Synthesis of Antimicrobial Chitosan-Silver Nanoparticles Mediated by Reusable Chitosan Fungal Beads
Abstract
:1. Introduction
2. Results and Discussion
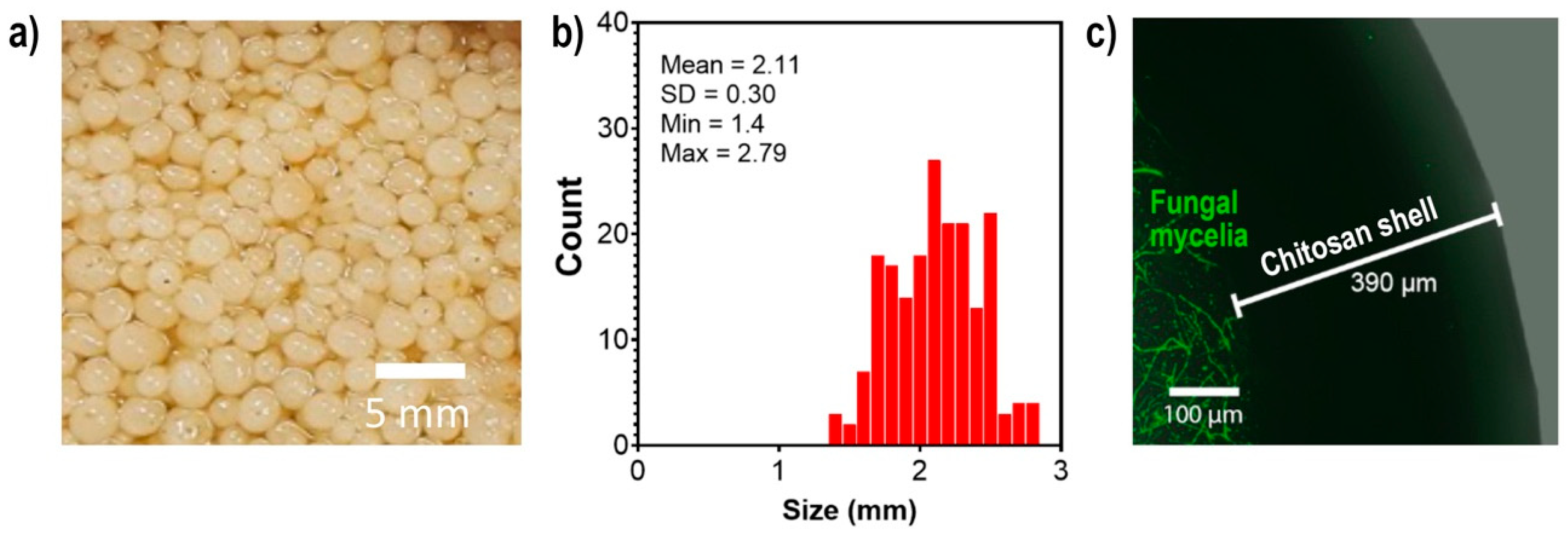
2.1. Encapsulation of S. hirsutum in Chitosan Beads
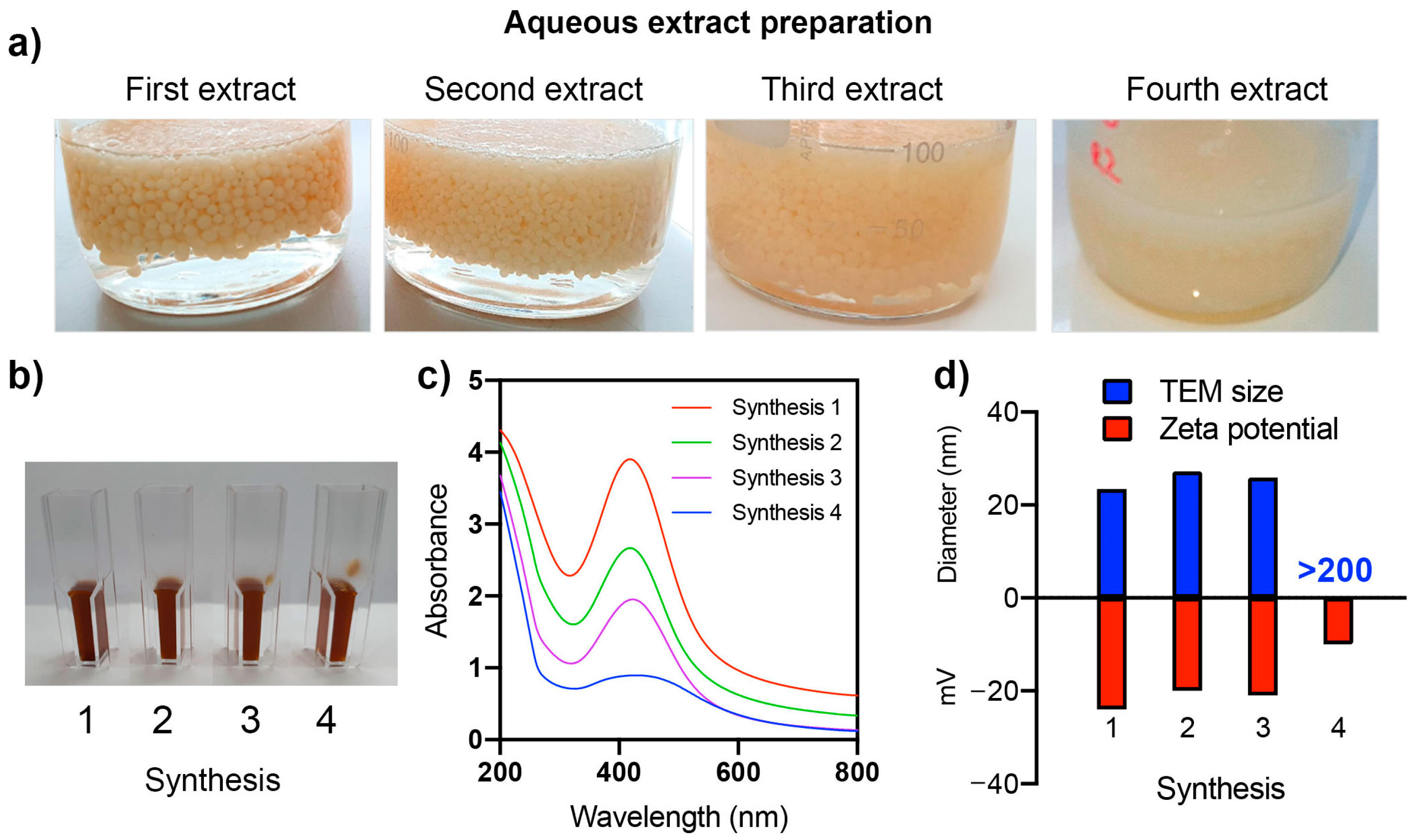
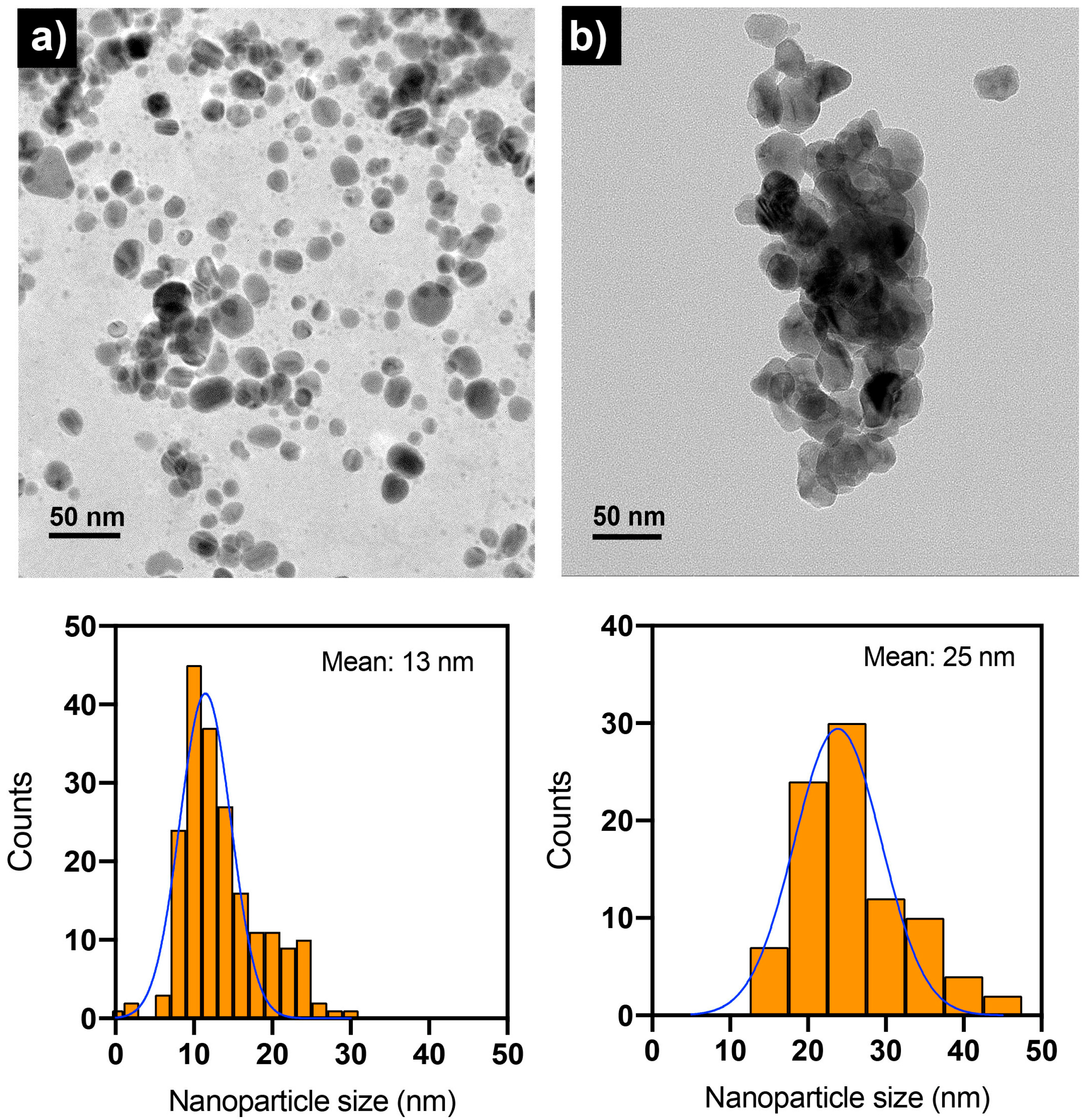
2.2. Synthesis of Nanoparticles Using the Aqueous Extract of Chitosan Fungal Beads
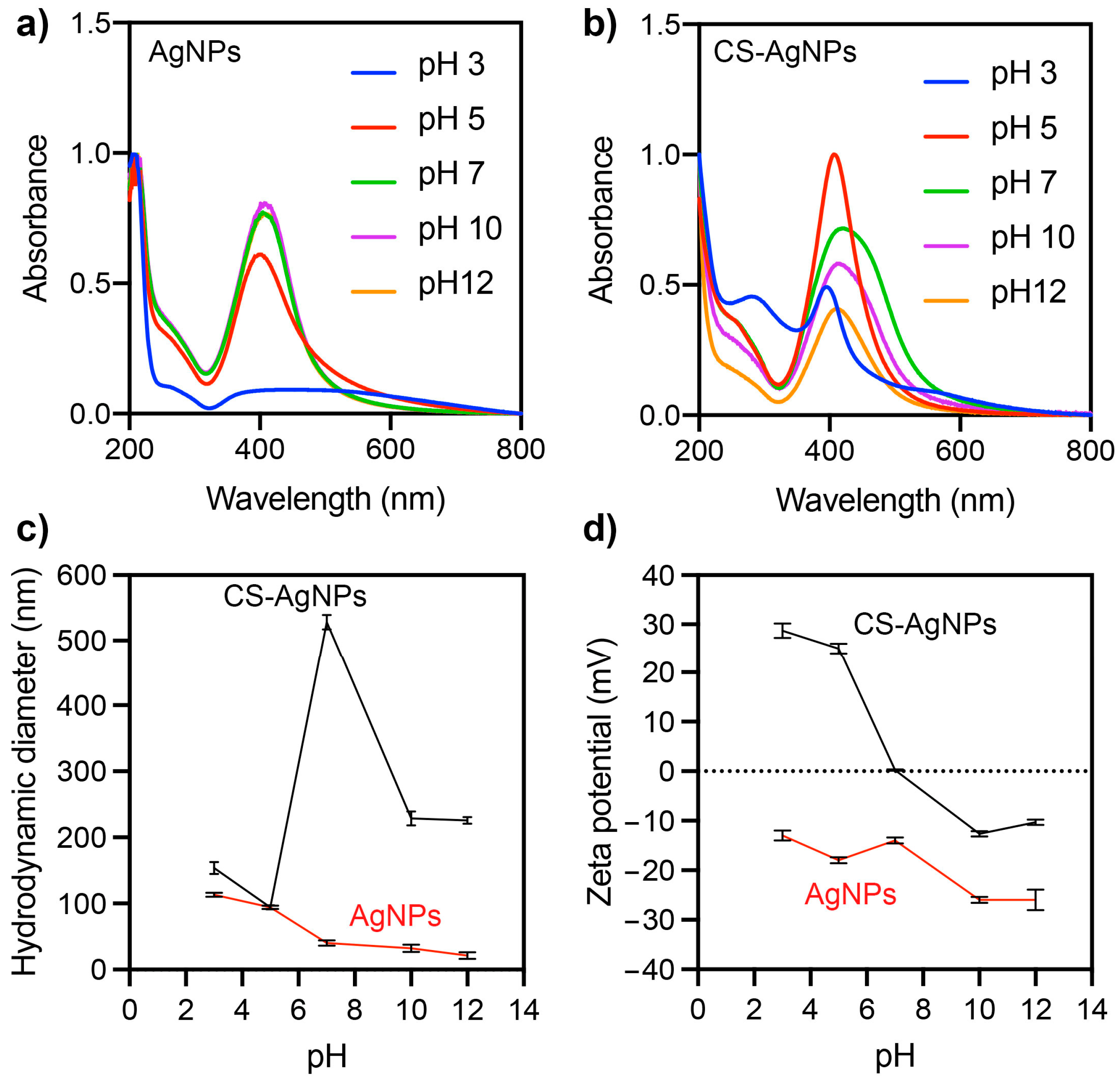
2.3. Effects of pH on the Stability of Ag NPs and CS-Ag NPs
2.4. Antimicrobial Activity of Ag NPs and CS-Ag NPs
3. Materials and Methods
3.1. Materials
3.2. Microorganism
3.3. Fungal Biomass Obtention
3.4. Preparation of Chitosan Fungal Beads
3.5. Synthesis of Nanoparticles Using Extract of Chitosan Fungal Beads
3.6. Synthesis of Nanoparticles Using Extract of Unencapsulated Fungal Biomass
3.7. Characterization of Synthesized Nanoparticles
3.8. Antimicrobial Activity of Ag NPs and CS-Ag NPs against Human Pathogen Bacteria
3.9. Antimicrobial Activity of Ag NPs and CS-Ag NPs against Human Pathogen Yeast
3.10. Antimicrobial Activity of Ag NPs and CS-Ag NPs against Phytopathogen Bacterium
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haktaniyan, M.; Bradley, M. Polymers Showing Intrinsic Antimicrobial Activity. Chem. Soc. Rev. 2022, 51, 8584–8611. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Xiang, S.; Lv, X.; Wang, X.; Li, F.; Liu, W.; Liu, C.; Ran, M.; Huang, J.; Xu, X.; et al. Biosynthesized Silver Nanoparticles Inhibit Pseudomonas syringae pv. tabaci by Directly Destroying Bacteria and Inducing Plant Resistance in Nicotiana benthamiana. Phytopathol. Res. 2022, 4, 43. [Google Scholar] [CrossRef]
- Shahryari, F.; Rabiei, Z.; Sadighian, S. Antibacterial Activity of Synthesized Silver Nanoparticles by Sumac Aqueous Extract and Silver-Chitosan Nanocomposite against Pseudomonas syringae pv. syringae. J. Plant Pathol. 2020, 102, 469–475. [Google Scholar] [CrossRef]
- Cisternas, C.; Tortella, G.; Seabra, A.B.; Pieretti, J.C.; Araya-Castro, K.; Hermosilla, E.; Cristina Diez, M.; Rubilar, O. Development of a New Biomimetic Method for the Synthesis of Silver Nanoparticles Based on Fungal Metabolites: Optimization and Antibacterial Activity. J. Chem. Technol. Biotechnol. 2021, 96, 1981–1990. [Google Scholar] [CrossRef]
- Kanwar, R.; Fatima, R.; Kanwar, R.; Javid, M.T.; Muhammad, U.W.; Ashraf, Z.; Khalid, A.; Tariq Javid, M.; Biological, A.K. Biological, Physical and Chemical Synthesis of Silver Nanoparticles and Their Non-Toxic Bio-Chemical Application: A Brief Review. Pure Appl. Biol. 2022, 11, 421–438. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of Silver Nanoparticles: Chemical, Physical and Biological Methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar] [PubMed]
- Rodríguez-Félix, F.; Graciano-Verdugo, A.Z.; Moreno-Vásquez, M.J.; Lagarda-Díaz, I.; Barreras-Urbina, C.G.; Armenta-Villegas, L.; Olguín-Moreno, A.; Tapia-Hernández, J.A. Trends in Sustainable Green Synthesis of Silver Nanoparticles Using Agri-Food Waste Extracts and Their Applications in Health. J. Nanomater. 2022, 2022, 8874003. [Google Scholar] [CrossRef]
- Liaqat, N.; Jahan, N.; Khalil-ur-Rahman; Anwar, T.; Qureshi, H. Green Synthesized Silver Nanoparticles: Optimization, Characterization, Antimicrobial Activity, and Cytotoxicity Study by Hemolysis Assay. Front. Chem. 2022, 10, 952006. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Ayaz, M.; Ahmad, I.; Nethi, S.K.; Mukherjee, S. Biosynthesis of Metal Nanoparticles via Microbial Enzymes: A Mechanistic Approach. Int. J. Mol. Sci. 2018, 19, 4100. [Google Scholar] [CrossRef] [Green Version]
- Elgorban, A.M.; Aref, S.M.; Seham, S.M.; Elhindi, K.M.; Bahkali, A.H.; Sayed, S.R.; Manal, M.A. Extracellular Synthesis of Silver Nanoparticles Using Aspergillus versicolor and Evaluation of Their Activity on Plant Pathogenic Fungi. Mycosphere 2016, 7, 844–852. [Google Scholar] [CrossRef]
- Chowdhury, S.; Basu, A.; Kundu, S. Green Synthesis of Protein Capped Silver Nanoparticles from Phytopathogenic Fungus Macrophomina phaseolina (Tassi) Goid with Antimicrobial Properties against Multidrug-Resistant Bacteria. Nanoscale Res. Lett. 2014, 9, 365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asadollahzadeh, M.; Mahboubi, A.; Taherzadeh, M.J.; Åkesson, D.; Lennartsson, P.R. Application of Fungal Biomass for the Development of New Polylactic Acid-Based Biocomposites. Polymers 2022, 14, 1738. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.G.H.; Park, H.J. Preparation and Characterization of Drug-Loaded Chitosan-Tripolyphosphate Microspheres by Spray Drying. Drug Dev. Res. 2005, 64, 114–128. [Google Scholar] [CrossRef]
- Hsu, J.S.; Yu, T.Y.; Wei, D.J.; Jane, W.N.; Chang, Y.T. Degradation of Decabromodiphenyl Ether in an Aerobic Clay Slurry Microcosm Using a Novel Immobilization Technique. Microorganisms 2022, 10, 402. [Google Scholar] [CrossRef]
- Fernández, M.; Pagnussat, L.A.; Borrajo, M.P.; Perez Bravo, J.J.; Francois, N.J.; Creus, C.M. Chitosan/Starch Beads as Bioinoculants Carrier: Long-Term Survival of Bacteria and Plant Growth Promotion. Appl. Microbiol. Biotechnol. 2022, 106, 7963–7972. [Google Scholar] [CrossRef]
- Hsieh, F.M.; Huang, C.; Lin, T.F.; Chen, Y.M.; Lin, J.C. Study of Sodium Tripolyphosphate-Crosslinked Chitosan Beads Entrapped with Pseudomonas putida for Phenol Degradation. Process Biochem. 2008, 43, 83–92. [Google Scholar] [CrossRef]
- Hermosilla, E.; Díaz, M.; Vera, J.; Seabra, A.B.; Tortella, G.; Parada, J.; Rubilar, O. Molecular Weight Identification of Compounds Involved in the Fungal Synthesis of AgNPs: Effect on Antimicrobial and Photocatalytic Activity. Antibiotics 2022, 11, 622. [Google Scholar] [CrossRef]
- Velgosová, O.; Mrazíková, A. Limitations and Possibilities of Green Synthesis and Long-Term Stability of Colloidal Ag Nanoparticles. In Proceedings of the 1st International Conference on Mechanical Engineering and Applied Science, Dhaka, Bangladesh, 22–23 February 2017; American Institute of Physics Inc.: College Park, MD, USA, 2017; Volume 1918. [Google Scholar]
- Restrepo, C.V.; Villa, C.C. Synthesis of Silver Nanoparticles, Influence of Capping Agents, and Dependence on Size and Shape: A Review. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100428. [Google Scholar] [CrossRef]
- Shnoudeh, A.J.; Hamad, I.; Abdo, R.W.; Qadumii, L.; Jaber, A.Y.; Surchi, H.S.; Alkelany, S.Z. Synthesis, Characterization, and Applications of Metal Nanoparticles. Biomater. Bionanotechnol. 2019, 527–612. [Google Scholar] [CrossRef]
- Wang, L.S.; Wang, C.Y.; Yang, C.H.; Hsieh, C.L.; Chen, S.Y.; Shen, C.Y.; Wang, J.J.; Huang, K.S. Synthesis and Anti-Fungal Effect of Silver Nanoparticles–Chitosan Composite Particles. Int. J. Nanomed. 2015, 10, 2685–2696. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial Properties of Chitosan and Mode of Action: A State of the Art Review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Williams, D.W. Escherichia Coli. Microbiol. Waterborne Dis. Second Ed. 2014, 89–117. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus Aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motta, E.P.; Farias, J.R.; Costa, A.A.C.d.; Silva, A.F.d.; Oliveira Lopes, A.J.; Cartágenes, M.d.S.S.; Nicolete, R.; Abreu, A.G.; Fernandes, E.S.; Nascimento, F.R.F.; et al. The Anti-Virulence Effect of Vismia guianensis against Candida albicans and Candida glabrata. Antibiotics 2022, 11, 1834. [Google Scholar] [CrossRef]
- Ahamad, I.; Bano, F.; Anwer, R.; Srivastava, P.; Kumar, R.; Fatma, T. Antibiofilm Activities of Biogenic Silver Nanoparticles Against Candida albicans. Front. Microbiol. 2022, 12, 741493. [Google Scholar] [CrossRef]
- Mare, A.D.; Ciurea, C.N.; Man, A.; Mareș, M.; Toma, F.; Berța, L.; Tanase, C. In Vitro Antifungal Activity of Silver Nanoparticles Biosynthesized with Beech Bark Extract. Plants 2021, 10, 2153. [Google Scholar] [CrossRef]
- Sherif, H.H.A.; Khalil, S.K.H.; Hegazi, A.G.; Khalil, W.A.; Moharram, M.A. Factors Affecting the Antibacterial Activity of Chitosan-Silver Nanocomposite. IET Nanobiotechnol. 2017, 11, 731–737. [Google Scholar] [CrossRef]
- Qiu, Y.; Hamilton, S.K.; Temenoff, J. Improving Mechanical Properties of Injectable Polymers and Composites. In Injectable Biomaterials; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2011; pp. 61–91. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [Green Version]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green Synthesis of Silver Nanoparticles: Biomolecule-Nanoparticle Organizations Targeting Antimicrobial Activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef]
- Tortella, G.; Rubilar, O.; Fincheira, P.; Pieretti, J.C.; Duran, P.; Lourenço, I.M.; Seabra, A.B.; Fernanda, M.; Carvalho, N.N. Antibiotics Bactericidal and Virucidal Activities of Biogenic Metal-Based Nanoparticles: Advances and Perspectives. Antibiotics 2021, 10, 783. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Azeredo, H.M.C.; Otoni, C.G.; Assis, O.B.G.; Corrêa, D.S.; de Moura, M.R.; Mattoso, L.H.C. Nanoparticles and Antimicrobial Food Packaging. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–13. [Google Scholar] [CrossRef]


| Strains | Minimum Inhibitory Concentration (MIC µg mL−1) | |||
|---|---|---|---|---|
| Antibiotic Control | Ag NPs (pH 10) | CS-Ag NPs (pH 10) | CS-Ag NPs (pH 5) | |
| P. syringae CCCT22.02 a | 1.0 TC | 40.0 | 1.5 (27-fold) | 1.5 (27-fold) |
| E. coli ATCC 25922 a | 0.25 CAZ | 12.5 | 6.2 (2-fold) | 1.6 (7.8-fold) |
| S. aureus ATCC 25923 b | 8.0 CAZ | 3.1 | 3.1 | 3.1 |
| C. albicans ATCC90028 c | 0.03 ANF | 16 | 4 (4-fold) | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hermosilla, E.; Díaz, M.; Vera, J.; Contreras, M.J.; Leal, K.; Salazar, R.; Barrientos, L.; Tortella, G.; Rubilar, O. Synthesis of Antimicrobial Chitosan-Silver Nanoparticles Mediated by Reusable Chitosan Fungal Beads. Int. J. Mol. Sci. 2023, 24, 2318. https://doi.org/10.3390/ijms24032318
Hermosilla E, Díaz M, Vera J, Contreras MJ, Leal K, Salazar R, Barrientos L, Tortella G, Rubilar O. Synthesis of Antimicrobial Chitosan-Silver Nanoparticles Mediated by Reusable Chitosan Fungal Beads. International Journal of Molecular Sciences. 2023; 24(3):2318. https://doi.org/10.3390/ijms24032318
Chicago/Turabian StyleHermosilla, Edward, Marcela Díaz, Joelis Vera, María José Contreras, Karla Leal, Rodrigo Salazar, Leticia Barrientos, Gonzalo Tortella, and Olga Rubilar. 2023. "Synthesis of Antimicrobial Chitosan-Silver Nanoparticles Mediated by Reusable Chitosan Fungal Beads" International Journal of Molecular Sciences 24, no. 3: 2318. https://doi.org/10.3390/ijms24032318
APA StyleHermosilla, E., Díaz, M., Vera, J., Contreras, M. J., Leal, K., Salazar, R., Barrientos, L., Tortella, G., & Rubilar, O. (2023). Synthesis of Antimicrobial Chitosan-Silver Nanoparticles Mediated by Reusable Chitosan Fungal Beads. International Journal of Molecular Sciences, 24(3), 2318. https://doi.org/10.3390/ijms24032318










