Increased Hemichannel Activity Displayed by a Connexin43 Mutation Causing a Familial Connexinopathy Exhibiting Hypotrichosis with Follicular Keratosis and Hyperostosis
Abstract
1. Introduction
2. Results
2.1. Cx43-G38E Is Translated in Xenopus Oocytes at Higher Levels Than Wild-Type Cx43
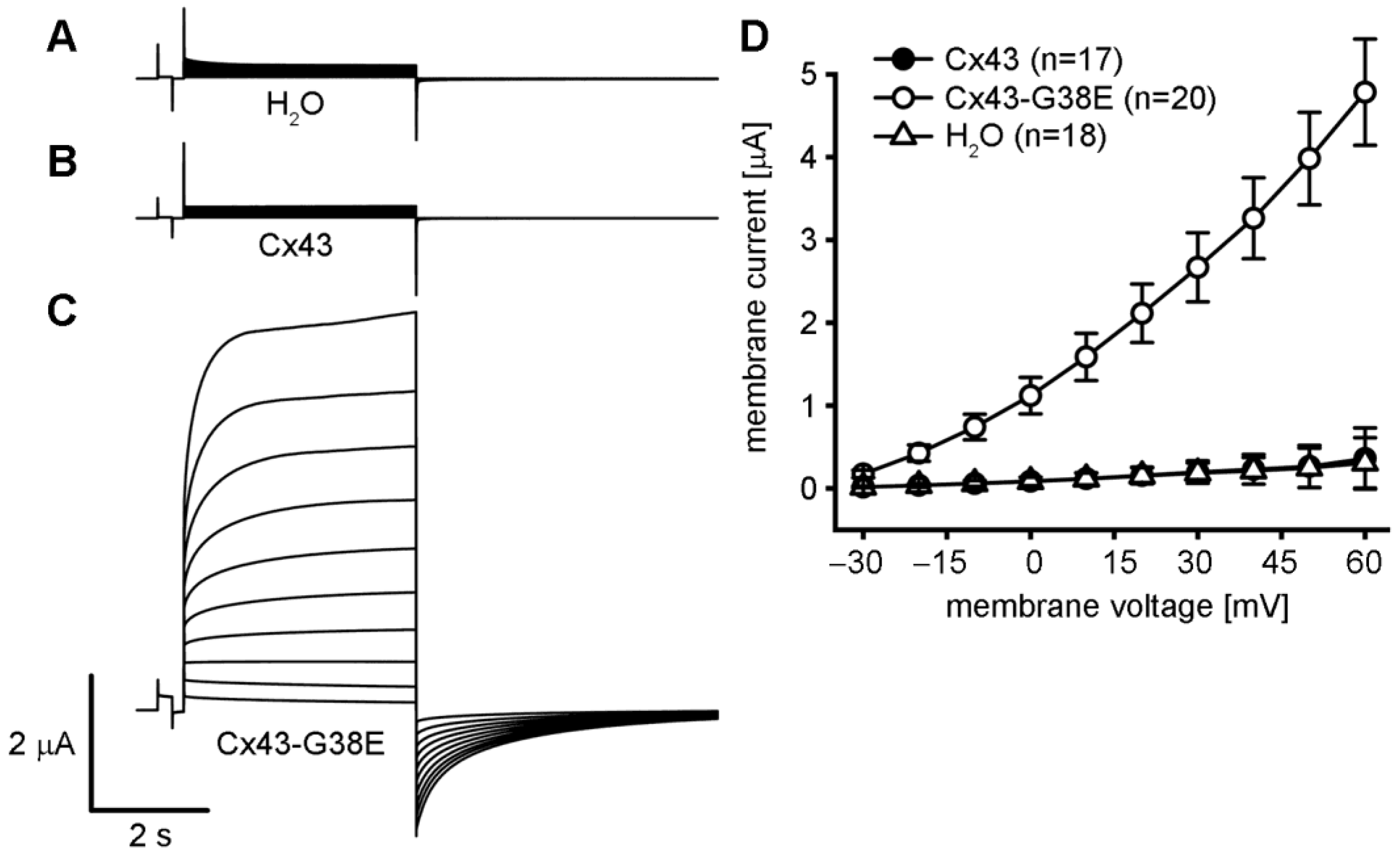
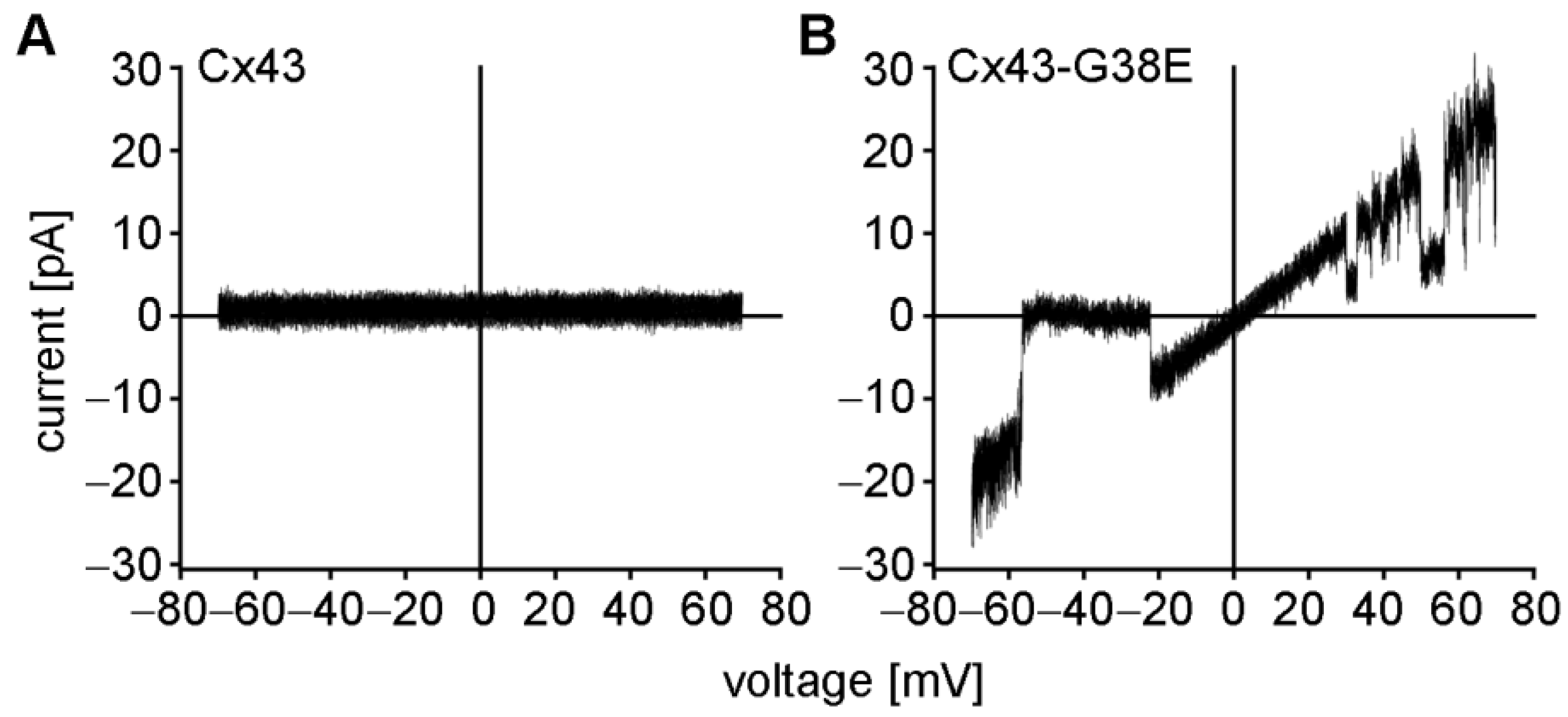
2.2. The Cx43-G38E Mutation Displays Increased Hemichannel Activity
2.3. The Cx43-G38E Mutation Localized to Cellular Interfaces in Transfected HeLa Cells
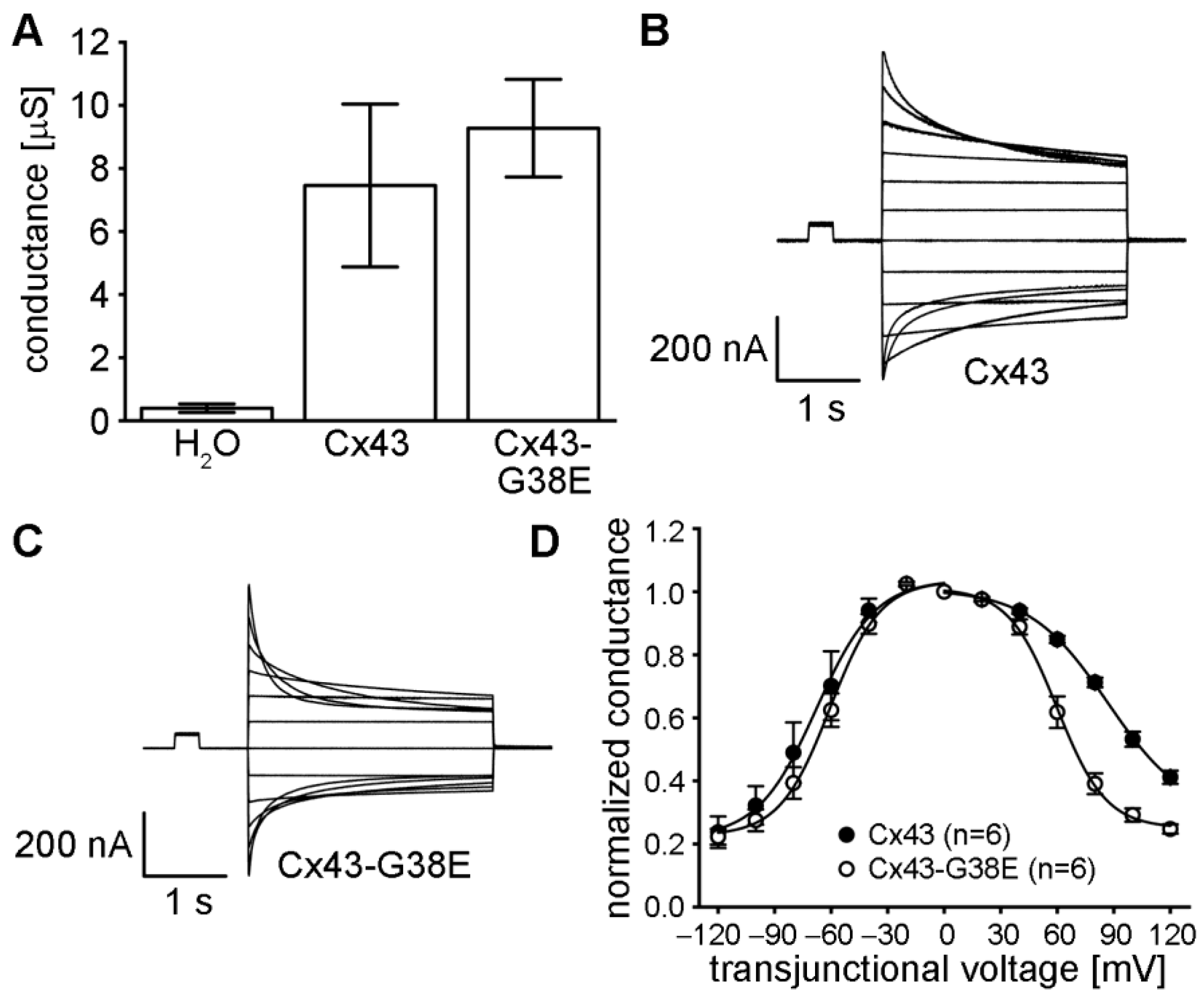
2.4. The Cx43-G38E Mutation Forms Functional Gap Junction Channels That Display Altered Voltage Gating
3. Discussion
4. Materials and Methods
4.1. Molecular Cloning
4.2. In Vitro Transcription and Xenopus Oocyte Preparation
4.3. Hemichannel Current Recording
4.4. Measurement of Gap Junctional Conductance
4.5. Western Blotting
4.6. Cell Transfection and Immunofluorescent Staining
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goodenough, D.A. Bulk isolation of mouse hepatocyte gap junctions. Characterization of the principal protein, connexin. J. Cell Biol. 1974, 61, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.M.; Gilula, N.B. Cloning and characterization of human and rat liver cDNAs coding for a gap junction protein. J. Cell Biol. 1986, 103, 767–776. [Google Scholar] [CrossRef] [PubMed]
- DeVries, S.H.; Schwartz, E.A. Hemi-gap-junction channels in solitary horizontal cells of the catfish retina. J. Physiol. 1992, 445, 201–230. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, L.; Steiner, E. Properties of a nonjunctional current expressed from a rat connexin46 cDNA in Xenopus oocytes. J. Gen. Physiol. 1993, 102, 59–74. [Google Scholar] [CrossRef]
- Trexler, E.B.; Bennett, M.V.; Bargiello, T.A.; Verselis, V.K. Voltage gating and permeation in a gap junction hemichannel. Proc. Natl. Acad. Sci. USA 1996, 93, 5836–5841. [Google Scholar] [CrossRef]
- Van Campenhout, R.; Gomes, A.R.; De Groof, T.W.M.; Muyldermans, S.; Devoogdt, N.; Vinken, M. Mechanisms Underlying Connexin Hemichannel Activation in Disease. Int. J. Mol. Sci. 2021, 22, 3503. [Google Scholar] [CrossRef]
- Lampe, P.D.; Laird, D.W. Recent advances in connexin gap junction biology. Fac. Rev. 2022, 11, 14. [Google Scholar] [CrossRef]
- Retamal, M.A.; Reyes, E.P.; Garcia, I.E.; Pinto, B.; Martinez, A.D.; Gonzalez, C. Diseases associated with leaky hemichannels. Front. Cell Neurosci. 2015, 9, 267. [Google Scholar] [CrossRef]
- Srinivas, M.; Verselis, V.K.; White, T.W. Human diseases associated with connexin mutations. Biochim. Biophys. Acta 2018, 1860, 192–201. [Google Scholar] [CrossRef]
- Delmar, M.; Laird, D.W.; Naus, C.C.; Nielsen, M.S.; Verselis, V.K.; White, T.W. Connexins and Disease. Cold Spring Harb. Perspect. Biol. 2018, 10, a029348. [Google Scholar] [CrossRef]
- Qiu, Y.; Zheng, J.; Chen, S.; Sun, Y. Connexin Mutations and Hereditary Diseases. Int. J. Mol. Sci. 2022, 23, 4255. [Google Scholar] [CrossRef]
- Garcia, I.E.; Bosen, F.; Mujica, P.; Pupo, A.; Flores-Munoz, C.; Jara, O.; Gonzalez, C.; Willecke, K.; Martinez, A.D. From Hyperactive Connexin26 Hemichannels to Impairments in Epidermal Calcium Gradient and Permeability Barrier in the Keratitis-Ichthyosis-Deafness Syndrome. J. Investig. Dermatol. 2016, 136, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Sellitto, C.; Li, L.; White, T.W. Connexin hemichannel inhibition ameliorates epidermal pathology in a mouse model of keratitis ichthyosis deafness syndrome. Sci. Rep. 2021, 11, 24118. [Google Scholar] [CrossRef]
- Levit, N.A.; White, T.W. Connexin hemichannels influence genetically determined inflammatory and hyperproliferative skin diseases. Pharmacol. Res. 2015, 99, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Zorzie, V.; Burattoa, D.; Ziraldoe, G.; Mazzardae, F.; Perese, C.; Nardine, C.; Salvatoree, A.M.; Chianie, F.; Scavizzie, F.; et al. A potent antagonist antibody targeting connexin hemichannels alleviates Clouston syndrome symptoms in mutant mice. EBioMedicine 2020, 57, 102825. [Google Scholar] [CrossRef]
- Li, L.; Fan, D.B.; Zhao, Y.T.; Li, Y.; Yang, Z.B.; Zheng, G.Y. GJA8 missense mutation disrupts hemichannels and induces cell apoptosis in human lens epithelial cells. Sci. Rep. 2019, 9, 19157. [Google Scholar] [CrossRef]
- Hongo, H.; Miyawaki, S.; Teranishi, Y.; Mitsui, J.; Katoh, H.; Komura, D.; Tsubota, K.; Matsukawa, T.; Watanabe, M.; Kurita, M.; et al. Somatic GJA4 gain-of-function mutation in orbital cavernous venous malformations. Angiogenesis 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Paznekas, W.A.; Boyadjiev, S.A.; Shapiro, R.E.; Daniels, O.; Wollnik, B.; Keegan, C.E.; Innis, J.W.; Dinulos, M.B.; Christian, C.; Hannibal, M.C.; et al. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am. J. Hum. Genet. 2003, 72, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.W. Syndromic and non-syndromic disease-linked Cx43 mutations. FEBS Lett. 2014, 588, 1339–1348. [Google Scholar] [CrossRef]
- Richardson, R.J.; Joss, S.; Tomkin, S.; Ahmed, M.; Sheridan, E.; Dixon, M.J. A nonsense mutation in the first transmembrane domain of connexin 43 underlies autosomal recessive oculodentodigital syndrome. J. Med. Genet. 2006, 43, e37. [Google Scholar] [CrossRef]
- Paznekas, W.A.; Karczeski, B.; Vermeer, S.; Lowry, R.B.; Delatycki, M.; Laurence, F.; Koivisto, P.A.; Van Maldergem, L.; Boyadjiev, S.A.; Bodurtha, J.N.; et al. GJA1 mutations, variants, and connexin 43 dysfunction as it relates to the oculodentodigital dysplasia phenotype. Hum. Mutat. 2009, 30, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Kogame, T.; Dainichi, T.; Shimomura, Y.; Tanioka, M.; Kabashima, K.; Miyachi, Y. Palmoplantar keratosis in oculodentodigital dysplasia with a GJA1 point mutation out of the C-terminal region of connexin 43. J. Dermatol. 2014, 41, 1095–1097. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, I.P.; de Almeida, S.; Tiziani, V.; Do Amaral, C.M.; Gowrishankar, K.; Passos-Bueno, M.R.; Reichenberger, E.J. A novel autosomal recessive GJA1 missense mutation linked to Craniometaphyseal dysplasia. PLoS ONE 2013, 8, e73576. [Google Scholar] [CrossRef] [PubMed]
- Elcioglu, N.; Hall, C.M. Temporal aspects in craniometaphyseal dysplasia: Autosomal recessive type. Am. J. Med. Genet. 1998, 76, 245–251. [Google Scholar] [CrossRef]
- Faruqi, T.; Dhawan, N.; Bahl, J.; Gupta, V.; Vohra, S.; Tu, K.; Abdelmagid, S.M. Molecular, phenotypic aspects and therapeutic horizons of rare genetic bone disorders. Biomed. Res. Int. 2014, 2014, 670842. [Google Scholar] [CrossRef]
- Boyden, L.M.; Craiglow, B.G.; Zhou, J.; Hu, R.; Loring, E.C.; Morel, K.D.; Lauren, C.T.; Lifton, R.P.; Bilguvar, K.; Yale Center for Mendelian, G.; et al. Dominant De Novo Mutations in GJA1 Cause Erythrokeratodermia Variabilis et Progressiva, without Features of Oculodentodigital Dysplasia. J. Investig. Dermatol. 2015, 135, 1540–1547. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cao, X.; Lin, Z.; Lee, M.; Jia, X.; Ren, Y.; Dai, L.; Guan, L.; Zhang, J.; Lin, X.; et al. Exome sequencing reveals mutation in GJA1 as a cause of keratoderma-hypotrichosis-leukonychia totalis syndrome. Hum. Mol. Genet. 2015, 24, 243–250. [Google Scholar] [CrossRef]
- Ishida-Yamamoto, A. Erythrokeratodermia variabilis et progressiva. J. Dermatol. 2016, 43, 280–285. [Google Scholar] [CrossRef]
- Basaran, E.; Yilmaz, E.; Alpsoy, E.; Yilmaz, G.G. Keratoderma, hypotrichosis and leukonychia totalis: A new syndrome? Br. J. Dermatol. 1995, 133, 636–638. [Google Scholar] [CrossRef]
- Cocozzelli, A.G.; White, T.W. Connexin 43 Mutations Lead to Increased Hemichannel Functionality in Skin Disease. Int. J. Mol. Sci. 2019, 20, 6186. [Google Scholar] [CrossRef]
- Srinivas, M.; Jannace, T.F.; Cocozzelli, A.G.; Li, L.; Slavi, N.; Sellitto, C.; White, T.W. Connexin43 mutations linked to skin disease have augmented hemichannel activity. Sci. Rep. 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Essenfelder, G.M.; Bruzzone, R.; Lamartine, J.; Charollais, A.; Blanchet-Bardon, C.; Barbe, M.T.; Meda, P.; Waksman, G. Connexin30 mutations responsible for hidrotic ectodermal dysplasia cause abnormal hemichannel activity. Hum. Mol. Genet. 2004, 13, 1703–1714. [Google Scholar] [CrossRef]
- Gerido, D.A.; DeRosa, A.M.; Richard, G.; White, T.W. Aberrant hemichannel properties of Cx26 mutations causing skin disease and deafness. Am. J. Physiol. Cell Physiol. 2007, 293, C337–C345. [Google Scholar] [CrossRef] [PubMed]
- Lilly, E.; Sellitto, C.; Milstone, L.M.; White, T.W. Connexin channels in congenital skin disorders. Semin. Cell Dev. Biol. 2016, 50, 4–12. [Google Scholar] [CrossRef]
- Shuja, Z.; Li, L.; Gupta, S.; Mese, G.; White, T.W. Connexin26 Mutations Causing Palmoplantar Keratoderma and Deafness Interact with Connexin43, Modifying Gap Junction and Hemichannel Properties. J. Investig. Dermatol. 2016, 136, 225–235. [Google Scholar] [CrossRef]
- Kelly, J.J.; Simek, J.; Laird, D.W. Mechanisms linking connexin mutations to human diseases. Cell Tissue Res. 2015, 360, 701–721. [Google Scholar] [CrossRef] [PubMed]
- Kozoriz, M.G.; Lai, S.; Vega, J.L.; Saez, J.C.; Sin, W.C.; Bechberger, J.F.; Naus, C.C. Cerebral ischemic injury is enhanced in a model of oculodentodigital dysplasia. Neuropharmacology 2013, 75, 549–556. [Google Scholar] [CrossRef]
- Dobrowolski, R.; Sommershof, A.; Willecke, K. Some oculodentodigital dysplasia-associated Cx43 mutations cause increased hemichannel activity in addition to deficient gap junction channels. J. Membr. Biol. 2007, 219, 9–17. [Google Scholar] [CrossRef]
- Bursztejn, A.C.; Magdelaine, C.; Mortemousque, B.; Zerah, M.; Schmutz, J.L.; Leheup, B. Hypotrichosis with keratosis follicular and hyperostosis: A new phenotype due to GJA1 mutation. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e219–e221. [Google Scholar] [CrossRef]
- Barrio, L.C.; Suchyna, T.; Bargiello, T.; Xu, L.X.; Roginski, R.S.; Bennett, M.V.; Nicholson, B.J. Gap junctions formed by connexins 26 and 32 alone and in combination are differently affected by applied voltage. Proc. Natl. Acad. Sci. USA 1991, 88, 8410–8414. [Google Scholar] [CrossRef]
- Bruzzone, R.; Haefliger, J.A.; Gimlich, R.L.; Paul, D.L. Connexin40, a component of gap junctions in vascular endothelium, is restricted in its ability to interact with other connexins. Mol. Biol. Cell 1993, 4, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, L. Xenopus connexin38 forms hemi-gap-junctional channels in the nonjunctional plasma membrane of Xenopus oocytes. Biophys. J. 1996, 71, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Mhaske, P.V.; Levit, N.A.; Li, L.; Wang, H.Z.; Lee, J.R.; Shuja, Z.; Brink, P.R.; White, T.W. The human Cx26-D50A and Cx26-A88V mutations causing keratitis-ichthyosis-deafness syndrome display increased hemichannel activity. Am. J. Physiol. Cell Physiol. 2013, 304, C1150–C1158. [Google Scholar] [CrossRef]
- White, T.W.; Deans, M.R.; O’Brien, J.; Al-Ubaidi, M.R.; Goodenough, D.A.; Ripps, H.; Bruzzone, R. Functional characteristics of skate connexin35, a member of the gamma subfamily of connexins expressed in the vertebrate retina. Eur. J. Neurosci. 1999, 11, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Bukauskas, F.F.; Bukauskiene, A.; Bennett, M.V.; Verselis, V.K. Gating properties of gap junction channels assembled from connexin43 and connexin43 fused with green fluorescent protein. Biophys. J. 2001, 81, 137–152. [Google Scholar] [CrossRef]
- White, T.W.; Bruzzone, R.; Wolfram, S.; Paul, D.L.; Goodenough, D.A. Selective interactions among the multiple connexin proteins expressed in the vertebrate lens: The second extracellular domain is a determinant of compatibility between connexins. J. Cell Biol. 1994, 125, 879–892. [Google Scholar] [CrossRef]
- Valiunas, V.; Gemel, J.; Brink, P.R.; Beyer, E.C. Gap junction channels formed by coexpressed connexin40 and connexin43. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H1675–H1689. [Google Scholar] [CrossRef]
- Bruzzone, R.; White, T.W.; Paul, D.L. Expression of chimeric connexins reveals new properties of the formation and gating behavior of gap junction channels. J. Cell Sci. 1994, 107 Pt 4, 955–967. [Google Scholar] [CrossRef]
- Spray, D.C.; Harris, A.L.; Bennett, M.V. Equilibrium properties of a voltage-dependent junctional conductance. J. Gen. Physiol. 1981, 77, 77–93. [Google Scholar] [CrossRef]
- Hansen, D.B.; Braunstein, T.H.; Nielsen, M.S.; MacAulay, N. Distinct permeation profiles of the connexin 30 and 43 hemichannels. FEBS Lett. 2014, 588, 1446–1457. [Google Scholar] [CrossRef]
- Hansen, D.B.; Ye, Z.C.; Calloe, K.; Braunstein, T.H.; Hofgaard, J.P.; Ransom, B.R.; Nielsen, M.S.; MacAulay, N. Activation, permeability, and inhibition of astrocytic and neuronal large pore (hemi)channels. J. Biol. Chem. 2014, 289, 26058–26073. [Google Scholar] [CrossRef]
- Valiunas, V. Cyclic nucleotide permeability through unopposed connexin hemichannels. Front. Pharmacol. 2013, 4, 75. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.X.; Siller-Jackson, A.J.; Burra, S. Roles of gap junctions and hemichannels in bone cell functions and in signal transmission of mechanical stress. Front. Biosci. 2007, 12, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- Hua, R.; Zhang, J.; Riquelme, M.A.; Jiang, J.X. Connexin Gap Junctions and Hemichannels Link Oxidative Stress to Skeletal Physiology and Pathology. Curr. Osteoporos. Rep. 2021, 19, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Hua, R.; Gu, S.; Jiang, J.X. Connexin 43 Hemichannels Regulate Osteoblast to Osteocyte Differentiation. Front. Cell Dev. Biol. 2022, 10, 892229. [Google Scholar] [CrossRef]
- Garcia, I.E.; Maripillan, J.; Jara, O.; Ceriani, R.; Palacios-Munoz, A.; Ramachandran, J.; Olivero, P.; Perez-Acle, T.; Gonzalez, C.; Saez, J.C.; et al. Keratitis-ichthyosis-deafness syndrome-associated cx26 mutants produce nonfunctional gap junctions but hyperactive hemichannels when co-expressed with wild type cx43. J. Investig. Dermatol. 2015, 135, 1338–1347. [Google Scholar] [CrossRef]
- Montgomery, J.R.; White, T.W.; Martin, B.L.; Turner, M.L.; Holland, S.M. A novel connexin 26 gene mutation associated with features of the keratitis-ichthyosis-deafness syndrome and the follicular occlusion triad. J. Am. Acad. Dermatol. 2004, 51, 377–382. [Google Scholar] [CrossRef]
- Chi, J.; Li, L.; Liu, M.; Tan, J.; Tang, C.; Pan, Q.; Wang, D.; Zhang, Z. Pathogenic connexin-31 forms constitutively active hemichannels to promote necrotic cell death. PLoS ONE 2012, 7, e32531. [Google Scholar] [CrossRef]
- Buratto, D.; Donati, V.; Zonta, F.; Mammano, F. Harnessing the therapeutic potential of antibodies targeting connexin hemichannels. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166047. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.J.; Khan, U.; Haddad, B.G.; Minogue, P.J.; Beyer, E.C.; Berthoud, V.M.; Reichow, S.L.; Ebihara, L. Molecular mechanisms underlying enhanced hemichannel function of a cataract-associated Cx50 mutant. Biophys. J. 2021, 120, 5644–5656. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.J.; Minogue, P.J.; Kobeszko, M.; Beyer, E.C.; Berthoud, V.M.; Ebihara, L. The connexin46 mutant, Cx46T19M, causes loss of gap junction function and alters hemi-channel gating. J. Membr. Biol. 2015, 248, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.E.; Prado, P.; Pupo, A.; Jara, O.; Rojas-Gomez, D.; Mujica, P.; Flores-Munoz, C.; Gonzalez-Casanova, J.; Soto-Riveros, C.; Pinto, B.I.; et al. Connexinopathies: A structural and functional glimpse. BMC Cell Biol. 2016, 17 (Suppl. 1), 17. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Carrer, A.; Zonta, F.; Qu, Z.; Ma, P.; Li, S.; Ceriani, F.; Buratto, D.; Crispino, G.; Zorzi, V.; et al. Design and Characterization of a Human Monoclonal Antibody that Modulates Mutant Connexin 26 Hemichannels Implicated in Deafness and Skin Disorders. Front. Mol. Neurosci. 2017, 10, 298. [Google Scholar] [CrossRef]
- Willebrords, J.; Maes, M.; Crespo Yanguas, S.; Vinken, M. Inhibitors of connexin and pannexin channels as potential therapeutics. Pharmacol. Ther. 2017, 180, 144–160. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.W.; Lampe, P.D. Therapeutic strategies targeting connexins. Nat. Rev. Drug Discov. 2018, 17, 905–921. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.L.; Weintraub, H. Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev. 1994, 8, 1434–1447. [Google Scholar] [CrossRef]
- Horton, R.M.; Cai, Z.L.; Ho, S.N.; Pease, L.R. Gene splicing by overlap extension: Tailor-made genes using the polymerase chain reaction. Biotechniques 1990, 8, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.R.; Derosa, A.M.; White, T.W. Connexin mutations causing skin disease and deafness increase hemichannel activity and cell death when expressed in Xenopus oocytes. J. Investig. Dermatol. 2009, 129, 870–878. [Google Scholar] [CrossRef]
- White, T.W.; Bruzzone, R.; Goodenough, D.A.; Paul, D.L. Mouse Cx50, a functional member of the connexin family of gap junction proteins, is the lens fiber protein MP70. Mol. Biol. Cell 1992, 3, 711–720. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Mese, G.; Valiunas, V.; Brink, P.R.; White, T.W. Connexin26 deafness associated mutations show altered permeability to large cationic molecules. Am. J. Physiol. Cell Physiol. 2008, 295, C966–C974. [Google Scholar] [CrossRef] [PubMed]
- Mese, G.; Sellitto, C.; Li, L.; Wang, H.Z.; Valiunas, V.; Richard, G.; Brink, P.R.; White, T.W. The Cx26-G45E mutation displays increased hemichannel activity in a mouse model of the lethal form of keratitis-ichthyosis-deafness syndrome. Mol. Biol. Cell 2011, 22, 4776–4786. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crouthamel, O.E.; Li, L.; Dilluvio, M.T.; White, T.W. Increased Hemichannel Activity Displayed by a Connexin43 Mutation Causing a Familial Connexinopathy Exhibiting Hypotrichosis with Follicular Keratosis and Hyperostosis. Int. J. Mol. Sci. 2023, 24, 2222. https://doi.org/10.3390/ijms24032222
Crouthamel OE, Li L, Dilluvio MT, White TW. Increased Hemichannel Activity Displayed by a Connexin43 Mutation Causing a Familial Connexinopathy Exhibiting Hypotrichosis with Follicular Keratosis and Hyperostosis. International Journal of Molecular Sciences. 2023; 24(3):2222. https://doi.org/10.3390/ijms24032222
Chicago/Turabian StyleCrouthamel, Olivia E., Leping Li, Michael T. Dilluvio, and Thomas W. White. 2023. "Increased Hemichannel Activity Displayed by a Connexin43 Mutation Causing a Familial Connexinopathy Exhibiting Hypotrichosis with Follicular Keratosis and Hyperostosis" International Journal of Molecular Sciences 24, no. 3: 2222. https://doi.org/10.3390/ijms24032222
APA StyleCrouthamel, O. E., Li, L., Dilluvio, M. T., & White, T. W. (2023). Increased Hemichannel Activity Displayed by a Connexin43 Mutation Causing a Familial Connexinopathy Exhibiting Hypotrichosis with Follicular Keratosis and Hyperostosis. International Journal of Molecular Sciences, 24(3), 2222. https://doi.org/10.3390/ijms24032222





