Cardiovascular Disease in Obstructive Sleep Apnea: Putative Contributions of Mineralocorticoid Receptors
Abstract
:1. Introduction
2. OSA and CVD
3. OSA Management with CPAP
4. MR Structure and Function
5. Pathological Mechanisms of MR Activation
6. Cardiovascular Consequences of MR Activation
7. Is MR a Mediator of OSA-Induced CVD?
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| α-SMC | Alpha-smooth muscle actin |
| AF | Atrial fibrillation |
| AHI | Apnea/hypopnea index |
| Akt | Protein kinase B |
| AP-1 | Activator protein 1 |
| Ang II | Angiotensin II |
| AT1R | Type I angiotensin receptors |
| BH4 | Tetrahydrobiopterin |
| BNP | B-type natriuretic peptide |
| CAD | Coronary artery disease |
| Cav1 | Caveolin 1 |
| Cav 1.2 | L-type calcium channel alpha 1C |
| COX-2 | Cyclooxygenase 2 |
| CPAP | Continuous positive airway pressure |
| CREB | cAMP-response element-binding protein |
| CRP | C-reactive protein |
| CTGF | Connective tissue growth factor |
| EGFR | Epidermal growth factor receptor |
| ENaC | Epithelial sodium channel |
| eNOS | Endothelial nitric oxide synthase |
| ERK | Extracellular signal-regulated kinase |
| FMD | Flow-mediated dilatation |
| GLUT4 | Glucose transporter 4 |
| GPER | G protein-coupled estrogen receptor 1 |
| GR | Glucocorticoid receptor |
| HF | Heart failure |
| HfpEF | Heart failure with preserved ejection fraction |
| HfrEF | Heart failure with reduced ejection fraction |
| HOMA | Homeostatic model assessment |
| HSD11B2 | 11-beta-hydroxysteroid dehydrogenase type 2 |
| ICAM1 | Intracellular adhesion molecule 1 |
| IGF1R | Insulin-like growth factor receptor 1 |
| IH | Intermittent hypoxia |
| IL1β | Interleukin 1 beta |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| IL-18 | Interleukin 18 |
| IR | Insulin resistance |
| IRS1 | Insulin receptor substrate 1 |
| JNK | c-Jun N-terminal kinase |
| MAPK | Mitogen-associated protein kinases |
| MCP-1 | Monocyte chemoattractant protein 1 |
| MHC-β | Myosin heavy chain beta |
| MI | Myocardial infarction |
| MLC | Myosin light chain |
| mTOR | Mammalian target of rapamycin |
| NGAL | Neutrophil gelatinase-associated lipocalin |
| NHE-1 | Sodium–hydrogen antiporter 1 |
| NFAT | Nuclear factor of activated T-cells |
| NF-ƙB | Nuclear factor kappa B |
| NLRP3 | NLR family pyrin domain containing 3 |
| NO | Nitric oxide |
| NOX | NADPH oxidases |
| OSA | Obstructive sleep apnea |
| PA | Primary aldosteronism |
| PAI-1 | Plasminogen activator inhibitor 1 |
| PDGFR | Platelet-derived growth factor receptor |
| PI3K | Phosphatidylinositide 3-kinases |
| PKC | Protein kinase C |
| PP2A | Protein phosphatase 2A |
| PWV | Pulse wave velocity |
| PVN | Paraventricular nucleus |
| RAAS | Renin–angiotensin–aldosterone system |
| Rac1 | Ras-related C3 botulinum toxin substrate |
| RCT | Randomized control trial |
| ROS | Reactive oxygen species |
| S6K1 | Ribosomal protein S6 kinase beta 1 |
| SF | Sleep fragmentation |
| SGK1 | Serine/threonine-protein kinase 1 |
| TGF-β | Transforming growth factor beta |
| TNF-α | Tumor necrosis factor alpha |
| VEGF | Vascular endothelial growth factor |
| VEGFR1 | Vascular endothelial growth factor receptor 1 |
| VSMCs | Vascular smooth muscle cells |
References
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pepin, J.L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef] [Green Version]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef]
- Javaheri, S.; Barbe, F.; Campos-Rodriguez, F.; Dempsey, J.A.; Khayat, R.; Javaheri, S.; Malhotra, A.; Martinez-Garcia, M.A.; Mehra, R.; Pack, A.I.; et al. Sleep Apnea: Types, Mechanisms, and Clinical Cardiovascular Consequences. J. Am. Coll Cardiol. 2017, 69, 841–858. [Google Scholar] [CrossRef]
- Bradley, T.D.; Floras, J.S. Obstructive sleep apnoea and its cardiovascular consequences. Lancet 2009, 373, 82–93. [Google Scholar] [CrossRef]
- Golbidi, S.; Badran, M.; Ayas, N.; Laher, I. Cardiovascular consequences of sleep apnea. Lung 2012, 190, 113–132. [Google Scholar] [CrossRef]
- Badran, M.; Yassin, B.A.; Fox, N.; Laher, I.; Ayas, N. Epidemiology of Sleep Disturbances and Cardiovascular Consequences. Can. J. Cardiol. 2015, 31, 873–879. [Google Scholar] [CrossRef]
- Badran, M.; Ayas, N.; Laher, I. Insights into obstructive sleep apnea research. Sleep Med. 2014, 15, 485–495. [Google Scholar] [CrossRef]
- Carreras, A.; Zhang, S.X.; Peris, E.; Qiao, Z.; Gileles-Hillel, A.; Li, R.C.; Wang, Y.; Gozal, D. Chronic sleep fragmentation induces endothelial dysfunction and structural vascular changes in mice. Sleep 2014, 37, 1817–1824. [Google Scholar] [CrossRef] [Green Version]
- Farre, R.; Almendros, I.; Martinez-Garcia, M.A.; Gozal, D. Experimental Models to Study End-Organ Morbidity in Sleep Apnea: Lessons Learned and Future Directions. Int. J. Mol. Sci. 2022, 23, 14430. [Google Scholar] [CrossRef]
- Khalyfa, A.; Wang, Y.; Zhang, S.X.; Qiao, Z.; Abdelkarim, A.; Gozal, D. Sleep fragmentation in mice induces nicotinamide adenine dinucleotide phosphate oxidase 2-dependent mobilization, proliferation, and differentiation of adipocyte progenitors in visceral white adipose tissue. Sleep 2014, 37, 999–1009. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Thorne, L.N.; Punjabi, N.M.; Sun, C.K.; Schwartz, A.R.; Smith, P.L.; Marino, R.L.; Rodriguez, A.; Hubbard, W.C.; O’Donnell, C.P.; et al. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ. Res. 2005, 97, 698–706. [Google Scholar] [CrossRef] [Green Version]
- Ip, M.S.; Lam, B.; Ng, M.M.; Lam, W.K.; Tsang, K.W.; Lam, K.S. Obstructive sleep apnea is independently associated with insulin resistance. Am. J. Respir. Crit. Care Med. 2002, 165, 670–676. [Google Scholar] [CrossRef] [Green Version]
- Kheirandish-Gozal, L.; Gozal, D. Obstructive Sleep Apnea and Inflammation: Proof of Concept Based on Two Illustrative Cytokines. Int. J. Mol. Sci. 2019, 20, 459. [Google Scholar] [CrossRef] [Green Version]
- Gozal, D.; Kheirandish-Gozal, L. Cardiovascular morbidity in obstructive sleep apnea: Oxidative stress, inflammation, and much more. Am. J. Respir Crit. Care Med. 2008, 177, 369–375. [Google Scholar] [CrossRef] [Green Version]
- Gileles-Hillel, A.; Alonso-Alvarez, M.L.; Kheirandish-Gozal, L.; Peris, E.; Cordero-Guevara, J.A.; Teran-Santos, J.; Martinez, M.G.; Jurado-Luque, M.J.; Corral-Penafiel, J.; Duran-Cantolla, J.; et al. Inflammatory markers and obstructive sleep apnea in obese children: The NANOS study. Mediators Inflamm. 2014, 2014, 605280. [Google Scholar] [CrossRef] [Green Version]
- Dyugovskaya, L.; Lavie, P.; Lavie, L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am. J. Respir Crit. Care Med. 2002, 165, 934–939. [Google Scholar] [CrossRef]
- Maniaci, A.; Iannella, G.; Cocuzza, S.; Vicini, C.; Magliulo, G.; Ferlito, S.; Cammaroto, G.; Meccariello, G.; De Vito, A.; Nicolai, A.; et al. Oxidative Stress and Inflammation Biomarker Expression in Obstructive Sleep Apnea Patients. J. Clin. Med. 2021, 10, 277. [Google Scholar] [CrossRef]
- Jun, J.; Reinke, C.; Bedja, D.; Berkowitz, D.; Bevans-Fonti, S.; Li, J.; Barouch, L.A.; Gabrielson, K.; Polotsky, V.Y. Effect of intermittent hypoxia on atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis 2010, 209, 381–386. [Google Scholar] [CrossRef] [Green Version]
- Hakim, F.; Gozal, D.; Kheirandish-Gozal, L. Sympathetic and catecholaminergic alterations in sleep apnea with particular emphasis on children. Front. Neurol. 2012, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Badran, M.; Yassin, B.A.; Lin, D.T.S.; Kobor, M.S.; Ayas, N.; Laher, I. Gestational intermittent hypoxia induces endothelial dysfunction, reduces perivascular adiponectin and causes epigenetic changes in adult male offspring. J. Physiol. 2019, 597, 5349–5364. [Google Scholar] [CrossRef]
- Badran, M.; Khalyfa, A.; Ericsson, A.; Puech, C.; McAdams, Z.; Bender, S.B.; Gozal, D. Gut microbiota mediate vascular dysfunction in a murine model of sleep apnea: Effect of probiotics. Eur. Respir. J. 2022, 61, 2200002. [Google Scholar] [CrossRef]
- Badran, M.; Gozal, D. PAI-1: A Major Player in the Vascular Dysfunction in Obstructive Sleep Apnea? Int. J. Mol. Sci. 2022, 23, 5516. [Google Scholar] [CrossRef]
- Badran, M.; Golbidi, S.; Devlin, A.; Ayas, N.; Laher, I. Chronic intermittent hypoxia causes endothelial dysfunction in a mouse model of diet-induced obesity. Sleep Med. 2014, 15, 596–602. [Google Scholar] [CrossRef]
- Badran, M.; Golbidi, S.; Ayas, N.; Laher, I. Nitric Oxide Bioavailability in Obstructive Sleep Apnea: Interplay of Asymmetric Dimethylarginine and Free Radicals. Sleep Disord. 2015, 2015, 387801. [Google Scholar] [CrossRef] [Green Version]
- Badran, M.; Bender, S.B.; Khalyfa, A.; Padilla, J.; Martinez-Lemus, L.A.; Gozal, D. Temporal changes in coronary artery function and flow velocity reserve in mice exposed to chronic intermittent hypoxia. Sleep 2022, 45, zsac131. [Google Scholar] [CrossRef]
- Badran, M.; Ayas, N.; Laher, I. Cardiovascular complications of sleep apnea: Role of oxidative stress. Oxid. Med. Cell Longev. 2014, 2014, 985258. [Google Scholar] [CrossRef]
- Badran, M.; Abuyassin, B.; Golbidi, S.; Ayas, N.; Laher, I. Alpha Lipoic Acid Improves Endothelial Function and Oxidative Stress in Mice Exposed to Chronic Intermittent Hypoxia. Oxid. Med. Cell Longev. 2019, 2019, 4093018. [Google Scholar] [CrossRef] [Green Version]
- Badran, M.; Abuyassin, B.; Golbidi, S.; Ayas, N.; Laher, I. Uncoupling of Vascular Nitric Oxide Synthase Caused by Intermittent Hypoxia. Oxid. Med. Cell Longev. 2016, 2016, 2354870. [Google Scholar] [CrossRef] [Green Version]
- Badran, M.; Abuyassin, B.; Ayas, N.; Laher, I. Intermittent hypoxia impairs uterine artery function in pregnant mice. J. Physiol. 2019, 597, 2639–2650. [Google Scholar] [CrossRef]
- Porto, F.; Sakamoto, Y.S.; Salles, C. Association between Obstructive Sleep Apnea and Myocardial Infarction: A Systematic Review. Arq. Bras. Cardiol. 2017, 108, 361–369. [Google Scholar] [CrossRef]
- Saeed, S.; Romarheim, A.; Solheim, E.; Bjorvatn, B.; Lehmann, S. Cardiovascular remodeling in obstructive sleep apnea: Focus on arterial stiffness, left ventricular geometry and atrial fibrillation. Expert Rev. Cardiovasc. Ther. 2022, 20, 455–464. [Google Scholar] [CrossRef]
- Nakashima, H.; Kurobe, M.; Minami, K.; Furudono, S.; Uchida, Y.; Amenomori, K.; Nunohiro, T.; Takeshita, S.; Maemura, K. Effects of moderate-to-severe obstructive sleep apnea on the clinical manifestations of plaque vulnerability and the progression of coronary atherosclerosis in patients with acute coronary syndrome. Eur. Heart J. Acute Cardiovasc. Care 2015, 4, 75–84. [Google Scholar] [CrossRef]
- Hao, W.; Wang, X.; Fan, J.; Zeng, Y.; Ai, H.; Nie, S.; Wei, Y. Association between apnea-hypopnea index and coronary artery calcification: A systematic review and meta-analysis. Ann. Med. 2021, 53, 302–317. [Google Scholar] [CrossRef]
- Hoffstein, V.; Mateika, S. Cardiac arrhythmias, snoring, and sleep apnea. Chest 1994, 106, 466–471. [Google Scholar] [CrossRef] [Green Version]
- Gami, A.S.; Pressman, G.; Caples, S.M.; Kanagala, R.; Gard, J.J.; Davison, D.E.; Malouf, J.F.; Ammash, N.M.; Friedman, P.A.; Somers, V.K. Association of atrial fibrillation and obstructive sleep apnea. Circulation 2004, 110, 364–367. [Google Scholar] [CrossRef]
- Oldenburg, O.; Lamp, B.; Faber, L.; Teschler, H.; Horstkotte, D.; Topfer, V. Sleep-disordered breathing in patients with symptomatic heart failure: A contemporary study of prevalence in and characteristics of 700 patients. Eur. J. Heart Fail 2007, 9, 251–257. [Google Scholar] [CrossRef]
- Sanchez-de-la-Torre, M.; Sanchez-de-la-Torre, A.; Bertran, S.; Abad, J.; Duran-Cantolla, J.; Cabriada, V.; Mediano, O.; Masdeu, M.J.; Alonso, M.L.; Masa, J.F.; et al. Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): A randomised controlled trial. Lancet Respir. Med. 2020, 8, 359–367. [Google Scholar] [CrossRef]
- Peker, Y.; Glantz, H.; Eulenburg, C.; Wegscheider, K.; Herlitz, J.; Thunstrom, E. Effect of Positive Airway Pressure on Cardiovascular Outcomes in Coronary Artery Disease Patients with Nonsleepy Obstructive Sleep Apnea. The RICCADSA Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2016, 194, 613–620. [Google Scholar] [CrossRef]
- McEvoy, R.D.; Antic, N.A.; Heeley, E.; Luo, Y.; Ou, Q.; Zhang, X.; Mediano, O.; Chen, R.; Drager, L.F.; Liu, Z.; et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N. Engl. J. Med. 2016, 375, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Nehme, A.; Zibara, K. Efficiency and specificity of RAAS inhibitors in cardiovascular diseases: How to achieve better end-organ protection? Hypertens. Res. 2017, 40, 903–909. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Bharadwaj, D.; Prasad, G.; Grechko, A.V.; Sazonova, M.A.; Orekhov, A.N. Renin-Angiotensin System in Pathogenesis of Atherosclerosis and Treatment of CVD. Int. J. Mol. Sci. 2021, 22, 6702. [Google Scholar] [CrossRef] [PubMed]
- Pacurari, M.; Kafoury, R.; Tchounwou, P.B.; Ndebele, K. The Renin-Angiotensin-aldosterone system in vascular inflammation and remodeling. Int. J. Inflam. 2014, 2014, 689360. [Google Scholar] [CrossRef] [Green Version]
- Ferrario, C.M.; Strawn, W.B. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am. J. Cardiol. 2006, 98, 121–128. [Google Scholar] [CrossRef] [PubMed]
- DuPont, J.J.; Jaffe, I.Z. 30 YEARS OF THE MINERALOCORTICOID RECEPTOR: The role of the mineralocorticoid receptor in the vasculature. J. Endocrinol. 2017, 234, T67–T82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCurley, A.; Jaffe, I.Z. Mineralocorticoid receptors in vascular function and disease. Mol. Cell Endocrinol. 2012, 350, 256–265. [Google Scholar] [CrossRef] [Green Version]
- Gorini, S.; Kim, S.K.; Infante, M.; Mammi, C.; La Vignera, S.; Fabbri, A.; Jaffe, I.Z.; Caprio, M. Role of Aldosterone and Mineralocorticoid Receptor in Cardiovascular Aging. Front. Endocrinol. 2019, 10, 584. [Google Scholar] [CrossRef]
- Ong, G.S.; Young, M.J. Mineralocorticoid regulation of cell function: The role of rapid signalling and gene transcription pathways. J. Mol. Endocrinol. 2017, 58, R33–R57. [Google Scholar] [CrossRef] [Green Version]
- Pippal, J.B.; Fuller, P.J. Structure-function relationships in the mineralocorticoid receptor. J. Mol. Endocrinol. 2008, 41, 405–413. [Google Scholar] [CrossRef] [Green Version]
- Alexandre, J.; Dolladille, C.; Douesnel, L.; Font, J.; Dabrowski, R.; Shavit, L.; Legallois, D.; Funck-Brentano, C.; Champ-Rigot, L.; Ollitrault, P.; et al. Effects of Mineralocorticoid Receptor Antagonists on Atrial Fibrillation Occurrence: A Systematic Review, Meta-Analysis, and Meta-Regression to Identify Modifying Factors. J. Am. Heart Assoc. 2019, 8, e013267. [Google Scholar] [CrossRef] [PubMed]
- Lofman, I.; Szummer, K.; Olsson, H.; Carrero, J.J.; Lund, L.H.; Jernberg, T. Association Between Mineralocorticoid Receptor Antagonist Use and Outcome in Myocardial Infarction Patients With Heart Failure. J. Am. Heart Assoc. 2018, 7, e009359. [Google Scholar] [CrossRef] [Green Version]
- Nagata, K.; Obata, K.; Xu, J.; Ichihara, S.; Noda, A.; Kimata, H.; Kato, T.; Izawa, H.; Murohara, T.; Yokota, M. Mineralocorticoid receptor antagonism attenuates cardiac hypertrophy and failure in low-aldosterone hypertensive rats. Hypertension 2006, 47, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, T.; Ikejima, H.; Tsujioka, H.; Kuroi, A.; Kobayashi, K.; Muragaki, Y.; Mochizuki, S.; Goto, M.; Yoshida, K.; Akasaka, T. Addition of eplerenone to an angiotensin-converting enzyme inhibitor effectively improves nitric oxide bioavailability. Hypertension 2008, 51, 734–741. [Google Scholar] [CrossRef] [Green Version]
- Sartorio, C.L.; Fraccarollo, D.; Galuppo, P.; Leutke, M.; Ertl, G.; Stefanon, I.; Bauersachs, J. Mineralocorticoid receptor blockade improves vasomotor dysfunction and vascular oxidative stress early after myocardial infarction. Hypertension 2007, 50, 919–925. [Google Scholar] [CrossRef] [Green Version]
- Rajagopalan, S.; Duquaine, D.; King, S.; Pitt, B.; Patel, P. Mineralocorticoid receptor antagonism in experimental atherosclerosis. Circulation 2002, 105, 2212–2216. [Google Scholar] [CrossRef] [Green Version]
- Kosmas, C.E.; Silverio, D.; Sourlas, A.; Montan, P.D.; Guzman, E. Role of spironolactone in the treatment of heart failure with preserved ejection fraction. Ann. Transl. Med. 2018, 6, 461. [Google Scholar] [CrossRef]
- Young, M.J. Targeting the mineralocorticoid receptor in cardiovascular disease. Expert Opin. Ther. Targets 2013, 17, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.A. Aldosterone inhibition and cardiovascular protection: More important than it once appeared. Cardiovasc. Drugs Ther 2010, 24, 345–350. [Google Scholar] [CrossRef]
- Moore, T.D.; Nawarskas, J.J.; Anderson, J.R. Eplerenone: A selective aldosterone receptor antagonist for hypertension and heart failure. Heart Dis. 2003, 5, 354–363. [Google Scholar] [CrossRef]
- Loh, H.H.; Sukor, N. Primary aldosteronism and obstructive sleep apnea: What do we know thus far? Front. Endocrinol. 2022, 13, 976979. [Google Scholar] [CrossRef]
- Fiori, C.Z.; Martinez, D.; Goncalves, S.C.; Montanari, C.C.; Fuchs, F.D. Effect of diuretics and sodium-restricted diet on sleep apnea severity: Study protocol for a randomized controlled trial. Trials 2015, 16, 188. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Zhang, H.; Cai, M.; Zou, Y.; Jiang, X.; Song, L.; Liang, E.; Bian, J.; Wu, H.; Hui, R. Effect of spironolactone on patients with resistant hypertension and obstructive sleep apnea. Clin. Exp. Hypertens. 2016, 38, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Goodfriend, T.L.; Calhoun, D.A. Resistant hypertension, obesity, sleep apnea, and aldosterone: Theory and therapy. Hypertension 2004, 43, 518–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeghiazarians, Y.; Jneid, H.; Tietjens, J.R.; Redline, S.; Brown, D.L.; El-Sherif, N.; Mehra, R.; Bozkurt, B.; Ndumele, C.E.; Somers, V.K. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e56–e67. [Google Scholar] [CrossRef]
- Kim, R.D.; Kapur, V.K.; Redline-Bruch, J.; Rueschman, M.; Auckley, D.H.; Benca, R.M.; Foldvary-Schafer, N.R.; Iber, C.; Zee, P.C.; Rosen, C.L.; et al. An Economic Evaluation of Home Versus Laboratory-Based Diagnosis of Obstructive Sleep Apnea. Sleep 2015, 38, 1027–1037. [Google Scholar] [CrossRef]
- Thornton, C.S.; Tsai, W.H.; Santana, M.J.; Penz, E.D.; Flemons, W.W.; Fraser, K.L.; Hanly, P.J.; Pendharkar, S.R. Effects of Wait Times on Treatment Adherence and Clinical Outcomes in Patients With Severe Sleep-Disordered Breathing: A Secondary Analysis of a Noninferiority Randomized Clinical Trial. JAMA Netw. Open. 2020, 3, e203088. [Google Scholar] [CrossRef]
- Khawaja, I.S.; Olson, E.J.; van der Walt, C.; Bukartyk, J.; Somers, V.; Dierkhising, R.; Morgenthaler, T.I. Diagnostic accuracy of split-night polysomnograms. J. Clin. Sleep Med. 2010, 6, 357–362. [Google Scholar] [CrossRef] [Green Version]
- Chiu, H.Y.; Chen, P.Y.; Chuang, L.P.; Chen, N.H.; Tu, Y.K.; Hsieh, Y.J.; Wang, Y.C.; Guilleminault, C. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: A bivariate meta-analysis. Sleep Med. Rev. 2017, 36, 57–70. [Google Scholar] [CrossRef]
- Rosen, I.M.; Kirsch, D.B.; Chervin, R.D.; Carden, K.A.; Ramar, K.; Aurora, R.N.; Kristo, D.A.; Malhotra, R.K.; Martin, J.L.; Olson, E.J.; et al. Clinical Use of a Home Sleep Apnea Test: An American Academy of Sleep Medicine Position Statement. J. Clin. Sleep Med. 2017, 13, 1205–1207. [Google Scholar] [CrossRef] [Green Version]
- Kump, K.; Whalen, C.; Tishler, P.V.; Browner, I.; Ferrette, V.; Strohl, K.P.; Rosenberg, C.; Redline, S. Assessment of the validity and utility of a sleep-symptom questionnaire. Am. J. Respir. Crit. Care Med. 1994, 150, 735–741. [Google Scholar] [CrossRef]
- Silverberg, D.S.; Oksenberg, A.; Iaina, A. Sleep-related breathing disorders as a major cause of essential hypertension: Fact or fiction? Curr. Opin. Nephrol. Hypertens. 1998, 7, 353–357. [Google Scholar] [CrossRef]
- Fletcher, E.C.; DeBehnke, R.D.; Lovoi, M.S.; Gorin, A.B. Undiagnosed sleep apnea in patients with essential hypertension. Ann. Int. Med. 1985, 103, 190–195. [Google Scholar] [CrossRef]
- Lavie, P.; Ben-Yosef, R.; Rubin, A.E. Prevalence of sleep apnea syndrome among patients with essential hypertension. Am. Heart J. 1984, 108, 373–376. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Palta, M.; Skatrud, J. Prospective study of the association between sleep-disordered breathing and hypertension. N. Engl. J. Med. 2000, 342, 1378–1384. [Google Scholar] [CrossRef]
- Logan, A.G.; Perlikowski, S.M.; Mente, A.; Tisler, A.; Tkacova, R.; Niroumand, M.; Leung, R.S.; Bradley, T.D. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J. Hypertens. 2001, 19, 2271–2277. [Google Scholar] [CrossRef]
- Grote, L.; Hedner, J.; Peter, J.H. Sleep-related breathing disorder is an independent risk factor for uncontrolled hypertension. J. Hypertens. 2000, 18, 679–685. [Google Scholar] [CrossRef]
- Portaluppi, F.; Provini, F.; Cortelli, P.; Plazzi, G.; Bertozzi, N.; Manfredini, R.; Fersini, C.; Lugaresi, E. Undiagnosed sleep-disordered breathing among male nondippers with essential hypertension. J. Hypertens. 1997, 15, 1227–1233. [Google Scholar] [CrossRef]
- Arzt, M.; Woehrle, H.; Oldenburg, O.; Graml, A.; Suling, A.; Erdmann, E.; Teschler, H.; Wegscheider, K.; Schla, H.F.I. Prevalence and Predictors of Sleep-Disordered Breathing in Patients With Stable Chronic Heart Failure: The SchlaHF Registry. JACC Heart Fail 2016, 4, 116–125. [Google Scholar] [CrossRef]
- Chan, J.; Sanderson, J.; Chan, W.; Lai, C.; Choy, D.; Ho, A.; Leung, R. Prevalence of sleep-disordered breathing in diastolic heart failure. Chest 1997, 111, 1488–1493. [Google Scholar] [CrossRef]
- Oldenburg, O.; Wellmann, B.; Buchholz, A.; Bitter, T.; Fox, H.; Thiem, U.; Horstkotte, D.; Wegscheider, K. Nocturnal hypoxaemia is associated with increased mortality in stable heart failure patients. Eur. Heart J. 2016, 37, 1695–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, B.; Liu, H.; Scherlag, B.J.; Sun, L.; Xing, S.; Xu, J.; Luo, M.; Guo, Y.; Cao, G.; Jiang, H. Atrial fibrillation in obstructive sleep apnea: Neural mechanisms and emerging therapies. Trends Cardiovasc. Med. 2021, 31, 127–132. [Google Scholar] [CrossRef]
- Otto, M.E.; Belohlavek, M.; Romero-Corral, A.; Gami, A.S.; Gilman, G.; Svatikova, A.; Amin, R.S.; Lopez-Jimenez, F.; Khandheria, B.K.; Somers, V.K. Comparison of cardiac structural and functional changes in obese otherwise healthy adults with versus without obstructive sleep apnea. Am. J. Cardiol. 2007, 99, 1298–1302. [Google Scholar] [CrossRef]
- Zaqqa, M.; Afshar, H.; Rasekh, A.; Khoshnevis, R.; Vaughn, W.K.; Massumi, A. Predictors of conversion to sinus rhythm using ibutilide for atrial fibrillation or flutter. Am. J. Cardiol. 2000, 85, 112–114, A119. [Google Scholar] [CrossRef] [PubMed]
- Gami, A.S.; Hodge, D.O.; Herges, R.M.; Olson, E.J.; Nykodym, J.; Kara, T.; Somers, V.K. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J. Am. Coll. Cardiol. 2007, 49, 565–571. [Google Scholar] [CrossRef] [Green Version]
- Defaye, P.; de la Cruz, I.; Marti-Almor, J.; Villuendas, R.; Bru, P.; Senechal, J.; Tamisier, R.; Pepin, J.L. A pacemaker transthoracic impedance sensor with an advanced algorithm to identify severe sleep apnea: The DREAM European study. Heart Rhythm. 2014, 11, 842–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simantirakis, E.N.; Schiza, S.I.; Marketou, M.E.; Chrysostomakis, S.I.; Chlouverakis, G.I.; Klapsinos, N.C.; Siafakas, N.S.; Vardas, P.E. Severe bradyarrhythmias in patients with sleep apnoea: The effect of continuous positive airway pressure treatment: A long-term evaluation using an insertable loop recorder. Eur. Heart J. 2004, 25, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.A.; Yaggi, H.K.; Concato, J.; Mohsenin, V. Obstructive sleep apnea as a risk factor for coronary events or cardiovascular death. Sleep Breath 2010, 14, 131–136. [Google Scholar] [CrossRef]
- Punjabi, N.M.; Caffo, B.S.; Goodwin, J.L.; Gottlieb, D.J.; Newman, A.B.; O’Connor, G.T.; Rapoport, D.M.; Redline, S.; Resnick, H.E.; Robbins, J.A.; et al. Sleep-disordered breathing and mortality: A prospective cohort study. PLoS Med. 2009, 6, e1000132. [Google Scholar] [CrossRef] [Green Version]
- Yeboah, J.; Redline, S.; Johnson, C.; Tracy, R.; Ouyang, P.; Blumenthal, R.S.; Burke, G.L.; Herrington, D.M. Association between sleep apnea, snoring, incident cardiovascular events and all-cause mortality in an adult population: MESA. Atherosclerosis 2011, 219, 963–968. [Google Scholar] [CrossRef]
- Gunta, S.P.; Jakulla, R.S.; Ubaid, A.; Mohamed, K.; Bhat, A.; Lopez-Candales, A.; Norgard, N. Obstructive Sleep Apnea and Cardiovascular Diseases: Sad Realities and Untold Truths regarding Care of Patients in 2022. Cardiovasc. Ther. 2022, 2022, 6006127. [Google Scholar] [CrossRef] [PubMed]
- Imani, M.M.; Sadeghi, M.; Khazaie, H.; Emami, M.; Sadeghi Bahmani, D.; Brand, S. Evaluation of Serum and Plasma Interleukin-6 Levels in Obstructive Sleep Apnea Syndrome: A Meta-Analysis and Meta-Regression. Front. Immunol. 2020, 11, 1343. [Google Scholar] [CrossRef]
- Schulz, R.; Mahmoudi, S.; Hattar, K.; Sibelius, U.; Olschewski, H.; Mayer, K.; Seeger, W.; Grimminger, F. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Impact of continuous positive airway pressure therapy. Am. J. Respir. Crit. Care Med. 2000, 162, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Kizawa, T.; Nakamura, Y.; Takahashi, S.; Sakurai, S.; Yamauchi, K.; Inoue, H. Pathogenic role of angiotensin II and oxidised LDL in obstructive sleep apnoea. Eur. Respir. J. 2009, 34, 1390–1398. [Google Scholar] [CrossRef]
- Vatansever, E.; Surmen-Gur, E.; Ursavas, A.; Karadag, M. Obstructive sleep apnea causes oxidative damage to plasma lipids and proteins and decreases adiponectin levels. Sleep Breath 2011, 15, 275–282. [Google Scholar] [CrossRef]
- Yamauchi, M.; Nakano, H.; Maekawa, J.; Okamoto, Y.; Ohnishi, Y.; Suzuki, T.; Kimura, H. Oxidative stress in obstructive sleep apnea. Chest 2005, 127, 1674–1679. [Google Scholar] [CrossRef] [Green Version]
- Rosa, D.P.; Martinez, D.; Picada, J.N.; Semedo, J.G.; Marroni, N.P. Hepatic oxidative stress in an animal model of sleep apnoea: Effects of different duration of exposure. Comp. Hepatol. 2011, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Castro-Grattoni, A.L.; Suarez-Giron, M.; Benitez, I.; Torres, M.; Almendros, I.; Farre, R.; Montserrat, J.M.; Dalmases, M.; Gozal, D.; Sanchez-de-la-Torre, M.; et al. Effect of age on the cardiovascular remodelling induced by chronic intermittent hypoxia as a murine model of sleep apnoea. Respirology 2020, 25, 312–320. [Google Scholar] [CrossRef]
- Farre, N.; Otero, J.; Falcones, B.; Torres, M.; Jorba, I.; Gozal, D.; Almendros, I.; Farre, R.; Navajas, D. Intermittent Hypoxia Mimicking Sleep Apnea Increases Passive Stiffness of Myocardial Extracellular Matrix. A Multiscale Study. Front. Physiol. 2018, 9, 1143. [Google Scholar] [CrossRef] [PubMed]
- Bourdier, G.; Detrait, M.; Bouyon, S.; Lemarie, E.; Brasseur, S.; Doutreleau, S.; Pepin, J.L.; Godin-Ribuot, D.; Belaidi, E.; Arnaud, C. Intermittent Hypoxia Triggers Early Cardiac Remodeling and Contractile Dysfunction in the Time-Course of Ischemic Cardiomyopathy in Rats. J. Am. Heart Assoc. 2020, 9, e016369. [Google Scholar] [CrossRef]
- Bao, Q.; Zhang, B.; Suo, Y.; Liu, C.; Yang, Q.; Zhang, K.; Yuan, M.; Yuan, M.; Zhang, Y.; Li, G. Intermittent hypoxia mediated by TSP1 dependent on STAT3 induces cardiac fibroblast activation and cardiac fibrosis. eLife 2020, 9, e49923. [Google Scholar] [CrossRef] [PubMed]
- Abuyassin, B.; Badran, M.; Ayas, N.T.; Laher, I. Intermittent hypoxia causes histological kidney damage and increases growth factor expression in a mouse model of obstructive sleep apnea. PLoS ONE 2018, 13, e0192084. [Google Scholar] [CrossRef] [Green Version]
- Ding, W.X.; Dong, Y.B.; Ding, N.; Zhang, X.F.; Zhang, S.J.; Zhang, X.L.; Liu, J.N.; Lu, G. Adiponectin protects rat heart from left ventricular remodeling induced by chronic intermittent hypoxia via inhibition of TGF-beta/smad2/3 pathway. J. Thorac. Dis. 2014, 6, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.P.; Lin, Y.N.; Li, N.; Sun, X.W.; Ding, Y.J.; Yan, Y.R.; Zhang, L.; Li, Q.Y. Angiotensin-(1-7) Rescues Chronic Intermittent Hypoxia-Aggravated Transforming Growth Factor-beta-Mediated Airway Remodeling in Murine and Cellular Models of Asthma. J. Pharmacol. Exp. Ther. 2020, 375, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Song, Y.; Ning, P.; Zhang, L.; Wu, S.; Quan, J.; Li, Q. Association between tumor necrosis factor alpha and obstructive sleep apnea in adults: A meta-analysis update. BMC Pulm. Med. 2020, 20, 215. [Google Scholar] [CrossRef] [PubMed]
- Htoo, A.K.; Greenberg, H.; Tongia, S.; Chen, G.; Henderson, T.; Wilson, D.; Liu, S.F. Activation of nuclear factor kappaB in obstructive sleep apnea: A pathway leading to systemic inflammation. Sleep Breath 2006, 10, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan, D.; Jun, J.; Polotsky, V. Inflammation in sleep apnea: An update. Rev. Endocr. Metab. Disord. 2015, 16, 25–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Zheng, X. Tumor necrosis factor alpha is a promising circulating biomarker for the development of obstructive sleep apnea syndrome: A meta-analysis. Oncotarget 2017, 8, 27616–27626. [Google Scholar] [CrossRef] [Green Version]
- Barros, D.; Garcia-Rio, F. Obstructive sleep apnea and dyslipidemia: From animal models to clinical evidence. Sleep 2019, 42, zsy236. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Dong, Y.; Somers, V.K.; Peterson, T.E.; Zhang, Y.; Wang, S.; Li, G.; Singh, P. Intermittent hypoxia regulates vasoactive molecules and alters insulin-signaling in vascular endothelial cells. Sci. Rep. 2018, 8, 14110. [Google Scholar] [CrossRef] [Green Version]
- Thomas, A.; Belaidi, E.; Moulin, S.; Horman, S.; van der Zon, G.C.; Viollet, B.; Levy, P.; Bertrand, L.; Pepin, J.L.; Godin-Ribuot, D.; et al. Chronic Intermittent Hypoxia Impairs Insulin Sensitivity but Improves Whole-Body Glucose Tolerance by Activating Skeletal Muscle AMPK. Diabetes 2017, 66, 2942–2951. [Google Scholar] [CrossRef] [Green Version]
- Tkacova, R.; Dorkova, Z.; Molcanyiova, A.; Radikova, Z.; Klimes, I.; Tkac, I. Cardiovascular risk and insulin resistance in patients with obstructive sleep apnea. Med. Sci. Monit. 2008, 14, CR438–CR444. [Google Scholar]
- Bhushan, B.; Maddalozzo, J.; Sheldon, S.H.; Haymond, S.; Rychlik, K.; Lales, G.C.; Billings, K.R. Metabolic alterations in children with obstructive sleep apnea. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Parish, J.M.; Adam, T.; Facchiano, L. Relationship of metabolic syndrome and obstructive sleep apnea. J. Clin. Sleep Med. 2007, 3, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.P.; Ayappa, I.A.; Caples, S.M.; Kimoff, R.J.; Patel, S.R.; Harrod, C.G. Treatment of Adult Obstructive Sleep Apnea With Positive Airway Pressure: An American Academy of Sleep Medicine Systematic Review, Meta-Analysis, and GRADE Assessment. J. Clin. Sleep Med. 2019, 15, 301–334. [Google Scholar] [CrossRef] [PubMed]
- da Silva Paulitsch, F.; Zhang, L. Continuous positive airway pressure for adults with obstructive sleep apnea and cardiovascular disease: A meta-analysis of randomized trials. Sleep Med. 2019, 54, 28–34. [Google Scholar] [CrossRef]
- Mokhlesi, B.; Finn, L.A.; Hagen, E.W.; Young, T.; Hla, K.M.; Van Cauter, E.; Peppard, P.E. Obstructive sleep apnea during REM sleep and hypertension. results of the Wisconsin Sleep Cohort. Am. J. Respir. Crit. Care Med. 2014, 190, 1158–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortese, R.; Gileles-Hillel, A.; Khalyfa, A.; Almendros, I.; Akbarpour, M.; Khalyfa, A.A.; Qiao, Z.; Garcia, T.; Andrade, J.; Gozal, D. Aorta macrophage inflammatory and epigenetic changes in a murine model of obstructive sleep apnea: Potential role of CD36. Sci. Rep. 2017, 7, 43648. [Google Scholar] [CrossRef] [Green Version]
- Arriza, J.L.; Weinberger, C.; Cerelli, G.; Glaser, T.M.; Handelin, B.L.; Housman, D.E.; Evans, R.M. Cloning of human mineralocorticoid receptor complementary DNA: Structural and functional kinship with the glucocorticoid receptor. Science 1987, 237, 268–275. [Google Scholar] [CrossRef]
- Huyet, J.; Pinon, G.M.; Fay, M.R.; Rafestin-Oblin, M.E.; Fagart, J. Structural determinants of ligand binding to the mineralocorticoid receptor. Mol. Cell Endocrinol. 2012, 350, 187–195. [Google Scholar] [CrossRef]
- Rogerson, F.M.; Brennan, F.E.; Fuller, P.J. Mineralocorticoid receptor binding, structure and function. Mol. Cell Endocrinol. 2004, 217, 203–212. [Google Scholar] [CrossRef]
- Galigniana, M.D.; Echeverria, P.C.; Erlejman, A.G.; Piwien-Pilipuk, G. Role of molecular chaperones and TPR-domain proteins in the cytoplasmic transport of steroid receptors and their passage through the nuclear pore. Nucleus 2010, 1, 299–308. [Google Scholar] [CrossRef] [Green Version]
- Grossmann, C.; Ruhs, S.; Langenbruch, L.; Mildenberger, S.; Stratz, N.; Schumann, K.; Gekle, M. Nuclear shuttling precedes dimerization in mineralocorticoid receptor signaling. Chem. Biol. 2012, 19, 742–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Billan, F.; Khan, J.A.; Lamribet, K.; Viengchareun, S.; Bouligand, J.; Fagart, J.; Lombes, M. Cistrome of the aldosterone-activated mineralocorticoid receptor in human renal cells. FASEB J. 2015, 29, 3977–3989. [Google Scholar] [CrossRef] [Green Version]
- Cannavo, A.; Bencivenga, L.; Liccardo, D.; Elia, A.; Marzano, F.; Gambino, G.; D’Amico, M.L.; Perna, C.; Ferrara, N.; Rengo, G.; et al. Aldosterone and Mineralocorticoid Receptor System in Cardiovascular Physiology and Pathophysiology. Oxid. Med. Cell Longev. 2018, 2018, 1204598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loffing, J.; Korbmacher, C. Regulated sodium transport in the renal connecting tubule (CNT) via the epithelial sodium channel (ENaC). Pflugers. Arch. 2009, 458, 111–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Sanchez, E.; Gomez-Sanchez, C.E. The multifaceted mineralocorticoid receptor. Compr. Physiol. 2014, 4, 965–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funder, J.W.; Pearce, P.T.; Smith, R.; Smith, A.I. Mineralocorticoid action: Target tissue specificity is enzyme, not receptor, mediated. Science 1988, 242, 583–585. [Google Scholar] [CrossRef]
- Mullins, L.J.; Kenyon, C.J.; Bailey, M.A.; Conway, B.R.; Diaz, M.E.; Mullins, J.J. Mineralocorticoid Excess or Glucocorticoid Insufficiency: Renal and Metabolic Phenotypes in a Rat Hsd11b2 Knockout Model. Hypertension 2015, 66, 667–673. [Google Scholar] [CrossRef] [Green Version]
- Mihailidou, A.S.; Loan Le, T.Y.; Mardini, M.; Funder, J.W. Glucocorticoids activate cardiac mineralocorticoid receptors during experimental myocardial infarction. Hypertension 2009, 54, 1306–1312. [Google Scholar] [CrossRef] [Green Version]
- Rossier, M.F.; Lenglet, S.; Vetterli, L.; Python, M.; Maturana, A. Corticosteroids and redox potential modulate spontaneous contractions in isolated rat ventricular cardiomyocytes. Hypertension 2008, 52, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Newfell, B.G.; Iyer, L.K.; Mohammad, N.N.; McGraw, A.P.; Ehsan, A.; Rosano, G.; Huang, P.L.; Mendelsohn, M.E.; Jaffe, I.Z. Aldosterone regulates vascular gene transcription via oxidative stress-dependent and -independent pathways. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1871–1880. [Google Scholar] [CrossRef] [Green Version]
- Turchin, A.; Guo, C.Z.; Adler, G.K.; Ricchiuti, V.; Kohane, I.S.; Williams, G.H. Effect of acute aldosterone administration on gene expression profile in the heart. Endocrinology 2006, 147, 3183–3189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gekle, M.; Freudinger, R.; Mildenberger, S.; Schenk, K.; Marschitz, I.; Schramek, H. Rapid activation of Na+/H+-exchange in MDCK cells by aldosterone involves MAP-kinase ERK1/2. Pflugers. Arch. 2001, 441, 781–786. [Google Scholar] [CrossRef]
- Blazer-Yost, B.L.; Paunescu, T.G.; Helman, S.I.; Lee, K.D.; Vlahos, C.J. Phosphoinositide 3-kinase is required for aldosterone-regulated sodium reabsorption. Am. J. Physiol. 1999, 277, C531–C536. [Google Scholar] [CrossRef]
- McEneaney, V.; Harvey, B.J.; Thomas, W. Aldosterone regulates rapid trafficking of epithelial sodium channel subunits in renal cortical collecting duct cells via protein kinase D activation. Mol. Endocrinol. 2008, 22, 881–892. [Google Scholar] [CrossRef] [Green Version]
- Mihailidou, A.S.; Mardini, M.; Funder, J.W. Rapid, nongenomic effects of aldosterone in the heart mediated by epsilon protein kinase C. Endocrinology 2004, 145, 773–780. [Google Scholar] [CrossRef] [Green Version]
- Ruhs, S.; Nolze, A.; Hubschmann, R.; Grossmann, C. 30 YEARS OF THE MINERALOCORTICOID RECEPTOR: Nongenomic effects via the mineralocorticoid receptor. J. Endocrinol. 2017, 234, T107–T124. [Google Scholar] [CrossRef] [Green Version]
- Ashton, A.W.; Le, T.Y.; Gomez-Sanchez, C.E.; Morel-Kopp, M.C.; McWhinney, B.; Hudson, A.; Mihailidou, A.S. Role of Nongenomic Signaling Pathways Activated by Aldosterone During Cardiac Reperfusion Injury. Mol. Endocrinol. 2015, 29, 1144–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coutinho, P.; Vega, C.; Pojoga, L.H.; Rivera, A.; Prado, G.N.; Yao, T.M.; Adler, G.; Torres-Grajales, M.; Maldonado, E.R.; Ramos-Rivera, A.; et al. Aldosterone’s rapid, nongenomic effects are mediated by striatin: A modulator of aldosterone’s effect on estrogen action. Endocrinology 2014, 155, 2233–2243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossmann, C.; Wuttke, M.; Ruhs, S.; Seiferth, A.; Mildenberger, S.; Rabe, S.; Schwerdt, G.; Gekle, M. Mineralocorticoid receptor inhibits CREB signaling by calcineurin activation. FASEB J. 2010, 24, 2010–2019. [Google Scholar] [CrossRef]
- Grossmann, C.; Ruhs, S.; Seiferth, A.; Gekle, M. Interaction between mineralocorticoid receptor and cAMP/CREB signaling. Steroids 2010, 75, 539–543. [Google Scholar] [CrossRef]
- Ozbaki-Yagan, N.; Liu, X.; Bodnar, A.J.; Ho, J.; Butterworth, M.B. Aldosterone-induced microRNAs act as feedback regulators of mineralocorticoid receptor signaling in kidney epithelia. FASEB J. 2020, 34, 11714–11728. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Kong, Q.; Kone, B.C. Aldosterone reprograms promoter methylation to regulate alphaENaC transcription in the collecting duct. Am. J. Physiol. Renal. Physiol. 2013, 305, F1006–F1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santillo, M.; Colantuoni, A.; Mondola, P.; Guida, B.; Damiano, S. NOX signaling in molecular cardiovascular mechanisms involved in the blood pressure homeostasis. Front. Physiol. 2015, 6, 194. [Google Scholar] [CrossRef]
- Ying, W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: Regulation and biological consequences. Antioxid. Redox Signal 2008, 10, 179–206. [Google Scholar] [CrossRef] [Green Version]
- Fiebeler, A.; Schmidt, F.; Muller, D.N.; Park, J.K.; Dechend, R.; Bieringer, M.; Shagdarsuren, E.; Breu, V.; Haller, H.; Luft, F.C. Mineralocorticoid receptor affects AP-1 and nuclear factor-kappab activation in angiotensin II-induced cardiac injury. Hypertension 2001, 37, 787–793. [Google Scholar] [CrossRef] [Green Version]
- Queisser, N.; Oteiza, P.I.; Stopper, H.; Oli, R.G.; Schupp, N. Aldosterone induces oxidative stress, oxidative DNA damage and NF-kappaB-activation in kidney tubule cells. Mol. Carcinog. 2011, 50, 123–135. [Google Scholar] [CrossRef]
- Hayashi, H.; Kobara, M.; Abe, M.; Tanaka, N.; Gouda, E.; Toba, H.; Yamada, H.; Tatsumi, T.; Nakata, T.; Matsubara, H. Aldosterone nongenomically produces NADPH oxidase-dependent reactive oxygen species and induces myocyte apoptosis. Hypertens. Res. 2008, 31, 363–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannavo, A.; Liccardo, D.; Eguchi, A.; Elliott, K.J.; Traynham, C.J.; Ibetti, J.; Eguchi, S.; Leosco, D.; Ferrara, N.; Rengo, G.; et al. Myocardial pathology induced by aldosterone is dependent on non-canonical activities of G protein-coupled receptor kinases. Nat. Commun. 2016, 7, 10877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montezano, A.C.; Callera, G.E.; Yogi, A.; He, Y.; Tostes, R.C.; He, G.; Schiffrin, E.L.; Touyz, R.M. Aldosterone and angiotensin II synergistically stimulate migration in vascular smooth muscle cells through c-Src-regulated redox-sensitive RhoA pathways. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1511–1518. [Google Scholar] [CrossRef] [Green Version]
- Nagase, M.; Ayuzawa, N.; Kawarazaki, W.; Ishizawa, K.; Ueda, K.; Yoshida, S.; Fujita, T. Oxidative stress causes mineralocorticoid receptor activation in rat cardiomyocytes: Role of small GTPase Rac1. Hypertension 2012, 59, 500–506. [Google Scholar] [CrossRef] [Green Version]
- Stas, S.; Whaley-Connell, A.; Habibi, J.; Appesh, L.; Hayden, M.R.; Karuparthi, P.R.; Qazi, M.; Morris, E.M.; Cooper, S.A.; Link, C.D.; et al. Mineralocorticoid receptor blockade attenuates chronic overexpression of the renin-angiotensin-aldosterone system stimulation of reduced nicotinamide adenine dinucleotide phosphate oxidase and cardiac remodeling. Endocrinology 2007, 148, 3773–3780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, L.; Beswick, R.A.; Yamamoto, T.; Palmer, T.; Taylor, T.A.; Pollock, J.S.; Pollock, D.M.; Brands, M.W.; Webb, R.C. Increased reactive oxygen species contributes to kidney injury in mineralocorticoid hypertensive rats. J. Physiol. Pharmacol. 2006, 57, 343–357. [Google Scholar] [PubMed]
- Kamalov, G.; Ahokas, R.A.; Zhao, W.; Zhao, T.; Shahbaz, A.U.; Johnson, P.L.; Bhattacharya, S.K.; Sun, Y.; Gerling, I.C.; Weber, K.T. Uncoupling the coupled calcium and zinc dyshomeostasis in cardiac myocytes and mitochondria seen in aldosteronism. J. Cardiovasc. Pharmacol. 2010, 55, 248–254. [Google Scholar] [CrossRef] [Green Version]
- Ndisang, J.F.; Jadhav, A. The heme oxygenase system attenuates pancreatic lesions and improves insulin sensitivity and glucose metabolism in deoxycorticosterone acetate hypertension. Am. J. Physiol. Regul. Integr. Comp Physiol. 2010, 298, R211–R223. [Google Scholar] [CrossRef]
- Park, Y.M.; Lim, B.H.; Touyz, R.M.; Park, J.B. Expression of NAD(P)H oxidase subunits and their contribution to cardiovascular damage in aldosterone/salt-induced hypertensive rat. J. Korean Med. Sci. 2008, 23, 1039–1045. [Google Scholar] [CrossRef] [Green Version]
- Schupp, N.; Kolkhof, P.; Queisser, N.; Gartner, S.; Schmid, U.; Kretschmer, A.; Hartmann, E.; Oli, R.G.; Schafer, S.; Stopper, H. Mineralocorticoid receptor-mediated DNA damage in kidneys of DOCA-salt hypertensive rats. FASEB J. 2011, 25, 968–978. [Google Scholar] [CrossRef]
- Nagata, D.; Takahashi, M.; Sawai, K.; Tagami, T.; Usui, T.; Shimatsu, A.; Hirata, Y.; Naruse, M. Molecular mechanism of the inhibitory effect of aldosterone on endothelial NO synthase activity. Hypertension 2006, 48, 165–171. [Google Scholar] [CrossRef]
- Taye, A.; Morawietz, H. Spironolactone inhibits NADPH oxidase-induced oxidative stress and enhances eNOS in human endothelial cells. Iran J. Pharm. Res. 2011, 10, 329–337. [Google Scholar]
- Virdis, A.; Neves, M.F.; Amiri, F.; Viel, E.; Touyz, R.M.; Schiffrin, E.L. Spironolactone improves angiotensin-induced vascular changes and oxidative stress. Hypertension 2002, 40, 504–510. [Google Scholar] [CrossRef] [Green Version]
- Mayyas, F.A.; Aljohmani, A.I.; Alzoubi, K.H. The Impact of Spironolactone on Markers of Myocardial Oxidative Status, Inflammation and Remodeling in Hyperthyroid Rats. Curr. Mol. Pharmacol. 2020, 13, 206–215. [Google Scholar] [CrossRef]
- Mayyas, F.; Alzoubi, K.H.; Bonyan, R. The role of spironolactone on myocardial oxidative stress in rat model of streptozotocin-induced diabetes. Cardiovasc. Ther. 2017, 35, e12242. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.A.; Leopold, J.A. Mineralocorticoid receptor antagonists and endothelial function. Curr. Opin. Investig. Drugs 2008, 9, 963–969. [Google Scholar] [PubMed]
- Stehr, C.B.; Mellado, R.; Ocaranza, M.P.; Carvajal, C.A.; Mosso, L.; Becerra, E.; Solis, M.; Garcia, L.; Lavandero, S.; Jalil, J.; et al. Increased levels of oxidative stress, subclinical inflammation, and myocardial fibrosis markers in primary aldosteronism patients. J. Hypertens. 2010, 28, 2120–2126. [Google Scholar] [CrossRef]
- Laffer, C.L.; Bolterman, R.J.; Romero, J.C.; Elijovich, F. Effect of salt on isoprostanes in salt-sensitive essential hypertension. Hypertension 2006, 47, 434–440. [Google Scholar] [CrossRef] [Green Version]
- Keidar, S.; Gamliel-Lazarovich, A.; Kaplan, M.; Pavlotzky, E.; Hamoud, S.; Hayek, T.; Karry, R.; Abassi, Z. Mineralocorticoid receptor blocker increases angiotensin-converting enzyme 2 activity in congestive heart failure patients. Circ. Res. 2005, 97, 946–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renke, M.; Tylicki, L.; Knap, N.; Rutkowski, P.; Neuwelt, A.; Larczynski, W.; Wozniak, M.; Rutkowski, B. Spironolactone attenuates oxidative stress in patients with chronic kidney disease. Hypertension 2008, 52, e132–e133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, N.J. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat. Rev. Nephrol. 2013, 9, 459–469. [Google Scholar] [CrossRef]
- Munoz-Durango, N.; Vecchiola, A.; Gonzalez-Gomez, L.M.; Simon, F.; Riedel, C.A.; Fardella, C.E.; Kalergis, A.M. Modulation of Immunity and Inflammation by the Mineralocorticoid Receptor and Aldosterone. Biomed. Res. Int. 2015, 2015, 652738. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, N.S.; Tostes, R.C.; Paradis, P.; Schiffrin, E.L. Aldosterone, Inflammation, Immune System, and Hypertension. Am. J. Hypertens. 2021, 34, 15–27. [Google Scholar] [CrossRef]
- Gilbert, K.C.; Brown, N.J. Aldosterone and inflammation. Curr. Opin. Endocrinol. Diabetes. Obes. 2010, 17, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Callera, G.E.; Yogi, A.; Briones, A.M.; Montezano, A.C.; He, Y.; Tostes, R.C.; Schiffrin, E.L.; Touyz, R.M. Vascular proinflammatory responses by aldosterone are mediated via c-Src trafficking to cholesterol-rich microdomains: Role of PDGFR. Cardiovasc. Res. 2011, 91, 720–731. [Google Scholar] [CrossRef] [Green Version]
- Ding, W.; Yang, L.; Zhang, M.; Gu, Y. Chronic inhibition of nuclear factor kappa B attenuates aldosterone/salt-induced renal injury. Life Sci. 2012, 90, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cui, R.; Cheng, X.; Du, J. Antiapoptotic effect of serum and glucocorticoid-inducible protein kinase is mediated by novel mechanism activating IkappaB kinase. Cancer Res. 2005, 65, 457–464. [Google Scholar] [CrossRef]
- Rickard, A.J.; Morgan, J.; Bienvenu, L.A.; Fletcher, E.K.; Cranston, G.A.; Shen, J.Z.; Reichelt, M.E.; Delbridge, L.M.; Young, M.J. Cardiomyocyte mineralocorticoid receptors are essential for deoxycorticosterone/salt-mediated inflammation and cardiac fibrosis. Hypertension 2012, 60, 1443–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usher, M.G.; Duan, S.Z.; Ivaschenko, C.Y.; Frieler, R.A.; Berger, S.; Schutz, G.; Lumeng, C.N.; Mortensen, R.M. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J. Clin. Investig. 2010, 120, 3350–3364. [Google Scholar] [CrossRef] [Green Version]
- McGraw, A.P.; Bagley, J.; Chen, W.S.; Galayda, C.; Nickerson, H.; Armani, A.; Caprio, M.; Carmeliet, P.; Jaffe, I.Z. Aldosterone increases early atherosclerosis and promotes plaque inflammation through a placental growth factor-dependent mechanism. J. Am. Heart Assoc. 2013, 2, e000018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rickard, A.J.; Morgan, J.; Chrissobolis, S.; Miller, A.A.; Sobey, C.G.; Young, M.J. Endothelial cell mineralocorticoid receptors regulate deoxycorticosterone/salt-mediated cardiac remodeling and vascular reactivity but not blood pressure. Hypertension 2014, 63, 1033–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caprio, M.; Newfell, B.G.; la Sala, A.; Baur, W.; Fabbri, A.; Rosano, G.; Mendelsohn, M.E.; Jaffe, I.Z. Functional mineralocorticoid receptors in human vascular endothelial cells regulate intercellular adhesion molecule-1 expression and promote leukocyte adhesion. Circ. Res. 2008, 102, 1359–1367. [Google Scholar] [CrossRef] [Green Version]
- Briet, M.; Barhoumi, T.; Mian, M.O.R.; Coelho, S.C.; Ouerd, S.; Rautureau, Y.; Coffman, T.M.; Paradis, P.; Schiffrin, E.L. Aldosterone-Induced Vascular Remodeling and Endothelial Dysfunction Require Functional Angiotensin Type 1a Receptors. Hypertension 2016, 67, 897–905. [Google Scholar] [CrossRef]
- Lemarie, C.A.; Simeone, S.M.; Nikonova, A.; Ebrahimian, T.; Deschenes, M.E.; Coffman, T.M.; Paradis, P.; Schiffrin, E.L. Aldosterone-induced activation of signaling pathways requires activity of angiotensin type 1a receptors. Circ. Res. 2009, 105, 852–859. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Liu, J.; Pang, X.; Zhao, J.; Wang, S.; Wu, D. Aldosterone induces C-reactive protein expression via MR-ROS-MAPK-NF-kappaB signal pathway in rat vascular smooth muscle cells. Mol. Cell Endocrinol. 2014, 395, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Bruder-Nascimento, T.; Ferreira, N.S.; Zanotto, C.Z.; Ramalho, F.; Pequeno, I.O.; Olivon, V.C.; Neves, K.B.; Alves-Lopes, R.; Campos, E.; Silva, C.A.; et al. NLRP3 Inflammasome Mediates Aldosterone-Induced Vascular Damage. Circulation 2016, 134, 1866–1880. [Google Scholar] [CrossRef]
- Doi, T.; Doi, S.; Nakashima, A.; Ueno, T.; Yokoyama, Y.; Kohno, N.; Masaki, T. Mizoribine ameliorates renal injury and hypertension along with the attenuation of renal caspase-1 expression in aldosterone-salt-treated rats. PLoS ONE 2014, 9, e93513. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Zhang, H.; Zhang, J.; Xie, C.; Fan, C.; Zhang, H.; Wu, P.; Wei, Q.; Tan, W.; Xu, L.; et al. Inflammation and Fibrosis in Perirenal Adipose Tissue of Patients With Aldosterone-Producing Adenoma. Endocrinology 2018, 159, 227–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savoia, C.; Touyz, R.M.; Amiri, F.; Schiffrin, E.L. Selective mineralocorticoid receptor blocker eplerenone reduces resistance artery stiffness in hypertensive patients. Hypertension 2008, 51, 432–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silvestre, J.S.; Robert, V.; Heymes, C.; Aupetit-Faisant, B.; Mouas, C.; Moalic, J.M.; Swynghedauw, B.; Delcayre, C. Myocardial production of aldosterone and corticosterone in the rat. Physiological regulation. J. Biol. Chem. 1998, 273, 4883–4891. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, D.M.; Shaqura, M.; Li, X.; Shakibaei, M.; Beyer, A.; Treskatsch, S.; Schafer, M.; Mousa, S.A. Aldosterone Synthase in Peripheral Sensory Neurons Contributes to Mechanical Hypersensitivity during Local Inflammation in Rats. Anesthesiology 2020, 132, 867–880. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Sanchez, C.E.; Zhou, M.Y.; Cozza, E.N.; Morita, H.; Foecking, M.F.; Gomez-Sanchez, E.P. Aldosterone biosynthesis in the rat brain. Endocrinology 1997, 138, 3369–3373. [Google Scholar] [CrossRef]
- Malik, M. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Wang, W. Chronic administration of aldosterone depresses baroreceptor reflex function in the dog. Hypertension 1994, 24, 571–575. [Google Scholar] [CrossRef] [Green Version]
- Struthers, A.D. Evidence for myocardial synthesis of aldosterone producing myocardial fibrosis in man. Clin. Sci. 2002, 102, 387. [Google Scholar] [CrossRef]
- MacFadyen, R.J.; Barr, C.S.; Struthers, A.D. Aldosterone blockade reduces vascular collagen turnover, improves heart rate variability and reduces early morning rise in heart rate in heart failure patients. Cardiovasc. Res. 1997, 35, 30–34. [Google Scholar] [CrossRef] [Green Version]
- Burke, S.L.; Barzel, B.; Jackson, K.L.; Gueguen, C.; Young, M.J.; Head, G.A. Role of Mineralocorticoid and Angiotensin Type 1 Receptors in the Paraventricular Nucleus in Angiotensin-Induced Hypertension. Front. Physiol. 2021, 12, 640373. [Google Scholar] [CrossRef]
- Huang, B.S.; Ahmadi, S.; Ahmad, M.; White, R.A.; Leenen, F.H. Central neuronal activation and pressor responses induced by circulating ANG II: Role of the brain aldosterone-"ouabain" pathway. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H422–H430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehe, L.; Mousa, S.A.; Aboryag, N.; Shaqura, M.; Beyer, A.; Schafer, M.; Treskatsch, S. Identification of Mineralocorticoid Receptors, Aldosterone, and Its Processing Enzyme CYP11B2 on Parasympathetic and Sympathetic Neurons in Rat Intracardiac Ganglia. Front. Neuroanat. 2021, 15, 802359. [Google Scholar] [CrossRef]
- Alexander, Y.; Osto, E.; Schmidt-Trucksass, A.; Shechter, M.; Trifunovic, D.; Duncker, D.J.; Aboyans, V.; Back, M.; Badimon, L.; Cosentino, F.; et al. Endothelial function in cardiovascular medicine: A consensus paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc. Res. 2021, 117, 29–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirsch, T.; Beese, M.; Wyss, K.; Klinge, U.; Haller, H.; Haubitz, M.; Fiebeler, A. Aldosterone modulates endothelial permeability and endothelial nitric oxide synthase activity by rearrangement of the actin cytoskeleton. Hypertension 2013, 61, 501–508. [Google Scholar] [CrossRef] [Green Version]
- Fiebeler, A.; Luft, F.C. The mineralocorticoid receptor and oxidative stress. Heart Fail Rev. 2005, 10, 47–52. [Google Scholar] [CrossRef]
- Briet, M.; Schiffrin, E.L. Vascular actions of aldosterone. J. Vasc. Res. 2013, 50, 89–99. [Google Scholar] [CrossRef]
- Maron, B.A.; Zhang, Y.Y.; Handy, D.E.; Beuve, A.; Tang, S.S.; Loscalzo, J.; Leopold, J.A. Aldosterone increases oxidant stress to impair guanylyl cyclase activity by cysteinyl thiol oxidation in vascular smooth muscle cells. J. Biol. Chem. 2009, 284, 7665–7672. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Ding, M.L.; Wu, F.; He, W.; Li, J.; Zhang, X.Y.; Xie, W.L.; Duan, S.Z.; Xia, W.H.; Tao, J. Impaired Endothelial Repair Capacity of Early Endothelial Progenitor Cells in Hypertensive Patients With Primary Hyperaldosteronemia: Role of 5,6,7,8-Tetrahydrobiopterin Oxidation and Endothelial Nitric Oxide Synthase Uncoupling. Hypertension 2016, 67, 430–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eatman, D.; Peagler, K.; Watson, J.; Rollins-Hairston, A.; Bayorh, M.A. The involvement of prostaglandins in the contractile function of the aorta by aldosterone. BMC Res. Notes 2011, 4, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco-Rivero, J.; Cachofeiro, V.; Lahera, V.; Aras-Lopez, R.; Marquez-Rodas, I.; Salaices, M.; Xavier, F.E.; Ferrer, M.; Balfagon, G. Participation of prostacyclin in endothelial dysfunction induced by aldosterone in normotensive and hypertensive rats. Hypertension 2005, 46, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Feletou, M.; Huang, Y.; Vanhoutte, P.M. Endothelium-mediated control of vascular tone: COX-1 and COX-2 products. Br. J. Pharmacol. 2011, 164, 894–912. [Google Scholar] [CrossRef] [Green Version]
- Leopold, J.A.; Dam, A.; Maron, B.A.; Scribner, A.W.; Liao, R.; Handy, D.E.; Stanton, R.C.; Pitt, B.; Loscalzo, J. Aldosterone impairs vascular reactivity by decreasing glucose-6-phosphate dehydrogenase activity. Nat. Med. 2007, 13, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Oberleithner, H.; Riethmuller, C.; Ludwig, T.; Hausberg, M.; Schillers, H. Aldosterone remodels human endothelium. Acta Physiol. 2006, 187, 305–312. [Google Scholar] [CrossRef]
- Jia, G.; Habibi, J.; Aroor, A.R.; Hill, M.A.; Yang, Y.; Whaley-Connell, A.; Jaisser, F.; Sowers, J.R. Epithelial Sodium Channel in Aldosterone-Induced Endothelium Stiffness and Aortic Dysfunction. Hypertension 2018, 72, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Jeggle, P.; Callies, C.; Tarjus, A.; Fassot, C.; Fels, J.; Oberleithner, H.; Jaisser, F.; Kusche-Vihrog, K. Epithelial sodium channel stiffens the vascular endothelium in vitro and in Liddle mice. Hypertension 2013, 61, 1053–1059. [Google Scholar] [CrossRef] [Green Version]
- Bienvenu, L.A.; Bell, J.R.; Weeks, K.L.; Delbridge, L.M.D.; Young, M.J. New Perspectives on Sex Steroid and Mineralocorticoid Receptor Signaling in Cardiac Ischemic Injury. Front. Physiol. 2022, 13, 896425. [Google Scholar] [CrossRef]
- Mueller, K.B.; Bender, S.B.; Hong, K.; Yang, Y.; Aronovitz, M.; Jaisser, F.; Hill, M.A.; Jaffe, I.Z. Endothelial Mineralocorticoid Receptors Differentially Contribute to Coronary and Mesenteric Vascular Function Without Modulating Blood Pressure. Hypertension 2015, 66, 988–997. [Google Scholar] [CrossRef] [Green Version]
- Davel, A.P.; Jaffe, I.Z.; Tostes, R.C.; Jaisser, F.; Belin de Chantemele, E.J. New roles of aldosterone and mineralocorticoid receptors in cardiovascular disease: Translational and sex-specific effects. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H989–H999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davel, A.P.; Lu, Q.; Moss, M.E.; Rao, S.; Anwar, I.J.; DuPont, J.J.; Jaffe, I.Z. Sex-Specific Mechanisms of Resistance Vessel Endothelial Dysfunction Induced by Cardiometabolic Risk Factors. J. Am. Heart Assoc. 2018, 7, e007675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannemann, A.; Wallaschofski, H.; Ludemann, J.; Volzke, H.; Markus, M.R.; Rettig, R.; Lendeckel, U.; Reincke, M.; Felix, S.B.; Empen, K.; et al. Plasma aldosterone levels and aldosterone-to-renin ratios are associated with endothelial dysfunction in young to middle-aged subjects. Atherosclerosis 2011, 219, 875–879. [Google Scholar] [CrossRef]
- Nishizaka, M.K.; Zaman, M.A.; Green, S.A.; Renfroe, K.Y.; Calhoun, D.A. Impaired endothelium-dependent flow-mediated vasodilation in hypertensive subjects with hyperaldosteronism. Circulation 2004, 109, 2857–2861. [Google Scholar] [CrossRef] [Green Version]
- Thum, T.; Schmitter, K.; Fleissner, F.; Wiebking, V.; Dietrich, B.; Widder, J.D.; Jazbutyte, V.; Hahner, S.; Ertl, G.; Bauersachs, J. Impairment of endothelial progenitor cell function and vascularization capacity by aldosterone in mice and humans. Eur. Heart J. 2011, 32, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.C.; Lo, S.C.; Chen, Y.L.; Huang, P.H.; Tsai, C.T.; Liang, C.J.; Kuo, C.C.; Kuo, Y.S.; Lee, B.C.; Wu, E.L.; et al. Endothelial progenitor cells in primary aldosteronism: A biomarker of severity for aldosterone vasculopathy and prognosis. J. Clin. Endocrinol. Metab. 2011, 96, 3175–3183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, T.; Oki, K.; Kajikawa, M.; Nakashima, A.; Maruhashi, T.; Iwamoto, Y.; Iwamoto, A.; Oda, N.; Hidaka, T.; Kihara, Y.; et al. Effect of aldosterone-producing adenoma on endothelial function and Rho-associated kinase activity in patients with primary aldosteronism. Hypertension 2015, 65, 841–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakima, A.; Arima, H.; Matayoshi, T.; Ishida, A.; Ohya, Y. Effect of Mineralocorticoid Receptor Blockade on Arterial Stiffness and Endothelial Function: A Meta-Analysis of Randomized Trials. Hypertension 2021, 77, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Stockand, J.D.; Meszaros, J.G. Aldosterone stimulates proliferation of cardiac fibroblasts by activating Ki-RasA and MAPK1/2 signaling. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H176–H184. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, J.A.; Kim, K.L.; Jang, H.S.; Kim, J.M.; Lee, J.Y.; Shin, I.S.; Lee, J.S.; Suh, W.; Choi, J.H.; et al. Aldosterone upregulates connective tissue growth factor gene expression via p38 MAPK pathway and mineralocorticoid receptor in ventricular myocytes. J. Korean Med. Sci. 2004, 19, 805–811. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.F.; Yang, S.F.; Chu, H.J.; Ueng, K.C. Cross-talk between mineralocorticoid receptor/angiotensin II type 1 receptor and mitogen-activated protein kinase pathways underlies aldosterone-induced atrial fibrotic responses in HL-1 cardiomyocytes. Int. J. Cardiol. 2013, 169, 17–28. [Google Scholar] [CrossRef] [PubMed]
- De Giusti, V.C.; Nolly, M.B.; Yeves, A.M.; Caldiz, C.I.; Villa-Abrille, M.C.; Chiappe de Cingolani, G.E.; Ennis, I.L.; Cingolani, H.E.; Aiello, E.A. Aldosterone stimulates the cardiac Na(+)/H(+) exchanger via transactivation of the epidermal growth factor receptor. Hypertension 2011, 58, 912–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallon, V.; Wyatt, A.W.; Klingel, K.; Huang, D.Y.; Hussain, A.; Berchtold, S.; Friedrich, B.; Grahammer, F.; Belaiba, R.S.; Gorlach, A.; et al. SGK1-dependent cardiac CTGF formation and fibrosis following DOCA treatment. J. Mol. Med. 2006, 84, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Azibani, F.; Benard, L.; Schlossarek, S.; Merval, R.; Tournoux, F.; Fazal, L.; Polidano, E.; Launay, J.M.; Carrier, L.; Chatziantoniou, C.; et al. Aldosterone inhibits antifibrotic factors in mouse hypertensive heart. Hypertension 2012, 59, 1179–1187. [Google Scholar] [CrossRef] [Green Version]
- Fraccarollo, D.; Berger, S.; Galuppo, P.; Kneitz, S.; Hein, L.; Schutz, G.; Frantz, S.; Ertl, G.; Bauersachs, J. Deletion of cardiomyocyte mineralocorticoid receptor ameliorates adverse remodeling after myocardial infarction. Circulation 2011, 123, 400–408. [Google Scholar] [CrossRef] [Green Version]
- Lacolley, P.; Challande, P.; Osborne-Pellegrin, M.; Regnault, V. Genetics and pathophysiology of arterial stiffness. Cardiovasc. Res. 2009, 81, 637–648. [Google Scholar] [CrossRef] [Green Version]
- McCurley, A.; Pires, P.W.; Bender, S.B.; Aronovitz, M.; Zhao, M.J.; Metzger, D.; Chambon, P.; Hill, M.A.; Dorrance, A.M.; Mendelsohn, M.E.; et al. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat. Med. 2012, 18, 1429–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvier, L.; Miana, M.; Reboul, P.; Cachofeiro, V.; Martinez-Martinez, E.; de Boer, R.A.; Poirier, F.; Lacolley, P.; Zannad, F.; Rossignol, P.; et al. Galectin-3 mediates aldosterone-induced vascular fibrosis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Tarjus, A.; Martinez-Martinez, E.; Amador, C.; Latouche, C.; El Moghrabi, S.; Berger, T.; Mak, T.W.; Fay, R.; Farman, N.; Rossignol, P.; et al. Neutrophil Gelatinase-Associated Lipocalin, a Novel Mineralocorticoid Biotarget, Mediates Vascular Profibrotic Effects of Mineralocorticoids. Hypertension 2015, 66, 158–166. [Google Scholar] [CrossRef]
- Harvey, A.P.; Montezano, A.C.; Hood, K.Y.; Lopes, R.A.; Rios, F.; Ceravolo, G.; Graham, D.; Touyz, R.M. Vascular dysfunction and fibrosis in stroke-prone spontaneously hypertensive rats: The aldosterone-mineralocorticoid receptor-Nox1 axis. Life Sci. 2017, 179, 110–119. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, K.; Chen, J.; Wang, M.H.; Wang, J.; Liu, P.; Huang, H. Roles of aldosterone in vascular calcification: An update. Eur. J. Pharmacol. 2016, 786, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.W.; He, W.B.; Xie, C.M.; Gao, M.; Feng, L.Y.; Liu, Z.Y.; Wang, J.F.; Huang, H.; Liu, P.M. Aldosterone enhances high phosphate-induced vascular calcification through inhibition of AMPK-mediated autophagy. J. Cell Mol. Med. 2020, 24, 13648–13659. [Google Scholar] [CrossRef] [PubMed]
- Voelkl, J.; Alesutan, I.; Leibrock, C.B.; Quintanilla-Martinez, L.; Kuhn, V.; Feger, M.; Mia, S.; Ahmed, M.S.; Rosenblatt, K.P.; Kuro, O.M.; et al. Spironolactone ameliorates PIT1-dependent vascular osteoinduction in klotho-hypomorphic mice. J. Clin. Investig. 2013, 123, 812–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaffe, I.Z.; Tintut, Y.; Newfell, B.G.; Demer, L.L.; Mendelsohn, M.E. Mineralocorticoid receptor activation promotes vascular cell calcification. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 799–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatsumoto, N.; Yamada, S.; Tokumoto, M.; Eriguchi, M.; Noguchi, H.; Torisu, K.; Tsuruya, K.; Kitazono, T. Spironolactone ameliorates arterial medial calcification in uremic rats: The role of mineralocorticoid receptor signaling in vascular calcification. Am. J. Physiol. Renal. Physiol. 2015, 309, F967–F979. [Google Scholar] [CrossRef] [Green Version]
- Gkaliagkousi, E.; Anyfanti, P.; Triantafyllou, A.; Gavriilaki, E.; Nikolaidou, B.; Lazaridis, A.; Vamvakis, A.; Douma, S. Aldosterone as a mediator of microvascular and macrovascular damage in a population of normotensive to early-stage hypertensive individuals. J. Am. Soc. Hypertens. 2018, 12, 50–57. [Google Scholar] [CrossRef]
- Mahmud, A.; Feely, J. Aldosterone-to-renin ratio, arterial stiffness, and the response to aldosterone antagonism in essential hypertension. Am. J. Hypertens. 2005, 18, 50–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambrosino, P.; Lupoli, R.; Tortora, A.; Cacciapuoti, M.; Lupoli, G.A.; Tarantino, P.; Nasto, A.; Di Minno, M.N. Cardiovascular risk markers in patients with primary aldosteronism: A systematic review and meta-analysis of literature studies. Int. J. Cardiol. 2016, 208, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Strauch, B.; Petrak, O.; Zelinka, T.; Wichterle, D.; Holaj, R.; Kasalicky, M.; Safarik, L.; Rosa, J.; Widimsky, J., Jr. Adrenalectomy improves arterial stiffness in primary aldosteronism. Am. J. Hypertens. 2008, 21, 1086–1092. [Google Scholar] [CrossRef] [Green Version]
- Yaribeygi, H.; Farrokhi, F.R.; Butler, A.E.; Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell Physiol. 2019, 234, 8152–8161. [Google Scholar] [CrossRef]
- Ginsberg, H.N. Insulin resistance and cardiovascular disease. J. Clin. Investig. 2000, 106, 453–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeMarco, V.G.; Habibi, J.; Jia, G.; Aroor, A.R.; Ramirez-Perez, F.I.; Martinez-Lemus, L.A.; Bender, S.B.; Garro, M.; Hayden, M.R.; Sun, Z.; et al. Low-Dose Mineralocorticoid Receptor Blockade Prevents Western Diet-Induced Arterial Stiffening in Female Mice. Hypertension 2015, 66, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Habibi, J.; Chen, D.; Hulse, J.L.; Whaley-Connell, A.; Sowers, J.R.; Jia, G. Targeting mineralocorticoid receptors in diet-induced hepatic steatosis and insulin resistance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022, 322, R253–R262. [Google Scholar] [CrossRef]
- Jia, G.; Aroor, A.R.; Sowers, J.R. The role of mineralocorticoid receptor signaling in the cross-talk between adipose tissue and the vascular wall. Cardiovasc. Res. 2017, 113, 1055–1063. [Google Scholar] [CrossRef] [Green Version]
- Jia, G.; Habibi, J.; Aroor, A.R.; Martinez-Lemus, L.A.; DeMarco, V.G.; Ramirez-Perez, F.I.; Sun, Z.; Hayden, M.R.; Meininger, G.A.; Mueller, K.B.; et al. Endothelial Mineralocorticoid Receptor Mediates Diet-Induced Aortic Stiffness in Females. Circ. Res. 2016, 118, 935–943. [Google Scholar] [CrossRef] [Green Version]
- Igbekele, A.E.; Jia, G.; Hill, M.A.; Sowers, J.R.; Jia, G. Mineralocorticoid Receptor Activation in Vascular Insulin Resistance and Dysfunction. Int. J. Mol. Sci. 2022, 23, 8954. [Google Scholar] [CrossRef]
- Jia, G.; Lockette, W.; Sowers, J.R. Mineralocorticoid receptors in the pathogenesis of insulin resistance and related disorders: From basic studies to clinical disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 320, R276–R286. [Google Scholar] [CrossRef]
- Preiss, D.; Zetterstrand, S.; McMurray, J.J.; Ostergren, J.; Michelson, E.L.; Granger, C.B.; Yusuf, S.; Swedberg, K.; Pfeffer, M.A.; Gerstein, H.C.; et al. Predictors of development of diabetes in patients with chronic heart failure in the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity (CHARM) program. Diabetes Care 2009, 32, 915–920. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.V.; Xu, L.; Lin, S.L.; Schooling, C.M. Spironolactone and glucose metabolism, a systematic review and meta-analysis of randomized controlled trials. J. Am. Soc. Hypertens. 2016, 10, 671–682. [Google Scholar] [CrossRef]
- Rossing, P.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Chan, J.C.N.; Kooy, A.; McCafferty, K.; Schernthaner, G.; et al. Finerenone in Predominantly Advanced CKD and Type 2 Diabetes With or Without Sodium-Glucose Cotransporter-2 Inhibitor Therapy. Kidney Int. Rep. 2022, 7, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Lipscombe, D. L-type calcium channels: Highs and new lows. Circ. Res. 2002, 90, 933–935. [Google Scholar] [CrossRef] [Green Version]
- Boixel, C.; Gavillet, B.; Rougier, J.S.; Abriel, H. Aldosterone increases voltage-gated sodium current in ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H2257–H2266. [Google Scholar] [CrossRef]
- Aronsen, J.M.; Swift, F.; Sejersted, O.M. Cardiac sodium transport and excitation-contraction coupling. J. Mol. Cell Cardiol. 2013, 61, 11–19. [Google Scholar] [CrossRef]
- Matsui, S.; Satoh, H.; Kawashima, H.; Nagasaka, S.; Niu, C.F.; Urushida, T.; Katoh, H.; Watanabe, Y.; Hayashi, H. Non-genomic effects of aldosterone on intracellular ion regulation and cell volume in rat ventricular myocytes. Can. J. Physiol. Pharmacol. 2007, 85, 264–273. [Google Scholar] [CrossRef] [Green Version]
- Mattiazzi, A. Positive inotropic effect of angiotensin II. Increases in intracellular Ca2+ or changes in myofilament Ca2+ responsiveness? J. Pharmacol. Toxicol. Methods 1997, 37, 205–214. [Google Scholar] [CrossRef]
- Denham, N.C.; Pearman, C.M.; Caldwell, J.L.; Madders, G.W.P.; Eisner, D.A.; Trafford, A.W.; Dibb, K.M. Calcium in the Pathophysiology of Atrial Fibrillation and Heart Failure. Front. Physiol. 2018, 9, 1380. [Google Scholar] [CrossRef] [Green Version]
- Ouvrard-Pascaud, A.; Sainte-Marie, Y.; Benitah, J.P.; Perrier, R.; Soukaseum, C.; Nguyen Dinh Cat, A.; Royer, A.; Le Quang, K.; Charpentier, F.; Demolombe, S.; et al. Conditional mineralocorticoid receptor expression in the heart leads to life-threatening arrhythmias. Circulation 2005, 111, 3025–3033. [Google Scholar] [CrossRef] [Green Version]
- Diaz, R.G.; Perez, N.G.; Morgan, P.E.; Villa-Abrille, M.C.; Caldiz, C.I.; Nolly, M.B.; Portiansky, E.L.; Ennis, I.L.; Cingolani, H.E. Myocardial mineralocorticoid receptor activation by stretching and its functional consequences. Hypertension 2014, 63, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgozoglu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Jaffe, I.Z.; Mendelsohn, M.E. Angiotensin II and aldosterone regulate gene transcription via functional mineralocortocoid receptors in human coronary artery smooth muscle cells. Circ. Res. 2005, 96, 643–650. [Google Scholar] [CrossRef] [Green Version]
- Marzolla, V.; Armani, A.; Mammi, C.; Moss, M.E.; Pagliarini, V.; Pontecorvo, L.; Antelmi, A.; Fabbri, A.; Rosano, G.; Jaffe, I.Z.; et al. Essential role of ICAM-1 in aldosterone-induced atherosclerosis. Int. J. Cardiol. 2017, 232, 233–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, R.; Rudolph, A.E.; Frierdich, G.E.; Nachowiak, D.A.; Kekec, B.K.; Blomme, E.A.; McMahon, E.G.; Delyani, J.A. Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1802–H1810. [Google Scholar] [CrossRef]
- Keidar, S.; Hayek, T.; Kaplan, M.; Pavlotzky, E.; Hamoud, S.; Coleman, R.; Aviram, M. Effect of eplerenone, a selective aldosterone blocker, on blood pressure, serum and macrophage oxidative stress, and atherosclerosis in apolipoprotein E-deficient mice. J. Cardiovasc. Pharmacol. 2003, 41, 955–963. [Google Scholar] [CrossRef]
- Suzuki, J.; Iwai, M.; Mogi, M.; Oshita, A.; Yoshii, T.; Higaki, J.; Horiuchi, M. Eplerenone with valsartan effectively reduces atherosclerotic lesion by attenuation of oxidative stress and inflammation. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 917–921. [Google Scholar] [CrossRef] [Green Version]
- Gueret, A.; Harouki, N.; Favre, J.; Galmiche, G.; Nicol, L.; Henry, J.P.; Besnier, M.; Thuillez, C.; Richard, V.; Kolkhof, P.; et al. Vascular Smooth Muscle Mineralocorticoid Receptor Contributes to Coronary and Left Ventricular Dysfunction After Myocardial Infarction. Hypertension 2016, 67, 717–723. [Google Scholar] [CrossRef] [Green Version]
- Inoue, K.; Goldwater, D.; Allison, M.; Seeman, T.; Kestenbaum, B.R.; Watson, K.E. Serum Aldosterone Concentration, Blood Pressure, and Coronary Artery Calcium: The Multi-Ethnic Study of Atherosclerosis. Hypertension 2020, 76, 113–120. [Google Scholar] [CrossRef]
- Ivanes, F.; Susen, S.; Mouquet, F.; Pigny, P.; Cuilleret, F.; Sautiere, K.; Collet, J.P.; Beygui, F.; Hennache, B.; Ennezat, P.V.; et al. Aldosterone, mortality, and acute ischaemic events in coronary artery disease patients outside the setting of acute myocardial infarction or heart failure. Eur. Heart J. 2012, 33, 191–202. [Google Scholar] [CrossRef] [Green Version]
- Beygui, F.; Collet, J.P.; Benoliel, J.J.; Vignolles, N.; Dumaine, R.; Barthelemy, O.; Montalescot, G. High plasma aldosterone levels on admission are associated with death in patients presenting with acute ST-elevation myocardial infarction. Circulation 2006, 114, 2604–2610. [Google Scholar] [CrossRef] [Green Version]
- van der Heijden, C.; Smeets, E.M.M.; Aarntzen, E.; Noz, M.P.; Monajemi, H.; Kersten, S.; Kaffa, C.; Hoischen, A.; Deinum, J.; Joosten, L.A.B.; et al. Arterial Wall Inflammation and Increased Hematopoietic Activity in Patients With Primary Aldosteronism. J. Clin. Endocrinol. Metab. 2020, 105, e1967–e1980. [Google Scholar] [CrossRef]
- Bucerius, J.; Hyafil, F.; Verberne, H.J.; Slart, R.H.; Lindner, O.; Sciagra, R.; Agostini, D.; Ubleis, C.; Gimelli, A.; Hacker, M.; et al. Position paper of the Cardiovascular Committee of the European Association of Nuclear Medicine (EANM) on PET imaging of atherosclerosis. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 780–792. [Google Scholar] [CrossRef] [Green Version]
- Monticone, S.; D’Ascenzo, F.; Moretti, C.; Williams, T.A.; Veglio, F.; Gaita, F.; Mulatero, P. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018, 6, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Hundemer, G.L.; Curhan, G.C.; Yozamp, N.; Wang, M.; Vaidya, A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: A retrospective cohort study. Lancet Diabetes Endocrinol. 2018, 6, 51–59. [Google Scholar] [CrossRef]
- Montalescot, G.; Pitt, B.; Lopez de Sa, E.; Hamm, C.W.; Flather, M.; Verheugt, F.; Shi, H.; Turgonyi, E.; Orri, M.; Vincent, J.; et al. Early eplerenone treatment in patients with acute ST-elevation myocardial infarction without heart failure: The Randomized Double-Blind Reminder Study. Eur. Heart J. 2014, 35, 2295–2302. [Google Scholar] [CrossRef] [Green Version]
- Beygui, F.; Cayla, G.; Roule, V.; Roubille, F.; Delarche, N.; Silvain, J.; Van Belle, E.; Belle, L.; Galinier, M.; Motreff, P.; et al. Early Aldosterone Blockade in Acute Myocardial Infarction: The ALBATROSS Randomized Clinical Trial. J. Am. Coll Cardiol. 2016, 67, 1917–1927. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, G.; Anker, S.D.; Agarwal, R.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Schloemer, P.; Tornus, I.; Joseph, A.; et al. Finerenone and Cardiovascular Outcomes in Patients With Chronic Kidney Disease and Type 2 Diabetes. Circulation 2021, 143, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.D.; Frangogiannis, N.G. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef]
- Tsai, C.H.; Pan, C.T.; Chang, Y.Y.; Chen, Z.W.; Wu, V.C.; Hung, C.S.; Lin, Y.H. Left ventricular remodeling and dysfunction in primary aldosteronism. J. Hum. Hypertens. 2021, 35, 131–147. [Google Scholar] [CrossRef]
- He, B.J.; Joiner, M.L.; Singh, M.V.; Luczak, E.D.; Swaminathan, P.D.; Koval, O.M.; Kutschke, W.; Allamargot, C.; Yang, J.; Guan, X.; et al. Oxidation of CaMKII determines the cardiotoxic effects of aldosterone. Nat. Med. 2011, 17, 1610–1618. [Google Scholar] [CrossRef] [Green Version]
- Hung, C.S.; Chou, C.H.; Liao, C.W.; Lin, Y.T.; Wu, X.M.; Chang, Y.Y.; Chen, Y.H.; Wu, V.C.; Su, M.J.; Ho, Y.L.; et al. Aldosterone Induces Tissue Inhibitor of Metalloproteinases-1 Expression and Further Contributes to Collagen Accumulation: From Clinical to Bench Studies. Hypertension 2016, 67, 1309–1320. [Google Scholar] [CrossRef] [Green Version]
- Sakamuri, S.S.; Valente, A.J.; Siddesha, J.M.; Delafontaine, P.; Siebenlist, U.; Gardner, J.D.; Bysani, C. TRAF3IP2 mediates aldosterone/salt-induced cardiac hypertrophy and fibrosis. Mol. Cell Endocrinol. 2016, 429, 84–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Martinez, E.; Calvier, L.; Fernandez-Celis, A.; Rousseau, E.; Jurado-Lopez, R.; Rossoni, L.V.; Jaisser, F.; Zannad, F.; Rossignol, P.; Cachofeiro, V.; et al. Galectin-3 blockade inhibits cardiac inflammation and fibrosis in experimental hyperaldosteronism and hypertension. Hypertension 2015, 66, 767–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mummidi, S.; Das, N.A.; Carpenter, A.J.; Kandikattu, H.; Krenz, M.; Siebenlist, U.; Valente, A.J.; Chandrasekar, B. Metformin inhibits aldosterone-induced cardiac fibroblast activation, migration and proliferation in vitro, and reverses aldosterone+salt-induced cardiac fibrosis in vivo. J. Mol. Cell Cardiol. 2016, 98, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, J.; Lu, L.; Chen, S.S.; Quinn, M.T.; Weber, K.T. Aldosterone-induced inflammation in the rat heart: Role of oxidative stress. Am. J. Pathol. 2002, 161, 1773–1781. [Google Scholar] [CrossRef]
- Brilla, C.G.; Pick, R.; Tan, L.B.; Janicki, J.S.; Weber, K.T. Remodeling of the rat right and left ventricles in experimental hypertension. Circ. Res. 1990, 67, 1355–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fletcher, E.K.; Morgan, J.; Kennaway, D.R.; Bienvenu, L.A.; Rickard, A.J.; Delbridge, L.M.D.; Fuller, P.J.; Clyne, C.D.; Young, M.J. Deoxycorticosterone/Salt-Mediated Cardiac Inflammation and Fibrosis Are Dependent on Functional CLOCK Signaling in Male Mice. Endocrinology 2017, 158, 2906–2917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oestreicher, E.M.; Martinez-Vasquez, D.; Stone, J.R.; Jonasson, L.; Roubsanthisuk, W.; Mukasa, K.; Adler, G.K. Aldosterone and not plasminogen activator inhibitor-1 is a critical mediator of early angiotensin II/NG-nitro-L-arginine methyl ester-induced myocardial injury. Circulation 2003, 108, 2517–2523. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Andres, N.; Martin-Fernandez, B.; Rossignol, P.; Zannad, F.; Lahera, V.; Fortuno, M.A.; Cachofeiro, V.; Diez, J. A role for cardiotrophin-1 in myocardial remodeling induced by aldosterone. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2372–H2382. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.M.; Park, M.Y.; Suh, Y.L.; Park, J.B. NAD(P)H oxidase inhibitor prevents blood pressure elevation and cardiovascular hypertrophy in aldosterone-infused rats. Biochem. Biophys. Res. Commun. 2004, 313, 812–817. [Google Scholar] [CrossRef]
- Freel, E.M.; Mark, P.B.; Weir, R.A.; McQuarrie, E.P.; Allan, K.; Dargie, H.J.; McClure, J.D.; Jardine, A.G.; Davies, E.; Connell, J.M. Demonstration of blood pressure-independent noninfarct myocardial fibrosis in primary aldosteronism: A cardiac magnetic resonance imaging study. Circ. Cardiovasc. Imaging 2012, 5, 740–747. [Google Scholar] [CrossRef] [Green Version]
- Rossi, G.P.; Di Bello, V.; Ganzaroli, C.; Sacchetto, A.; Cesari, M.; Bertini, A.; Giorgi, D.; Scognamiglio, R.; Mariani, M.; Pessina, A.C. Excess aldosterone is associated with alterations of myocardial texture in primary aldosteronism. Hypertension 2002, 40, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Ohno, Y.; Sone, M.; Inagaki, N.; Kawashima, A.; Takeda, Y.; Yoneda, T.; Kurihara, I.; Itoh, H.; Tsuiki, M.; Ichijo, T.; et al. Nadir Aldosterone Levels After Confirmatory Tests Are Correlated With Left Ventricular Hypertrophy in Primary Aldosteronism. Hypertension 2020, 75, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.W.; Huang, K.C.; Lee, J.K.; Lin, L.C.; Chen, C.W.; Chang, Y.Y.; Liao, C.W.; Wu, V.C.; Hung, C.S.; Lin, Y.H.; et al. Aldosterone induces left ventricular subclinical systolic dysfunction: A strain imaging study. J. Hypertens. 2018, 36, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.P.; Sacchetto, A.; Visentin, P.; Canali, C.; Graniero, G.R.; Palatini, P.; Pessina, A.C. Changes in left ventricular anatomy and function in hypertension and primary aldosteronism. Hypertension 1996, 27, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Rev. Esp. Cardiol. 2022, 75, 523. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, P.; Cleland, J.G.; Bhandari, S.; Tala, S.; Gustafsson, F.; Fay, R.; Lamiral, Z.; Dobre, D.; Pitt, B.; Zannad, F. Determinants and consequences of renal function variations with aldosterone blocker therapy in heart failure patients after myocardial infarction: Insights from the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study. Circulation 2012, 125, 271–279. [Google Scholar] [CrossRef]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef] [Green Version]
- Filippatos, G.; Anker, S.D.; Bohm, M.; Gheorghiade, M.; Kober, L.; Krum, H.; Maggioni, A.P.; Ponikowski, P.; Voors, A.A.; Zannad, F.; et al. A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur. Heart J. 2016, 37, 2105–2114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosmala, W.; Rojek, A.; Przewlocka-Kosmala, M.; Wright, L.; Mysiak, A.; Marwick, T.H. Effect of Aldosterone Antagonism on Exercise Tolerance in Heart Failure With Preserved Ejection Fraction. J. Am. Coll Cardiol. 2016, 68, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Edelmann, F.; Wachter, R.; Schmidt, A.G.; Kraigher-Krainer, E.; Colantonio, C.; Kamke, W.; Duvinage, A.; Stahrenberg, R.; Durstewitz, K.; Loffler, M.; et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: The Aldo-DHF randomized controlled trial. JAMA 2013, 309, 781–791. [Google Scholar] [CrossRef] [Green Version]
- Pitt, B.; Pfeffer, M.A.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Claggett, B.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; et al. Spironolactone for heart failure with preserved ejection fraction. N. Engl. J. Med. 2014, 370, 1383–1392. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, J.P.; Cleland, J.G.; Girerd, N.; Bozec, E.; Rossignol, P.; Pellicori, P.; Cosmi, F.; Mariottoni, B.; Solomon, S.D.; Pitt, B.; et al. Spironolactone effect on cardiac structure and function of patients with heart failure and preserved ejection fraction: A pooled analysis of three randomized trials. Eur. J. Heart Fail 2022. Online Version of Record. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.M.; Rueda, A.; Sainte-Marie, Y.; Pereira, L.; Zissimopoulos, S.; Zhu, X.; Schaub, R.; Perrier, E.; Perrier, R.; Latouche, C.; et al. Mineralocorticoid modulation of cardiac ryanodine receptor activity is associated with downregulation of FK506-binding proteins. Circulation 2009, 119, 2179–2187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benitah, J.P.; Perrier, E.; Gomez, A.M.; Vassort, G. Effects of aldosterone on transient outward K+ current density in rat ventricular myocytes. J. Physiol. 2001, 537, 151–160. [Google Scholar] [CrossRef]
- Reil, J.C.; Hohl, M.; Selejan, S.; Lipp, P.; Drautz, F.; Kazakow, A.; Munz, B.M.; Muller, P.; Steendijk, P.; Reil, G.H.; et al. Aldosterone promotes atrial fibrillation. Eur. Heart J. 2012, 33, 2098–2108. [Google Scholar] [CrossRef] [Green Version]
- Lammers, C.; Dartsch, T.; Brandt, M.C.; Rottlander, D.; Halbach, M.; Peinkofer, G.; Ockenpoehler, S.; Weiergraeber, M.; Schneider, T.; Reuter, H.; et al. Spironolactone prevents aldosterone induced increased duration of atrial fibrillation in rat. Cell Physiol. Biochem. 2012, 29, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, Y.; Ramirez, R.J.; Kaur, K.; Salvador-Montanes, O.; Ponce-Balbuena, D.; Ramos-Mondragon, R.; Ennis, S.R.; Guerrero-Serna, G.; Berenfeld, O.; Jalife, J. Eplerenone Reduces Atrial Fibrillation Burden Without Preventing Atrial Electrical Remodeling. J. Am. Coll Cardiol. 2017, 70, 2893–2905. [Google Scholar] [CrossRef]
- Mulatero, P.; Monticone, S.; Deinum, J.; Amar, L.; Prejbisz, A.; Zennaro, M.C.; Beuschlein, F.; Rossi, G.P.; Nishikawa, T.; Morganti, A.; et al. Genetics, prevalence, screening and confirmation of primary aldosteronism: A position statement and consensus of the Working Group on Endocrine Hypertension of The European Society of Hypertension. J. Hypertens. 2020, 38, 1919–1928. [Google Scholar] [CrossRef]
- Seccia, T.M.; Letizia, C.; Muiesan, M.L.; Lerco, S.; Cesari, M.; Bisogni, V.; Petramala, L.; Maiolino, G.; Volpin, R.; Rossi, G.P. Atrial fibrillation as presenting sign of primary aldosteronism: Results of the Prospective Appraisal on the Prevalence of Primary Aldosteronism in Hypertensive (PAPPHY) Study. J. Hypertens. 2020, 38, 332–339. [Google Scholar] [CrossRef]
- Rossi, G.P.; Maiolino, G.; Flego, A.; Belfiore, A.; Bernini, G.; Fabris, B.; Ferri, C.; Giacchetti, G.; Letizia, C.; Maccario, M.; et al. Adrenalectomy Lowers Incident Atrial Fibrillation in Primary Aldosteronism Patients at Long Term. Hypertension 2018, 71, 585–591. [Google Scholar] [CrossRef]
- Swedberg, K.; Zannad, F.; McMurray, J.J.; Krum, H.; van Veldhuisen, D.J.; Shi, H.; Vincent, J.; Pitt, B.; Investigators, E.-H.S. Eplerenone and atrial fibrillation in mild systolic heart failure: Results from the EMPHASIS-HF (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure) study. J. Am. Coll Cardiol. 2012, 59, 1598–1603. [Google Scholar] [CrossRef] [Green Version]
- Rienstra, M.; Hobbelt, A.H.; Alings, M.; Tijssen, J.G.P.; Smit, M.D.; Brugemann, J.; Geelhoed, B.; Tieleman, R.G.; Hillege, H.L.; Tukkie, R.; et al. Targeted therapy of underlying conditions improves sinus rhythm maintenance in patients with persistent atrial fibrillation: Results of the RACE 3 trial. Eur. Heart J. 2018, 39, 2987–2996. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstrom-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Buffolo, F.; Tetti, M.; Mulatero, P.; Monticone, S. Aldosterone as a Mediator of Cardiovascular Damage. Hypertension 2022, 79, 1899–1911. [Google Scholar] [CrossRef] [PubMed]
- Funder, J.W.; Carey, R.M.; Mantero, F.; Murad, M.H.; Reincke, M.; Shibata, H.; Stowasser, M.; Young, W.F., Jr. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol. Metab. 2016, 101, 1889–1916. [Google Scholar] [CrossRef]
- Barcelo, A.; Pierola, J.; Esquinas, C.; de la Pena, M.; Arque, M.; Alonso-Fernandez, A.; Bauca, J.M.; Robles, J.; Barcelo, B.; Barbe, F. Relationship between aldosterone and the metabolic syndrome in patients with obstructive sleep apnea hypopnea syndrome: Effect of continuous positive airway pressure treatment. PLoS ONE 2014, 9, e84362. [Google Scholar] [CrossRef]
- Marzolla, V.; Armani, A.; Zennaro, M.C.; Cinti, F.; Mammi, C.; Fabbri, A.; Rosano, G.M.; Caprio, M. The role of the mineralocorticoid receptor in adipocyte biology and fat metabolism. Mol. Cell Endocrinol. 2012, 350, 281–288. [Google Scholar] [CrossRef]
- Raff, H.; Roarty, T.P. Renin, ACTH, and aldosterone during acute hypercapnia and hypoxia in conscious rats. Am. J. Physiol. 1988, 254, R431–R435. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, L.; Guo, L. Changes of aldosterone levels in patients with type 2 diabetes complicated by moderate to severe obstructive sleep apnea-hypopnea syndrome before and after treatment with continuous positive airway pressure. J. Int. Med. Res. 2019, 47, 4723–4733. [Google Scholar] [CrossRef]
- Svatikova, A.; Olson, L.J.; Wolk, R.; Phillips, B.G.; Adachi, T.; Schwartz, G.L.; Somers, V.K. Obstructive sleep apnea and aldosterone. Sleep 2009, 32, 1589–1592. [Google Scholar] [CrossRef] [Green Version]
- Wolk, R.; Shamsuzzaman, A.S.; Somers, V.K. Obesity, sleep apnea, and hypertension. Hypertension 2003, 42, 1067–1074. [Google Scholar] [CrossRef]
- Vecchiola, A.; Lagos, C.F.; Carvajal, C.A.; Baudrand, R.; Fardella, C.E. Aldosterone Production and Signaling Dysregulation in Obesity. Curr. Hypertens. Rep. 2016, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Goodfriend, T.L.; Egan, B.M.; Kelley, D.E. Plasma aldosterone, plasma lipoproteins, obesity and insulin resistance in humans. Prostaglandins. Leukot. Essent. Fatty Acids 1999, 60, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Gonzaga, C.C.; Gaddam, K.K.; Ahmed, M.I.; Pimenta, E.; Thomas, S.J.; Harding, S.M.; Oparil, S.; Cofield, S.S.; Calhoun, D.A. Severity of obstructive sleep apnea is related to aldosterone status in subjects with resistant hypertension. J. Clin. Sleep Med. 2010, 6, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Pratt-Ubunama, M.N.; Nishizaka, M.K.; Boedefeld, R.L.; Cofield, S.S.; Harding, S.M.; Calhoun, D.A. Plasma aldosterone is related to severity of obstructive sleep apnea in subjects with resistant hypertension. Chest 2007, 131, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Dudenbostel, T.; Calhoun, D.A. Resistant hypertension, obstructive sleep apnoea and aldosterone. J. Hum. Hypertens. 2012, 26, 281–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.H.; Yu, Y.; Kang, Y.M.; Wei, S.G.; Felder, R.B. Aldosterone acts centrally to increase brain renin-angiotensin system activity and oxidative stress in normal rats. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1067–H1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krasinska, B.; Cofta, S.; Szczepaniak-Chichel, L.; Rzymski, P.; Trafas, T.; Paluszkiewicz, L.; Tykarski, A.; Krasinski, Z. The Effects of Eplerenone on the Circadian Blood Pressure Pattern and Left Ventricular Hypertrophy in Patients with Obstructive Sleep Apnea and Resistant Hypertension-A Randomized, Controlled Trial. J. Clin. Med. 2019, 8, 1671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krasinska, B.; Miazga, A.; Cofta, S.; Szczepaniak-Chichel, L.; Trafas, T.; Krasinski, Z.; Pawlaczyk-Gabriel, K.; Tykarski, A. Effect of eplerenone on the severity of obstructive sleep apnea and arterial stiffness in patients with resistant arterial hypertension. Pol. Arch. Med. Wewn 2016, 126, 330–339. [Google Scholar] [CrossRef] [Green Version]
- Rodenstein, D.O.; D’Odemont, J.P.; Pieters, T.; Aubert-Tulkens, G. Diurnal and nocturnal diuresis and natriuresis in obstructive sleep apnea. Effects of nasal continuous positive airway pressure therapy. Am. Rev. Respir. Dis. 1992, 145, 1367–1371. [Google Scholar] [CrossRef]
- Pedrosa, R.P.; Drager, L.F.; de Paula, L.K.G.; Amaro, A.C.S.; Bortolotto, L.A.; Lorenzi-Filho, G. Effects of OSA treatment on BP in patients with resistant hypertension: A randomized trial. Chest 2013, 144, 1487–1494. [Google Scholar] [CrossRef]
- Moller, D.S.; Lind, P.; Strunge, B.; Pedersen, E.B. Abnormal vasoactive hormones and 24-hour blood pressure in obstructive sleep apnea. Am. J. Hypertens. 2003, 16, 274–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meston, N.; Davies, R.J.; Mullins, R.; Jenkinson, C.; Wass, J.A.; Stradling, J.R. Endocrine effects of nasal continuous positive airway pressure in male patients with obstructive sleep apnoea. J. Intern. Med. 2003, 254, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Joyeux-Faure, M.; Baguet, J.P.; Barone-Rochette, G.; Faure, P.; Sosner, P.; Mounier-Vehier, C.; Levy, P.; Tamisier, R.; Pepin, J.L. Continuous Positive Airway Pressure Reduces Night-Time Blood Pressure and Heart Rate in Patients With Obstructive Sleep Apnea and Resistant Hypertension: The RHOOSAS Randomized Controlled Trial. Front. Neurol. 2018, 9, 318. [Google Scholar] [CrossRef] [PubMed] [Green Version]

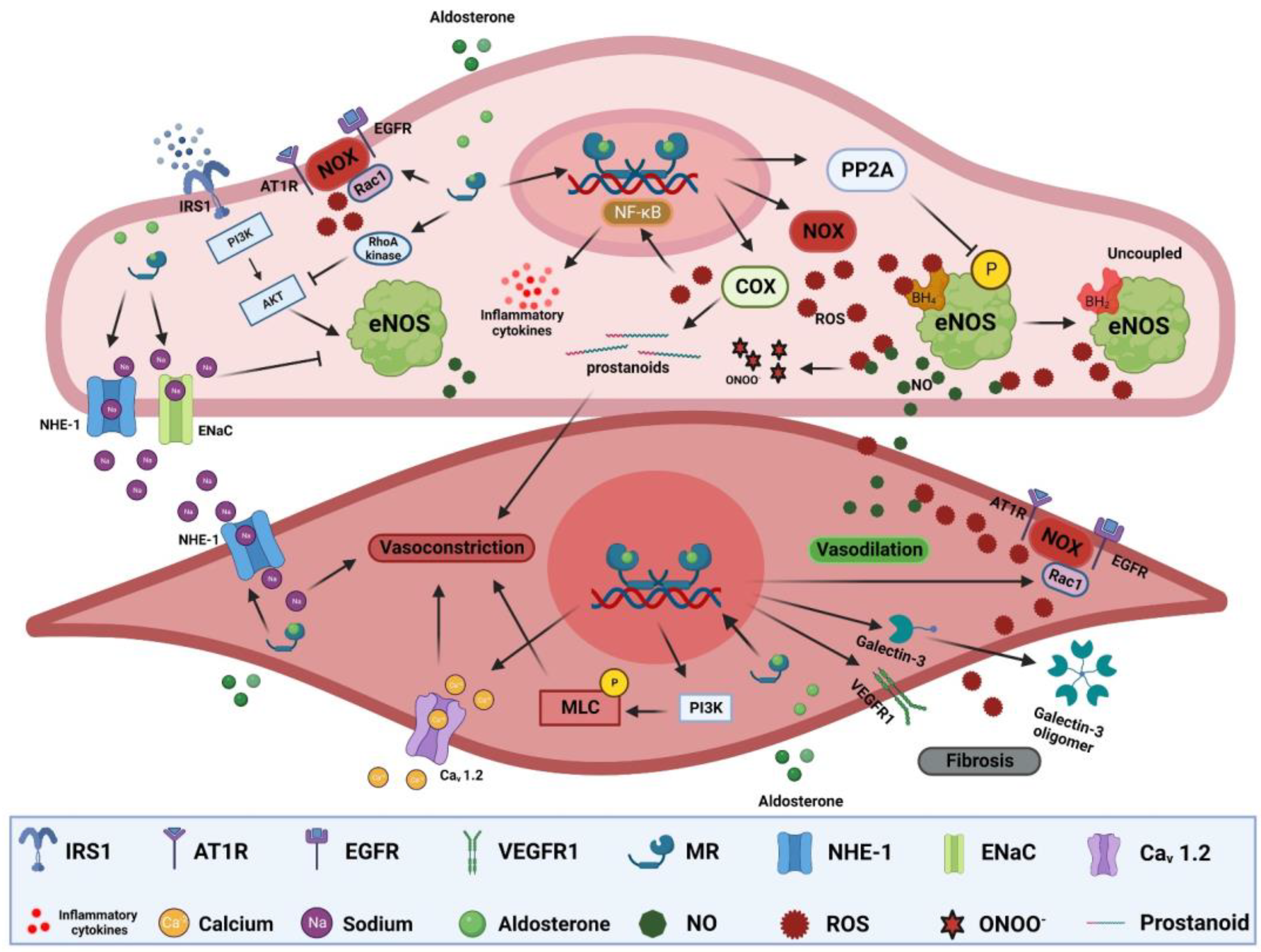
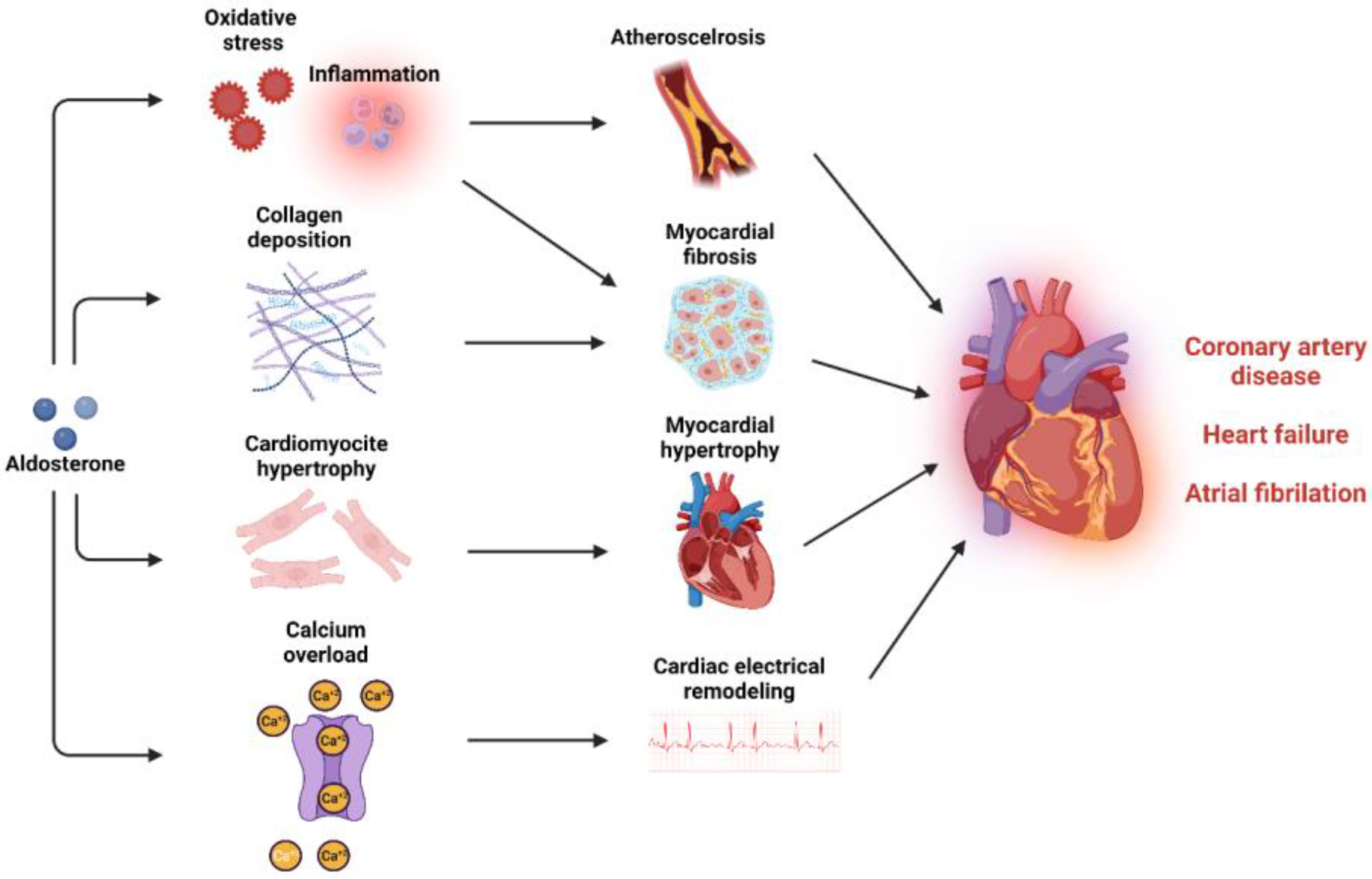

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badran, M.; Bender, S.B.; Gozal, D. Cardiovascular Disease in Obstructive Sleep Apnea: Putative Contributions of Mineralocorticoid Receptors. Int. J. Mol. Sci. 2023, 24, 2245. https://doi.org/10.3390/ijms24032245
Badran M, Bender SB, Gozal D. Cardiovascular Disease in Obstructive Sleep Apnea: Putative Contributions of Mineralocorticoid Receptors. International Journal of Molecular Sciences. 2023; 24(3):2245. https://doi.org/10.3390/ijms24032245
Chicago/Turabian StyleBadran, Mohammad, Shawn B. Bender, and David Gozal. 2023. "Cardiovascular Disease in Obstructive Sleep Apnea: Putative Contributions of Mineralocorticoid Receptors" International Journal of Molecular Sciences 24, no. 3: 2245. https://doi.org/10.3390/ijms24032245






