Abstract
DNA damage is induced by many factors, some of which naturally occur in the environment. Because of their sessile nature, plants are especially exposed to unfavorable conditions causing DNA damage. In response to this damage, the DDR (DNA damage response) pathway is activated. This pathway is highly conserved between eukaryotes; however, there are some plant-specific DDR elements, such as SOG1—a transcription factor that is a central DDR regulator in plants. In general, DDR signaling activates transcriptional and epigenetic regulators that orchestrate the cell cycle arrest and DNA repair mechanisms upon DNA damage. The cell cycle halts to give the cell time to repair damaged DNA before replication. If the repair is successful, the cell cycle is reactivated. However, if the DNA repair mechanisms fail and DNA lesions accumulate, the cell enters the apoptotic pathway. Thereby the proper maintenance of DDR is crucial for plants to survive. It is particularly important for agronomically important species because exposure to environmental stresses causing DNA damage leads to growth inhibition and yield reduction. Thereby, gaining knowledge regarding the DDR pathway in crops may have a huge agronomic impact—it may be useful in breeding new cultivars more tolerant to such stresses. In this review, we characterize different genotoxic agents and their mode of action, describe DDR activation and signaling and summarize DNA repair mechanisms in plants.
1. Introduction
All living organisms are continuously exposed to various environmental stresses, some of which may cause DNA damage, either directly or indirectly (e.g., by increasing the level of reactive oxygen species—ROS). Due to their immobile nature, plants are not able to avoid these stresses. Moreover, cell metabolism itself may also introduce DNA lesions through, e.g., replication errors or ROS production. Therefore, proper sensing of DNA damage and precise activation and functioning of the DNA repair machinery is of great importance in preserving genome integrity. The signal of DNA damage is processed through pathways collectively termed DDR (DNA damage response), which form a complex multilevel signaling network leading to the activation of processes related to DNA repair. Simultaneously, in the case of proliferating cells, DDR leads to cell cycle arrest, through control checkpoints, for as long as the DNA is not repaired. Various genotoxic agents causing different DNA lesions have been used to study DDR in plants. It is well known that distinct DNA repair mechanisms are activated depending on the type of DNA damage. The aim of this review is to summarize the current knowledge regarding the DNA damage response pathway in plants.
2. Wide Range of Genotoxic Agents Activating DDR in Plants
The activity of genotoxic factors leads to the generation and accumulation of DNA damage, which triggers the DDR pathway. Unrepaired or not correctly repaired DNA damage gives rise to mutations that can lead to many abnormalities in the cell metabolism or even cell death. There are diverse types of DNA damage that can be grouped into two main categories: (1) disturbing only one DNA strand and (2) affecting both DNA strands. The first group includes lesions such as single-strand breaks (SSBs), oxidized/alkylated bases, DNA adducts, intra-strand crosslinks (CLs) between adjacent guanines, and DNA photoadducts. The second group includes inter-strand CLs between opposite DNA strands, and double-strand breaks (DSBs) (Figure 1) [1]. Among them, DSBs are the most harmful because they can lead to the loss of genetic material through chromosomal fragmentation, and as a consequence, they can lead to cell death [2,3].
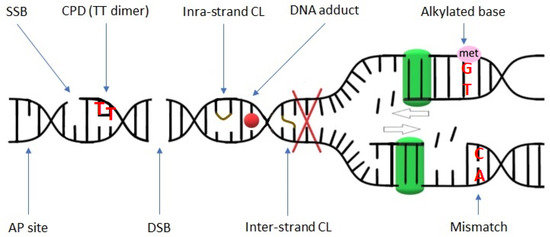
Figure 1.
Schematic representation of various DNA lesions. Red X indicates the blockage of the replication fork (replication stress) that may be caused by DNA damage. The green squares symbolize polymerase performing replication. SSB—single-strand break; CPD—cyclobutene pyrimidine dimer; CL—crosslink; AP site—apurinic/apyrimidinic site; DSB—double-strand break.
Diverse groups of exogenous genotoxic agents causing different types of DNA damage can be used to study distinct elements of the DDR pathway and DNA repair mechanisms. One of the groups of chemical genotoxins comprises alkylating agents. The main targets of monofunctional alkylators are guanines and adenines that can be methylated at O and N positions, leading to the generation of DNA lesions such as O6-methylguanine N7-methylguanine, N3-methyladenine, or others [4,5]. The alkylated base is not appropriately recognized during replication, which leads to mispairing with incorrect nucleotides. It can also be removed (base loss), which creates an abasic site (AP-Apurinic/APyrimidinic site) [6]. Alkylating agents, such as EMS (Ethyl MethaneSulfonate), MMS (Methyl MethaneSulphonate), or MNU (N-Methyl-N-NitrosoUrea), are used to study DDR, but they are also widely used to induce mutations for plant breeding and reverse genetic studies, e.g., through the creation of TILLING populations [7,8,9,10,11,12,13,14,15]. Apart from monofunctional alkylators, there are also bi- and polyfunctional ones, such as nitrogen mustards or platinum drugs. They damage DNA by generating bulky adducts to nucleotides, intra-strand crosslinks, as well as inter-strand crosslinks that block DNA-related processes, such as replication and transcription. Because of their ability to block these processes in tumor cells with a high proliferation rate, they are widely used in cancer chemotherapy [16,17,18]. In plant studies, the most frequently used DNA crosslinking agents are cisplatin (cis-diamminedichloroplatinum II, CDDP) and mitomycin C [19,20,21,22,23,24,25].
Another group of chemical genotoxins includes radiomimetics, whose name indicates that they affect DNA similarly to ionizing radiation. They induce double-strand breaks through oxidative damage. The most common radiomimetic used to generate DSBs in plants’ genomes is bleomycin (BLM) [26,27,28,29]. This glycopeptidic antibiotic isolated from Streptococcus verticillus is also used as an antitumor agent. It is known to directly produce ROS by forming a complex with molecular oxygen and divalent ions such as iron. The bleomycin/iron complex binds to the DNA helix through a bithiazol ring, which causes a DNA strand scission and lipid peroxidation [18,30]. Zeocin is another radiomimetic antibiotic that belongs to the bleomycin family and has recently been used most often to study DDR pathways in plants [31,32,33,34].
Genotoxic stress can also be induced in plant cells by other chemical agents, such as hydroxyurea (HU), which acts at the S-phase of the cell cycle, as it is a ribonucleotide reductase (RNR) inhibitor. RNR is an enzyme crucial for the formation of deoxyribonucleotides—it induces the conversion of ribonucleotides to deoxyribonucleotides by catalyzing the removal of a 2′OH group from ribonucleoside diphosphates. Therefore HU causes DNA replication stoppage. HU has been used in hematological disorders and cancer therapy as an antiproliferative drug since the 1960s [35,36]. Its action is easily reversible; therefore, short HU treatment is often used in scientific research to synchronize the cell cycle. However, higher concentrations and more prolonged treatment may lead to the accumulation of DNA damage [37]. Camptothecin (CPT) is another genotoxic factor that has been used to study DDR in plants, e.g., in Medicago truncatula [38]. Its cytotoxic effects have been long known for plants [39]. CPT causes the induction of both SSBs and DSBs and enhances the level of cell death in plants [40,41]. It is also widely used in anticancer therapies—it inhibits the activity of topoisomerase I (TopI), which is an enzyme required for the relaxation of DNA supercoiling after replication, transcription, etc. CPT acts through intercalating between DNA breaks flanking the TopI–cleavage complex [42,43]. Among the chemical factors used for the creation of DNA damage in plants is also zebularine, which is a cytidine analog leading to the formation of DNA–protein crosslinks. It has been recently used to study DDR, i.e., in Arabidopsis [44].
There are two main physical factors causing DNA damage: ionizing and non-ionizing radiation. The most common physical agent used in plant DDR studies and mutation breeding is ionizing radiation (IR) with, e.g., γ-rays, X-rays, or ion beams. IR is known to induce DNA damage directly by ionizing DNA molecules, which may cause DSBs and hence DNA fragmentation. It also acts indirectly through the radiolysis of water that leads to ROS production, e.g., hydroxyl and hydrogen radicals (OH● and H●) and free electrons (e−). The most frequent DNA lesions caused by thus-induced oxidative stress are oxidized bases (e.g., thymine glycols and 8-oxoguanine), base loss (abasic sites), and SSBs. Among IR-induced DNA damages are also DNA–protein crosslinks [1,45]. The non-ionizing radiation also leads to DNA damage. Plants need sunlight for photosynthesis and survival. However, the UV light that is a part of solar energy is harmful to DNA (UV-B in particular). It generates photoadducts, mainly cyclobutene pyrimidine dimers (CPDs) and pyrimidine (6-4) pyrimidone dimers (6-4PPs). In CPDs, there are covalent bonds between the C-5 and C-6 carbon atoms of neighboring pyrimidines (mainly TT, less often TC and CC), forming a four-member ring structure. In 6-4PPs, there is a bond between the C-6 and C-4 carbon atoms in TC dinucleotide. The proportion and distribution of these photoadducts in the genome depend on the nucleotide composition of DNA and chromatin structure [1,46]. However, it has been estimated that in plants, CPDs are the major types of UV-induced DNA lesions (approximately 75%, up to 90%) [47,48]. Both CPDs and 6-4PPs can block the transcription and replication processes. The replication blockage can lead to the collapse of replication forks that can generate DSBs [49,50]. Additionally, UV radiation can induce ROS production and oxidative damage (pyrimidine hydrates) and DNA–DNA as well as DNA–protein crosslinks [48].
UV light is the most ubiquitous genome-damaging factor worldwide, but there are also other environmental stresses, which plants are constantly exposed to, that may damage DNA. Abiotic stresses such as drought, heat, cold, or soil salinity are known to promote ROS formation and accumulation, which results in oxidative stress that can cause DNA damage [51,52]. For example, it is long known that cold stress induces chromatin fragmentation and apoptotic changes in tobacco [53]. Later work on Arabidopsis treated with low temperature (4 °C) has shown that chilling stress provokes DNA damage mainly in root stem cells and their early descendants [54]. On the other hand, DNA integrity may also be disrupted by heat stress that triggers nucleotide modifications and single-strand as well as double-strand breaks, and changes in chromatin architecture [55,56,57]. Salinity stress has also been proven to induce DNA strand breaks, e.g., in Arabidopsis [58] and rice [59], predominantly through the production of ROS. The application of ROS-specific antioxidants significantly reduces the amount of DNA breaks caused by NaCl [60,61,62]. Much evidence shows that heavy metals, such as cadmium (Cd) and lead (Pb), cause oxidative and genotoxic stress [63]. Aluminum (Al), the most common metal in the Earth’s crust, is highly phytotoxic in acidic soils (at pH below 5.5), which now comprise more than 50% of arable lands. Under acidic conditions, Al is known to affect genome integrity, induce DSBs in root meristems and activate the DDR pathway [64,65,66]. It is worth mentioning that low pH itself induces oxidative stress in plant cells and hence, through the production of ROS, it may also lead to DNA damage [67]. So, plants are continuously exposed to conditions harmful to their DNA. It has to be noted that DNA damage can also be induced by endogenous factors that are the products of cell metabolism. All processes associated with DNA, such as replication, recombination, etc., are not free of mistakes and can introduce DNA lesions. It has been estimated that the DNA of each living organism accumulates thousands of lesions every single day [68]. Thus, the importance of DDR mechanisms in maintaining genome integrity and stability cannot be overestimated. Exploring the details of the DDR pathways may be essential for fully understanding plant responses to environmental stresses and breeding new cultivars tolerant to them.
3. DDR–Sensing and Signaling the DNA Damage
Cells are constantly subjected to DNA damage and all organisms, including plants, have evolved efficient mechanisms for sensing this damage (Figure 2). Two key factors involved in the recognition of DNA lesions are ATM (Ataxia Telangiectasia Mutated) and ATR (ATM and Rad3-related), protein kinases belonging to the phosphatidylinositol 3-kinase-like family. In principle, they play distinct and additive roles—ATM recognizes DSBs, whereas ATR is predominantly recruited to ssDNA and stalled replication forks—the hallmarks of replication stress [69]. These kinases are activated by different DNA damage sensors. Similarly, as in the case of animals and yeast, in plants, ATM is activated by the MRN complex (MRE11-RAD50-NBS1) that senses DSBs [70]. ATM is targeted to the DSBs sites by the C-terminus of NBS1 (NIJMEGEN BREAKAGE SYNDROME 1) [71]. ATR is recruited to ssDNA by a different mechanism. ATR is inactive in complex with ATRIP (ATR interacting protein) and is recruited to ssDNA through interaction with RPA (replication protein A) that senses and coats ssDNA. In parallel, another group of proteins is also recruited by RPA to the ssDNA site—a group that comprises DNA polymerase α, RFC (RAD17-replication factor C), and 9-1-1 complex (RAD9, RAD1, and HUS1). This complex is involved in ATR activation [72]. The DDR pathway has been extensively studied in animals because its malfunction is related to cancer development. Nevertheless, in plant research, it has also been studied in detail for the last 20 years. The elements involved in sensing DNA damage are highly conserved among all eukaryotes. Arabidopsis homologs of ATM and ATR kinases were identified in the early 2000s [73,74], and plant homologs of DNA damage sensors mentioned above were identified shortly thereafter [75,76,77].
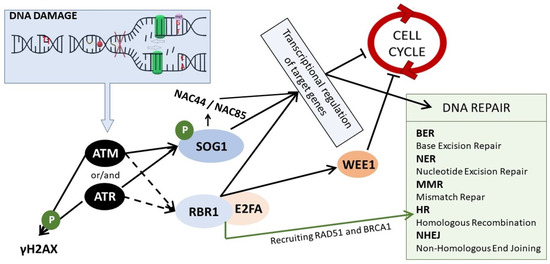
Figure 2.
The scheme of DDR signaling in plants. In response to DNA damage, ATM and/or ATR kinases are activated. ATM is activated upon DSBs, whereas ATR upon SSBs and replication stress. They phosphorylate and activate SOG1, which regulates genes responsible for cell cycle stoppage and DNA repair. This regulation is both direct and indirect, i.e., through other NAC TFs, such as NAC44 and NAC85. ATM and ATR kinases lead also to the phosphorylation of histone H2AX, and they induce the assembly of the RBR1/E2FA complex that regulates the expression of genes related to the cell cycle arrest and is also involved in the DNA repair through HR by recruiting RAD51 and BRCA1 to the DNA lesions.
The activated ATM and ATR kinases start a signaling cascade and phosphorylate a plethora of downstream elements of the DDR pathway. One of these elements is γH2AX (a histone variant phosphorylated at Ser-139 residue) that is accumulated at DNA damage sites in an ATM/ATR-dependent manner [70]. γH2AX is commonly known as a sensitive marker of DNA damage [78]. A recently identified XIP protein (γH2AX-INTERACTING PROTEIN) was found to interact directly with γH2AX, as well as with RAD51, the key recombinase involved DNA repair through HR (homologous recombination) [79]. Both ATM and ATR kinases phosphorylate and activate SOG1 (Suppressor Of Gamma 1)—the master regulator of DDR [80,81]. SOG1 was first identified from a gamma radiation-induced DNA damage suppressor screen in Arabidopsis (where uvh1–UV hypersensitive mutant was mutagenized) [82]. SOG1 is a transcription factor belonging to the NAC (NAM, ATAF1/2, and CUC2) family, which is specific to plants. It is considered to be a functional equivalent of p53—a tumor suppressor that controls DNA repair and cell cycle stoppage in response to DNA damage in animals. However, p53 and SOG1 are not true homologs, because they do not share any sequence similarity [83]. SOG1 is activated via phosphorylation of conserved C-terminal serine-glutamine (SQ) motifs [80]. Thus-activated SOG1 orchestrates the mechanisms of DNA repair, cell cycle arrest, endoreduplication, and PCD through transcriptional regulation [84]. An enormous number of genes were found to be directly or indirectly controlled by SOG1; e.g., ChIP-seq analysis in Arabidopsis revealed that more than 140 genes are its direct targets [85]. Very sophisticated DDR transcriptional models have been created by Bourbousse et al., confirming that SOG1 is a major activator of genes upregulated upon DNA damage. It was calculated that SOG1 directly controls ~8% of the transcriptional response to DNA damage caused by γ-radiation in Arabidopsis [86]. It is confirmed by many studies that SOG1 is a direct positive regulator of genes related to DNA repair (e.g., RAD51 and BRCA1 involved in HR or PARP1 and PARP2 involved in NHEJ), but also of the genes required for the cell cycle arrest (such as SMR5 and SMR7–SIAMESE-RELATED cyclin-dependent kinase inhibitors) [81,85,86,87]. SOG1 is also known to induce the expression of other transcription factors, belonging to different TF families (i.e., WRKY or Zn Finger), including its own homologs from the NAC family, such as NAC044 and NAC085 [86].
Recent studies have revealed additional pathways of DDR in plants. The new player, whose function depends on ATM/ATR activity, is RBR1 (RETINOBLASTOMA-RELATED1), which is the only Arabidopsis homolog of animal tumor suppressor pRb. However, RBR was not found to be a direct target of ATM/ATR, hence the process of its activation upon DNA stress remains to be elucidated [88]. In general, RBR1 forms complexes with E2F transcription factors that are regulators of genes involved in entrance into the S phase during the cell cycle. The E2F transcription factors activate the expression of S-phase genes, and the RBR1 inhibits the E2F activity [89]. Upon genotoxic stress, RBR1, together with its binding partner E2FA, was found to accumulate at damage sites in nuclei and, together with BRCA1, it was involved in the recruitment of the DNA repair machinery [90,91]. Importantly, RBR1/E2FA complex regulates the expression of many DDR genes (e.g., WEE1) by association with their promoters, in a SOG1-independent manner [92].
4. DNA Repair Mechanisms
In principle, when a DNA lesion occurs it is important to repair it before replication and cell division. Numerous different mechanisms of DNA repair may be activated, depending on the type of DNA damage. DSBs may be repaired through homologous recombination (HR) or non-homologous end-joining (NHEJ); SSBs, AP sites, and some alkylated bases may be fixed by base excision repair (BER); changes caused by UV radiation (such as 6-4PP, CPD) may be corrected through direct reversal repair or nucleotide excision repair (NER); and polymerase mistakes may be removed by the mismatch repair mechanism (MMR) reviewed in [1,93,94,95]. These mechanisms are highly conserved among all eukaryotes.
4.1. Repair Mechanisms of Damage in Single DNA Strand
If damage occurs in one DNA strand only, the sequence information from the second, complementary strand may be used for its repair. The three most common pathways for the repair of these types of damages are BER, NER, and MMR.
BER (base excision repair) is triggered by a broad range of DNA lesions—by damaged or modified (alkylated, oxidized, or deaminated) DNA bases [96]. BER is initiated by DNA glycosylases that remove the damaged DNA bases and create AP sites. The AP sites may be formed after the accumulation of uracil in the DNA strand, which is caused by hydrolytic deamination of 5-methylcytosine. As a result, the U-G pairs are created, which, if not repaired before replication, will lead to C/G to T/A mutations. In this case, the BER mechanism is activated by UDG (URACIL DNA GLYCOSYLASE), which cuts the N-glycosidic bond and then cleaves the uracil out, leading to the creation of AP sites [97,98]. In Arabidopsis, there are several enzymes from the uracil-DNA glycosylase family involved in the removal of uracil from DNA [99,100]. In the case of other damaged or modified bases, the proper lesion-specific DNA glycosylases initiate BER by removing the affected base and thus generating the AP site. The first identified glycosylase involved in the removal of alkylated DNA bases in plants was 3-METHYLADENINE-DNA GLYCOSYLASE [101]. AP sites are then processed by AP endonucleases and/or AP lyases, which cleave the sugar-phosphate backbone at the AP site. The AP lyase activity is associated with bifunctional DNA glycosylases. As it follows, during the BER pathway the single-strand breaks are created, which may also be substrates for other repair mechanisms, such as NER [102]. The next steps of BER, following the base removal and AP site incision, include cleaning of DNA termini, and filling the gap by proper polymerases. Depending on the number of nucleotides incorporated, there are two types of BER–short-patch (SP-BER) with insertion of only one nucleotide and long-patch (LP-BER) with insertion of several (usually 2–13) nucleotides. In mammals, different polymerases are used in SP-BER and LP-BER (pol β and pol δ/ε, respectively) [103,104]. The last step of BER is DNA nick ligation. In mammals, during SP-BER, the ligation is proceeded by a complex consisting of XRCC1 and LigIIIa, whereas in LP-BER by LIG1 [104,105]. Plants possess the orthologs of most BER genes found in other kingdoms, however with some exceptions; e.g., plants do not have orthologs of pol β or LigIII related to SP-BER in animals. Additionally, some plant-specific BER proteins have also been identified, which indicates that some plant-specific characteristics emerged along BER evolution reviewed in [106]. Both mono- and bifunctional glycosylases have been found in Arabidopsis. Among bifunctional ones, with glycosylase and lyase activity, are, e.g., AtFPG and AtOGG1. Together with ZDP 3′ DNA phosphatase and ARP endonuclease, they are confirmed to be involved in the repair of oxidized bases [107]. Recently, it was shown that APE2 endonuclease and ZDP phosphatase play overlapping roles in maintenance of epigenome and genome stability in Arabidopsis [108]. Initially, because of the lack of plant orthologs of pol β and LigIII, which are the main players involved in SP-BER in mammals, it was assumed that plants do not repair damaged bases through this subpathway of BER [109]. However, it has been confirmed that in Arabidopsis uracil and AP sites may be repaired by both LP- as well as SP-BER [81,82]. Plant Pol λ, which belongs to the same family as Pol β (family X), is probably implicated in the synthesis step of the BER pathway in Arabidopsis [99,110]. On the other hand, plants possess orthologs of Pol δ and ε, which are involved in LP-BER in mammals. Nevertheless, their potential function in LP-BER needs to be further investigated [106]. Arabidopsis genome encodes three ligases—AtLIG1, AtLIG4, and AtLIG6. The last one is plant-specific and together with AtLIG4 is critical for seed longevity [111]. AtLIG4 is confirmed to be implicated in DSB repair [112,113]. AtLIG1 is the only ligase known to be responsible for the final ligation of DNA ends in both BER subpathways (SP- and LP-) in Arabidopsis [100]. AtXRCC1 stimulates 3′-end cleaning by ZDP and enhances the ligation step, probably by interaction with AtLIG1 [114]. In plants, the proper functioning of the BER pathway is crucial for seed longevity, since seed storage leads to the accumulation of oxidative changes, and BER is involved in repairing oxidative DNA lesions in germinating embryos [107,115]. The list of BER-related proteins in plants is provided in Table 1.

Table 1.
BER-related proteins in Arabidopsis thaliana.
NER (nucleotide excision repair) is used to repair various bulky DNA adducts, such as UV-induced photoproducts, that cause helix distortion in the DNA structure. In humans, deficiencies in NER-related genes lead to several disorders, e.g., xeroderma pigmentosum. Interestingly, NER-related proteins were found to be more conserved among bacteria, yeast, plant, and animal species than proteins from other DNA repair pathways [116]. There are two NER subpathways in mammals: (1) global genomic repair (GGR or GG-NER) and (2) transcriptional-coupled repair (TCR or TC-NER), which differ in the mode of damage recognition, but use the same machinery to correct the lesions [94,117]. In the GG-NER subpathway, damage that occurs anywhere in the genome may be detected. In animals, the repair is initiated by the XPC-HR23B-CEN2 (Xeroderma Pigmentosum group C-Homolog of Rad23B–Centrin2) complex. This complex scans DNA and detects helix distortion. This process is very often enhanced by the activity of the DDB complex (damaged DNA-binding complex), composed of DDB1 and DDB2 subunits, which helps to find lesions that only slightly alter the structure of DNA, such as CPDs. In the TC-NER, only the damage that occurs in the transcribed DNA strand of highly expressed genes is detected. The repair is initiated by RNA polymerase, which is stalled at the lesion site. Then, the TCR-specific factors bind to stalled RNA polymerase, such as CSA, CSB, and XAB2 [118]. These factors initiate the assembly of other factors that result in the displacement of the polymerase complex and induces chromatin modifications that allow the exposure of lesions for further processing [119]. After DNA damage recognition a stable pre-incision complex is formed around the lesion. The multiprotein TFIIH (Transcription Factor IIH) complex is recruited to the lesion. This complex comprises of two sub-complexes: (1)–XPB DNA helicase, p62, p52, p44, p34, and p8; (2) CDK7 kinase, cyclin H, and MAT1 assembly factor. The subcomplexes are linked by XPD DNA helicase. The activity of the TFIIH complex induces the unwinding of the DNA helix, which leads to the recruitment of XPA, RPA, and XPG endonuclease. The next step is the excision of a short DNA fragment containing damaged nucleotide with the use of specific enzymes—the incision 5′ to the lesion is catalyzed by XPF/ERCC1 endonuclease that interacts with XPA and incision 3′ to the lesion by XPG endonuclease. Then, the DNA synthesis may be performed by three various polymerases (δ, ε, or κ), and DNA ligation is accomplished by Lig I or Lig III. In plants, the homologs of NER genes were identified and confirmed to be involved in this mechanism of DNA repair. For example, Arabidopsis plants defective in DDB1A, DDB1B, DDB2, CSA, and XPD are characterized by altered UV sensitivity/tolerance [120,121,122,123,124,125]. The list of NER-related proteins in Arabidopsis is provided in Table 2. The UV-induced photoadducts may be also repaired directly, not by NER, through the photoreactivation (light-dependent process), which is performed by photolyases that require light (360–420 nm) to be active [126]. There are two types of these repair enzymes in plants: Class II CPD photolyase and (6-4) photolyase [127,128].

Table 2.
NER-related proteins in Arabidopsis thaliana.
As its name indicates, the MMR (mismatch repair) mechanism is used to fix not only mismatches but also small insertion/deletion loops (IDLs) that arise from the incorrect incorporation of nucleotides or accidental insertion/deletion of nucleotides during DNA replication in the S phase. The first step of this mechanism is the recognition of lesions by MutS complexes that have DNA binding activities. In general, in eukaryotes, mismatches are detected by specific MutSα (MSH2/MSH6 heterodimer) and MutSβ (MSH2/MSH3 heterodimer) complexes. However, plants possess an additional complex involved in this step—MutSγ (MSH2/MSH7 heterodimer) [129,130]. MutSα recognizes single-base mismatches (including oxidative and methylated mispairs) and very short IDLs, MutSβ recognizes longer IDLs (with up to 16 unpaired nucleotides), and MutSγ detects few types of single-base mismatches in plants [131,132,133]. Moreover, MutSα can also recognize UV-induced lesions such as CPD and 6-4 PPs and initiate MMR for their repair; however, these types of lesions are usually repaired through NER mechanisms [134,135]. MSH2 protein, which is a component of each known MutS complex involved in MMR, has the ability to activate ATR kinase [135,136]. The MutS complexes recruit the MutLα complex to the lesion site in an ATP-dependent manner. MutLα complex has endonuclease activity and it initiates a repair reaction by nicking the daughter strand 5′ to the mismatch. In humans, the MutLα complex is a heterodimer of MLH1 and PMS2 proteins, whereas in plants this heterodimer is composed of MLH1 and PMS1. MutLα recruits Exo1 to the nick. Exo1 is the exonuclease that conducts 5′ → 3′ excision in the daughter strand. When the excision reaches the mismatch, the MutS has to dissociate from the lesion site to allow Exo1 to continue excision [137]. Interestingly, there is strong evidence that Exo1-independent subpathway of MMR also exists, where DNA2 protein with helicase and nuclease domains plays an important role [138,139,140]. Arabidopsis homolog of human and yeast DNA2-JHS1 (JING HE SHENG 1) was confirmed to be involved in DNA repair, cell cycle regulation, and meristem maintenance in plants [141]. The MMR pathway is completed by a proper DNA re-synthesis by DNA Pol δ assisted by RFC, PCNA, and RPA, and then the nick is ligated by Lig 1 reviewed in [94,142]. The list of MMR-related proteins in Arabidopsis is provided in Table 3.

Table 3.
MMR-related proteins in Arabidopsis thaliana.
4.2. DSB Repair–Homologous Recombination (HR) and Non-Homologous End Joining (NHEJ)
The efficient functioning of HR and NHEJ pathways is of great importance because they are used to repair DSBs, the most harmful of all lesions. The basic principles of these two repair mechanisms are conserved among all eukaryotes.
The first step of DSB repair through HR is the resection of DNA ends, which is mediated by the MRN complex (MRE11-RAD50-NBS1) recruited at the DSB site [27,143]. MRN complex is known to bind to DNA ends and preserve them in close proximity to each other. It also recruits and activates ATM and induces excision of 5′ ends to form long single-stranded 3′ overhangs that are coated with RPA to protect them from exonucleolytic degradation [144]. In the case of DSB repair through homologous recombination, another DNA molecule (donor molecule) is needed as a repair template to recover genetic information. Its sequence should be identical or nearly identical to the damaged sequence. Depending on the cell phase it might come from a sister chromatid or homologous chromosome. To initiate homologous recombination, the BRCA1/2 (Breast Cancer 1/2) interacts with and recruits RAD51 recombinase that replaces RPA coating ssDNA. RAD51 initiates homology search and facilitates strand invasion into the homologous template. After recognition of the donor molecule, the 3′ overhang invades the dsDNA template by displacing the noncomplementary template strand and base pair with the other template strand, forming the opened structure called “D-loop” (displacement loop) [145]. In this structure, the DNA polymerase can start the elongation of the free 3′ end of the overhang, based on the homologous donor locus as a template. As the elongation proceeds, the D-loop may be enlarged by a DNA helicase or migrates together with the polymerase along the donor molecule [146]. There are two main models of DSB repair through HR: (1) DSBR–double-strand break repair, the classical HR model, which involves the formation of double Holliday junction (dHJ) intermediates; and (2) SDSA–synthesis-dependent strand annealing. (1) DSBR model may result in different end products (crossover and non-crossover) and it occurs mainly during meiosis when a non-sister chromatid from a homologous chromosome is used as a donor template. In the process of DSBR, two strands of the donor molecule are simultaneously used as templates for the elongation of two 3′ overhangs [147]. It leads to the creation of the double Holliday junction, the structure containing two interconnected DNA molecules. The dHJ intermediates are resolved (separated into two double-strand molecules) by specific endonucleases called resolvases, which may cut both crossed and non-crossed strands, resulting in the crossover or non-crossover products, respectively [2]. (2) In the case of the SDSA model, only non-crossover products arise. It is based on the elongation of only one 3′ overhang with the use of the donor strand. The elongation proceeds until it is possible to realign with the other side of the DSB in the originally damaged DNA molecule [144,148]. These two models of HR are flawless and do not lead to any loss of DNA sequence. There is also a third model of DSB repair by HR called SSA–single strand annealing. SSA can be used to repair DSBs localized between tandemly repeated sequences. The homologous repeats are used to bridge DSB ends. SSA is not flawless as it leads to a deletion rearrangement between the repeats [149]. The list of HR-related proteins in plants is provided in Table 4.

Table 4.
HR-related proteins in Arabidopsis thaliana.
Contrary to HR, in the NHEJ pathway, the re-joining of broken DNA strands is straightforward and does not need any donor template. Because the sequence information is not used in this repair mechanism, it is error-prone [27]. There are two subpathways of NHEJ: cNHEJ (canonical NHEJ) and aNHEJ (alternative NHEJ) [150]. In the cNHEJ pathway, the DSB is recognized by Ku70/Ku80 heterodimer that forms a ring that keeps the DNA ends in close proximity and protects them from degradation. The binding of Ku70/Ku80 initiates the NHEJ repair [151]. Then, several factors involved in the resection and processing of DNA ends are recruited at the DSBs site such as PARP (POLY ADP-RIBOSE POLYMERASE) proteins, SNM1 (SENSITIVE TO NITROGEN MUSTARD), ZDP (ZINC 4 FINGER DNA 3′-PHOSPHOESTERASE), Pol λ, Rad9, and MRN complex. In the end, the complex consisting of Ligase 4 and XRCC4 performs the final ligation [152]. In the aNHEJ subpathway, which is activated in the absence of cNHEJ factors, the 3′-resection of the broken ends occurs by various exonucleases, making it similar to the SSA mechanism. When produced 3′ overhangs from both sides of DSBs have some complementary nucleotides, they can simply anneal to each other. After trimming the remaining ends, re-ligation occurs. However, the precise molecular mechanisms underlying aNHEJ in plants remain unclear [153]. The list of NHEJ-related proteins in plants is provided in Table 5. In general, in somatic cells, the NHEJ pathway plays a major role in the repair of plant DSBs, however, the HR pathway may be also widely involved in DSB repair in dividing cells, during the S and G2 phases of the cell cycle, due to the availability of sister chromatids [134]. During meiosis, where the formation of DSBs is highly controlled, the HR mechanism (precisely DSBR) is the main mode of their repair, where RAD51 acts together with meiosis specific DMC1 protein (DISRUPTED MEIOTIC CDNA1) and SMC5/6 (STRUCTURAL MAINTENANCE OF CHROMOSOME 5/6) complex [154,155].

Table 5.
NHEJ-related proteins in Arabidopsis thaliana.
Obviously, the DSB repair mechanisms in plants are not as well described as in humans. However, recently the DNA repair mechanisms became of special interest in plant studies because they are used in genetic engineering to achieve controlled modification of plant genomes (gene editing, i.e., through CRISPR/Cas-based methods) [152,156,157,158]. Most of the HR and NHEJ proteins have been identified and characterized in plants, and many plant mutants, especially Arabidopsis, have been studied, showing high evolutionary conservation of these mechanisms between plants and animals reviewed in [94].
Interestingly, studies performed on Arabidopsis thaliana established an important role for specific small RNAs (called diRNAs for DSB-induced small RNAs) in efficient DSB repair. DiRNAs are produced from sequences flanking DSB. The proposed role of diRNAs in DSB repair is that they are guide molecules directing chromatin modification and involved in recruitment of proper complexes, such as SMC5/6, a chromosomal ATPase involved in DSB repair, to DSBs. However, their precise role remains to be elucidated [159,160]. SMC5/6 complex is known to play a role in the maintenance of genome integrity; however, its mode of function is not fully understood yet [161]. It has already been revealed that several factors are involved in the recruitment of SMC5/6 to DSB site. Recently, it has been shown that SWI3B subunit of SWI/SNF (SWITCH/SUCROSE NON- FERMENTABLE) chromatin remodeling complex enhances dissociation of SMC5 from chromosomes for its further recruitment at DSB site [162].
5. Cell Cycle Stoppage—Giving the Cell Time to Repair
The main regulator of the DDR pathway in plants, SOG1, activates directly and indirectly not only many genes related to DNA repair, but also those related to cell cycle regulation. Dividing tissues are the most sensitive to DNA-damaging agents. Cell cycle arrest is the first effect of DDR activation and is crucial to allow time to repair to avoid transmission of lesions to daughter cells. In general, the cell can be arrested in the G2/M or S phase of the cell cycle (by activation of G2/M and replication checkpoints, respectively). The arrest depends on the phase at which DNA damage occurs [60].
The progression of the cell cycle (through G1-S-G2-M phases) in plants relies on an enormous number of cell cycle regulators, very often encoded by multiple loci. In principle, the cell cycle is controlled by various cyclin-dependent kinases (CDKs) that are active only when properly phosphorylated and in complex with appropriate cyclins. Their activity is highly increased at the transition from G1 to S and from G2 to M. At the G1/S transition, they phosphorylate many proteins important for DNA synthesis, whereas at the G2/M transition, they phosphorylate proteins related to chromosome segregation. There are many mechanisms modulating CDK-cyclin activity, such as transcriptional regulation, protein degradation, phosphorylation of Thr residues, and binding of specific inhibitors [163,164].
In response to DNA damage, SOG1 activates the transcription of genes encoding inhibitors of cyclin-dependent kinases. Two of them, SMR5 and SMR7 (Siamese Related 5/7), are confirmed to be direct targets of SOG1 in Arabidopsis [87]. Upon DNA stress, SOG1 represses the transcription of specific B-type CDKs—CDKB2s that are mitotic regulators in Arabidopsis [83,165]. This suppression leads to the activation of the G2/M checkpoint and blockage of the cell cycle. On the other hand, SOG1 enhances the activity of plant-specific CDKB1 by stimulation of CYCB1 (which forms a complex with CDKB1 and is widely used as a marker for cell proliferation). This conundrum was solved by confirming that CDKB1-CYCB1 also mediates homologous recombination (HR), showing the dual face of cyclin B1 [21,166].
SOG1 activates the G2/M checkpoint also via stimulation of some MYB3R genes—MYB3R1, MYB3R3, and MYB3R5 (collectively called Rep-MYB3R)—that encode major repressors of genes related to the regulation of G2/M transition and are required for M phase onset [86]. Principally, CDK complexes phosphorylate Rep-MYB3R and directs them to proteosomal degradation, which allows G2/M progression. However, under genotoxic stress, CDK activity is inhibited by SOG1 (via activation of SMR5 and SMR7), which leads to the accumulation of Rep-MYB3R—this halts G2/M progression [32]. Recent studies in Arabidopsis have shown that two NAC transcription factors closely related to SOG1, ANAC044 and ANAC085 (that are also regulated by SOG1), are involved in Rep-MYB3R stabilization and accumulation, and hence the activation of the G2/M checkpoint [167]. Additionally, ANAC044, together with homologs of the human DREAM (DIMERIZATION PARTNER, RB-LIKE, E2F AND MULTI-VULVAL CLASS B) complex, was found to be a part of the RBR1 interactome. DREAM complex is very well studied in humans as a repressive regulator of the cell cycle; however, in plants, the full assembly of this complex was not known until recently. The newest data indicate the existence of multiple DREAM complexes in plants that mediate growth arrest upon DNA stress in conjunction with ANAC044 [168].
The existence of DNA lesions may perturb the course of DNA replication (replication stress), causing the cell cycle arrest in the S phase. The evolutionary conserved WEE1 kinase is a key Intra-S checkpoint protein [164]. It is confirmed in Arabidopsis that this kinase is accumulated in S-phase nuclei upon replication stress [169]. The WEE1 kinase, regulated by both SOG1 and RBR1/E2FA complex, is negatively controlling the activity of CDKs by their inhibitory phosphorylation (at Thr-14 and Tyr-15 positions), which leads to delay of S-phase progression [85,170]. Recently, it has been suggested that WEE1 can induce cell cycle arrest by phosphorylation of other proteins, e.g., FBL17 (F-box-like 17). FBL17 is an E3 ubiquitin ligase that promotes the degradation of CDK inhibitors. In Arabidopsis, phosphorylation of FBL17, driven by WEE1, directs it to degradation and hence leads to the accumulation of CDK inhibitors [171]. A novel cell cycle control mechanism regulated by WEE1 has been lately revealed in Arabidopsis by Wang et al. During replication stress, WEE1 was found to directly phosphorylate PRL1-the core protein of MAC (MOS4-associated complex) involved in alternative splicing. This phosphorylation leads to proteosomal degradation of PRL1, which in consequence induces intron retention of cell cycle genes (e.g., CYCD1;1 and CYCD3;1) contributing to cell cycle arrest in the S phase [172]. Such a delay of S-phase progression empowers DNA repair during replication stress.
6. Endoreduplication and Programmed Cell Death
In the case of an extreme number of DNA lesions, when DNA repair machinery is not able to fix them, the endoreduplication (endoreplication) may be activated. In the regular cell cycle, one DNA replication round is followed by mitotic division. Endoreduplication is a situation when the cell is replicating its DNA several times without the following mitosis, which causes an increase in ploidy level and usually leads to enlargement and differentiation of the cell [173]. Endoreduplication is known to be implicated in various stress responses in plants [174]. In general, the arrest of the cell cycle in the G2 phase triggers the transition from mitotic division to endoreduplication [175]. One of the most important factors required for the progression of endoreduplication in plants is the DNA topoisomerase VI complex, that in Arabidopsis is composed of AtSPO11-3 (one of the homologs of TOPOISOMERASE VIA), AtTOP6B (TOPOISOMERASE 6 SB), RHL1 (ROOT HAIRLESS 1), and MIDGET proteins [176,177,178]. The programmed induction of endoreduplication in response to DSBs in Arabidopsis helps to prevent the transmission of damaged DNA to daughter cells through proliferation, but, on the other hand, it maintains growth [31]. However, in some plant species, such as rice (Oryza sativa), endoreduplication does not occur as a response to extreme DNA damage [179].
In order to erase damaged cells, in the case of severe genotoxic stress, programmed cell death (PCD) may be activated. It is a genetically regulated biochemical pathway of organized cell destruction [180]. It is an essential element of normal plant development [181], but additionally, it may be activated upon different stresses, both biotic and abiotic, including genotoxic stress [182]. In humans, PCD induced by DNA stress is governed by p53 and caspase cascade. Caspases are cysteine proteases that cleave a set of target proteins causing disassembly of the cell [183]. However, in plants, there are no homologs of either p53 or caspases. SOG1, as a functional homolog of p53, is the key player in PCD in response to severe genotoxic stress, together with its two targets—also NAC transcription factors—ANAC044 and ANAC085 [167]. However, the downstream PCD effectors are not yet fully elucidated in plants [182].
7. Concluding Remarks
The proper functioning of the DDR pathway is crucial to preserve genome integrity. It is responsible for orchestrating the processes of DNA repair, cell cycle stoppage, and cell death upon genotoxic stress that may be caused by many factors, including environmental ones. Our understanding of plant DDR has greatly increased over recent years; however, it still lags behind that of animal and human DDR. Most of the knowledge collected for plants concerns a model species Arabidopsis thaliana, and the new challenge is to reveal the details of these processes in crops. Plants exposed to DNA-damaging conditions display a significant reduction in productivity and yield, which can have a huge agronomic and economic impact. Detailed knowledge about maintaining the DDR pathway in crops may be useful in the breeding of new cultivars more tolerant to stresses causing DNA damage.
Author Contributions
Writing—original draft preparation, M.S.-Z.; writing—review and editing, I.S.; figures preparation and mauscript editing, P.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Science Centre, Poland (grant Beethoven Life1 2018/31/F/NZ2/03952).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Manova, V.; Gruszka, D. DNA damage and repair in plants—From models to crops. Front. Plant Sci. 2015, 6, 885. [Google Scholar] [CrossRef]
- Dudáš, A.; Chovanec, M. DNA double-strand break repair by homologous recombination. Mutat. Res. Rev. Mutat. Res. 2004, 566, 131. [Google Scholar] [CrossRef]
- Sonoda, E.; Hochegger, H. Differential usage of non-homologous end-joining and homologous recombina-tion in double strand break repair. DNA Repair 2006, 5, 1021. [Google Scholar] [CrossRef]
- Kleibl, K. Molecular mechanisms of adaptive response to alkylating agents in Escherichia coli and some remarks on O(6)-methylguanine DNA-methyltransferase in other organisms. Mutat. Res. 2002, 512, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Shrivastav, N.; Li, D.; Essigmann, J.M. Chemical biology of mutagenesis and DNA repair: Cellular responses to DNA alkylation. Carcinogenesis 2010, 31, 59–70. [Google Scholar] [CrossRef]
- Oladosu, Y.; Rafii, M.Y.; Abdullah, N.; Hussin, G.; Ramli, A.; Rahim, H.A.; Miah, G.; Usman, M. Principle and application of plant mutagenesis in crop improvement: A review. Biotechnol. Biotechnol. Equip. 2016, 30, 1–16. [Google Scholar] [CrossRef]
- Till, B.J.; Cooper, J.; Tai, T.H.; Colowit, P.; Greene, E.A.; Henikoff, S.; Comai, L. Discovery of chemically induced mutations in rice by TILLING. BMC Plant Biol. 2007, 7, 19. [Google Scholar] [CrossRef]
- Kurowska, M.; Daszkowska-Golec, A.; Gruszka, D.; Marzec, M.; Szurman, M.; Szarejko, I.; Maluszynski, M. TILLING—A shortcut in functional genomics. J. Appl. Genet. 2011, 52, 317. [Google Scholar] [CrossRef]
- Jankowicz-Cieslak, J.; Huynh, O.A.; Brozynska, M.; Nakitandwe, J.; Till, B.J. Induction, rapid fixation and retention of mutations in vegetatively propagated banana. Plant Biotechnol. J. 2012, 10, 1056–1066. [Google Scholar] [CrossRef]
- Chen, L.; Huang, L.; Min, D.; Phillips, A.; Wang, S.; Madgwick, P.J.; Parry, M.A.; Hu, Y.G. Development and characterization of a new TILLING population of common bread wheat (Triticum aestivum L.). PLoS ONE 2012, 7, e41570. [Google Scholar] [CrossRef]
- Tadele, Z. Mutagenesis and TILLING to dissect gene function in plants. Curr. Genom. 2016, 17, 499–508. [Google Scholar] [CrossRef]
- Espina, M.J.; Ahmed, C.M.S.; Bernardini, A.; Adeleke, E.; Yadegari, Z.; Arelli, P.; Pantalone, V.; Taheri, A. Development and Phenotypic Screening of an Ethyl Methane Sulfonate Mutant Population in Soybean. Front. Plant Sci. 2018, 9, 394. [Google Scholar] [CrossRef]
- Szurman-Zubrzycka, M.; Zbieszczyk, J.; Marzec, M.; Jelonek, J.; Chmielewska, B.; Kurowska, M.; Krok, M.; Daszkowska-Golec, A.; Guzy-Wrobelska, J.; Gruszka, D.; et al. HorTILLUS—A rich and renewable source of induced mutations for forward/reverse genetics and pre-breeding programs in barley (Hordeum vulgare L.). Front. Plant Sci. 2018, 9, 216. [Google Scholar] [CrossRef]
- Siddique, M.I.; Back, S.; Lee, J.H.; Jo, J.; Jang, S.; Han, K.; Venkatesh, J.; Kwon, J.K.; Jo, Y.D.; Kang, B.C. Development and Characterization of an Ethyl Methane Sulfonate (EMS) Induced Mutant Population in Capsicum annuum L. Plants 2020, 9, 396. [Google Scholar] [CrossRef]
- Karaman, K.; Kizil, S.; Başak, M.; Uzun, B.; Yol, E. Development of EMS-induced Mutagenized Groundnut Population and Discovery of Point Mutations in the ahFAD2 and Ara h 1 Genes by TILLING. J. Oleo Sci. 2021, 70, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- De Silva, I.U.; McHugh, P.J.; Clingen, P.H.; Hartley, J.A. Defining the roles of nucleotide excision repair and recombination in the repair of DNA interstrand crosslinks in mammalian cells. Mol. Cell Biol. 2000, 20, 7980–7990. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, L. DNA crosslinking damage and cancer—A tale of friend and foe. Transl. Cancer Res. 2013, 2, 144–154. [Google Scholar] [CrossRef]
- Weber, G.F. DNA Damaging Drugs. Molecular Therapies of Cancer; Springer: Berlin/Heidelberg, Germany, 2014; pp. 9–112. [Google Scholar] [CrossRef]
- Menke, M.; Chen, I.P.; Angelis, K.J.; Schubert, I. DNA damage and repair in Arabidopsis thaliana as measured by the comet assay after treatment with different classes of genotoxins. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2001, 493, 87–93. [Google Scholar] [CrossRef]
- Chen, I.P.; Haehnel, U.; Altschmied, L.; Schubert, I.; Puchta, H. The transcriptional response of Arabidopsis to genotoxic stress—A high-density colony array study (HDCA). Plant J. 2003, 35, 771–786. [Google Scholar] [CrossRef]
- Weimer, A.K.; Biedermann, S.; Harashima, H.; Roodbarkelari, F.; Takahashi, N.; Foreman, J.; Guan, Y.; Pochon, G.; Heese, M.; Van Damme, D.; et al. The plant-specific CDKB1-CYCB1 complex mediates homologous recombination repair in Arabidopsis. EMBO J. 2016, 35, 2068–2086. [Google Scholar] [CrossRef]
- Papadia, P.; Barozzi, F.; Hoeschele, J.D.; Piro, G.; Margiotta, N.; Di Sansebastiano, G.P. Cisplatin, oxaliplatin, and kiteplatin subcellular effects compared in a plant model. Int. J. Plant Sci. 2017, 18, 306. [Google Scholar] [CrossRef]
- Dorn, A.; Puchta, H. Analyzing Somatic DNA Repair in Arabidopsis Meiotic Mutants. In Plant Meiosis; Pradillo, M., Heckmann, S., Eds.; Humana: New York, NY, USA, 2007; Volume 2061, pp. 359–366. [Google Scholar] [CrossRef]
- Parra-Nunez, P.; Cooper, C.; Sanchez-Moran, E. The Role of DNA Topoisomerase Binding Protein 1 (TopBP1) in Genome Stability in Arabidopsis. Plants 2021, 10, 2568. [Google Scholar] [CrossRef]
- Rosa, M.; Scheid, O.M. DNA Damage Sensitivity Assays with Arabidopsis Seedlings. Bio Protoc. 2014, 4, e1093. [Google Scholar] [CrossRef]
- West, C.E.; Waterworth, W.M.; Sunderland, P.A.; Bray, C.M. Arabidopsis DNA double-strand break repair pathways. Biochem. Soc. Trans. 2004, 32, 964–966. [Google Scholar] [CrossRef]
- Charbonnel, C.; Gallego, M.E.; White, C.I. Xrcc1-dependent and Ku-dependent DNA double-strand break repair kinetics in Arabidopsis plants. Plant J. 2010, 64, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Stolarek, M.; Gruszka, D.; Braszewska-Zalewska, A.; Maluszynski, M. Functional analysis of the new barley gene HvKu80 indicates that it plays a key role in double-strand DNA break repair and telomere length regulation. Mutagenesis 2015, 30, 785–797. [Google Scholar] [CrossRef][Green Version]
- Stolarek, M.; Gruszka, D.; Braszewska-Zalewska, A.; Maluszynski, M. Alleles of newly identified barley gene HvPARP3 exhibit changes in efficiency of DNA repair. DNA Repair 2015, 28, 116–130. [Google Scholar] [CrossRef]
- Allawzi, A.; Elajaili, H.; Redente, E.F.; Nozik-Grayck, E. Oxidative toxicology of bleomycin: Role of the extracellular redox environment. Curr. Opin. Toxicol. 2019, 13, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Adachi, S.; Minamisawa, K.; Okushima, Y.; Inagaki, S.; Yoshiyama, K.; Kondou, Y.; Kaminuma, E.; Ka-washima, M.; Toyoda, T.; Matsui, M.; et al. Programmed induction of endoreduplication by DNA double-strand breaks in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 10004–10009. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Takatsuka, H.; Takahashi, N.; Kurata, R.; Fukao, Y.; Kobayashi, K.; Ito, M.; Umeda, M. Arabidopsis R1R2R3-Myb proteins are essential for inhibiting cell division in response to DNA damage. Nat. Commun. 2017, 8, 635. [Google Scholar] [CrossRef] [PubMed]
- Yoshiyama, K.O.; Kaminoyama, K.; Sakamoto, T.; Kimura, S. Increased Phosphorylation of Ser-Gln Sites on SUPPRESSOR OF GAMMA RESPONSE1 Strengthens the DNA Damage Response in Arabidopsis thaliana. Plant Cell 2017, 29, 3255–3268. [Google Scholar] [CrossRef] [PubMed]
- Čížková, M.; Slavková, M.; Vítovám, M.; Zachleder, V.; Bišová, K. Response of the Green Alga Chlamydomonas reinhardtii to the DNA Damaging Agent Zeocin. Cells 2019, 8, 735. [Google Scholar] [CrossRef] [PubMed]
- Saban, N.; Bujak, M. Hydroxyurea and hydroxamic acid derivatives as antitumor drugs. Cancer Chemotheraphy Pharmacol. 2009, 64, 213–221. [Google Scholar] [CrossRef]
- Madaan, K.; Kaushik, D.; Verma, T. Hydroxyurea: A key player in cancer chemotherapy. Expert Rev. Anticancer Ther. 2012, 1, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Musiałek, M.W.; Rybaczek, D. Hydroxyurea—The Good, the Bad and the Ugly. Genes 2021, 12, 1096. [Google Scholar] [CrossRef]
- Gualtieri, C.; Gianella, M.; Pagano, A.; Cadeddu, T.; Araújo, S.; Balestrazzi, A.; Macovei, A. Exploring microRNA Signatures of DNA Damage Response Using an Innovative System of Genotoxic Stress in Medicago truncatula Seedlings. Front. Plant Sci. 2021, 12, 645323. [Google Scholar] [CrossRef]
- Buta, J.G.; Worley, J.F. Camptothecin, a selective plant growth regulator. J. Agric. Food Chem. 1976, 24, 1085–1086. [Google Scholar] [CrossRef]
- Locato, V.; Balestrazzi, A.; De Gara, L.; Carbonera, D. Reduced expression of top1beta gene induces programmed cell death and alters ascorbate metabolism in Daucus carota cultured cells. J. Exp. Bot. 2006, 57, 1667–1676. [Google Scholar] [CrossRef]
- Iakimova, E.T.; Yordanova, Z.P.; Cristescu, S.M.; Harren, F.F.M.; Woltering, E.J. Cell death associated release of volatile organic sulphur compounds with antioxidant properties in chemical-challenged tobacco BY-2 suspension cultured cells. J. Plant Physiol. 2020, 251, 153223. [Google Scholar] [CrossRef]
- Pommier, Y. Topoisomerase I inhibitors: Camptothecins and beyond. Nat. Rev. Cancer 2006, 6, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y.; Leo, E.; Zhang, H.; Marchand, C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010, 17, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Prochazkova, K.; Finke, A.; Tomaštíková, E.D.; Filo, J.; Bente, H.; Dvořák, P.; Ovečka, M.; Šamaj, J.; Pecinka, A. Zebularine induces enzymatic DNA–protein crosslinks in 45S rDNA heterochromatin of Arabidopsis nuclei. Nucleic Acids Res. 2022, 50, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ryu, T.; Lee, S.; Lee, S.; Chung, B. Ionizing Radiation manifesting DNA damage response in plants: An overview of DNA damage signaling and repair mechanisms in plants. Plant Sci. 2019, 278, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Kumar, A.; Tyagi, M.; Sinha, R. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J. Nucleic Acids 2010, 29, 592980. [Google Scholar] [CrossRef] [PubMed]
- Dany, A.-L.; Douki, T.; Triantaphylides, C.; Cadet, J. Repair of the main UV-induced thymine dimeric lesions within Arabidopsisi thaliana DNA: Evidence for the major involvement of photoreactivation pathways. J. Photochem. Photobiol. B Biol. 2001, 65, 127–135. [Google Scholar] [CrossRef]
- Gill, S.; Anjum, M.; Gill, R.; Jka, M.; Tuteja, N. DNA damage and repair in plants under ultraviolet and ionizing radiations. Sci. World J. 2015, 2015, 250158. [Google Scholar] [CrossRef]
- Limoli, C.L.; Giedzinski, E.; Bonner, W.M.; Cleaver, J.E. UV-induced replication arrest in the xeroderma pigmentosum variant leads to DNA doublestrand breaks, γ-H2AX formation, and Mre11 relocalization. Proc. Natl. Acad. Sci. USA 2002, 99, 233–238. [Google Scholar] [CrossRef]
- Batista, L.F.Z.; Kaina, B.; Meneghini, R.; Menck, C.F.M. How DNA lesions are turned into powerful killing structures: Insights from UV-induced apoptosis. Mutat. Res. 2009, 681, 197–208. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Chan, Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef]
- Nisa, M.-U.; Huang, Y.; Benhamed, M.; Raynaud, C. The plant DNA Damage Response: Signaling pathways leading to growth inhibition and putative role in response to stress conditions. Front. Plant Sci. 2019, 10, 653. [Google Scholar] [CrossRef]
- Koukalová, B.; Kovarík, A.; Fajkus, J.; Siroký, J. Chromatin fragmentation associated with apoptotic changes in tobacco cells exposed to cold stress. FEBS Lett. 1997, 414, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Savina, M.; Du, J.; Devendran, A.; Kannivadi Ramakanth, K.; Tian, X.; Sim, W.S.; Mironova, V.V.; Xu, J. A Sacrifice-for-Survival Mechanism Protects Root Stem Cell Niche from Chilling Stress. Cell 2017, 170, 102–113.e14. [Google Scholar] [CrossRef] [PubMed]
- Pecinka, A.; Dinh, H.Q.; Baubec, T.; Rosa, M.; Lettner, N.; Mittelsten Scheid, O. Epigenetic regulation of repetitive elements is attenuated by prolonged heat stress in Arabidopsis. Plant Cell 2010, 22, 3118–3129. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Kajihara, A.; Kirita, T.; Mori, E. Heat meets DNA: DNA damage and repair. Therm. Med. 2018, 34, 15–22. [Google Scholar] [CrossRef]
- Han, S.H.; Kim, J.Y.; Lee, J.H.; Park, C.M. Safeguarding genome integrity under heat stress in plants. J. Exp. Bot. 2021, 3, erab355. [Google Scholar] [CrossRef]
- Boyko, A.; Golubov, A.; Bilichak, A.; Kovalchuk, I. Chlorine ions but not sodium ions alter genome stability of Arabidopsis thaliana. Plant Cell Physiol. 2010, 51, 1066–1078. [Google Scholar] [CrossRef]
- Sihi, S.; Bakshi, S.; Maiti, S.; Nayak, A.; Sengupta, D.N. Analysis of DNA polymerase λ activity and gene expression in response to salt and drought stress in Oryza sativa Indica rice cultivars. J. Plant Growth Regul. 2022, 41, 1499–1515. [Google Scholar] [CrossRef]
- Pedroza-Garcia, J.A.; Xiang, Y.; De Veylder, L. Cell cycle checkpoint control in response to DNA damage by environmental stresses. Plant J. 2022, 109, 490–507. [Google Scholar] [CrossRef]
- Saha, P.; Mukherjee, A.; Biswas, A.K. Modulation of NaCl induced DNA damage and oxidative stress in mungbean by pretreatment with sublethal dose. Biol. Plant. 2014, 59, 139–146. [Google Scholar] [CrossRef]
- Zvanarou, S.; Vágnerová, R.; Mackievic, V.; Usnich, S.; Smolich, I.; Sokolik, A.; Yu, M.; Huang, X.; Angelis, K.J.; Demidchik, V. Salt stress triggers generation of oxygen free radicals and DNA breaks in Physcomitrella patens protonema. Environ. Exp. Bot. 2020, 180, 104236. [Google Scholar] [CrossRef]
- Dutta, S.; Mitra, M.; Agarwal, P.; Mahapatra, K.; De, S.; Sett, U.; Roy, S. Oxidative and genotoxic damages in plants in response to heavy metal stress and maintenance of genome stability. Plant Signal. Behav. 2018, 13, e1460048. [Google Scholar] [CrossRef] [PubMed]
- Nezames, C.; Sjogren, C.; Barajas, J.; Larsen, P. The Arabidopsis cell cycle checkpoint regulators TANMEI/ALT2 and ATR mediate the active process of aluminum-dependent root growth inhibition. Plant Cell 2012, 24, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Jaskowiak, J.; Tkaczyk, O.; Slota, M.; Kwasniewksa, J.; Szarejko, I. Analysis of aluminium toxicity in Hordeum vulgare roots with an emphasis on DNA integrity and cell cycle. PLoS ONE 2018, 13, e0193156. [Google Scholar] [CrossRef] [PubMed]
- Szurman-Zubrzycka, M.; Nawrot, M.; Jelonek, J.; Dziekanowski, M.; Kwasniewska, J.; Szarejko, I. ATR, a DNA damage signaling kinase, is involved in aluminium response in barley. Front. Plant Sci. 2019, 10, 1299. [Google Scholar] [CrossRef] [PubMed]
- Szurman-Zubrzycka, M.; Chwailkowska, K.; Niemira, M.; Kwasniewski, M.; Nawrot, M.; Gajecka, M.; Larsen, P.; Szarejko, I. Aluminum or low pH—which is the bigger enemy of barley? Transcriptome analysis of barley root meristem under Al and low pH stress. Front. Genet. 2021, 12, 675260. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, T.; Barnes, D. Repair of endogenous DNA damage. Cold Spring Harb. Symp. Quant. Biol. 2000, 65, 127–133. [Google Scholar] [CrossRef]
- Maréchal, A.; Zou, L. DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb. Perspect. Biol. 2013, 5, a012716. [Google Scholar] [CrossRef]
- Amiard, S.; Charbonnel, C.; Allain, E.; Depeiges, A.; White, C.I.; Gallego, M.E. Distinct roles of the ATR kinase and the Mre11-Rad50-Nbs1 complex in the maintenance of chromosomal stability in Arabidopsis. Plant Cell 2010, 22, 3020–3033. [Google Scholar] [CrossRef]
- Falck, J.; Coates, J.; Jackson, S.P. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature 2005, 434, 605–611. [Google Scholar] [CrossRef]
- Saldivar, J.C.; Cortez, D.; Cimprich, K.A. The essential kinase ATTR: Ensuring faithful duplication of a challenging genome. Nat. Rev. Mol. Cell Biol. 2017, 18, 622–636. [Google Scholar] [CrossRef]
- Garcia, V.; Bruchet, H.; Camescasse, D.; Granier, F.; Bouchez, D.; Tissier, A. AtATM is essential for meiosis and the somatic response to DNA damage in plants. Plant Cell 2003, 15, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Culligan, K.; Tissier, A.; Britt, A. ATR regulates a G2-phase cell-cycle checkpoint in Arabidopsis thaliana. Plant Cell 2004, 16, 1091–1104. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, P.R.; Britt, A.B.; Culligan, K.M. The Arabidopsis ATRIP ortholog is required for a programmed response to replication inhibitors. Plant J. 2009, 60, 518–526. [Google Scholar] [CrossRef]
- Heitzeberg, F.; Chen, I.-P.; Hartung, F.; Orel, N.; Angelis, K.J.; Puchta, H. The Rad17 homologue of Ara-bidopsis is involved in the regulation of DNA damage repair and homologous recombination. Plant J. 2004, 38, 954–968. [Google Scholar] [CrossRef] [PubMed]
- Aklilu, B.B.; Soderquist, R.S.; Culligan, K.M. Genetic analysis of the replication protein A large subunit family in Arabidopsis reveals unique and overlapping roles in DNA repair, meiosis and DNA replication. Nucleic Acids Res. 2014, 42, 3104–3118. [Google Scholar] [CrossRef]
- Mah, L.J.; El-Osta, A.; Karagiannis, T. γH2AX: A sensitive molecular marker of DNA damage and repair. Leukemia 2010, 24, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Kang, H.; Wu, D.; Zhu, X.; Huang, L.; Wu, J.; Zhu, Y. Arabidopsis γ-H2A. X-INTERACTING PROTEIN participates in DNA damage response and safeguards chromatin stability. Nat. Commun. 2022, 13, 1–14. [Google Scholar] [CrossRef]
- Yoshiyama, K.O.; Kobayashi, J.; Ogita, N.; Uead, M.; Kimura, S.; Maki, H.; Umeda, M. ATM-mediated phosphorylation of SOG1 is essential for the DNA damage response in Arabidopsis. EMBO Rep. 2013, 14, 817–822. [Google Scholar] [CrossRef]
- Sjogren, C.A.; Bolaris, S.C.; Larsen, P.B. Aluminum-Dependent Terminal Differentiation of the Arabidopsis Root Tip Is Mediated through an ATR-, ALT2-, and SOG1-Regulated Transcriptional Response. Plant Cell 2015, 27, 2501–2515. [Google Scholar] [CrossRef]
- Preuss, S.B.; Britt, A.B. A DNA-damage-induced cell cycle checkpoint in Arabidopsis. Genetics 2003, 164, 323–334. [Google Scholar] [CrossRef]
- Yoshiyama, K.; Conklin, P.; Huefner, N.D.; Britt, A.B. Suppressor of gamma response 1 (SOG1) encodes a putative transcription factor governing multiple responses to DNA damage. Proc. Natl. Acad. Sci. USA 2009, 106, 12843–12848. [Google Scholar] [CrossRef] [PubMed]
- Yoshiyama, K. SOG1: A master regulator of the DNA damage response in plants. Genes Genet. Syst. 2016, 90, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Ogita, N.; Okushima, Y.; Tokizawa, M.; Yamamoto, Y.Y.; Tanaka, M.; Seki, M.; Makita, Y.; Matsui, M.; Okamoto-Yoshiyama, K.; Sakamoto, T.; et al. Identifying the target genes of SUPPRESSOR OF GAMMA RESPONSE 1, a master transcription factor controlling DNA damage response in Arabidopsis. Plant J. 2018, 94, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Bourbousse, C.; Vegesna, N.; Law, J.A. SOG1 activator and MYB3R repressors regulate a complex DNA damage network in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, E12453–E12462. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.; Kamei, C.L.A.; Cools, T.; Vanderauwera, S.; Takahashi, N.; Okushima, Y.; Eekhout, T.; Yoshiyama, K.O.; Larkin, J.; Van den Daele, H.; et al. The Arabidopsis SIAMESE-RELATED Cyclin-Dependent Kinase Inhibitors SMR5 and SMR7 Regulate the DNA Damage Checkpoint in Response to Reactive Oxygen Species. Plant Cell 2014, 26, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Roitinger, E.; Hofer, M.; Köcher, T.; Pichler, P.; Novatchkova, M.; Yang, J.; Schlögelhofer, P.; Mechtler, K. Quantitative phosphoproteomics of the ataxia telangiectasia-mutated (ATM) and ataxia telangiectasia-mutated and rad3-related (ATR) dependent DNA damage response in Arabidopsis thaliana. Mol. Cell Proteom. 2015, 14, 556–571. [Google Scholar] [CrossRef] [PubMed]
- Berckmans, B.; De Veylder, L. Transcriptional control of the cell cycle. Curr. Opin. Plant Biol. 2009, 12, 599–605. [Google Scholar] [CrossRef]
- Horvath, B.M.; Kourova, H.; Nagy, S.; Nemeth, E.; Magyar, Z.; Papdi, C.; Ahmad, Z.; Sanchez-Perez, G.F.; Perilli, S.; Blilou, I.; et al. Arabidopsis RETINOBLASTOMA RELATED directly regulates DNA damage responses through functions beyond cell cycle control. EMBO J. 2017, 36, 1261–1278. [Google Scholar] [CrossRef]
- Biedermann, S.; Harashima, H.; Chen, P.; Heese, M.; Bouyer, D.; Sofroni, K.; Schnittger, A. The retinoblas-toma homolog RBR1 mediates localization of the repair protein RAD51 to DNA lesions in Arabidopsis. EMBO J. 2017, 36, 1279–1297. [Google Scholar] [CrossRef]
- Bouyer, D.; Heese, M.; Chen, P.; Harashima, H.; Roudier, F.; Grüttner, C.; Schnittger, A. Genome-wide identification of RETINOBLASTOMA RELATED 1 binding sites in Arabidopsis reveals novel DNA damage regulators. PLoS Genet. 2018, 14, e1007797. [Google Scholar] [CrossRef]
- De Medeiros, N.M.C.; De Medeiros, A.L.M.; Silva, H.C.; Scortecci, K.C. Recent advances in plant DNA repair. In Advances in Plant DNA Repair; IntechOpen: London, UK, 2015. [Google Scholar] [CrossRef]
- Spampinato, C.P. Protecting DNA from errors and damage: An overview of DNA repair mechanisms in plants compared to mammals. Cell Mol. Life Sci. 2017, 74, 1693–1709. [Google Scholar] [CrossRef] [PubMed]
- Raina, A.; Sahu, P.K.; Laskar, R.A.; Rajora, N.; Sao, R.; Khan, S.; Ganai, R.A. Mechanisms of Genome Maintenance in Plants: Playing It Safe with Breaks and Bumps. Front Genet. 2021, 12, 675686. [Google Scholar] [CrossRef] [PubMed]
- Krokan, H.E.; Bjoras, M. Base excision repair. Cold Spring Harb. Perspect. Biol. 2013, 5, a012583. [Google Scholar] [CrossRef] [PubMed]
- Krokan, H.E.; Drablos, F.; Slupphaug, G. Uracil in DNA—Occurrence, consequences and repair. Oncogene 2002, 21, 8935–8938. [Google Scholar] [CrossRef]
- Córdoba-Cañero, D.; Dubois, E.; Ariza, R.R.; Doutriaux, M.-P.; Roldán-Arjona, T. Arabidopsis Uracil DNA Glycosylase (UNG) Is Required for Base Excision Repair of Uracil and Increases Plant Sensitivity to 5-Fluorouracil. J. Biol. Chem. 2010, 285, 7475–7483. [Google Scholar] [CrossRef]
- Córdoba-Cañero, D.; Morales-Ruiz, T.; Roldán-Arjona, T.; Ariza, R.R. Single-nucleotide and long-patch base excision repair of DNA damage in plants. Plant J. 2009, 60, 716–728. [Google Scholar] [CrossRef]
- Córdoba-Cañero, D.; Roldán-Arjona, T.; Ariza, R.R. Arabidopsis ARP endonuclease functions in a branched base excision DNA repair pathway completed by LIG1. Plant J. 2011, 68, 693–702. [Google Scholar] [CrossRef]
- Santerre, A.; Britt, A.B. Cloning of a 3-methyladenine-DNA glycosylase from Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1994, 91, 2240–2244. [Google Scholar] [CrossRef]
- Memisoglu, A.; Samson, L. Base excision repair in yeast and mammals. Mutat. Res 2000, 451, 39–51. [Google Scholar] [CrossRef]
- Srivastava, D.K.; Berg, B.J.; Prasad, R.; Molina, J.T.; Beard, W.A.; Tomkinson, A.E.; Wilson, S.H. Mammalian abasic site base excision repair. Identification of the reaction sequence and rate-determining steps. J. Biol. Chem. 1998, 273, 21203–21209. [Google Scholar] [CrossRef]
- Levin, D.S.; Bai, W.; Yao, N.; O’Donnell, M.; Tomkinson, A.E. An interaction between DNA ligase I and proliferating cell nuclear antigen: Implications for Okazaki fragment synthesis and joining. Proc. Natl. Acad. Sci. USA 1997, 94, 12863–12868. [Google Scholar] [CrossRef]
- Nash, R.A.; Caldecott, K.W.; Barnes, D.E.; Lindahl, T. XRCC1 protein interacts with one of two distinct forms of DNA ligase III. Biochemistry 1997, 36, 5207–5211. [Google Scholar] [CrossRef] [PubMed]
- Roldán-Arjona, T.; Ariza, R.R.; Córdoba-Cañero, D. DNA Base Excision Repair in Plants: An Unfolding Story with Familiar and Novel Characters. Front. Plant Sci. 2019, 10, 1055. [Google Scholar] [CrossRef] [PubMed]
- Córdoba-Cañero, D.; Roldán-Arjona, T.; Ariza, R.R. Arabidopsis ZDP DNA 3′-phosphatase and ARP en-donuclease function in 8-oxoG repair initiated by FPG and OGG1 DNA glycosylases. Plant J. 2014, 79, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liang, W.; Li, Y.; Qian, W. Apurinic/apyrimidinic endonuclease2 and zinc finger dna 3′-phosphoesterase play overlapping roles in the maintenance of epigenome and genome stability. Plant Cell 2018, 30, 1954–1970. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, Y.; Suzuki, Y.; Sakaguchi, K. Characterization of plant XRCC1 and its interaction with proliferating cell nuclear antigen. Planta 2008, 227, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, Y.; Takeuchi, R.; Kodera, H.; Sakaguchi, K. Distribution and roles of X-family DNA polymerases in eukaryotes. Biochimie 2009, 91, 165–170. [Google Scholar] [CrossRef]
- Waterworth, W.M.; Masnavi, G.; Bhardwaj, R.M.; Jiang, Q.; Bray, C.M.; West, C.E. A plant DNA ligase is an important determinant of seed longevity. Plant J. 2010, 63, 848–860. [Google Scholar] [CrossRef]
- West, C.E.; Waterworth, W.M.; Jiang, Q.; Bray, C.M. Arabidopsis DNA ligase IV is induced by gamma-irradiation and interacts with an Arabidopsis homologue of the double strand break repair protein XRCC4. Plant J. 2000, 24, 67–78. [Google Scholar] [CrossRef]
- van Attikum, H.; Bundock, P.; Overmeer, R.M.; Lee, L.Y.; Gelvin, S.B.; Hooykaas, P.J. The Arabidopsis AtLIG4 gene is required for the repair of DNA damage, but not for the integration of Agrobacterium T-DNA. Nucleic Acids Res. 2003, 31, 4247–4255. [Google Scholar] [CrossRef]
- Martínez-Macías, M.I.; Córdoba-Cañero, D.; Ariza, R.R.; Roldán-Arjona, T. The DNA repair protein XRCC1 functions in the plant DNA demethylation pathway by stimulating cytosine methylation (5-meC) excision, gap tailoring, and DNA ligation. J. Biol. Chem. 2013, 288, 5496–5505. [Google Scholar] [CrossRef]
- Macovei, A.; Balestrazzi, A.; Confalonieri, M.; Faè, M.; Carbonera, D. New insights on the barrel medic MtOGG1 and MtFPG functions in relation to oxidative stress response in planta and during seed imbibition. Plant Physiol. Biochem. 2011, 49, 1040. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Roy, S.; Choudhury, S.R.; Sengupta, D.N. DNA repair and recombination in higher plants: Insights from comparative genomics of Arabidopsis and rice. BMC Genom. 2010, 11, 443. [Google Scholar] [CrossRef] [PubMed]
- Hanawalt, P.C.; Spivak, G. Transcription-coupled DNA repair: Two decades of progress and surprises. Nat. Rev. Mol. Cell Biol. 2008, 9, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Schärer, O.D. Nucleotide excision repair in eukaryotes. Cold Spring Harb. Perspect. Biol. 2013, 5, a012609. [Google Scholar] [CrossRef]
- Reed, S.H. Nucleotide excision repair in chromatin: Damage removal at the drop of a HAT. DNA Repair 2011, 10, 734–742. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, H.; Zhang, J.; Guo, G.; Schumaker, K.; Guo, Y. Arabidopsis cockayne syndrome A-like proteins 1A and 1B form a complex with CULLIN4 and damage DNA binding protein 1A and regulate the response to UV irradiation. Plant Cell 2010, 22, 2353–2369. [Google Scholar] [CrossRef]
- Biedermann, S.; Hellmann, H. The DDB1a interacting proteins ATCSA-1 and DDB2 are critical factors for UV-B tolerance and genomic integrity in Arabidopsis thaliana. Plant J. 2010, 62, 404–415. [Google Scholar] [CrossRef]
- Molinier, J.; Lechner, E.; Dumbliauskas, E.; Genschik, P. Regulation and role of Arabidopsis CUL4-DDB1A-DDB2 in maintaining genome integrity upon UV stress. PLoS Genet. 2008, 4, e1000093. [Google Scholar] [CrossRef]
- Ganpudi, A.; Schroeder, D. Genetic interactions of Arabidopsis thaliana damaged DNA binding protein 1B (DDB1B) with DDB1A, DET1, and COP1. Genes Genomes Genet. 2013, 3, 493–503. [Google Scholar] [CrossRef]
- Koga, A.; Ishibashi, T.; Kimura, S.; Uchiyama, Y.; Sakaguchi, K. Characterization of T-DNA insertion mutants and RNAi silenced plants of Arabidopsis thaliana UV-damaged DNA binding protein 2 (AtUV-DDB2). Plant Mol. Biol. 2006, 61, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Ly, V.; Hatherell, A.; Kim, E.; Chan, A.; Belmonte, M.; Schroeder, D. Interactions between Arabidopsis DNA repair genes UVH6, DDB1A, and DDB2 during abiotic stress tolerance and floral development. Plant Sci. 2013, 213, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Brettel, K.; Byrdin, M. Reaction mechanisms of DNA photolyase. Curr. Opin. Struct. Biol. 2010, 20, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Hitomi, K.; DiTacchio, L.; Arvai, A.S.; Yamamoto, J.; Kim, S.T.; Todo, T.; Tainer, J.A.; Iwai, S.; Panda, S.; Getzoff, E.D. Functional motifs in the (6-4) photolyase crystal structure make a comparative framework for DNA repair photolyases and clock cryptochromes. Proc. Natl. Acad. Sci. USA 2009, 106, 6962–6967. [Google Scholar] [CrossRef]
- Hitomi, K.; Arvai, A.S.; Yamamoto, J.; Hitomi, C.; Teranishi, M.; Hirouchi, T.; Yamamoto, K.; Iwai, S.; Tainer, J.A.; Hidema, J.; et al. Eukaryotic class II cyclobutane pyrimidine dimer photolyase structure reveals basis for improved ultraviolet tolerance in plants. J. Biol. Chem. 2012, 287, 12060–12069. [Google Scholar] [CrossRef]
- Chirinos-Arias, M.C.; Spampinato, C.P. Growth and development of AtMSH7 mutants in Arabidopsis thaliana. Plant Physiol. Biochem. 2020, 146, 329–336. [Google Scholar] [CrossRef]
- Verma, P.; Tandon, R.; Yadav, G.; Gaur, V. Structural Aspects of DNA Repair and Recombination in Crop Improvement. Front. Genet. 2020, 11, 574549. [Google Scholar] [CrossRef]
- Marti, T.M.; Kunz, C.; Fleck, O. DNA mismatch repair and mutation avoidance pathways. J. Cell. Physiol. 2002, 191, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Culligan, K.; Lamers, M.; Hays, J. Dissimilar mispair-recognition spectra of arabidopsis DNA-mismatch-repair proteins MSH2·MSH6 (MutSα) and MSH2·MSH7 (MutSγ). Nucleic Acids Res. 2003, 31, 6027–6034. [Google Scholar] [CrossRef]
- Tian, L.; Gu, L.; Li, G.M. Distinct nucleotide binding/hydrolysis properties and molar ratio of MutSalpha and MutSbeta determine their differential mismatch binding activities. J. Biol. Chem. 2009, 284, 11557–11562. [Google Scholar] [CrossRef]
- Wang, H.; Lawrence, C.; Li, G.-M.; Hays, J. Specific Binding of Human MSH2·MSH6 Mismatch-Repair Protein Heterodimers to DNA Incorporating Thymine- or Uracil-containing UV Light Photoproducts Opposite Mismatched Bases. J. Biol. Chem. 1999, 274, 16894–16900. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cao, Q.; Zhao, Q.; Arfan, M.; Liu, W. Mechanisms used by DNA MMR system to cope with Cadmium-induced DNA damage in plants. Chemosphere 2020, 246, 25614. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qin, J. MSH2 and ATR form a signaling module and regulate two branches of the damage response to DNA methylation. Proc. Natl. Acad. Sci. USA 2003, 100, 15387–15392. [Google Scholar] [CrossRef] [PubMed]
- Ortega, J.; Lee, G.S.; Gu, L.; Yang, W.; Li, G.M. Mispair-bound human MutS-MutL complex triggers DNA incisions and activates mismatch repair. Cell Res. 2021, 31, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Goellner, E.M.; Putnam, C.D.; Kolodner, R.D. Exonuclease 1-dependent and independent mismatch repair. DNA Repair. 2015, 32, 24–32. [Google Scholar] [CrossRef]
- Gujar, V.V.; Dahal, B.K.; Kadyrova, L.K.; Kadyrov, F. Exo1-independent MMR at euchromatin is errorprone and involves Pol ζ and Rev1. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Kadyrova, L.Y.; Dahal, B.K.; Gujar, V.; Daley, J.M.; Sung, P.; Kadyrov, F.A. The nuclease activity of DNA2 promotes exonuclease 1-independent mismatch repair. J. Biol. Chem. 2022, 298, 101831. [Google Scholar] [CrossRef]
- Jia, N.; Liu, X.; Gao, H. A DNA2 Homolog Is Required for DNA Damage Repair, Cell Cycle Regulation, and Meristem Maintenance in Plants. Plant Physiol. 2016, 171, 318–333. [Google Scholar] [CrossRef]
- Modrich, P. Mechanisms in eukaryotic mismatch repair. J. Biol. Chem. 2006, 281, 30305–30309. [Google Scholar] [CrossRef]
- Qiu, S.; Huang, J. MRN complex is an essential effector of DNA damage repair. J. Zhejiang Univ. Sci. B 2021, 22, 31–37. [Google Scholar] [CrossRef]
- Schmidt, C.; Pacher, M.; Puchta, H. DNA break repair in plant and its application for genome engineering. In Transgenic Plants. Methods in Molecular Biology; Kumar, S., Barone, P., Smith, M., Eds.; Humana Press: New York, NY, USA, 2019; Volume 1864, pp. 237–266. [Google Scholar] [CrossRef]
- Mannuss, A.; Trapp, O.; Puchta, H. Gene regulation in response to DNA damage. Biochim. Biophys. Acta 2012, 1819, 154. [Google Scholar] [CrossRef] [PubMed]
- Schröpfer, S.; Knoll, A.; Trapp, O.; Puchta, H. DNA Repair and Recombination in Plants. In Molecular Biology; Plant Sciences book series; Springer: New York, NY, USA, 2014; Volume 2, pp. 51–93. [Google Scholar] [CrossRef]
- Gorbunova, V.; Levy, A. How plants make ends meet: DNA double-strand break repair. Trends Plant Sci. 1999, 4, 263–269. [Google Scholar] [CrossRef]
- Bray, C.M.; West, C.E. DNA repair mechanisms in plants: Crucial sensors and effectors for the maintenance of genome integrity. New Phytol. 2005, 168, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, R.; Onyango, D.; Stark, J. Regulation of Single Strand Annealing and its role in genome maintenance. Trends Genet. 2016, 32, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Mladenov, E.; Iliakis, G. Induction and repair of DNA double strand breaks: The increasing spectrum of non-homologous end joining pathways. Mutat. Res. 2011, 711, 61–72. [Google Scholar] [CrossRef]
- Waterworth, W.; Drury, G.; Bray, C.; West, C. Repairing breaks in the plant genome: The importance of keeping it together. New Phytol. 2011, 192, 805–822. [Google Scholar] [CrossRef]
- Shen, H.; Li, Z. DNA double-strand break repairs and their application in plant DNA integration. Genes 2022, 13, 322. [Google Scholar] [CrossRef] [PubMed]
- Knoll, A.; Fauser, F.; Puchta, H. DNA recombination in somatic plant cells: Mechanisms and evolutionary consequences. Chromosome Res. 2014, 22, 191–201. [Google Scholar] [CrossRef]
- Sanchez-Moran, E.; Armstrong, S.J. Meiotic chromosome synapsis and recombination in Arabidopsis thali-ana: New ways of integrating cytological and molecular approaches. Chromosome Res. 2014, 22, 179–190. [Google Scholar] [CrossRef]
- Chen, H.; He, C.; Wang, C.; Wang, X.; Ruan, F.; Yan, J.; Yin, P.; Wang, Y.; Yan, S. RAD51 supports DMC1 by inhibiting the SMC5/6 complex during meiosis. Plant Cell 2021, 33, 2869–2882. [Google Scholar] [CrossRef]
- Gaj, T.; Gersbach, C.; Barbas, C.F., 3rd. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013, 31, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Pacher, M.; Puchta, H. From classical mutagenesis to nuclease-based breeding–directing natural DNA repair for a natural end-product. Plant J. 2017, 90, 819–833. [Google Scholar] [CrossRef]
- Liu, G.; Lin, Q.; Jin, S.; Gao, C. The CRISPR-Cas toolbox and gene editing technologies. Mol. Cell 2022, 82, 333–347. [Google Scholar] [CrossRef]
- Wei, W.; Ba, Z.; Gao, M.; Wu, Y.; Ma, Y.; Amiard, S.; White, C.; Danielsen, J.; Yang, Y.; Qi, Y. A role for small RNAs in DNA double-strand break repair. Cell 2012, 149, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Ou, X.; Han, D.; He, Z.; Liu, S.; Mao, N.; Zhang, Z.; Peng, C.L.; Lai, J.; Yang, C. A diRNA–protein scaffold module mediates SMC5/6 recruitment in plant DNA repair. Plant Cell 2022, 34, 3899–3914. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.; Cordero, G.; Kawamura, R.; Sverzhinsky, A.; Sarker, M.; Roy, S.; Malo, C.; Pascal, J.M.; Marko, J.F.; D’Amours, D. The Smc5/6 Core Complex Is a Structure-Specific DNA Binding and Compacting Machine. Mol. Cell. 2020, 80, 1025–1038.e5. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Mao, N.; Hu, H.; Tang, J.; Han, D.; Liu, S.; Wu, Q.; Liu, Y.; Peng, C.; Lai, J.; et al. A SWI/SNF subunit regulates chromosomal dissociation of structural maintenance complex 5 during DNA repair in plant cells. Proc. Natl. Acad. Sci. USA 2019, 116, 15288–15296. [Google Scholar] [CrossRef]
- Inagaki, S.; Umeda, M. Cell-cycle control and plant development. Int. Rev. Cell Mol. Biol. 2011, 291, 227–261. [Google Scholar] [CrossRef]
- Gentric, N.; Genschik, P.; Noir, S. Connections between the Cell Cycle and the DNA Damage Response in Plants. Int. J. Mol. Sci. 2021, 22, 9558. [Google Scholar] [CrossRef]
- Andersen, S.U.; Buechel, S.; Zhao, Z.; Ljung, K.; Novák, O.; Busch, W.; Schuster, C.; Lohmann, J.U. Requirement of B2-type cyclin-dependent kinases for meristem integrity in Arabidopsis thaliana. Plant Cell 2008, 20, 88–100. [Google Scholar] [CrossRef]
- Schnittger, A.; De Veylder, L. The Dual Face of Cyclin B1. Trends Plant Sci. 2018, 23, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Ogita, N.; Takahashi, T.; Taniguchi, S.; Tanaka, M.; Seki, M.; Umeda, M. A regulatory module controlling stress-induced cell cycle arrest in Arabidopsis. eLife 2019, 9, e43944. [Google Scholar] [CrossRef] [PubMed]
- Lang, L.; Pettkó-Szandtner, A.; Tunçay Elbaşı, H.; Takatsuka, H.; Nomoto, Y.; Zaki, A.; Dorokhov, S.; De Jaeger, G.; Eeckhout, D.; Ito, M.; et al. The DREAM complex represses growth in response to DNA damage in Arabidopsis. Life Sci. Alliance. 2021, 4, e202101141. [Google Scholar] [CrossRef] [PubMed]
- Cools, T.; Iantcheva, A.; Weimer, A.K.; Boens, S.; Takahashi, N.; Maes, S.; Van den Daele, H.; Van Isterdael, G.; Schnittger, A.; De Veylder, L. The Arabidopsis thaliana checkpoint kinase WEE1 protects against premature vascular differentiation during replication stress. Plant Cell 2011, 23, 1435–1448. [Google Scholar] [CrossRef] [PubMed]
- De Schutter, K.; Joubès, J.; Cools, T.; Verkest, A.; Corellou, F.; Babiychuk, E.; Van Der Schueren, E.; Beeckman, T.; Kushnir, S.; Inzé, D.; et al. Arabidopsis WEE1 kinase controls cell cycle arrest in response to activation of the DNA integrity checkpoint. Plant Cell 2007, 19, 211–225. [Google Scholar] [CrossRef]
- Pan, T.; Qin, Q.; Nong, C.; Gao, S.; Wang, L.; Cai, B.; Zhang, M.; Wu, C.; Chen, H.; Li, T.; et al. A novel WEE1 pathway for replication stress responses. Nat. Plants 2021, 7, 209–218. [Google Scholar] [CrossRef]
- Wang, L.; Zhan, L.; Zhao, Y.; Huang, Y.; Wu, C.; Pan, T.; Qin, Q.; Xu, Y.; Deng, Z.; Li, J.; et al. The ATR-WEE1 kinase module inhibits the MAC complex to regulate replication stress response. Nucleic Acids Res. 2021, 49, 1411–1425. [Google Scholar] [CrossRef]
- De Veylder, L.; Larkin, J.C.; Schnittger, A. Molecular control and function of endoreplication in development and physiology. Trends Plant Sci. 2011, 16, 624–634. [Google Scholar] [CrossRef]
- Lang, L.; Schnittger, A. Endoreplication—A means to an end in cell growth and stress response. Curr. Opin. Plant Biol. 2020, 54, 85–92. [Google Scholar] [CrossRef]
- Fox, D.T.; Duronio, R.J. Endoreplication and polyploidy: Insights into development and disease. Development 2013, 140, 3–12. [Google Scholar] [CrossRef]
- Sugimoto-Shirasu, K.; Stacey, N.J.; Corsar, J.; Roberts, K.; McCann, M.C. DNA topoisomerase VI is essential for endoreduplication in Arabidopsis. Curr. Biol. 2002, 12, 1782–1786. [Google Scholar] [CrossRef]
- Sugimoto-Shirasu, K.; Roberts, G.R.; Stacey, N.J.; McCann, M.C.; Maxwell, A.; Roberts, K. RHL1 is an essential component of the plant DNA topoisomerase VI complex and is required for ploidy-dependent cell growth. Proc. Natl. Acad. Sci. USA 2005, 102, 18736–18741. [Google Scholar] [CrossRef]
- Kirik, V.; Schrader, A.; Uhrig, J.F.; Hulskamp, M. MIDGET unravels functions of the Arabidopsis topoiso-merase VI complex in DNA endoreduplication, chromatin condensation, and transcriptional silencing. Plant Cell. 2007, 19, 3100–3110. [Google Scholar] [CrossRef]
- Endo, M.; Nakayama, S.; Umeda-Hara, C.; Ohtsuki, N.; Saika, H.; Umeda, M.; Toki, S. CDKB2 is involved in mitosis and DNA damage response in rice. Plant J. 2012, 69, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Danon, A.; Delorme, V.; Mailhac, N.; Gallois, P. Plant programmed cell death: A common way to die. Plant Physiol. Biochem. 2000, 38, 647–655. [Google Scholar] [CrossRef]
- Daneva, A.; Gao, Z.; Van Durme, M.; Nowack, M.K. Functions and Regulation of Programmed Cell Death in Plant Development. Annu. Rev. Cell Dev. Biol. 2016, 32, 441–468. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.; Schwarze, J.; Sherwood, O.L.; Jnaid, Y.; McCabe, P.F.; Kacprzyk, J. Stressed to Death: The Role of Transcription Factors in Plant Programmed Cell Death Induced by Abiotic and Biotic Stimuli. Front. Plant Sci. 2020, 11, 1235. [Google Scholar] [CrossRef]
- Chowdhury, I.; Tharakan, B.; Bhat, G.K. Current concepts in apoptosis: The physiological suicide program revisited. Cell Mol. Biol. Lett. 2006, 11, 506–525. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).