Transcriptional Analysis of the Differences between ToLCNDV-India and ToLCNDV-ES Leading to Contrary Symptom Development in Cucumber
Abstract
1. Introduction
2. Results
2.1. Infectivity of Two ToLCNDV Clones in Cucumber
2.2. Determination of the DNA Component Responsible for Viral Disease Symptoms in Cucumber
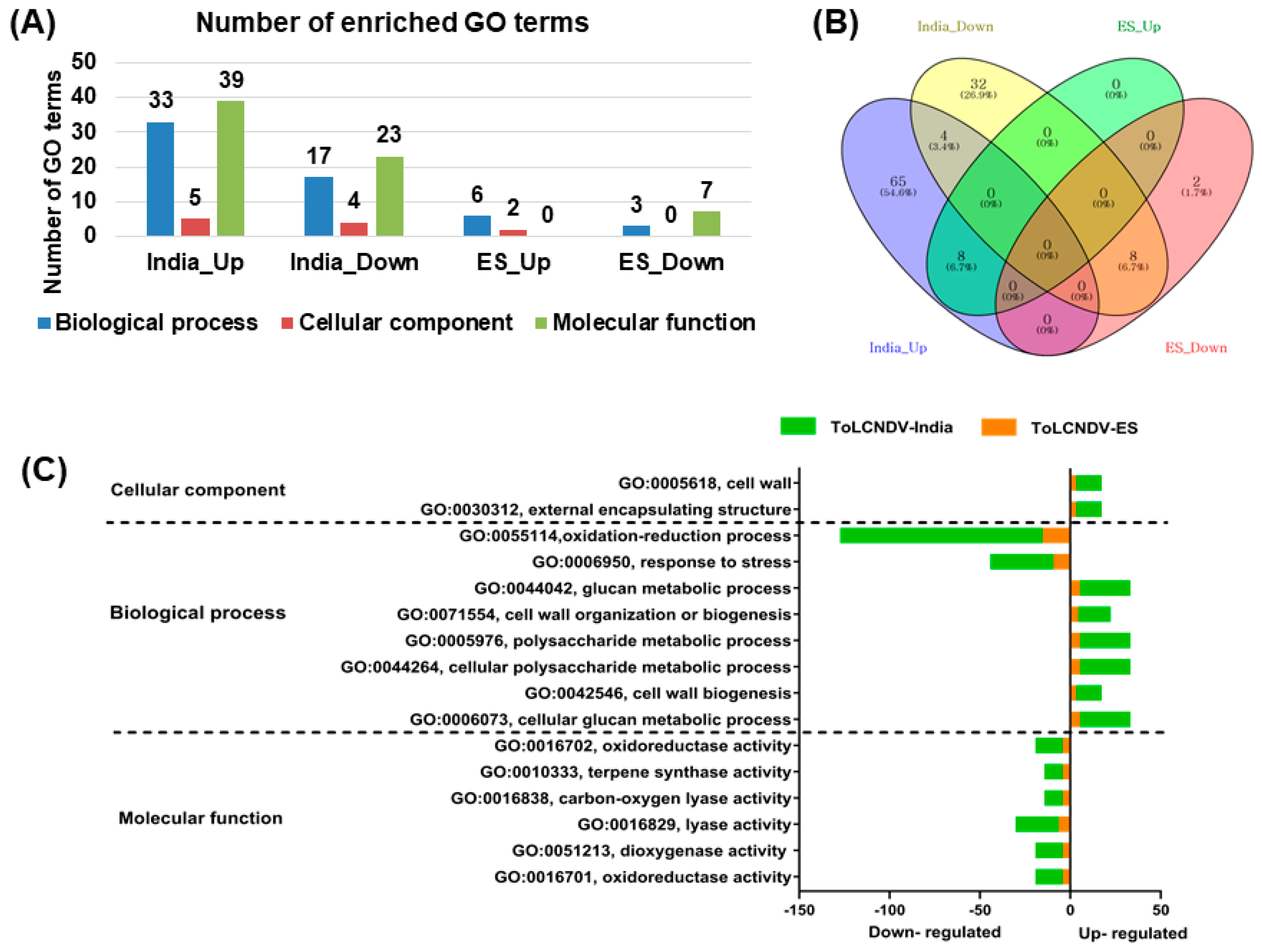
2.3. Identification of Differentially Expressed Genes
2.4. GO Enrichment Analysis of Identified DEGs
2.5. Pathway Annotation of DEGs
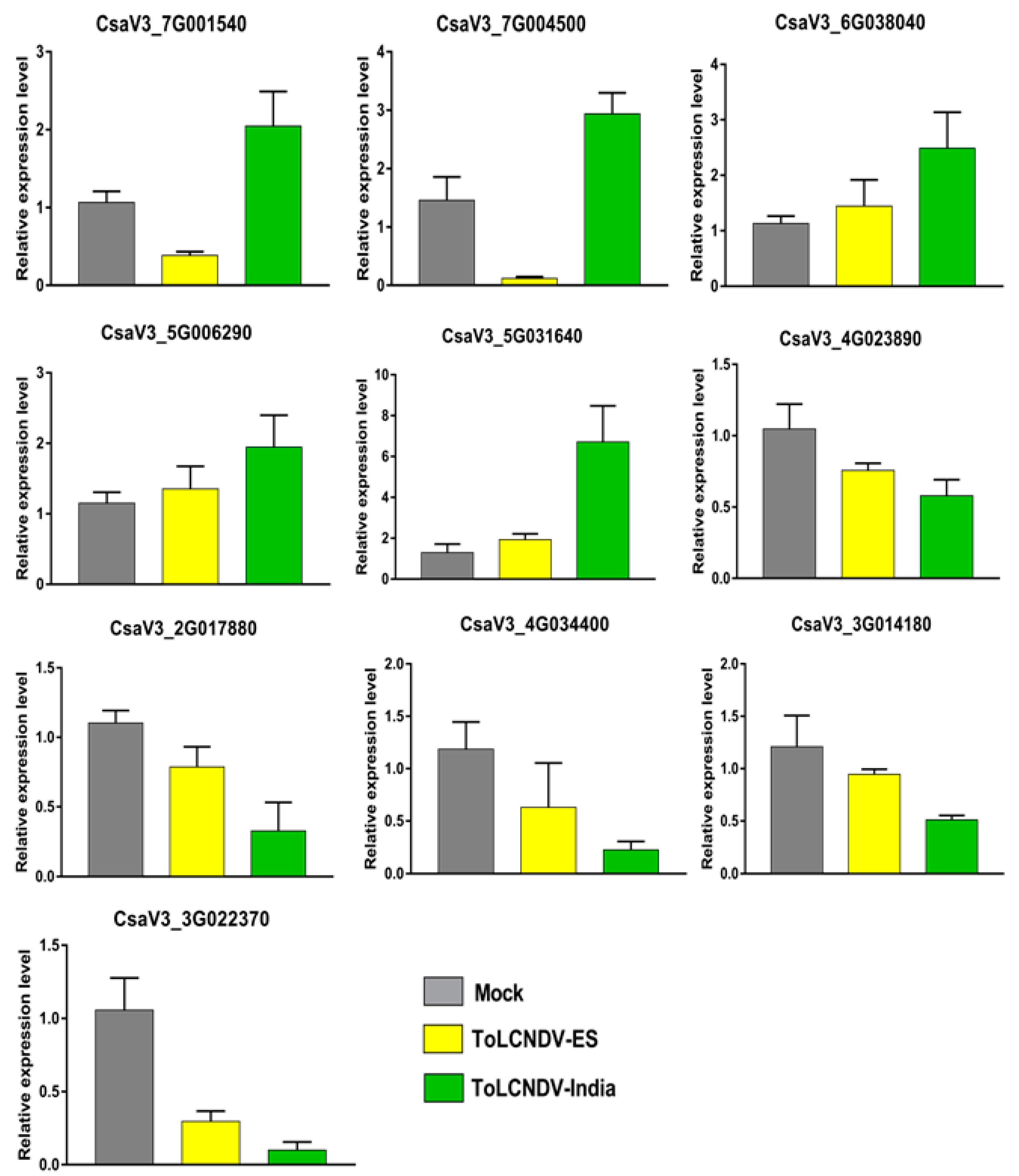
2.6. Validation of the Transcriptome Data by qRT-PCR
3. Discussion
4. Materials and Methods
4.1. Plant Growth Condition and Inoculation of Infectious Clones
4.2. Total RNA Extraction and Illumina Sequencing
4.3. RNA Sequencing Data Analysis
4.4. Analysis of Differentially Expressed Genes
4.5. Gene Ontology and Pathway Enrichment Analysis
4.6. Quantitative Real-Time Polymerase Chain Reaction
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fiallo-Olivé, E.; Lett, J.-M.; Martin, D.P.; Roumagnac, P.; Varsani, A.; Zerbini, F.M.; Navas-Castillo, J. ICTV Virus Taxonomy Profile: Geminiviridae 2021. J. Gen. Virol 2021, 102, 001696. [Google Scholar] [CrossRef] [PubMed]
- Fondong, V.N. Geminivirus protein structure and function. Mol. Plant Pathol. 2013, 14, 635–649. [Google Scholar] [CrossRef]
- Böttcher, B.; Unseld, S.; Ceulemans, H.; Russell, R.B.; Jeske, H. Geminate structures of African cassava mosaic virus. J. Virol. 2004, 78, 6758–6765. [Google Scholar] [CrossRef] [PubMed]
- Padidam, M.; Beachy, R.N.; Fauquet, C.M. Tomato leaf curl geminivirus from India has a bipartite genome and coat protein is not essential for infectivity. J. Gen. Virol 1995, 76 Pt 1, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Kil, E.J.; Vo, T.T.B.; Fadhila, C.; Ho, P.T.; Lal, A.; Troiano, E.; Parrella, G.; Lee, S. Seed Transmission of Tomato Leaf Curl New Delhi Virus from Zucchini Squash in Italy. Plants 2020, 9, 563. [Google Scholar] [CrossRef]
- López, C.; Ferriol, M.; Picó, M.B. Mechanical transmission of Tomato leaf curl New Delhi virus to cucurbit germplasm: Selection of tolerance sources in Cucumis melo. Euphytica 2015, 204, 679–691. [Google Scholar] [CrossRef]
- Sangeetha, B.; Malathi, V.G.; Alice, D.; Suganthy, M.; Renukadevi, P. A distinct seed-transmissible strain of tomato leaf curl New Delhi virus infecting Chayote in India. Virus Res. 2018, 258, 81–91. [Google Scholar] [CrossRef]
- Moriones, E.; Praveen, S.; Chakraborty, S. Tomato leaf curl New Delhi virus: An emerging virus complex threatening vegetable and fiber crops. Viruses 2017, 9, 264. [Google Scholar] [CrossRef]
- Zaidi, S.S.; Martin, D.P.; Amin, I.; Farooq, M.; Mansoor, S. Tomato leaf curl New Delhi virus: A widespread bipartite begomovirus in the territory of monopartite begomoviruses. Mol. Plant Pathol. 2017, 18, 901–911. [Google Scholar] [CrossRef]
- Srivastava, A.; Kumar, S.; Jaidi, M.; Raj, S.K.; Shukla, S.K. First Report of Tomato leaf curl New Delhi virus on Opium Poppy (Papaver somniferum) in India. Plant Dis. 2015, 100, 232. [Google Scholar] [CrossRef]
- Jamil, N.; Rehman, A.; Hamza, M.; Hafeez, A.; Ismail, H.; Zubair, M.; Mansoor, S.; Amin, I. First Report of Tomato leaf curl New Delhi virus, a Bipartite Begomovirus, Infecting Soybean (Glycine max). Plant Dis. 2016, 101, 845. [Google Scholar] [CrossRef]
- Sharma, J.; Lager, P.; Kumar, Y. First report of Tomato leaf curl New Delhi virus infecting Ricinus communis. New Dis. Rep 2021, 44, e12053. [Google Scholar] [CrossRef]
- Venkataravanappa, V.; Lakshminarayana Reddy, C.N.; Jalali, S.; Krishna Reddy, M. Association of tomato leaf curl New Delhi virus DNA-B with bhendi yellow vein mosaic virus in okra showing yellow vein mosaic disease symptoms. Acta Virol. 2015, 59, 125–139. [Google Scholar] [CrossRef]
- Juárez, M.; Tovar, R.; Fiallo-Olivé, E.; Aranda, M.A.; Gosálvez, B.; Castillo, P.; Moriones, E.; Navas-Castillo, J. First Detection of Tomato leaf curl New Delhi virus Infecting Zucchini in Spain. Plant Dis. 2014, 98, 857. [Google Scholar] [CrossRef]
- Mnari-Hattab, M.; Zammouri, S.; Belkadhi, M.; Doña, D.B.; Ben Nahia, E.; Hajlaoui, M. First report of Tomato leaf curl New Delhi virus infecting cucurbits in Tunisia. New Dis. Rep. 2015, 31, 21. [Google Scholar] [CrossRef]
- Panno, S.; Iacono, G.; Davino, M.; Marchione, S.; Zappardo, V.; Bella, P.; Tomassoli, L.; Accotto, G.; Davino, S. First report of Tomato leaf curl New Delhi virus affecting zucchini squash in an important horticultural area of southern Italy. New Dis. Rep. 2016, 33, 6. [Google Scholar] [CrossRef]
- Sifres, A.; Sáez, C.; Ferriol, M.; Selmani, E.A.; Riado, J.; Picó, B.; López, C. First Report of Tomato leaf curl New Delhi virus Infecting Zucchini in Morocco. Plant Dis. 2017, 102, 1045. [Google Scholar] [CrossRef]
- Orfanidou, C.G.; Malandraki, I.; Beris, D.; Kektsidou, O.; Vassilakos, N.; Varveri, C.; Katis, N.I.; Maliogka, V.I. First report of tomato leaf curl New Delhi virus in zucchini crops in Greece. J. Plant Pathol. 2019, 101, 799. [Google Scholar] [CrossRef]
- Desbiez, C.; Gentit, P.; Cousseau-Suhard, P.; Renaudin, I.; Verdin, E. First report of Tomato leaf curl New Delhi virus infecting courgette in France. New Dis. Rep. 2021, 43, e12006. [Google Scholar] [CrossRef]
- Takács, A.; Horváth, J.; Gáborjányi, R.; Kazinczi, G.; Mikulás, J. Chapter 5—Hosts and non-hosts in plant virology and the effects of plant viruses on host plants. In Plant Virus–Host Interaction; Gaur, R.K., Hohn, T., Sharma, P., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 105–124. [Google Scholar]
- Dodds, P.N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Gao, S.; Muegge, K.; Zhang, W.; Zhou, B. Advanced Applications of RNA Sequencing and Challenges. Bioinform. Biol. Insights 2015, 9 (Suppl. 1), 29–46. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, Z.; Gu, Q.; Li, H.; Han, W.; Shi, Y. Transcriptome analysis of Cucumis sativus infected by Cucurbit chlorotic yellows virus. Virol. J. 2017, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Šubr, Z.; Predajňa, L.; Šoltys, K.; Bokor, B.; Budiš, J.; Glasa, M. Comparative Transcriptome Analysis of Two Cucumber Cultivars with Different Sensitivity to Cucumber Mosaic Virus Infection. Pathogens 2020, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Jeevalatha, A.; Siddappa, S.; Kumar, A.; Kaundal, P.; Guleria, A.; Sharma, S.; Nagesh, M.; Singh, B.P. An insight into differentially regulated genes in resistant and susceptible genotypes of potato in response to tomato leaf curl New Delhi virus-[potato] infection. Virus Res. 2017, 232, 22–33. [Google Scholar] [CrossRef]
- Sáez, C.; Flores-León, A.; Montero-Pau, J.; Sifres, A.; Dhillon, N.P.S.; López, C.; Picó, B. RNA-Seq Transcriptome Analysis Provides Candidate Genes for Resistance to Tomato Leaf Curl New Delhi Virus in Melon. Front. Plant Sci. 2022, 12, 798858. [Google Scholar] [CrossRef]
- Patel, C.; Panigrahi, J. Starch glucose coating-induced postharvest shelf-life extension of cucumber. Food Chem. 2019, 288, 208–214. [Google Scholar] [CrossRef]
- Hao, J.; Li, Q.; Yu, H.; Wang, H.; Chai, L.; Miao, T.; Jiang, W. Comparative proteomic analysis of cucumber fruits under nitrogen deficiency at the fruiting stage. Hortic. Plant J. 2021, 7, 59–72. [Google Scholar] [CrossRef]
- Sáez, C.; Ambrosio, L.G.M.; Miguel, S.M.; Valcárcel, J.V.; Díez, M.J.; Picó, B.; López, C. Resistant Sources and Genetic Control of Resistance to ToLCNDV in Cucumber. Microorganisms 2021, 9, 913. [Google Scholar] [CrossRef]
- Liu, X.; Ma, Y.; Zhang, M. Research advances in expansins and expansion-like proteins involved in lignocellulose degradation. Biotechnol. Lett. 2015, 37, 1541–1551. [Google Scholar] [CrossRef]
- Figueiredo, A.; Monteiro, F.; Sebastiana, M. Subtilisin-like proteases in plant–pathogen recognition and immune priming: A perspective. Front. Plant Sci. 2014, 5, 739. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Sharoni, A.M.; Kikuchi, S. Roles of NAC transcription factors in the regulation of biotic and abiotic stress responses in plants. Front. Microbiol. 2013, 4, 248. [Google Scholar] [CrossRef] [PubMed]
- Von Arnim, A.; Stanley, J. Determinants of tomato golden mosaic virus symptom development located on DNA B. Virology 1992, 186, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Mansoor, S.; Iram, S.; Fatima, A.N.; Zafar, Y. The nuclear shuttle protein of Tomato leaf curl New Delhi virus is a pathogenicity determinant. J. Virol. 2005, 79, 4434–4439. [Google Scholar] [CrossRef]
- Ghosh, D.; Chakraborty, S. Molecular interplay between phytohormones and geminiviruses: A saga of a never-ending arms race. J. Exp. Bot 2021, 72, 2903–2917. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Vicente, O.; Boscaiu, M. Flavonoids: Antioxidant compounds for plant defence... and for a healthy human diet. Not. Bot. Horti Agrobot. 2018, 46, 14–21. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as Important Molecules of Plant Interactions with the Environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Fofana, B.; Benhamou, N.; McNally, D.J.; Labbé, C.; Séguin, A.; Bélanger, R.R. Suppression of Induced Resistance in Cucumber Through Disruption of the Flavonoid Pathway. Phytopathology 2005, 95, 114–123. [Google Scholar] [CrossRef]
- Lozano-Durán, R.; Rosas-Díaz, T.; Luna, A.P.; Bejarano, E.R. Identification of host genes involved in geminivirus infection using a reverse genetics approach. PLoS ONE 2011, 6, e22383. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.S.; Naqvi, R.Z.; Asif, M.; Strickler, S.; Shakir, S.; Shafiq, M.; Khan, A.M.; Amin, I.; Mishra, B.; Mukhtar, M.S.; et al. Molecular insight into cotton leaf curl geminivirus disease resistance in cultivated cotton (Gossypium hirsutum). Plant Biotechnol. J. 2020, 18, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.T.B.; Lal, A.; Ho, P.T.; Troiano, E.; Parrella, G.; Kil, E.-J.; Lee, S. Different Infectivity of Mediterranean and Southern Asian Tomato Leaf Curl New Delhi Virus Isolates in Cucurbit Crops. Plants 2022, 11, 704. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.T.B.; Troiano, E.; Lal, A.; Hoang, P.T.; Kil, E.-J.; Lee, S.; Parrella, G. ToLCNDV-ES infection in tomato is enhanced by TYLCV: Evidence from field survey and agroinoculation. Front. Microbiol. 2022, 13, 954460. [Google Scholar] [CrossRef]
- Kucukural, A.; Yukselen, O.; Ozata, D.M.; Moore, M.J.; Garber, M. DEBrowser: Interactive differential expression analysis and visualization tool for count data. BMC Genom. 2019, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

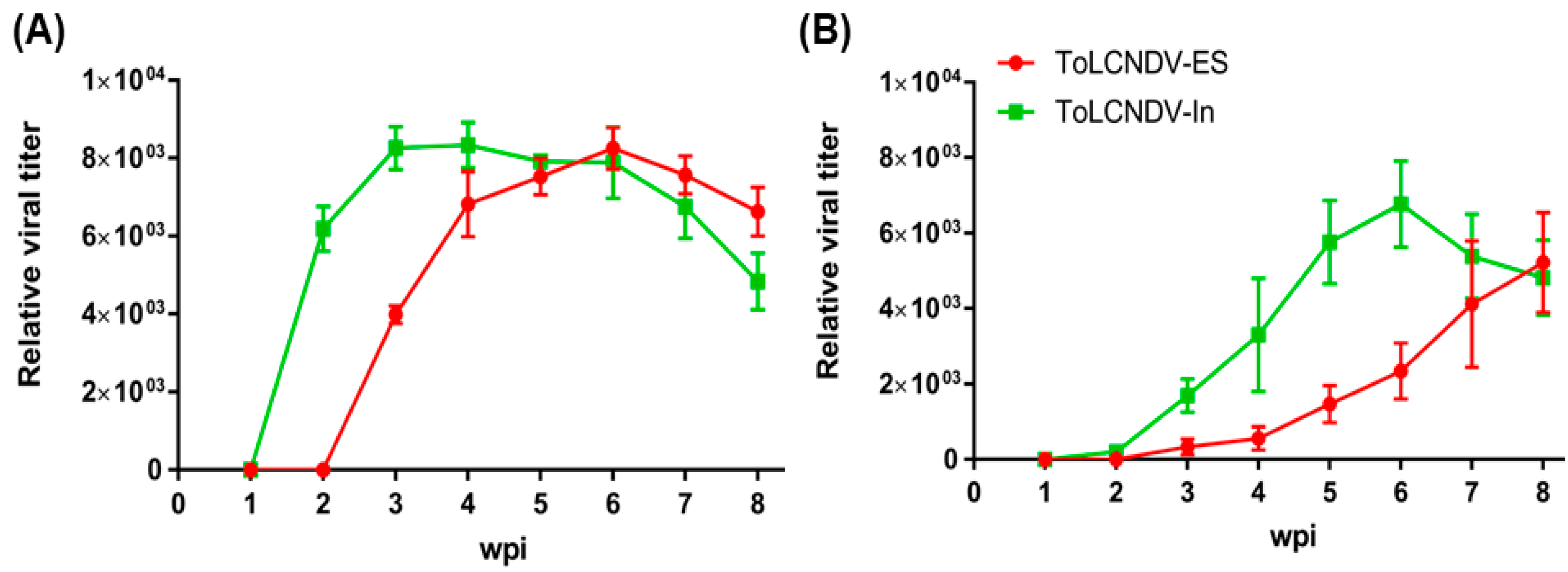
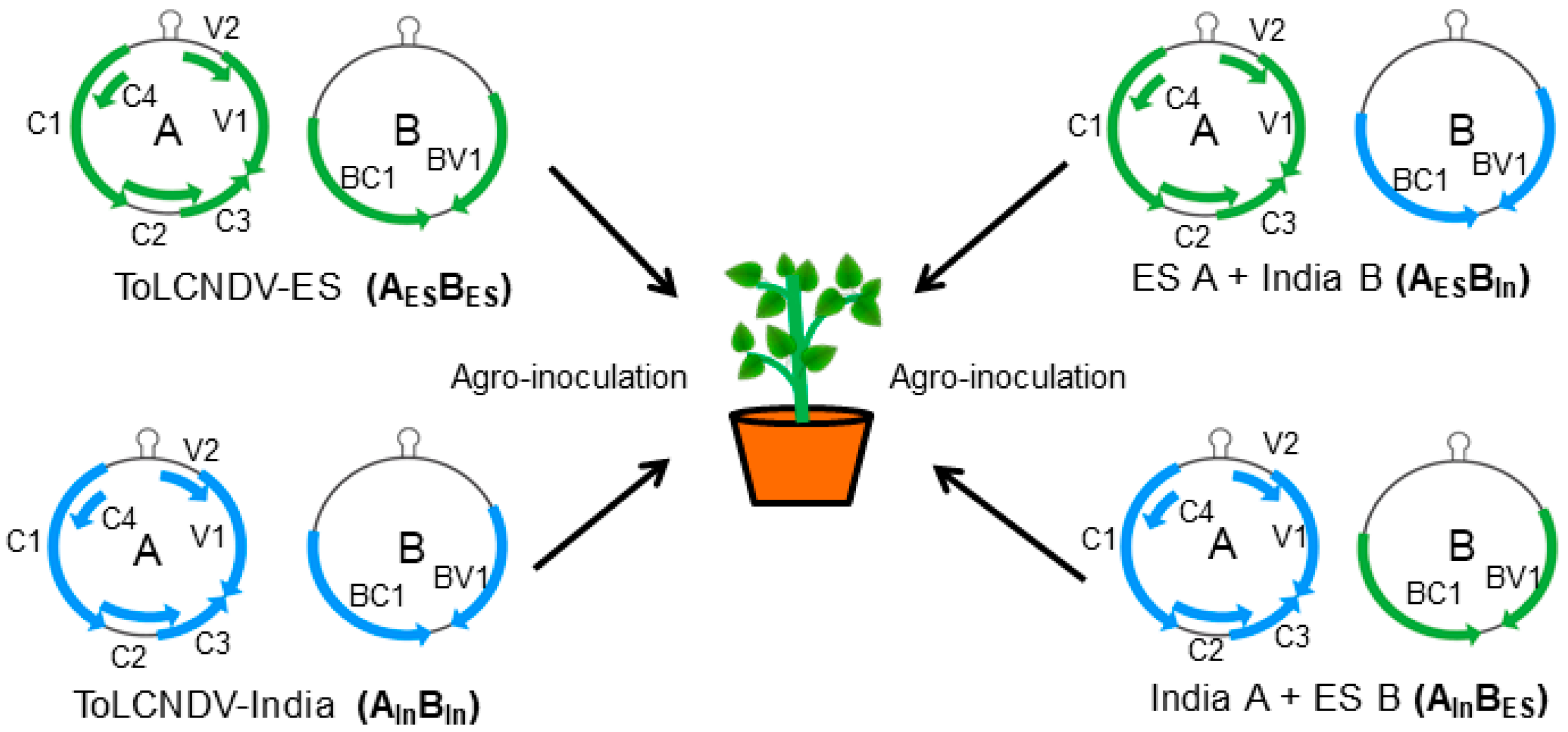




| IC | N. benthamiana | Cucumber | ||||
|---|---|---|---|---|---|---|
| Infectivity | Severity * | Symptoms | Infectivity | Severity * | Symptoms | |
| Mock | 0/9 | - | - | 0/7 | - | - |
| AESBES | 9/9 | +++ | Severe leaf curl, mosaic, stunting | 5/7 | - | No symptoms |
| AInBIn | 9/9 | +++ | Severe leaf curl, mosaic, stunting | 7/7 | ++ | Leaf curl, yellow mosaic |
| AESBIn | 9/9 | +++ | Severe leaf curl, mosaic, stunting | 4/7 | + | Yellow mosaic on leaf |
| AInBES | 9/9 | +++ | Severe leaf curl, mosaic, stunting | 3/7 | + | Yellow mosaic on leaf |
| Index | Infected Condition | Sample ID | Total Read Bases | Total Reads | GC (%) | Q20 (%) | Q30 (%) |
|---|---|---|---|---|---|---|---|
| 1 | ToLCNDV-ES | I-NDV-1-1 | 4,258,032,538 | 42,158,738 | 45.16 | 98.9 | 96.45 |
| 2 | ToLCNDV-ES | I-NDV-1-2 | 4,264,281,004 | 42,220,604 | 45.62 | 98.95 | 96.54 |
| 3 | ToLCNDV-ES | I-NDV-2-1 | 3,917,026,238 | 38,782,438 | 45.41 | 98.9 | 96.41 |
| 4 | ToLCNDV-ES | I-NDV-2-2 | 3,668,776,722 | 36,324,522 | 45.09 | 98.98 | 96.54 |
| 5 | Mock | Mock 1-1 | 3,895,794,422 | 38,572,222 | 45 | 98.89 | 96.38 |
| 6 | Mock | Mock 1-2 | 3,596,973,600 | 35,613,600 | 44.75 | 98.89 | 96.34 |
| 7 | Mock | Mock 2-1 | 3,797,215,190 | 37,596,190 | 44.95 | 98.84 | 96.22 |
| 8 | Mock | Mock 2-2 | 3,931,060,996 | 38,921,396 | 45 | 98.88 | 96.29 |
| 9 | ToLCNDV-India | Pa-NDV-1-1 | 3,523,436,914 | 34,885,514 | 44.79 | 97.54 | 92.9 |
| 10 | ToLCNDV-India | Pa-NDV-1-2 | 4,123,268,238 | 40,824,438 | 44.95 | 97.74 | 93.41 |
| 11 | ToLCNDV-India | Pa-NDV-2-1 | 4,111,628,796 | 40,709,196 | 44.98 | 97.58 | 93.02 |
| 12 | ToLCNDV-India | Pa-NDV-2-2 | 3,201,276,608 | 31,695,808 | 44.97 | 97.42 | 92.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vo, T.T.B.; Cho, W.K.; Jo, Y.; Lal, A.; Nattanong, B.; Qureshi, M.A.; Tabssum, M.; Troiano, E.; Parrella, G.; Kil, E.-J.; et al. Transcriptional Analysis of the Differences between ToLCNDV-India and ToLCNDV-ES Leading to Contrary Symptom Development in Cucumber. Int. J. Mol. Sci. 2023, 24, 2181. https://doi.org/10.3390/ijms24032181
Vo TTB, Cho WK, Jo Y, Lal A, Nattanong B, Qureshi MA, Tabssum M, Troiano E, Parrella G, Kil E-J, et al. Transcriptional Analysis of the Differences between ToLCNDV-India and ToLCNDV-ES Leading to Contrary Symptom Development in Cucumber. International Journal of Molecular Sciences. 2023; 24(3):2181. https://doi.org/10.3390/ijms24032181
Chicago/Turabian StyleVo, Thuy T. B., Won Kyong Cho, Yeonhwa Jo, Aamir Lal, Bupi Nattanong, Muhammad Amir Qureshi, Marjia Tabssum, Elisa Troiano, Giuseppe Parrella, Eui-Joon Kil, and et al. 2023. "Transcriptional Analysis of the Differences between ToLCNDV-India and ToLCNDV-ES Leading to Contrary Symptom Development in Cucumber" International Journal of Molecular Sciences 24, no. 3: 2181. https://doi.org/10.3390/ijms24032181
APA StyleVo, T. T. B., Cho, W. K., Jo, Y., Lal, A., Nattanong, B., Qureshi, M. A., Tabssum, M., Troiano, E., Parrella, G., Kil, E.-J., Lee, T.-K., & Lee, S. (2023). Transcriptional Analysis of the Differences between ToLCNDV-India and ToLCNDV-ES Leading to Contrary Symptom Development in Cucumber. International Journal of Molecular Sciences, 24(3), 2181. https://doi.org/10.3390/ijms24032181







