Abstract
Autoimmune thyroid disease (AITD), including Graves’ disease (GD) or Hashimoto’s thyroiditis (HT), occurs due to genetic susceptibility and environmental factors, among which the role of stressful events remains controversial. This study investigated the relationship between the number and impact of stressful life events in AITD patients with selenium status, and the Th1/Th2/Th17 immune response. The study population included three groups: HT (n = 47), GD (n = 13), and a control group (n = 49). Thyroid function parameters, autoantibody levels, and the plasma levels of cytokines, selenium, selenoprotein P (SeP), and glutathione peroxidase 3 (GPx) activity were measured. Participants filled out the Life Experiences Survey. No significant differences in the number of stressful life events were found among the patients with HT, GD, and the controls. A higher (median (interquartile range)) negative stress level (8 (4–12)) than a positive stress level (3 (1–9)) was found in the HT group. The HT group showed a correlation between SeP and the positive stress level: rs = −0.296, p = 0.048, and the GD group between GPx and the negative stress level (rs = −0.702, p = 0.011). Significant positive correlations between thyroid peroxidase antibody level and the total number of major life events (p = 0.023), the number of major life events in the last 7–12 months, and the number of major life events with no impact and a negative stress level were found. We suggest that the measurements of Th2-related cytokines and selenoproteins could be used as biomarkers for the development of AITD in cases where stress is considered a component cause of the pathogenic mechanism of the disease.
1. Introduction
Autoimmune thyroid disease (AITD), including Graves’ disease (GD) or Hashimoto’s thyroiditis (HT), occurs due to an interaction between genetic susceptibility, which contributes approximately 70–80% in the development of thyroid autoimmunity, and various environmental factors, such as increased iodine intake, selenium deficiency, smoking status, alcohol consumption, infections, drug side effects, microbiota, and stressful life events [1,2,3]. Numerous studies on psychological stress have been inconclusive [4,5,6,7,8], but several excellent mechanism-based studies have examined the pathways the immune deviation [9,10,11] and have named stress as an exacerbating factor for autoimmune disease activity. Stress plays an important role in the response to therapy [12,13,14], and perhaps, in the aetiology as well [15,16].
HT and GD have distinct and predominant T helper (Th) 1- and Th2-mediated immune responses, respectively [17,18]. Thus, GD is characterised by increased levels of Th2-released cytokines, such as interleukin (IL)-4, IL-5, IL-6, and IL-13, which mainly mediate the humoral response-induced production of autoantibodies to the thyroid-stimulating hormone (TSH) receptor. Whereas in HT, the upregulation of Th1 cytokines, such as IL-2, IL-1β, interferon (IFN)-γ, and tumour necrosis factor (TNF)-α, leads to cell-mediated immunity and causes thyroid cell apoptosis [19]. Recently, it was discovered that Th17 cells also play a role in the pathogenesis of thyroid autoimmunity [20,21,22].
Regardless of the early paradigm that considered the stress-related effects as primarily immunosuppressive, more recent findings provide evidence that both acute and chronic stress might affect the homeostasis of the human immune system and induce the development of an autoimmune disease. During stress, activation of the hypothalamic-pituitary-adrenal axis and the sympathoadrenal system causes an oversecretion of glucocorticoids and catecholamines, which both have a similar immunoregulatory effect and induce the Th1/Th2 imbalance characteristic of GD. Stress hormones suppress IL-2 production by antigen-presenting cells and enhance the secretion of IL-4 and IL-10 by Th2 cells, thereby directing immunity towards Th2 responses [9,10,23]. In addition, glucocorticoids inhibit the synthesis of IL-1, IL-2, TNFα, and IFNγ, and stimulate IL-4, IL-10, and IL-13 production [24]. Altogether, this may trigger the development of GD, which is considered to be a Th2-dependent autoimmune disease. Accordingly, the role of psychosocial stress in the etiopathogenesis of GD has been suggested by numerous studies. Stressful life events trigger both the onset and recurrence of hyperthyroidism in GD patients who were followed up for at least 5 years after antithyroid drug withdrawal [25]. Several anecdotal GD cases reported an acute stress event prior to the occurrence of the disease [26]. However, a prospective 5-year follow-up study with 790 participants who had positive family anamnesis for AITD, found no causal association between stress and GD [27]. Thus, the role of stressful events in the development of GD remains controversial.
The relationship between stress and HT has not been thoroughly explored. According to several prospective and cross-sectional studies and observations, stress does not have any triggering role in HT [7,28]. In contrast, a connection between stress and HT was found in other studies, addressing the impact of chronic stress-induced trauma in childhood [29,30,31]. However, it has been hypothesised that in individuals with a susceptibility to thyroid autoimmunity, an enhancement of cell-mediated immune function due to a “return shift” from the Th2 to Th1 immune response could occur following stress [32], so there is a hypothetical mechanism by which stress can contribute to the pathogenesis of HT.
Another point of intersection between psychosocial stress and AITD is the status of selenium, an essential trace element necessary for the synthesis of the amino acid selenocysteine, which is integrated into selenoproteins [33]. The physiological roles of selenoproteins are wide-ranging. The most important examples could be the glutathione peroxidase family and the thioredoxin reductases that protect against oxidative stress damage, endoplasmic reticulum stress, and inflammatory reactions [34]. It is well-established that psychological stress induces neuronal damage and dysfunction via oxidative stress. In addition, during stress, elevated levels of glucocorticoids can downregulate antioxidant enzymes [35], exacerbating the neurobehavioural consequences of stress. On the other hand, the thyroid gland has the highest concentration of selenium in the body. Notably, thyroid hormone synthesis produces an excess of hydrogen peroxide, which can modify antigens and damage thyrocytes [36], thus representing a potential initiating pathway in the pathogenesis of AITD. Therefore, low selenium levels and declining selenoprotein biosynthesis might aggravate the damage to thyrocytes. One of the most reliable parameters of selenium status is selenoprotein P (SeP), which is rich in selenocysteine and comprises approximately 25% of the body selenium, thereby maintaining selenium homeostasis [37,38].
In this study, we aimed to investigate the relationship between the number and impact of stressful life events in AITD patients, the Th1/Th2/Th17 immune response, and the selenium parameters, to better understand the effects of stress-related mechanisms on the immune system.
2. Results
The demographic and biochemical characteristics of the participants are presented in Table 1. All three study groups–HT, GD, and controls–were similar in age and gender. Thyroid function (thyroid stimulating hormone (TSH), free thyroxine (FT4),and free triiodothyronine (FT3)) and thyroid autoimmunity (thyroid peroxidase antibody (TPO-Ab), thyroglobulin antibody (Tg-Ab), and TSH-receptor antibody (TR-Ab) level) results showed significant differences between the groups (all p < 0.001 and the Kruskal–Wallis test) in accordance with the disease characteristics. Post hoc tests showed significant differences in these parameters between all groups, except FT3 in controls vs. HT (p = 0.676), TPO-Ab in GD vs. HT (p = 0.964), and Tg-Ab in GD vs. HT (p = 0.921). No significant differences in serum selenium (Se), glutathione peroxidase (GPx), or selenoprotein P (SeP) levels were found among the patients with HT, the patients with GD, and the controls (all p > 0.05). A low serum selenium, as defined by the selenium level <80 µg/L, was found in 38.8% of the controls, 42.6% of the HT group patients, and 61.5% of the GD group patients. Furthermore, a significant positive correlation between selenium and selenoprotein P was found in the HT patient group (rs = 0.392 and p = 0.007). No significant differences in the number of stressful life events were found among the patients with HT, the patients with GD, and the controls; however, negative stress levels tended to be higher in the patients with HT and GD than in the controls (Table 1). Furthermore, a significantly higher negative stress level compared to the positive stress level was found in the HT group (p = 0.023), but the difference was not significant in the other study groups (p = 0.406 in the control group and p = 0.505 in the GD group).

Table 1.
Demographic and biochemical characteristics of the study groups. Numerical data presented as median (interquartile range).
Further correlations between the number of major life events from the Life Experiences Survey and selenium parameters were analysed within the three study groups. There were no significant correlations in the control group (all p > 0.05). The HT group showed significant negative correlations between SeP and positive stress levels (Figure 1a). The GD group showed significant negative correlations between GPx and negative stress levels (Figure 1b).
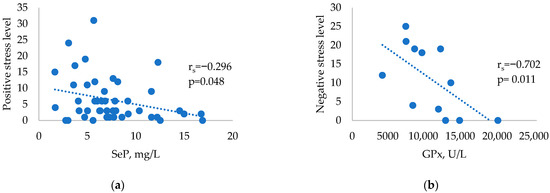
Figure 1.
Correlations between selenium parameters and stressful events in HT and GD patients: (a) Correlation between SeP and positive stress levels in HT patients. (b) Correlation between GPx and negative stress levels in patients with GD.
Plasma levels of Th17-related cytokines, Th17-promoting cytokines, Treg-associated cytokines, Th1-related cytokines, and Th2-related cytokines did not differ significantly between the study groups (Table 2).

Table 2.
Plasma levels of cytokines.
Cytokine levels were analysed in relation to stressful life events in the three study groups and showed no significant correlations in the GD patients (p > 0.05). There was a significant negative correlation between the Th17-related cytokine IL-22 and stressful life events 7–12 months prior to the HT diagnosis (Figure 2a). Two significant Th2-related cytokine correlations with positive stress scores were found in the control group (Figure 2b,c).
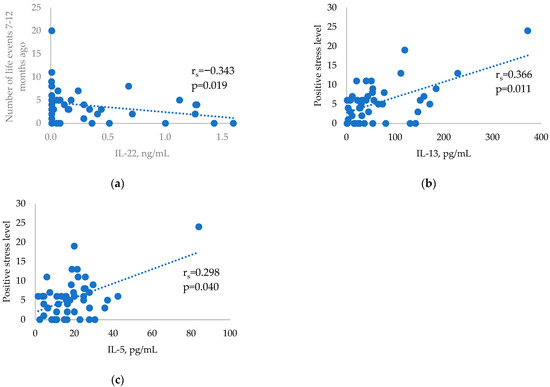
Figure 2.
Correlations between selected cytokines and stressful life events in the HT and control groups: (a) Correlation between IL-22 and stressful life events occurring 7–12 months before HT diagnosis. (b) Correlation between positive stress levels and IL-13 in the control group. (c) Correlation between positive stress levels and IL-5 in the control group.
Thyroid autoantibody level correlations with different parameters of the Life Experiences Survey were also analysed. Significant correlations between various stress parameters and TPO-Ab and Tg-Ab were found (Table 3).

Table 3.
Correlations between various stress parameters and TPO-Ab and Tg-Ab.
When cytokines IL-13, IL-22, and IL-5 were mutually adjusted in the logistic regression analysis, only IL-22 showed a significant association with GD (p = 0.031). The higher level of IL-22 was associated with lower odds of GD (the OR of one unit IL-22 increase was 0.03 (95%CI 0.01–0.73)), which became non-significant after the other factors were added to the model. The total number of major life events was neither a significant factor itself, nor did it affect the strength of the association between cytokines and AITDs. Negative life events were associated with higher odds of HT: the OR associated with one event increase in the number of negative events was 1.10 (95%CI 1.01–1.19). The timing of the stressful life events, in the last six months or earlier, did not have any effect. GPx did not show any association with AITDs, but selenium showed a borderline significant (p = 0.05) association with a lower probability of GD: OR 0.98 (95%CI 0.95–1.00). The final model is presented in Table 4.

Table 4.
Odds ratio of AITDs in association with selected cytokines, the number of negative major life events, and serum selenium: multiple regression model.
3. Discussion
Chronic stress has been shown to be associated with systemic inflammation, leading to increased proinflammatory cytokine levels, while stress-induced cytokine changes may be implicated in the development of autoimmune diseases, including AITD [39]. Although some authors indicate that only undesirable events lead to psychological impairment [40,41], it can be argued that in the same way, all life changes require adaptation and may be stressful at the individual level. We analysed possible relationships between the number and impact of stressful life events and the plasma levels of several Th1, Th2, and Th17 cytokines in patients with new-onset AITD. No significant differences in the Th1/Th2/Th17/Treg cytokine levels were found among the HT patients, GD patients, and controls. In contrast to AITD, which is an organ-specific autoimmune disorder, the aforementioned cytokines are secreted by a broad spectrum of immune and nonimmune cells throughout the body; therefore, their production is not organ-specific. Recently, after analysing the expression patterns of Th17-associated cytokines within the thyroid tissue, the expression level of IL-17 in thyrocytes was found to be significantly higher in the HT and GD patients than in controls [42]. However, our results provide evidence for a negative correlation between the plasma IL-22 levels and the number of stressful life events that took place 7–12 months prior to a HT diagnosis.
IL-22, a member of the IL-10 family of cytokines, is produced by different types of lymphocytes, including Th17 and Th22 cells, natural killer T cells, and γδ T cells [43]. Although IL-22 has been associated with the development of autoimmune and inflammatory diseases, such as psoriasis and rheumatoid arthritis, it is a cytokine with dual pro- and anti-inflammatory activities. IL-22 maintains the barrier function and hosts the defence against pathogens by inducing the expression of antimicrobial peptides at mucosal surfaces [44]. Recently, IL-22 has been named a potent endogenous suppressor of oxidative and endoplasmic reticulum stress [45]. On the other hand, higher IL-22 serum levels were found in untreated euthyroid HT patients than in those patients with nodular goitre or in healthy subjects [46]. It is well-known that all life event stressors stimulate physiological responses, releasing glucocorticoids and catecholamines, and promoting a shift in the cytokine balance. Glucocorticoids were shown to inhibit IL-22 production in the human and mice group three innate lymphocytes, and this suppression was glucocorticoid-dependent [47]. The correlation between IL-22 levels and stressful life events is complex, and so it requires further investigation.
In addition, positive relationships between IL-13 and IL-5 and life events with positive stress levels were found in the control group, indicating that a positive stress level also affects Th2 proinflammatory cytokine responses. An elevated IL-13 is able to suppress the synthesis of Th1 proinflammatory cytokines, such as TNFα and IFNγ, as well as the production of several macrophage- and monocyte-derived inflammatory cytokines [48]. In addition, IL-13 protects beta-cells against IL-1β-induced apoptosis. For this reason, IL-13 is classified as an anti-inflammatory interleukin [49]. In this study, we also found a negative correlation between GPx and negative stress levels, which was only significant in patients with GD, a predominantly Th2 autoimmune disease. Lower levels of GPx have been described to shift the cytokine production towards the Th2 cytokine response [50], and vice versa, higher levels of GSH induce the differentiation of naïve CD4+ T cells to Th1 cells [51]. Together, these considerations suggest that both positive and negative stress may play a role in the pathogenic mechanism of Th2-mediated diseases.
Although no association between the presence of TPO antibodies and self-reported stress in 759 euthyroid subjects was previously observed in the study by Strieder et al. [28], we found positive correlations between the number of major life events and life events with a negative impact on both TPO and Tg antibody levels, suggesting that stress might be involved in the progression/course of HT. Because the precise onset of the disease is generally unknown, it is difficult to determine the mechanism of stress for triggering HT.
In contrast to GD, the development of HT is mainly associated with the cellular immune responses mediating cytotoxicity and inflammation via increased Th1 and Th17 activity; however, humoral-mediated immune mechanisms are also implicated in the pathogenesis of HT [19]. TPO antibodies have demonstrated several pathogenic effects, such as the complement system activation and the induction of oxidative stress. They are involved in thyrocyte damage by both antibody- and complement-dependent cytotoxicity mechanisms [52]. Moreover, lower antioxidant parameters and higher oxidant parameters were detected in euthyroid untreated HT patients, suggesting that thyroid antibodies are independent risk factors for developing oxidative stress, irrespective of the thyroid function [53]. Selenium, in the form of selenoproteins, has relevant antioxidant properties, and an adequate selenium status not only reduces the level of thyroid antibodies [54,55], but also lowers the risk of AITD [56]. We did not observe any significant differences in plasma selenium, SeP, or GPx levels among the groups, but it is important to highlight that the average selenium status in all groups (87.2 µg/L 71.3 µg/L and 84.6 µg/L) was lower than the recommended optimal level of 125 µg/L [57]. Optimal and effective synthesis of GPx requires a plasma Se level of at least 100 µg/L [58], but concentrations of approximately 120 µg/L are required for the maximal expression of SeP, which reflects the functional selenium pool [59]. Therefore, our data demonstrate selenium concentrations that are considered deficient for optimal antioxidant enzyme expression and function. Various exogenous selenium-containing compounds with immunomodulatory and anti-inflammatory properties are described to contribute to the regulation of diseases of the immune system [60]. Recently, a source of Se that is receiving increased interest is nanoparticles (SeNPs), which have shown potential therapeutic benefits in various diseases mediated by oxidative stress and inflammation systems and have demonstrated the ability to modulate the functional state of neutrophils in mice [61]. Another interesting aspect of our study is the negative correlation between the number of stressful events and the concentration of selenoproteins, which supports the taking of the measurements for selenoprotein levels/activity instead of plasma selenium because the selenium content also includes the functionally inactive selenium incorporated within the protein structure as selenmethionine [62].
Interestingly, the effects of stress management were recently studied in HT patients, and this study demonstrated a significant reduction in the anti-TG antibody titres and stress levels after an 8-week stress management intervention [12]. Although the implementation of stress management in the treatment strategy for HT patients is still questionable, we believe that patients might benefit from such interventions.
4. Materials and Methods
This cross-sectional study was implemented at the Riga East University Hospital, from September to December 2020. The study was carried out after receiving approval by the ethics committee of Riga East University Hospital Research Committee and the Central Medical Ethics Committee, Latvia (Decision No. 01-29.1/5033).
This study enrolled 109 adult participants (16 males and 93 females). The inclusion criteria were: (1) newly diagnosed cases of AITD: Hashimoto’s thyroiditis (positive circulating antibodies to thyroid antigens: Thyroid peroxidase antibody (TPO-Ab) and/or Thyroglobulin antibody (Tg-Ab) values greater than 100 IU/mL were considered diagnostic) or Graves’ disease (decreased TSH levels and positive TSH-receptor antibodies (TR-Ab); (2) healthy controls–healthy individuals who do not have the following diseases or special diets: liver or kidney disorders, a vegetarian or vegan diet, any other autoimmune disorder, and a participant positive for antinuclear antibodies (ANA) or tissue transglutaminase IgA (tTg-IgA) antibodies; (3) the patient has not used medications affecting the thyroid function. The exclusion criteria were: (1) pregnancy; (2) if the participant is less than 18 years old; (3) if the patient is taking medications that can affect the thyroid/immune function–Amiodarone, for example, corticosteroids, antidepressants, etc. The study population was divided into 3 groups: Hashimoto’s thyroiditis group (n = 47), Graves’ disease group (n = 13), and control group (n = 49). All participants of the study provided written informed consent before enrolment and had a blood sample collected.
4.1. Materials of the Study
Peripheral blood samples were collected from all 109 participants. Thyroid function tests (the levels of serum-free thyroxine (FT4), free triiodothyronine (FT3), and TSH) and the levels of TPO-Ab and Tg-Ab were measured using chemiluminescence immunoassays (Siemens, Malvern, PA, USA). Reference values were as follows: FT4, 0.7–1.48 ng/dL; FT3, 0.2–0.44 ng/dL; TSH, 0.35–4.94 μIU/mL; TPO-Ab, 0–60 IU/mL; and Tg-Ab, 0–40 U/mL. Plasma levels of TR-Ab, ANAs, and tTG-IgA autoantibodies were detected by ELISA (Pharmacia Diagnostics Freiburg, Germany), reference range: TR-Ab, 0–1.58 IU/L; ANA, reference range was “negative”; tTG-IgA, 0–10.0 U/mL. Analyses of clinical variables were performed in an accredited diagnostic hospital laboratory using automated diagnostic equipment.
4.2. Plasma Levels of Cytokines
Th17-related cytokines (IL-17a and IL-22), Th17-promoting cytokines (IL-23 and IL-6), Treg-associated cytokines (IL-10), Th1-related cytokines (IFN-γ and IL-2), and Th2-related cytokines (IL-4, IL-5, and IL-13) were analysed in duplicates with the plasma samples in EDTA, and were analysed for immunological markers using xMAP technology (Magpix system, Luminex Corporation, Austin, TX, USA).
4.3. The Plasma Selenium
Concentration was determined fluorometrically using a fluorescence spectrophotometer “Cary Eclipse” (Varian, Inc., Houten, The Netherlands). Interlaboratory quality control was conducted by employing two standards—selenium AAS solution (Aldrich, St. Louis, MO, USA, Cat# 24, 792-8) and Seronorm TE Serum Level I (Sero AS, Cat# 201 405, Billingstad, Norway)—for the Seronorm™ Trace Elements-Controls Programme. External Quality Assessment Services were performed by Labquality Oy, Finland.
4.4. Determination of Glutathione Peroxidase (GPx) 3 and (EC) 1.11.1.9 Activity
The activity of GPx was evaluated in heparinised whole blood by the conventional method of Paglia and Valentine (50), using commercial tests manufactured by Randox Laboratories (UK, Antrium) in a RXDaytona analyser. GPx from the sample of heparinised whole blood catalysed the oxidation of GSH (4 mmol/L) by cumene hydroperoxide (0.18 mmol/L). Oxidised glutathione (GSSG) was converted to GSH in the reaction catalysed by glutathione reductase (>0.5 U/L) in the presence of NADPH (reduced form of nicotinamide adenine dinucleotide phosphate) (0.34 mmol/L), which led to a decrease in absorbance, which was further measured at 340 nm. One unit corresponds to the amount of enzyme produced by 1.0 μMNADPH oxidation at NADP+1 min at 340 nm at 37 °C, and GPx activity was expressed as U/L of the haemolysate.
4.5. Selenoprotein P (SeP)
Concentrations were measured using Spark® (Tecan Group Ltd.) multimode microplate reader by a validated commercial SELENOP-specific ELISA (Cusabio, Wuhan, China) for human cells and a rat selenoprotein P (Selenop) ELISA kit (Cusabio), according to the instructions of supplier.
4.6. Stress Parameters
To assess the parameters of stress, participants were asked to fill out the Life Experiences Survey [63] to quantify the emotional impact of important life events. The Life Experiences Survey counts the total number of major life events experienced in the last 12 months (checklist of 47 possible events). The participant separately scored the positive or negative impact of each experienced life event, rated on a scale of zero (meaning no impact at all) to three (significant positive or negative impact). From this scale, the total scores of positive (e.g., outstanding personal achievement) and negative (e.g., death of a close family member) impacts were summarised and thus, the total scores of self-reported psychosocial stresses were calculated. The parameters of self-reported psychosocial stress were both the number of stressful life events (total, 0–6 months ago, and 7–12 months ago) and the score of the self-assessed impact of these life events (positive, negative, and none).
4.7. Statistics
For descriptive statistics, the data are presented as the median values and interquartile ranges (IQRs). A nonparametric Kruskal–Wallis test was used to detect the differences between the groups. A post hoc analysis to compare the two independent samples was performed using the Mann–Whitney test. The Wilcoxon signed-rank test was used to compare the sets of scores without a normal distribution that came from the same participants. A nonparametric Spearman’s correlation analysis was performed to determine the relationships of the studied parameters in each subgroup of the study cohort. A multiple logistic regression analysis was used to explore the associations between measures of stressful life events, cytokines, and selenium as the independent factors and the presence of AITDs as the dependent variable. A p-value < 0.05 was considered statistically significant for all statistical tests. Statistical analysis of the data was performed in IBM SPSS version 26.0.
5. Conclusions
Even though the autoimmune thyroid disease patients did not differ in the number of stressful life events compared to the control group, a significantly higher negative stress level was found in the Hashimoto’s thyroiditis group. In addition, the thyroid peroxidase antibody level correlated with the number of stressful life events, providing evidence for stress-induced changes in immune pathways. We suggest measurements of Th2-related cytokines and selenoproteins as biomarkers for the development of autoimmune thyroid disease in cases where stress is considered a component cause of the pathogenic mechanism of the disease.
Author Contributions
Conceptualisation, I.V., I.K. and T.Z.; methodology, M.D., I.K., T.Z. and A.S.; investigation, G.G., M.D. and A.S.; data curation, I.S.; writing—original draft preparation, I.V., T.Z., I.S., S.U.-E. and D.G.; writing—review and editing, I.K.; visualisation, I.S. and D.G.; supervision, I.K.; funding acquisition, I.K., T.Z., S.U.-E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Latvian Council of Science, grant number lzp-2018/2-0059.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Riga East University Hospital Research Committee and the Central Medical Ethics Committee (Decision No. 01-29.1/5033).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tomer, Y.; Davies, T. Searching for the Autoimmune Thyroid Disease Susceptibility Genes: From Gene Mapping to Gene Function. Endocr. Rev. 2003, 24, 694–717. [Google Scholar] [CrossRef]
- Hansen, P.S.; Brix, T.H.; Iachine, I.; Kyvik, K.O.; Hegedüs, L. The relative importance of genetic and environmental effects for the early stages of thyroid autoimmunity: A study of healthy Danish twins. Eur. J. Endocrinol. 2006, 154, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Docimo, G.; Cangiano, A.; Romano, R.M.; Pignatelli, M.F.; Offi, C.; Paglionico, V.A.; Galdiero, M.; Donnarumma, G.; Nigro, V.; Esposito, D.; et al. The Human Microbiota in Endocrinology: Implications for Pathophysiology, Treatment, and Prognosis in Thyroid Diseases. Front. Endocrinol. 2020, 11, 586529. [Google Scholar] [CrossRef]
- Matos-Santos, A.; Nobre, E.L.; Costa, J.G.; Nogueira, P.J.; Macedo, A.; Galvão-Teles, A.; de Castro, J.J. Relationship between the number and impact of stressful life events and the onset of Graves’ disease and toxic nodular goitre. Clin. Endocrinol. 2001, 55, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Tsatsoulis, A.; Johnson, E.; Kalogera, C.; Seferiadis, K.; Tsolas, O. The effect of thyrotoxicosis on adrenocortical reserve. Eur. J. Endocrinol. 2000, 142, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Fukao, A.; Takamatsu, J.; Murakami, Y.; Sakane, S.; Miyauchi, A.; Kuma, K.; Hayashi, S.; Hanafusa, T. The relationship of psychological factors to the prognosis of hyperthyroidism in antithyroid drug-treated patients with Graves’ disease. Clin. Endocrinol. 2003, 58, 550–555. [Google Scholar] [CrossRef]
- Effraimidis, G.; Tijssen, J.G.; Brosschot, J.F.; Wiersinga, W.M. Involvement of stress in the pathogenesis of autoimmune thyroid disease: A prospective study. Psychoneuroendocrinology 2012, 37, 1191–1198. [Google Scholar] [CrossRef]
- Damian, L.; Ghiciuc, C.M.; Dima-Cozma, L.C.; Ungureanu, M.C.; Cozma, S.; Patacchioli, F.R.; Lupusoru, C.E. No definitive evidence for a connection between autoimmune thyroid diseases and stress in women. Neuro Endocrinol. Lett. 2016, 37, 155–162. [Google Scholar]
- Blotta, M.H.; DeKruyff, R.H.; Umetsu, D.T. Corticosteroids inhibit IL-12 production in human monocytes and enhance their capacity to induce IL-4 synthesis in CD4+ lymphocytes. J. Immunol. 1997, 158, 5589–5595. [Google Scholar] [CrossRef]
- DeKruyff, R.H.; Fang, Y.; Umetsu, D.T. Corticosteroids enhance the capacity of macrophages to induce Th2 cytokine synthesis in CD4+ lymphocytes by inhibiting IL-12 production. J. Immunol. 1998, 160, 2231–2237. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Miyara, M.; Costantino, C.; Hafler, D.A. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 2010, 10, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Markomanolaki, Z.S.; Tigani, X.; Siamatras, T.; Bacopoulou, F.; Tsartsalis, A.; Artemiadis, A.; Megalooikonomou, V.; Vlachakis, D.; Chrousos, G.P.; Darviri, C. Stress Management in Women with Hashimoto’s thyroiditis: A Randomized Controlled Trial. J. Mol. Biochem. 2019, 8, 3–12. [Google Scholar] [PubMed]
- Moncayo, R.; Moncayo, H. The WOMED model of benign thyroid disease: Acquired magnesium deficiency due to physical and psychological stressors relates to dysfunction of oxidative phosphorylation. BBA Clin. 2014, 3, 44–64. [Google Scholar] [CrossRef] [PubMed]
- Yoshiuchi, K.; Kumano, H.; Nomura, S.; Yoshimura, H.; Ito, K.; Kanaji, Y.; Kuboki, T.; Suematsu, H. Psychosocial factors influencing the short-term outcome of antithyroid drug therapy in Graves’ disease. Psychosom. Med. 1998, 60, 592–596. [Google Scholar] [CrossRef]
- Porcelli, B.; Pozza, A.; Bizzaro, N.; Fagiolini, A.; Costantini, M.C.; Terzuoli, L.; Ferretti, F. Association between stressful life events and autoimmune diseases: A systematic review and meta-analysis of retrospective case-control studies. Autoimmun. Rev. 2016, 15, 325–334. [Google Scholar] [CrossRef]
- Radosavljević, V.R.; Janković, S.M.; Marinković, J.M. Stressful life events in the pathogenesis of Graves’ disease. Eur. J. Endocrinol. 1996, 134, 699–701. [Google Scholar] [CrossRef]
- Effraimidis, G.; Wiersinga, W.M. Mechanisms in endocrinology: Autoimmune thyroid disease: Old and new players. Eur. J. Endocrinol. 2014, 170, 241–252. [Google Scholar] [CrossRef]
- Fröhlich, E.; Wahl, R. Thyroid Autoimmunity: Role of Anti-thyroid Antibodies in Thyroid and Extra-Thyroidal Diseases. Front. Immunol. 2017, 8, 521. [Google Scholar] [CrossRef]
- Ramos-Leví, A.M.; Marazuela, M. Pathogenesis of thyroid autoimmune disease: The role of cellular mechanisms. Endocrinol. Nutr. 2016, 63, 421–429. [Google Scholar] [CrossRef]
- Figueroa-Vega, N.; Alfonso-Pérez, M.; Benedicto, I.; Sánchez-Madrid, F.; González-Amaro, R.; Marazuela, M. Increased circulating pro-inflammatory cytokines and Th17 lymphocytes in Hashimoto’s thyroiditis. J. Clin. Endocrinol. Metab. 2010, 95, 953–962. [Google Scholar] [CrossRef]
- Peng, D.; Xu, B.; Wang, Y.; Guo, H.; Jiang, Y. A high frequency of circulating Th22 and Th17 cells in patients with new onset graves’ disease. PLoS ONE 2013, 8, e68446. [Google Scholar] [CrossRef]
- Esfahanian, F.; Ghelich, R.; Rashidian, H.; Jadali, Z. Increased Levels of Serum Interleukin-17 in Patients with Hashimoto’s Thyroiditis. Indian J. Endocrinol. Metab. 2017, 21, 551–554. [Google Scholar]
- Chrousos, G.P.; Elenkov, I.J. Interactions of the endocrine and immune systems. In Endocrinology, 5th ed.; De Groot, L.J., Jameson, J.L., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2006; Volume 1, pp. 799–818. [Google Scholar]
- Dhabhar, F.S. Effects of stress on immune function: The good, the bad, and the beautiful. Immunol. Res. 2014, 58, 193–210. [Google Scholar] [CrossRef]
- Vita, R.; Lapa, D.; Trimarchi, F.; Benvenga, S. Stress triggers the onset and the recurrences of hyperthyroidism in patients with Graves’ disease. Endocrine 2015, 48, 254–263. [Google Scholar] [CrossRef]
- Matsubayashi, S.; Tamaí, H.; Matsumoto, Y.; Tamagawa, K.; Mukuta, T.; Morita, T.; Kubo, C. Graves’disease after the onset of panic disorder. Psychother. Psychosom. 1996, 65, 277–280. [Google Scholar] [CrossRef]
- Effraimidis, G.; Strieder, T.G.; Tijssen, J.G.; Wiersinga, W.M. Natural history of the transition from euthyroidism to overt autoimmune hypo- or hyperthyroidism: A prospective study. Eur. J. Endocrinol. 2011, 164, 107–113. [Google Scholar] [CrossRef]
- Strieder, T.G.; Prummel, M.F.; Tijssen, J.G.; Brosschot, J.F.; Wiersinga, W.M. Stress is not associated with thyroid peroxidase autoantibodies in euthyroid women. Brain Behav. Immun. 2005, 19, 203–206. [Google Scholar] [CrossRef]
- Terzidis, K.; Panoutsopoulos, A.; Mantzou, A.; Tourli, P.; Papageorgiou, G.; Saltiki, K.; Mara, C.; Alevizaki, M. Lower early morning plasma cortisol levels are associated with thyroid autoimmunity in the elderly. Eur. J. Endocrinol. 2010, 162, 307–313. [Google Scholar] [CrossRef]
- Müssig, K.; Künle, A.; Säuberlich, A.L.; Weinert, C.; Ethofer, T.; Saur, R.; Klein, R.; Häring, H.U.; Klingberg, S.; Gallwitz, B.; et al. Thyroid peroxidase antibody positivity is associated with symptomatic distress in patients with Hashimoto’s thyroiditis. Brain Behav. Immun. 2012, 26, 559–563. [Google Scholar] [CrossRef]
- Plaza, A.; Garcia-Esteve, L.; Torres, A. Childhood physical abuse as a common risk factor for depression and thyroid dysfunction in the earlier postpartum. Psychiatry Res. 2012, 200, 329–335. [Google Scholar] [CrossRef]
- Tsatsoulis, A. The role of stress in the clinical expression of thyroid autoimmunity. Ann. N. Y. Acad. Sci. 2006, 1088, 382–395. [Google Scholar] [CrossRef] [PubMed]
- Arnér, E.S.J. Common modifications of selenocysteine in selenoproteins. Essays Biochem. 2020, 64, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef] [PubMed]
- Torres, D.J.; Alfulaij, N.; Berry, M.J. Stress and the Brain: An Emerging Role for Selenium. Front. Neurosci. 2021, 15, 666601. [Google Scholar] [CrossRef]
- Schomburg, L.; Köhrle, J. On the importance of selenium and iodine metabolism for thyroid hormone biosynthesis and human health. Mol. Nutr. Food Res. 2008, 52, 1235–1246. [Google Scholar] [CrossRef]
- Brodin, O.; Hackler, J.; Misra, S.; Wendt, S.; Sun, Q.; Laaf, E.; Stoppe, C.; Björnstedt, M.; Schomburg, L. Selenoprotein P as Biomarker of Selenium Status in Clinical Trials with Therapeutic Dosages of Selenite. Nutrients 2020, 12, 1067. [Google Scholar] [CrossRef]
- Xia, Y.; Hill, K.E.; Li, P.; Xu, J.; Zhou, D.; Motley, A.K.; Wang, L.; Byrne, D.W.; Burk, R.F. Optimization of selenoprotein P and other plasma selenium biomarkers for the assessment of the selenium nutritional requirement: A placebo-controlled, double-blind study of selenomethionine supplementation in selenium-deficient Chinese subjects. Am. J. Clin. Nutr. 2010, 92, 525–531. [Google Scholar] [CrossRef]
- Sharif, K.; Watad, A.; Coplan, L.; Lichtbroun, B.; Krosser, A.; Lichtbroun, M.; Bragazzi, N.L.; Amital, H.; Afek, A.; Shoenfeld, Y. The role of stress in the mosaic of autoimmunity: An overlooked association. Autoimmun. Rev. 2018, 17, 967–983. [Google Scholar] [CrossRef]
- Goodyer, I.M. Recent undesirable life events: Their influence on subsequent psychopathology. Eur. Child. Adolesc. Psychiatry 1996, 5 (Suppl. 1), 33–37. [Google Scholar] [CrossRef]
- Vinokur, A.; Caplan, R.D. Cognitive and affective components of life events: Their relations and effects on well-being. Am. J. Community Psychol. 1986, 14, 351–370. [Google Scholar] [CrossRef]
- Zake, T.; Skuja, S.; Kalere, I.; Konrade, I.; Groma, V. Upregulated tissue expression of T helper (Th) 17 pathogenic interleukin (IL)-23 and IL-1β in Hashimoto’s thyroiditis but not in Graves’ disease. Endocr. J. 2019, 66, 423–430. [Google Scholar] [CrossRef]
- Ouyang, W.; Rutz, S.; Crellin, N.K.; Valdez, P.A.; Hymowitz, S.G. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 2011, 29, 71–109. [Google Scholar] [CrossRef]
- Sonnenberg, G.F.; Fouser, L.A.; Artis, D. Border patrol: Regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat. Immunol. 2011, 12, 383–390. [Google Scholar] [CrossRef]
- Gulhane, M.; Murray, L.; Lourie, R.; Tong, H.; Sheng, Y.H.; Wang, R.; Kang, A.; Schreiber, V.; Wong, K.Y.; Magor, G.; et al. High Fat Diets Induce Colonic Epithelial Cell Stress and Inflammation that is Reversed by IL-22. Sci. Rep. 2016, 6, 28990. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Minciullo, P.; Saitta, S.; Giovinazzo, S.; Certo, R.; Campennì, A.; Trimarchi, F.; Gangemi, S.; Benvenga, S. Serum interleukin-22 (IL-22) is increased in the early stage of Hashimoto’s thyroiditis compared to non-autoimmune thyroid disease and healthy controls. Hormones 2014, 13, 338–344. [Google Scholar] [CrossRef]
- Seshadri, S.; Pope, R.L.; Zenewicz, L.A. Glucocorticoids Inhibit Group 3 Innate Lymphocyte IL-22 Production. J. Immunol. 2018, 201, 1267–1274. [Google Scholar] [CrossRef]
- Mori, S.; Maher, P.; Conti, B. Neuroimmunology of the Interleukins 13 and 4. Brain Sci. 2016, 6, 18. [Google Scholar] [CrossRef]
- Rütti, S.; Howald, C.; Arous, C.; Dermitzakis, E.; Halban, P.A.; Bouzakri, K. IL-13 improves beta-cell survival and protects against IL-1beta-induced beta-cell death. Mol. Metab. 2015, 5, 122–131. [Google Scholar] [CrossRef]
- Peterson, J.D.; Herzenberg, L.A.; Vasquez, K.; Waltenbaugh, C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc. Natl. Acad. Sci. USA 1998, 95, 3071–3076. [Google Scholar] [CrossRef]
- Fraternale, A.; Brundu, S.; Magnani, M. Glutathione and glutathione derivatives in immunotherapy. Biol. Chem. 2017, 398, 261–275. [Google Scholar] [CrossRef]
- Rebuffat, S.A.; Nguyen, B.; Robert, B.; Castex, F.; Peraldi-Roux, S. Antithyroperoxidase antibody-dependent cytotoxicity in autoimmune thyroid disease. J. Clin. Endocrinol. Metab. 2008, 93, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.M.; Vicchio, T.M.; Cristani, M.; Certo, R.; Caccamo, D.; Alibrandi, A.; Giovinazzo, S.; Saija, A.; Campennì, A.; Trimarchi, F.; et al. Oxidative Stress and Advanced Glycation End Products in Hashimoto’s Thyroiditis. Thyroid 2016, 26, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Szkróbka, W.; Okopień, B. The Effect of Vitamin D on Thyroid Autoimmunity in Levothyroxine-Treated Women with Hashimoto’s Thyroiditis and Normal Vitamin D Status. Exp. Clin. Endocrinol. Diabetes 2017, 125, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Duntas, L.H.; Mantzou, E.; Koutras, D.A. Effects of a six month treatment with selenomethionine in patients with autoimmune thyroiditis. Eur. J. Endocrinol. 2003, 148, 389–393. [Google Scholar] [CrossRef]
- Wu, Q.; Rayman, M.P.; Lv, H.; Schomburg, L.; Cui, B.; Gao, C.; Chen, P.; Zhuang, G.; Zhang, Z.; Peng, X.; et al. Low Population Selenium Status Is Associated with Increased Prevalence of Thyroid Disease. J. Clin. Endocrinol. Metab. 2015, 100, 4037–4047. [Google Scholar] [CrossRef]
- Winther, K.H.; Rayman, M.P.; Bonnema, S.J.; Hegedüs, L. Selenium in thyroid disorders—Essential knowledge for clinicians. Nat. Rev. Endocrinol. 2020, 16, 165–176. [Google Scholar] [CrossRef]
- Thomson, C.D.; Rea, H.M.; Doesburg, V.M.; Robinson, M.F. Selenium concentrations and glutathione peroxidase activities in whole blood of New Zealand residents. Br. J. Nutr. 1977, 37, 457–460. [Google Scholar] [CrossRef]
- Kipp, A.P.; Strohm, D.; Brigelius-Flohé, R.; Schomburg, L.; Bechthold, A.; Leschik-Bonnet, E.; Heseker, H.; German Nutrition Society (DGE). Revised reference values for selenium intake. J. Trace Elem. Med. Biol. 2015, 32, 195–199. [Google Scholar] [CrossRef]
- Mal’tseva, V.N.; Goltyaev, M.V.; Turovsky, E.A.; Varlamova, E.G. Immunomodulatory and Anti-Inflammatory Properties of Selenium-Containing Agents: Their Role in the Regulation of Defense Mechanisms against COVID-19. Int. J. Mol. Sci. 2022, 23, 2360. [Google Scholar] [CrossRef]
- Mal’tseva, V.N.; Gudkov, S.V.; Turovsky, E.A. Modulation of the Functional State of Mouse Neutrophils by Selenium Nanoparticles In Vivo. Int. J. Mol. Sci. 2022, 23, 13651. [Google Scholar] [CrossRef]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- Sarason, I.G.; Johnson, J.H.; Siegel, J.M. Assessing the impact of life changes: Development of the Life Experiences Survey. J. Consult. Clin. Psychol. 1978, 46, 932–946. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).