Antineoplastic Activity of Pazopanib in Anaplastic Thyroid Cancer in Primary Culture
Abstract
1. Introduction
2. Results
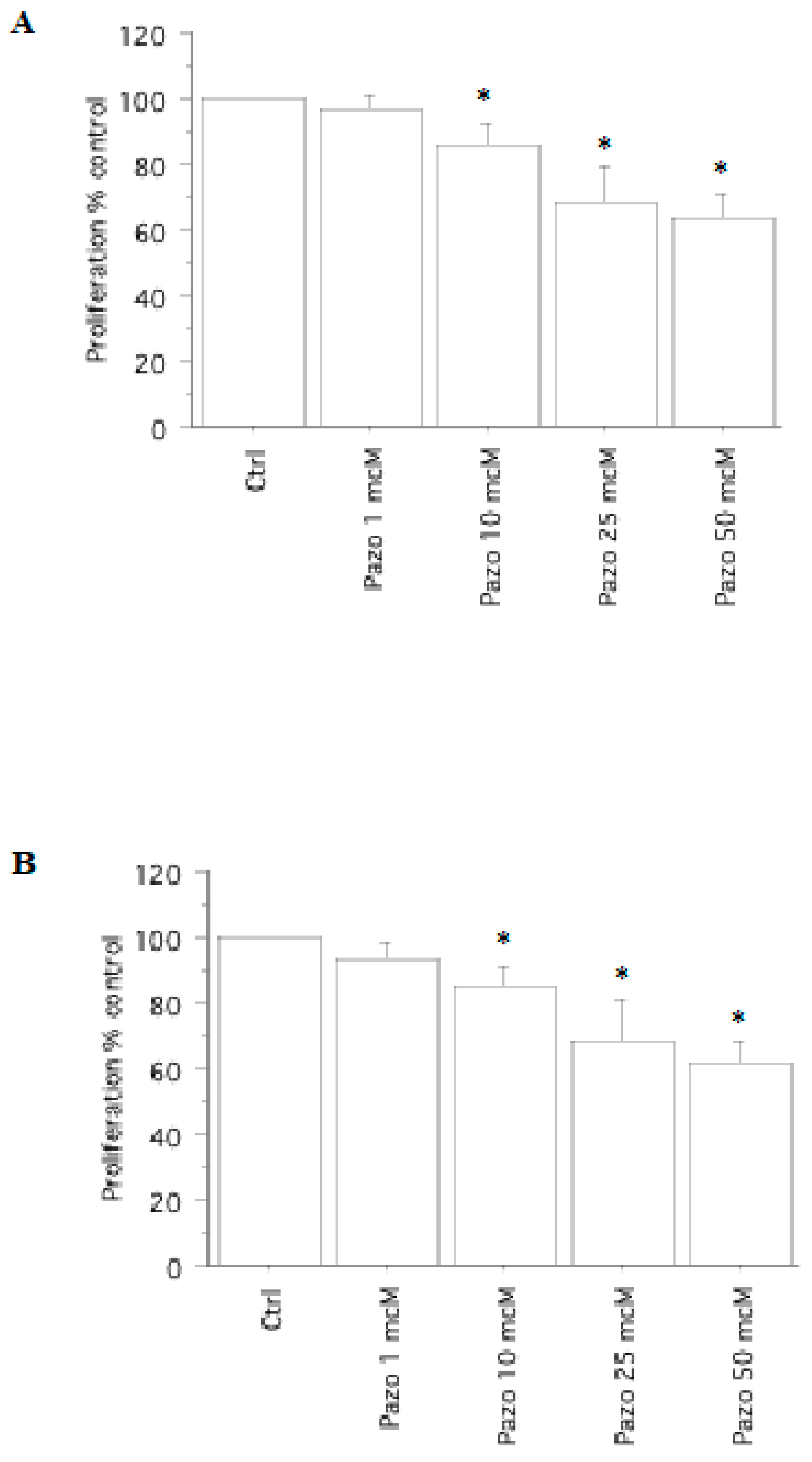
2.1. Cell Viability and Proliferation Assay
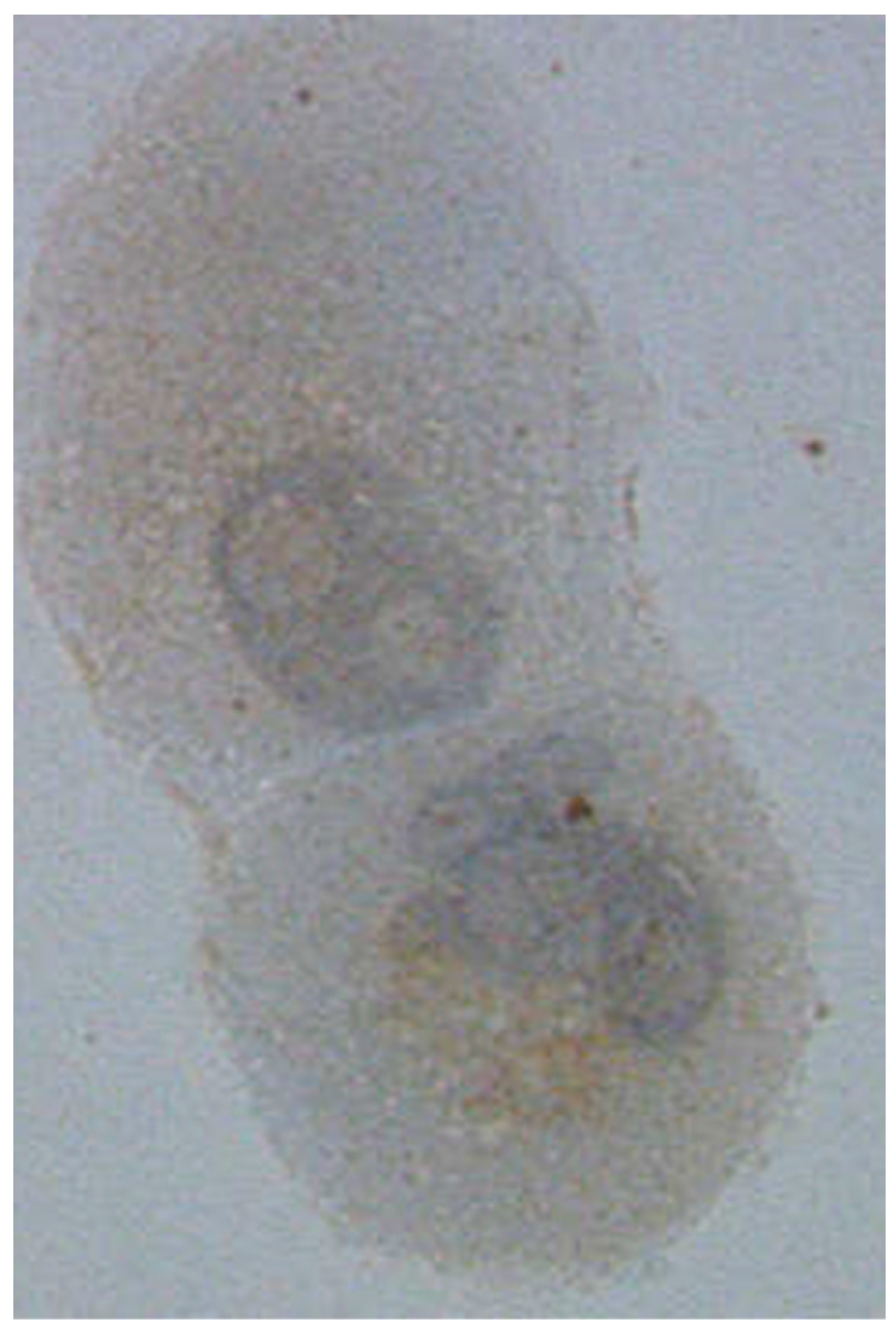
2.2. Apoptosis
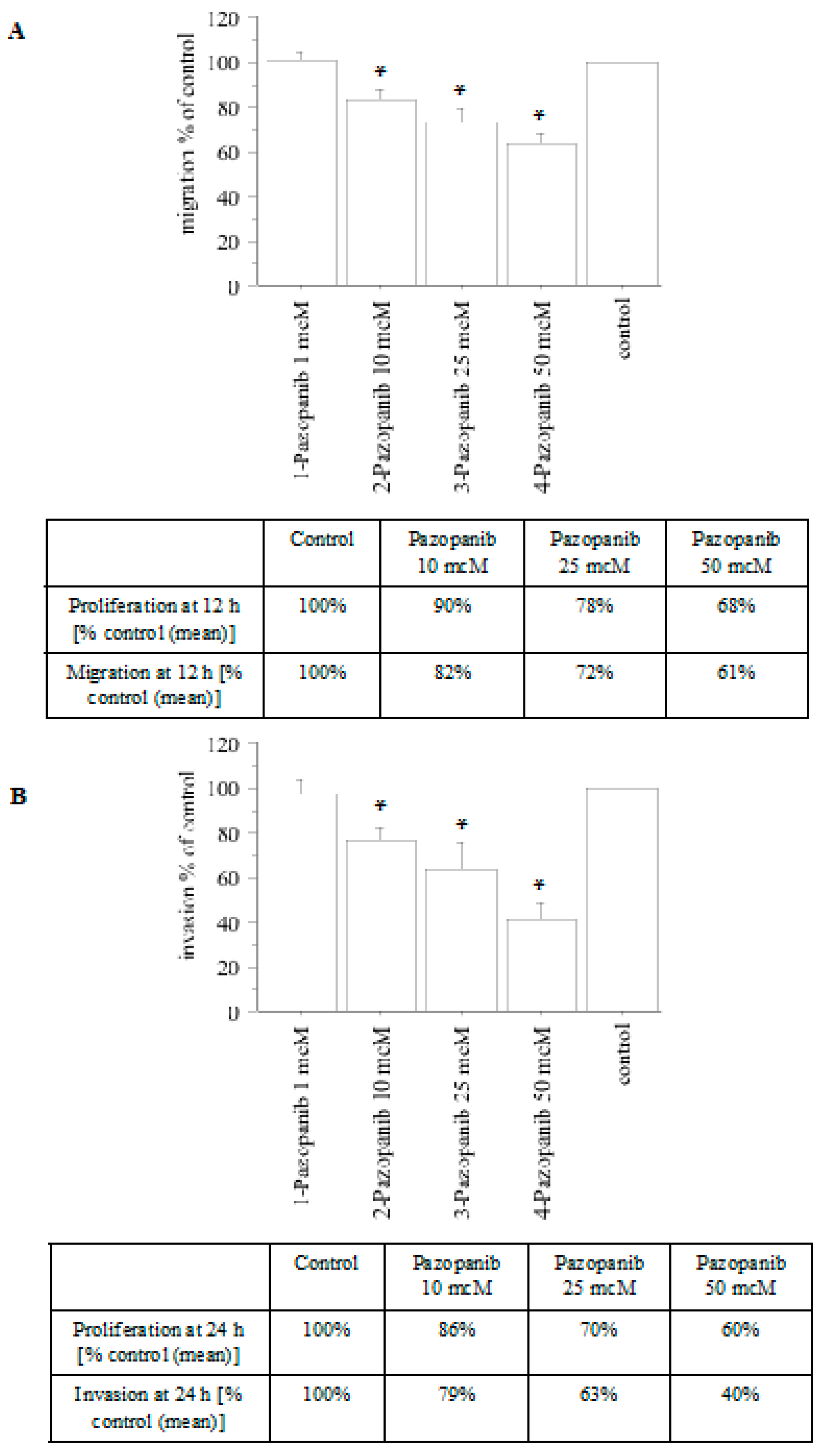
2.3. Migration and Invasion
2.4. VEGF Expression
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.1.1. Patients Source for Thyroidal Tissue Samples
4.1.2. Primary ATC Cell Culture
4.1.3. Characterization of Thyroidal Samples
4.2. Cell Proliferation and Viability Assay
4.3. Cell Counting
4.4. Apoptosis Determination
4.4.1. Hoechst Uptake
4.4.2. Annexin V Binding Test
4.5. Migration and Invasion
4.6. Immunocytochemistry for VEGF Expression
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fallahi, P.; Ferrari, S.M.; Galdiero, M.R.; Varricchi, G.; Elia, G.; Ragusa, F.; Paparo, S.R.; Benvenga, S.; Antonelli, A. Molecular targets of tyrosine kinase inhibitors in thyroid cancer. Semin. Cancer Biol. 2022, 79, 180–196. [Google Scholar] [CrossRef]
- Ferrari, S.M.; Fallahi, P.; Galdiero, M.R.; Ruffilli, I.; Elia, G.; Ragusa, F.; Paparo, S.R.; Patrizio, A.; Mazzi, V.; Varricchi, G.; et al. Immune and Inflammatory Cells in Thyroid Cancer Microenvironment. Int. J. Mol. Sci. 2019, 20, 4413. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; La Motta, C. Novel therapeutic clues in thyroid carcinomas: The role of targeting cancer stem cells. Med. Res. Rev. 2017, 37, 1299–1317. [Google Scholar] [CrossRef]
- De Crevoisier, R.; Baudin, E.; Bachelot, A.; Leboulleux, S.; Travagli, J.P.; Caillou, B.; Schlumberger, M. Combined treatment of anaplastic thyroid carcinoma with surgery, chemotherapy, and hyperfractionated accelerated external radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 1137–1143. [Google Scholar] [CrossRef]
- Miccoli, P.; Antonelli, A.; Spinelli, C.; Ferdeghini, M.; Fallahi, P.; Baschieri, L. Completion total thyroidectomy in children with thyroid cancer secondary to the Chernobyl accident. Arch. Surg. 1998, 133, 89–93. [Google Scholar] [CrossRef]
- Ferrari, S.M.; Elia, G.; Ragusa, F.; Ruffilli, I.; La Motta, C.; Paparo, S.R.; Patrizio, A.; Vita, R.; Benvenga, S.; Materazzi, G.; et al. Novel treatments for anaplastic thyroid carcinoma. Gland. Surg. 2020, 9, S28–S42. [Google Scholar] [CrossRef]
- Wartofsky, L. Highlights of the American Thyroid Association Guidelines for patients with thyroid nodules or differentiated thyroid carcinoma: The 2009 revision. Thyroid 2009, 19, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.M.; Ruffilli, I.; Centanni, M.; Virili, C.; Materazzi, G.; Alexopoulou, M.; Miccoli, M.; Antonelli, A.; Fallahi, P. Lenvatinib in the Therapy of Aggressive Thyroid Cancer: State of the Art and New Perspectives with Patents Recently Applied. Recent Pat. Anticancer. Drug Discov. 2018, 13, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.M.; Centanni, M.; Virili, C.; Miccoli, M.; Ferrari, P.; Ruffilli, I.; Ragusa, F.; Antonelli, A.; Fallahi, P. Sunitinib in the Treatment of Thyroid Cancer. Curr. Med. Chem. 2019, 26, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Fallahi, P.; Ferrari, S.M.; Ruffilli, I.; Santini, F.; Minuto, M.; Galleri, D.; Miccoli, P. New targeted therapies for thyroid cancer. Curr. Genom. 2011, 12, 626–631. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 131–133. [Google Scholar] [CrossRef]
- Nair, A.; Lemery, S.J.; Yang, J.; Marathe, A.; Zhao, L.; Zhao, H.; Jiang, X.; He, K.; Ladouceur, G.; Mitra, A.K.; et al. FDA Approval Summary: Lenvatinib for Progressive, Radio-iodine-Refractory Differentiated Thyroid Cancer. Clin. Cancer Res. 2015, 21, 5205–5208. [Google Scholar] [CrossRef]
- FDA Approves Cabozantinib for Patients With Previously Treated Radioactive Iodine–Refractory Differentiated Thyroid Cancer. Available online: https://ascopost.com/issues/october-10-2021/fda-approves-cabozantinib-for-patients-with-previously-treated-radioactive-iodine-refractory-differentiated-thyroid-cancer/ (accessed on 25 October 2022).
- Kurzrock, R.; Sherman, S.I.; Ball, D.W.; Forastiere, A.A.; Cohen, R.B.; Mehra, R.; Pfister, D.G.; Cohen, E.E.; Janisch, L.; Nauling, F.; et al. Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J. Clin. Oncol. 2011, 29, 2660–2666. [Google Scholar] [CrossRef]
- Wells, S.A., Jr.; Gosnell, J.E.; Gagel, R.F.; Moley, J.; Pfister, D.; Sosa, J.A.; Skinner, M.; Krebs, A.; Vasselli, J.; Schlumberger, M. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J. Clin. Oncol. 2010, 28, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Thornton, K.; Kim, G.; Maher, V.E.; Chattopadhyay, S.; Tang, S.; Moon, Y.J.; Song, P.; Marathe, A.; Balakrishnan, S.; Zhu, H.; et al. Vandetanib for the treatment of symptomatic or progressive medullary thyroid cancer in patients with unresectable locally advanced or metastatic disease: U.S. Food and Drug Administration drug approval summary. Clin. Cancer Res. 2012, 18, 3722–3730. [Google Scholar] [CrossRef] [PubMed]
- Iyer, P.C.; Dadu, R.; Ferrarotto, R.; Busaidy, N.L.; Habra, M.A.; Zafereo, M.; Gross, N.; Hess, K.R.; Gule-Monroe, M.; Williams, M.D.; et al. Real-World Experience with Targeted Therapy for the Treatment of Anaplastic Thyroid Carcinoma. Thyroid 2018, 28, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Kreitman, R.J.; Wainberg, Z.A.; Cho, J.Y.; Schellens, J.H.M.; Soria, J.C.; Wen, P.Y.; Zielinski, C.; Cabanillas, M.E.; Urbanowitz, G.; et al. Dabrafenib and Trametinib Treatment in Patients With Locally Advanced or Metastatic BRAF V600-Mutant Anaplastic Thyroid Cancer. J. Clin. Oncol. 2018, 36, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.M.; Bocci, G.; Di Desidero, T.; Elia, G.; Ruffilli, I.; Ragusa, F.; Orlandi, P.; Paparo, S.R.; Patrizio, A.; Piaggi, S.; et al. Lenvatinib exhibits antineoplastic activity in anaplastic thyroid cancer in vitro and in vivo. Oncol. Rep. 2018, 39, 2225–2234. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.M.; Bocci, G.; Di Desidero, T.; Ruffilli, I.; Elia, G.; Ragusa, F.; Fioravanti, A.; Orlandi, P.; Paparo, S.R.; Patrizio, A.; et al. Vandetanib has antineoplastic activity in anaplastic thyroid cancer, in vitro and in vivo. Oncol. Rep. 2018, 39, 2306–2314. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.M.; La Motta, C.; Elia, G.; Ragusa, F.; Ruffilli, I.; Quattrini, L.; Paparo, S.R.; Piaggi, S.; Patrizio, A.; Ulisse, S.; et al. Antineoplastic Effect of Lenvatinib and Vandetanib in Primary Anaplastic Thyroid Cancer Cells Obtained From Biopsy or Fine Needle Aspiration. Front. Endocrinol. 2018, 9, 764. [Google Scholar] [CrossRef]
- Antonelli, A.; Bocci, G.; Fallahi, P.; La Motta, C.; Ferrari, S.M.; Mancusi, C.; Fioravanti, A.; Di Desidero, T.; Sartini, S.; Corti, A.; et al. CLM3, a multitarget tyrosine kinase inhibitor with antiangiogenic properties, is active against primary anaplastic thyroid cancer in vitro and in vivo. J. Clin. Endocrinol. Metab. 2014, 99, E572–E581. [Google Scholar] [CrossRef]
- Antonelli, A.; Bocci, G.; La Motta, C.; Ferrari, S.M.; Fallahi, P.; Ruffilli, I.; Di Domenicantonio, A.; Fioravanti, A.; Sartini, S.; Minuto, M.; et al. CLM94, a novel cyclic amide with anti-VEGFR-2 and antiangiogenic properties, is active against primary anaplastic thyroid cancer in vitro and in vivo. J. Clin. Endocrinol. Metab. 2012, 97, E528–E536. [Google Scholar] [CrossRef]
- Fallahi, P.; Ferrari, S.M.; La Motta, C.; Materazzi, G.; Bocci, G.; Da Settimo, F.; Miccoli, P.; Antonelli, A. CLM29 and CLM24, pyrazolopyrimidine derivatives, have antitumoral activity in vitro in anaplastic thyroid cancer, with or without BRAF mutation. Endocrine 2016, 53, 136–144. [Google Scholar] [CrossRef]
- Newell, D.R. Flasks fibres and flanks—Pre-clinical tumour models for predicting clinical antitumour activity. Br. J. Cancer 2001, 84, 1289–1290. [Google Scholar] [CrossRef] [PubMed]
- Dollner, R.; Granzow, C.; Werner, J.A.; Dietz, A. Is there a role for chemosensitivity tests in head and neck cancer? Onkologie 2004, 27, 310–315. [Google Scholar] [CrossRef]
- Schroyens, W.; Tueni, E.; Dodion, P.; Bodecker, R.; Stoessel, F.; Klastersky, J. Validation of clinical predictive value of in vitro colorimetric chemosensitivity assay in head and neck cancer. Eur. J. Cancer 1990, 26, 834–838. [Google Scholar] [CrossRef]
- Fallahi, P.; Ferrari, S.M.; Elia, G.; Ragusa, F.; Patrizio, A.; Paparo, S.R.; Marone, G.; Galdiero, M.R.; Guglielmi, G.; Foddis, R.; et al. Primary cell cultures for the personalized therapy in aggressive thyroid cancer of follicular origin. Semin. Cancer Biol. 2022, 79, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Bible, K.C.; Suman, V.J.; Molina, J.R.; Smallridge, R.C.; Maples, W.J.; Menefee, M.E.; Rubin, J.; Sideras, K.; Morris, J.C., 3rd; McIver, B.; et al. Endocrine Malignancies Disease Oriented Group; Mayo Clinic Cancer Center; Mayo Phase 2 Consortium. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: Results of a phase 2 consortium study. Lancet Oncol. 2010, 11, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Bible, K.C.; Menefee, M.E.; Lin, C.J.; Millward, M.J.; Maples, W.J.; Goh, B.C.; Karlin, N.J.; Kane, M.A.; Adkins, D.R.; Molina, J.R.; et al. An International Phase 2 Study of Pazopanib in Progressive and Metastatic Thyroglobulin Antibody Negative Radioactive Iodine Refractory Differentiated Thyroid Cancer. Thyroid 2020, 30, 1254–1262. [Google Scholar] [CrossRef]
- de la Fouchardière, C.; Godbert, Y.; Dalban, C.; Illouz, F.; Wassermann, J.; Do Cao, C.; Bardet, S.; Zerdoud, S.; Chougnet, C.N.; Zalzali, M.; et al. PAZOTHYR investigators. Intermittent versus continuous administration of pazopanib in progressive radioiodine refractory thyroid carcinoma: Final results of the randomised, multicenter, open-label phase II trial PAZOTHYR. Eur. J. Cancer 2021, 157, 153–164. [Google Scholar] [CrossRef]
- Isham, C.R.; Bossou, A.R.; Negron, V.; Fisher, K.E.; Kumar, R.; Marlow, L.; Lingle, W.L.; Smallridge, R.C.; Sherman, E.J.; Suman, V.J.; et al. Pazopanib enhances paclitaxel-induced mitotic catastrophe in anaplastic thyroid cancer. Sci. Transl. Med. 2013, 5, 166ra3. [Google Scholar] [CrossRef] [PubMed]
- Di Desidero, T.; Orlandi, P.; Gentile, D.; Bocci, G. Effects of Pazopanib Monotherapy vs. Pazopanib and Topotecan Combination on Anaplastic Thyroid Cancer Cells. Front. Oncol. 2019, 9, 1202. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, W.; Phay, J.E.; Shen, R.; Pellegata, N.S.; Saji, M.; Ringel, M.D.; de la Chapelle, A.; He, H. Primary Cell Culture Systems for Human Thyroid Studies. Thyroid 2016, 26, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Pilli, T.; Prasad, K.V.; Jayarama, S.; Pacini, F.; Prabhakar, B.S. Potential utility and limitations of thyroid cancer cell lines as models for studying thyroid cancer. Thyroid 2009, 19, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Ferrari, S.M.; Fallahi, P.; Berti, P.; Materazzi, G.; Minuto, M.; Giannini, R.; Marchetti, I.; Barani, L.; Basolo, F.; et al. Thiazolidinediones and antiblastics in primary human anaplastic thyroid cancer cells. Clin. Endocrinol. 2009, 70, 946–953. [Google Scholar] [CrossRef]
- Antonelli, A.; Ferrari, S.M.; Fallahi, P.; Berti, P.; Materazzi, G.; Marchetti, I.; Ugolini, C.; Basolo, F.; Miccoli, P.; Ferrannini, E. Evaluation of the sensitivity to chemotherapeutics or thiazolidinediones of primary anaplastic thyroid cancer cells obtained by fine-needle aspiration. Eur. J. Endocrinol. 2008, 159, 283–291. [Google Scholar] [CrossRef]
- Antonelli, A.; Ferrari, S.M.; Fallahi, P.; Berti, P.; Materazzi, G.; Barani, L.; Marchetti, I.; Ferrannini, E.; Miccoli, P. Primary cell cultures from anaplastic thyroid cancer obtained by fine-needle aspiration used for chemosensitivity tests. Eur. J. Endocrinol. 2008, 69, 148–152. [Google Scholar] [CrossRef]
- Antonelli, A.; Ferrari, S.M.; Fallahi, P.; Frascerra, S.; Piaggi, S.; Gelmini, S.; Lupi, C.; Minuto, M.; Berti, P.; Benvenga, S.; et al. Dysregulation of secretion of CXC alpha-chemokine CXCL10 in papillary thyroid cancer: Modulation by peroxisome proliferator-activated receptor-gamma agonists. Endocr. Relat. Cancer 2009, 16, 1299–1311. [Google Scholar] [CrossRef]
- Antonelli, A.; Bocci, G.; La Motta, C.; Ferrari, S.M.; Fallahi, P.; Fioravanti, A.; Sartini, S.; Minuto, M.; Piaggi, S.; Corti, A.; et al. Novel pyrazolopyrimidine derivatives as tyrosine kinase inhibitors with antitumoral activity in vitro and in vivo in papillary dedifferentiated thyroid cancer. J. Clin. Endocrinol. Metab. 2011, 96, E288–E296. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, S.M.; Elia, G.; Ragusa, F.; Paparo, S.R.; Mazzi, V.; Patrizio, A.; Piaggi, S.; Baldini, E.; Centanni, M.; La Motta, C.; et al. Antineoplastic Activity of Pazopanib in Anaplastic Thyroid Cancer in Primary Culture. Int. J. Mol. Sci. 2023, 24, 2398. https://doi.org/10.3390/ijms24032398
Ferrari SM, Elia G, Ragusa F, Paparo SR, Mazzi V, Patrizio A, Piaggi S, Baldini E, Centanni M, La Motta C, et al. Antineoplastic Activity of Pazopanib in Anaplastic Thyroid Cancer in Primary Culture. International Journal of Molecular Sciences. 2023; 24(3):2398. https://doi.org/10.3390/ijms24032398
Chicago/Turabian StyleFerrari, Silvia Martina, Giusy Elia, Francesca Ragusa, Sabrina Rosaria Paparo, Valeria Mazzi, Armando Patrizio, Simona Piaggi, Enke Baldini, Marco Centanni, Concettina La Motta, and et al. 2023. "Antineoplastic Activity of Pazopanib in Anaplastic Thyroid Cancer in Primary Culture" International Journal of Molecular Sciences 24, no. 3: 2398. https://doi.org/10.3390/ijms24032398
APA StyleFerrari, S. M., Elia, G., Ragusa, F., Paparo, S. R., Mazzi, V., Patrizio, A., Piaggi, S., Baldini, E., Centanni, M., La Motta, C., Antonelli, A., & Fallahi, P. (2023). Antineoplastic Activity of Pazopanib in Anaplastic Thyroid Cancer in Primary Culture. International Journal of Molecular Sciences, 24(3), 2398. https://doi.org/10.3390/ijms24032398







