Development of Reverse Transcription Recombinase Polymerase Amplification (RT-RPA): A Methodology for Quick Diagnosis of Potato Leafroll Viral Disease in Potato
Abstract
1. Introduction
2. Results
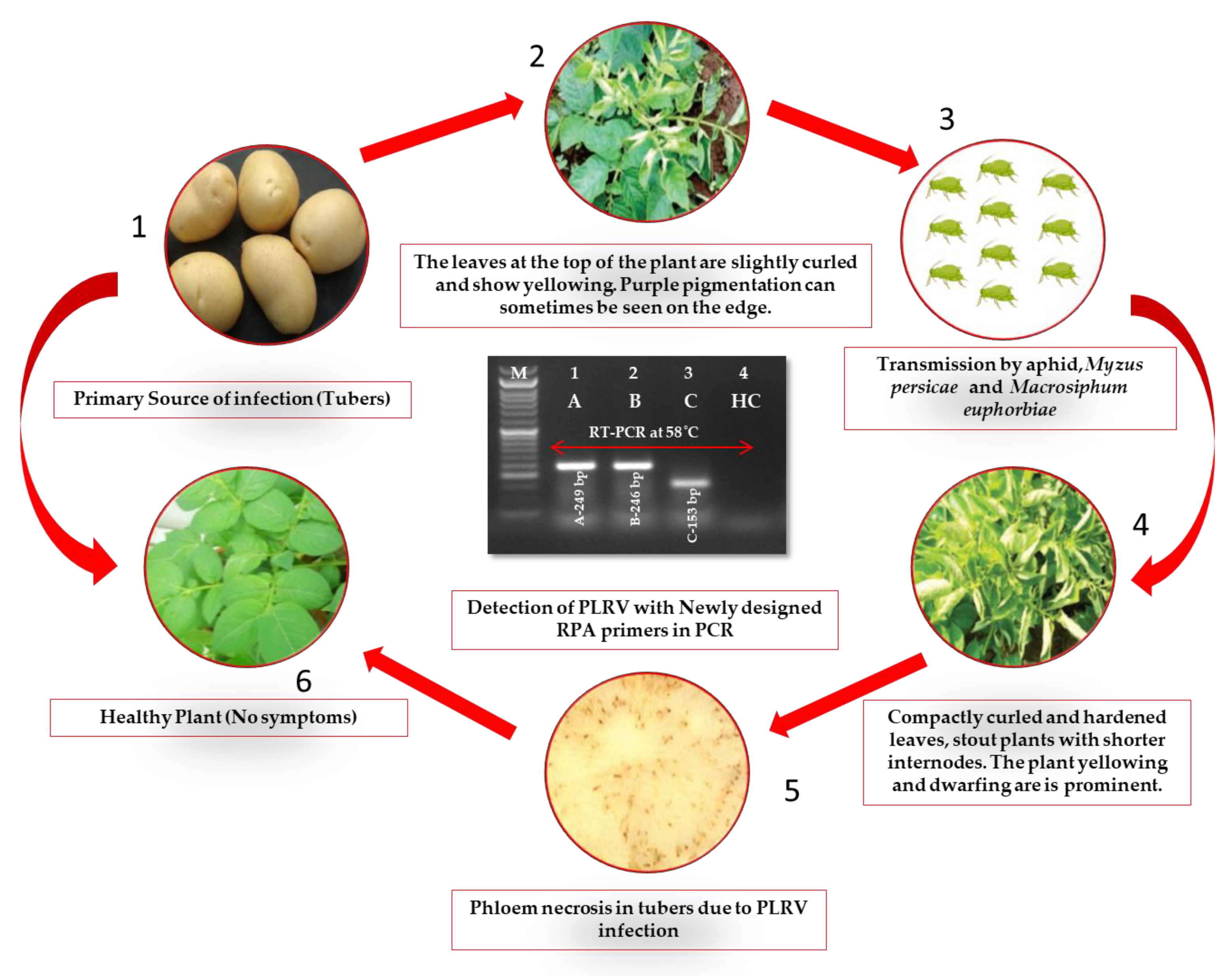
2.1. Evaluation of Plant Samples and RT-RPA Primers for PLRV Detection
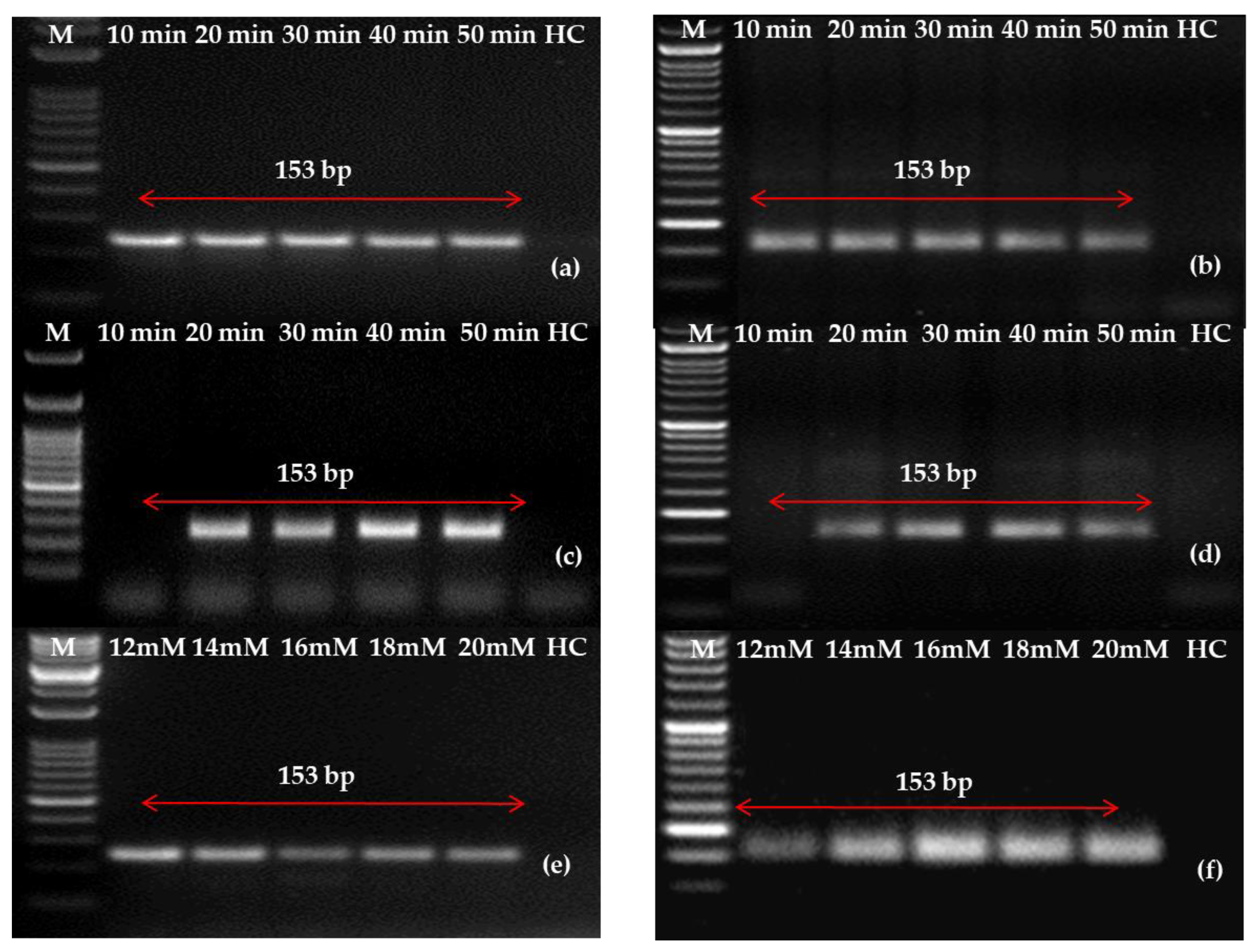
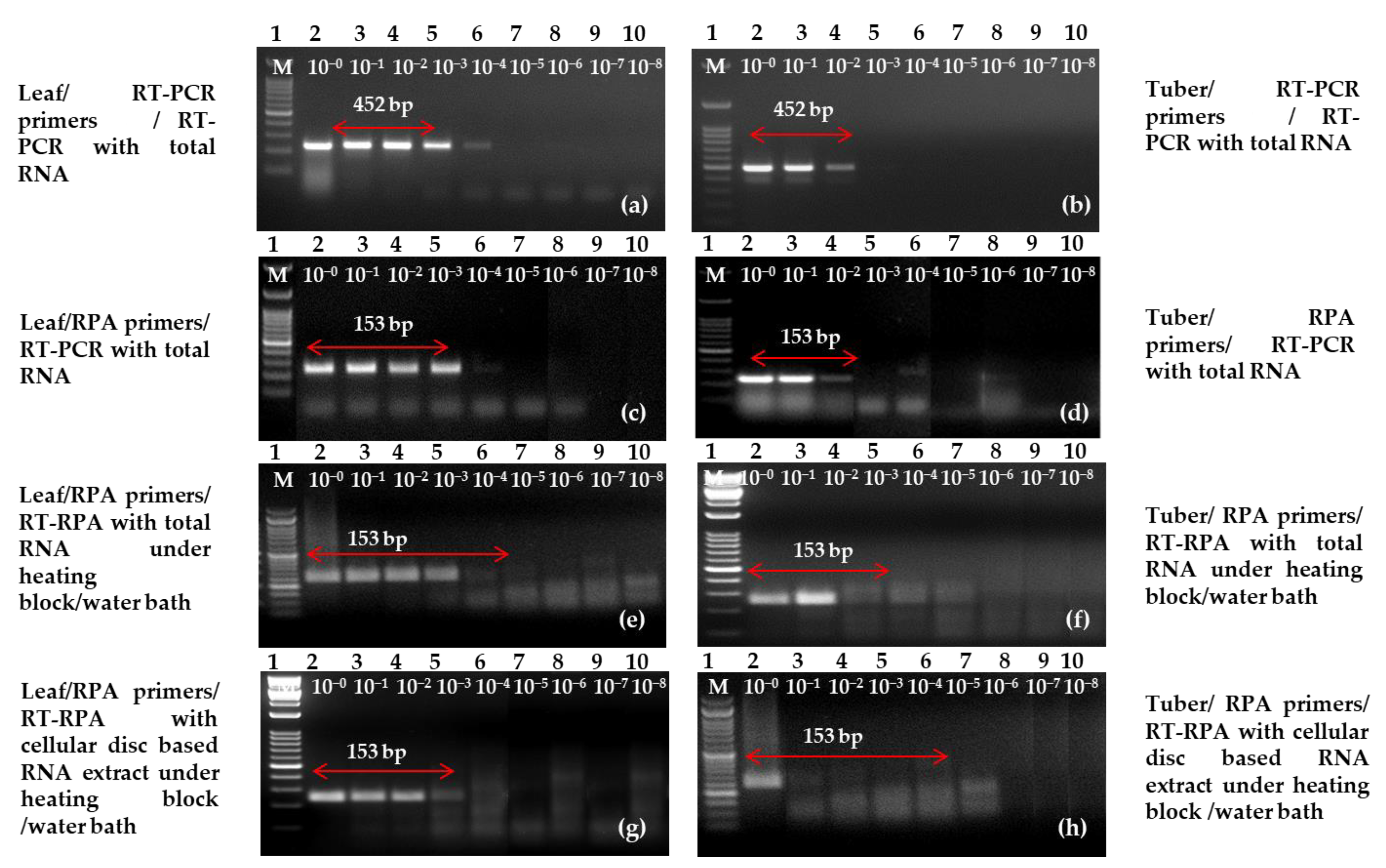
2.2. Optimization of One-Step RT-RPA Method for the Detection of PLRV Using Optimized Primers
2.3. Detection of PLRV Using Selected Primers and Optimization of One-Step RT-RPA
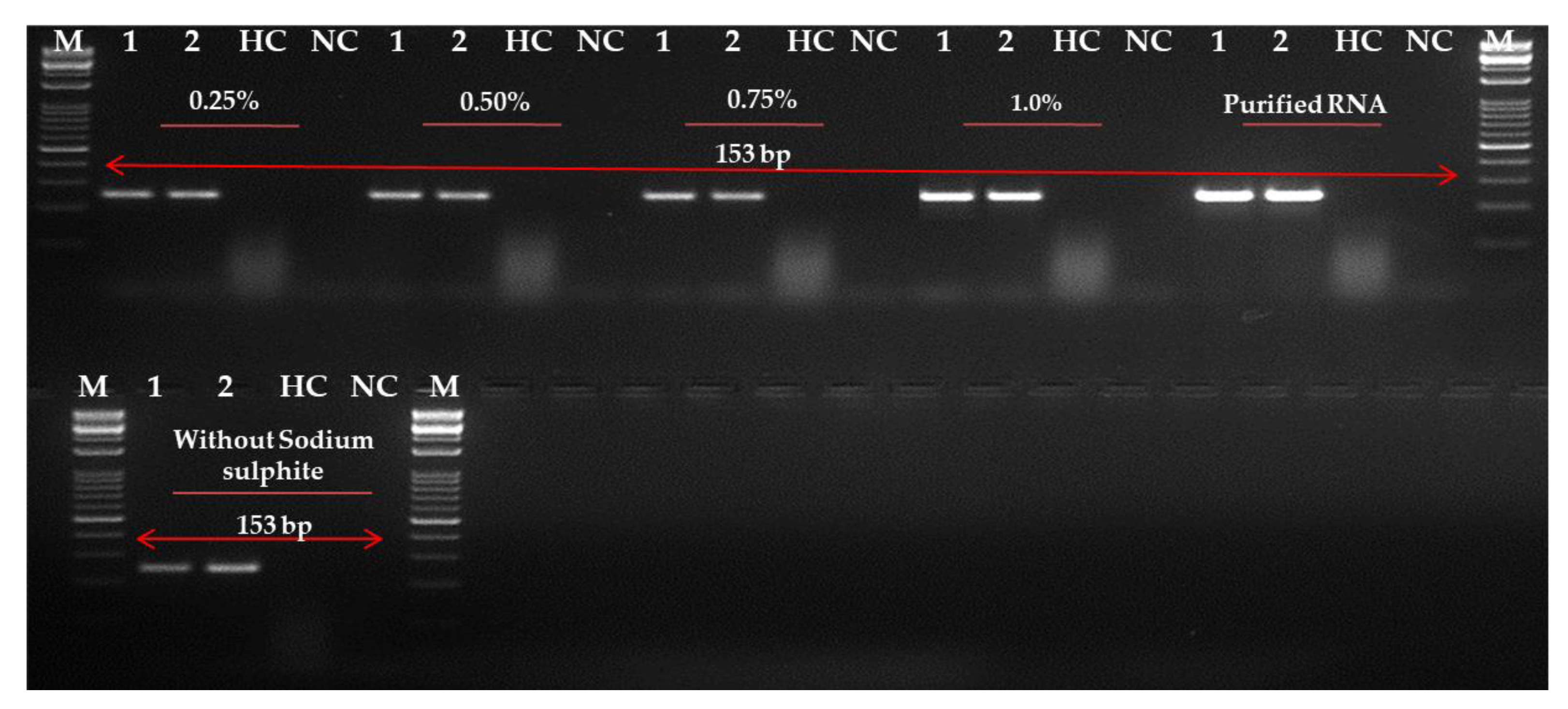
2.4. Evaluation and Optimization of RNA Extraction Method
2.5. Sensitivity and Specificity Analysis
2.6. Cloning and Sequencing of Amplicons for Primer Accuracy
2.7. Validation of One-Step RT-RPA Results on Diverse Field Samples
3. Discussion
4. Materials and Methods
4.1. Source of Viral Pure Culture and Plant Materials
4.2. The Process of RNA Isolation and cDNA Synthesis
4.3. Configuring the Reaction for One-Step RT-PCR
4.4. The Designing and Synthesis of Primers
4.5. Optimization of RT-RPA and Primer Selection
4.6. The Optimization of RT-RPA in One Step for the Detection of PLRV
4.7. Evaluation and Improvement of RNA Extraction Techniques in Leaves
4.8. Analysis of the Sensitivity and Specificity of One-Step RT-PCR/RT-RPA
4.9. Elution, Cloning and Sequencing for Specificity Confirmation
4.10. Natural Infection Validation Using a Single RT-RPA Technique
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Altaf, M.A.; Mandal, S.; Behera, B.; Mangal, V.; Naz, S.; Kumar, R.; Kumar, A.; Ghorai, M.; Singh, B.; Dey, A.; et al. Salinity Stress Tolerance in Solanaceous Crops: Current Understanding and Its Prospects in Genome Editing. J. Plant Growth Regul. 2022, 1–17. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Kumar, R.; Sharma, S.; Sagar, V.; Aggarwal, R.; Naga, K.C.; Lal, M.K.; Chourasia, K.N.; Kumar, D.; Kumar, M. Potato Dry Rot Disease: Current Status, Pathogenomics and Management. 3 Biotech 2020, 10, 503. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.A.; Shahid, R.; Altaf, M.M.; Kumar, R.; Naz, S.; Kumar, A.; Alam, P.; Tiwari, R.K.; Lal, M.K.; Ahmad, P. Melatonin: First-Line Soldier in Tomato under Abiotic Stress Current and Future Perspective. Plant Physiol. Biochem. 2022, 185, 188–197. [Google Scholar] [CrossRef]
- Mangal, V.; Lal, M.K.; Tiwari, R.K.; Altaf, M.A.; Sood, S.; Kumar, D.; Bharadwaj, V.; Singh, B.; Singh, R.K.; Aftab, T. Molecular Insights into the Role of Reactive Oxygen, Nitrogen and Sulphur Species in Conferring Salinity Stress Tolerance in Plants. J. Plant Growth Regul. 2022, 1–21. [Google Scholar] [CrossRef]
- Kumar, R.; Tiwari, R.K.; Sundaresha, S.; Kaundal, P.; Raigond, B. Potato Viruses and Their Management. In Sustainable Management of Potato Pests and Diseases; Springer: Singapore, 2022; pp. 309–335. [Google Scholar] [CrossRef]
- Devaux, A.; Goffart, J.P.; Petsakos, A.; Kromann, P.; Gatto, M.; Okello, J.; Suarez, V.; Hareau, G. Global Food Security, Contributions from Sustainable Potato Agri-Food Systems. In The Potato Crop: Its Agricultural, Nutritional and Social Contribution to Humankind; Springer: Cham, Switzerland, 2019; pp. 3–35. [Google Scholar] [CrossRef]
- Lal, M.K.; Tiwari, R.K.; Kumar, A.; Dey, A.; Kumar, R.; Kumar, D.; Jaiswal, A.; Changan, S.S.; Raigond, P.; Dutt, S.; et al. Mechanistic Concept of Physiological, Biochemical, and Molecular Responses of the Potato Crop to Heat and Drought Stress. Plants 2022, 11, 2857. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Bashyal, B.M.; Shanmugam, V.; Lal, M.K.; Kumar, R.; Sharma, S.; Naga, K.C.; Chourasia, K.N.; Aggarwal, R. First Report of Dry Rot of Potato Caused by Fusarium Proliferatum in India. J. Plant Dis. Prot. 2022, 129, 173–179. [Google Scholar] [CrossRef]
- Lal, M.K.; Singh, B.; Sharma, S.; Singh, M.P.; Kumar, A. Glycemic Index of Starchy Crops and Factors Affecting Its Digestibility: A Review. Trends Food Sci. Technol. 2021, 111, 741–755. [Google Scholar] [CrossRef]
- Lal, M.K.; Kumar, A.; Raigond, P.; Dutt, S.; Changan, S.S.; Chourasia, K.N.; Tiwari, R.K.; Kumar, D.; Sharma, S.; Chakrabarti, S.K.; et al. Impact of Starch Storage Condition on Glycemic Index and Resistant Starch of Cooked Potato (Solanum Tuberosum) Tubers. Starch-Stärke 2021, 73, 1900281. [Google Scholar] [CrossRef]
- Lal, M.K.; Tiwari, R.K.; Jaiswal, A.; Luthra, S.K.; Singh, B.; Kumar, S.; Gopalakrishnan, S.; Gaikwad, K.; Kumar, A.; Paul, V.; et al. Combinatorial Interactive Effect of Vegetable and Condiments with Potato on Starch Digestibility and Estimated in Vitro Glycemic Response. J. Food Meas. Charact. 2022, 16, 2446–2458. [Google Scholar] [CrossRef]
- Kreuze, J.F.; Souza-Dias, J.A.C.; Jeevalatha, A.; Figueira, A.R.; Valkonen, J.P.T.; Jones, R.A.C. Viral Diseases in Potato. In The Potato Crop: Its Agricultural, Nutritional and Social Contribution to Humankind; Springer: Cham, Switzerland, 2019; pp. 389–430. [Google Scholar] [CrossRef]
- Lal, M.K.; Sharma, N.; Adavi, S.B.; Sharma, E.; Altaf, M.A.; Tiwari, R.K.; Kumar, R.; Kumar, A.; Dey, A.; Paul, V.; et al. From Source to Sink: Mechanistic Insight of Photoassimilates Synthesis and Partitioning under High Temperature and Elevated [CO2]. Plant Mol. Biol. 2022, 110, 305–324. [Google Scholar] [CrossRef]
- Behera, B.; Kancheti, M.; Raza, M.B.; Shiv, A.; Mangal, V.; Rathod, G.; Altaf, M.A.; Kumar, A.; Aftab, T.; Kumar, R.; et al. Mechanistic Insight on Boron-Mediated Toxicity in Plant Vis-a-Vis Its Mitigation Strategies: A Review. Int. J. Phytoremediation 2022, 25, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Lal, M.K.; Tiwari, R.K.; Jaiswal, A.; Behera, B.; Shiv, A.; Kumar, A.; Kumar, S.; Paul, V.; Singh, M.P.; Singh, B.; et al. Physiological and Biochemical Mechanisms and Adaptation Strategies of Plants under Boron Deficiency Conditions. In Boron in Plants and Agriculture; Academic Press: Cambridge, MA, USA, 2022; pp. 127–146. [Google Scholar] [CrossRef]
- Kumar, R.; Tiwari, R.K.; Jeevalatha, A.; Siddappa, S.; Shah, M.A.; Sharma, S.; Sagar, V.; Kumar, M.; Chakrabarti, S.K. Potato Apical Leaf Curl Disease: Current Status and Perspectives on a Disease Caused by Tomato Leaf Curl New Delhi Virus. J. Plant Dis. Prot. 2021, 128, 897–911. [Google Scholar] [CrossRef]
- Shoala, T.; Al-Karmalawy, A.A.; Germoush, M.O.; Alshamrani, S.M.; Abdein, M.A.; Awad, N.S. Nanobiotechnological Approaches to Enhance Potato Resistance against Potato Leafroll Virus (PLRV) Using Glycyrrhizic Acid Ammonium Salt and Salicylic Acid Nanoparticles. Horticulturae 2021, 7, 402. [Google Scholar] [CrossRef]
- Kumar, R.; Jeevalatha, A.; Baswaraj, R.; Tiwari, R.K. Viral and Viroid Diseases of Potato and Their Management. In Potato Science & Technology for Sub Tropics, 1st ed.; New India Publishing Agency: New Delhi, India, 2020; pp. 267–292. [Google Scholar]
- Jailani, A.A.K.; Shilpi, S.; Mandal, B. Rapid Demonstration of Infectivity of a Hybrid Strain of Potato Virus Y Occurring in India through Overlapping Extension PCR. Physiol. Mol. Plant Pathol. 2017, 98, 62–68. [Google Scholar] [CrossRef]
- Shoala, T.; Eid, K.E.; EL-Fiki, I.A.I. Impact of Chemotherapy and Thermotherapy Treatments on the Presence of Potato Viruses PVY, PVX and PLRV in Tissue-Cultured Shoot Tip Meristem. J. Plant Prot. Pathol. 2019, 10, 581–585. [Google Scholar] [CrossRef]
- Kumar, R.; Tiwari, R.K.; Kaundal, P.; Sharma, S.; Chakrabarti, S. Potato Viruses and Their Diagnostic Techniques: An Overview. J. Pharm. Phytochem. 2019, 8, 1932–1944. [Google Scholar]
- Shah, M.A.; Sharma, S.; Kumar, R.; Singh, R.K. Evaluation of Polypropylene Row Covers for Excluding Virus Vectors and Their Effect on the Incidence of Diseases and Yield in Potato. Indian Phytopathol. 2020, 73, 751–757. [Google Scholar] [CrossRef]
- Kumar, R.; Jeevalatha, A.; Sharma, N.N.; Sharma, S.; Chakrabarti, S.K.; Singh, B.P. Development of PCR Based Methods for Detection of Potato Aucuba Mosaic Virus in India. Potato J. 2014, 41, 166–174. [Google Scholar]
- Onozuka, N.; Ohki, T.; Oka, N.; Maoka, T. Detection of Four Major Potato Viruses in Japan Using a Simple RNA Preparation and One-Step Multiplex RT-PCR. J. Gen. Plant Pathol. 2020, 86, 290–299. [Google Scholar] [CrossRef]
- Kumar, R.; Jeevalatha, A.; Raigond, B.; Kumar, R.; Sharma, S.; Nagesh, M. A Multiplex RT-PCR Assay for Simultaneous Detection of Five Viruses in Potato. J. Plant Pathol. 2017, 99, 37–45. [Google Scholar]
- Rashid, M.-O.; Wang, Y.; Han, C.-G. Molecular Detection of Potato Viruses in Bangladesh and Their Phylogenetic Analysis. Plants 2020, 9, 1413. [Google Scholar] [CrossRef] [PubMed]
- Halabi, M.H.; Oladokun, J.O.; Nath, P.D. Rapid Detection of Potato Leafroll Virus and Potato Virus Y by Reverse Transcription Loop-Mediated Isothermal Amplification Method in North-East India. J. Virol. Methods 2022, 300, 114363. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kaundal, P.; Arjunan, J.; Sharma, S.; Chakrabarti, S.K. Development of a Visual Detection Method for Potato Virus S by Reverse Transcription Loop-Mediated Isothermal Amplification. 3 Biotech 2020, 10, 213. [Google Scholar] [CrossRef]
- Kumar, R.; Kaundal, P.; Tiwari, R.K.; Siddappa, S.; Kumari, H.; Chandra Naga, K.; Sharma, S.; Kumar, M. Rapid and Sensitive Detection of Potato Virus X by One-Step Reverse Transcription-Recombinase Polymerase Amplification Method in Potato Leaves and Dormant Tubers. Mol. Cell. Probes 2021, 58, 101743. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.; Srivastava, N.; Kumar, S.; Saritha, R.K.; Sharma, S.K.; Jain, R.K.; Baranwal, V.K. Development of a Recombinase Polymerase Amplification Assay for the Diagnosis of Banana Bunchy Top Virus in Different Banana Cultivars. Arch. Virol. 2017, 162, 2791–2796. [Google Scholar] [CrossRef]
- Naveen, K.P.; Bhat, A.I. Reverse Transcriptase Loop-Mediated Isothermal Amplification and Reverse Transcriptase Recombinase Amplification Assays for Rapid and Sensitive Detection of Cardamom Vein Clearing Virus. 3 Biotech 2020, 10, 250. [Google Scholar] [CrossRef]
- Zeng, R.; Luo, J.; Gao, S.; Xu, L.; Song, Z.; Dai, F. Rapid Detection of Cucumber Green Mottle Mosaic Virus by Reverse Transcription Recombinase Polymerase Amplification. Mol. Cell. Probes 2019, 43, 84–85. [Google Scholar] [CrossRef]
- Srivastava, N.; Kapoor, R.; Kumar, R.; Kumar, S.; Saritha, R.K.; Kumar, S.; Baranwal, V.K. Rapid Diagnosis of Cucumber Mosaic Virus in Banana Plants Using a Fluorescence-Based Real-Time Isothermal Reverse Transcription-Recombinase Polymerase Amplification Assay. J. Virol. Methods 2019, 270, 52–58. [Google Scholar] [CrossRef]
- Kumar, R.; Kaundal, P.; Tiwari, R.K.; Siddappa, S.; Kumari, H.; Lal, M.K.; Naga, K.C.; Sharma, S.; Sagar, V.; Kumar, M. Establishment of a One-Step Reverse Transcription Recombinase Polymerase Amplification Assay for the Detection of Potato Virus S. J. Virol. Methods 2022, 307, 114568. [Google Scholar] [CrossRef]
- Babujee, L.; Witherell, R.A.; Mikami, K.; Aiuchi, D.; Charkowski, A.O.; Rakotondrafara, A.M. Optimization of an Isothermal Recombinase Polymerase Amplification Method for Real-Time Detection of Potato Virus Y O and N Types in Potato. J. Virol. Methods 2019, 267, 16–21. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, R.; Nie, X.; Zhong, Z.; Li, C.; Li, K.; Huang, W.; Fu, X.; Liu, J.; Nie, B. Rapid and Sensitive Detection of Potato Virus Y by Isothermal Reverse Transcription-Recombinase Polymerase Amplification Assay in Potato. Mol. Cell. Probes 2020, 50, 101505. [Google Scholar] [CrossRef] [PubMed]
- Babu, B.; Washburn, B.K.; Miller, S.H.; Poduch, K.; Sarigul, T.; Knox, G.W.; Ochoa-Corona, F.M.; Paret, M.L. A Rapid Assay for Detection of Rose Rosette Virus Using Reverse Transcription-Recombinase Polymerase Amplification Using Multiple Gene Targets. J. Virol. Methods 2017, 240, 78–84. [Google Scholar] [CrossRef]
- Jiao, Y.; Jiang, J.; An, M.; Xia, Z.; Wu, Y. Recombinase Polymerase Amplification Assay for Rapid Detection of Maize Chlorotic Mottle Virus in Maize. Arch. Virol. 2019, 164, 2581–2584. [Google Scholar] [CrossRef] [PubMed]
- Mohandas, A.; Bhat, A.I. Recombinase Polymerase Amplification Assay for the Detection of Piper Yellow Mottle Virus Infecting Black Pepper. Virusdisease 2020, 31, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.; Bömer, M.; Nkere, C.; Lava Kumar, P.; Seal, S.E. Rapid and Specific Detection of Yam Mosaic Virus by Reverse-Transcription Recombinase Polymerase Amplification. J. Virol. Methods 2015, 222, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Onozuka, N.; Ohki, T.; Oka, N.; Maoka, T. One-Step Real-Time Multiplex Reverse Transcription-Polymerase Chain Reaction Assay with Melt Curve Analysis for Detection of Potato Leafroll Virus, Potato Virus S, Potato Virus X, and Potato Virus Y. Virol. J. 2021, 18, 131. [Google Scholar] [CrossRef]
- Lobato, I.M.; O’Sullivan, C.K. Recombinase Polymerase Amplification: Basics, Applications and Recent Advances. Trends Anal. Chem. 2018, 98, 19. [Google Scholar] [CrossRef]
- Yamanaka, E.S.; Tortajada-Genaro, L.A.; Maquieira, Á. Low-Cost Genotyping Method Based on Allele-Specific Recombinase Polymerase Amplification and Colorimetric Microarray Detection. Microchim. Acta 2017, 184, 1453–1462. [Google Scholar] [CrossRef]
- Mayboroda, O.; Benito, A.G.; del Rio, J.S.; Svobodova, M.; Julich, S.; Tomaso, H.; O’Sullivan, C.K.; Katakis, I. Isothermal Solid-Phase Amplification System for Detection of Yersinia Pestis. Anal. Bioanal. Chem. 2016, 408, 671–676. [Google Scholar] [CrossRef]
- Bhat, A.I.; Aman, R.; Mahfouz, M. Onsite Detection of Plant Viruses Using Isothermal Amplification Assays. Plant Biotechnol. J. 2022, 20, 1859–1873. [Google Scholar] [CrossRef]
- James, A.; MacDonald, J. Recombinase Polymerase Amplification: Emergence as a Critical Molecular Technology for Rapid, Low-Resource Diagnostics. Expert Rev. Mol. Diagn. 2015, 15, 1475–1489. [Google Scholar] [CrossRef] [PubMed]
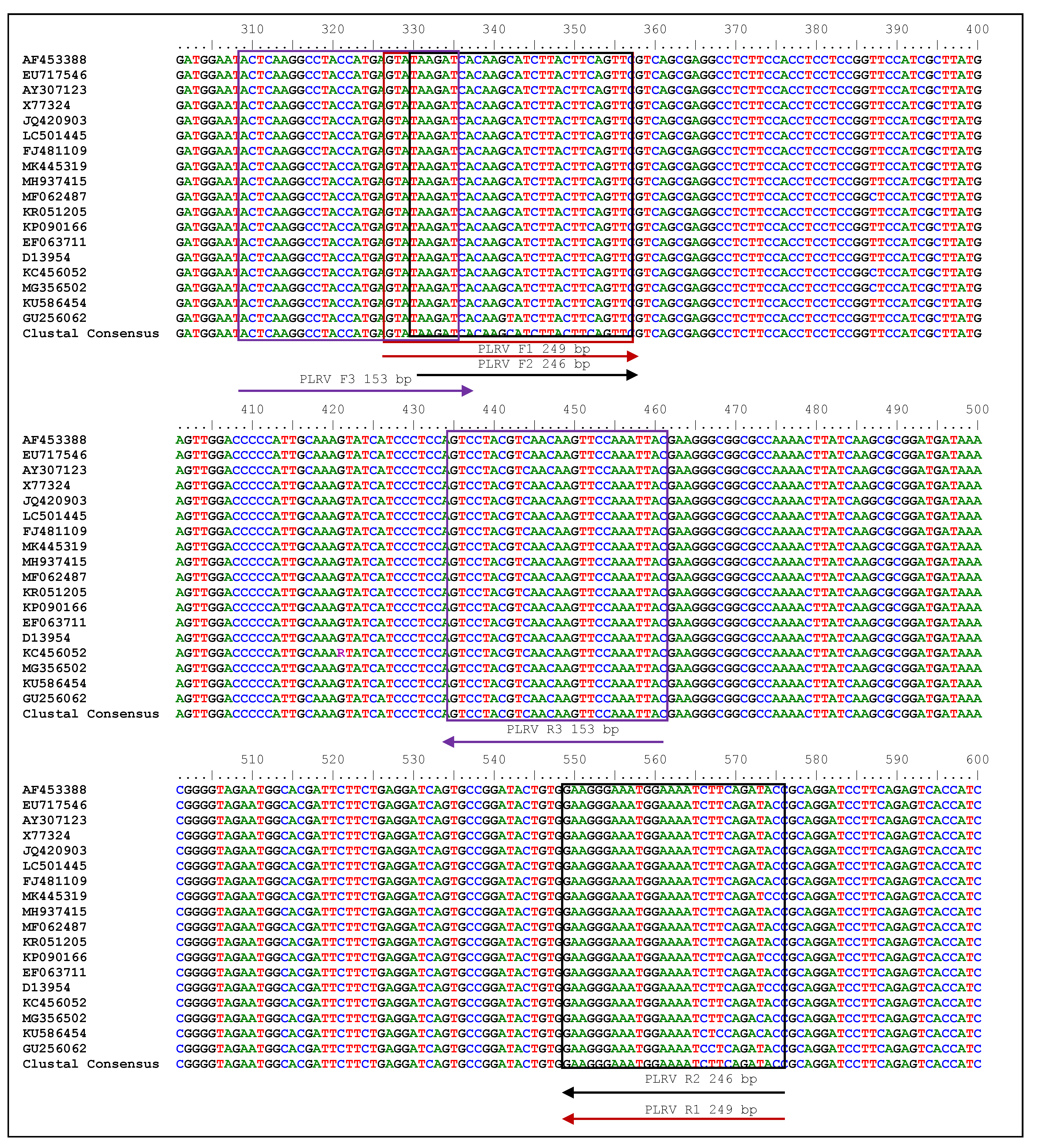




| Target Virus | Primer Set No. | Primers | Polarity | Sequence 5′–3′ | Bases | Target Position on Coat Protein Gene | Position on Accession Number in NCBI GeneBank (NC_001747.1) | GC (%) | Tm (°C) | Amplicon Size (bp) |
|---|---|---|---|---|---|---|---|---|---|---|
| PLRV | A | LRRPAF1 | Sense | GTATAAGATCACAAGCATCTTACTTCAGTTC | 31 | 327–357 | 4019–4267 | 35.5 | 61.3 | 249 |
| LRRPAR1 | Antisense | GTATCTGAAGATTTTCCATTTCCCTTC | 27 | 548–575 | 37 | 62.5 | ||||
| B | LRRPAF2 | Sense | TAAGATCACAAGCATCTTACTTCAGTTC | 28 | 330–357 | 4022–4267 | 35.7 | 60.5 | 246 | |
| LRRPAR2 | Antisense | GTATCTGAAGATTTTCCATTTCCCTTC | 27 | 548–575 | 37 | 62.5 | ||||
| C | LRRPAF3 | Sense | ACTCAAGGCCTACCATGAGTATAAGAT | 27 | 309–335 | 4001–4183 | 40.7 | 60.9 | 153 * | |
| LRRPAR3 | Antisense | GTAATTTGGAACTTGTTGACGTAGGACT | 28 | 434–461 | 39.3 | 63 |
| Field Sample No. | One-Step RT-PCR [12] | One Step RT-RPA with Primer Pair C (LRRPAF3/R3) under Heating Block/Water Bath | One Step RT-RPA with Primer Pair C (LRRPAF3/R3) under Heating Block/Water Bath |
|---|---|---|---|
| Leaves | Purified RNA | Purified RNA | Crude RNA (Cellular Disc-Mediated RNA Extract with Added 1.0% Sodium Sulphite) |
| 1 | + | + | + |
| 2 | + | + | + |
| 3 | + | + | − |
| 4 | − | − | − |
| 5 | + | + | + |
| 6 | + | + | + |
| 7 | + | + | + |
| 8 | + | + | + |
| 9 | + | + | + |
| 10 | + | + | + |
| 11 | − | − | − |
| 12 | + | + | + |
| 13 | + | + | + |
| 14 | + | + | + |
| 15 | − | − | − |
| Healthy Control | − | − | − |
| Negative Control | − | − | − |
| Positive Control | + | + | + |
| Field Sample No. | One-step RT-PCR [12] | One-step RT-RPA with primer pair C (LRRPAF3/R3) under thermal Cycler | One step RT-RPA with primer pair C (LRRPAF3/R3) under heating block/water bath |
| Tubers | Purified RNA | Purified RNA | Purified RNA |
| 1 | + | + | + |
| 2 | + | + | + |
| 3 | + | + | + |
| 4 | + | + | − |
| 5 | − | − | − |
| 6 | + | + | + |
| 7 | + | + | + |
| 8 | + | + | + |
| 9 | + | + | + |
| 10 | + | + | + |
| Healthy Control | − | − | − |
| Positive Control | + | + | + |
| Sample No. | Location/State | Cultivar/Variety | DAS-ELISA | One-Step RT-PCR [12] | One Step RT-RPA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RNA | RNA | Crude RNA | |||||||||
| Leaves | Dormant Tubers | Sprouted Tubers | Leaves | Dormant Tubers | Leaves | Dormant Tubers | Leaves | Dormant Tubers | |||
| 1 | Gujarat | Kufri Pukhraj | − | − | − | − | − | + | − | + | − |
| 2 | Gujarat | Kufri Jyoti | + | − | − | + | + | + | + | + | + |
| 3 | Punjab | Kufri Pukhraj | − | − | − | − | − | + | − | + | − |
| 4 | Punjab | Kufri Badshah | − | − | − | + | + | + | + | + | − |
| 5 | Haryana | Kufri Chandramukhi | + | − | − | + | + | + | + | + | + |
| 6 | Haryana | Kufri Jyoti | + | − | + | + | + | + | + | + | + |
| 7 | Haryana | Kufri Bahar | − | − | − | − | − | + | − | + | − |
| 8 | Himachal Pradesh | Kufri Jyoti | − | − | − | − | − | + | − | + | − |
| 9 | Himachal Pradesh | Kufri Himalini | − | − | − | − | − | + | − | + | − |
| 10 | Himachal Pradesh | Kufri Chandramukhi | + | − | − | + | + | + | + | + | + |
| 11 | Uttar Pradesh | Kufri Pukhraj | + | − | − | + | − | + | − | + | + |
| 12 | Uttar Pradesh | Kufri Chipsona 1 | + | − | + | + | + | + | + | + | + |
| 13 | Uttar Pradesh | Kufri Chipsona 4 | − | − | − | + | − | + | − | + | − |
| 14 | Uttar Pradesh | Kufri Bahar | − | − | − | + | − | + | + | + | + |
| 15 | Bihar | Kufri Sindhuri | − | − | − | − | − | + | + | + | − |
| 16 | Bihar | Kufri Chandramukhi | − | − | − | + | − | + | + | + | − |
| 17 | Bihar | Kufri Anand | − | − | − | − | − | − | − | − | − |
| 18 | Madhya Pradesh | Kufri Pukhraj | + | − | + | + | + | + | + | + | + |
| 19 | Madhya Pradesh | Kufri Lauvkar | − | − | − | − | − | + | − | + | − |
| 20 | Madhya Pradesh | Kufri Chandramukhi | − | − | − | − | − | + | − | + | − |
| 21 | West Bengal | Kufri Jyoti | + | − | − | + | + | + | + | + | + |
| 22 | West Bengal | Kufri Chandramukhi | − | − | − | − | − | + | + | + | − |
| 23 | West Bengal | Kufri Pukhraj | + | − | − | + | + | + | + | + | + |
| 24 | West Bengal | Kufri Ashoka | + | − | + | + | + | + | + | + | + |
| 25 | Meghalaya | Kufri Megha | + | − | − | + | + | + | + | + | + |
| 26 | Meghalaya | Kufri Giriraj | − | − | − | − | − | + | − | + | − |
| 27 | Meghalaya | Kufri Jyoti | + | − | − | + | + | + | + | + | + |
| Total | 13 | 0 | 04 | 16 | 12 | 26 | 16 | 26 | 13 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, R.; Kaundal, P.; Tiwari, R.K.; Lal, M.K.; Kumari, H.; Kumar, R.; Naga, K.C.; Kumar, A.; Singh, B.; Sagar, V.; et al. Development of Reverse Transcription Recombinase Polymerase Amplification (RT-RPA): A Methodology for Quick Diagnosis of Potato Leafroll Viral Disease in Potato. Int. J. Mol. Sci. 2023, 24, 2511. https://doi.org/10.3390/ijms24032511
Kumar R, Kaundal P, Tiwari RK, Lal MK, Kumari H, Kumar R, Naga KC, Kumar A, Singh B, Sagar V, et al. Development of Reverse Transcription Recombinase Polymerase Amplification (RT-RPA): A Methodology for Quick Diagnosis of Potato Leafroll Viral Disease in Potato. International Journal of Molecular Sciences. 2023; 24(3):2511. https://doi.org/10.3390/ijms24032511
Chicago/Turabian StyleKumar, Ravinder, Priyanka Kaundal, Rahul Kumar Tiwari, Milan Kumar Lal, Hema Kumari, Rakesh Kumar, Kailash Chandra Naga, Awadhesh Kumar, Brajesh Singh, Vinay Sagar, and et al. 2023. "Development of Reverse Transcription Recombinase Polymerase Amplification (RT-RPA): A Methodology for Quick Diagnosis of Potato Leafroll Viral Disease in Potato" International Journal of Molecular Sciences 24, no. 3: 2511. https://doi.org/10.3390/ijms24032511
APA StyleKumar, R., Kaundal, P., Tiwari, R. K., Lal, M. K., Kumari, H., Kumar, R., Naga, K. C., Kumar, A., Singh, B., Sagar, V., & Sharma, S. (2023). Development of Reverse Transcription Recombinase Polymerase Amplification (RT-RPA): A Methodology for Quick Diagnosis of Potato Leafroll Viral Disease in Potato. International Journal of Molecular Sciences, 24(3), 2511. https://doi.org/10.3390/ijms24032511







