545. [Google Scholar] [CrossRef]
- Nersesyan, Y.; Demirkhanyan, L.; Cabezas-Bratesco, D.; Oakes, V.; Kusuda, R.; Dawson, T.; Sun, X.; Cao, C.; Cohen, A.M.; Chelluboina, B. Oxytocin modulates nociception as an agonist of pain-sensing TRPV1. Cell Rep. 2017, 21, 1681–1691. [Google Scholar] [CrossRef]
- Gonzalez-Hernandez, A.; Charlet, A. Oxytocin, GABA, and TRPV1, the analgesic triad? Front. Mol. Neurosci. 2018, 11, 398. [Google Scholar] [CrossRef]
- Trayhurn, P. Origins and early development of the concept that brown adipose tissue thermogenesis is linked to energy balance and obesity. Biochimie 2017, 134, 62–70. [Google Scholar] [CrossRef]
- Kasahara, Y.; Sato, K.; Takayanagi, Y.; Mizukami, H.; Ozawa, K.; Hidema, S.; So, K.H.; Kawada, T.; Inoue, N.; Ikeda, I. Oxytocin receptor in the hypothalamus is sufficient to rescue normal thermoregulatory function in male oxytocin receptor knockout mice. Endocrinology 2013, 154, 4305–4315. [Google Scholar] [CrossRef]
- Blondin, D.P.; Haman, F. Shivering and nonshivering thermogenesis in skeletal muscles. Handb. Clin. Neurol. 2018, 156, 153–173. [Google Scholar]
- Palmer, B.F.; Clegg, D.J. Non-shivering thermogenesis as a mechanism to facilitate sustainable weight loss. Obes. Rev. 2017, 18, 819–831. [Google Scholar] [CrossRef]
- Jurek, B.; Neumann, I.D. The oxytocin receptor: From intracellular signaling to behavior. Physiol. Rev. 2018, 98, 1805–1908. [Google Scholar] [CrossRef]
- Breton, C.; Haenggeli, C.; Barberis, C.; Heitz, F.; Bader, C.R.; Bernheim, L.; Tribollet, E. Presence of functional oxytocin receptors in cultured human myoblasts. J. Clin. Endocrinol. Metab. 2002, 87, 1415–1418. [Google Scholar] [CrossRef]
- Qin, J.; Feng, M.; Wang, C.; Ye, Y.; Wang, P.S.; Liu, C. Oxytocin receptor expressed on the smooth muscle mediates the excitatory effect of oxytocin on gastric motility in rats. Neurogastroenterol. Motil. 2009, 21, 430–438. [Google Scholar] [CrossRef]
- Baribeau, D.A.; Anagnostou, E. Oxytocin and vasopressin: Linking pituitary neuropeptides and their receptors to social neurocircuits. Front. Neurosci. 2015, 9, 335. [Google Scholar] [CrossRef]
- Ding, C.; Magkos, F. Oxytocin and vasopressin systems in obesity and metabolic health: Mechanisms and perspectives. Curr. Obes. Rep. 2019, 8, 301–316. [Google Scholar] [CrossRef]
- Sun, L.; Lizneva, D.; Ji, Y.; Colaianni, G.; Hadelia, E.; Gumerova, A.; Ievleva, K.; Kuo, T.C.; Korkmaz, F.; Ryu, V. Oxytocin regulates body composition. Proc. Natl. Acad. Sci. USA 2019, 116, 26808–26815. [Google Scholar] [CrossRef]
- Camerino, C. Oxytocin Involvement in Body Composition Unveils the True Identity of Oxytocin. Int. J. Mol. Sci. 2021, 22, 6383. [Google Scholar] [CrossRef]
- Costa, D.M.; da Cruz-Filho, J.; Vasconcelos, A.B.S.; Gomes-Santos, J.V.; Reis, L.C.; de Lucca, W., Jr.; Camargo, E.A.; Lauton-Santos, S.; Zanon, N.M.; do Carmo Kettelhut, Í. Oxytocin induces anti-catabolic and anabolic effects on protein metabolism in the female rat oxidative skeletal muscle. Life Sci. 2021, 279, 119665. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, P.; Stefanovic, B.; Spasojevic, N.; Puskas, N.; Dronjak, S. Effects of oxytocin on adreno-medullary catecholamine synthesis, uptake and storage in rats exposed to chronic isolation stress. Endocr. Res. 2016, 41, 124–131. [Google Scholar] [CrossRef]
- Khan, M.M.; Lustrino, D.; Silveira, W.A.; Wild, F.; Straka, T.; Issop, Y.; O’Connor, E.; Cox, D.; Reischl, M.; Marquardt, T. Sympathetic innervation controls homeostasis of neuromuscular junctions in health and disease. Proc. Natl. Acad. Sci. USA 2016, 113, 746–750. [Google Scholar] [CrossRef]
- Gajdosechova, L.; Krskova, K.; Olszanecki, R.; Zorad, S. Differential regulation of oxytocin receptor in various adipose tissue depots and skeletal muscle types in obese Zucker rats. Horm. Metab. Res. 2015, 47, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, A.; Radu, B.M.; Radu, M.; Cretoiu, S.M. Muscle changes during atrophy. Adv. Exp. Med. Biol. 2018, 1088, 73–92. [Google Scholar] [PubMed]
- Gutkowska, J.; Jankowski, M.; Lambert, C.; Mukaddam-Daher, S.; Zingg, H.H.; McCann, S.M. Oxytocin releases atrial natriuretic peptide by combining with oxytocin receptors in the heart. Proc. Natl. Acad. Sci. USA 1997, 94, 11704–11709. [Google Scholar] [CrossRef]
- Tsingotjidou, A.S. Oxytocin: A Multi-Functional Biomolecule with Potential Actions in Dysfunctional Conditions; From Animal Studies and Beyond. Biomolecules 2022, 12, 1603. [Google Scholar] [CrossRef]
- Jankowski, M.; Broderick, T.L.; Gutkowska, J. The role of oxytocin in cardiovascular protection. Front. Psychol. 2020, 11, 2139. [Google Scholar] [CrossRef]
- Wang, P.; Wang, S.C.; Yang, H.; Lv, C.; Jia, S.; Liu, X.; Wang, X.; Meng, D.; Qin, D.; Zhu, H. Therapeutic potential of oxytocin in atherosclerotic cardiovascular disease: Mechanisms and signaling pathways. Front. Neurosci. 2019, 13, 454. [Google Scholar] [CrossRef]
- Olszewski, P.K.; Noble, E.E.; Paiva, L.; Ueta, Y.; Blevins, J.E. Oxytocin as a potential pharmacological tool to combat obesity. J. Neuroendocrinol. 2022, 34, e13106. [Google Scholar] [CrossRef]
- Swaab, D.F. Prader—Willi syndrome and the hypothalamus. Acta Paediatr. 1997, 86, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Anekonda, V.T.; Thompson, B.W.; Ho, J.M.; Roberts, Z.S.; Edwards, M.M.; Nguyen, H.K.; Dodson, A.D.; Wolden-Hanson, T.; Chukri, D.W.; Herbertson, A.J.; et al. Hindbrain Administration of Oxytocin Reduces Food Intake, Weight Gain and Activates Catecholamine Neurons in the Hindbrain Nucleus of the Solitary Tract in Rats. J. Clin. Med. 2021, 10, 5078. [Google Scholar] [CrossRef]
- Kerem, L.; Hadjikhani, N.; Holsen, L.; Lawson, E.A.; Plessow, F. Oxytocin reduces the functional connectivity between brain regions involved in eating behavior in men with overweight and obesity. Int. J. Obes. 2020, 44, 980–989. [Google Scholar] [CrossRef]
- Liu, C.M.; Spaulding, M.O.; Rea, J.J.; Noble, E.E.; Kanoski, S.E. Oxytocin and food intake control: Neural, behavioral, and signaling mechanisms. Int. J. Mol. Sci. 2021, 22, 10859. [Google Scholar] [CrossRef]
- Carrel, A.L.; Moerchen, V.; Myers, S.E.; Bekx, M.T.; Whitman, B.Y.; Allen, D.B. Growth hormone improves mobility and body composition in infants and toddlers with Prader-Willi syndrome. J. Pediatr. 2004, 145, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Wronski, M.L.; Plessow, F.; Kerem, L.; Asanza, E.; O’Donoghue, M.L.; Stanford, F.C.; Bredella, M.A.; Torriani, M.; Soukas, A.A.; Kheterpal, A.; et al. A randomized, double-blind, placebo-controlled clinical trial of 8-week intranasal oxytocin administration in adults with obesity: Rationale, study design, and methods. Contemp. Clin. Trials 2022, 122, 106909. [Google Scholar] [CrossRef] [PubMed]
- Elfers, C.T.; Blevins, J.E.; Lawson, E.A.; Pittner, R.; Silva, D.; Kiselyov, A.; Roth, C.L. Robust Reductions of Body Weight and Food Intake by an Oxytocin Analog in Rats. Front. Physiol. 2021, 12, 726411. [Google Scholar] [CrossRef]
- Elfers, C.T.; Blevins, J.E.; Salameh, T.S.; Lawson, E.A.; Silva, D.; Kiselyov, A.; Roth, C.L. Novel Long-Acting Oxytocin Analog with Increased Efficacy in Reducing Food Intake and Body Weight. Int. J. Mol. Sci. 2022, 23, 11249. [Google Scholar] [CrossRef]
- Ermisch, A.; Rühle, H.J.; Landgraf, R.; Hess, J. Blood—Brain barrier and peptides. J. Cereb. Blood Flow Metab. 1985, 5, 350–357. [Google Scholar] [CrossRef]
- Butler, M.G. Management of obesity in Prader-Willi syndrome. Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 592–593. [Google Scholar] [CrossRef]
- Labyb, M.; Chrétien, C.; Caillon, A.; Rohner-Jeanrenaud, F.; Altirriba, J. Oxytocin administration alleviates acute but not chronic leptin resistance of diet-induced obese mice. Int. J. Mol. Sci. 2018, 20, 88. [Google Scholar] [CrossRef]
- Miller, J.L.; Tamura, R.; Butler, M.G.; Kimonis, V.; Sulsona, C.; Gold, J.A.; Driscoll, D.J. Oxytocin treatment in children with Prader-Willi syndrome: A double-blind, placebo-controlled, crossover study. Am. J. Med. Genet. A 2017, 173, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Damen, L.; Grootjen, L.N.; Juriaans, A.F.; Donze, S.H.; Huisman, T.M.; Visser, J.A.; Delhanty, P.J.D.; Hokken-Koelega, A.C.S. Oxytocin in young children with Prader-Willi syndrome: Results of a randomized, double-blind, placebo-controlled, crossover trial investigating 3 months of oxytocin. Clin. Endocrinol. 2021, 94, 774–785. [Google Scholar] [CrossRef]
- Butler, M.G. Prader-Willi syndrome: Obesity due to genomic imprinting. Curr. Genom. 2011, 12, 204–215. [Google Scholar] [CrossRef]
- Bittel, D.C.; Butler, M.G. Prader–Willi syndrome: Clinical genetics, cytogenetics and molecular biology. Expert Rev. Mol. Med. 2005, 7, 1–20. [Google Scholar] [CrossRef]
- Johnson, L.; Manzardo, A.M.; Miller, J.L.; Driscoll, D.J.; Butler, M.G. Elevated plasma oxytocin levels in children with Prader–Willi syndrome compared with healthy unrelated siblings. Am. J. Med. Genet. Part A 2016, 170, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.G. Prader-Willi syndrome: Current understanding of cause and diagnosis. Am. J. Med. Genet. 1990, 35, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Elefteriou, F.; Ahn, J.D.; Takeda, S.; Starbuck, M.; Yang, X.; Liu, X.; Kondo, H.; Richards, W.G.; Bannon, T.W.; Noda, M. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 2005, 434, 514–520. [Google Scholar] [CrossRef]
- Lawson, E.A.; Olszewski, P.K.; Weller, A.; Blevins, J.E. The role of oxytocin in regulation of appetitive behaviour, body weight and glucose homeostasis. J. Neuroendocrinol. 2020, 32, e12805. [Google Scholar] [CrossRef]
- Niu, J.; Tong, J.; Blevins, J.E. Oxytocin as an Anti-obesity Treatment. Front. Neurosci. 2021, 15, 743546. [Google Scholar] [CrossRef]
- Leng, G.; Sabatier, N. Oxytocin–the sweet hormone? Trends Endocrinol. Metabol. 2017, 28, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S.M.; Samineni, S.; Allen, P.C.; Stockinger, D.; Bales, K.L.; Hwa, G.G.; Roberts, J.A. Plasma and CSF oxytocin levels after intranasal and intravenous oxytocin in awake macaques. Psychoneuroendocrinology 2016, 66, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Kievit, P.; Halem, H.; Marks, D.L.; Dong, J.Z.; Glavas, M.M.; Sinnayah, P.; Pranger, L.; Cowley, M.A.; Grove, K.L.; Culler, M.D. Chronic treatment with a melanocortin-4 receptor agonist causes weight loss, reduces insulin resistance, and improves cardiovascular function in diet-induced obese rhesus macaques. Diabetes 2013, 62, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Insel, T.R.; Winslow, J.T.; Witt, D.M. Homologous regulation of brain oxytocin receptors. Endocrinology 1992, 130, 2602–2608. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Slattery, D.A.; Uschold-Schmidt, N.; Reber, S.O.; Neumann, I.D. Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice. Psychoneuroendocrinology 2014, 42, 225–236. [Google Scholar] [CrossRef]
- Chepurny, O.G.; Bonaccorso, R.L.; Leech, C.A.; Wöllert, T.; Langford, G.M.; Schwede, F.; Roth, C.L.; Doyle, R.P.; Holz, G.G. Chimeric peptide EP45 as a dual agonist at GLP-1 and NPY2R receptors. Sci. Rep. 2018, 8, 3749. [Google Scholar] [CrossRef]
- Maejima, Y.; Iwasaki, Y.; Yamahara, Y.; Kodaira, M.; Sedbazar, U.; Yada, T. Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging 2011, 3, 1169. [Google Scholar] [CrossRef]
- Enebo, L.B.; Berthelsen, K.K.; Kankam, M.; Lund, M.T.; Rubino, D.M.; Satylganova, A.; Lau, D.C. Safety, tolerability, pharmacokinetics, and pharmacodynamics of concomitant administration of multiple doses of cagrilintide with semaglutide 2· 4 mg for weight management: A randomised, controlled, phase 1b trial. Lancet 2021, 397, 1736–1748. [Google Scholar] [CrossRef] [PubMed]
- Allison, D.B.; Gadde, K.M.; Garvey, W.T.; Peterson, C.A.; Schwiers, M.L.; Najarian, T.; Tam, P.Y.; Troupin, B.; Day, W.W. Controlled-release phentermine/topiramate in severely obese adults: A randomized controlled trial (EQUIP). Obesity 2012, 20, 330–342. [Google Scholar] [CrossRef]
- Edwards, M.M.; Nguyen, H.K.; Dodson, A.D.; Herbertson, A.J.; Wietecha, T.A.; Wolden-Hanson, T.; Graham, J.L.; Honeycutt, M.K.; Slattery, J.D.; O’Brien, K.D. Effects of combined oxytocin and beta-3 receptor agonist (CL 316243) treatment on body weight and adiposity in male diet-induced obese rats. Front. Physiol. 2021, 12, 725912. [Google Scholar] [CrossRef]
- Baskin, A.S.; Linderman, J.D.; Brychta, R.J.; McGehee, S.; Anflick-Chames, E.; Cero, C.; Johnson, J.W.; O’Mara, A.E.; Fletcher, L.A.; Leitner, B.P. Regulation of human adipose tissue activation, gallbladder size, and bile acid metabolism by a β3-adrenergic receptor agonist. Diabetes 2018, 67, 2113–2125. [Google Scholar] [CrossRef]
- Himms-Hagen, J.; Cui, E.; Danforth, E., Jr.; Taatjes, D.J.; Lang, S.S.; Waters, B.L.; Claus, T.H. Effect of CL-316,243, a thermogenic beta 3-agonist, on energy balance and brown and white adipose tissues in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1994, 266, R1371–R1382. [Google Scholar] [CrossRef]
- Hsu, E.A.; Miller, J.L.; Perez, F.A.; Roth, C.L. Oxytocin and naltrexone successfully treat hypothalamic obesity in a boy post-craniopharyngioma resection. J. Clin. Endocrinol. Metab. 2018, 103, 370–375. [Google Scholar] [CrossRef]
- Dal Monte, O.; Piva, M.; Anderson, K.M.; Tringides, M.; Holmes, A.J.; Chang, S.W. Oxytocin under opioid antagonism leads to supralinear enhancement of social attention. Proc. Natl. Acad. Sci. USA 2017, 114, 5247–5252. [Google Scholar] [CrossRef]
- Snider, B.; Geiser, A.; Yu, X.P.; Beebe, E.C.; Willency, J.A.; Qing, K.; Guo, L.; Lu, J.; Wang, X.; Yang, Q. Long-acting and selective oxytocin peptide analogs show antidiabetic and antiobesity effects in male mice. J. Endocr. Soc. 2019, 3, 1423–1444. [Google Scholar] [CrossRef]
- Rault, J.L.; Carter, C.S.; Garner, J.P.; Marchant-Forde, J.N.; Richert, B.T.; Lay Jr., D.C. Repeated intranasal oxytocin administration in early life dysregulates the HPA axis and alters social behavior. Physiol. Behav. 2013, 112, 40–48. [Google Scholar] [CrossRef]
- Liu, C.M.; Davis, E.A.; Suarez, A.N.; Wood, R.I.; Noble, E.E.; Kanoski, S.E. Sex differences and estrous influences on oxytocin control of food intake. Neuroscience 2020, 447, 63–73. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camerino, C. The Long Way of Oxytocin from the Uterus to the Heart in 70 Years from Its Discovery. Int. J. Mol. Sci. 2023, 24, 2556. https://doi.org/10.3390/ijms24032556
Camerino C. The Long Way of Oxytocin from the Uterus to the Heart in 70 Years from Its Discovery. International Journal of Molecular Sciences. 2023; 24(3):2556. https://doi.org/10.3390/ijms24032556
Chicago/Turabian StyleCamerino, Claudia. 2023. "The Long Way of Oxytocin from the Uterus to the Heart in 70 Years from Its Discovery" International Journal of Molecular Sciences 24, no. 3: 2556. https://doi.org/10.3390/ijms24032556
APA StyleCamerino, C. (2023). The Long Way of Oxytocin from the Uterus to the Heart in 70 Years from Its Discovery. International Journal of Molecular Sciences, 24(3), 2556. https://doi.org/10.3390/ijms24032556




