The Cell-Specific Role of SHP2 in Regulating Bone Homeostasis and Regeneration Niches
Abstract
1. Introduction
2. Somatic SHP2 Mutation and Bone Diseases
3. SHP2 in Osteoblast Lineage Cells
3.1. Mesenchymal Stem Cells
3.2. Osteoblasts
3.3. Osteoclast Progenitors/Osteoclasts
3.4. Chondrocytes
4. SHP2 in Bone Niche Cells
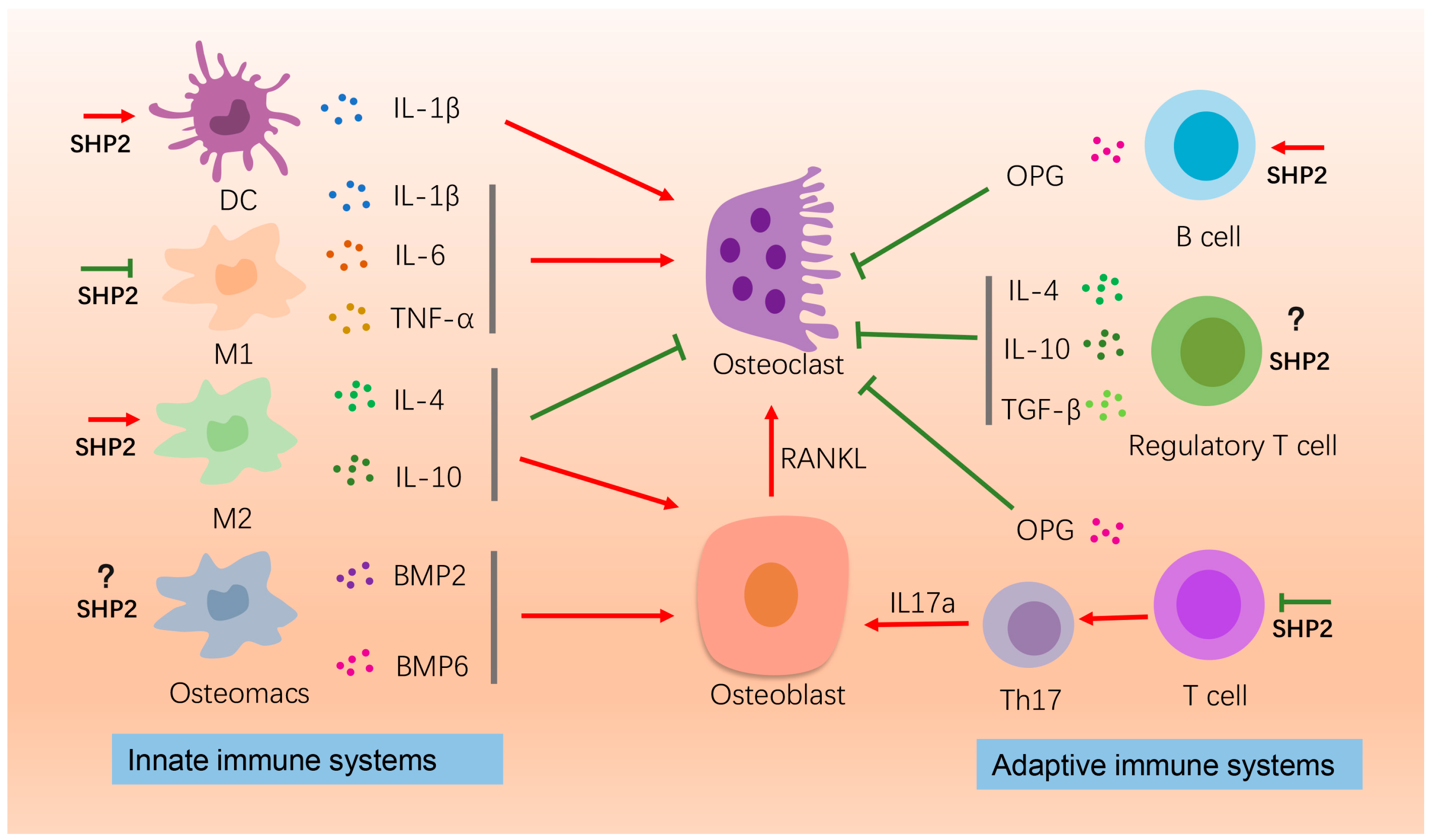
4.1. Immune Cells
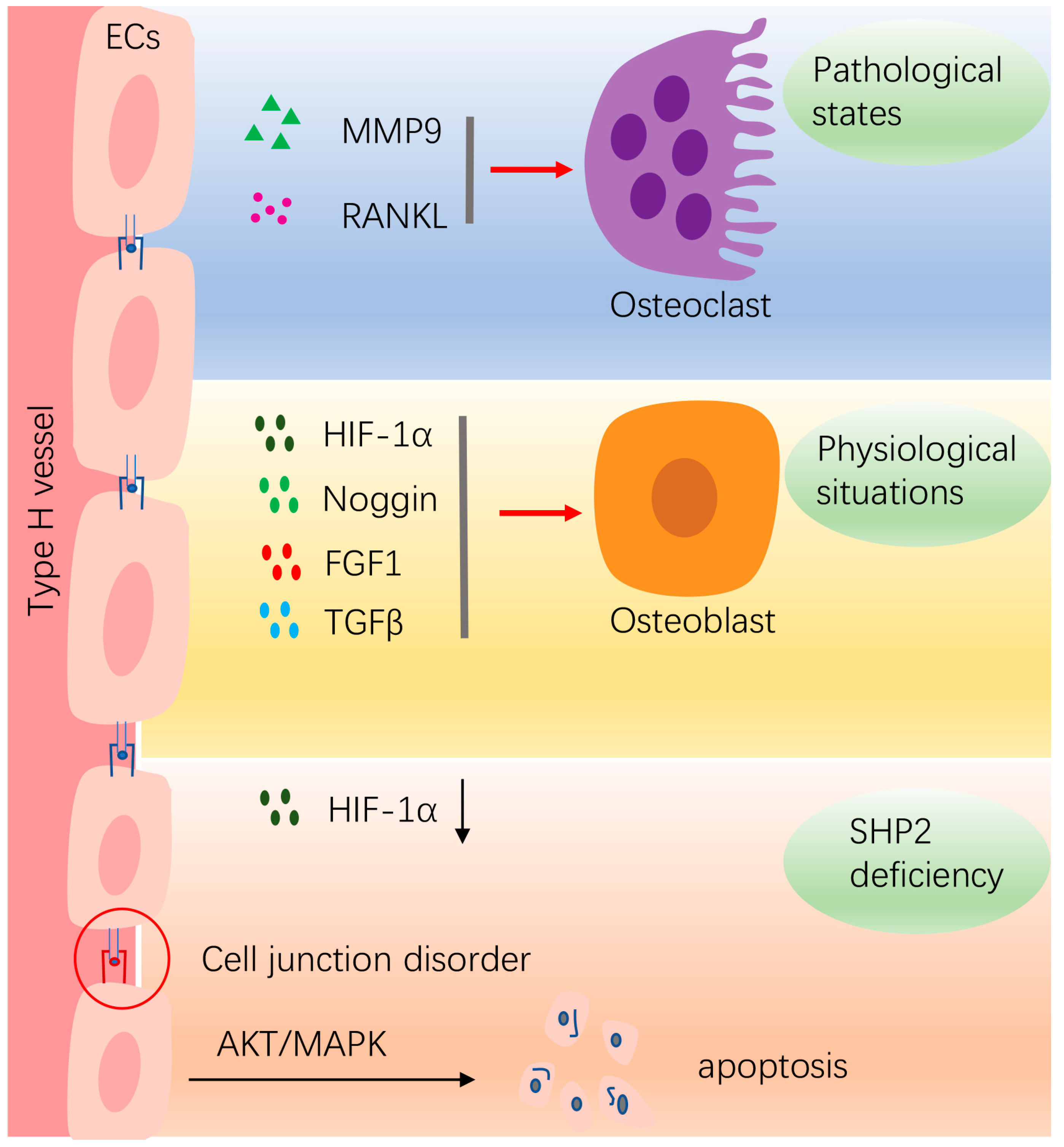
4.2. Vasculature Endothelial Cells
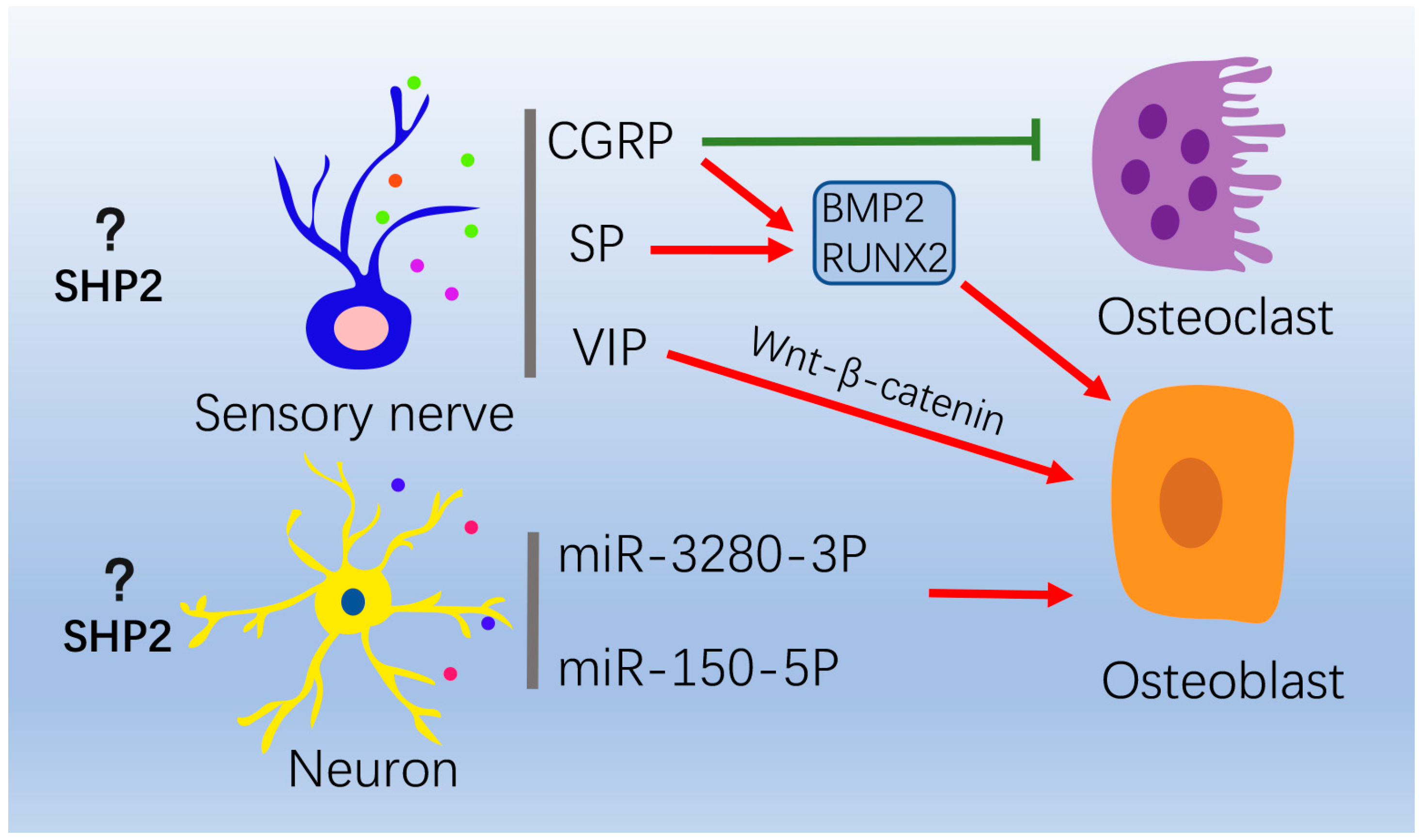
4.3. Nervous System
5. Prospects of SHP2 Agonists and Inhibitors in Treating Bone-Related Diseases
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sommerfeldt, D.W.; Rubin, C.T. Biology of bone and how it orchestrates the form and function of the skeleton. Eur. Spine J. 2001, 10, S86–S95. [Google Scholar] [CrossRef] [PubMed]
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, A.D.; Olsen, B.R. Bone development. Bone 2015, 80, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.; Martins-Cruz, C.; Oliveira, M.B.; Mano, J.F. Bone physiology as inspiration for tissue regenerative therapies. Biomaterials 2018, 185, 240–275. [Google Scholar] [CrossRef]
- Qin, Q.; Lee, S.; Patel, N.; Walden, K.; Gomez-Salazar, M.; Levi, B.; James, A.W. Neurovascular coupling in bone regeneration. Exp. Mol. Med. 2022, 54, 1844–1849. [Google Scholar] [CrossRef]
- Kamiya, N.; Kim, H.K.; King, P.D. Regulation of bone and skeletal development by the SHP-2 protein tyrosine phosphatase. Bone 2014, 69, 55–60. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, W.; Zhao, Q.; Chen, J.; Wang, T.; Ji, J. The role of the protein tyrosine phosphatase SHP2 in ossification. Dev. Dyn. 2021, 251, 748–758. [Google Scholar] [CrossRef]
- Yang, W.; Wang, J.; Moore, D.C.; Liang, H.; Dooner, M.; Wu, Q.; Terek, R.; Chen, Q.; Ehrlich, M.G.; Quesenberry, P.J.; et al. Ptpn11 deletion in a novel progenitor causes metachondromatosis by inducing hedgehog signalling. Nature 2013, 499, 491–495. [Google Scholar] [CrossRef]
- Chan, G.; Kalaitzidis, D.; Neel, B.G. The tyrosine phosphatase Shp2 (PTPN11) in cancer. Cancer Metastasis Rev. 2008, 27, 179–192. [Google Scholar] [CrossRef]
- Yu, Z.-H.; Zhang, R.-Y.; Walls, C.D.; Chen, L.; Zhang, S.; Wu, L.; Liu, S.; Zhang, Z.-Y. Molecular Basis of Gain-of-Function LEOPARD Syndrome-Associated SHP2 Mutations. Biochemistry 2014, 53, 4136–4151. [Google Scholar] [CrossRef]
- Dong, L.; Han, D.; Meng, X.; Xu, M.; Zheng, C.; Xia, Q. Activating Mutation of SHP2 Establishes a Tumorigenic Phonotype Through Cell-Autonomous and Non-Cell-Autonomous Mechanisms. Front. Cell Dev. Biol. 2021, 9, 630712. [Google Scholar] [CrossRef]
- Bowen, M.; Boyden, E.D.; Holm, I.; Campos-Xavier, B.; Bonafé, L.; Superti-Furga, A.; Ikegawa, S.; Cormier-Daire, V.; Bovee, J.; Pansuriya, T.C.; et al. Loss-of-Function Mutations in PTPN11 Cause Metachondromatosis, but Not Ollier Disease or Maffucci Syndrome. PLoS Genet. 2011, 7, e1002050. [Google Scholar] [CrossRef] [PubMed]
- Tajan, M.; Serra, A.D.R.; Valet, P.; Edouard, T.; Yart, A. SHP2 sails from physiology to pathology. Eur. J. Med. Genet. 2015, 58, 509–525. [Google Scholar] [CrossRef]
- Serra-Nédélec, A.D.R.; Edouard, T.; Tréguer, K.; Tajan, M.; Araki, T.; Dance, M.; Mus, M.; Montagner, A.; Tauber, M.; Salles, J.-P.; et al. Noonan syndrome-causing SHP2 mutants inhibit insulin-like growth factor 1 release via growth hormone-induced ERK hyperactivation, which contributes to short stature. Proc. Natl. Acad. Sci. USA 2012, 109, 4257–4262. [Google Scholar] [CrossRef] [PubMed]
- Bowen, M.E.; Ayturk, U.M.; Kurek, K.C.; Yang, W.; Warman, M.L. SHP2 Regulates Chondrocyte Terminal Differentiation, Growth Plate Architecture and Skeletal Cell Fates. PLoS Genet. 2014, 10, e1004364. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Feng, G.-S.; Chen, D.; King, P.D.; Kamiya, N. Targeted Disruption of Shp2 in Chondrocytes Leads to Metachondromatosis With Multiple Cartilaginous Protrusions. J. Bone Miner. Res. 2013, 29, 761–769. [Google Scholar] [CrossRef]
- Wang, L.; Huang, J.; Moore, D.C.; Song, Y.; Ehrlich, M.G.; Yang, W. SHP2 regulates intramembranous ossification by modifying the TGFβ and BMP2 signaling pathway. Bone 2019, 120, 327–335. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.; Huang, J.; Pei, S.; Feng, J.Q.; Jing, D.; Zhao, H.; Kronenberg, H.M.; Moore, D.C.; Yang, W. Targeted Ptpn11 deletion in mice reveals the essential role of SHP2 in osteoblast differentiation and skeletal homeostasis. Bone Res. 2021, 9, 6. [Google Scholar] [CrossRef]
- Guo, W.; Liu, W.; Chen, Z.; Gu, Y.; Peng, S.; Shen, L.; Shen, Y.; Wang, X.; Feng, G.-S.; Sun, Y.; et al. Tyrosine phosphatase SHP2 negatively regulates NLRP3 inflammasome activation via ANT1-dependent mitochondrial homeostasis. Nat. Commun. 2017, 8, 2168. [Google Scholar] [CrossRef]
- Heun, Y.; Pogoda, K.; Anton, M.; Pircher, J.; Pfeifer, A.; Woernle, M.; Ribeiro, A.; Kameritsch, P.; Mykhaylyk, O.; Plank, C.; et al. HIF-1α Dependent Wound Healing Angiogenesis In Vivo Can Be Controlled by Site-Specific Lentiviral Magnetic Targeting of SHP-2. Mol. Ther. 2017, 25, 1616–1627. [Google Scholar] [CrossRef]
- Nakamura, T.; Gulick, J.; Colbert, M.C.; Robbins, J. Protein tyrosine phosphatase activity in the neural crest is essential for normal heart and skull development. Proc. Natl. Acad. Sci. USA 2009, 106, 11270–11275. [Google Scholar] [CrossRef] [PubMed]
- Van Der Burgt, I. Noonan syndrome. Orphanet J. Rare Dis. 2007, 2, 4. [Google Scholar] [CrossRef]
- Yart, A.; Edouard, T. Noonan syndrome: An update on growth and development. Curr. Opin. Endocrinol. Diabetes 2018, 25, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.E.; Allanson, J.E.; Tartaglia, M.; Gelb, B.D. Noonan syndrome. Lancet 2013, 381, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Sarkozy, A.; Digilio, M.C.; Dallapiccola, B. Leopard syndrome. Orphanet J. Rare Dis. 2008, 3, 13. [Google Scholar] [CrossRef]
- Aboh, I.V.; Chisci, G.; Gennaro, P.; Gabriele, G.; Cascino, F.; Ginori, A.; Giovannetti, F.; Iannetti, G. LEOPARD Syndrome: Maxillofacial care. J. Craniofacial Surg. 2014, 25, 1094–1095. [Google Scholar] [CrossRef] [PubMed]
- Dzemeshkevich, S.; Frolova, J.; Betekhtin, M.; Shapieva, A.; Rizun, L. The case of 17-year-old male with LEOPARD syndrome. J. Cardiol. Cases 2012, 7, e37–e41. [Google Scholar] [CrossRef]
- Fisher, T.J.; Williams, N.; Morris, L.; Cundy, P.J. Metachondromatosis: More than just multiple osteochondromas. J. Child. Orthop. 2013, 7, 455–464. [Google Scholar] [CrossRef]
- Jamshidi, K.; Shooshtarizadeh, T.; Bahrabadi, M. Chondrosarcoma in Metachondromatosis: A Rare Case Report. Acta Med. Iran. 2017, 55, 793–799. [Google Scholar]
- Tartaglia, M.; Gelb, B.D. Germ-line and somatic PTPN11 mutations in human disease. Eur. J. Med. Genet. 2005, 48, 81–96. [Google Scholar] [CrossRef]
- Hasle, H. Malignant Diseases in Noonan Syndrome and Related Disorders. Horm. Res. Paediatr. 2009, 72, 8–14. [Google Scholar] [CrossRef]
- Moore, E.R.; Chen, J.C.; Jacobs, C.R. Prx1-Expressing Progenitor Primary Cilia Mediate Bone Formation in response to Mechanical Loading in Mice. Stem Cells Int. 2019, 2019, 3094154. [Google Scholar] [CrossRef]
- de Lageneste, O.D.; Julien, A.; Abou-Khalil, R.; Frangi, G.; Carvalho, C.; Cagnard, N.; Cordier, C.; Conway, S.J.; Colnot, C. Periosteum contains skeletal stem cells with high bone regenerative potential controlled by Periostin. Nat. Commun. 2018, 9, 773. [Google Scholar] [CrossRef]
- Zuo, C.; Wang, L.; Kamalesh, R.M.; Bowen, M.E.; Moore, D.C.; Dooner, M.S.; Reginato, A.M.; Wu, Q.; Schorl, C.; Song, Y.; et al. SHP2 regulates skeletal cell fate by modifying SOX9 expression and transcriptional activity. Bone Res. 2018, 6, 12. [Google Scholar] [CrossRef]
- Zhou, Y.; Mohan, A.; Moore, D.C.; Lin, L.; Zhou, F.L.; Cao, J.; Wu, Q.; Qin, Y.; Reginato, A.M.; Ehrlich, M.G.; et al. SHP2 regulates osteoclastogenesis by promoting preosteoclast fusion. FASEB J. 2015, 29, 1635–1645. [Google Scholar] [CrossRef]
- Kim, H.K.W.; Aruwajoye, O.; Sucato, D.; Richards, B.S.; Feng, G.-S.; Chen, D.; King, P.D.; Kamiya, N. Induction of SHP2 Deficiency in Chondrocytes Causes Severe Scoliosis and Kyphosis in Mice. Spine 2013, 38, E1307–E1312. [Google Scholar] [CrossRef]
- Kamiya, N.; Shen, J.; Noda, K.; Kitami, M.; Feng, G.-S.; Chen, D.; Komatsu, Y. SHP2-Deficiency in Chondrocytes Deforms Orofacial Cartilage and Ciliogenesis in Mice. J. Bone Miner. Res. 2015, 30, 2028–2032. [Google Scholar] [CrossRef]
- Wang, L.J.; Huang, J.H.; Moore, U.C.; Zuo, C.L.; Wu, Q.; Xie, L.Q.; Von Der Mark, K.; Yuan, X.; Chen, D.; Warman, M.L.; et al. SHP2 Regulates the Osteogenic Fate of Growth Plate Hypertrophic Chondrocytes. Sci. Rep. 2017, 7, 12699. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.; Liu, Q.; Zhu, Y.; Fan, Z.; Chen, W.; Liu, S.; Li, X.; Guo, W.; Feng, G.-S.; Yu, H.; et al. Targeting chondrocytes for arresting bony fusion in ankylosing spondylitis. Nat. Commun. 2021, 12, 6540. [Google Scholar] [CrossRef]
- Miah, S.M.S.; Jayasuriya, C.T.; Salter, A.; Reilly, E.C.; Fugere, C.; Yang, W.; Chen, Q.; Brossay, L. Ptpn11 Deletion in CD4+ Cells Does Not Affect T Cell Development and Functions but Causes Cartilage Tumors in a T Cell-Independent Manner. Front. Immunol. 2017, 8, 132. [Google Scholar] [CrossRef]
- Fan, D.; Liu, S.; Jiang, S.; Li, Z.; Mo, X.; Ruan, H.; Zou, G.-M.; Fan, C. The use of SHP-2 gene transduced bone marrow mesenchymal stem cells to promote osteogenic differentiation and bone defect repair in rat. J. Biomed. Mater. Res. Part A 2016, 104, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.-P.; Deng, M.; Chen, L.-Y.; Fang, N.; Du, C.; Chen, L.; Zou, Y.; Dai, J.; Zhu, M.; Wang, W.; et al. Shp2 regulates chlorogenic acid-induced proliferation and adipogenic differentiation of bone marrow-derived mesenchymal stem cells in adipogenesis. Mol. Med. Rep. 2015, 11, 4489–4495. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.P.; Lin, S.J.; Wan, W.B.; Zuo, H.L.; Yao, F.F.; Ruan, H.B.; Xu, J.; Song, W.; Zhou, Y.C.; Wen, S.Y.; et al. Chlorogenic Acid Prevents Osteoporosis by Shp2/PI3K/Akt Pathway in Ovariectomized Rats. PLoS ONE 2016, 11, e0166751. [Google Scholar] [CrossRef]
- Roubelakis, M.G.; Tsaknakis, G.; Lyu, F.-J.; Trohatou, O.; Zannettino, A.C.W.; Watt, S.M. P0-Related Protein Accelerates Human Mesenchymal Stromal Cell Migration by Modulating VLA-5 Interactions with Fibronectin. Cells 2020, 9, 1100. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jing, H.; Kou, X.; Chen, C.; Liu, D.; Jin, Y.; Lu, L.; Shi, S. PD-1 is required to maintain stem cell properties in human dental pulp stem cells. Cell Death Differ. 2018, 25, 1350–1360. [Google Scholar] [CrossRef]
- Lapinski, P.E.; Meyer, M.F.; Feng, G.-S.; Kamiya, N.; King, P.D. Deletion of SHP-2 in mesenchymal stem cells causes growth retardation, limb and chest deformity, and calvarial defects in mice. Dis. Model. Mech. 2013, 6, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- Bauler, T.; Kamiya, N.; Lapinski, P.E.; Langewisch, E.; Mishina, Y.; Wilkinson, J.E.; Feng, G.-S.; King, P.D. Development of severe skeletal defects in induced SHP-2-deficient adult mice: A model of skeletal malformation in humans with SHP-2 mutations. Dis. Model. Mech. 2011, 4, 228–239. [Google Scholar] [CrossRef]
- Wang, L.; Iorio, C.; Yan, K.; Yang, H.; Takeshita, S.; Kang, S.; Neel, B.G.; Yang, W. A ERK/RSK-mediated negative feedback loop regulates M-CSF–evoked PI3K/AKT activation in macrophages. FASEB J. 2018, 32, 875–887. [Google Scholar] [CrossRef]
- Sims, N.A.; Jenkins, B.J.; Quinn, J.M.; Nakamura, A.; Glatt, M.; Gillespie, M.T.; Ernst, M.; Martin, T.J. Glycoprotein 130 regulates bone turnover and bone size by distinct downstream signaling pathways. J. Clin. Investig. 2004, 113, 379–389. [Google Scholar] [CrossRef]
- Tao, T.; Luo, D.; Gao, C.; Liu, H.; Lei, Z.; Liu, W.; Zhou, C.; Qi, D.; Deng, Z.; Sun, X.; et al. Src Homology 2 Domain-Containing Protein Tyrosine Phosphatase Promotes Inflammation and Accelerates Osteoarthritis by Activating β-Catenin. Front. Cell Dev. Biol. 2021, 9, 646386. [Google Scholar] [CrossRef]
- Liu, Q.; Zhai, L.; Han, M.; Shi, D.; Sun, Z.; Peng, S.; Wang, M.; Zhang, C.; Gao, J.; Yan, W.; et al. SH2 Domain–Containing Phosphatase 2 Inhibition Attenuates Osteoarthritis by Maintaining Homeostasis of Cartilage Metabolism via the Docking Protein 1/Uridine Phosphorylase 1/Uridine Cascade. Arthritis Rheumatol. 2022, 74, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Kang, L.; Li, C.; Chen, S.; Wang, Q.; Yang, J.; Long, Y.; Li, J.; Zhao, K.; Xu, W.; et al. A self-powered implantable and bioresorbable electrostimulation device for biofeedback bone fracture healing. Proc. Natl. Acad. Sci. USA 2021, 118, e2100772118. [Google Scholar] [CrossRef]
- Walsh, M.C.; Takegahara, N.; Kim, H.; Choi, Y. Updating osteoimmunology: Regulation of bone cells by innate and adaptive immunity. Nat. Rev. Rheumatol. 2018, 14, 146–156. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, X.; Liu, J.; Zheng, Y.; Wu, Y.; Yang, W.; Yi, Y.; Liu, J.; Wang, J. d-mannose attenuates lipopolysaccharide-induced osteolysis via CPT1A-Mediated lipid metabolic regulation in macrophages. Biochem. Biophys. Res. Commun. 2021, 583, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Graney, P.L.; Roohani-Esfahani, S.I.; Zreiqat, H.; Spiller, K.L. In vitro response of macrophages to ceramic scaffolds used for bone regeneration. J. R. Soc. Interface 2016, 13, 20160346. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, K.M.; Reddy, G.B.; Hyzy, S.L.; Schwartz, Z.; Boyan, B.D.; Olivares-Navarrete, R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2015, 31, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Zhao, Y.; Zhang, Y.; Ruan, Z. The Macrophage Polarization Regulates MSC Osteoblast Differentiation in vitro. Ann. Clin. Lab. Sci. 2016, 46, 65–71. [Google Scholar] [PubMed]
- von Kaeppler, E.P.; Wang, Q.; Raghu, H.; Bloom, M.S.; Wong, H.; Robinson, W.H. Interleukin 4 promotes anti-inflammatory macrophages that clear cartilage debris and inhibits osteoclast development to protect against osteoarthritis. Clin. Immunol. 2021, 229, 108784. [Google Scholar] [CrossRef]
- Xiao, P.; Guo, Y.; Zhang, H.; Zhang, X.; Cheng, H.; Cao, Q.; Ke, Y. Myeloid-restricted ablation of Shp2 restrains melanoma growth by amplifying the reciprocal promotion of CXCL9 and IFN-γ production in tumor microenvironment. Oncogene 2018, 37, 5088–5100. [Google Scholar] [CrossRef]
- Wang, Y.; Mohseni, M.; Grauel, A.; Diez, J.E.; Guan, W.; Liang, S.; Choi, J.E.; Pu, M.; Chen, D.; Laszewski, T.; et al. SHP2 blockade enhances anti-tumor immunity via tumor cell intrinsic and extrinsic mechanisms. Sci. Rep. 2021, 11, 1399. [Google Scholar] [CrossRef]
- Batoon, L.; Millard, S.; Raggatt, L.J.; Pettit, A.R. Osteomacs and Bone Regeneration. Curr. Osteoporos. Rep. 2017, 15, 385–395. [Google Scholar] [CrossRef]
- Batoon, L.; Millard, S.M.; Wullschleger, M.E.; Preda, C.; Wu, A.C.-K.; Kaur, S.; Tseng, H.-W.; Hume, D.A.; Levesque, J.-P.; Raggatt, L.J.; et al. CD169+ macrophages are critical for osteoblast maintenance and promote intramembranous and endochondral ossification during bone repair. Biomaterials 2019, 196, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, L.; Abou-Ezzi, G.; Ciucci, T.; Amiot, V.; Belaïd, N.; Obino, D.; Mansour, A.; Rouleau, M.; Wakkach, A.; Blin-Wakkach, C. Inflammatory Osteoclasts Prime TNFα-Producing CD4+T Cells and Express CX3CR1. J. Bone Miner. Res. 2016, 31, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, Y.; Yang, Y.; Liu, Y.; Tian, Q.; Liu, P.; Ding, Z.; Cheng, H.; Zhang, X.; Ke, Y. Tyrosine phosphatase Shp2 regulates p115RhoGEF/Rho-dependent dendritic cell migration. Cell. Mol. Immunol. 2020, 18, 755–757. [Google Scholar] [CrossRef]
- Weitzmann, M.N.; Ofotokun, I. Physiological and pathophysiological bone turnover—Role of the immune system. Nat. Rev. Endocrinol. 2016, 12, 518–532. [Google Scholar] [CrossRef]
- Li, Y.; Toraldo, G.; Li, A.; Yang, X.; Zhang, H.; Qian, W.-P.; Weitzmann, M.N. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood 2007, 109, 3839–3848. [Google Scholar] [CrossRef] [PubMed]
- Weitzmann, M.N. Bone and the Immune System. Toxicol. Pathol. 2017, 45, 911–924. [Google Scholar] [CrossRef]
- Friederichs, K.; Schmitz, J.; Weissenbach, M.; Heinrich, P.C.; Schaper, F. Interleukin-6-induced proliferation of pre-B cells mediated by receptor complexes lacking the SHP2/SOCS3 recruitment sites revisited. JBIC J. Biol. Inorg. Chem. 2001, 268, 6401–6407. [Google Scholar] [CrossRef]
- Tamir, I.; Porto, J.M.D.; Cambier, J.C. Cytoplasmic protein tyrosine phosphatases SHP-1 and SHP-2: Regulators of B cell signal transduction. Curr. Opin. Immunol. 2000, 12, 307–315. [Google Scholar] [CrossRef]
- Bozec, A.; Zaiss, M.M. T Regulatory Cells in Bone Remodelling. Curr. Osteoporos. Rep. 2017, 15, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Yokosuka, T.; Takamatsu, M.; Kobayashi-Imanishi, W.; Hashimoto-Tane, A.; Azuma, M.; Saito, T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J. Exp. Med. 2012, 209, 1201–1217. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, E.; Partap, S.; Azevedo, M.M.; Jell, G.; Stevens, M.M.; O’Brien, F.J. Hypoxia-mimicking bioactive glass/collagen glycosaminoglycan composite scaffolds to enhance angiogenesis and bone repair. Biomaterials 2015, 52, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.-Y.; Lin, R.-B.; Huang, S.-H.; Liang, Y.-J.; Li, X.; Zhang, S.-E.; Ouyang, D.-Q.; Li, K.; Zheng, G.-S.; Liao, G.-Q. PDGF-BB exhibited therapeutic effects on rat model of bisphosphonate-related osteonecrosis of the jaw by enhancing angiogenesis and osteogenesis. Bone 2019, 144, 115117. [Google Scholar] [CrossRef]
- Kusumbe, A.P.; Ramasamy, S.K.; Adams, R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 2014, 507, 323–328, Erratum in Nature 2014, 513, 574. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, J.; Jing, W. Motivating role of type H vessels in bone regeneration. Cell Prolif. 2020, 53, e12874. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, S.; Li, Y.; Crane, J.L. Type H blood vessels in bone modeling and remodeling. Theranostics 2020, 10, 426–436. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, H.; Cai, D.; Zeng, C.; Lai, P.; Shao, Y.; Fang, H.; Li, D.; Ouyang, J.; Zhao, C.; et al. Positive-Feedback Regulation of Subchondral H-Type Vessel Formation by Chondrocyte Promotes Osteoarthritis Development in Mice. J. Bone Miner. Res. 2018, 33, 909–920. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, J.; Qi, T.; Huang, Y.; Lu, Y.; Zhan, T.; Gong, H.; Zhu, Z.; Shi, Y.; Zhou, J.; et al. SHP2 protects endothelial cell barrier through suppressing VE-cadherin internalization regulated by MET-ARF1. FASEB J. 2018, 33, 1124–1137. [Google Scholar] [CrossRef]
- Rieck, S.; Heun, Y.; Heidsieck, A.; Mykhaylyk, O.; Pfeifer, A.; Gleich, B.; Mannell, H.; Wenzel, D. Local anti-angiogenic therapy by magnet-assisted downregulation of SHP2 phosphatase. J. Control. Release 2019, 305, 155–164. [Google Scholar] [CrossRef]
- Wang, Y.; Salvucci, O.; Ohnuki, H.; Tran, A.D.; Ha, T.; Feng, J.; DiPrima, M.; Kwak, H.; Wang, D.; Yu, Y.; et al. Targeting the SHP2 phosphatase promotes vascular damage and inhibition of tumor growth. EMBO Mol. Med. 2021, 13, e14089. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Guo, C.; Ye, Q.; Shi, Y.; Sun, Y.; Zhang, J.; Huang, J.; Huang, Y.; Zeng, C.; Zhang, X.; et al. Endothelial deletion of SHP2 suppresses tumor angiogenesis and promotes vascular normalization. Nat. Commun. 2021, 12, 6310. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Xie, J.; Cai, Z.; Liu, X.; Wen, J.; Cui, Z.-K.; Zhao, R.; Zhou, X.; Chen, J.; Mao, X.; et al. Damaged brain accelerates bone healing by releasing small extracellular vesicles that target osteoprogenitors. Nat. Commun. 2021, 12, 6043. [Google Scholar] [CrossRef] [PubMed]
- Idelevich, A.; Baron, R. Brain to bone: What is the contribution of the brain to skeletal homeostasis? Bone 2018, 115, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Elefteriou, F.; Takeda, S.; Ebihara, K.; Magre, J.; Patano, N.; Kim, C.A.; Ogawa, Y.; Liu, X.; Ware, S.M.; Craigen, W.J.; et al. Serum leptin level is a regulator of bone mass. Proc. Natl. Acad. Sci. USA 2004, 101, 3258–3263. [Google Scholar] [CrossRef] [PubMed]
- Offley, S.C.; Guo, T.Z.; Wei, T.; Clark, J.D.; Vogel, H.; Lindsey, D.P.; Jacobs, C.R.; Yao, W.; Lane, N.E.; Kingery, W.S. Capsaicin-Sensitive Sensory Neurons Contribute to the Maintenance of Trabecular Bone Integrity. J. Bone Miner. Res. 2004, 20, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Matsusue, Y. Neuronal regulation of bone metabolism and anabolism: Calcitonin gene-related peptide-, substance P-, and tyrosine hydroxylase-containing nerves and the bone. Microsc. Res. Tech. 2002, 58, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Orita, S.; Ohtori, S.; Koshi, T.; Yamashita, M.; Yamauchi, K.; Inoue, G.; Suzuki, M.; Eguchi, Y.; Kamoda, H.; Arai, G.; et al. The Effects of Risedronate and Exercise on Osteoporotic Lumbar Rat Vertebrae and Their Sensory Innervation. Spine 2010, 35, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, J.; Chen, X.; Li, Y.; Mi, J.; Qin, L. The Effects of Calcitonin Gene-Related Peptide on Bone Homeostasis and Regeneration. Curr. Osteoporos. Rep. 2020, 18, 621–632. [Google Scholar] [CrossRef]
- Li, J.; Kreicbergs, A.; Bergström, J.; Stark, A.; Ahmed, M. Site-specific CGRP innervation coincides with bone formation during fracture healing and modeling: A study in rat angulated tibia. J. Orthop. Res. 2007, 25, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Park, B.-W.; Kim, J.-R.; Lee, J.-H.; Byun, J.-H. Expression of nerve growth factor and vascular endothelial growth factor in the inferior alveolar nerve after distraction osteogenesis. Int. J. Oral Maxillofac. Surg. 2006, 35, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Tuzmen, C.; Campbell, P.G. Crosstalk between neuropeptides SP and CGRP in regulation of BMP2-induced bone differentiation. Connect. Tissue Res. 2018, 59, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Feng, L.; Zhu, M.-L.; Yang, Z.-M.; Wu, T.-Y.; Xu, J.; Liu, Y.; Lin, W.-P.; Lo, J.H.T.; Zhang, J.-F.; et al. Vasoactive Intestinal Peptide Stimulates Bone Marrow-Mesenchymal Stem Cells Osteogenesis Differentiation by Activating Wnt/β-Catenin Signaling Pathway and Promotes Rat Skull Defect Repair. Stem Cells Dev. 2020, 29, 655–666. [Google Scholar] [CrossRef]
- Ke, Y.; Zhang, E.E.; Hagihara, K.; Wu, D.; Pang, Y.; Klein, R.; Curran, T.; Ranscht, B.; Feng, G.-S. Deletion of Shp2 in the Brain Leads to Defective Proliferation and Differentiation in Neural Stem Cells and Early Postnatal Lethality. Mol. Cell. Biol. 2007, 27, 6706–6717. [Google Scholar] [CrossRef]
- Hagihara, K.; Zhang, E.; Ke, Y.-H.; Liu, G.; Liu, J.-J.; Rao, Y.; Feng, G.-S. Shp2 acts downstream of SDF-1α/CXCR4 in guiding granule cell migration during cerebellar development. Dev. Biol. 2009, 334, 276–284. [Google Scholar] [CrossRef]
- He, Z.; Zhang, S.S.; Meng, Q.; Li, S.; Zhu, H.H.; Raquil, M.-A.; Alderson, N.; Zhang, H.; Wu, J.; Rui, L.; et al. Shp2 Controls Female Body Weight and Energy Balance by Integrating Leptin and Estrogen Signals. Mol. Cell. Biol. 2012, 32, 1867–1878. [Google Scholar] [CrossRef]
- Krajewska, M.; Banares, S.; Zhang, E.E.; Huang, X.; Scadeng, M.; Jhala, U.S.; Feng, G.-S.; Krajewski, S. Development of Diabesity in Mice with Neuronal Deletion of Shp2 Tyrosine Phosphatase. Am. J. Pathol. 2008, 172, 1312–1324. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, M.; Shen, L.; Zhu, Y.; Ma, H.; Liu, B.; Ouyang, L.; Guo, W.; Xu, Q.; Sun, Y. SHP2-mediated mitophagy boosted by lovastatin in neuronal cells alleviates parkinsonism in mice. Signal Transduct. Target. Ther. 2021, 6, 34. [Google Scholar] [CrossRef]
- Xie, L.; Zhu, G.; Shang, J.; Chen, X.; Zhang, C.; Ji, X.; Zhang, Q.; Wei, Y. An overview on the biological activity and anti-cancer mechanism of lovastatin. Cell. Signal. 2021, 87, 110122. [Google Scholar] [CrossRef]
- Garrett, I.; Gutierrez, G.; Rossini, G.; Nyman, J.; McCluskey, B.; Flores, A.; Mundy, G. Locally delivered lovastatin nanoparticles enhance fracture healing in rats. J. Orthop. Res. 2007, 25, 1351–1357. [Google Scholar] [CrossRef]
- Bleedorn, J.A.; Sullivan, R.; Lu, Y.; Nemke, B.; Kalscheur, V.; Markel, M.D. Percutaneous lovastatin accelerates bone healing but is associated with periosseous soft tissue inflammation in a canine tibial osteotomy model. J. Orthop. Res. 2013, 32, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Montoya, C.J.; Jaimes, F.; Higuita, E.A.; Convers-Paez, S.; Estrada, S.; Gutierrez, F.; Amariles, P.; Giraldo, N.; Penaloza, C.; Rugeles, M.T. Antiretroviral effect of lovastatin on HIV-1-infected individuals without highly active antiretroviral therapy (The LIVE study): A phase-II randomized clinical trial. Trials 2009, 10, 41. [Google Scholar] [CrossRef]
- Amadasu, E.; Kang, R.; Usmani, A.; Borlongan, C.V. Effects of Lovastatin on Brain Cancer Cells. Cell Transplant. 2022, 31, 9636897221102903. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-N.P.; Lamarche, M.J.; Chan, H.M.; Fekkes, P.; Garcia-Fortanet, J.; Acker, M.G.; Antonakos, B.; Chen, C.H.-T.; Chen, Z.; Cooke, V.G.; et al. Allosteric inhibition of SHP2 phosphatase inhibits cancers driven by receptor tyrosine kinases. Nature 2016, 535, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Miao, L.; Lin, H.; Cheng, J.; Li, M.; Zhuo, Z.; He, J. Targeting RAS in neuroblastoma: Is it possible? Pharmacol. Ther. 2022, 236, 108054. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wu, Z.; Zhao, M.; Zhang, R.; Li, M.; Sun, D.; Cheng, H.; Qi, X.; Shen, Y.; Xu, Q.; et al. Allosteric inhibition reveals SHP2-mediated tumor immunosuppression in colon cancer by single-cell transcriptomics. Acta Pharm. Sin. B 2021, 12, 149–166. [Google Scholar] [CrossRef]
- Kano, H.; Ichihara, E.; Watanabe, H.; Nishii, K.; Ando, C.; Nakasuka, T.; Ninomiya, K.; Kato, Y.; Kubo, T.; Rai, K.; et al. SHP2 Inhibition Enhances the Effects of Tyrosine Kinase Inhibitors in Preclinical Models of Treatment-naïve ALK-, ROS1-, or EGFR-altered Non–small Cell Lung Cancer. Mol. Cancer Ther. 2021, 20, 1653–1662. [Google Scholar] [CrossRef]
- Wang, J.; Huang, L.; Huang, Y.; Jiang, Y.; Zhang, L.; Feng, G.; Liu, L. Therapeutic effect of the injectable thermosensitive hydrogel loaded with SHP099 on intervertebral disc degeneration. Life Sci. 2020, 266, 118891. [Google Scholar] [CrossRef]
- Yin, H.; Huang, J.; Cao, X.; Wang, Z.-X.; Cao, J.; Hu, Y.; Luo, J.; Tan, Y.-J.; Chen, T.-H.; Chen, C.-Y.; et al. Inhibition of Src Homology 2 Domain-Containing Protein Tyrosine Phosphatase-2 Facilitates CD31hiEndomucinhi Blood Vessel and Bone Formation in Ovariectomized Mice. Cell. Physiol. Biochem. 2018, 50, 1068–1083. [Google Scholar] [CrossRef]
- LaMarche, M.J.; Acker, M.G.; Argintaru, A.; Bauer, D.; Boisclair, J.; Chan, H.; Chen, C.H.-T.; Chen, Y.-N.P.; Chen, Z.; Deng, Z.; et al. Identification of TNO155, an Allosteric SHP2 Inhibitor for the Treatment of Cancer. J. Med. Chem. 2020, 63, 13578–13594. [Google Scholar] [CrossRef]
- Shen, D.; Chen, W.; Zhu, J.; Wu, G.; Shen, R.; Xi, M.; Sun, H. Therapeutic potential of targeting SHP2 in human developmental disorders and cancers. Eur. J. Med. Chem. 2020, 190, 112117. [Google Scholar] [CrossRef] [PubMed]
- Tajan, M.; Pernin-Grandjean, J.; Beton, N.; Gennero, I.; Capilla, F.; Neel, B.G.; Araki, T.; Valet, P.; Tauber, M.; Salles, J.-P.; et al. Noonan syndrome-causing SHP2 mutants impair ERK-dependent chondrocyte differentiation during endochondral bone growth. Hum. Mol. Genet. 2018, 27, 2276–2289. [Google Scholar] [CrossRef] [PubMed]
| Target Cell | Phenotype | Mechanism | Related Signaling Pathways | References |
|---|---|---|---|---|
| Prrx1+ mesenchyme stem cells | Skeletal dysplasia; impaired ossification in skull, long bones, ribs, limbs and joint; pectus excavatum and pectus carinatum; endochondral ossification; exostoses | Chondrogenic transcription factors: SOX9, Acan, Col2a1, Col10a1↑ Osteogenic transcription factors: ALP, Col1a1, Ctnnb1, Sp7, RUNX2↓ | TGF-β/SMAD2/3, BMP2/SMAD1/5/8↓ | [17,32,33,34] |
| Bglap+ osteoblasts | Scoliosis, osteoporosis, osteochondromas, enchondromas | Osteogenic transcription factors: RUNX2, Osterix7, Dmp1, Sost↓ | STAT3/RANKL↑ | [18] |
| LysM+ osteoclasts precursors | Age-related osteopetrosis | Osteoclastogenesis transcription factors: Nfatc1↓ | AKT↓, RAS /ERK↓ | [8] |
| CTSK+ osteoclasts | Osteopetrosis, scoliosis, exostoses and enchondromas | Reducing osteoclasts activity | MAPK↓, IHH↑ | [8,35] |
| Col2a1+ chondrocytes | Spinal deformities, scoliosis, kyphosis, lordosis, enchondroma and exostosis | Chondrogenic transcription factors: SOX9, BMP6↑ Osteogenic transcription factors: ALP↓ | IHH↑, MAPK↓, β-catenin↓ | [15,16,36,37] |
| Fsp1+ expressing fibroblasts | Exostosis | Inducing normal cells undergo chondrogenesis by paracrine | Unknow | [15] |
| Col10a-1+ chondrocytes | Bone mineral density reduction | Chondrogenic transcription factors: SOX9↑ Osteogenic transcription factors: Ibsp, RUNX2, Ctnnb1↓ | WNT/β-catenin↓ | [38] |
| CD4+ chondrocytes | Bone fusion and joint stiffness, ankylosing spondylitis, osteoporosis | Chondrogenic transcription factors: Col2a1, Col10a1, Acan, and Pthrp↑ Osteogenic transcription factors: RUNX2, Sp7, Ocn↑ | BMP6/Smad1/5↑, ERK1/2↓ IHH↓ | [39,40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Ye, C.; Zhu, Y.; Wang, J.; Liu, J. The Cell-Specific Role of SHP2 in Regulating Bone Homeostasis and Regeneration Niches. Int. J. Mol. Sci. 2023, 24, 2202. https://doi.org/10.3390/ijms24032202
Zhang J, Ye C, Zhu Y, Wang J, Liu J. The Cell-Specific Role of SHP2 in Regulating Bone Homeostasis and Regeneration Niches. International Journal of Molecular Sciences. 2023; 24(3):2202. https://doi.org/10.3390/ijms24032202
Chicago/Turabian StyleZhang, Jie, Chengxinyue Ye, Yufan Zhu, Jun Wang, and Jin Liu. 2023. "The Cell-Specific Role of SHP2 in Regulating Bone Homeostasis and Regeneration Niches" International Journal of Molecular Sciences 24, no. 3: 2202. https://doi.org/10.3390/ijms24032202
APA StyleZhang, J., Ye, C., Zhu, Y., Wang, J., & Liu, J. (2023). The Cell-Specific Role of SHP2 in Regulating Bone Homeostasis and Regeneration Niches. International Journal of Molecular Sciences, 24(3), 2202. https://doi.org/10.3390/ijms24032202







