Comparative Transcriptome Analysis of the Heterosis of Salt Tolerance in Inter-Subspecific Hybrid Rice
Abstract
1. Introduction
2. Results
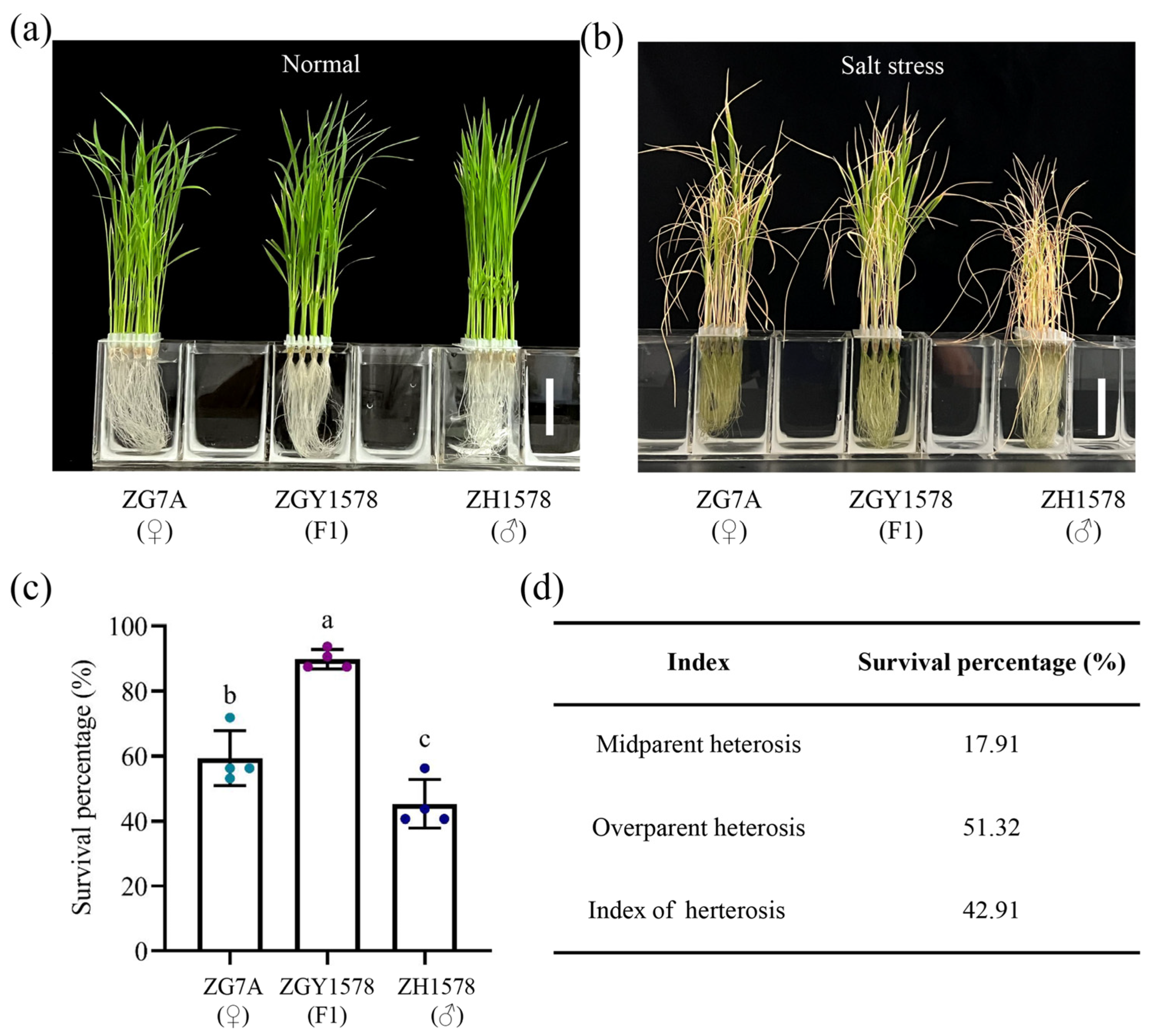
2.1. Heterosis of Salt Tolerance in Inter-Subspecific Hybrid Rice
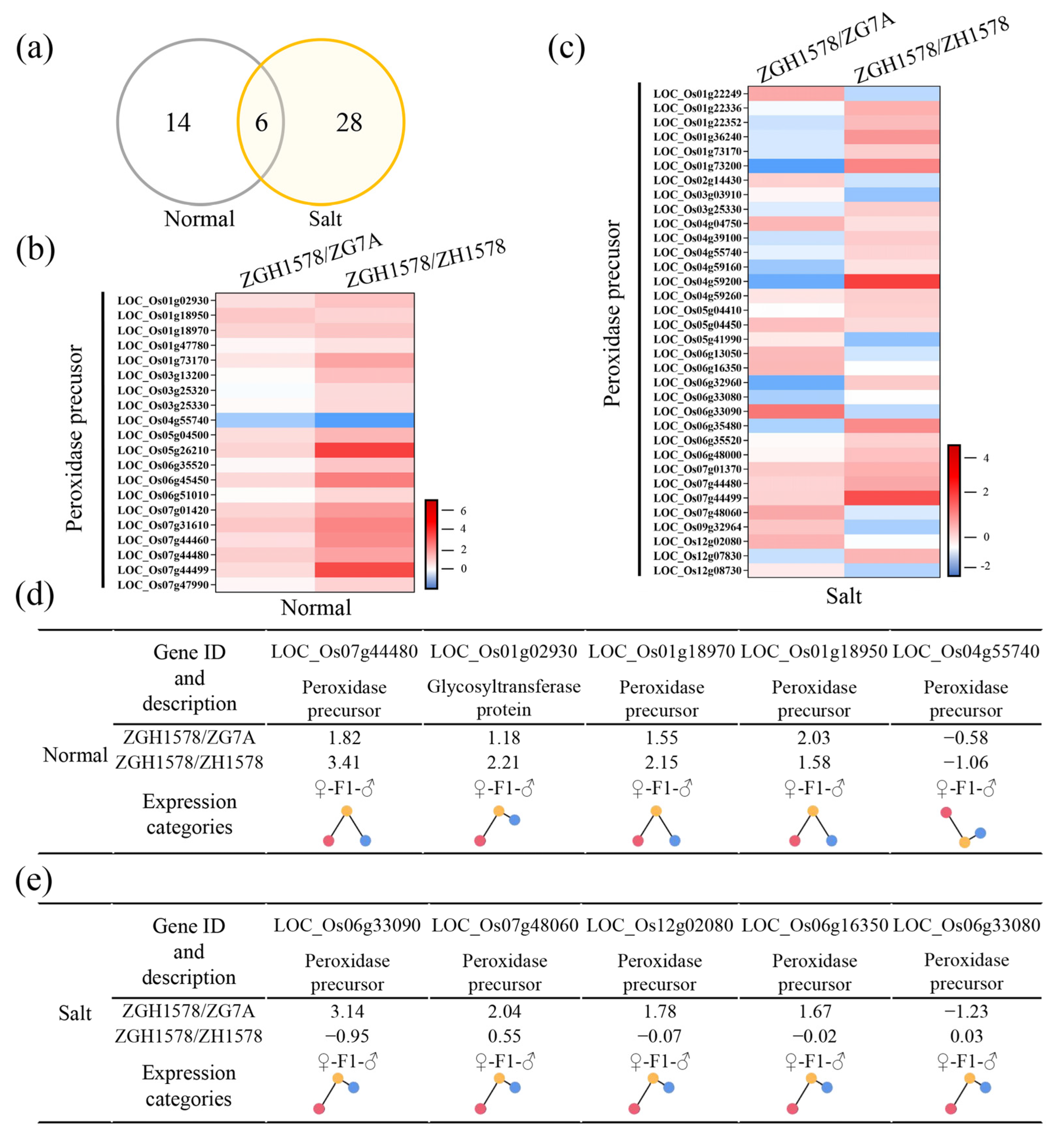
2.2. Differentially Expressed Genes among Hybrid and Its Parents
2.3. Expression Pattern of Differentially Expressed Genes
2.4. Transcription Factors Associated with Heterosis of Salt Tolerance
2.5. Hormone-Related Genes Associated with Heterosis of Salt Tolerance
2.6. ROS-Related Genes Associated with Heterosis of Salt Tolerance
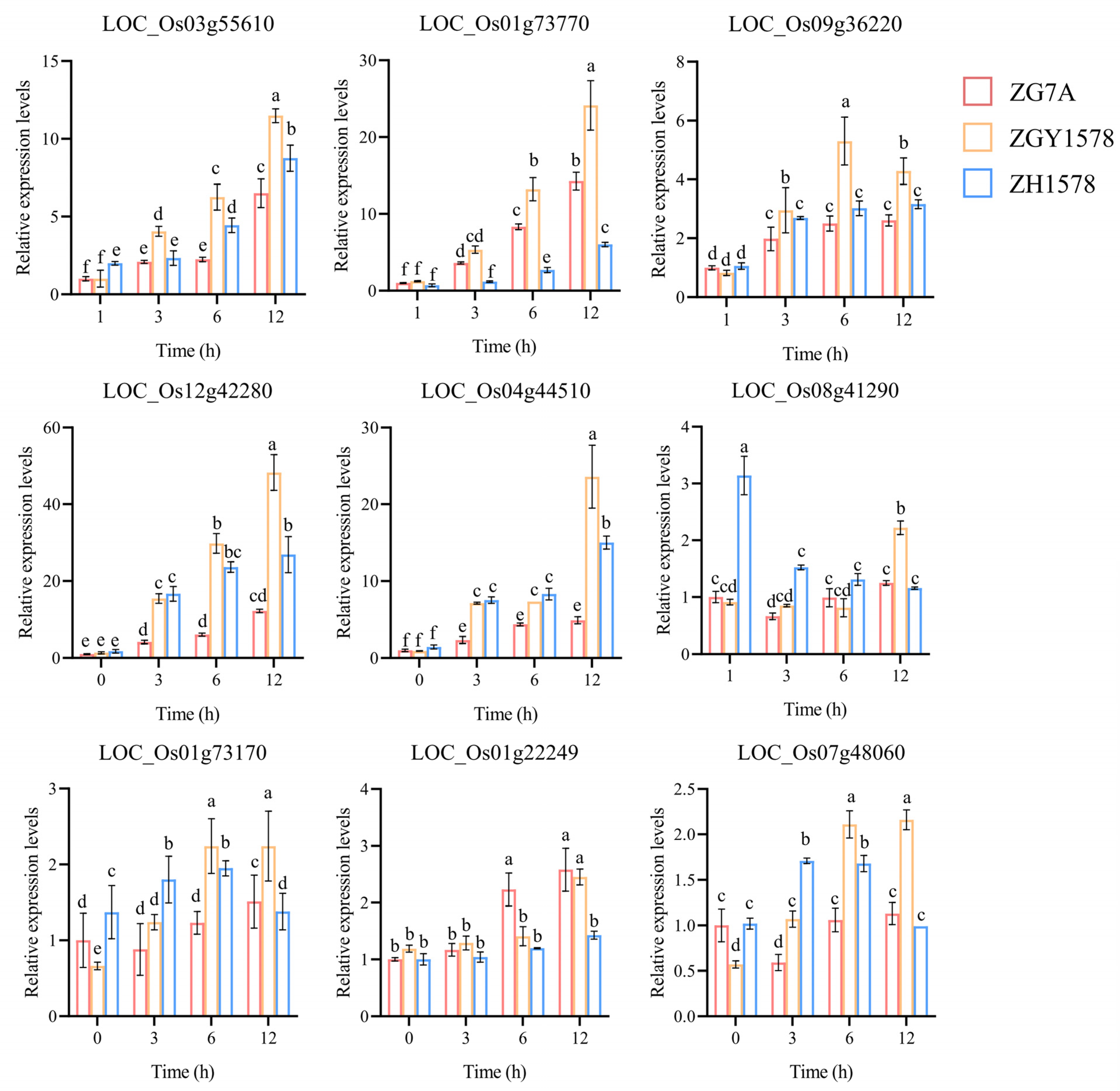
2.7. Validation of DEGs by Real-Time Quantitative PCR
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Evaluation of Salt Tolerance
4.3. RNA-Seq
4.4. Differentially Expressed Gene Analysis
4.5. Real-Time Quantitative RT-PCR Analysis
4.6. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, S.H.; Zhuang, J.Y.; Fan, Y.Y.; Du, J.H.; Cao, L.Y. Progress in research and development on hybrid rice: A super-domesticate in China. Ann. Bot. 2007, 100, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Chen, Y.; Yao, W.; Zhang, C.; Xie, W.; Hua, J.; Xing, Y.; Xiao, J.; Zhang, Q. Genetic composition of yield heterosis in an elite rice hybrid. Proc. Natl. Acad. Sci. USA 2012, 109, 15847–15852. [Google Scholar] [CrossRef]
- Zhai, R.; Feng, Y.; Wang, H.; Zhan, X.; Shen, X.; Wu, W.; Zhang, Y.; Chen, D.; Dai, G.; Yang, Z.; et al. Transcriptome analysis of rice root heterosis by RNA-Seq. BMC Genom. 2013, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ye, J.; Yang, Y.; Zhang, M.; Xu, Q.; Feng, Y.; Yuan, X.; Yu, H.; Wang, Y.; Yang, Y.; et al. Genome-wide association study of rice rooting ability at the seedling stage. Rice 2020, 13, 59. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Arremsetty, H.P.S.; Singh, A.K.; Khandekar, D.; Ulaganathan, K.; Shenoy, V.; Sinha, P.; Singh, V.K. Marker-assisted improvement of the elite maintainer line of rice, IR 58025B for wide compatibility S5n gene. Front. Plant Sci. 2018, 9, 1051. [Google Scholar] [CrossRef] [PubMed]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pol. Res. Int. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Ganie, S.A.; Wani, S.H.; Henry, R.; Hensel, G. Improving rice salt tolerance by precision breeding in a new era. Cur. Opin. Plant Biol. 2021, 60, 101996. [Google Scholar] [CrossRef]
- Bruce, A.B. The Mendelian theory of heredity and the augmentation of vigor. Science 1910, 32, 627–828. [Google Scholar] [CrossRef]
- Shull, G.H. Hybrid seed corn. Science 1946, 103, 547–550. [Google Scholar] [CrossRef]
- Minvielle, F. Dominance is not necessary for heterosis: A two-locus model. Genet. Res. 1987, 49, 245–247. [Google Scholar] [CrossRef]
- Schnell, F.W.; Cockerham, C.C. Multiplicative vs. arbitrary gene action in heterosis. Genetics 1992, 131, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.F. Dominance of linked factors as a means of accounting for heterosis. Proc. Natl. Acad. Sci. USA 1917, 3, 310–312. [Google Scholar] [CrossRef] [PubMed]
- East, E.M. Heterosis. Genetics 1936, 21, 375–397. [Google Scholar] [CrossRef]
- Huang, X.; Yang, S.; Gong, J.; Zhao, Q.; Feng, Q.; Zhan, Q.; Zhao, Y.; Li, W.; Cheng, B.; Xia, J.; et al. Genomic architecture of heterosis for yield traits in rice. Nature 2016, 537, 629–633. [Google Scholar] [CrossRef]
- Zhao, M.F.; Li, X.H.; Yang, J.B.; Xu, C.G.; Hu, R.Y.; Liu, D.; Zhang, Q. Relationship between molecular marker heterozygosity and hybrid performance in intra- and inter-subspecific crosses of rice. Plant Breed. 1999, 118, 139e144. [Google Scholar] [CrossRef]
- Wei, G.; Tao, Y.; Liu, G.; Chen, C.; Luo, R.; Xia, H.; Gan, Q.; Zeng, H.; Lu, Z.; Han, Y.; et al. A transcriptomic analysis of superhybrid rice LYP9 and its parents. Proc. Natl. Acad. Sci. USA 2009, 106, 7695–7701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, L.L.; Xing, F.; Kudrna, D.A.; Yao, W.; Copetti, D.; Mu, T.; Li, W.; Song, J.M.; Xie, W.; et al. Extensive sequence divergence between the reference genomes of two elite indica rice varieties Zhenshan 97 and Minghui 63. Proc. Natl. Acad. Sci. USA 2016, 113, E5163–E5171. [Google Scholar] [CrossRef]
- Chen, L.; Bian, J.; Shi, S.; Yu, J.; Khanzada, H.; Wassan, G.M.; Zhu, C.; Luo, X.; Tong, S.; Yang, X.; et al. Genetic analysis for the grain number heterosis of a super-hybrid rice WFYT025 combination using RNA-Seq. Rice 2018, 11, 37. [Google Scholar] [CrossRef]
- Matsumuro, T.; Miyake, H.; Takeoka, Y.J. Effects of salinity stress on the seminal root tip ultrastructures of rice seedlings. Plant Prod. Sci. 2001, 4, 103–111. [Google Scholar]
- Cartagena, J.A.; Yao, Y.; Mitsuya, S.; Tsuge, T. Comparative transcriptome analysis of root types in salt tolerant and sensitive rice varieties in response to salinity stress. Physiol. Plant. 2021, 173, 1629–1642. [Google Scholar] [CrossRef]
- Fujimoto, R.; Uezono, K.; Ishikura, S.; Osabe, K.; Peacock, W.J.; Dennis, E.S. Recent research on the mechanism of heterosis is important for crop and vegetable breeding systems. Breed. Sci. 2018, 68, 145–158. [Google Scholar] [CrossRef]
- He, G.; Zhu, X.; Elling, A.A.; Chen, L.; Wang, X.; Guo, L.; Liang, M.; He, H.; Zhang, H.; Chen, F.; et al. Global epigenetic and transcriptional trends among two rice subspecies and their reciprocal hybrids. Plant Cell 2010, 22, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Chodavarapu, R.K.; Feng, S.; Ding, B.; Simon, S.A.; Lopez, D.; Jia, Y.; Wang, G.L.; Meyers, B.C.; Jacobsen, S.E.; Pellegrini, M. Transcriptome and methylome interactions in rice hybrids. Proc. Natl. Acad. Sci. USA 2012, 109, 12040–12045. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yuan, J.; Wu, M.; Han, Z.; Li, L.; Jiang, H.; Jia, Y.; Han, X.; Liu, M.; Sun, D.; et al. Transcriptome and DNA methylome reveal insights into yield heterosis in the curds of broccoli Brassica oleracea L. var. italic. BMC Plant Biol. 2018, 18, 168. [Google Scholar] [CrossRef]
- Song, G.S.; Zhai, H.L.; Peng, Y.G.; Zhang, L.; Wei, G.; Chen, X.Y.; Xiao, Y.G.; Wang, L.; Chen, Y.J.; Wu, B.; et al. Comparative transcriptional profiling and preliminary study on heterosis mechanism of super-hybrid rice. Mol. Plant 2010, 3, 1012–1025. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, J. Transcriptome and DNA methylome analyses provide insight into the heterosis in flag leaf of inter-subspecific hybrid rice. Plant Mol. Biol. 2022, 108, 105–125. [Google Scholar] [CrossRef]
- Wang, Q.; Guan, Y.; Wu, Y.; Chen, H.; Chen, F.; Chu, C. Overexpression of a rice OsDREB1F gene increases salt, drought, and low temperature tolerance in both Arabidopsis and rice. Plant Mol. Biol. 2008, 67, 589–602. [Google Scholar] [CrossRef]
- Singh, B.P.; Jayaswal, P.K.; Singh, B.; Singh, P.K.; Kumar, V.; Mishra, S.; Singh, N.; Panda, K.; Singh, N.K. Natural allelic diversity in OsDREB1F gene in the Indian wild rice germplasm led to ascertain its association with drought tolerance. Plant Cell Rep. 2015, 34, 993–1004. [Google Scholar] [CrossRef]
- Kumimoto, R.W.; Zhang, Y.; Siefers, N.; Holt, B.F., 3rd. NF-YC3, NF-YC4 and NF-YC9 are required for CONSTANS-mediated, photoperiod-dependent flowering in Arabidopsis thaliana. Plant J. 2010, 63, 379–391. [Google Scholar] [CrossRef]
- Hwang, K.; Susila, H.; Nasim, Z.; Jung, J.Y.; Ahn, J.H. Arabidopsis ABF3 and ABF4 transcription factors act with the NF-YC complex to regulate SOC1 expression and mediate drought-accelerated flowering. Mol. Plant 2019, 12, 489–505. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, X.; Liu, X.; Li, Y.; Wu, K.; Hou, X. Arabidopsis NF-YCs mediate the light-controlled hypocotyl elongation via modulating histone acetylation. Mol. Plant 2017, 10, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, D.; Liu, X.; Ji, C.; Hao, L.; Zhao, X.; Li, X.; Chen, C.; Cheng, Z.; Zhu, L. OsMYB103L, an R2R3-MYB transcription factor, influences leaf rolling and mechanical strength in rice. BMC Plant Biol. 2014, 14, 158. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Liu, B.; Zhao, M.; Wu, K.; Cheng, W.; Chen, X.; Liu, Q.; Liu, Z.; Fu, X.; Wu, Y. CEF1/OsMYB103L is involved in GA-mediated regulation of secondary wall biosynthesis in rice. Plant Mol. Biol. 2015, 89, 385–401. [Google Scholar] [CrossRef]
- De Smet, I.; Signora, L.; Beeckman, T.; Inzé, D.; Foyer, C.H.; Zhang, H. An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis. Plant J. 2003, 33, 543–555. [Google Scholar] [CrossRef]
- Li, X.; Mo, X.; Shou, H.; Wu, P. Cytokinin-mediated cell cycling arrest of pericycle founder cells in lateral root initiation of Arabidopsis. Plant Cell Physiol. 2006, 47, 1112–1123. [Google Scholar] [CrossRef]
- Mao, C.; He, J.; Liu, L.; Deng, Q.; Yao, X.; Liu, C.; Qiao, Y.; Li, P.; Ming, F. OsNAC2 integrates auxin and cytokinin pathways to modulate rice root development. Plant Biotechnol. J. 2020, 18, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Liu, Y.N.; Cheng, J.F.; Guo, R.; Tian, L.; Wang, B. Dual roles of OsGH3.2 in modulating rice root morphology and affecting arbuscular mycorrhizal symbiosis. Front. Plant Sci. 2022, 13, 853435. [Google Scholar] [CrossRef]
- Fang, X.; Bo, C.; Wang, M.; Yuan, H.; Li, W.; Chen, H.; Ma, Q.; Cai, R. Overexpression of the maize WRKY114 gene in transgenic rice reduce plant height by regulating the biosynthesis of GA. Plant Signal. Behav. 2021, 16, 1967635. [Google Scholar] [CrossRef]
- Prakash, S.; Rai, R.; Zamzam, M.; Ahmad, O.; Peesapati, R.; Vijayraghavan, U. OsbZIP47 is an integrator for meristem regulators during rice plant growth and development. Front. Plant Sci. 2022, 13, 865928. [Google Scholar] [CrossRef]
- Jin, T.; Sun, Y.; Zhao, R.; Shan, Z.; Gai, J.; Li, Y. Overexpression of peroxidase gene GsPRX9 confers salt tolerance in soybean. Int. J. Mol. Sci. 2019, 20, 3745. [Google Scholar] [CrossRef]
- Su, P.; Yan, J.; Li, W.; Wang, L.; Zhao, J.; Ma, X.; Li, A.; Wang, H.; Kong, L. A member of wheat class III peroxidase gene family, TaPRX-2A, enhanced the tolerance of salt stress. BMC Plant Biol. 2020, 20, 392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Tang, Y.J.; Ma, Q.B.; Yang, C.; Mu, Y.H.; Suo, H.C.; Luo, L.H.; Nian, H. OsDREB2A, a rice transcription factor, significantly affects salt tolerance in transgenic soybean. PLoS ONE 2013, 8, e83011. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.G.; Shan, J.X.; Shi, M.; Gao, J.P.; Lin, H.X. DCA1 acts as a transcriptional co-activator of DST and contributes to drought and salt tolerance in rice. PLoS Genet. 2015, 11, e1005617. [Google Scholar] [CrossRef]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinfor. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, W.; Sun, S.; Peng, L.; Huang, Z.; He, Y.; Wang, Z. The rice small auxin-up RNA gene OsSAUR33 regulates seed vigor via sugar pathway during early seed germination. Int. J. Mol. Sci. 2021, 22, 1562. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Ye, J.; Zhai, R.; Wu, M.; Yu, F.; Zhu, G.; Wang, Z.; Zhang, X.; Ye, S. Comparative Transcriptome Analysis of the Heterosis of Salt Tolerance in Inter-Subspecific Hybrid Rice. Int. J. Mol. Sci. 2023, 24, 2212. https://doi.org/10.3390/ijms24032212
Huang Z, Ye J, Zhai R, Wu M, Yu F, Zhu G, Wang Z, Zhang X, Ye S. Comparative Transcriptome Analysis of the Heterosis of Salt Tolerance in Inter-Subspecific Hybrid Rice. International Journal of Molecular Sciences. 2023; 24(3):2212. https://doi.org/10.3390/ijms24032212
Chicago/Turabian StyleHuang, Zhibo, Jing Ye, Rongrong Zhai, Mingming Wu, Faming Yu, Guofu Zhu, Zhoufei Wang, Xiaoming Zhang, and Shenghai Ye. 2023. "Comparative Transcriptome Analysis of the Heterosis of Salt Tolerance in Inter-Subspecific Hybrid Rice" International Journal of Molecular Sciences 24, no. 3: 2212. https://doi.org/10.3390/ijms24032212
APA StyleHuang, Z., Ye, J., Zhai, R., Wu, M., Yu, F., Zhu, G., Wang, Z., Zhang, X., & Ye, S. (2023). Comparative Transcriptome Analysis of the Heterosis of Salt Tolerance in Inter-Subspecific Hybrid Rice. International Journal of Molecular Sciences, 24(3), 2212. https://doi.org/10.3390/ijms24032212






