Egln1Tie2Cre Mice Exhibit Similar Therapeutic Responses to Sildenafil, Ambrisentan, and Treprostinil as Pulmonary Arterial Hypertension (PAH) Patients, Supporting Egln1Tie2Cre Mice as a Useful PAH Model
Abstract
1. Introduction
2. Results
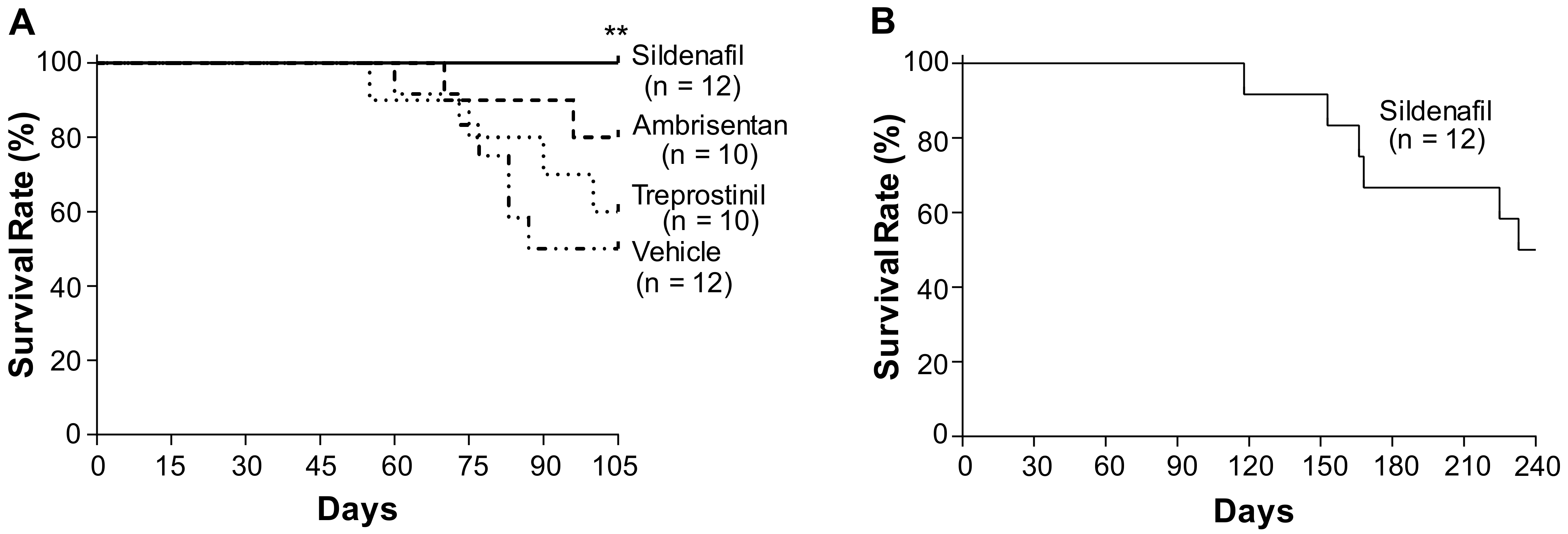
2.1. Effects of Sildenafil, Ambrisentan, and Treprostinil on the Survival of Egln1Tie2Cre Mice
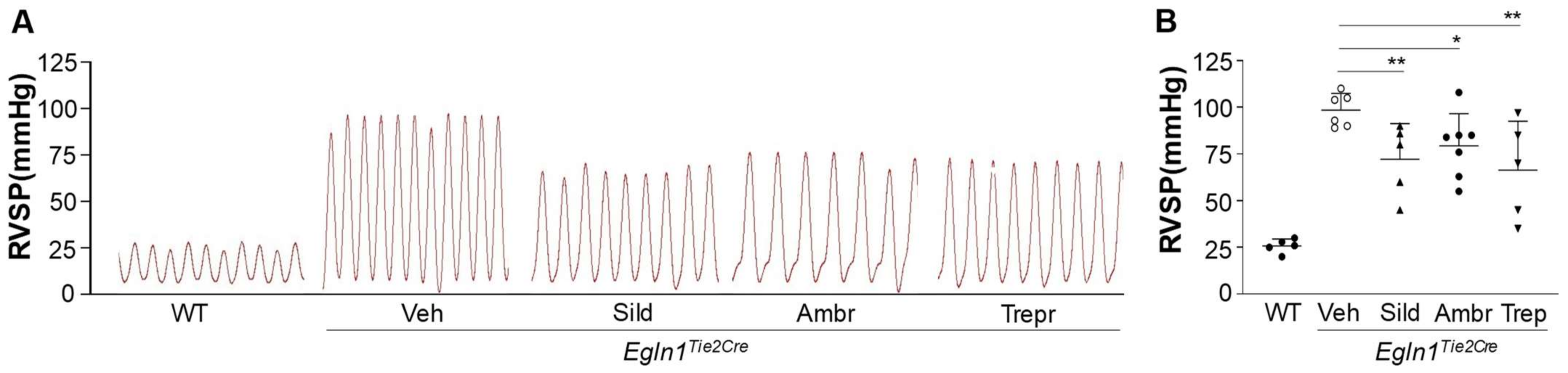
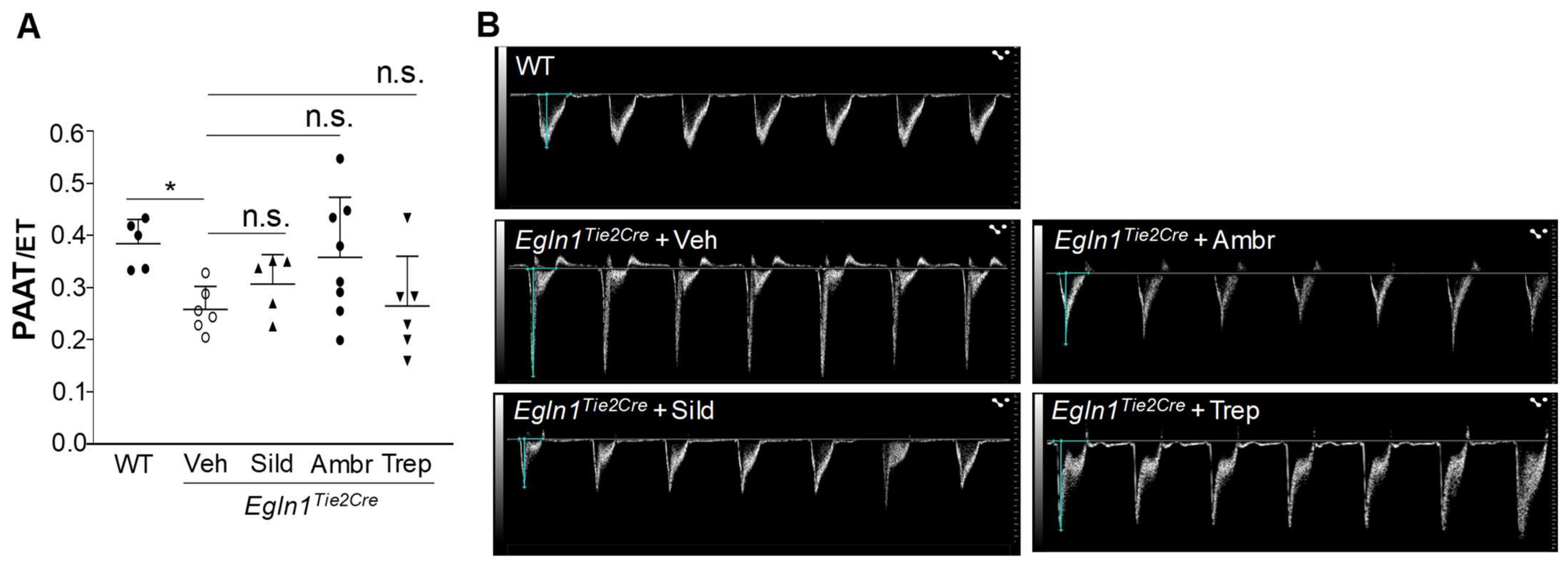
2.2. Sildenafil, Ambrisentan, or Treprostinil Treatment Attenuated Right Ventricular Systolic Pressure (RVSP) with Neglectable Effects on Pulmonary Artery (PA) Function in Egln1Tie2Cre Mice
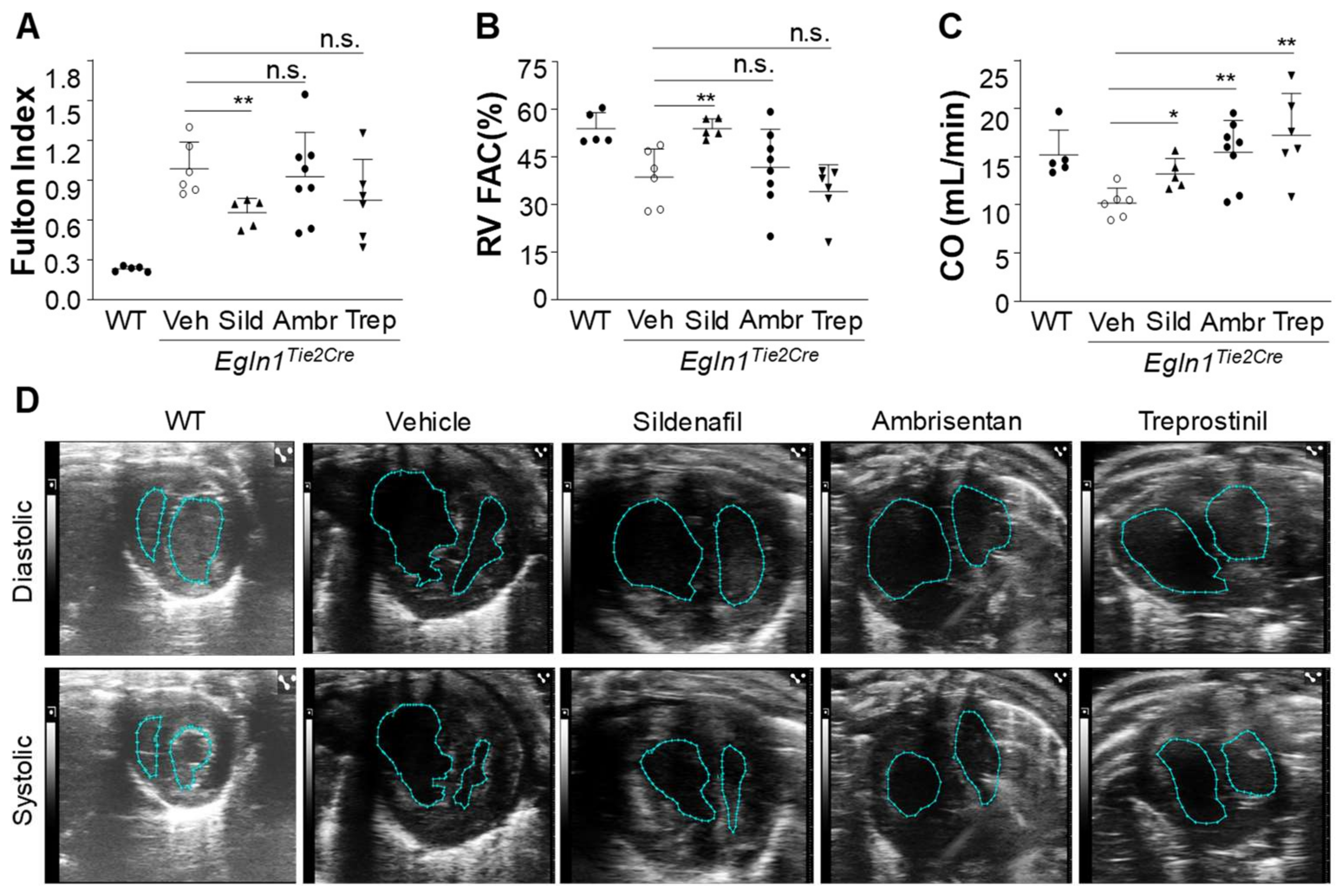
2.3. The Effects of Sildenafil, Ambrisentan, or Treprostinil Treatment on RV Hypertrophy and Function in Egln1Tie2Cre Mice
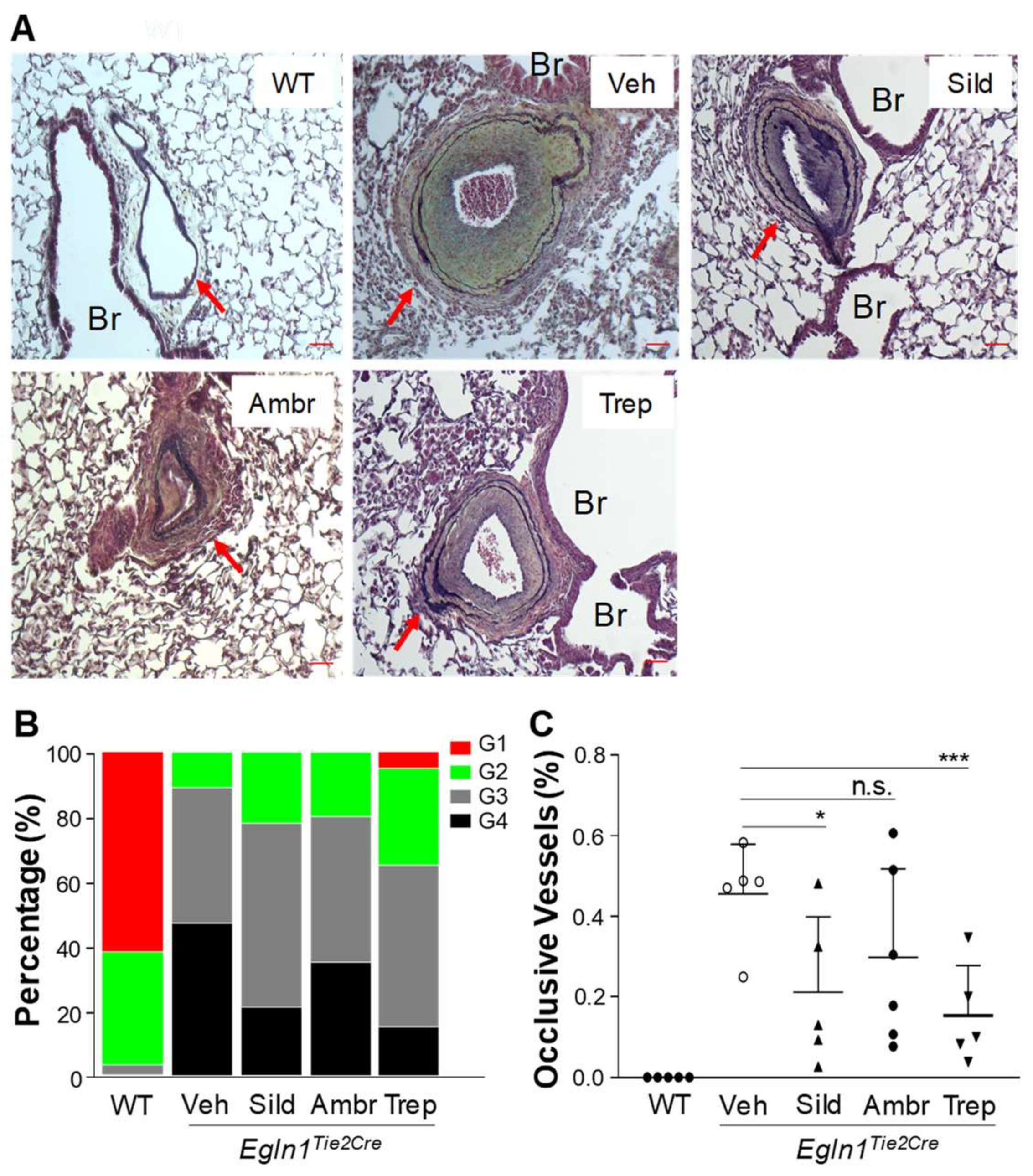
2.4. The Effects of Sildenafil, Ambrisentan, or Treprostinil Treatment on Obliterative Pulmonary Vascular Remodeling in Egln1Tie2 Mice
3. Discussion
4. Materials and Methods
4.1. Surgical Procedures for Osmotic Minipump Implantation in Mice
4.2. Echocardiography
4.3. RV Hemodynamic Measurements
4.4. Histological Assessment of Pulmonary Vascular Remodeling
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hassoun, P.M. Pulmonary Arterial Hypertension. N. Engl. J. Med. 2021, 385, 2361–2376. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.E.; Cober, N.D.; Dai, Z.; Stewart, D.J.; Zhao, Y.Y. Endothelial cells in the pathogenesis of pulmonary arterial hypertension. Eur. Respir. J. 2021, 58, 2003957. [Google Scholar] [CrossRef]
- Galiè, N.; Channick, R.N.; Frantz, R.P.; Grünig, E.; Jing, Z.C.; Moiseeva, O.; Preston, I.R.; Pulido, T.; Safdar, Z.; Tamura, Y.; et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur. Respir. J. 2019, 53, 1801889. [Google Scholar] [CrossRef] [PubMed]
- Ruopp, N.F.; Cockrill, B.A. Diagnosis and Treatment of Pulmonary Arterial Hypertension: A Review. JAMA 2022, 327, 1379–1391. [Google Scholar] [CrossRef]
- Maron, B.A.; Abman, S.H.; Elliott, C.G.; Frantz, R.P.; Hopper, R.K.; Horn, E.M.; Nicolls, M.R.; Shlobin, O.A.; Shah, S.J.; Kovacs, G.; et al. Pulmonary Arterial Hypertension: Diagnosis, Treatment, and Novel Advances. Am. J. Respir. Crit. Care Med. 2021, 203, 1472–1487. [Google Scholar] [CrossRef] [PubMed]
- Kay, J.M.; Harris, P.; Heath, D. Pulmonary hypertension produced in rats by ingestion of Crotalaria spectabilis seeds. Thorax 1967, 22, 176–179. [Google Scholar] [CrossRef]
- Taraseviciene-Stewart, L.; Kasahara, Y.; Alger, L.; Hirth, P.; Mc Mahon, G.; Waltenberger, J.; Voelkel, N.F.; Tuder, R.M. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2001, 15, 427–438. [Google Scholar] [CrossRef]
- Ruiter, G.; de Man, F.S.; Schalij, I.; Sairras, S.; Grunberg, K.; Westerhof, N.; van der Laarse, W.J.; Vonk-Noordegraaf, A. Reversibility of the monocrotaline pulmonary hypertension rat model. Eur. Respir. J. 2013, 42, 553–556. [Google Scholar] [CrossRef]
- de Raaf, M.A.; Schalij, I.; Gomez-Arroyo, J.; Rol, N.; Happé, C.; de Man, F.S.; Vonk-Noordegraaf, A.; Westerhof, N.; Voelkel, N.F.; Bogaard, H.J. SuHx rat model: Partly reversible pulmonary hypertension and progressive intima obstruction. Eur. Respir. J. 2014, 44, 160–168. [Google Scholar] [CrossRef]
- Gomez-Arroyo, J.; Saleem, S.J.; Mizuno, S.; Syed, A.A.; Bogaard, H.J.; Abbate, A.; Taraseviciene-Stewart, L.; Sung, Y.; Kraskauskas, D.; Farkas, D.; et al. A brief overview of mouse models of pulmonary arterial hypertension: Problems and prospects. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 302, L977–L991. [Google Scholar] [CrossRef]
- Dai, Z.; Zhao, Y.Y. Discovery of a murine model of clinical PAH: Mission impossible? Trends Cardiovasc. Med. 2017, 27, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Li, M.; Wharton, J.; Zhu, M.M.; Zhao, Y.Y. Prolyl-4 Hydroxylase 2 (PHD2) Deficiency in Endothelial Cells and Hematopoietic Cells Induces Obliterative Vascular Remodeling and Severe Pulmonary Arterial Hypertension in Mice and Humans Through Hypoxia-Inducible Factor-2α. Circulation 2016, 133, 2447–2458. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Zhu, M.M.; Peng, Y.; Machireddy, N.; Evans, C.E.; Machado, R.; Zhang, X.; Zhao, Y.Y. Therapeutic Targeting of Vascular Remodeling and Right Heart Failure in Pulmonary Arterial Hypertension with a HIF-2alpha Inhibitor. Am. J. Respir. Crit. Care Med. 2018, 198, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Peng, Y.; Yi, D.; Dai, J.; Vanderpool, R.; Zhu, M.M.; Dai, Z.; Zhao, Y.Y. Endothelial PHD2 deficiency induces nitrative stress via suppression of Caveolin-1 in pulmonary arterial hypertension. Eur. Respir. J. 2022, 60, 2102643. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, K.; Sydykov, A.; Tian, X.; Mamazhakypov, A.; Neupane, B.; Luitel, H.; Weissmann, N.; Seeger, W.; Grimminger, F.; Kretschmer, A.; et al. Soluble guanylate cyclase stimulator riociguat and phosphodiesterase 5 inhibitor sildenafil ameliorate pulmonary hypertension due to left heart disease in mice. Int. J. Cardiol. 2016, 216, 85–91. [Google Scholar] [CrossRef]
- Wagenaar, G.T.; el Laghmani, H.; de Visser, Y.P.; Sengers, R.M.; Steendijk, P.; Baelde, H.J.; Walther, F.J. Ambrisentan reduces pulmonary arterial hypertension but does not stimulate alveolar and vascular development in neonatal rats with hyperoxic lung injury. Am. J. Physiol. -Lung Cell. Mol. Physiol. 2013, 304, L264–L275. [Google Scholar] [CrossRef]
- Nikam, V.S.; Schermuly, R.T.; Dumitrascu, R.; Weissmann, N.; Kwapiszewska, G.; Morrell, N.; Klepetko, W.; Fink, L.; Seeger, W.; Voswinckel, R. Treprostinil inhibits the recruitment of bone marrow-derived circulating fibrocytes in chronic hypoxic pulmonary hypertension. Eur. Respir. J. 2010, 36, 1302–1314. [Google Scholar] [CrossRef]
- Parpia, T.; Burgess, G.; Branzi, A.; Galile, N.; Ghofrani, H.A.; Torbicki, A.; Brast, R.J.; Rubin, L.J.; Badesch, D.; Fleming, T.; et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N. Engl. J. Med. 2005, 353, 2148–2157. [Google Scholar] [CrossRef]
- Dai, Z.; Zhu, M.M.; Peng, Y.; Jin, H.; Machireddy, N.; Qian, Z.; Zhang, X.; Zhao, Y.Y. Endothelial and Smooth Muscle Cell Interaction via FoxM1 Signaling Mediates Vascular Remodeling and Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2018, 198, 788–802. [Google Scholar] [CrossRef]
- Scheuermann, T.H.; Li, Q.; Ma, H.W.; Key, J.; Zhang, L.; Chen, R.; Garcia, J.A.; Naidoo, J.; Longgood, J.; Frantz, D.E.; et al. Allosteric inhibition of hypoxia inducible factor-2 with small molecules. Nat. Chem. Biol. 2013, 9, 271–276. [Google Scholar] [CrossRef]
- Rubin, L.J.; Badesch, D.B.; Fleming, T.R.; Galie, N.; Simonneau, G.; Ghofrani, H.A.; Oakes, M.; Layton, G.; Serdarevic-Pehar, M.; McLaughlin, V.V.; et al. Long-term treatment with sildenafil citrate in pulmonary arterial hypertension: The SUPER-2 study. Chest 2011, 140, 1274–1283. [Google Scholar] [CrossRef]
- Barst, R.J.; Ivy, D.D.; Gaitan, G.; Szatmari, A.; Rudzinski, A.; Garcia, A.E.; Sastry, B.K.; Pulido, T.; Layton, G.R.; Serdarevic-Pehar, M.; et al. A randomized, double-blind, placebo-controlled, dose-ranging study of oral sildenafil citrate in treatment-naive children with pulmonary arterial hypertension. Circulation 2012, 125, 324–334. [Google Scholar] [CrossRef]
- Galiè, N.; Olschewski, H.; Oudiz, R.J.; Torres, F.; Frost, A.; Ghofrani, H.A.; Badesch, D.B.; McGoon, M.D.; McLaughlin, V.V.; Roecker, E.B.; et al. Ambrisentan for the treatment of pulmonary arterial hypertension: Results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation 2008, 117, 3010–3019. [Google Scholar] [CrossRef]
- Blalock, S.E.; Matulevicius, S.; Mitchell, L.C.; Reimold, S.; Warner, J.; Peshock, R.; Torres, F.; Chin, K.M. Long-term outcomes with ambrisentan monotherapy in pulmonary arterial hypertension. J. Card. Fail. 2010, 16, 121–127. [Google Scholar] [CrossRef]
- Oudiz, R.J.; Galie, N.; Olschewski, H.; Torres, F.; Frost, A.; Ghofrani, H.A.; Badesch, D.B.; McGoon, M.D.; McLaughlin, V.V.; Roecker, E.B.; et al. Long-term ambrisentan therapy for the treatment of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2009, 54, 1971–1981. [Google Scholar] [CrossRef]
- Simonneau, G.; Barst, R.J.; Galie, N.; Naeije, R.; Rich, S.; Bourge, R.C.; Keogh, A.; Oudiz, R.; Frost, A.; Blackburn, S.D.; et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: A double-blind, randomized, placebo-controlled trial. Am. J. Respir. Crit. Care Med. 2002, 165, 800–804. [Google Scholar] [CrossRef]
- Barst, R.J.; Galie, N.; Naeije, R.; Simonneau, G.; Jeffs, R.; Arneson, C.; Rubin, L.J. Long-term outcome in pulmonary arterial hypertension patients treated with subcutaneous treprostinil. Eur. Respir. J. 2006, 28, 1195–1203. [Google Scholar] [CrossRef]
- Tang, H.; Babicheva, A.; McDermott, K.M.; Gu, Y.; Ayon, R.J.; Song, S.; Wang, Z.; Gupta, A.; Zhou, T.; Sun, X.; et al. Endothelial HIF-2alpha contributes to severe pulmonary hypertension due to endothelial-to-mesenchymal transition. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L256–L275. [Google Scholar] [CrossRef]
- Girerd, B.; Weatherald, J.; Montani, D.; Humbert, M. Heritable pulmonary hypertension: From bench to bedside. Eur. Respir. Rev. 2017, 26, 170037. [Google Scholar] [CrossRef]
- Stenmark, K.R.; Meyrick, B.; Galie, N.; Mooi, W.J.; McMurtry, I.F. Animal models of pulmonary arterial hypertension: The hope for etiological discovery and pharmacological cure. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 297, L1013–L1032. [Google Scholar] [CrossRef]
- Vitali, S.H.; Hansmann, G.; Rose, C.; Fernandez-Gonzalez, A.; Scheid, A.; Mitsialis, S.A.; Kourembanas, S. The Sugen 5416/hypoxia mouse model of pulmonary hypertension revisited: Long-term follow-up. Pulm. Circ. 2014, 4, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Chenouard, V.; Remy, S.; Tesson, L.; Ménoret, S.; Ouisse, L.H.; Cherifi, Y.; Anegon, I. Advances in Genome Editing and Application to the Generation of Genetically Modified Rat Models. Front. Genet. 2021, 12, 615491. [Google Scholar] [CrossRef] [PubMed]
- Reichenberger, F.; Voswinckel, R.; Enke, B.; Rutsch, M.; El Fechtali, E.; Schmehl, T.; Olschewski, H.; Schermuly, R.; Weissmann, N.; Ghofrani, H.A.; et al. Long-term treatment with sildenafil in chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2007, 30, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Galie, N.; Badesch, D.; Oudiz, R.; Simonneau, G.; McGoon, M.D.; Keogh, A.M.; Frost, A.E.; Zwicke, D.; Naeije, R.; Shapiro, S.; et al. Ambrisentan therapy for pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2005, 46, 529–535. [Google Scholar] [CrossRef]
- Tapson, V.F.; Gomberg-Maitland, M.; McLaughlin, V.V.; Benza, R.L.; Widlitz, A.C.; Krichman, A.; Barst, R.J. Safety and efficacy of IV treprostinil for pulmonary arterial hypertension: A prospective, multicenter, open-label, 12-week trial. Chest 2006, 129, 683–688. [Google Scholar] [CrossRef]
- Hoeper, H.M.; Welte, T. Sildenafil citrate therapy for pulmonary hypertension. N. Engl. J. Med. 2006, 354, 1091–1093. [Google Scholar] [CrossRef]
- Sebkhi, A.; Strange, J.W.; Phillips, S.C.; Wharton, J.; Wilkins, M.R. Phosphodiesterase type 5 as a target for the treatment of hypoxia-induced pulmonary hypertension. Circulation 2003, 107, 3230–3235. [Google Scholar] [CrossRef]
- Schermuly, R.T.; Kreisselmeier, K.P.; Ghofrani, H.A.; Yilmaz, H.; Butrous, G.; Ermert, L.; Ermert, M.; Weissmann, N.; Rose, F.; Guenther, A.; et al. Chronic sildenafil treatment inhibits monocrotaline-induced pulmonary hypertension in rats. Am. J. Respir. Crit. Care Med. 2004, 169, 39–45. [Google Scholar] [CrossRef]
- He, Y.; Zuo, C.; Jia, D.; Bai, P.; Kong, D.; Chen, D.; Liu, G.; Li, J.; Wang, Y.; Chen, G.; et al. Loss of DP1 Aggravates Vascular Remodeling in Pulmonary Arterial Hypertension via mTORC1 Signaling. Am. J. Respir. Crit. Care Med. 2020, 201, 1263–1276. [Google Scholar] [CrossRef]
- Jasmin, J.F.; Lucas, M.; Cernacek, P.; Dupuis, J. Effectiveness of a nonselective ET(A/B) and a selective ET(A) antagonist in rats with monocrotaline-induced pulmonary hypertension. Circulation. 2001, 103, 314–318. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Dai, J.; Zhao, Y.-Y. Egln1Tie2Cre Mice Exhibit Similar Therapeutic Responses to Sildenafil, Ambrisentan, and Treprostinil as Pulmonary Arterial Hypertension (PAH) Patients, Supporting Egln1Tie2Cre Mice as a Useful PAH Model. Int. J. Mol. Sci. 2023, 24, 2391. https://doi.org/10.3390/ijms24032391
Peng Y, Dai J, Zhao Y-Y. Egln1Tie2Cre Mice Exhibit Similar Therapeutic Responses to Sildenafil, Ambrisentan, and Treprostinil as Pulmonary Arterial Hypertension (PAH) Patients, Supporting Egln1Tie2Cre Mice as a Useful PAH Model. International Journal of Molecular Sciences. 2023; 24(3):2391. https://doi.org/10.3390/ijms24032391
Chicago/Turabian StylePeng, Yi, Jingbo Dai, and You-Yang Zhao. 2023. "Egln1Tie2Cre Mice Exhibit Similar Therapeutic Responses to Sildenafil, Ambrisentan, and Treprostinil as Pulmonary Arterial Hypertension (PAH) Patients, Supporting Egln1Tie2Cre Mice as a Useful PAH Model" International Journal of Molecular Sciences 24, no. 3: 2391. https://doi.org/10.3390/ijms24032391
APA StylePeng, Y., Dai, J., & Zhao, Y.-Y. (2023). Egln1Tie2Cre Mice Exhibit Similar Therapeutic Responses to Sildenafil, Ambrisentan, and Treprostinil as Pulmonary Arterial Hypertension (PAH) Patients, Supporting Egln1Tie2Cre Mice as a Useful PAH Model. International Journal of Molecular Sciences, 24(3), 2391. https://doi.org/10.3390/ijms24032391




