The COPD-Associated Polymorphism Impairs the CFTR Function to Suppress Excessive IL-8 Production upon Environmental Pathogen Exposure
Abstract
1. Introduction
2. Results
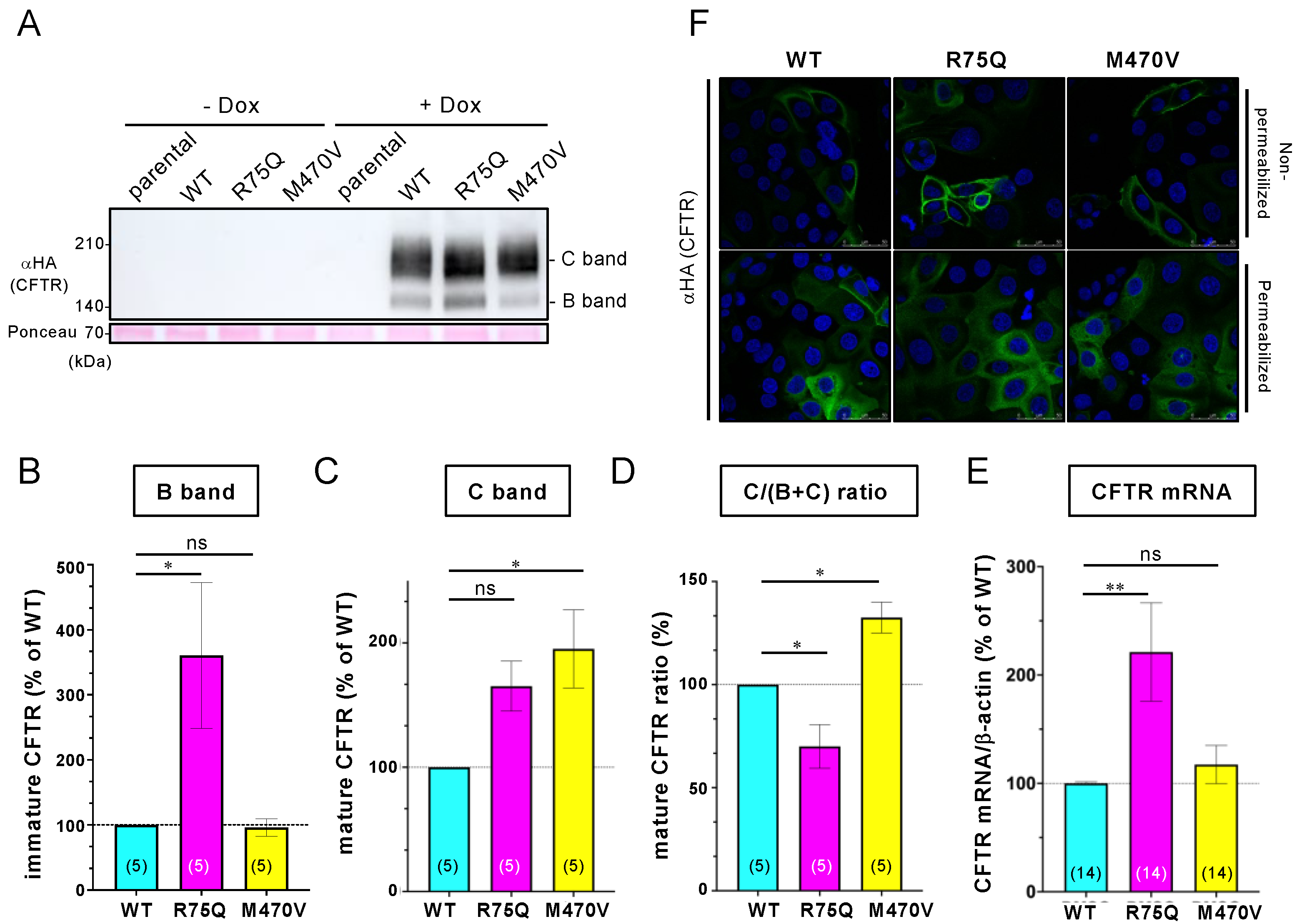
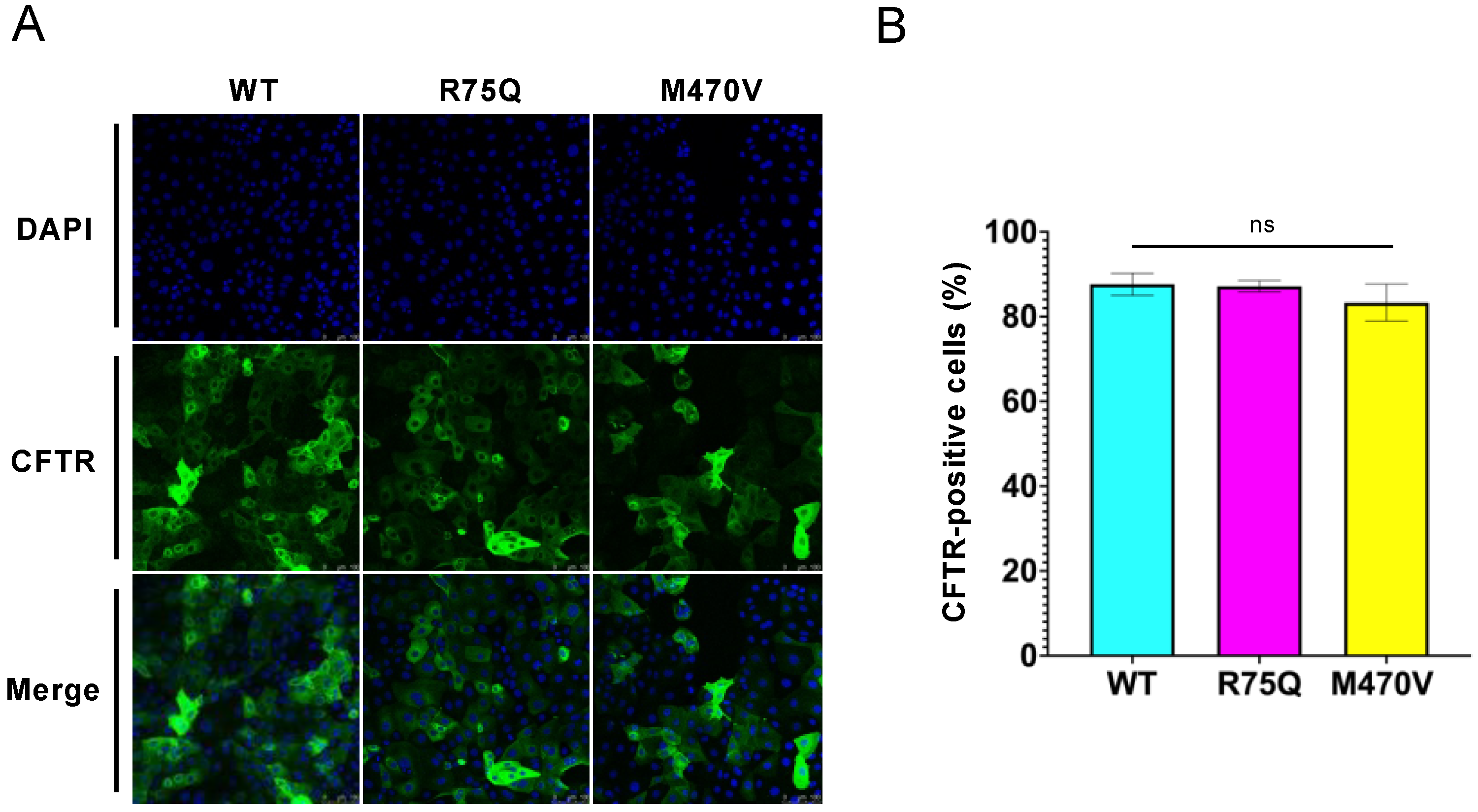
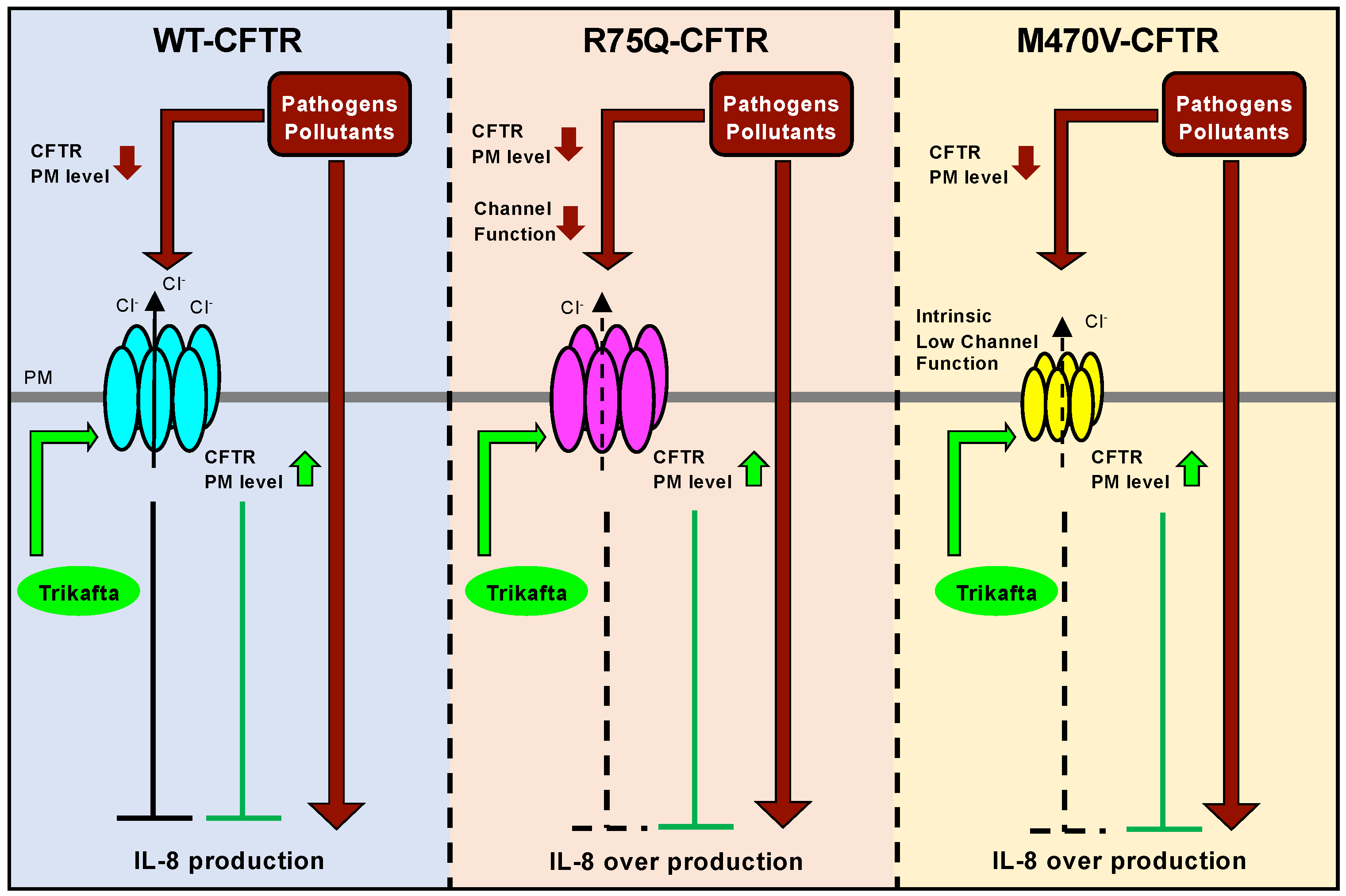
2.1. R75Q or M470V Polymorphism Loses CFTR’s Function to Suppress the Proinflammatory Cytokine Secretion
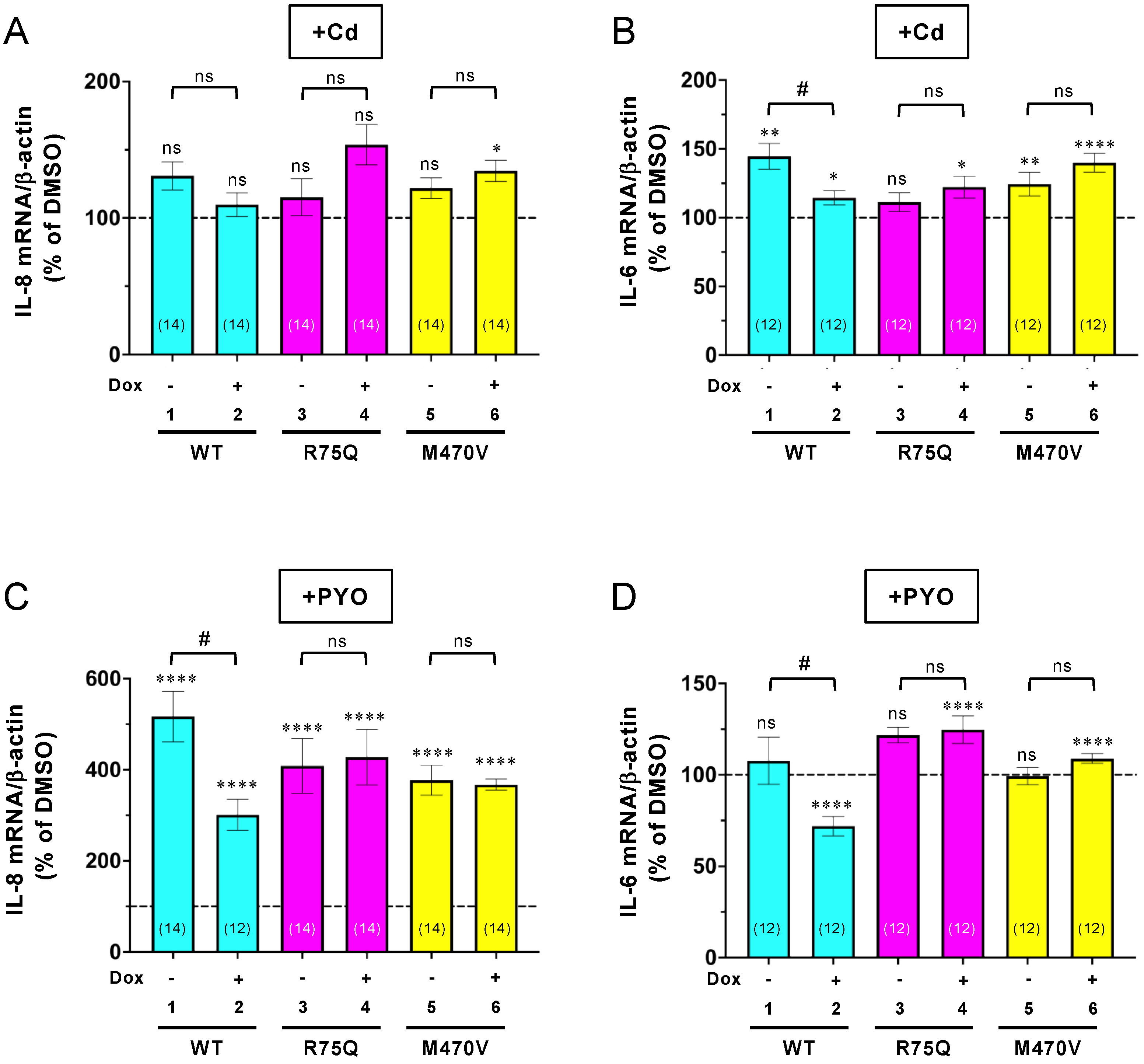
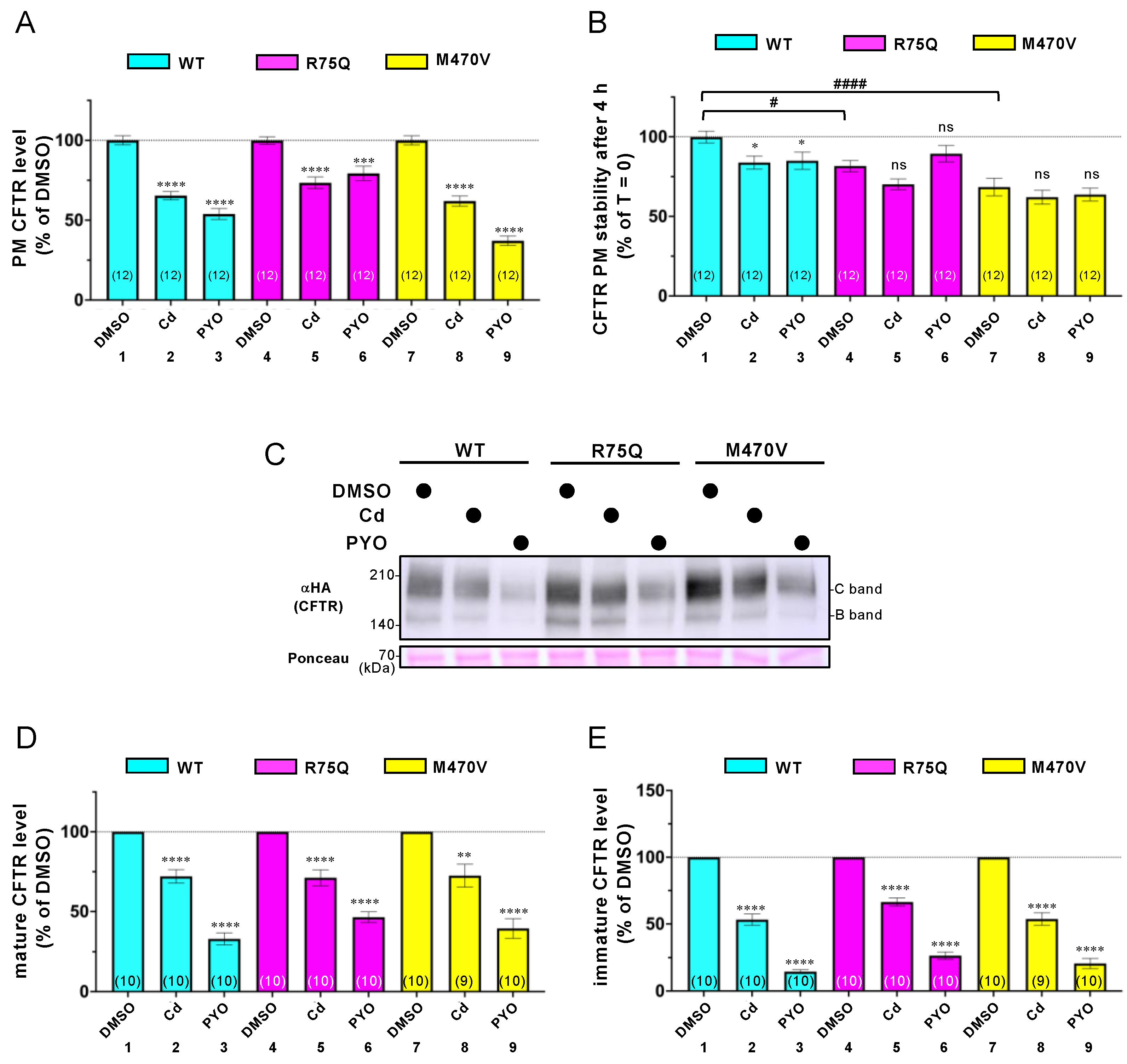
2.2. Sustained Exposure to Cd or PYO Reduces the PM Level of R75Q-CFTR and M470V-CFTR Proteins to the Same Extent as WT-CFTR
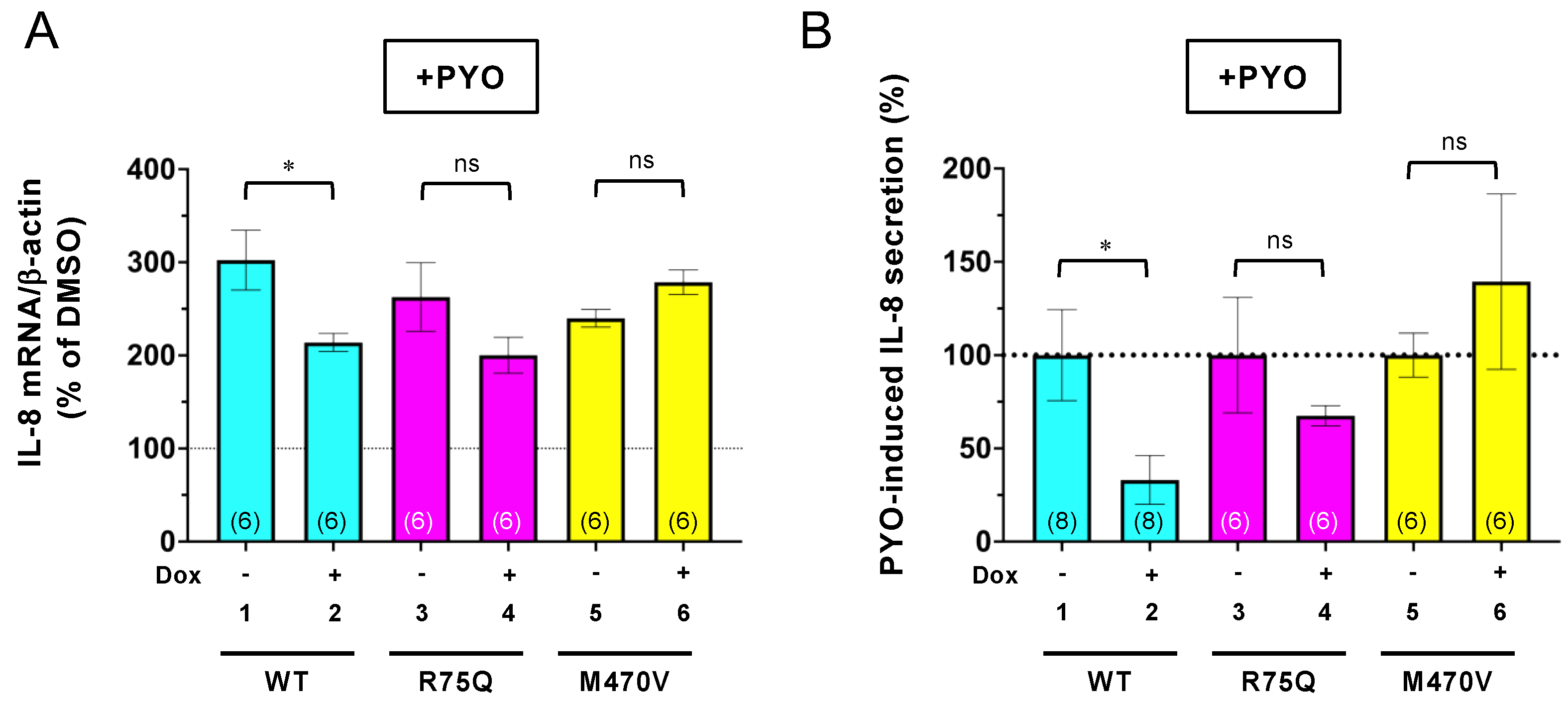
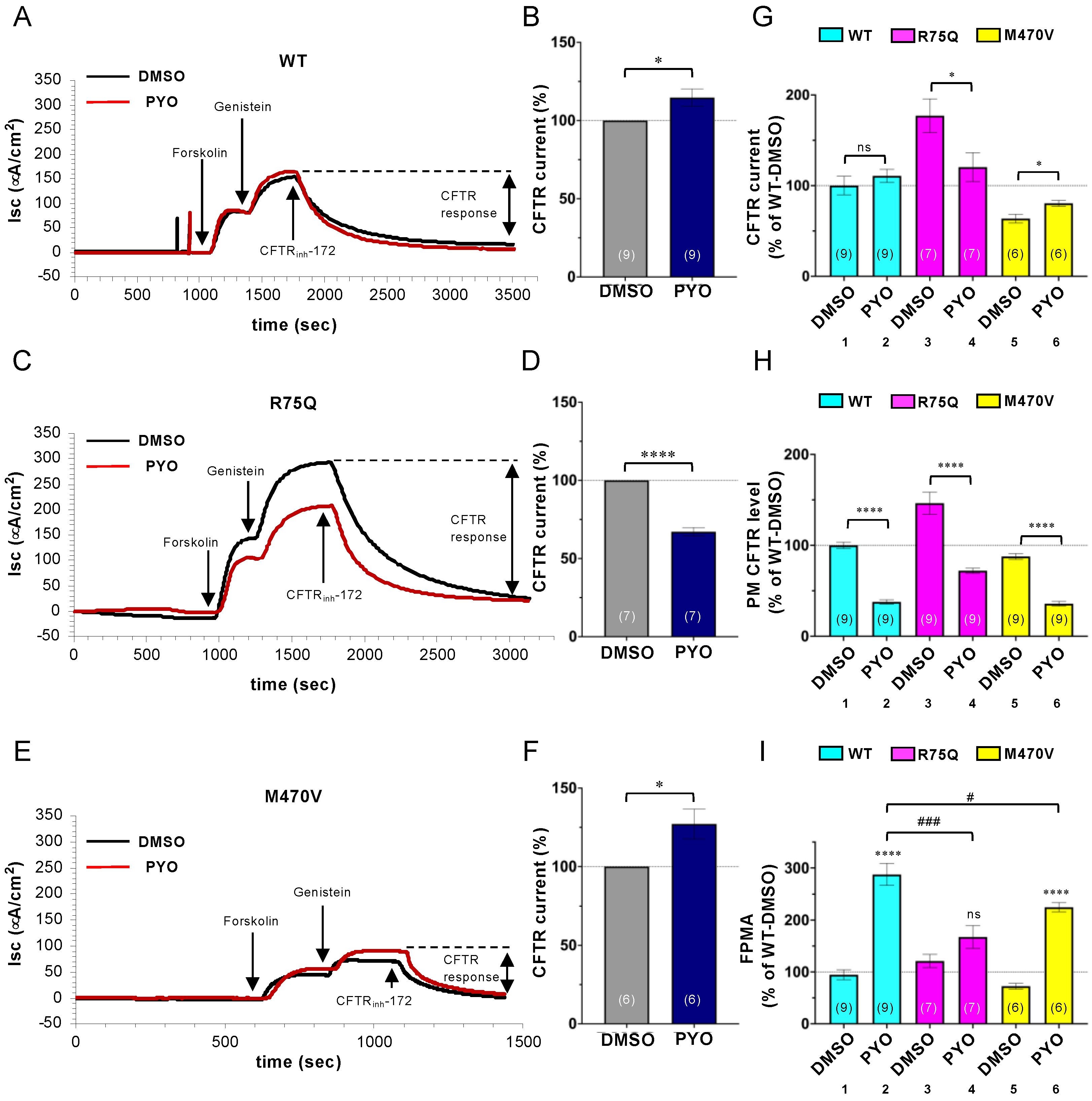
2.3. Chronic PYO Exposure Inactivates the R75Q-CFTR Channel
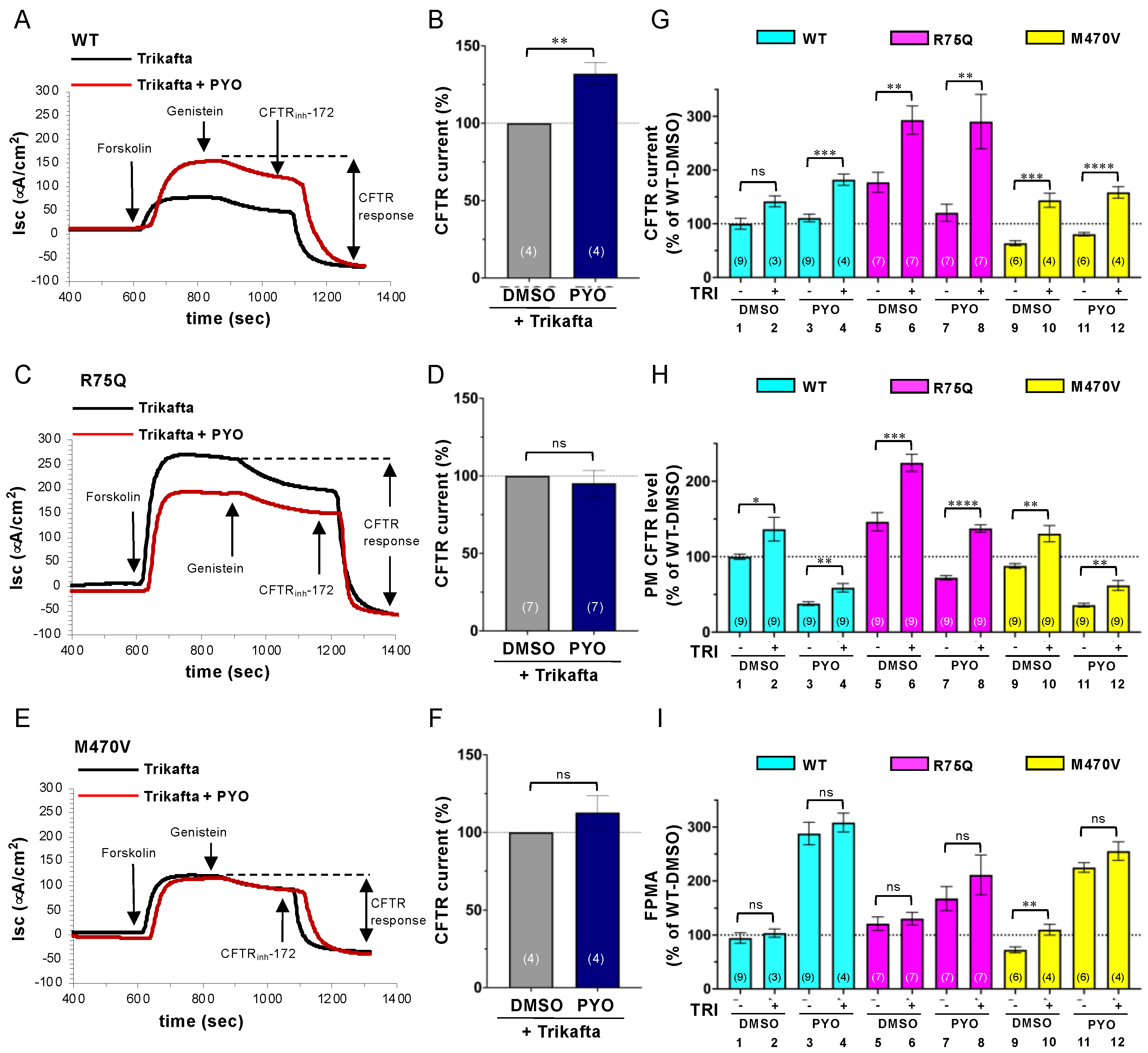
2.4. Trikafta Corrects the Cell Surface Expression of CFTR upon PYO Exposure
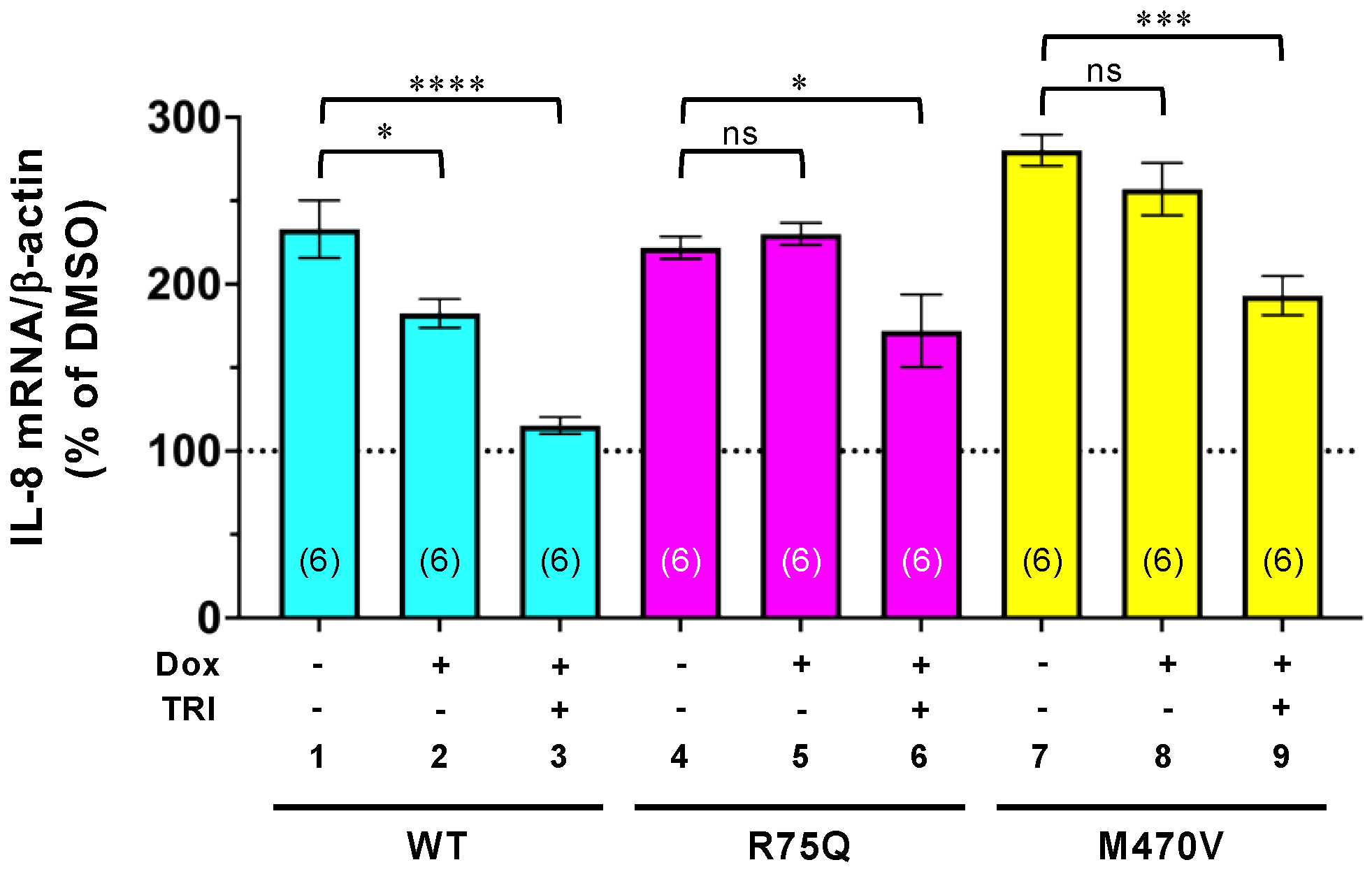
2.5. Trikafta Corrects the Function of R75Q- or M470V-CFTR to Suppress the PYO-induced IL-8 Production
3. Discussion
4. Materials and Methods
4.1. Constructs
4.2. Cell Culture and Reagents
4.3. Immunocytochemistry
4.4. Quantitative Real-Time PCR
4.5. IL-8 ELISA
4.6. Cell Surface Density and Cell Surface Stability Measurements of CFTR
4.7. Western Blotting
4.8. Short-Circuit Current Measurement
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christenson, S.; Smith, B.; Bafadhel, M.; Putcha, N. Chronic obstructive pulmonary disease. Lancet 2022, 399, 2227–2242. [Google Scholar] [CrossRef]
- WHO. Chronic Obstructive Pulmonary Disease (COPD); WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Dransfield, M.; Rowe, S.; Vogelmeier, C.; Wedzicha, J.; Criner, G.; Han, M.; Martinez, F.; Calverley, P. Cystic Fibrosis Transmembrane Conductance Regulator: Roles in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2022, 205, 631–640. [Google Scholar] [CrossRef]
- Riordan, J.; Rommens, J.; Kerem, B.; Alon, N.; Rozmahel, R.; Grzelczak, Z.; Zielenski, J.; Lok, S.; Plavsic, N.; Chou, J. Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science 1989, 245, 1066–1073. [Google Scholar] [CrossRef]
- Mall, M.; Grubb, B.; Harkema, J.; O’Neal, W.; Boucher, R. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat. Med. 2004, 10, 487–493. [Google Scholar] [CrossRef]
- Pilewski, J.; Frizzell, R. Role of CFTR in airway disease. Physiol. Rev. 1999, 79, S215–S255. [Google Scholar] [CrossRef]
- Cantin, A. Cystic Fibrosis Transmembrane Conductance Regulator. Implications in Cystic Fibrosis and Chronic Obstructive Pulmonary Disease. Ann. Am. Thorac. Soc. 2016, 13, S150–S155. [Google Scholar]
- Dransfield, M.; Wilhelm, A.; Flanagan, B.; Courville, C.; Tidwell, S.; Raju, S.; Gaggar, A.; Steele, C.; Tang, L.; Liu, B.; et al. Acquired cystic fibrosis transmembrane conductance regulator dysfunction in the lower airways in COPD. Chest 2013, 144, 498–506. [Google Scholar] [CrossRef]
- Bodas, M.; Min, T.; Mazur, S.; Vij, N. Critical modifier role of membrane-cystic fibrosis transmembrane conductance regulator-dependent ceramide signaling in lung injury and emphysema. J. Immunol. 2011, 186, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liang, X.; Zhao, M.; Liu, S.; Huang, Y.; Idell, S.; Li, X.; Ji, H. Correlation of apical fluid-regulating channel proteins with lung function in human COPD lungs. PLoS ONE 2014, 9, e109725. [Google Scholar] [CrossRef] [PubMed]
- Kreindler, J.; Jackson, A.; Kemp, P.; Bridges, R.; Danahay, H. Inhibition of chloride secretion in human bronchial epithelial cells by cigarette smoke extract. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 288, L894–L902. [Google Scholar] [CrossRef]
- Raju, S.V.; Jackson, P.; Courville, C.; McNicholas, C.; Sloane, P.; Sabbatini, G.; Tidwell, S.; Tang, L.; Liu, B.; Fortenberry, J.; et al. Cigarette smoke induces systemic defects in cystic fibrosis transmembrane conductance regulator function. Am. J. Respir. Crit. Care Med. 2013, 188, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Marklew, A.; Patel, W.; Moore, P.; Tan, C.; Smith, A.; Sassano, M.; Gray, M.; Tarran, R. Cigarette Smoke Exposure Induces Retrograde Trafficking of CFTR to the Endoplasmic Reticulum. Sci. Rep. 2019, 9, 13655. [Google Scholar] [CrossRef]
- Rasmussen, J.; Sheridan, J.; Polk, W.; Davies, C.; Tarran, R. Cigarette smoke-induced Ca2+ release leads to cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction. J. Biol. Chem. 2014, 289, 7671–7681. [Google Scholar] [CrossRef]
- Rennolds, J.; Butler, S.; Maloney, K.; Boyaka, P.; Davis, I.; Knoell, D.; Parinandi, N.; Cormet-Boyaka, E. Cadmium regulates the expression of the CFTR chloride channel in human airway epithelial cells. Toxicol. Sci. 2010, 116, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Hernández, L.; Quintana-Gallego, E.; Calero, C.; Reinoso-Arija, R.; Ruiz-Duque, B.; López-Campos, J. Dysfunction in the Cystic Fibrosis Transmembrane Regulator in Chronic Obstructive Pulmonary Disease as a Potential Target for Personalised Medicine. Biomedicines 2021, 9, 1437. [Google Scholar] [CrossRef]
- Hanrahan, J.; Abu-Arish, A.; Wong, F.; Turner, M.; Carlile, G.; Thomas, D.; Cantin, A. Chronic obstructive pulmonary disease and the modulation of CFTR by acute exposure to cigarette smoke. Am. J. Physiol. Cell Physiol. 2022, 323, C1374–C1392. [Google Scholar] [CrossRef] [PubMed]
- Fresquez, M.; Pappas, R.; Watson, C. Establishment of toxic metal reference range in tobacco from US cigarettes. J. Anal. Toxicol. 2013, 37, 298–304. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, J.; Leonard, S.; Rao, K. Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic. Biol. Med. 2004, 36, 1434–1443. [Google Scholar] [CrossRef]
- Jin, T.; Nordberg, M.; Frech, W.; Dumont, X.; Bernard, A.; Ye, T.; Kong, Q.; Wang, Z.; Li, P.; Lundström, N.; et al. Cadmium biomonitoring and renal dysfunction among a population environmentally exposed to cadmium from smelting in China (ChinaCad). Biometals 2002, 15, 397–410. [Google Scholar] [CrossRef]
- Nordberg, G. Lung cancer and exposure to environmental cadmium. Lancet Oncol. 2006, 7, 99–101. [Google Scholar] [CrossRef]
- Pääkkö, P.; Anttila, S.; Kokkonen, P.; Kalliomäki, P. Cadmium in lung tissue as marker for smoking. Lancet 1988, 1, 477. [Google Scholar] [CrossRef]
- Chambers, R.; Laurent, G.; Westergren-Thorsson, G. Cadmium inhibits proteoglycan and procollagen production by cultured human lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 1998, 19, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Schnúr, A.; Premchandar, A.; Bagdany, M.; Lukacs, G. Phosphorylation-dependent modulation of CFTR macromolecular signalling complex activity by cigarette smoke condensate in airway epithelia. Sci. Rep. 2019, 9, 12706. [Google Scholar] [CrossRef]
- Murphy, T. The many faces of Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Clin. Infect. Dis. 2008, 47, 1534–1536. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T.; Prince, A. Cystic fibrosis: A mucosal immunodeficiency syndrome. Nat. Med. 2012, 18, 509–519. [Google Scholar] [CrossRef]
- Hao, Y.; Kuang, Z.; Xu, Y.; Walling, B.; Lau, G. Pyocyanin-induced mucin production is associated with redox modification of FOXA2. Respir. Res. 2013, 14, 82. [Google Scholar] [CrossRef]
- Wilson, R.; Sykes, D.; Watson, D.; Rutman, A.; Taylor, G.; Cole, P. Measurement of Pseudomonas aeruginosa phenazine pigments in sputum and assessment of their contribution to sputum sol toxicity for respiratory epithelium. Infect. Immun. 1988, 56, 2515–2517. [Google Scholar] [CrossRef]
- Gloyne, L.; Grant, G.; Perkins, A.; Powell, K.; McDermott, C.; Johnson, P.; Anderson, G.; Kiefel, M.; Anoopkumar-Dukie, S. Pyocyanin-induced toxicity in A549 respiratory cells is causally linked to oxidative stress. Toxicol. In Vitro 2011, 25, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Young, L.; Chen, Y.; Ran, H.; Meyers, M.; Joseph, P.; Cho, Y.; Hassett, D.; Lau, G. Pseudomonas aeruginosa pyocyanin inactivates lung epithelial vacuolar ATPase-dependent cystic fibrosis transmembrane conductance regulator expression and localization. Cell Microbiol. 2006, 8, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, C.; Fischer, H.; Kim, E.-J. Oxidative stress caused by pyocyanin impairs CFTR Cl—Transport in human bronchial epithelial cells. Free. Radic. Biol. Med. 2008, 45, 1653–1662. [Google Scholar] [CrossRef]
- Domenech, A.; Puig, C.; Martí, S.; Santos, S.; Fernández, A.; Calatayud, L.; Dorca, J.; Ardanuy, C.; Liñares, J. Infectious etiology of acute exacerbations in severe COPD patients. J. Infect. 2013, 67, 516–523. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Almagro, P.; Romaní, V.; Rodríguez-Carballeira, M.; Cuchi, E.; Canales, L.; Blasco, D.; Heredia, J.; Garau, J. Pseudomonas aeruginosa in patients hospitalised for COPD exacerbation: A prospective study. Eur. Respir. J. 2009, 34, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo-Troyano, A.; Melo, V.; Marcos, P.; Laserna, E.; Peiro, M.; Suarez-Cuartin, G.; Perea, L.; Feliu, A.; Plaza, V.; Faverio, P.; et al. Pseudomonas aeruginosa in Chronic Obstructive Pulmonary Disease Patients with Frequent Hospitalized Exacerbations: A Prospective Multicentre Study. Respiration 2018, 96, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Tzetis, M.; Efthymiadou, A.; Strofalis, S.; Psychou, P.; Dimakou, A.; Pouliou, E.; Doudounakis, S.; Kanavakis, E. CFTR gene mutations—Including three novel nucleotide substitutions—And haplotype background in patients with asthma, disseminated bronchiectasis and chronic obstructive pulmonary disease. Hum. Genet. 2001, 108, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Larusch, J.; Sun, X.; Aloe, A.; Lamb, J.; Hawes, R.; Cotton, P.; Brand, R.; Anderson, M.; Money, M.; et al. Combined bicarbonate conductance-impairing variants in CFTR and SPINK1 variants are associated with chronic pancreatitis in patients without cystic fibrosis. Gastroenterology 2011, 140, 162–171. [Google Scholar] [CrossRef]
- Cuppens, H.; Lin, W.; Jaspers, M.; Costes, B.; Teng, H.; Vankeerberghen, A.; Jorissen, M.; Droogmans, G.; Reynaert, I.; Goossens, M.; et al. Polyvariant mutant cystic fibrosis transmembrane conductance regulator genes. The polymorphic (Tg)m locus explains the partial penetrance of the T5 polymorphism as a disease mutation. J. Clin. Investig. 1998, 101, 487–496. [Google Scholar] [CrossRef]
- Veit, G.; Bossard, F.; Goepp, J.; Verkman, A.S.; Galietta, L.J.; Hanrahan, J.W.; Lukacs, G.L. Proinflammatory cytokine secretion is suppressed by TMEM16A or CFTR channel activity in human cystic fibrosis bronchial epithelia. Mol. Biol. Cell 2012, 23, 4188–4202. [Google Scholar] [CrossRef]
- Stankovic, M.; Nikolic, A.; Divac, A.; Tomovic, A.; Petrovic-Stanojevic, N.; Andjelic, M.; Dopudja-Pantic, V.; Surlan, M.; Vujicic, I.; Ponomarev, D.; et al. The CFTR M470V gene variant as a potential modifier of COPD severity: Study of Serbian population. Genet. Test 2008, 12, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Divac, A.; Nikolic, A.; Mitic-Milikic, M.; Nagorni-Obradovic, L.; Petrovic-Stanojevic, N.; Dopudja-Pantic, V.; Nadaskic, R.; Savic, A.; Radojkovic, D. High frequency of the R75Q CFTR variation in patients with chronic obstructive pulmonary disease. J. Cyst. Fibros. 2004, 3, 189–191. [Google Scholar] [CrossRef]
- LaRusch, J.; Jung, J.; General, I. Mechanisms of CFTR Functional Variants That Impair Regulated Bicarbonate Permeation and Increase Risk for Pancreatitis but Not for Cystic Fibrosis. PLoS Genet. 2014, 10, e1004376. [Google Scholar] [CrossRef]
- Ehrhardt, C.; Collnot, E.; Baldes, C.; Becker, U.; Laue, M.; Kim, K.; Lehr, C. Towards an in vitro model of cystic fibrosis small airway epithelium: Characterisation of the human bronchial epithelial cell line CFBE41o-. Cell Tissue Res. 2006, 323, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Okiyoneda, T.; Veit, G.; Sakai, R.; Aki, M.; Fujihara, T.; Higashi, M.; Susuki-Miyata, S.; Miyata, M.; Fukuda, N.; Yoshida, A.; et al. Chaperone-Independent Peripheral Quality Control of CFTR by RFFL E3 Ligase. Dev. Cell 2018, 44, 694–708.e7. [Google Scholar] [CrossRef]
- Bebok, Z.; Collawn, J.; Wakefield, J.; Parker, W.; Li, Y.; Varga, K.; Sorscher, E.; Clancy, J. Failure of cAMP agonists to activate rescued deltaF508 CFTR in CFBE41o- airway epithelial monolayers. J. Physiol. 2005, 569, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Rosell, A.; Monsó, E.; Soler, N.; Torres, F.; Angrill, J.; Riise, G.; Zalacaín, R.; Morera, J.; Torres, A. Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease. Arch Intern. Med. 2005, 165, 891–897. [Google Scholar] [CrossRef]
- Miravitlles, M.; Espinosa, C.; Fernández-Laso, E.; Martos, J.; Maldonado, J.; Gallego, M. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Study Group of Bacterial Infection in COPD. Chest 1999, 116, 40–46. [Google Scholar] [CrossRef]
- Eller, J.; Ede, A.; Schaberg, T.; Niederman, M.; Mauch, H.; Lode, H. Infective exacerbations of chronic bronchitis: Relation between bacteriologic etiology and lung function. Chest 1998, 113, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.; Brauer, A.; Eschberger, K.; Lobbins, P.; Grove, L.; Cai, X.; Sethi, S. Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008, 177, 853–860. [Google Scholar] [CrossRef]
- Clunes, L.; Davies, C.; Coakley, R.; Aleksandrov, A.; Henderson, A.; Zeman, K.; Worthington, E.; Gentzsch, M.; Kreda, S.; Cholon, D.; et al. Cigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydration. FASEB J. 2012, 26, 533–545. [Google Scholar] [CrossRef]
- Bagdany, M.; Veit, G.; Fukuda, R.; Avramescu, R.; Okiyoneda, T.; Baaklini, I.; Singh, J.; Sovak, G.; Xu, H.; Apaja, P.; et al. Chaperones rescue the energetic landscape of mutant CFTR at single molecule and in cell. Nat. Commun. 2017, 8, 398. [Google Scholar] [CrossRef]
- Cowley, E.; Linsdell, P. Oxidant stress stimulates anion secretion from the human airway epithelial cell line Calu-3: Implications for cystic fibrosis lung disease. J. Physiol. 2002, 543, 201–209. [Google Scholar] [CrossRef]
- Hoy, S. Elexacaftor/Ivacaftor/Tezacaftor: First Approval. Drugs 2019, 79, 2001–2007. [Google Scholar] [CrossRef] [PubMed]
- Veit, G.; Roldan, A.; Hancock, M.; Da Fonte, D.; Xu, H.; Hussein, M.; Frenkiel, S.; Matouk, E.; Velkov, T.; Lukacs, G. Allosteric folding correction of F508del and rare CFTR mutants by elexacaftor-tezacaftor-ivacaftor (Trikafta) combination. JCI Insight 2020, 5, e139983. [Google Scholar] [CrossRef] [PubMed]
- Boncoeur, E.; Roque, T.; Bonvin, E.; Saint-Criq, V.; Bonora, M.; Clement, A.; Tabary, O.; Henrion-Caude, A.; Jacquot, J. Cystic fibrosis transmembrane conductance regulator controls lung proteasomal degradation and nuclear factor-kappaB activity in conditions of oxidative stress. Am. J. Pathol. 2008, 172, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Cantin, A.; Bilodeau, G.; Ouellet, C.; Liao, J.; Hanrahan, J. Oxidant stress suppresses CFTR expression. Am. J. Physiol. Cell Physiol. 2006, 290, C262–C270. [Google Scholar] [CrossRef]
- Bérubé, J.; Roussel, L.; Nattagh, L.; Rousseau, S. Loss of cystic fibrosis transmembrane conductance regulator function enhances activation of p38 and ERK MAPKs, increasing interleukin-6 synthesis in airway epithelial cells exposed to Pseudomonas aeruginosa. J. Biol. Chem. 2010, 285, 22299–22307. [Google Scholar] [CrossRef]
- Pezzulo, A.; Tang, X.; Hoegger, M.; Alaiwa, M.A.; Ramachandran, S.; Moninger, T.; Karp, P.; Wohlford-Lenane, C.; Haagsman, H.; van Eijk, M.; et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 2012, 487, 109–113. [Google Scholar] [CrossRef]
- Song, Y.; Salinas, D.; Nielson, D.; Verkman, A. Hyperacidity of secreted fluid from submucosal glands in early cystic fibrosis. Am. J. Physiol. Cell Physiol. 2006, 290, C741–C749. [Google Scholar] [CrossRef]
- Cho, S.; Urata, Y.; Iida, T.; Goto, S.; Yamaguchi, M.; Sumikawa, K.; Kondo, T. Glutathione downregulates the phosphorylation of I kappa B: Autoloop regulation of the NF-kappa B-mediated expression of NF-kappa B subunits by TNF-alpha in mouse vascular endothelial cells. Biochem. Biophys. Res. Commun. 1998, 253, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Huang, X.; Warren, J. Intracellular glutathione redox status modulates MCP-1 expression in pulmonary granulomatous vasculitis. Lab Invest. 1999, 79, 837–847. [Google Scholar]
- Christman, J.; Sadikot, R.; Blackwell, T. The role of nuclear factor-kappa B in pulmonary diseases. Chest 2000, 117, 1482–1487. [Google Scholar] [CrossRef]
- Hashimoto, S.; Gon, Y.; Matsumoto, K.; Takeshita, I.; Asai, Y.; Machino, T.; Horie, T. Regulation by intracellular glutathione of TNF-alpha-induced p38 MAP kinase activation and RANTES production by human pulmonary vascular endothelial cells. Allergy 2000, 55, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Gosset, P.; Wallaert, B.; Tonnel, A.; Fourneau, C. Thiol regulation of the production of TNF-alpha, IL-6 and IL-8 by human alveolar macrophages. Eur. Respir. J. 1999, 14, 98–105. [Google Scholar] [CrossRef]
- Kim, Y.; Jun, I.; Shin, D.; Yoon, J.; Piao, H.; Jung, J.; Park, H.; Cheng, M.; Bahar, I.; Whitcomb, D.; et al. Regulation of CFTR Bicarbonate Channel Activity by WNK1: Implications for Pancreatitis and CFTR-Related Disorders. Cell Mol. Gastroenterol. Hepatol. 2020, 9, 79–103. [Google Scholar] [CrossRef]
- Wong, F.; AbuArish, A.; Matthes, E.; Turner, M.; Greene, L.; Cloutier, A.; Robert, R.; Thomas, D.; Cosa, G.; Cantin, A.; et al. Cigarette smoke activates CFTR through ROS-stimulated cAMP signaling in human bronchial epithelial cells. Am. J. Physiol. Cell Physiol. 2018, 314, C118–C134. [Google Scholar] [CrossRef] [PubMed]
- Harrington, M.; Gunderson, K.; Kopito, R. Redox reagents and divalent cations alter the kinetics of cystic fibrosis transmembrane conductance regulator channel gating. J. Biol. Chem. 1999, 274, 27536–27544. [Google Scholar] [CrossRef]
- Harrington, M.; Kopito, R. Cysteine residues in the nucleotide binding domains regulate the conductance state of CFTR channels. Biophys. J. 2002, 82, 1278–1292. [Google Scholar] [CrossRef] [PubMed]
- Solomon, G.; Fu, L.; Rowe, S.; Collawn, J. The therapeutic potential of CFTR modulators for COPD and other airway diseases. Curr. Opin. Pharmacol. 2017, 34, 132–139. [Google Scholar] [CrossRef]
- Lambert, J.; Raju, S.; Tang, L.; McNicholas, C.; Li, Y.; Courville, C.; Farris, R.; Coricor, G.; Smoot, L.; Mazur, M.; et al. Cystic fibrosis transmembrane conductance regulator activation by roflumilast contributes to therapeutic benefit in chronic bronchitis. Am. J. Respir. Cell Mol. Biol. 2014, 50, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Sloane, P.; Shastry, S.; Wilhelm, A.; Courville, C.; Tang, L.; Backer, K.; Levin, E.; Raju, S.; Li, Y.; Mazur, M.; et al. A pharmacologic approach to acquired cystic fibrosis transmembrane conductance regulator dysfunction in smoking related lung disease. PLoS ONE 2012, 7, e39809. [Google Scholar] [CrossRef]
- Raju, S.; Lin, V.; Liu, L.; McNicholas, C.; Karki, S.; Sloane, P.; Tang, L.; Jackson, P.; Wang, W.; Wilson, L.; et al. The Cystic Fibrosis Transmembrane Conductance Regulator Potentiator Ivacaftor Augments Mucociliary Clearance Abrogating Cystic Fibrosis Transmembrane Conductance Regulator Inhibition by Cigarette Smoke. Am. J. Respir. Cell Mol. Biol. 2017, 56, 99–108. [Google Scholar] [CrossRef]
- Okiyoneda, T.; Barrière, H.; Bagdány, M.; Rabeh, W.; Du, K.; Höhfeld, J.; Young, J.; Lukacs, G. Peripheral protein quality control removes unfolded CFTR from the plasma membrane. Science 2010, 329, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Fiedorczuk, K.; Chen, J. Molecular structures reveal synergistic rescue of Δ508 CFTR by Trikafta modulators. Science 2022, 378, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Glozman, R.; Okiyoneda, T.; Mulvihill, C.; Rini, J.; Barriere, H.; Lukacs, G. N-glycans are direct determinants of CFTR folding and stability in secretory and endocytic membrane traffic. J. Cell Biol. 2009, 184, 847–862. [Google Scholar] [CrossRef] [PubMed]
| Primer | Orientation | Sequence |
|---|---|---|
| Human β-actin | Forward | 5′-ACTCTTCCAGCCTTCCTTCC-3′ |
| Reverse | 5′-GAGGAGCAATGATCTTGATCTTC-3′ | |
| Human IL-8 | Forward | 5′-TCCTGATTTCTGCAAGCTCTG-3′ |
| Reverse | 5′-GTCCACTCTCAATCACTCTCAG-3′ | |
| Human IL-6 | Forward | 5′-GCACTGGCAGAAAACAACCT-3′ |
| Reverse | 5′-CAGGGGTGGTTATTGCATCT-3′ | |
| Human CFTR | Forward | 5′-AGTGGAGGAAAGCCTTTGGAGT-3′ |
| Reverse | 5′-ACAGATCTGAGCCCAACCTCA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hinata, D.; Fukuda, R.; Okiyoneda, T. The COPD-Associated Polymorphism Impairs the CFTR Function to Suppress Excessive IL-8 Production upon Environmental Pathogen Exposure. Int. J. Mol. Sci. 2023, 24, 2305. https://doi.org/10.3390/ijms24032305
Hinata D, Fukuda R, Okiyoneda T. The COPD-Associated Polymorphism Impairs the CFTR Function to Suppress Excessive IL-8 Production upon Environmental Pathogen Exposure. International Journal of Molecular Sciences. 2023; 24(3):2305. https://doi.org/10.3390/ijms24032305
Chicago/Turabian StyleHinata, Daichi, Ryosuke Fukuda, and Tsukasa Okiyoneda. 2023. "The COPD-Associated Polymorphism Impairs the CFTR Function to Suppress Excessive IL-8 Production upon Environmental Pathogen Exposure" International Journal of Molecular Sciences 24, no. 3: 2305. https://doi.org/10.3390/ijms24032305
APA StyleHinata, D., Fukuda, R., & Okiyoneda, T. (2023). The COPD-Associated Polymorphism Impairs the CFTR Function to Suppress Excessive IL-8 Production upon Environmental Pathogen Exposure. International Journal of Molecular Sciences, 24(3), 2305. https://doi.org/10.3390/ijms24032305






