N6-methyladenosine Modification of Hepatitis B Virus RNA in the Coding Region of HBx
Abstract
1. Introduction
2. Results
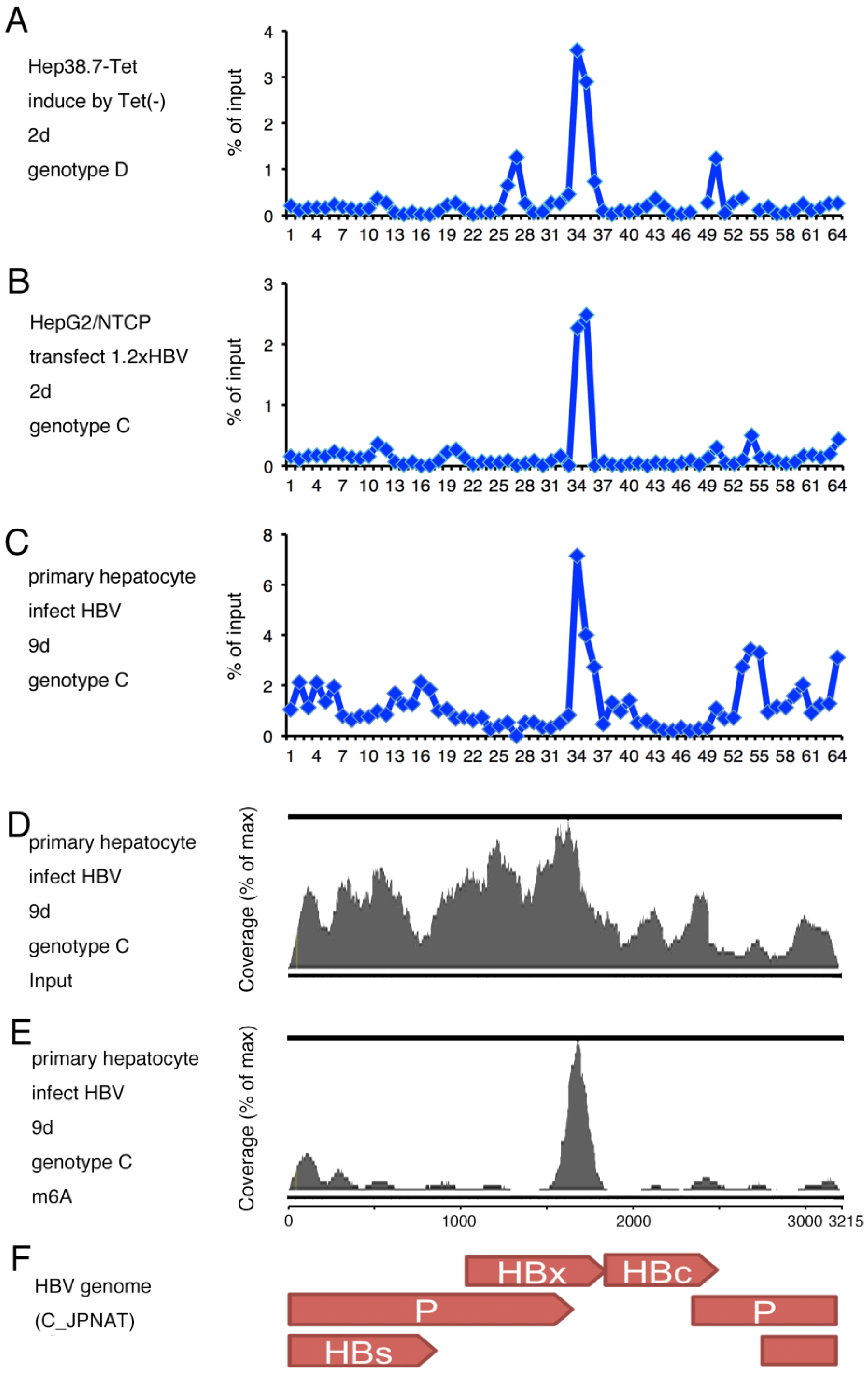
2.1. m6A Modification of HBV RNA
2.2. Identification of m6A Modified Residues in HBV RNA
2.3. Mutation of m6A Sites Decreased HBV RNA and HBs Levels
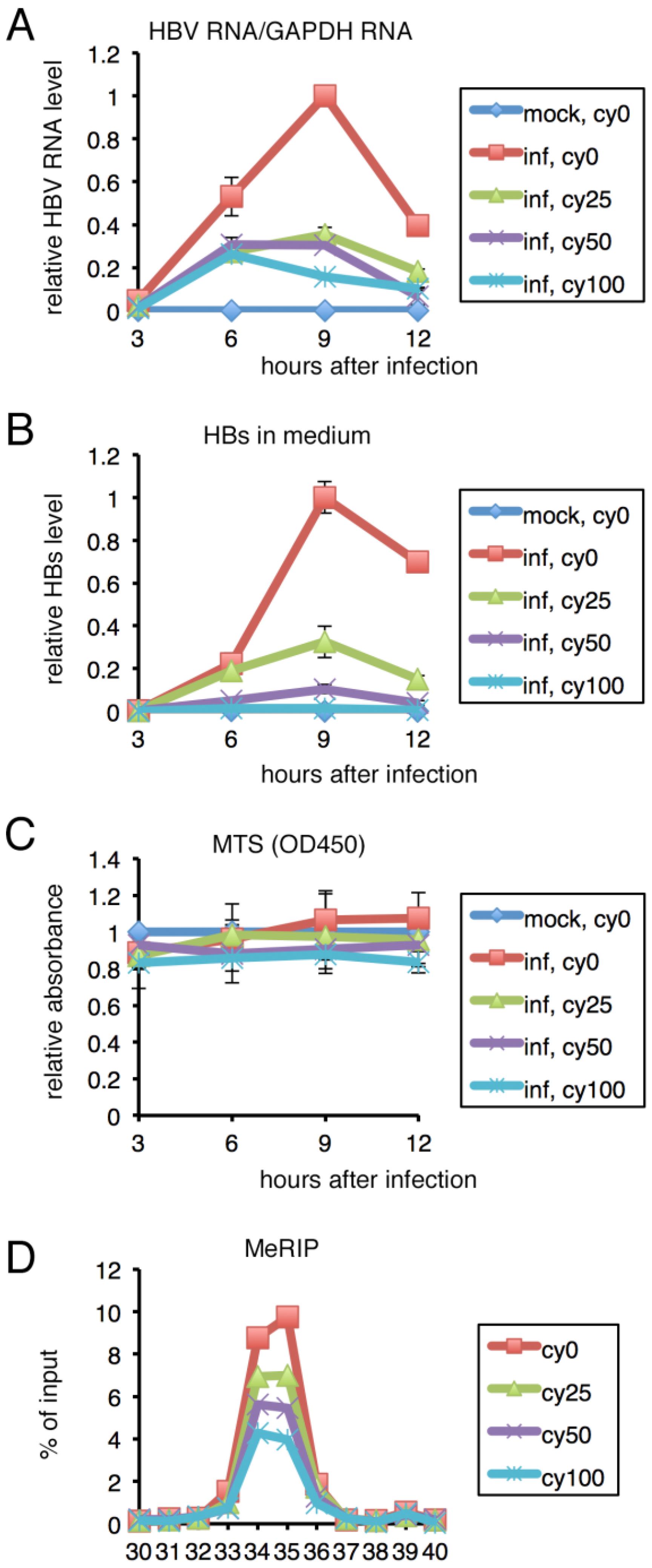
2.4. Inhibition of m6A Modification Resulted in Lower HBV RNA and HBs Levels in PHH
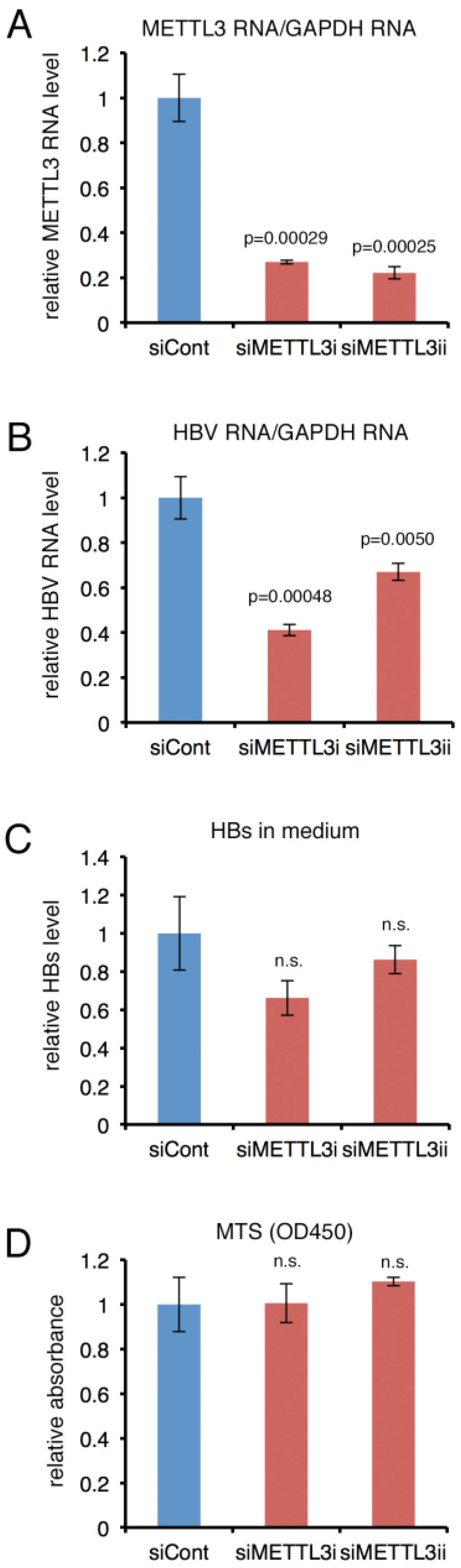
2.5. Knockdown of METTL3 Decreased HBV RNA and HBs Levels in PHH
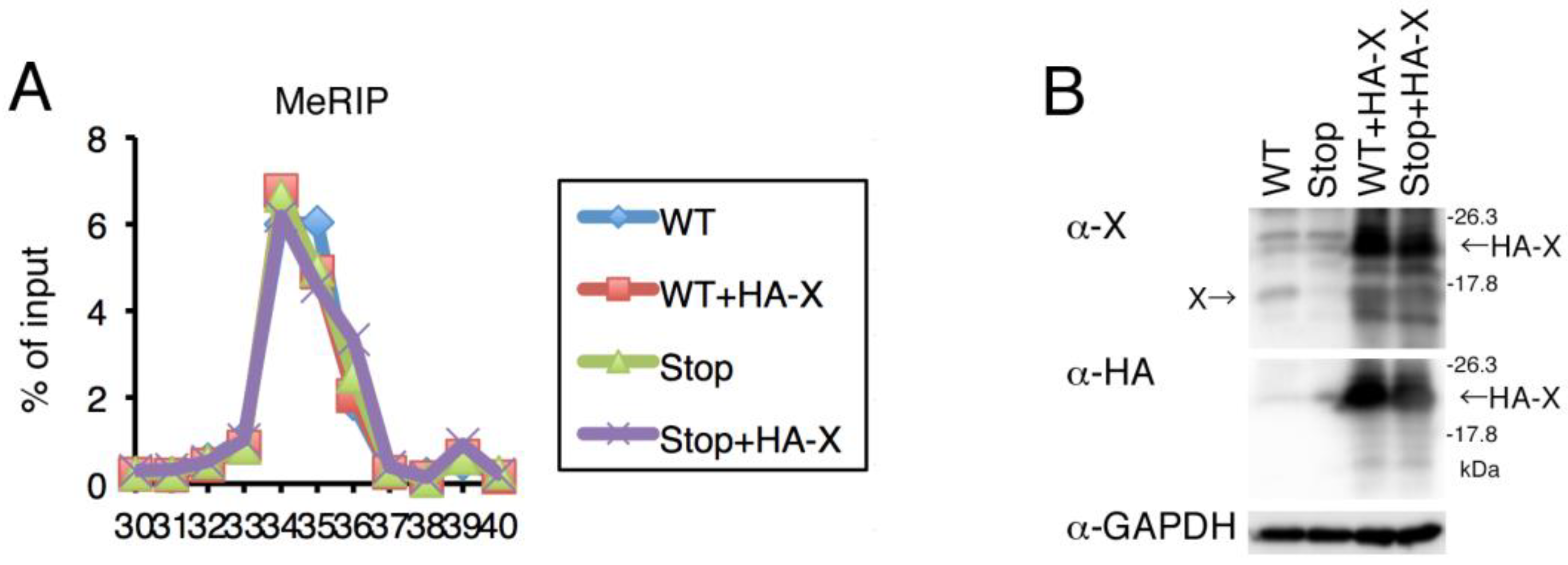
2.6. Expression of HBx Was Not Involved in m6A Modification
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Plasmids
4.3. qRT-PCR
4.4. MeRIP-qRT-PCR and MeRIP-RNAseq
4.4.1. ELISA
4.4.2. MTS Assay
4.4.3. Knockdown by siRNA
4.4.4. Western Blotting
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schweitzer, A.; Horn, J.; Mikolajczyk, R.T.; Krause, G.; Ott, J.J. Estimations of worldwide prevalence of chronic hepatitis B virus infection: A systematic review of data published between 1965 and 2013. Lancet 2015, 386, 1546–1555. [Google Scholar] [CrossRef]
- Trepo, C.; Chan, H.; Lok, A. Hepatitis B virus infection. Lancet 2014, 384, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Yuen, M.F.; Chen, D.S.; Dusheiko, G.M.; Janssen, H.L.; Lau, D.T.; Locarnini, S.A.; Peters, M.G.; Lai, C.L. Hepatitis B virus infection. Nat. Rev. Dis. Prim. 2018, 4, 18035. [Google Scholar] [CrossRef] [PubMed]
- Deny, P.; Zoulim, F. Hepatitis B virus: From diagnosis to treatment. Pathol. Biol. 2010, 58, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Lee, J.; Ahn, S. Hepatitis B Virus Cure: Targets and Future Therapies. Int. J. Mol. Sci. 2020, 22, 213. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhan, Y.; Zhuo, L.; Zhang, Q.; Li, G.; Li, Q.; Qi, S.; Zhu, J.; Lv, Q.; Shen, Y.; et al. Biological functions of m(6)A methyltransferases. Cell Biosci. 2021, 11, 15. [Google Scholar] [CrossRef]
- He, P.C.; He, C. m6A RNA methylation: From mechanisms to therapeutic potential. EMBO J. 2021, 40, e105977. [Google Scholar] [CrossRef]
- Dang, W.; Xie, Y.; Cao, P.; Xin, S.; Wang, J.; Li, S.; Li, Y.; Lu, J. N(6)-Methyladenosine and Viral Infection. Front. Microbiol. 2019, 10, 417. [Google Scholar] [CrossRef]
- Imam, H.; Kim, G.; Siddiqui, A. Epitranscriptomic(N6-methyladenosine) Modification of Viral RNA and Virus-Host Interactions. Front. Cell. Infect. Microbiol. 2020, 10, 584283. [Google Scholar] [CrossRef]
- Tan, B.; Gao, S. RNA epitranscriptomics: Regulation of infection of RNA and DNA viruses by N(6)-methyladenosine (m(6)A). Rev. Med. Virol. 2018, 28, e1983. [Google Scholar] [CrossRef]
- Williams, G.D.; Gokhale, N.; Horner, S. Regulation of Viral Infection by the RNA Modification N6-Methyladenosine. Annu. Rev. Virol. 2019, 6, 235–253. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.M.; Bogerd, H.P.; Kornepati, A.V.R.; Kang, D.; Ghoshal, D.; Marshall, J.B.; Poling, B.C.; Tsai, K.; Gokhale, N.S.; Horner, S.M.; et al. Posttranscriptional m(6)A Editing of HIV-1 mRNAs Enhances Viral Gene Expression. Cell Host Microbe 2016, 19, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Tirumuru, N.; Zhao, B.S.; Lu, W.; Lu, Z.; He, C.; Wu, L. N(6)-methyladenosine of HIV-1 RNA regulates viral infection and HIV-1 Gag protein expression. eLife 2016, 5, e15528. [Google Scholar] [CrossRef]
- Courtney, D.G.; Kennedy, E.M.; Dumm, R.E.; Bogerd, H.P.; Tsai, K.; Heaton, N.S.; Cullen, B.R. Epitranscriptomic Enhancement of Influenza A Virus Gene Expression and Replication. Cell Host Microbe 2017, 22, 377–386.e5. [Google Scholar] [CrossRef] [PubMed]
- Gokhale, N.S.; McIntyre, A.B.R.; McFadden, M.J.; Roder, A.E.; Kennedy, E.M.; Gandara, J.A.; Hopcraft, S.E.; Quicke, K.M.; Vazquez, C.; Willer, J.; et al. N6-Methyladenosine in Flaviviridae Viral RNA Genomes Regulates Infection. Cell Host Microbe 2016, 20, 654–665. [Google Scholar] [CrossRef]
- Tan, B.; Liu, H.; Zhang, S.; da Silva, S.R.; Zhang, L.; Meng, J.; Cui, X.; Yuan, H.; Sorel, O.; Zhang, S.W.; et al. Viral and cellular N(6)-methyladenosine and N(6),2′-O-dimethyladenosine epitranscriptomes in the KSHV life cycle. Nat. Microbiol. 2018, 3, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Chen, E.; Nilsen, T. Kaposi’s Sarcoma-Associated Herpesvirus Utilizes and Manipulates RNA N(6)-Adenosine Methylation To Promote Lytic Replication. J. Virol. 2017, 91, e00466-17. [Google Scholar] [CrossRef] [PubMed]
- Imam, H.; Khan, M.; Gokhale, N.S.; McIntyre, A.B.R.; Kim, G.-W.; Jang, J.Y.; Kim, S.-J.; Mason, C.E.; Horner, S.M.; Siddiqui, A. N6-methyladenosine modification of hepatitis B virus RNA differentially regulates the viral life cycle. Proc. Natl. Acad. Sci. USA 2018, 115, 8829–8834. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.W.; Siddiqui, A. Hepatitis B virus X protein recruits methyltransferases to affect cotranscriptional N6-methyladenosine modification of viral/host RNAs. Proc. Natl. Acad. Sci. USA 2021, 118, e2019455118. [Google Scholar] [CrossRef]
- Zhou, Y.; Zeng, P.; Li, Y.H.; Zhang, Z.; Cui, Q. SRAMP: Prediction of mammalian N6-methyladenosine (m6A) sites based on sequence-derived features. Nucleic Acids Res. 2016, 44, e91. [Google Scholar] [CrossRef]
- Meyer, K.D.; Saletore, Y.; Zumbo, P.; Elemento, O.; Mason, C.E.; Jaffrey, S.R. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell 2012, 149, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.M.; Nguyen, N.; Burrows, C. Colocalization of m(6)A and G-Quadruplex-Forming Sequences in Viral RNA (HIV, Zika, Hepatitis B, and SV40) Suggests Topological Control of Adenosine N (6)-Methylation. ACS Cent. Sci. 2019, 5, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, Y.; Mao, Q.; Jiang, X.; Jiang, W.; Chen, J.; Xu, W.; Zhong, L.; Sun, X. Overexpression of YTHDF1 is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Biomark. 2018, 21, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yin, Z.; Hou, B.; Yu, M.; Chen, R.; Jin, H.; Jian, Z. Expression profiles and prognostic significance of RNA N6-methyladenosine-related genes in patients with hepatocellular carcinoma: Evidence from independent datasets. Cancer Manag. Res. 2019, 11, 3921–3931. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wei, L.; Law, C.-T.; Tsang, F.H.-C.; Shen, J.; Cheng, C.L.-H.; Tsang, L.-H.; Ho, D.W.-H.; Chiu, D.K.-C.; Lee, J.M.-F.; et al. RNA N6-methyladenosine methyltransferase-like 3 promotes liver cancer progression through YTHDF2-dependent posttranscriptional silencing of SOCS2. Hepatology 2018, 67, 2254–2270. [Google Scholar] [CrossRef]
- Fang, Q.; Chen, H. The significance of m6A RNA methylation regulators in predicting the prognosis and clinical course of HBV-related hepatocellular carcinoma. Mol. Med. 2020, 26, 60. [Google Scholar] [CrossRef]
- Nishitsuji, H.; Ujino, S.; Shimizu, Y.; Harada, K.; Zhang, J.; Sugiyama, M.; Mizokami, M.; Shimotohno, K. Novel reporter system to monitor early stages of the hepatitis B virus life cycle. Cancer Sci. 2015, 106, 1616–1624. [Google Scholar] [CrossRef]
- Ogura, N.; Watashi, K.; Noguchi, T.; Wakita, T. Formation of covalently closed circular DNA in Hep38.7-Tet cells, a tetracycline inducible hepatitis B virus expression cell line. Biochem. Biophys. Res. Commun. 2014, 452, 315–321. [Google Scholar] [CrossRef]
- Ladner, S.K.; Otto, M.J.; Barker, C.S.; Zaifert, K.; Wang, G.H.; Guo, J.T.; Seeger, C.; King, R.W. Inducible expression of human hepatitis B virus (HBV) in stably transfected hepatoblastoma cells: A novel system for screening potential inhibitors of HBV replication. Antimicrob. Agents Chemother. 1997, 41, 1715–1720. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, M.; Tanaka, Y.; Kato, T.; Orito, E.; Ito, K.; Acharya, S.K.; Gish, R.G.; Kramvis, A.; Shimada, T.; Izumi, N.; et al. Influence of hepatitis B virus genotypes on the intra- and extracellular expression of viral DNA and antigens. Hepatology 2006, 44, 915–924. [Google Scholar] [CrossRef]
- Yanagi, Y.; Watanabe, T.; Hara, Y.; Sato, Y.; Kimura, H.; Murata, T. EBV Exploits RNA m(6)A Modification to Promote Cell Survival and Progeny Virus Production During Lytic Cycle. Front. Microbiol. 2022, 13, 870816. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murata, T.; Iwahori, S.; Okuno, Y.; Nishitsuji, H.; Yanagi, Y.; Watashi, K.; Wakita, T.; Kimura, H.; Shimotohno, K. N6-methyladenosine Modification of Hepatitis B Virus RNA in the Coding Region of HBx. Int. J. Mol. Sci. 2023, 24, 2265. https://doi.org/10.3390/ijms24032265
Murata T, Iwahori S, Okuno Y, Nishitsuji H, Yanagi Y, Watashi K, Wakita T, Kimura H, Shimotohno K. N6-methyladenosine Modification of Hepatitis B Virus RNA in the Coding Region of HBx. International Journal of Molecular Sciences. 2023; 24(3):2265. https://doi.org/10.3390/ijms24032265
Chicago/Turabian StyleMurata, Takayuki, Satoko Iwahori, Yusuke Okuno, Hironori Nishitsuji, Yusuke Yanagi, Koichi Watashi, Takaji Wakita, Hiroshi Kimura, and Kunitada Shimotohno. 2023. "N6-methyladenosine Modification of Hepatitis B Virus RNA in the Coding Region of HBx" International Journal of Molecular Sciences 24, no. 3: 2265. https://doi.org/10.3390/ijms24032265
APA StyleMurata, T., Iwahori, S., Okuno, Y., Nishitsuji, H., Yanagi, Y., Watashi, K., Wakita, T., Kimura, H., & Shimotohno, K. (2023). N6-methyladenosine Modification of Hepatitis B Virus RNA in the Coding Region of HBx. International Journal of Molecular Sciences, 24(3), 2265. https://doi.org/10.3390/ijms24032265





