Cannabidiol and Cannabigerol Exert Antimicrobial Activity without Compromising Skin Microbiota
Abstract
1. Introduction
2. Results
2.1. CBD and CBG Purity and Chemical Analysis
2.2. CBG and CBD Hinder Microbial Growth
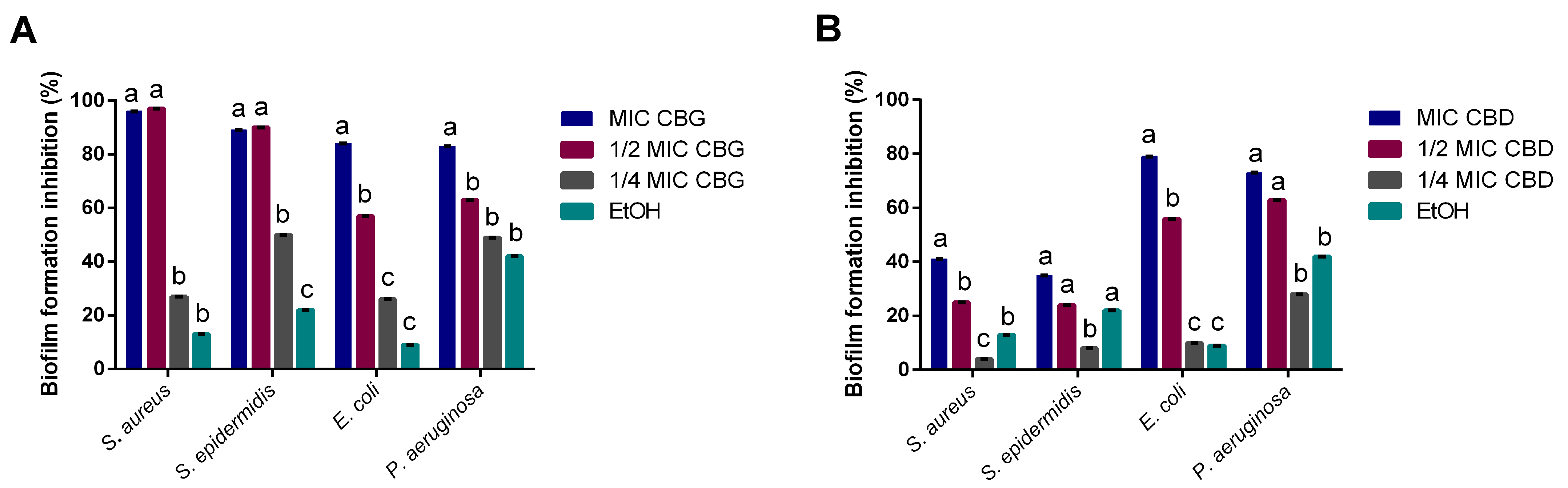
2.3. CBG and CBD Inhibit Biofilm formation
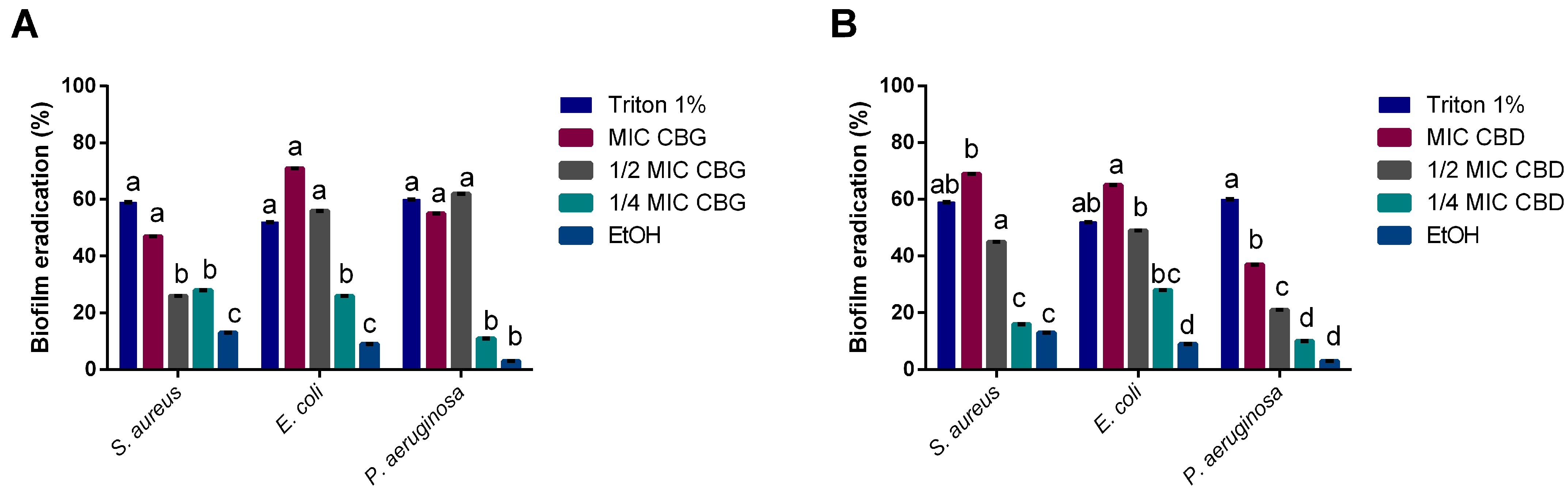
2.4. CBG and CBD Disrupt Mature Biofilms
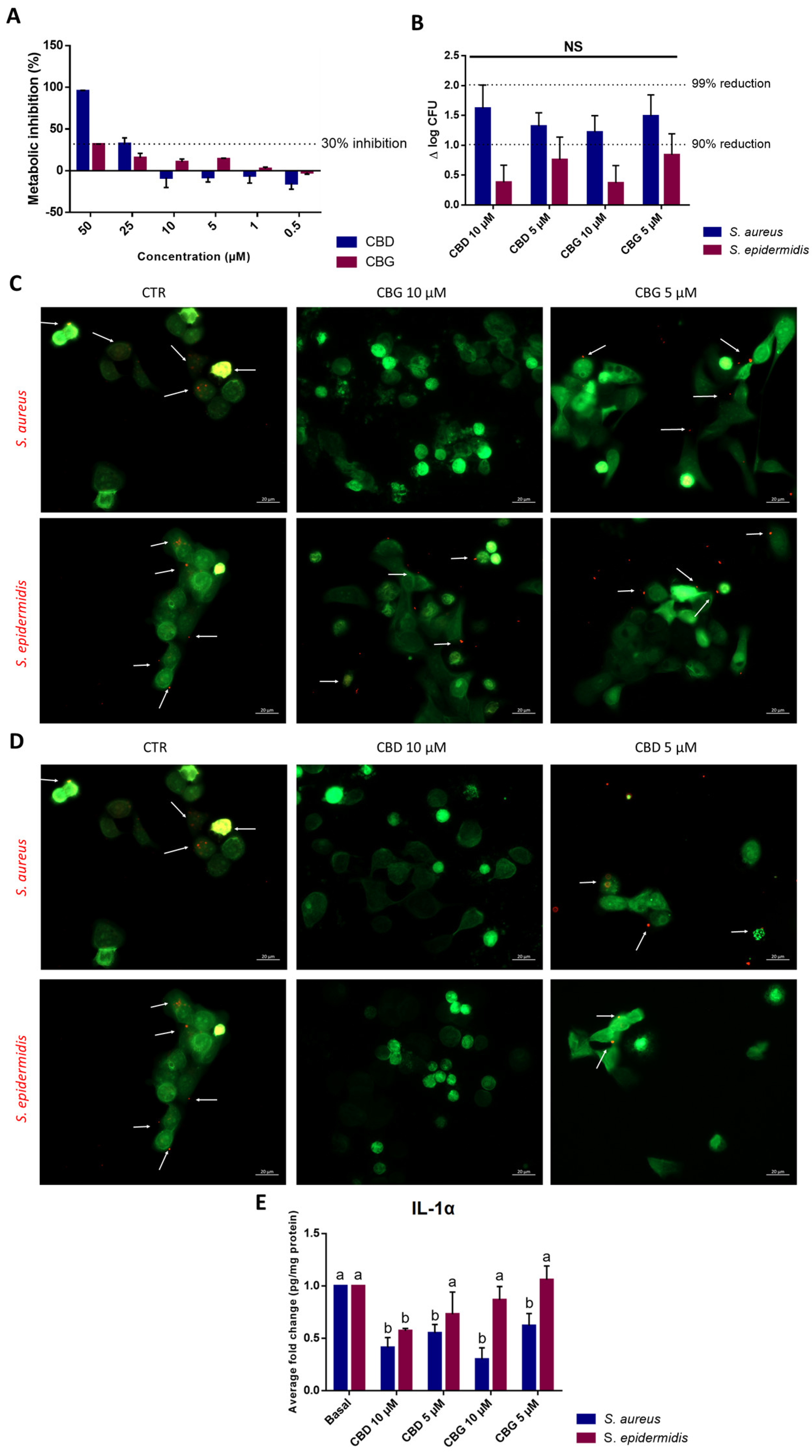
2.5. CBD and CBG Impaired S. aureus Adhesion to Keratinocytes
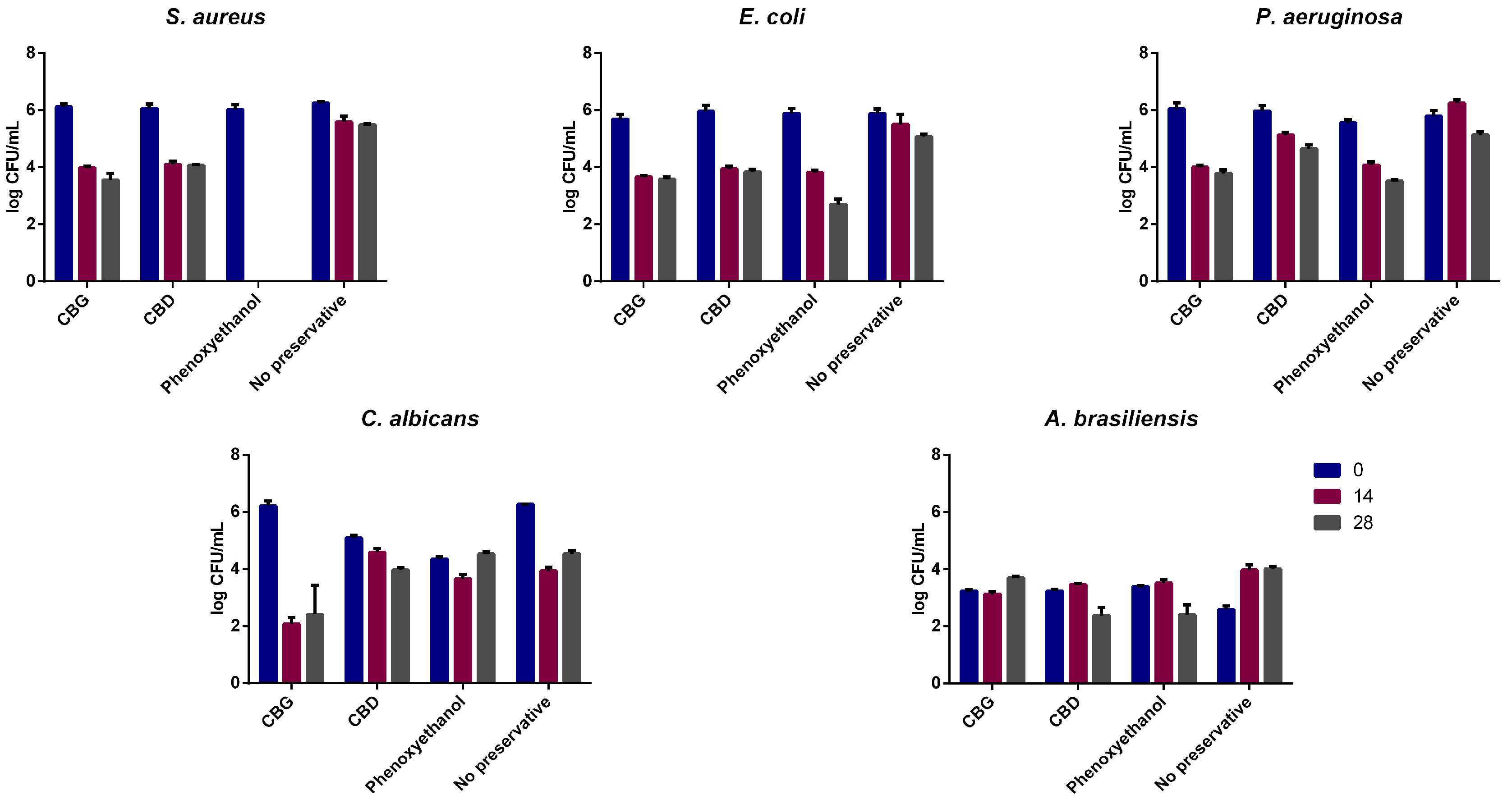
2.6. Evaluation of CBD and CBG as a Preservative in Cosmetic Formulations
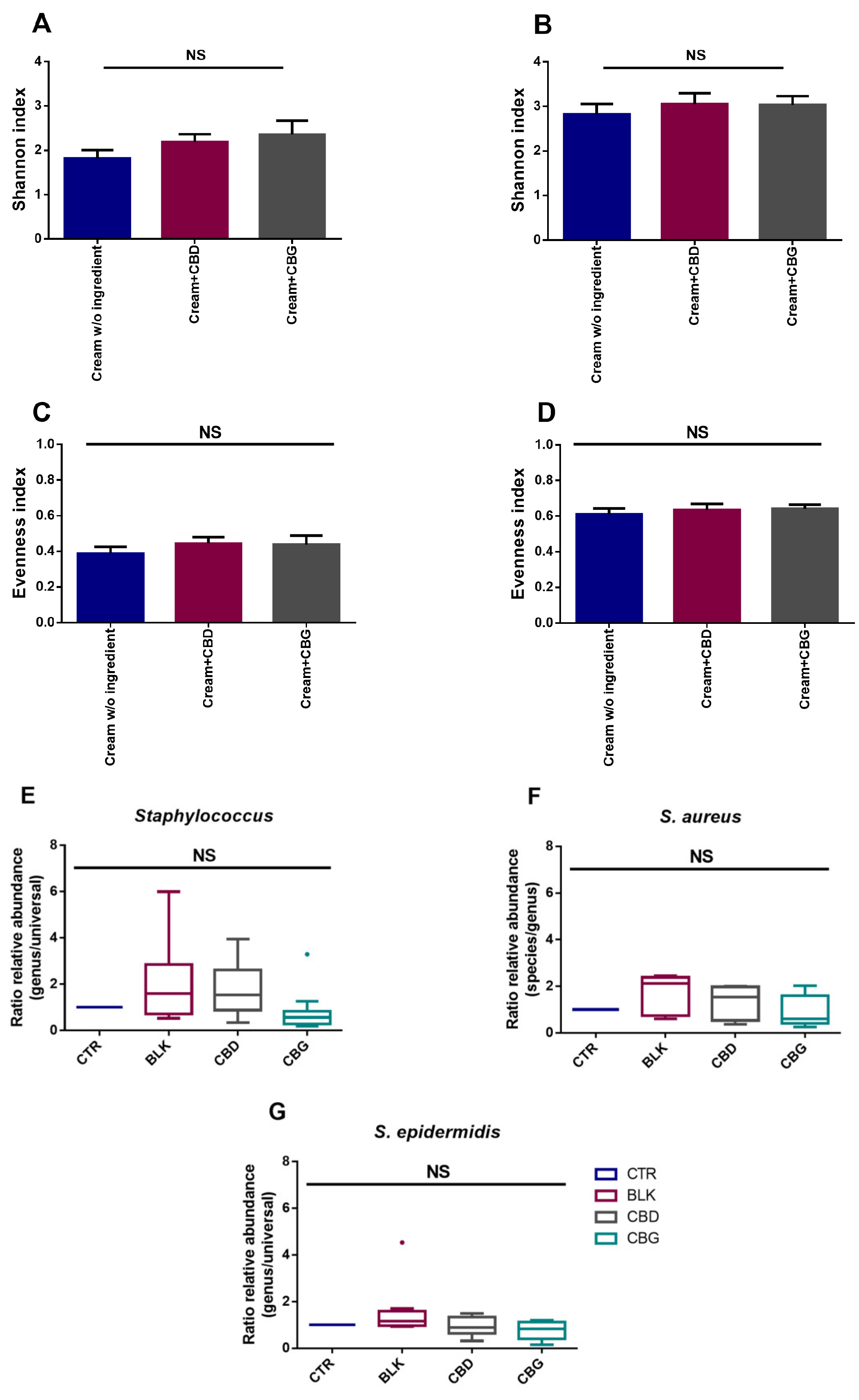
2.7. CBG and CBD Have No Significant Impact on Skin Microbiota
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Cannabinoids’ Preparation
Cannabinoids’ Analysis by GC-MS
5.2. Determination of MIC and MLC
5.3. Biofilm Formation Inhibition Assay
5.4. Mature Biofilm Eradication Assay
5.5. Staphylococcus spp. Infection on Keratinocytes
5.5.1. Keratinocytes’ Viability
5.5.2. Staphylococcus spp. Infection of Keratinocytes
5.5.3. Cytokines Quantification
5.6. Challenge Test
5.7. Evaluation of the Impact of Cannabinoids on the Skin Microbiota
5.7.1. 16S rRNA Gene and ITS2 Region Amplification and Sequencing
5.7.2. Sequencing Data Analysis
5.7.3. Determination of Relative Abundance of Staphylococcus Genus, and S. aureus and S. epidermidis Species
5.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Vendor | Phase I | (w/v) % |
|---|---|---|
| AAK (Malmö, Sweden) | Akoline PGPR | 5.00 |
| Aprinnova (USA) | Neossance Squalene | 5.00 |
| Acofarma (Madrid, Spain) | Caprylic/Capric triglyceride | 7.00 |
| Vaseline | 10.00 | |
| Lanoline | 10.00 | |
| Beeswax | 1.80 | |
| Magnesium Stearate | 1.00 | |
| Phase II | ||
| Deionized water | 55.95 | |
| Acofarma (Madrid, Spain) | Glycerin | 3.00 |
| Sodium Chloride | 0.75 | |
| Phase III | ||
| Preservative | 0.50 | |
| Total | 100.00 | |
| Bacteria | Not less than 2.0 log reduction from the initial count at 14 days, and no increase from the 14 days count at 28 days |
| Yeast and Moulds | No increase (not more than 0.5 log unit higher than the previous value measured) from the initial calculated count at 14 and 28 days. |
| Primer | Forward Primer (5′ ->3′) | Reverse Primer (5′->3′) | Reference |
|---|---|---|---|
| Universal Bacteria | TCCTACGGGAGGCAGCAGT | CGTATTACCGCGGCTGCTGGCAC | [48] |
| Staphylococcus | GGCCGTGTTGAACGTGGTCAAATCA | YATHACCATTTCWGTACCTTCTGGTAA | [49] |
| S. aureus | AGGACAATCATGGCAAGCGTAC | AACGGACAACATCTAAACTGGC | [50] |
| S. epidermidis | GGCAAATTTGTGGGTCAAGA | TGGCTAATGGTTTGTCACCA | [51] |
| Purity (%) | SD | |
|---|---|---|
| CBD Amyris | 98.63 | 0.37 |
| CBD Tocris | 98.44 | 1.02 |
| CBD Linnea | 99.36 | 0.40 |
| CBG Amyris | 99.35 | 0.02 |
| CBG Tocris | 99.48 | 0.19 |
References
- Mechoulam, R.; Hanuš, L.O.; Pertwee, R.; Howlett, A.C. Early phytocannabinoid chemistry to endocannabinoids and beyond. Nat. Rev. Neurosci. 2014, 15, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Hillard, C.J. Circulating endocannabinoids: From whence do they come and where are they going? Neuropsychopharmacology 2018, 43, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Gülck, T.; Møller, B.L. Phytocannabinoids: Origins and biosynthesis. Trends Plant Sci. 2020, 25, 985–1004. [Google Scholar] [CrossRef]
- Reekie, T.A.; Scott, M.P.; Kassiou, M. The evolving science of phytocannabinoids. Nat. Rev. Chem. 2017, 2, 0101. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2020, 9, 21. [Google Scholar] [CrossRef]
- Dos-Santos-Pereira, M.; Guimarães, F.S.; Del-Bel, E.; Raisman-Vozari, R.; Michel, P.P. Cannabidiol prevents LPS-induced microglial inflammation by inhibiting ROS/NF-κB-dependent signaling and glucose consumption. Glia 2020, 68, 561–573. [Google Scholar] [CrossRef]
- Junior, N.C.F.; Dos-Santos-Pereira, M.; Guimarães, F.S.; Del Bel, E. Cannabidiol and Cannabinoid Compounds as Potential Strategies for Treating Parkinson’s Disease and l-DOPA-Induced Dyskinesia. Neurotox. Res. 2019, 37, 12–29. [Google Scholar] [CrossRef]
- Franco, V.; Perucca, E. Pharmacological and Therapeutic Properties of Cannabidiol for Epilepsy. Drugs 2019, 13, 1435–1454. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, S.; Mammana, S.; Cavalli, E.; Bramanti, P.; Mazzon, E. Use of cannabidiol in the treatment of epilepsy: Efficacy and security in clinical trials. Molecules 2019, 24, 1459. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Borgelt, L.M.; Blackmer, A.B. Cannabidiol: A new hope for patients with Dravet or Lennox-Gastaut syndromes. Ann. Pharmacother. 2019, 53, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Blaskovich, M.A.; Kavanagh, A.M.; Elliott, A.G.; Zhang, B.; Ramu, S.; Amado, M.; Lowe, G.J.; Hinton, A.O.; Zuegg, J.; Beare, N. The antimicrobial potential of cannabidiol. Commun. Biol. 2021, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Martinenghi, L.D.; Jønsson, R.; Lund, T.; Jenssen, H. Isolation, purification, and antimicrobial characterization of cannabidiolic acid and cannabidiol from Cannabis sativa L. Biomolecules 2020, 10, 900. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial cannabinoids from Cannabis sativa: A structure−activity study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Iseppi, R.; Brighenti, V.; Licata, M.; Lambertini, A.; Sabia, C.; Messi, P.; Pellati, F.; Benvenuti, S. Chemical characterization and evaluation of the antibacterial activity of essential oils from fibre-type Cannabis sativa L. (Hemp). Molecules 2019, 24, 2302. [Google Scholar] [CrossRef]
- Van Klingeren, B.; Ten Ham, M. Antibacterial activity of Δ 9-tetrahydrocannabinol and cannabidiol. Antonie Van Leeuwenhoek 1976, 42, 9–12. [Google Scholar] [CrossRef]
- Cascio, M.G.; Gauson, L.A.; Stevenson, L.A.; Ross, R.A.; Pertwee, R.G. Evidence that the plant cannabinoid cannabigerol is a highly potent α2-adrenoceptor agonist and moderately potent 5HT1A receptor antagonist. Br. J. Pharmacol. 2010, 159, 129–141. [Google Scholar] [CrossRef]
- Gugliandolo, A.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. In vitro model of neuroinflammation: Efficacy of cannabigerol, a non-psychoactive cannabinoid. Int. J. Mol. Sci. 2018, 19, 1992. [Google Scholar] [CrossRef] [PubMed]
- Farha, M.A.; El-Halfawy, O.M.; Gale, R.T.; MacNair, C.R.; Carfrae, L.A.; Zhang, X.; Jentsch, N.G.; Magolan, J.; Brown, E.D. Uncovering the hidden antibiotic potential of cannabis. ACS Infect. Dis. 2020, 6, 338–346. [Google Scholar] [CrossRef]
- Aqawi, M.; Sionov, R.V.; Gallily, R.; Friedman, M.; Steinberg, D. Anti-Bacterial Properties of Cannabigerol Toward Streptococcus mutans. Front. Microbiol. 2021, 12, 922. [Google Scholar] [CrossRef]
- Aqawi, M.; Gallily, R.; Sionov, R.V.; Zaks, B.; Friedman, M.; Steinberg, D. Cannabigerol Prevents Quorum Sensing and Biofilm Formation of Vibrio harveyi. Front. Microbiol. 2020, 11, 858. [Google Scholar] [CrossRef]
- Rademaker, M.; Agnew, K.; Anagnostou, N.; Andrews, M.; Armour, K.; Baker, C.; Foley, P.; Gebauer, K.; Gupta, M.; Marshman, G. Psoriasis and infection. A clinical practice narrative. Australas. J. Dermatol. 2019, 60, 91–98. [Google Scholar] [CrossRef]
- Li, S.; Villarreal, M.; Stewart, S.; Choi, J.; Ganguli-Indra, G.; Babineau, D.; Philpot, C.; David, G.; Yoshida, T.; Boguniewicz, M. Altered composition of epidermal lipids correlates with Staphylococcus aureus colonization status in atopic dermatitis. Br. J. Dermatol. 2017, 177, e125. [Google Scholar] [CrossRef]
- Telfer, N.R.; Chalmers, R.J.; Whale, K.; Colman, G. The role of streptococcal infection in the initiation of guttate psoriasis. Arch. Dermatol. 1992, 128, 39–42. [Google Scholar] [CrossRef]
- Tomi, N.S.; Kränke, B.; Aberer, E. Staphylococcal toxins in patients with psoriasis, atopic dermatitis, and erythroderma, and in healthy control subjects. J. Am. Acad. Dermatol. 2005, 53, 67–72. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. 3rd ed. ISO: Geneva, Switzerland, 2009.
- Pharmacopoeia, U.S. US Pharmacopoeial Convention; Pharmacopoeia, U.S.: Rockville, MD, USA, 2002; p. 51. [Google Scholar]
- Schofs, L.; Sparo, M.D.; Sánchez Bruni, S.F. The antimicrobial effect behind Cannabis sativa. Pharmacol. Res. Perspect. 2021, 9, e00761. [Google Scholar] [CrossRef]
- Tahsin, K.N.; Watson, D.; Rizkalla, A.; Heinrichs, D.; Charpentier, P. Antimicrobial Studies of Cannabidiol as Biomaterials against superbug MRSA. CMBES Proc. 2021, 44. Available online: https://proceedings.cmbes.ca/index.php/proceedings/article/view/915 (accessed on 10 September 2022).
- Oláh, A.; Markovics, A.; Szabó-Papp, J.; Szabó, P.T.; Stott, C.; Zouboulis, C.C.; Bíró, T. Differential effectiveness of selected non-psychotropic phytocannabinoids on human sebocyte functions implicates their introduction in dry/seborrhoeic skin and acne treatment. Exp. Dermatol. 2016, 25, 701–707. [Google Scholar] [CrossRef]
- Kircik, L.H. What’s new in the management of acne vulgaris. Cutis 2019, 104, 48–52. [Google Scholar] [PubMed]
- Feldman, M.; Sionov, R.V.; Mechoulam, R.; Steinberg, D. Anti-Biofilm Activity of Cannabidiol against Candida albicans. Microorganisms 2021, 9, 441. [Google Scholar] [CrossRef]
- Pretzsch, C.M.; Voinescu, B.; Lythgoe, D.; Horder, J.; Mendez, M.A.; Wichers, R.; Ajram, L.; Ivin, G.; Heasman, M.; Edden, R.A. Effects of cannabidivarin (CBDV) on brain excitation and inhibition systems in adults with and without Autism Spectrum Disorder (ASD): A single dose trial during magnetic resonance spectroscopy. Transl. Psychiatry 2019, 9, 313. [Google Scholar] [CrossRef]
- Huizenga, M.N.; Sepulveda-Rodriguez, A.; Forcelli, P.A. Preclinical safety and efficacy of cannabidivarin for early life seizures. Neuropharmacology 2019, 148, 189–198. [Google Scholar] [CrossRef]
- Jiang, X.; Pace, J.L. Microbial biofilms. In Biofilms, Infection, and Antimicrobial Therapy; CRC Press: Boca Raton, FL, USA, 2005; pp. 21–38. [Google Scholar]
- Parsek, M.R.; Greenberg, E.P. Acyl-homoserine lactone quorum sensing in gram-negative bacteria: A signaling mechanism involved in associations with higher organisms. Proc. Natl. Acad. Sci. USA 2000, 97, 8789–8793. [Google Scholar] [CrossRef]
- Cogen, A.; Nizet, V.; Gallo, R. Skin microbiota: A source of disease or defence? Br. J. Dermatol. 2008, 158, 442–455. [Google Scholar] [CrossRef]
- Milando, R.; Friedman, A. Cannabinoids: Potential role in inflammatory and neoplastic skin diseases. Am. J. Clin. Dermatol. 2019, 20, 167–180. [Google Scholar] [CrossRef]
- Attard, T.M.; McElroy, C.R.; Rezende, C.A.; Polikarpov, I.; Clark, J.H.; Hunt, A.J. Sugarcane waste as a valuable source of lipophilic molecules. Ind. Crops Prod. 2015, 76, 95–103. [Google Scholar] [CrossRef]
- M11-A6; Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria, Approved Standard—9th Edition. CLSI: Berwyn, PA, USA, 2018; Volume 24.
- M07; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 11th Edition. CLSI: Berwyn, PA, USA, 2018; Volume 32.
- M27-A2; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, Approved Standard—Second Edition. CLSI: Berwyn, PA, USA, 2002; Volume 22, p. 51.
- Fernandes, J.C.; Tavaria, F.K.; Soares, J.C.; Ramos, Ó.S.; Monteiro, M.J.; Pintado, M.E.; Malcata, F.X. Antimicrobial effects of chitosans and chitooligosaccharides, upon Staphylococcus aureus and Escherichia coli, in food model systems. Food Microbiol. 2008, 25, 922–928. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Costa, M.R.; Pereira, M.F.; Pereira, J.O.; Soares, J.C.; Pintado, M.M. Aqueous extracts of Vaccinium corymbosum as inhibitors of Staphylococcus aureus. Food Control 2015, 51, 314–320. [Google Scholar] [CrossRef]
- Costa, E.; Silva, S.; Tavaria, F.; Pintado, M. Insights into chitosan antibiofilm activity against methicillin-resistant Staphylococcus aureus. J. Appl. Microbiol. 2017, 122, 1547–1557. [Google Scholar] [CrossRef]
- Carvalho, M.J.; Pinto-Ribeiro, I.; Castro, C.; Pedrosa, S.S.; Oliveira, A.L.S.; Pintado, M.; Madureira, A.R. Preclinical model to evaluate how beneficial are cosmetic ingredients for skin microbiota. In Proceedings of the 9th Beneficial Microbes Conference, Amsterdam, The Netherlands, 14–16 November 2022. [Google Scholar]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Horz, H.; Vianna, M.; Gomes, B.; Conrads, G. Evaluation of universal probes and primer sets for assessing total bacterial load in clinical samples: General implications and practical use in endodontic antimicrobial therapy. J. Clin. Microbiol. 2005, 43, 5332–5337. [Google Scholar] [CrossRef] [PubMed]
- Van Der Krieken, D.A.; Ederveen, T.H.; Van Hijum, S.A.; Jansen, P.A.; Melchers, W.J.; Scheepers, P.T.; Schalkwijk, J.; Zeeuwen, P.L. An in vitro model for bacterial growth on human stratum corneum. Acta Derm. Venereol. 2016, 96, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Wampach, L.; Heintz-Buschart, A.; Hogan, A.; Muller, E.E.; Narayanasamy, S.; Laczny, C.C.; Hugerth, L.W.; Bindl, L.; Bottu, J.; Andersson, A.F. Colonization and succession within the human gut microbiome by archaea, bacteria, and microeukaryotes during the first year of life. Front. Microbiol. 2017, 8, 738. [Google Scholar] [CrossRef]
- Byrne, F.J.; Waters, S.M.; Waters, P.S.; Curtin, W.; Kerin, M. Development of a molecular methodology to quantify Staphylococcus epidermidis in surgical wash-out samples from prosthetic joint replacement surgery. Eur. J. Orthop. Surg. Traumatol. 2007, 1–7. [Google Scholar] [CrossRef] [PubMed]

| Compound | S. aureus | S. epidermidis | S. pyogenes | C. acnes | P. innocua | P. aeruginosa | E. coli | C. albicans | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| CBG | 25 | 75 | 25 | 50 | 50 | 75 | 500 | 3180 | 10 | 50 | 400 | 5000 | 500 | 5000 | 200 | 400 |
| CBG Tocris | 10 | 25 | 25 | 75 | 75 | 100 | 1000 | 3180 | 10 | 25 | 400 | 3180 | 1000 | 3180 | 400 | 500 |
| CBD | 10 | 75 | 10 | 25 | 25 | 50 | 500 | 5000 | 25 | 75 | 750 | 5000 | 750 | 5000 | 200 | 400 |
| CBD Tocris | 75 | 100 | 50 | 75 | 50 | 100 | >1000 | >5000 | 75 | 100 | 1000 | 3180 | 3180 | 3180 | 250 | 500 |
| CBD Linnea | 10 | 25 | 5 | 10 | 10 | 25 | 300 | 5000 | 10 | 50 | 1000 | >5000 | 3180 | >5000 | 200 | 300 |
| Vancomycin | 0.34 | 0.7 | 0.17 | 0.34 | 0.17 | 0.34 | 0.7 | 1.4 | 0.09 | 0.17 | - | - | - | - | - | - |
| Ciprofloxacin | 3 | 6 | 0.3 | 0.75 | 1.5 | 3 | 0.3 | 0.75 | 0.3 | 0.3 | 3 | 6 | 6 | 15 | - | - |
| Colistin | - | - | - | - | - | - | - | - | - | - | 0.4 | 4.3 | 0.4 | 0.9 | - | - |
| Log Variation | Day 14 | Day 28 | ||||||
|---|---|---|---|---|---|---|---|---|
| CBG | CBD | No Preservative | Phenoxyethanol | CBG | CBD | No Preservative | Phenoxyethanol | |
| Staphylococcus aureus | 2.1 | 2.0 | 0.7 | 6.0 | No increase | |||
| Escherichia coli | 2.0 | 2.0 | 0.4 | 2.1 | ||||
| Pseudomonas aeruginosa | 2.0 | 0.8 | −0.5 | 1.5 | ||||
| Candida albicans | No increase | |||||||
| Aspergillus brasiliensis | No increase | Increase | No increase | No increase | Increase | No increase | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luz-Veiga, M.; Amorim, M.; Pinto-Ribeiro, I.; Oliveira, A.L.S.; Silva, S.; Pimentel, L.L.; Rodríguez-Alcalá, L.M.; Madureira, R.; Pintado, M.; Azevedo-Silva, J.; et al. Cannabidiol and Cannabigerol Exert Antimicrobial Activity without Compromising Skin Microbiota. Int. J. Mol. Sci. 2023, 24, 2389. https://doi.org/10.3390/ijms24032389
Luz-Veiga M, Amorim M, Pinto-Ribeiro I, Oliveira ALS, Silva S, Pimentel LL, Rodríguez-Alcalá LM, Madureira R, Pintado M, Azevedo-Silva J, et al. Cannabidiol and Cannabigerol Exert Antimicrobial Activity without Compromising Skin Microbiota. International Journal of Molecular Sciences. 2023; 24(3):2389. https://doi.org/10.3390/ijms24032389
Chicago/Turabian StyleLuz-Veiga, Mariana, Manuela Amorim, Inês Pinto-Ribeiro, Ana L. S. Oliveira, Sara Silva, Lígia L. Pimentel, Luís M. Rodríguez-Alcalá, Raquel Madureira, Manuela Pintado, João Azevedo-Silva, and et al. 2023. "Cannabidiol and Cannabigerol Exert Antimicrobial Activity without Compromising Skin Microbiota" International Journal of Molecular Sciences 24, no. 3: 2389. https://doi.org/10.3390/ijms24032389
APA StyleLuz-Veiga, M., Amorim, M., Pinto-Ribeiro, I., Oliveira, A. L. S., Silva, S., Pimentel, L. L., Rodríguez-Alcalá, L. M., Madureira, R., Pintado, M., Azevedo-Silva, J., & Fernandes, J. (2023). Cannabidiol and Cannabigerol Exert Antimicrobial Activity without Compromising Skin Microbiota. International Journal of Molecular Sciences, 24(3), 2389. https://doi.org/10.3390/ijms24032389











