In Vivo and In Silico Analgesic Activity of Ficus populifolia Extract Containing 2-O-β-D-(3′,4′,6′-Tri-acetyl)-glucopyranosyl-3-methyl Pentanoic Acid
Abstract
1. Introduction
2. Results and Discussion
2.1. Isolation and Structure Elucidation of the Constituents
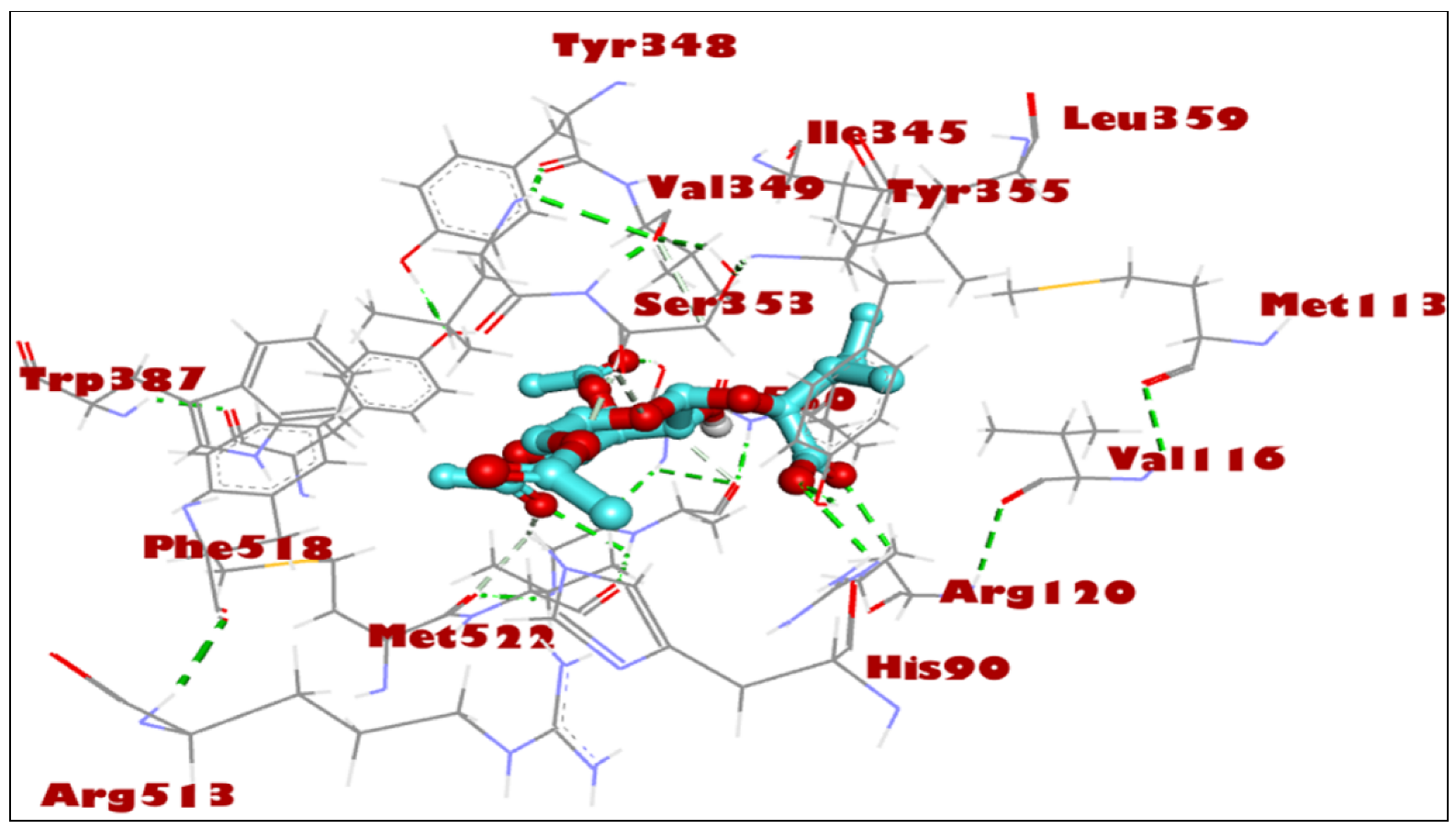
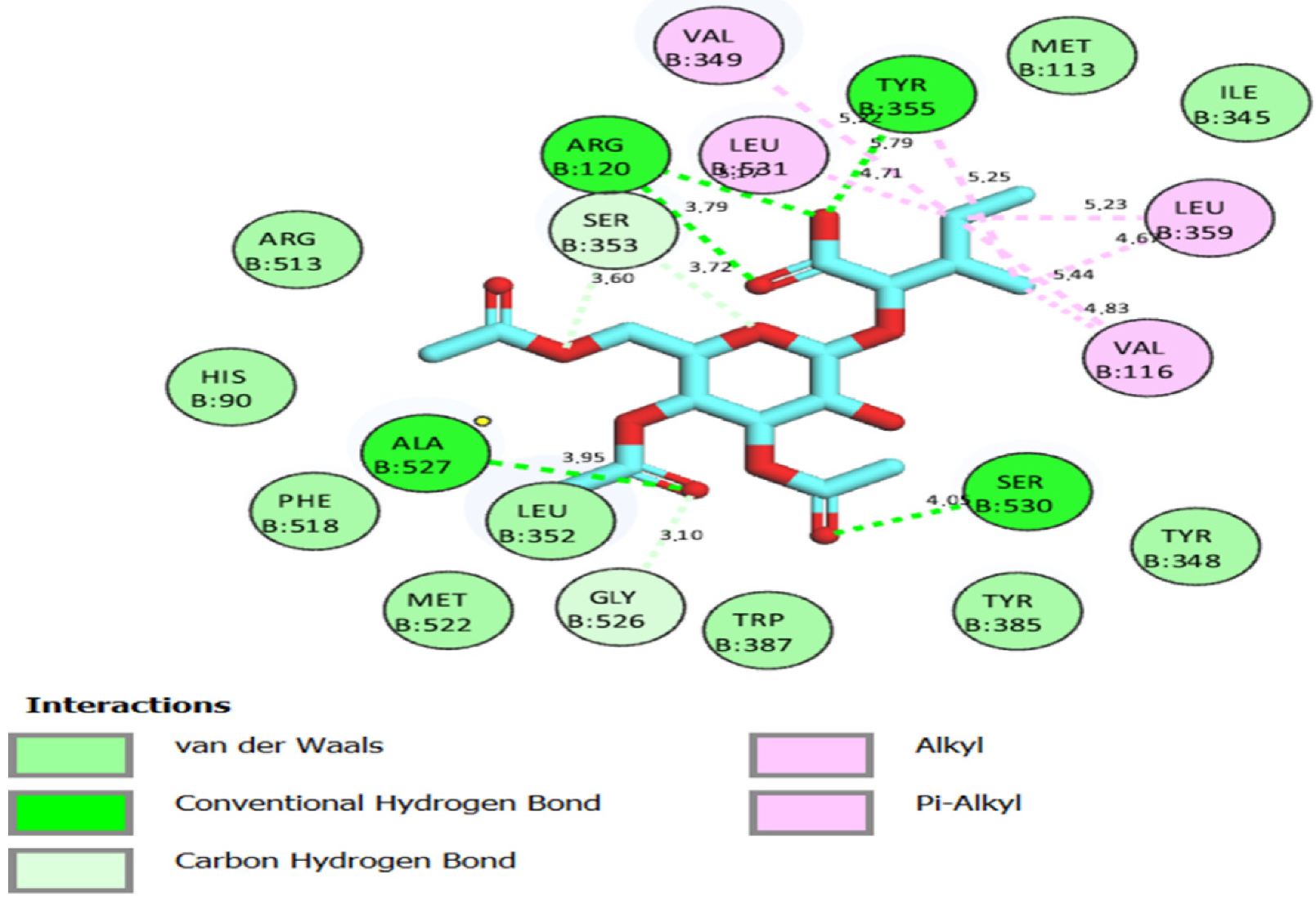
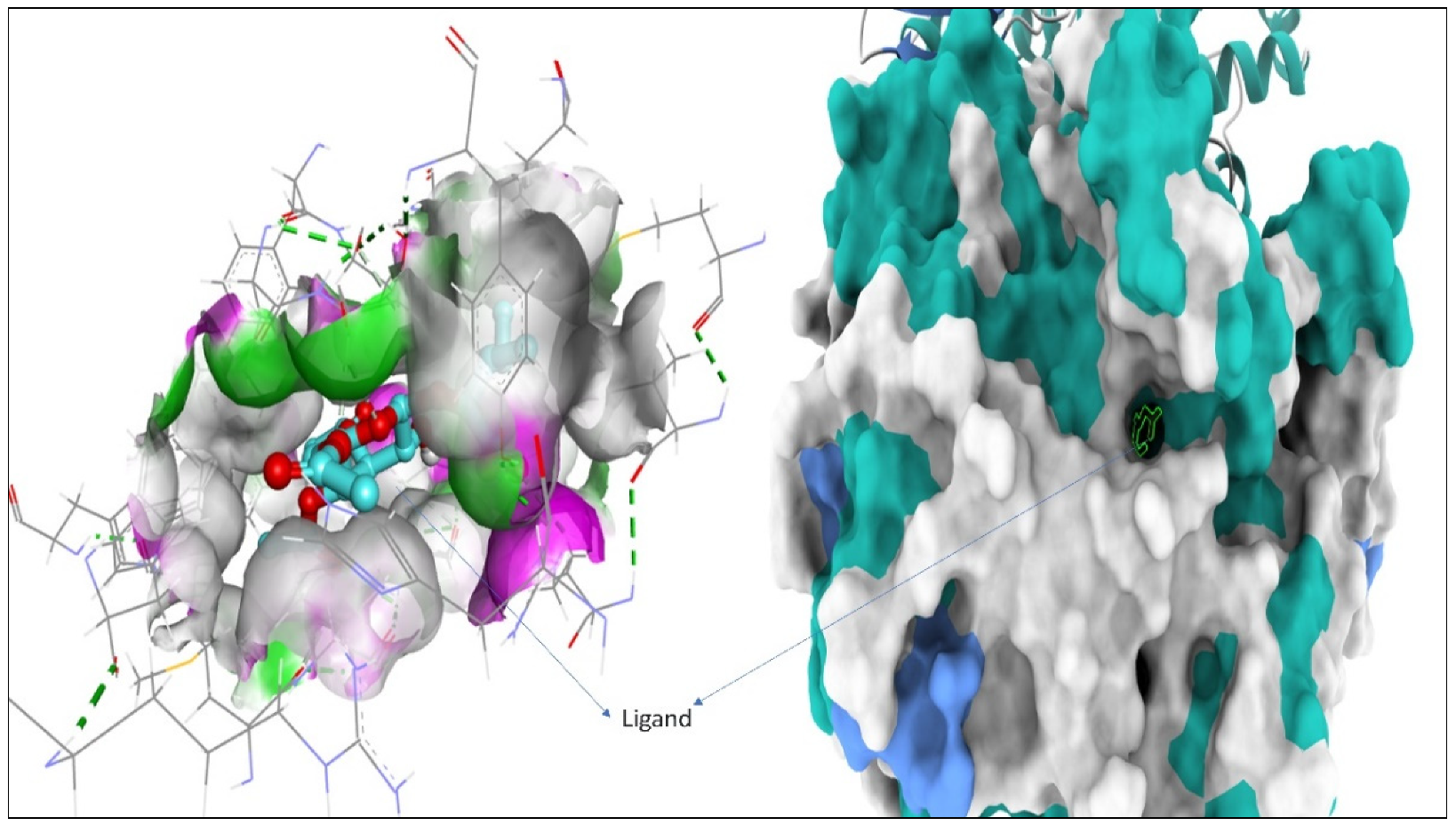
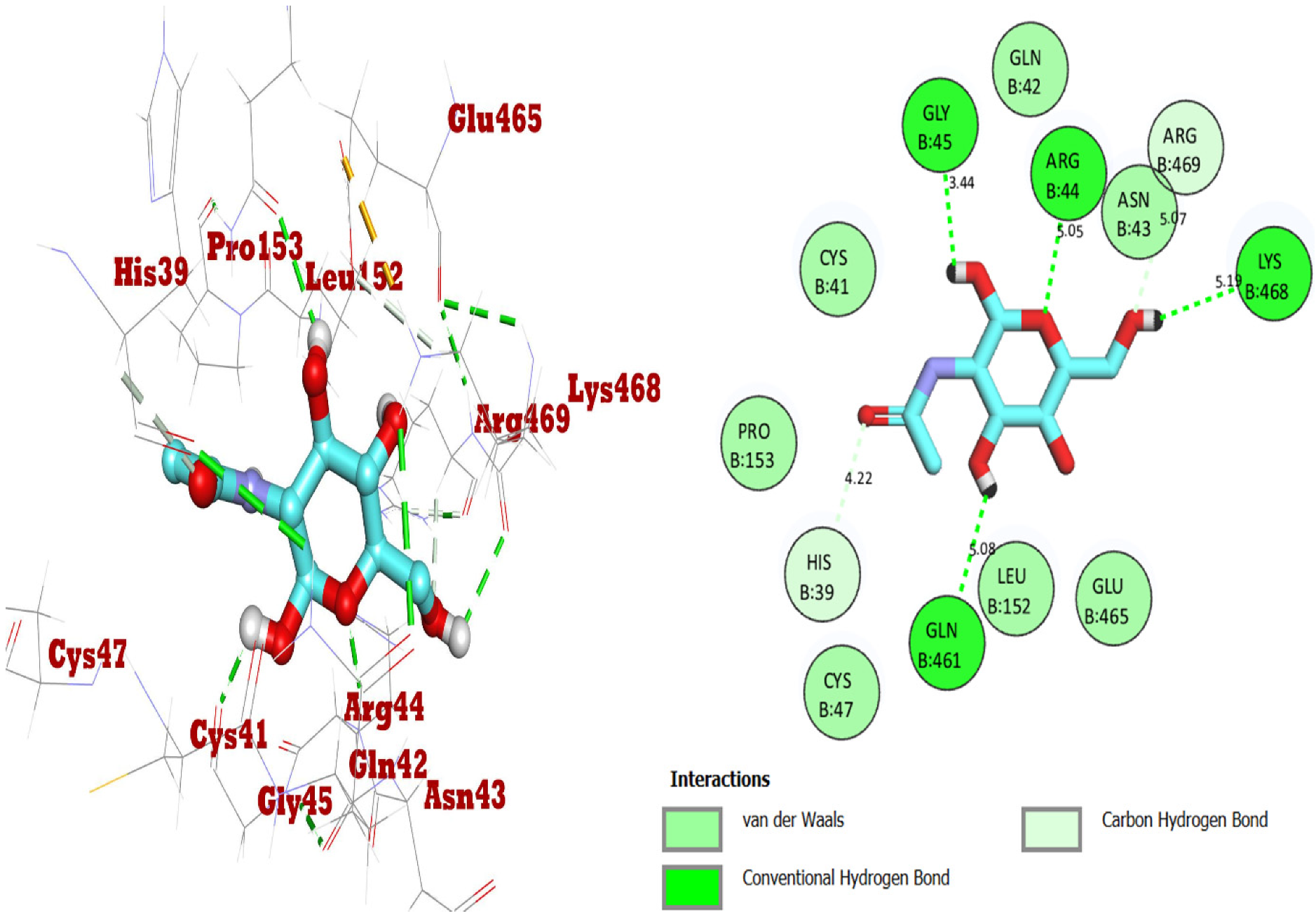
2.2. Docking Studies for Analgesic Activity
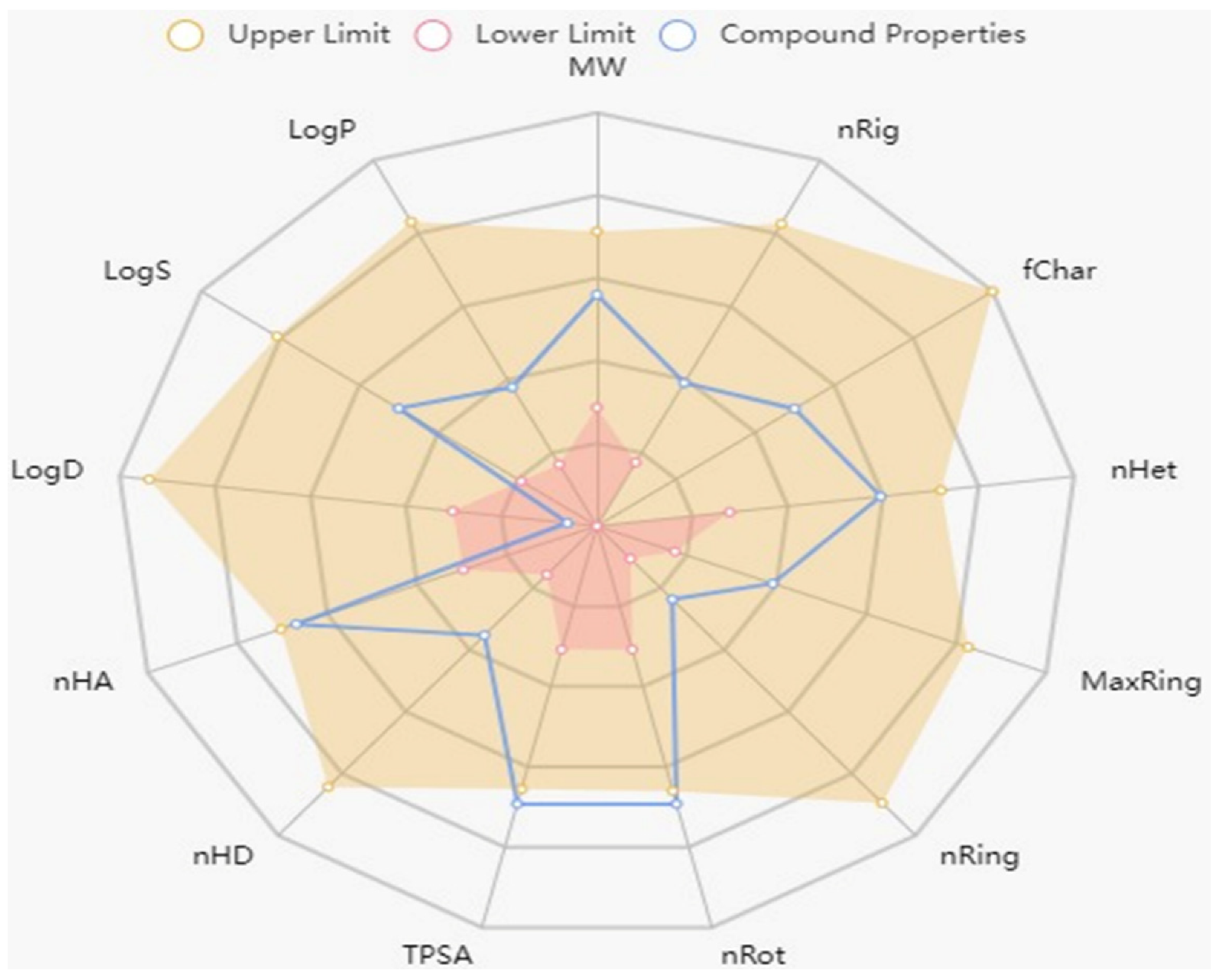
2.3. Physiochemical and ADMET Profiling
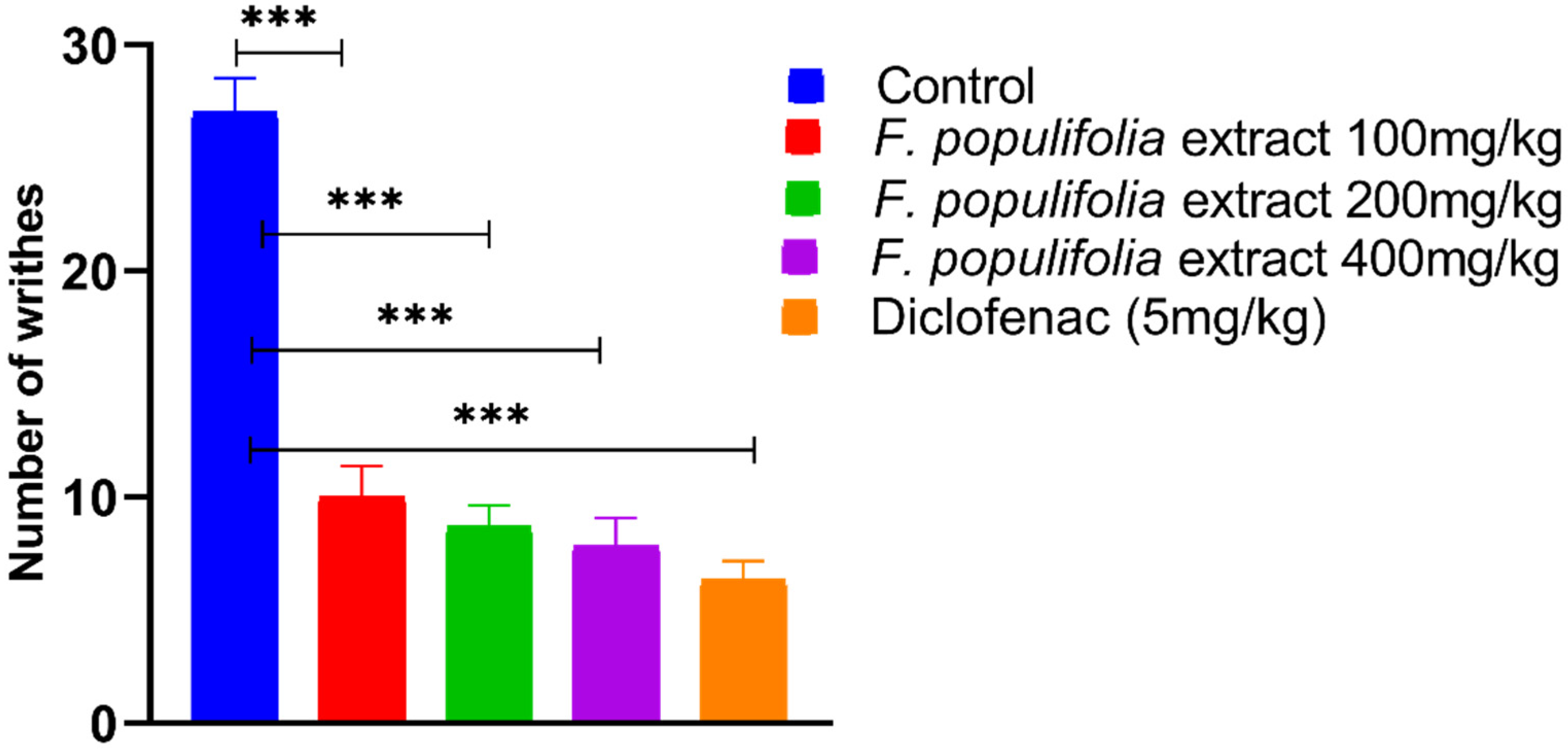
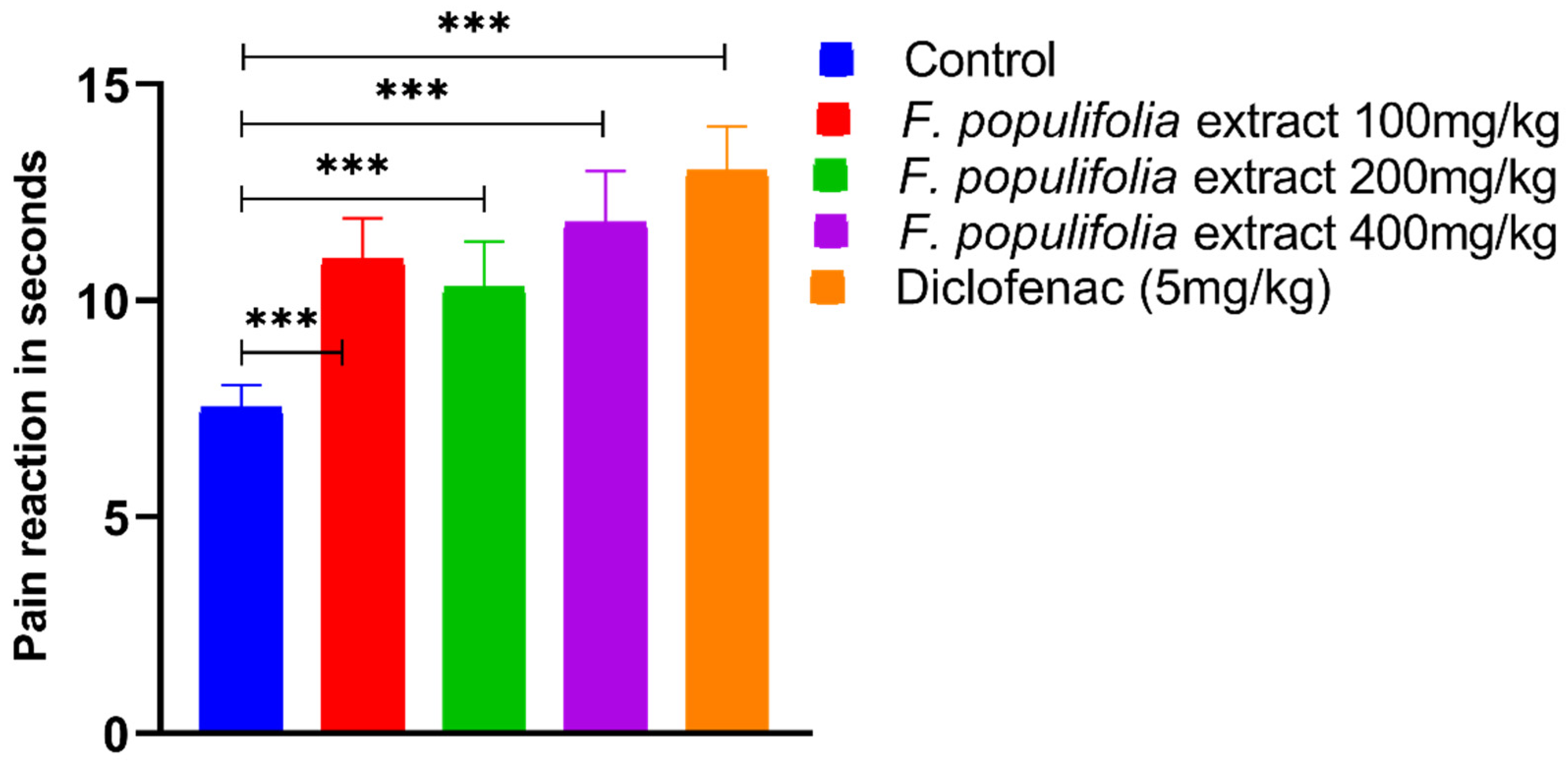
2.4. In Vivo Analgesic Activity Evaluations
3. Materials and Methods
3.1. Chemistry: Plant Material, Extraction, and Isolation of Products
3.2. Biological Activity: Animal Groups
3.3. Evaluation of Peripherally Mediated Analgesic Activity Using Acetic Acid-Induced Writhing Model
3.4. Evaluation of Centrally Mediated Analgesic Activity Using Analgesia Meter Method
3.5. In Silico Studies: COX-2 Docking
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirmani, S.A.K.; Ali, P.; Azam, F. Topological Indices and QSPR/QSAR Analysis of Some Antiviral Drugs Being Investigated for the Treatment of COVID-19 Patients. Int. J. Quantum Chem. 2021, 121, e26594. [Google Scholar] [CrossRef] [PubMed]
- Tahir ul Qamar, M.; Zhu, X.-T.; Chen, L.-L.; Alhussain, L.; Alshiekheid, M.A.; Theyab, A.; Algahtani, M. Target-Specific Machine Learning Scoring Function Improved Structure-Based Virtual Screening Performance for SARS-CoV-2 Drugs Development. Int. J. Mol. Sci. 2022, 23, 11003. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.A.; Khan, R.A.; Abdel-Hafez, A.A.; Abdel-Aziz, M.; Ahmed, E.; Enany, S.; Mahgoub, S.; Al-Rugaie, O.; Alsharidah, M.; Aly, M.S.A. Phytochemical Profiling, in Vitro and in Silico Anti-Microbial and Anti-Cancer Activity Evaluations and Staph GyraseB and h-TOP-IIβ Receptor-Docking Studies of Major Constituents of Zygophyllum coccineum L. Aqueous-Ethanolic Extract and Its Subsequent Fractions: An Approach to Validate Traditional Phytomedicinal Knowledge. Molecules 2021, 26, 577. [Google Scholar] [PubMed]
- Mohammed, H.A. The Valuable Impacts of Halophytic Genus Suaeda; Nutritional, Chemical, and Biological Values. Med. Chem. 2020, 16, 1044–1057. [Google Scholar] [CrossRef]
- Khan, R.A. Natural Products Chemistry: The Emerging Trends and Prospective Goals. Saudi Pharm. J. 2018, 26, 739–753. [Google Scholar] [CrossRef]
- Alharbi, S.A.; AL-Juhani, W.S.; Albokhari, E.J. Plastome Characterization, Phylogenetic Relationships, and Regional Conservation Status of Ficus populifolia Vahl. (Moraceae), a Peripherally Isolated Plant Population in the Arabian Peninsula. Forests 2022, 13, 2063. [Google Scholar] [CrossRef]
- Alfarhan, A.H.; Al-Turki, T.A.; Basahy, A.Y. Flora of Jizan Region; Final Report; Supported by King Abdulaziz City for Science and Technology: Riyadh, Saudi Arabia, 2005; Volume 1, p. 545. [Google Scholar]
- Bwalya, A.G. Evaluation of the In Vitro Biological Activities and Phytochemical Profiling of Eight Ficus Species Collected in Zambia. Ph.D. Thesis, University College London, London, UK, 2015. [Google Scholar]
- Odugbemi, T. A Textbook of Medicinal Plants from Nigeria; Tolu Odugbemi: Lagos, Nigeria, 2008; ISBN 9784871297. [Google Scholar]
- Augustino, S.; Gillah, P.R. Medicinal Plants in Urban Districts of Tanzania: Plants, Gender Roles and Sustainable Use. Int. For. Rev. 2005, 7, 44–58. [Google Scholar] [CrossRef]
- Lansky, E.P.; Paavilainen, H.M. Figs: The Genus Ficus; CRC Press: Boca Raton, FL, USA, 2010; ISBN 0429138202. [Google Scholar]
- Deepa, P.; Sowndhararajan, K.; Kim, S.; Park, S.J. A Role of Ficus Species in the Management of Diabetes Mellitus: A Review. J. Ethnopharmacol. 2018, 215, 210–232. [Google Scholar] [CrossRef]
- Kaur, A.; Rana, A.C.; Tiwari, V.; Sharma, R.; Kumar, S. Review on Ethanomedicinal and Pharmacological Properties of Ficus Religiosa. J. Appl. Pharm. Sci. 2011, 1, 6–11. [Google Scholar]
- Mousa, O.; Vuorela, P.; Kiviranta, J.; Wahab, S.A.; Hiltunen, R.; Vuorela, H. Bioactivity of Certain Egyptian Ficus Species. J. Ethnopharmacol. 1994, 41, 71–76. [Google Scholar] [CrossRef]
- Patil, M.S.; Patil, C.R.; Patil, S.W.; Jadhav, R.B. Anticonvulsant Activity of Aqueous Root Extract of Ficus Religiosa. J. Ethnopharmacol. 2011, 133, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Meshram, A.; Bhagyawant, S.S.; Srivastava, N. Ficus Religiosa-Potential Role in Pharmaceuticals. Int. J. Pharm. Sci. Res. 2014, 5, 1616. [Google Scholar]
- Subramanian, P.M.; Misra, G.S. Chemical Constituents of Ficus Bengalensis (Part II). Pol. J. Pharmacol. Pharm. 1978, 30, 559–562. [Google Scholar] [PubMed]
- Tsai, P.-W.; Castro-Cruz, D.; Shen, C.-C.; Chiou, C.-T.; Ragasa, C.Y. Chemical Constituents of Ficus Odorata. Pharm. Chem. J. 2012, 46, 225–227. [Google Scholar] [CrossRef]
- Almahy, H.A.; Rahmani, M.; Sukari, M.A.; Ali, A.M. The Chemical Constituents of Ficus benjamina Linn. and Their Biological Activities. Pertanika J. Sci. Technol 2003, 11, 73–81. [Google Scholar]
- Ragasa, C.Y.; Alimboyoguen, A.B.; Shen, C.-C. Chemical Constituents of Ficus Nota. Der Pharma Chem. 2014, 6, 98–101. [Google Scholar]
- Vaya, J.; Mahmood, S. Flavonoid Content in Leaf Extracts of the Fig (Ficus carica L.), Carob (Ceratonia siliqua L.) and Pistachio (Pistacia lentiscus L.). Biofactors 2006, 28, 169–175. [Google Scholar] [CrossRef]
- Kuete, V.; Ngameni, B.; Simo, C.C.F.; Tankeu, R.K.; Ngadjui, B.T.; Meyer, J.J.M.; Lall, N.; Kuiate, J.R. Antimicrobial Activity of the Crude Extracts and Compounds from Ficus Chlamydocarpa and Ficus Cordata (Moraceae). J. Ethnopharmacol. 2008, 120, 17–24. [Google Scholar] [CrossRef]
- Raga, D.D.; Cheng, C.L.C.; Lee, K.C.I.C.; Olaziman, W.Z.P.; De Guzman, V.J.A.; Shen, C.-C. Bioactivities of Triterpenes and a Sterol from Syzygium Samarangense. Z. Für Naturforsch. C 2011, 66, 235–244. [Google Scholar] [CrossRef]
- Dembele, O.; Haidara, M.; Ndong, A.; Barbosa, F.; Sy, G.Y.; Wélé, A. Potential Analgesic Activity of the Methanolic F4 Fraction of Leaf Extracts of Annona Senegalensis Pers. (Annonaceae). J. Drug Deliv. Ther. 2022, 12, 160–162. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Al-Omar, M.S.; Mohammed, S.A.A.; Alhowail, A.H.; Eldeeb, H.M.; Sajid, M.S.M.; Abd-Elmoniem, E.M.; Alghulayqeh, O.A.; Kandil, Y.I.; Khan, R.A. Phytochemical Analysis, Pharmacological and Safety Evaluations of Halophytic Plant, Salsola cyclophylla. Molecules 2021, 26, 2384. [Google Scholar] [CrossRef] [PubMed]
- Kariuki, D.K.; Kanui, T.I.; Mbugua, P.M.; Githinji, C.G. Analgesic and Anti-Inflammatory Activities of 9-Hexacosene and Stigmasterol Isolated from Mondia whytei. Phytopharmacology 2012, 2, 212–223. [Google Scholar]
- Nirmal, S.A.; Pal, S.C.; Mandal, S.C.; Patil, A.N. Analgesic and Anti-Inflammatory Activity of β-Sitosterol Isolated from Nyctanthes Arbortristis Leaves. Inflammopharmacology 2012, 20, 219–224. [Google Scholar] [CrossRef]
- Ahmed, F.; Rahman, M.Z.; Khan, M.F.; Rashid, M.A.; Rahman, M.S. β-Amyrin as an Analgesic Component of the Leaves of Callistemon citrinus (Curtis) Skeels: Chemical, Biological and in Silico Studies. Bangladesh J. Bot. 2019, 48, 379–385. [Google Scholar] [CrossRef]
- Jannat, T.; Hossain, M.J.; El-Shehawi, A.M.; Kuddus, M.R.; Rashid, M.A.; Albogami, S.; Jafri, I.; El-Shazly, M.; Haque, M.R. Chemical and Pharmacological Profiling of Wrightia coccinea (Roxb. Ex Hornem.) Sims Focusing Antioxidant, Cytotoxic, Antidiarrheal, Hypoglycemic, and Analgesic Properties. Molecules 2022, 27, 4024. [Google Scholar] [CrossRef] [PubMed]
- Thamaraiselvi, L.; Selvankumar, T.; Wesely, E.G.; Nathan, V.K. In-Silico Molecular Docking Analysis of Some Plant Derived Molecules for Anti-Inflammatory Inhibitory Activity. Curr. Bot 2021, 12, 22–27. [Google Scholar] [CrossRef]
- Akinloye, O.A.; Akinloye, D.I.; Onigbinde, S.B.; Metibemu, D.S. Phytosterols Demonstrate Selective Inhibition of COX-2: In-Vivo and In-Silico Studies of Nicotiana Tabacum. Bioorg. Chem. 2020, 102, 104037. [Google Scholar] [CrossRef]
- Noble, F.; Smadja, C.; Valverde, O.; Maldonado, R.; Coric, P.; Turcaud, S.; Fournié-Zaluski, M.-C.; Roques, B.P. Pain-Suppressive Effects on Various Nociceptive Stimuli (Thermal, Chemical, Electrical and Inflammatory) of the First Orally Active Enkephalin-Metabolizing Enzyme Inhibitor RB 120. Pain 1997, 73, 383–391. [Google Scholar] [CrossRef]
- Lanhers, M.-C.; Fleurentin, J.; Mortier, F.; Vinche, A.; Younos, C. Anti-Inflammatory and Analgesic Effects of an Aqueous Extract of Harpagophytum procumbens. Planta Med. 1992, 58, 117–123. [Google Scholar] [CrossRef]
- Bertram, G.K. Basic and Clinical Pharmacology: Appleton and Lange. Stamford Connect. 1998, 7, 579–588. [Google Scholar]
- Lee, Y.; Rodriguez, C.; Dionne, R.A. The Role of COX-2 in Acute Pain and the Use of Selective COX-2 Inhibitors for Acute Pain Relief. Curr. Pharm. Des. 2005, 11, 1737–1755. [Google Scholar] [CrossRef] [PubMed]
- Sadik, O.A.; Yazgan, I.; Eroglu, O.; Liu, P.; Olsen, S.T.; Moser, A.M.; Sander, P.G.; Tsiagbe, C.; Harada, K.; Bajwa, S.; et al. Objective Clinical Pain Analysis Using Serum Cyclooxygenase-2 and Inducible Nitric Oxide Synthase in American Patients. Clin. Chim. Acta 2018, 484, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Lenherr, A.; Mabry, T.J. Acetylated Allose-Containing Flavonoid Glucosides from Stachys anisochila. Phytochemistry 1987, 26, 1185–1188. [Google Scholar] [CrossRef]
- Lenherr, A.; Lahloub, M.F.; Sticher, O. Three Flavonoid Glycosides Containing Acetylated Allose from Stachys recta. Phytochemistry 1984, 23, 2343–2345. [Google Scholar] [CrossRef]
- Hui, Y.; Shuang-Xi, M.E.I.; Li-Yan, P.; Zhong-Wen, L.I.N.; Han-Dong, S.U.N. A New Glucoside from Rhodiola fastigiata (Crassulaceae). J. Integr. Plant Biol. 2002, 44, 224. [Google Scholar]
- Della Greca, M.; Monaco, P.; Previtera, L. Stigmasterols from Typha Latifolia. J. Nat. Prod. 1990, 53, 1430–1435. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Abdelwahab, M.F.; El-Ghaly, E.-S.M.; Ragab, E.A. Phytochemical Characterization, In Vitro Anti-Inflammatory, Anti-Diabetic, and Cytotoxic Activities of the Edible Aromatic Plant; Pulicaria jaubertii. Molecules 2021, 26, 203. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, A.K.; Dobhal, M.P.; Sharma, M.C.; Gupta, R.S. Antidiabetic and Antioxidant Potential of Β-Sitosterol in Streptozotocin-Induced Experimental Hyperglycemia. J. Diabetes 2011, 3, 29–37. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Al-Omar, M.S.; El-Readi, M.Z.; Alhowail, A.H.; Aldubayan, M.A.; Abdellatif, A.A.H. Formulation of Ethyl Cellulose Microparticles Incorporated Pheophytin A Isolated from Suaeda Vermiculata for Antioxidant and Cytotoxic Activities. Molecules 2019, 24, 1501. [Google Scholar] [CrossRef]
- Knight, S.A. Carbon-13 NMR Spectra of Some Tetra- and Pentacyclic Triterpenoids. Org. Magn. Reson. 1974, 6, 603–611. [Google Scholar] [CrossRef]
- Orlando, B.J.; Malkowski, M.G. Substrate-Selective Inhibition of Cyclooxygeanse-2 by Fenamic Acid Derivatives Is Dependent on Peroxide Tone. J. Biol. Chem. 2016, 291, 15069–15081. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Balanean, L.; Braicu, C.; Berindan-Neagoe, I.; Nastasa, C.; Tiperciuc, B.; Verite, P.; Oniga, O. Synthesis of Novel 2-Metylamino-4-Substituted-1, 3-Thiazoles with Antiproliferative Activity. Rev. Chim. 2014, 65, 1413–1417. [Google Scholar]
- Ertl, P.; Rohde, B.; Selzer, P. Fast Calculation of Molecular Polar Surface Area as a Sum of Fragment-Based Contributions and Its Application to the Prediction of Drug Transport Properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.D.; Blagg, J.; Price, D.A.; Bailey, S.; DeCrescenzo, G.A.; Devraj, R.V.; Ellsworth, E.; Fobian, Y.M.; Gibbs, M.E.; Gilles, R.W. Physiochemical Drug Properties Associated with in Vivo Toxicological Outcomes. Bioorg. Med. Chem. Lett. 2008, 18, 4872–4875. [Google Scholar] [CrossRef]
- Jain, A.K.; Singh, D.; Dubey, K.; Maurya, R.; Mittal, S.; Pandey, A.K. Models and Methods for In Vitro Toxicity. In In Vitro Toxicology; Academic Press: Cambridge, MA, USA, 2018; pp. 45–65. [Google Scholar] [CrossRef]
- Lee, H.M.; Yu, M.S.; Kazmi, S.R.; Oh, S.Y.; Rhee, K.H.; Bae, M.A.; Lee, B.H.; Shin, D.S.; Oh, K.S.; Ceong, H.; et al. Computational Determination of HERG-Related Cardiotoxicity of Drug Candidates. BMC Bioinform. 2019, 20, 67–73. [Google Scholar] [CrossRef]
- Nohmi, T. Thresholds of Genotoxic and Non-Genotoxic Carcinogens. Toxicol. Res. 2018, 34, 281. [Google Scholar] [CrossRef]
- Prakash, V.E.D. Terpenoids as Source of Anti-Inflammatory Compounds. Asian J. Pharm. Clin. Res. 2017, 10, 68–76. [Google Scholar] [CrossRef]
- Saudagar, R.B.; Saokar, S. Anti-Inflammatory Natural Compounds from Herbal and Marine Origin. J. Drug Deliv. Ther. 2019, 9, 669–672. [Google Scholar]
- Williamson, E.M.; Okpako, D.T.; Evans, F.J. Selection, Preparation and Pharmacological Evaluation of Plant Material, Volume 1; John Wiley & Sons: Hoboken, NJ, USA, 1996; Volume 1, ISBN 0471942170. [Google Scholar]
- Koster, R. Acetic Acid for Analgesic Screening. Fed. Proc. 1959, 18, 412. [Google Scholar]
- Gulecha, V.; Sivakumar, T.; Upaganlawar, A.; Mahajan, M.; Upasani, C. Screening of Ficus Religiosa Leaves Fractions for Analgesic and Anti-Inflammatory Activities. Indian J. Pharmacol. 2011, 43, 662. [Google Scholar]
- Liao, C.-R.; Kao, C.-P.; Peng, W.-H.; Chang, Y.-S.; Lai, S.-C.; Ho, Y.-L. Analgesic and Anti-Inflammatory Activities of Methanol Extract of Ficus pumila L. in Mice. Evid.-Based Complement. Altern. Med. 2012, 2012, 340141. [Google Scholar] [CrossRef] [PubMed]
- Das, P.C.; Das, A.; Mandal, S. Antimicrobial and Anti-Inflammatory Activities of the Seed Kernel of Mangifera indica. Fitoterapia 1989, 60, 235–240. [Google Scholar]
- Otimenyin, S.O.; Uguru, M.O.; Atang, B.L. Antiinflamatory and Analgesic Activities of Ficus Thonningii and Pseudocedrela Kotschyi Extracts. Niger. J. Pharm. Res. 2004, 3, 82–85. [Google Scholar] [CrossRef]
- Abdulmalik, I.A.; Sule, M.I.; Yaro, A.H.; Abdullahi, M.I.; Abdulkadir, M.E.; Yusuf, H. Evaluation of Analgesic and Anti-Inflammatory Effects of Ethanol Extract of Ficus iteophylla Leaves in Rodents. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 462–466. [Google Scholar] [CrossRef]
- Chemical Computing Group. Molecular Operating Environment (MOE); Chemical Computing Group Inc.: Montreal, QC, Canada, 2016. [Google Scholar]
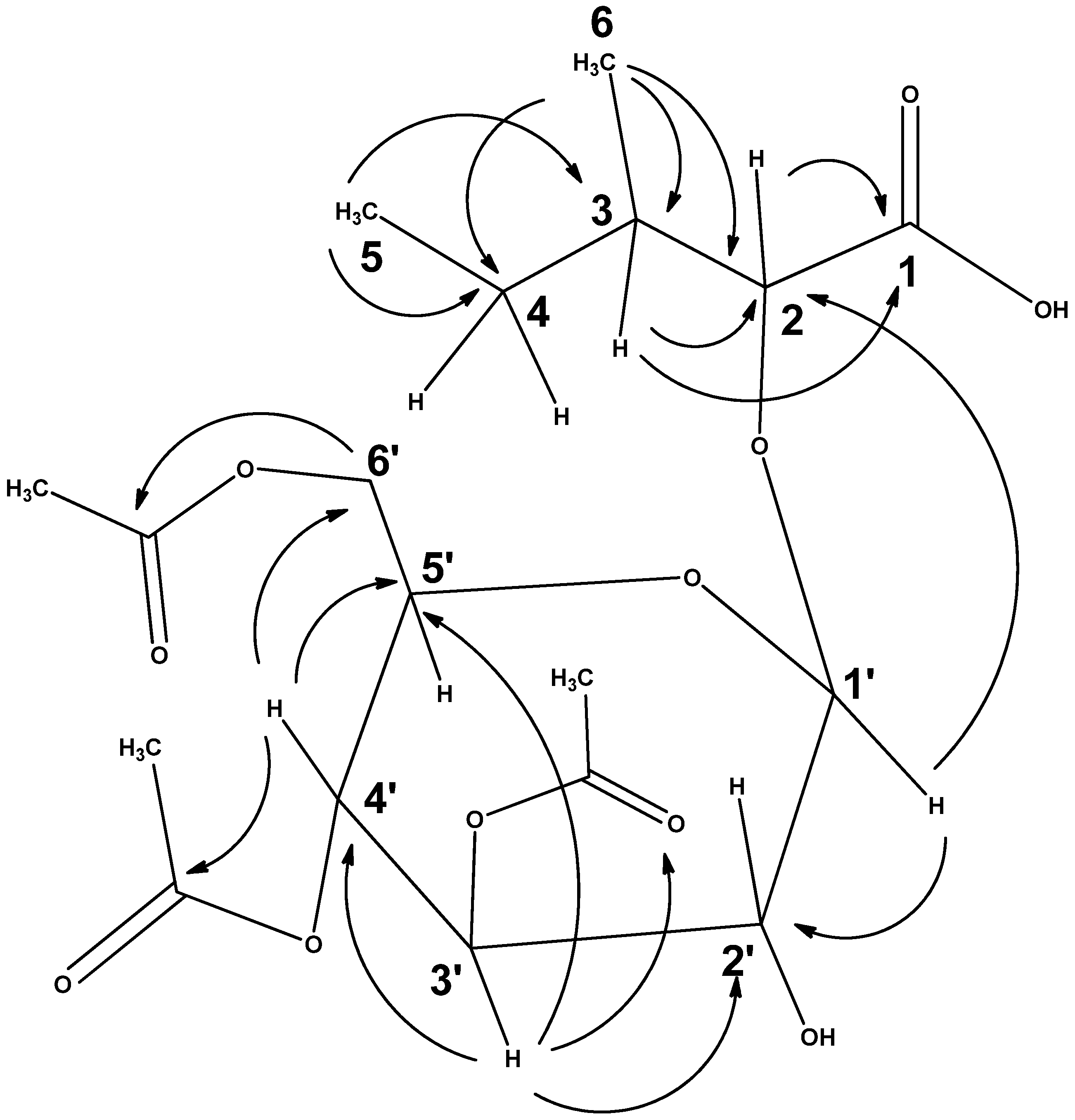
| Carbon Number | 1H δ-Value | 13C δ-Value | DEPT (135) | 1H-1H COSY | 1H-13C HMBC |
|---|---|---|---|---|---|
| 1 | 168.16 | C=O | |||
| 2 | 4.53, d, J 2.5 Hz | 76.80 | CH | H-3 | C-1 |
| 3 | 2.07, m | 36.92 | CH | H-4a and 4b, H-2 and H-6 | C-1 and C-2 |
| 4 | 1.47, m | 25.55 | CH2 | H-3 and H-5 | |
| 1.38, m | |||||
| 5 | 0.87, t. J 7.5 Hz | 11.58 | CH3 | H-4a and 4b | C-3 and C-4 |
| 6 | 0.93, d, J 7 Hz | 11.96 | CH3 | H-3 | C-2, C-3 and C-4 |
| 1’ | 4.76, d, J 8.5 Hz | 95.33 | CH | H-2’ | C-2 and C-2’ |
| 2’ | 4.24, m (overlapped) | 74.60 | CH | H-1’ and H-3 | |
| 3’ | 5.28, t, J 9.5 Hz | 71.27 | CH | H-2’ and H-4 | C-2’, 4’,5’ and C-3′ (acetoxy C=O) |
| 4’ | 5.04, t, J 9.5 Hz | 68.11 | CH | H-3’ and H-5’ | C-3’, 5’ 6’ and C-4′ (acetoxy C=O) |
| 5’ | 3.80, m | 73.20 | CH | H-4’ and H-6’a and 6’b | |
| 6’ | 4.26, dd, J 12.5, 1.5 Hz | 61.56 | CH2 | H-5’ and H-6’b | C-5’ and C-6′ (acetoxy C=O) |
| 4.10, dd, J 12.5, 3.0 Hz | H-5’ and H-6’a | C-4’, 5’ and C-6′ (acetoxy C=O) | |||
| 3′ | (acetoxy C=O) | 169.97 | C=O | ||
| 4′ | (acetoxy C=O) | 169.67 | C=O | ||
| 6′ | (acetoxy C=O) | 170.60 | C=O | ||
| 3′ | 1.97, s (acetoxy methyl) | 20.65 | CH3 | C-3′ | |
| 4′ | 2.01, s (acetoxy methyl) | 20.55 | CH3 | C-4′ | |
| 6′ | 2.06, s (acetoxy methyl) | 20.75 | CH3 | C-6′ |
| Compound | Binding Score (kcal/mol) | Hydrogen Bond Interactions | Distance (Å) | Hydrophobic Interactions | Distance (Å) |
|---|---|---|---|---|---|
| 3′,4′,6′-Triacetylated pentanoic acid glucoside | −8.1 | TYR 355 GLY 526 SER 530 | 2.34, 2.53 3.34 1.97 | VAL 349 LEU 352 TYR 355 LEU 359 TRP 387 PHE 518 VAL 523 LEU 531 | 3.42 3.67 3.78, 3.41 3.13 3.52 3.63 3.68 3.31 |
| (NAG)2-Acetamido-2-deoxy-β-D-glucopyranose | −7.6 | ARG 44 GLY 45 GLN 461 LYS 468 | 2.08 2.21 2.65 2.69 | CYS 41 ASN 43 | 3.50 3.24 |
| Molecular Weight | Number of Heavy Atoms | Number of Aromatic Heavy Atoms | Number of Rotatable Bonds | Number H-Bond Acceptor (HBA) | Number H-Bond Donors (HBD) | Topological Polar Surface Area (TPSA) | Lipophilicity (Log P) | Water Solubility (Log S) |
|---|---|---|---|---|---|---|---|---|
| 420.16 | 29 | 0.0 | 12 | 11 | 2 | 154.89 | 1.87 | −1.741 |
| GI Absorption | BBB Permeant | CYP1A2 Inhibitor | CYP2C19 Inhibitor | CYP2C9 Inhibitor | CYP2D6 Inhibitor | CYP3A4 Inhibitor | Lipinski |
|---|---|---|---|---|---|---|---|
| Low | No | No | No | No | No | No | Yes; 1 violation: NorO > 10 |
| hERG Blockers | H-HT | DILI | AMES Toxicity | Rat Oral Acute Toxicity | FDAMDD | Skin Sensitization | Carcinogenicity |
|---|---|---|---|---|---|---|---|
| 0.01 | 0.742 | 0.908 | 0.034 | 0.035 | 0.004 | 0.082 | 0.152 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, H.A.; Abouzied, A.S.; Mohammed, S.A.A.; Khan, R.A. In Vivo and In Silico Analgesic Activity of Ficus populifolia Extract Containing 2-O-β-D-(3′,4′,6′-Tri-acetyl)-glucopyranosyl-3-methyl Pentanoic Acid. Int. J. Mol. Sci. 2023, 24, 2270. https://doi.org/10.3390/ijms24032270
Mohammed HA, Abouzied AS, Mohammed SAA, Khan RA. In Vivo and In Silico Analgesic Activity of Ficus populifolia Extract Containing 2-O-β-D-(3′,4′,6′-Tri-acetyl)-glucopyranosyl-3-methyl Pentanoic Acid. International Journal of Molecular Sciences. 2023; 24(3):2270. https://doi.org/10.3390/ijms24032270
Chicago/Turabian StyleMohammed, Hamdoon A., Amr S. Abouzied, Salman A. A. Mohammed, and Riaz A. Khan. 2023. "In Vivo and In Silico Analgesic Activity of Ficus populifolia Extract Containing 2-O-β-D-(3′,4′,6′-Tri-acetyl)-glucopyranosyl-3-methyl Pentanoic Acid" International Journal of Molecular Sciences 24, no. 3: 2270. https://doi.org/10.3390/ijms24032270
APA StyleMohammed, H. A., Abouzied, A. S., Mohammed, S. A. A., & Khan, R. A. (2023). In Vivo and In Silico Analgesic Activity of Ficus populifolia Extract Containing 2-O-β-D-(3′,4′,6′-Tri-acetyl)-glucopyranosyl-3-methyl Pentanoic Acid. International Journal of Molecular Sciences, 24(3), 2270. https://doi.org/10.3390/ijms24032270







