Proteins Found in the Triple-Negative Breast Cancer Secretome and Their Therapeutic Potential
Abstract
1. Introduction
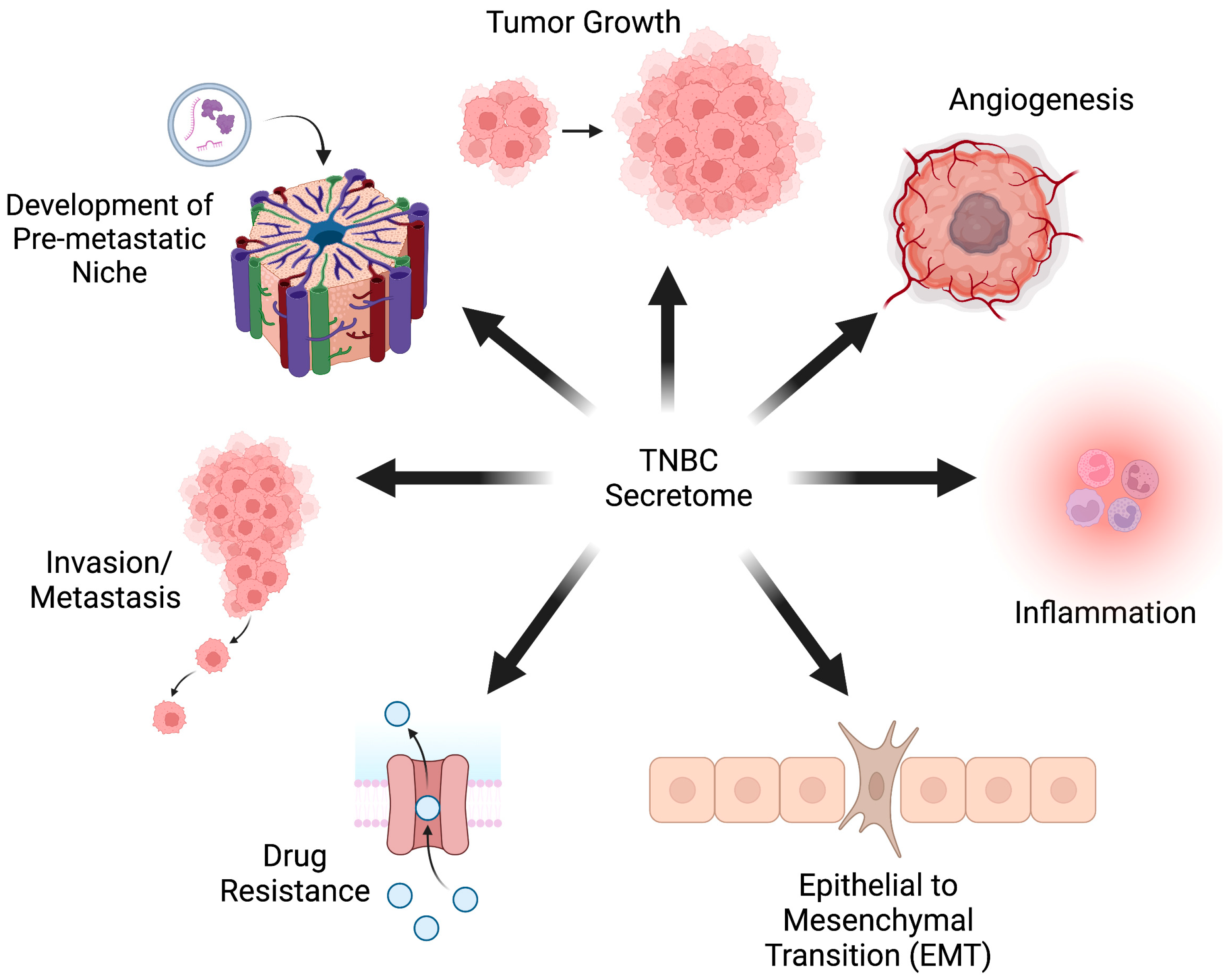
2. The TNBC Secretome
2.1. Cytokines and Growth Factors
2.2. Extracellular Matrix Proteins
2.3. Proteases and Protease Inhibitors
2.4. Other Proteins
2.4.1. Membrane and Extracellular Vesicle Proteins
2.4.2. Peptide Hormones
2.4.3. Metabolic Proteins
2.5. Drug-Induced Changes
3. Secreted Factors Outside of the Primary Tumor
3.1. Influence of TNBC on Surrounding Cells
3.2. Proteins Secreted from the TME
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APP | amyloid-β precursor protein |
| APLP2 | amyloid-β precursor-like protein 2 |
| BBB | blood–brain barrier |
| CAF | cancer-associated fibroblasts |
| CM | conditioned media |
| CTSD | cathepsin D |
| CTSZ | cathepsin Z |
| ECM | extracellular matrix |
| EFEMP1/FBLN3 | fibulin 3 |
| EMT | epithelial–mesenchymal transition |
| ER | estrogen receptor |
| EV | extracellular vesicle |
| FBLN1 | fibulin 1 |
| FN | fibronectin |
| FST | follistatin |
| GDF15 | growth differentiation factor 15 |
| IGFBP | insulin-like growth factor-binding protein |
| IRE1 | inositol-requiring enzyme 1 alpha |
| LCN2 | lipocalin 2 |
| LDL | low-density lipoprotein |
| LEC | lymphatic endothelial cells |
| LPA | lipoprotein A |
| MEC | microvascular endothelial cells |
| MIC-1 | macrophage inhibitory cytokine-1 |
| MMP | matrix metalloproteinase |
| MSC | mesenchymal stromal cell |
| MTA | microtubule-targeting agents |
| OGF | opioid growth factor |
| PAI1 | plasminogen activator inhibitor 1 |
| PENK | proenkephalin |
| PLAT | tissue plasminogen activator |
| PLG | plasminogen |
| PR | progesterone receptor |
| SASP | senescence-associated secretory phenotype |
| SERPINE1 | serine peptidase inhibitor E1 |
| SNPs | single-nucleotide polymorphisms |
| STX | sialyl transferase X |
| suPAR/PLAUR | soluble form of the urokinase receptor |
| TF | tissue factor |
| THBS1 | thrombospondin |
| TIMP | tissue inhibitor of metalloproteinases |
| TIS | therapeutic-induced senescent |
| TME | tumor microenvironment |
| TNBC | triple-negative breast cancer |
| TSPAN11 | tetraspanin 11 |
| uPA/PLAU | urokinase plasminogen activator |
| uPAR | urokinase plasminogen activator receptor |
| VEGF | vascular endothelial growth factor |
References
- Madden, E.C.; Gorman, A.M.; Logue, S.E.; Samali, A. Tumour Cell Secretome in Chemoresistance and Tumour Recurrence. Trends Cancer 2020, 6, 489–505. [Google Scholar] [CrossRef] [PubMed]
- Cheang, M.C.; Voduc, D.; Bajdik, C.; Leung, S.; McKinney, S.; Chia, S.K.; Perou, C.M.; Nielsen, T.O. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin. Cancer Res. 2008, 14, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Leidy, J.; Khan, A.; Kandil, D. Basal-like breast cancer: Update on clinicopathologic, immunohistochemical, and molecular features. Arch. Pathol. Lab. Med. 2014, 138, 37–43. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, E.A.; Gubbins, L.; Sharma, S.; Tully, R.; Guang, M.H.; Weiner-Gorzel, K.; McCaffrey, J.; Harrison, M.; Furlong, F.; Kell, M.; et al. The fate of chemoresistance in triple negative breast cancer (TNBC). BBA Clin. 2015, 3, 257–275. [Google Scholar] [CrossRef]
- Dong, M.; Liu, Q.; Xu, Y.; Zhang, Q. Extracellular Vesicles: The Landscape in the Progression, Diagnosis, and Treatment of Triple-Negative Breast Cancer. Front. Cell Dev. Biol. 2022, 10, 842898. [Google Scholar] [CrossRef]
- Gonzalez Suarez, N.; Fernandez-Marrero, Y.; Torabidastgerdooei, S.; Annabi, B. EGCG Prevents the Onset of an Inflammatory and Cancer-Associated Adipocyte-like Phenotype in Adipose-Derived Mesenchymal Stem/Stromal Cells in Response to the Triple-Negative Breast Cancer Secretome. Nutrients 2022, 14, 1099. [Google Scholar] [CrossRef]
- Hamester, F.; Sturken, C.; Saygi, C.; Qi, M.; Legler, K.; Gorzelanny, C.; Robador, J.R.; Schmalfeldt, B.; Laakmann, E.; Muller, V.; et al. Insights into the Steps of Breast Cancer-Brain Metastases Development: Tumor Cell Interactions with the Blood-Brain Barrier. Int. J. Mol. Sci. 2022, 23, 1900. [Google Scholar] [CrossRef]
- Pujals, M.; Resar, L.; Villanueva, J. HMGA1, Moonlighting Protein Function, and Cellular Real Estate: Location, Location, Location! Biomolecules 2021, 11, 1334. [Google Scholar] [CrossRef]
- Mendez, O.; Perez, J.; Soberino, J.; Racca, F.; Cortes, J.; Villanueva, J. Clinical Implications of Extracellular HMGA1 in Breast Cancer. Int. J. Mol. Sci. 2019, 20, 5950. [Google Scholar] [CrossRef]
- Di Agostino, S.; Vahabi, M.; Turco, C.; Fontemaggi, G. Secreted Non-Coding RNAs: Functional Impact on the Tumor Microenvironment and Clinical Relevance in Triple-Negative Breast Cancer. Noncoding RNA 2022, 8, 5. [Google Scholar] [CrossRef]
- Ziegler, Y.S.; Moresco, J.J.; Yates, J.R., 3rd; Nardulli, A.M. Integration of Breast Cancer Secretomes with Clinical Data Elucidates Potential Serum Markers for Disease Detection, Diagnosis, and Prognosis. PLoS ONE 2016, 11, e0158296. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, T.; Shah, K.; Gargani, S.; Lao, L.; Alsaleem, M.; Chen, J.; Ntafis, V.; Huang, P.; Ditsiou, A.; Vella, V.; et al. PIK3Cdelta expression by fibroblasts promotes triple-negative breast cancer progression. J. Clin. Investig. 2020, 130, 3188–3204. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.F.; Tseng, L.M.; Hsu, C.Y.; Yang, M.H.; Chiu, J.H.; Shyr, Y.M. Brain-derived neurotrophic factor (BDNF)-TrKB signaling modulates cancer-endothelial cells interaction and affects the outcomes of triple negative breast cancer. PLoS ONE 2017, 12, e0178173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Dietrich, U.M.; Geng, J.G.; Bicknell, R.; Esko, J.D.; Wang, L. Repulsive axon guidance molecule Slit3 is a novel angiogenic factor. Blood 2009, 114, 4300–4309. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, K.; Winkelmaier, G.; Andersen, C.; Yaswen, P.; Quilici, D.; Furuta, S.; Cheng, Q.; Parvin, B. Protein Ligands in the Secretome of CD36(+) Fibroblasts Induce Growth Suppression in a Subset of Breast Cancer Cell Lines. Cancers 2021, 13, 4521. [Google Scholar] [CrossRef]
- Nassar, E.; Hassan, N.; El-Ghonaimy, E.A.; Hassan, H.; Abdullah, M.S.; Rottke, T.V.; Kiesel, L.; Greve, B.; Ibrahim, S.A.; Gotte, M. Syndecan-1 Promotes Angiogenesis in Triple-Negative Breast Cancer through the Prognostically Relevant Tissue Factor Pathway and Additional Angiogenic Routes. Cancers 2021, 13, 2318. [Google Scholar] [CrossRef]
- Kumar, N.; Prasad, P.; Jash, E.; Jayasundar, S.; Singh, I.; Alam, N.; Murmu, N.; Somashekhar, S.P.; Goldman, A.; Sehrawat, S. cAMP regulated EPAC1 supports microvascular density, angiogenic and metastatic properties in a model of triple negative breast cancer. Carcinogenesis 2018, 39, 1245–1253. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Gadalla, R.; El-Ghonaimy, E.A.; Samir, O.; Mohamed, H.T.; Hassan, H.; Greve, B.; El-Shinawi, M.; Mohamed, M.M.; Gotte, M. Syndecan-1 is a novel molecular marker for triple negative inflammatory breast cancer and modulates the cancer stem cell phenotype via the IL-6/STAT3, Notch and EGFR signaling pathways. Mol. Cancer 2017, 16, 57. [Google Scholar] [CrossRef]
- Logue, S.E.; McGrath, E.P.; Cleary, P.; Greene, S.; Mnich, K.; Almanza, A.; Chevet, E.; Dwyer, R.M.; Oommen, A.; Legembre, P.; et al. Inhibition of IRE1 RNase activity modulates the tumor cell secretome and enhances response to chemotherapy. Nat. Commun. 2018, 9, 3267. [Google Scholar] [CrossRef]
- Mendez, O.; Peg, V.; Salvans, C.; Pujals, M.; Fernandez, Y.; Abasolo, I.; Perez, J.; Matres, A.; Valeri, M.; Gregori, J.; et al. Extracellular HMGA1 Promotes Tumor Invasion and Metastasis in Triple-Negative Breast Cancer. Clin. Cancer Res. 2018, 24, 6367–6382. [Google Scholar] [CrossRef]
- Chiefari, E.; Foti, D.P.; Sgarra, R.; Pegoraro, S.; Arcidiacono, B.; Brunetti, F.S.; Greco, M.; Manfioletti, G.; Brunetti, A. Transcriptional Regulation of Glucose Metabolism: The Emerging Role of the HMGA1 Chromatin Factor. Front. Endocrinol. 2018, 9, 357. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Kim, D.H.; Lim, J.M.; Lee, J.W.; Jeong, S.J.; Kim, K.P.; Surh, Y.J. Breast Cancer Cell-Derived Soluble CD44 Promotes Tumor Progression by Triggering Macrophage IL1beta Production. Cancer Res. 2020, 80, 1342–1356. [Google Scholar] [CrossRef] [PubMed]
- Motallebnejad, P.; Rajesh, V.V.; Azarin, S.M. Evaluating the Role of IL-1beta in Transmigration of Triple Negative Breast Cancer Cells Across the Brain Endothelium. Cell. Mol. Bioeng. 2022, 15, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Malone, M.K.; Smrekar, K.; Park, S.; Blakely, B.; Walter, A.; Nasta, N.; Park, J.; Considine, M.; Danilova, L.V.; Pandey, N.B.; et al. Cytokines secreted by stromal cells in TNBC microenvironment as potential targets for cancer therapy. Cancer Biol. 2020, 21, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Resmini, G.; Rizzo, S.; Franchin, C.; Zanin, R.; Penzo, C.; Pegoraro, S.; Ciani, Y.; Piazza, S.; Arrigoni, G.; Sgarra, R.; et al. HMGA1 regulates the Plasminogen activation system in the secretome of breast cancer cells. Sci. Rep. 2017, 7, 11768. [Google Scholar] [CrossRef] [PubMed]
- Campion, O.; Thevenard Devy, J.; Billottet, C.; Schneider, C.; Etique, N.; Dupuy, J.W.; Raymond, A.A.; Boulagnon Rombi, C.; Meunier, M.; Djermoune, E.H.; et al. LRP-1 Matricellular Receptor Involvement in Triple Negative Breast Cancer Tumor Angiogenesis. Biomedicines 2021, 9, 1430. [Google Scholar] [CrossRef]
- Dieters-Castator, D.; Dantonio, P.M.; Piaseczny, M.; Zhang, G.; Liu, J.; Kuljanin, M.; Sherman, S.; Jewer, M.; Quesnel, K.; Kang, E.Y.; et al. Embryonic protein NODAL regulates the breast tumor microenvironment by reprogramming cancer-derived secretomes. Neoplasia 2021, 23, 375–390. [Google Scholar] [CrossRef]
- Dore-Savard, L.; Lee, E.; Kakkad, S.; Popel, A.S.; Bhujwalla, Z.M. The Angiogenic Secretome in VEGF overexpressing Breast Cancer Xenografts. Sci. Rep. 2016, 6, 39460. [Google Scholar] [CrossRef]
- Geng, L.; Chaudhuri, A.; Talmon, G.; Wisecarver, J.L.; Wang, J. TGF-Beta suppresses VEGFA-mediated angiogenesis in colon cancer metastasis. PLoS ONE 2013, 8, e59918. [Google Scholar] [CrossRef]
- Jarad, M.; Kuczynski, E.A.; Morrison, J.; Viloria-Petit, A.M.; Coomber, B.L. Release of endothelial cell associated VEGFR2 during TGF-beta modulated angiogenesis in vitro. BMC Cell Biol. 2017, 18, 10. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, W.; Zhu, M.; Li, M.; Yang, Z. miR-185 inhibits prostate cancer angiogenesis induced by the nodal/ALK4 pathway. BMC Urol. 2020, 20, 49. [Google Scholar] [CrossRef] [PubMed]
- Hueng, D.Y.; Lin, G.J.; Huang, S.H.; Liu, L.W.; Ju, D.T.; Chen, Y.W.; Sytwu, H.K.; Chang, C.; Huang, S.M.; Yeh, Y.S.; et al. Inhibition of Nodal suppresses angiogenesis and growth of human gliomas. J. Neurooncol. 2011, 104, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, J.; Zhou, J.; Lu, L.; Wang, H.; Zhang, G.; Wan, G.; Cai, S.; Du, J. Nodal Facilitates Differentiation of Fibroblasts to Cancer-Associated Fibroblasts that Support Tumor Growth in Melanoma and Colorectal Cancer. Cells 2019, 8, 538. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Pandey, N.B.; Popel, A.S. Simultaneous blockade of IL-6 and CCL5 signaling for synergistic inhibition of triple-negative breast cancer growth and metastasis. Breast Cancer Res. 2018, 20, 54. [Google Scholar] [CrossRef]
- Sayyad, M.R.; Puchalapalli, M.; Vergara, N.G.; Wangensteen, S.M.; Moore, M.; Mu, L.; Edwards, C.; Anderson, A.; Kall, S.; Sullivan, M.; et al. Syndecan-1 facilitates breast cancer metastasis to the brain. Breast Cancer Res. Treat. 2019, 178, 35–49. [Google Scholar] [CrossRef]
- Sanchez-Bailon, M.P.; Calcabrini, A.; Mayoral-Varo, V.; Molinari, A.; Wagner, K.U.; Losada, J.P.; Ciordia, S.; Albar, J.P.; Martin-Perez, J. Cyr61 as mediator of Src signaling in triple negative breast cancer cells. Oncotarget 2015, 6, 13520–13538. [Google Scholar] [CrossRef]
- Ndoye, A.; Miskin, R.P.; DiPersio, C.M. Integrin α3β1 Represses Reelin Expression in Breast Cancer Cells to Promote Invasion. Cancers 2021, 13, 344. [Google Scholar] [CrossRef]
- Kong, Y.; Lyu, N.; Wu, J.; Tang, H.; Xie, X.; Yang, L.; Li, X.; Wei, W.; Xie, X. Breast cancer stem cell markers CD44 and ALDH1A1 in serum: Distribution and prognostic value in patients with primary breast cancer. J. Cancer 2018, 9, 3728–3735. [Google Scholar] [CrossRef]
- Thomas, S.N.; Zhu, F.; Schnaar, R.L.; Alves, C.S.; Konstantopoulos, K. Carcinoembryonic antigen and CD44 variant isoforms cooperate to mediate colon carcinoma cell adhesion to E- and L-selectin in shear flow. J. Biol. Chem. 2008, 283, 15647–15655. [Google Scholar] [CrossRef]
- Pramanik, A.; Xu, Z.; Shamsuddin, S.H.; Khaled, Y.S.; Ingram, N.; Maisey, T.; Tomlinson, D.; Coletta, P.L.; Jayne, D.; Hughes, T.A.; et al. Affimer Tagged Cubosomes: Targeting of Carcinoembryonic Antigen Expressing Colorectal Cancer Cells Using In Vitro and In Vivo Models. ACS Appl. Mater. Interfaces 2022, 14, 11078–11091. [Google Scholar] [CrossRef]
- Pramanik, A.; Xu, Z.; Ingram, N.; Coletta, P.L.; Millner, P.A.; Tyler, A.I.I.; Hughes, T.A. Hyaluronic-Acid-Tagged Cubosomes Deliver Cytotoxics Specifically to CD44-Positive Cancer Cells. Mol. Pharm. 2022, 19, 4601–4611. [Google Scholar] [CrossRef] [PubMed]
- Nabil, G.; Alzhrani, R.; Alsaab, H.O.; Atef, M.; Sau, S.; Iyer, A.K.; Banna, H.E. CD44 Targeted Nanomaterials for Treatment of Triple-Negative Breast Cancer. Cancers 2021, 13, 898. [Google Scholar] [CrossRef] [PubMed]
- Kalyane, D.; Polaka, S.; Vasdev, N.; Tekade, R.K. CD44-Receptor Targeted Gold-Doxorubicin Nanocomposite for Pulsatile Chemo-Photothermal Therapy of Triple-Negative Breast Cancer Cells. Pharmaceutics 2022, 14, 2734. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Wang, T.; Han, M.; Gu, Y.; Sun, G.; Peng, X.; Shou, Q.; Song, H.; Liu, W.; Nian, R. Dual CEA/CD44 targeting to colorectal cancer cells using nanobody-conjugated hyaluronic acid-modified mesoporous silica nanoparticles with pH- and redox-sensitivity. Mater. Adv. 2022, 3, 4707–4717. [Google Scholar] [CrossRef]
- Finnson, K.W.; Tam, B.Y.; Liu, K.; Marcoux, A.; Lepage, P.; Roy, S.; Bizet, A.A.; Philip, A. Identification of CD109 as part of the TGF-beta receptor system in human keratinocytes. FASEB J. 2006, 20, 1525–1527. [Google Scholar] [CrossRef]
- Li, C.; Hancock, M.A.; Sehgal, P.; Zhou, S.; Reinhardt, D.P.; Philip, A. Soluble CD109 binds TGF-beta and antagonizes TGF-beta signalling and responses. Biochem. J. 2016, 473, 537–547. [Google Scholar] [CrossRef]
- Tao, J.; Li, H.; Li, Q.; Yang, Y. CD109 is a potential target for triple-negative breast cancer. Tumour Biol. 2014, 35, 12083–12090. [Google Scholar] [CrossRef]
- Hockla, A.; Radisky, D.C.; Radisky, E.S. Mesotrypsin promotes malignant growth of breast cancer cells through shedding of CD109. Breast Cancer Res. Treat. 2010, 124, 27–38. [Google Scholar] [CrossRef]
- Han, Z.; Ni, J.; Smits, P.; Underhill, C.B.; Xie, B.; Chen, Y.; Liu, N.; Tylzanowski, P.; Parmelee, D.; Feng, P.; et al. Extracellular matrix protein 1 (ECM1) has angiogenic properties and is expressed by breast tumor cells. FASEB J. 2001, 15, 988–994. [Google Scholar] [CrossRef]
- Lee, K.M.; Nam, K.; Oh, S.; Lim, J.; Kim, Y.P.; Lee, J.W.; Yu, J.H.; Ahn, S.H.; Kim, S.B.; Noh, D.Y.; et al. Extracellular matrix protein 1 regulates cell proliferation and trastuzumab resistance through activation of epidermal growth factor signaling. Breast Cancer Res. 2014, 16, 479. [Google Scholar] [CrossRef]
- Lal, G.; Hashimi, S.; Smith, B.J.; Lynch, C.F.; Zhang, L.; Robinson, R.A.; Weigel, R.J. Extracellular matrix 1 (ECM1) expression is a novel prognostic marker for poor long-term survival in breast cancer: A Hospital-based Cohort Study in Iowa. Ann. Surg. Oncol. 2009, 16, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.W.; Lee, K.M. ECM1 is associated with endocrine resistance in ER(+) breast cancers. Anim. Cells Syst. 2022, 26, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Steinhaeuser, S.S.; Morera, E.; Budkova, Z.; Schepsky, A.; Wang, Q.; Rolfsson, O.; Riedel, A.; Krueger, A.; Hilmarsdottir, B.; Maelandsmo, G.M.; et al. ECM1 secreted by HER2-overexpressing breast cancer cells promotes formation of a vascular niche accelerating cancer cell migration and invasion. Lab. Investig. 2020, 100, 928–944. [Google Scholar] [CrossRef] [PubMed]
- Fararjeh, A.S.; Kaddumi, E.; Al Khader, A.; Aloliqi, A.A. The Diagnostic and Prognostic Significance of EFEMP1 in Breast Cancer: An Immunohistochemistry Study. Int. J. Surg. Pathol. 2022. [Google Scholar] [CrossRef]
- Noonan, M.M.; Dragan, M.; Mehta, M.M.; Hess, D.A.; Brackstone, M.; Tuck, A.B.; Viswakarma, N.; Rana, A.; Babwah, A.V.; Wondisford, F.E.; et al. The matrix protein Fibulin-3 promotes KISS1R induced triple negative breast cancer cell invasion. Oncotarget 2018, 9, 30034–30052. [Google Scholar] [CrossRef]
- Cosentino, G.; Romero-Cordoba, S.; Plantamura, I.; Cataldo, A.; Iorio, M.V. miR-9-Mediated Inhibition of EFEMP1 Contributes to the Acquisition of Pro-Tumoral Properties in Normal Fibroblasts. Cells 2020, 9, 2143. [Google Scholar] [CrossRef]
- Bardin, A.; Moll, F.; Margueron, R.; Delfour, C.; Chu, M.L.; Maudelonde, T.; Cavailles, V.; Pujol, P. Transcriptional and posttranscriptional regulation of fibulin-1 by estrogens leads to differential induction of messenger ribonucleic acid variants in ovarian and breast cancer cells. Endocrinology 2005, 146, 760–768. [Google Scholar] [CrossRef]
- Marano, F.; Zunino, V.; Frairia, R.; Arvat, E.; Castellano, I.; Bosco, O.; Catalano, M.G.; Fortunati, N. Fibulin-1 interacts with Sex Hormone Binding Globulin and is linked to less aggressive estrogen-dependent breast cancers. Life Sci. 2018, 207, 372–380. [Google Scholar] [CrossRef]
- Pupa, S.M.; Argraves, W.S.; Forti, S.; Casalini, P.; Berno, V.; Agresti, R.; Aiello, P.; Invernizzi, A.; Baldassari, P.; Twal, W.O.; et al. Immunological and pathobiological roles of fibulin-1 in breast cancer. Oncogene 2004, 23, 2153–2160. [Google Scholar] [CrossRef]
- Ramdas, P.; Radhakrishnan, A.K.; Abdu Sani, A.A.; Abdul-Rahman, P.S. Tocotrienols Modulate Breast Cancer Secretomes and Affect Cancer-Signaling Pathways in MDA-MB-231 Cells: A Label-Free Quantitative Proteomic Analysis. Nutr. Cancer 2019, 71, 1263–1271. [Google Scholar] [CrossRef]
- Pupa, S.M.; Giuffre, S.; Castiglioni, F.; Bertola, L.; Cantu, M.; Bongarzone, I.; Baldassari, P.; Mortarini, R.; Argraves, W.S.; Anichini, A.; et al. Regulation of breast cancer response to chemotherapy by fibulin-1. Cancer Res. 2007, 67, 4271–4277. [Google Scholar] [CrossRef] [PubMed]
- Allard, J.B.; Duan, C. IGF-Binding Proteins: Why Do They Exist and Why Are There So Many? Front. Endocrinol. 2018, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.C.; Falkenberg, N.; Hauck, S.M.; Priller, M.; Braselmann, H.; Feuchtinger, A.; Walch, A.; Schmitt, M.; Aubele, M. Cyr61 and YB-1 are novel interacting partners of uPAR and elevate the malignancy of triple-negative breast cancer. Oncotarget 2016, 7, 44062–44075. [Google Scholar] [CrossRef] [PubMed]
- Babic, A.M.; Kireeva, M.L.; Kolesnikova, T.V.; Lau, L.F. CYR61, a product of a growth factor-inducible immediate early gene, promotes angiogenesis and tumor growth. Proc. Natl. Acad. Sci. USA 1998, 95, 6355–6360. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, I.; Kurapaty, C.; Park, C.H.; Vander Steen, T.; Kleer, C.G.; Wiley, E.; Rademaker, A.; Cuyas, E.; Verdura, S.; Buxo, M.; et al. Depletion of CCN1/CYR61 reduces triple-negative/basal-like breast cancer aggressiveness. Am. J. Cancer Res. 2022, 12, 839–851. [Google Scholar]
- Winiarski, B.K.; Wolanska, K.I.; Rai, S.; Ahmed, T.; Acheson, N.; Gutowski, N.J.; Whatmore, J.L. Epithelial ovarian cancer-induced angiogenic phenotype of human omental microvascular endothelial cells may occur independently of VEGF signaling. Transl. Oncol. 2013, 6, 703–714. [Google Scholar] [CrossRef]
- Lee, L.Y.; Thaysen-Andersen, M.; Baker, M.S.; Packer, N.H.; Hancock, W.S.; Fanayan, S. Comprehensive N-glycome profiling of cultured human epithelial breast cells identifies unique secretome N-glycosylation signatures enabling tumorigenic subtype classification. J. Proteome Res. 2014, 13, 4783–4795. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Zeng, Y.N.; He, F.; Yang, X.M.; Guan, F. Enhanced expression of polysialic acid correlates with malignant phenotype in breast cancer cell lines and clinical tissue samples. Int. J. Mol. Med. 2016, 37, 197–206. [Google Scholar] [CrossRef]
- Rochefort, H. Cathepsin D in breast cancer. Breast Cancer Res. Treat. 1990, 16, 3–13. [Google Scholar] [CrossRef]
- Benes, P.; Vetvicka, V.; Fusek, M. Cathepsin D—Many functions of one aspartic protease. Crit. Rev. Oncol. Hematol. 2008, 68, 12–28. [Google Scholar] [CrossRef]
- Pranjol, Z.I.; Whatmore, J.L. Cathepsin D in the Tumor Microenvironment of Breast and Ovarian Cancers. Adv. Exp. Med. Biol. 2020, 1259, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Anantaraju, H.S.; Battu, M.B.; Viswanadha, S.; Sriram, D.; Yogeeswari, P. Cathepsin D inhibitors as potential therapeutics for breast cancer treatment: Molecular docking and bioevaluation against triple-negative and triple-positive breast cancers. Mol. Divers. 2016, 20, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Dumas, J.; Brittelli, D.; Chen, J.; Dixon, B.; Hatoum-Mokdad, H.; Konig, G.; Sibley, R.; Witowsky, J.; Wong, S. Synthesis and structure activity relationships of novel small molecule cathepsin D inhibitors. Bioorg. Med. Chem. Lett. 1999, 9, 2531–2536. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Uttamchandani, M.; Yao, S.Q. Applying small molecule microarrays and resulting affinity probe cocktails for proteome profiling of mammalian cell lysates. Chem. Asian J. 2011, 6, 2803–2815. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Z.; Lin, L.; Sun, H.; White, L.V.; Ding, K.; Li, Z. Quantitative Proteomics Reveals Cellular Off-Targets of a DDR1 Inhibitor. ACS Med. Chem. Lett. 2020, 11, 535–540. [Google Scholar] [CrossRef]
- Ashraf, Y.; Mansouri, H.; Laurent-Matha, V.; Alcaraz, L.B.; Roger, P.; Guiu, S.; Derocq, D.; Robin, G.; Michaud, H.A.; Delpech, H.; et al. Immunotherapy of triple-negative breast cancer with cathepsin D-targeting antibodies. J. Immunother. Cancer 2019, 7, 29. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Li, Y.; Guan, X.Y. Overexpression of cathepsin Z contributes to tumor metastasis by inducing epithelial-mesenchymal transition in hepatocellular carcinoma. PLoS ONE 2011, 6, e24967. [Google Scholar] [CrossRef]
- Sharif-Kashani, B.; Ghanbari Motlagh, A.; Mafi, A.R.; Esnaashari, O.; Ramzi, M.; Taghizadeh, A.; Najafi, S. The Incidence of Deep Vein Thrombosis in Breast Cancer Patients Receiving Outpatient Cancer Therapy in Iran. Tanaffos 2019, 18, 244–253. [Google Scholar]
- Okazaki, M.; Bando, H.; Ichioka, E.; Iguchi-Manaka, A.; Nasu, K.; Hara, H. Four cases of Trousseau syndrome associated with breast cancer that exhibited central nervous system manifestations. Int. Cancer Conf. J. 2020, 9, 146–150. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Z.; Zhou, P.; Yu, M.; Li, B.; Liu, Y.; Jin, J.; Liu, W.; Jing, H.; Du, J.; et al. Phosphatidylserine-exposing tumor-derived microparticles exacerbate coagulation and cancer cell transendothelial migration in triple-negative breast cancer. Theranostics 2021, 11, 6445–6460. [Google Scholar] [CrossRef]
- Ismail, A.A.; Shaker, B.T.; Bajou, K. The Plasminogen-Activator Plasmin System in Physiological and Pathophysiological Angiogenesis. Int. J. Mol. Sci. 2021, 23, 337. [Google Scholar] [CrossRef] [PubMed]
- de Meis, E.; Azambuja, D.; Ayres-Silva, J.P.; Zamboni, M.; Pinheiro, V.R.; Levy, R.A.; Monteiro, R.Q. Increased expression of tissue factor and protease-activated receptor-1 does not correlate with thrombosis in human lung adenocarcinoma. Braz. J. Med. Biol. Res. 2010, 43, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Bromberg, M. Tissue factor as a novel target for treatment of breast cancer. Oncologist 2013, 18, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Q.; Zhao, H.; Ma, L.; Meng, T.; Qian, J.; Jin, R.; Shen, J.; Yu, K. Pathological expression of tissue factor confers promising antitumor response to a novel therapeutic antibody SC1 in triple negative breast cancer and pancreatic adenocarcinoma. Oncotarget 2017, 8, 59086–59102. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cao, D.; Zheng, X.; Wang, G.; Liu, M. Tissue factor as a new target for tumor therapy-killing two birds with one stone: A narrative review. Ann. Transl. Med. 2022, 10, 1250. [Google Scholar] [CrossRef] [PubMed]
- Kuentzel, S.L.; Ali, S.M.; Altman, R.A.; Greenberg, B.D.; Raub, T.J. The Alzheimer beta-amyloid protein precursor/protease nexin-II is cleaved by secretase in a trans-Golgi secretory compartment in human neuroglioma cells. Biochem J. 1993, 295 Pt 2, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Krishnamurthy, P.T.; Pinduprolu, S.; Sola, P. DR-5 and DLL-4 mAb Functionalized SLNs of Gamma-Secretase Inhibitors—An Approach for TNBC Treatment. Adv. Pharm. Bull. 2021, 11, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Kandasamy, T.; Ghosh, S.S. Multi-targeting TACE/ADAM17 and gamma-secretase of notch signalling pathway in TNBC via drug repurposing approach using Lomitapide. Cell Signal. 2023, 102, 110529. [Google Scholar] [CrossRef]
- Li, Z.L.; Chen, C.; Yang, Y.; Wang, C.; Yang, T.; Yang, X.; Liu, S.C. Gamma secretase inhibitor enhances sensitivity to doxorubicin in MDA-MB-231 cells. Int. J. Clin. Exp. Pathol. 2015, 8, 4378–4387. [Google Scholar]
- Pandey, P.; Sliker, B.; Peters, H.L.; Tuli, A.; Herskovitz, J.; Smits, K.; Purohit, A.; Singh, R.K.; Dong, J.; Batra, S.K.; et al. Amyloid precursor protein and amyloid precursor-like protein 2 in cancer. Oncotarget 2016, 7, 19430–19444. [Google Scholar] [CrossRef]
- Lee, H.N.; Jeong, M.S.; Jang, S.B. Molecular Characteristics of Amyloid Precursor Protein (APP) and Its Effects in Cancer. Int. J. Mol. Sci. 2021, 22, 4999. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, S.; Lu, C. Amyloid precursor protein promotes the migration and invasion of breast cancer cells by regulating the MAPK signaling pathway. Int. J. Mol. Med. 2020, 45, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Chitayat, S.; Dattilo, B.M.; Schiefner, A.; Diez, J.; Chazin, W.J.; Fritz, G. Structural basis for ligand recognition and activation of RAGE. Structure 2010, 18, 1342–1352. [Google Scholar] [CrossRef] [PubMed]
- Placencio, V.R.; Ichimura, A.; Miyata, T.; DeClerck, Y.A. Small Molecule Inhibitors of Plasminogen Activator Inhibitor-1 Elicit Anti-Tumorigenic and Anti-Angiogenic Activity. PLoS ONE 2015, 10, e0133786. [Google Scholar] [CrossRef]
- Akhter, H.; Huang, W.T.; van Groen, T.; Kuo, H.C.; Miyata, T.; Liu, R.M. A Small Molecule Inhibitor of Plasminogen Activator Inhibitor-1 Reduces Brain Amyloid-beta Load and Improves Memory in an Animal Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 64, 447–457. [Google Scholar] [CrossRef]
- Kubala, M.H.; DeClerck, Y.A. The plasminogen activator inhibitor-1 paradox in cancer: A mechanistic understanding. Cancer Metastasis Rev. 2019, 38, 483–492. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, J.; Fang, H.; Tang, L.; Chen, W.; Sun, Q.; Zhang, Q.; Yang, F.; Sun, Z.; Cao, L.; et al. Endothelial cells promote triple-negative breast cancer cell metastasis via PAI-1 and CCL5 signaling. FASEB J. 2018, 32, 276–288. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuna, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Schoeps, B.; Fradrich, J.; Kruger, A. Cut loose TIMP-1: An emerging cytokine in inflammation. Trends Cell Biol. 2022. [Google Scholar] [CrossRef]
- Knight, B.E.; Kozlowski, N.; Havelin, J.; King, T.; Crocker, S.J.; Young, E.E.; Baumbauer, K.M. TIMP-1 Attenuates the Development of Inflammatory Pain Through MMP-Dependent and Receptor-Mediated Cell Signaling Mechanisms. Front. Mol. Neurosci. 2019, 12, 220. [Google Scholar] [CrossRef]
- Ries, C. Cytokine functions of TIMP-1. Cell. Mol. Life Sci. 2014, 71, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Fan, X.; Hao, M.; Wang, J.; Zhou, X.; Sun, X. Higher levels of TIMP-1 expression are associated with a poor prognosis in triple-negative breast cancer. Mol. Cancer 2016, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, R.C.; Liu, X.W.; Najy, A.J.; Jung, Y.S.; Won, J.; Chai, K.X.; Fridman, R.; Kim, H.R. TIMP-1 via TWIST1 induces EMT phenotypes in human breast epithelial cells. Mol. Cancer Res. 2014, 12, 1324–1333. [Google Scholar] [CrossRef] [PubMed]
- Schoeps, B.; Eckfeld, C.; Fluter, L.; Keppler, S.; Mishra, R.; Knolle, P.; Bayerl, F.; Bottcher, J.; Hermann, C.D.; Haussler, D.; et al. Identification of invariant chain CD74 as a functional receptor of tissue inhibitor of metalloproteinases-1 (TIMP-1). J. Biol. Chem. 2021, 297, 101072. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.S.; Gu, Q.; Tzschentke, S.; Lin, K.; Ganig, N.; Thepkaysone, M.L.; Wong, F.C.; Polster, H.; Seifert, L.; Seifert, A.M.; et al. Extravesicular TIMP-1 is a non-invasive independent prognostic marker and potential therapeutic target in colorectal liver metastases. Oncogene 2022, 41, 1809–1820. [Google Scholar] [CrossRef]
- Abdollahi, A.; Nozarian, Z.; Nazar, E. Association between Expression of Tissue Inhibitors of Metalloproteinases-1, Matrix Metalloproteinase-2, and Matrix Metalloproteinase-9 Genes and Axillary Lymph Nodes Metastasis in Patients with Breast Cancer. Int. J. Prev. Med. 2019, 10, 127. [Google Scholar] [CrossRef]
- Jezierska, A.; Motyl, T. Matrix metalloproteinase-2 involvement in breast cancer progression: A mini-review. Med. Sci. Monit. 2009, 15, RA32–RA40. [Google Scholar]
- Chien, Y.C.; Liu, L.C.; Ye, H.Y.; Wu, J.Y.; Yu, Y.L. EZH2 promotes migration and invasion of triple-negative breast cancer cells via regulating TIMP2-MMP-2/-9 pathway. Am. J. Cancer Res. 2018, 8, 422–434. [Google Scholar]
- Wang, K.; Wang, G.; Huang, S.; Luo, A.; Jing, X.; Li, G.; Zhou, Y.; Zhao, X. Association between TIMP-2 gene polymorphism and breast cancer in Han Chinese women. BMC Cancer 2019, 19, 446. [Google Scholar] [CrossRef]
- Peeney, D.; Jensen, S.M.; Castro, N.P.; Kumar, S.; Noonan, S.; Handler, C.; Kuznetsov, A.; Shih, J.; Tran, A.D.; Salomon, D.S.; et al. TIMP-2 suppresses tumor growth and metastasis in murine model of triple-negative breast cancer. Carcinogenesis 2020, 41, 313–325. [Google Scholar] [CrossRef]
- Su, C.W.; Lin, C.W.; Yang, W.E.; Yang, S.F. TIMP-3 as a therapeutic target for cancer. Adv. Med. Oncol. 2019, 11, 1758835919864247. [Google Scholar] [CrossRef] [PubMed]
- Jackson, H.W.; Hojilla, C.V.; Weiss, A.; Sanchez, O.H.; Wood, G.A.; Khokha, R. Timp3 deficient mice show resistance to developing breast cancer. PLoS ONE 2015, 10, e0120107. [Google Scholar] [CrossRef] [PubMed]
- Klenotic, P.A.; Munier, F.L.; Marmorstein, L.Y.; Anand-Apte, B. Tissue inhibitor of metalloproteinases-3 (TIMP-3) is a binding partner of epithelial growth factor-containing fibulin-like extracellular matrix protein 1 (EFEMP1). Implications for macular degenerations. J. Biol. Chem. 2004, 279, 30469–30473. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, Y.; Yin, Y.; Jia, X.; Mao, L. Mechanism of cargo sorting into small extracellular vesicles. Bioengineered 2021, 12, 8186–8201. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Jeon, Y.R.; Kim, S.Y.; Lee, E.J.; Kim, Y.N.; Noh, D.Y.; Park, S.Y.; Moon, A. Identification of annexin II as a novel secretory biomarker for breast cancer. Proteomics 2013, 13, 3145–3156. [Google Scholar] [CrossRef]
- Beykou, M.; Arias-Garcia, M.; Roumeliotis, T.I.; Choudhary, J.S.; Moser, N.; Georgiou, P.; Bakal, C. Proteomic characterisation of triple negative breast cancer cells following CDK4/6 inhibition. Sci. Data 2022, 9, 395. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Yang, S.; Pi, H.; Li, Z.; Yao, P.; Zhang, Q.; Wang, Q.; Shen, P.; Li, X.; et al. Proteomic Landscape of Exosomes Reveals the Functional Contributions of CD151 in Triple-Negative Breast Cancer. Mol. Cell. Proteom. 2021, 20, 100121. [Google Scholar] [CrossRef]
- Lopatina, T.; Favaro, E.; Danilova, L.; Fertig, E.J.; Favorov, A.V.; Kagohara, L.T.; Martone, T.; Bussolati, B.; Romagnoli, R.; Albera, R.; et al. Extracellular Vesicles Released by Tumor Endothelial Cells Spread Immunosuppressive and Transforming Signals Through Various Recipient Cells. Front. Cell Dev. Biol. 2020, 8, 698. [Google Scholar] [CrossRef]
- Sung, J.S.; Kang, C.W.; Kang, S.; Jang, Y.; Chae, Y.C.; Kim, B.G.; Cho, N.H. ITGB4-mediated metabolic reprogramming of cancer-associated fibroblasts. Oncogene 2020, 39, 664–676. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Kannan, A.; Philley, J.V.; Hertweck, K.L.; Ndetan, H.; Singh, K.P.; Sivakumar, S.; Wells, R.B.; Vadlamudi, R.K.; Dasgupta, S. Cancer Testis Antigen Promotes Triple Negative Breast Cancer Metastasis and is Traceable in the Circulating Extracellular Vesicles. Sci. Rep. 2019, 9, 11632. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Gonzalez-Prieto, R.; Zhang, M.; Geurink, P.P.; Kooij, R.; Iyengar, P.V.; van Dinther, M.; Bos, E.; Zhang, X.; Le Devedec, S.E.; et al. Deubiquitinase Activity Profiling Identifies UCHL1 as a Candidate Oncoprotein That Promotes TGFbeta-Induced Breast Cancer Metastasis. Clin. Cancer Res. 2020, 26, 1460–1473. [Google Scholar] [CrossRef] [PubMed]
- Hedger, M.P.; Winnall, W.R.; Phillips, D.J.; de Kretser, D.M. The regulation and functions of activin and follistatin in inflammation and immunity. Vitam. Horm. 2011, 85, 255–297. [Google Scholar] [CrossRef] [PubMed]
- Zabkiewicz, C.; Resaul, J.; Hargest, R.; Jiang, W.G.; Ye, L. Increased Expression of Follistatin in Breast Cancer Reduces Invasiveness and Clinically Correlates with Better Survival. Cancer Genom. Proteom. 2017, 14, 241–251. [Google Scholar] [CrossRef]
- Liu, S.; Liu, B.; Zhao, Q.; Shi, J.; Gu, Y.; Guo, Y.; Li, Y.; Liu, Y.; Cheng, Y.; Qiao, Y.; et al. Down-regulated FST expression is involved in the poor prognosis of triple-negative breast cancer. Cancer Cell Int. 2021, 21, 267. [Google Scholar] [CrossRef] [PubMed]
- Zagon, I.S.; Porterfield, N.K.; McLaughlin, P.J. Opioid growth factor—Opioid growth factor receptor axis inhibits proliferation of triple negative breast cancer. Exp. Biol. Med. 2013, 238, 589–599. [Google Scholar] [CrossRef]
- Hamann, M.; Grill, S.; Struck, J.; Bergmann, A.; Hartmann, O.; Polcher, M.; Kiechle, M. Detection of early breast cancer beyond mammographic screening: A promising biomarker panel. Biomark. Med. 2019, 13, 1107–1117. [Google Scholar] [CrossRef]
- Fridman, E.S.; Ginini, L.; Gil, Z. The Role of Extracellular Vesicles in Metabolic Reprogramming of the Tumor Microenvironment. Cells 2022, 11, 1433. [Google Scholar] [CrossRef]
- Dykstra, H.; LaRose, C.; Fisk, C.; Waldhart, A.; Meng, X.; Zhao, G.; Wu, N. TXNIP interaction with GLUT1 depends on PI(4,5)P(2). Biochim. Biophys. Acta Biomembr. 2021, 1863, 183757. [Google Scholar] [CrossRef]
- Jawi, M.M.; Frohlich, J.; Chan, S.Y. Lipoprotein(a) the Insurgent: A New Insight into the Structure, Function, Metabolism, Pathogenicity, and Medications Affecting Lipoprotein(a) Molecule. J. Lipids 2020, 2020, 3491764. [Google Scholar] [CrossRef] [PubMed]
- Edelberg, J.M.; Pizzo, S.V. Lipoprotein(a) inhibits plasminogen activation in a template-dependent manner. Blood Coagul. Fibrinolysis 1991, 2, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Yano, Y.; Seishima, M.; Tokoro, Y.; Noma, A. Stimulatory effects of lipoprotein(a) and low-density lipoprotein on human umbilical vein endothelial cell migration and proliferation are partially mediated by fibroblast growth factor-2. Biochim. Biophys. Acta 1998, 1393, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Kalaivani, V.; Jaleel, A. Apolipoprotein(a), an enigmatic anti-angiogenic glycoprotein in human plasma: A curse or cure? Pharm. Res. 2020, 158, 104858. [Google Scholar] [CrossRef]
- Kavanagh, E.L.; Lindsay, S.; Halasz, M.; Gubbins, L.C.; Weiner-Gorzel, K.; Guang, M.H.Z.; McGoldrick, A.; Collins, E.; Henry, M.; Blanco-Fernandez, A.; et al. Protein and chemotherapy profiling of extracellular vesicles harvested from therapeutic induced senescent triple negative breast cancer cells. Oncogenesis 2017, 6, e388. [Google Scholar] [CrossRef]
- Borodkina, A.V.; Deryabin, P.I.; Giukova, A.A.; Nikolsky, N.N. “Social Life” of Senescent Cells: What Is SASP and Why Study It? Acta Nat. 2018, 10, 4–14. [Google Scholar] [CrossRef]
- Bhola, N.E.; Balko, J.M.; Dugger, T.C.; Kuba, M.G.; Sanchez, V.; Sanders, M.; Stanford, J.; Cook, R.S.; Arteaga, C.L. TGF-beta inhibition enhances chemotherapy action against triple-negative breast cancer. J. Clin. Investig. 2013, 123, 1348–1358. [Google Scholar] [CrossRef]
- Bellio, C.; Emperador, M.; Castellano, P.; Gris-Oliver, A.; Canals, F.; Sanchez-Pla, A.; Zamora, E.; Arribas, J.; Saura, C.; Serra, V.; et al. GDF15 Is an Eribulin Response Biomarker also Required for Survival of DTP Breast Cancer Cells. Cancers 2022, 14, 2562. [Google Scholar] [CrossRef]
- Blache, U.; Horton, E.R.; Xia, T.; Schoof, E.M.; Blicher, L.H.; Schonenberger, A.; Snedeker, J.G.; Martin, I.; Erler, J.T.; Ehrbar, M. Mesenchymal stromal cell activation by breast cancer secretomes in bioengineered 3D microenvironments. Life Sci. Alliance 2019, 2, e201900304. [Google Scholar] [CrossRef]
- Valkenburg, K.C.; de Groot, A.E.; Pienta, K.J. Targeting the tumour stroma to improve cancer therapy. Nat. Rev. Clin. Oncol. 2018, 15, 366–381. [Google Scholar] [CrossRef]
- Gonzalez Suarez, N.; Rodriguez Torres, S.; Ouanouki, A.; El Cheikh-Hussein, L.; Annabi, B. EGCG Inhibits Adipose-Derived Mesenchymal Stem Cells Differentiation into Adipocytes and Prevents a STAT3-Mediated Paracrine Oncogenic Control of Triple-Negative Breast Cancer Cell Invasive Phenotype. Molecules 2021, 26, 1506. [Google Scholar] [CrossRef] [PubMed]
- Beebe, J.D.; Liu, J.Y.; Zhang, J.T. Two decades of research in discovery of anticancer drugs targeting STAT3, how close are we? Pharmacol. Ther. 2018, 191, 74–91. [Google Scholar] [CrossRef] [PubMed]
- Fertig, E.J.; Lee, E.; Pandey, N.B.; Popel, A.S. Analysis of gene expression of secreted factors associated with breast cancer metastases in breast cancer subtypes. Sci. Rep. 2015, 5, 12133. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.T.; Phuong, T.N.T.; Tien, N.L.B.; Tran, D.K.; Nguyen, T.T.; Thanh, V.V.; Quang, T.L.; Minh, L.B.; Pham, V.H.; Ngoc, V.T.N.; et al. The Effects of Adipocytes on the Regulation of Breast Cancer in the Tumor Microenvironment: An Update. Cells 2019, 8, 857. [Google Scholar] [CrossRef]
- Santiago-Sanchez, G.S.; Noriega-Rivera, R.; Hernandez-O’Farrill, E.; Valiyeva, F.; Quinones-Diaz, B.; Villodre, E.S.; Debeb, B.G.; Rosado-Albacarys, A.; Vivas-Mejia, P.E. Targeting Lipocalin-2 in Inflammatory Breast Cancer Cells with Small Interference RNA and Small Molecule Inhibitors. Int. J. Mol. Sci. 2021, 22, 8581. [Google Scholar] [CrossRef]
- Mezi, S.; Botticelli, A.; Pomati, G.; Cerbelli, B.; Scagnoli, S.; Amirhassankhani, S.; d’Amati, G.; Marchetti, P. Standard of Care and Promising New Agents for the Treatment of Mesenchymal Triple-Negative Breast Cancer. Cancers 2021, 13, 1080. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, D.; Yao, Z.; Lin, X.; Liu, J.; Gu, Q.; Dong, X.; Liu, F.; Wang, Y.; Yao, N.; et al. Anti-angiogenic treatment promotes triple-negative breast cancer invasion via vasculogenic mimicry. Cancer Biol. Ther. 2017, 18, 205–213. [Google Scholar] [CrossRef]
- Thomas, R.; Al-Khadairi, G.; Decock, J. Immune Checkpoint Inhibitors in Triple Negative Breast Cancer Treatment: Promising Future Prospects. Front. Oncol. 2020, 10, 600573. [Google Scholar] [CrossRef]
- Chen, W.; Shen, L.; Jiang, J.; Zhang, L.; Zhang, Z.; Pan, J.; Ni, C.; Chen, Z. Antiangiogenic therapy reverses the immunosuppressive breast cancer microenvironment. Biomark. Res. 2021, 9, 59. [Google Scholar] [CrossRef]
- Wang, S.E. Extracellular Vesicles and Metastasis. Cold Spring Harb. Perspect. Med. 2020, 10, a037275. [Google Scholar] [CrossRef]
| Cytokines | ||||
|---|---|---|---|---|
| Protein | Aliases | Source | Evidence | Refs. |
| CCL2 | Chemokine (C-C motif) ligand 2 | Adipose-derived MSCs | ADMSCs treated with TNBC CM | [6] |
| CCL5 | Chemokine (C-C motif) ligand 5, RANTES | Adipose-derived MSCs | ADMSCs treated with TNBC CM | [6] |
| COX2 | Cyclooxygenase 2, Prostaglandin synthase 2 | Adipose-derived MSCs | ADMSCs treated with TNBC CM | [6] |
| CXCL1/GRO | CXC motif chemokine ligand 1, growth-related oncogene | TNBC cells | Secretome profiling of CM; TNBC cells cocultured with hBMECS; inhibition of IRE1 in TNBC cells | [7,18,19] |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor | TNBC cells | Inhibition of IRE1 in TNBC cells | [19] |
| HIF-1α | Hypoxia-inducible factor-1 subunit alpha | Adipose-derived MSCs | ADMSCs treated with TNBC CM | [6] |
| HMGA1 | High-mobility group A1 | TNBC cells and tumors | TNBC tumors | [20,21] |
| IL-1β | Interleukin-1 beta | Macrophages, adipose-derived MSCs, TNBC cells | CM and patient serum in mouse model; ADMSCs treated with TNBC CM; TNBC cells in model of blood–brain barrier | [6,22,23] |
| IL-6 | Interleukin-6 | TNBC cells; adipose-derived MSCs | Secretome profiling of CM; ADMSCs treated with TNBC CM; Inhibition of IRE1 in TNBC cells | [6,18,19] |
| IL-8 | Interleukin-8 | TNBC cells | Secretome profiling of CM; Inhibition of IRE1 in TNBC cells | [18,19] |
| LCN2 | Lipocalin-2 | Stromal cells | TNBC cells cocultured with four types of stromal cells | [24] |
| PD-L1 | Programmed death ligand 1 | Adipose-derived MSCs | ADMSCs treated with TNBC CM | [6] |
| PLAUR | suPAR, soluble urokinase receptor | TNBC cells | TNBC cells (inducible silencing of HMGA1) | [25] |
| SAA1 | Serum amyloid A1 | TNBC cells | MS analysis of CM | [11] |
| SLIT3 | Slit guidance ligand 3 | Fibroblasts | TNBC cells treated with CAF CM | [15] |
| THBS1 | Thrombospondin 1 | TNBC cells | MS analysis of CM | [11] |
| Growth Factors | ||||
| BDNF | Brain-derived neurotrophic factor | Fibroblasts | Coculture of fibroblasts with TNBC cells | [12,13] |
| END1 | Endothelin-1 | TNBC cells | CM from TNBC cell lines depleted of Syndecan-1 | [16] |
| GRN | Granulin | TNBC cells | TNBC cell line depleted of LRP-1 | [26] |
| NODAL | Nodal growth differentiation factor (TGFβ superfamily) | TNBC tumors | IHC of human tumors | [27] |
| PLGF | Placental growth factor | Fibroblasts | Coculture of fibroblasts with TNBC cells | [12] |
| TGFβ1 | Transforming growth factor beta 1 | TNBC cells | TNBC cell line depleted of LRP-1; MS analysis of CM | [11,26] |
| TGFβ2 | Transforming growth factor beta 2 | TNBC cells | Inhibition of IRE1 in TNBC cells | [19] |
| VEGF-A | Vascular endothelial growth factor A | TNBC cells and xenograft tumors; Adipose-derived MSCs | CM from TNBC cell lines depleted of Syndecan-1; interstitial fluid of xenograft tumors; ADMSCs treated with TNBC CM | [6,16,28] |
| Protein | Aliases | Source | Evidence | Refs. |
|---|---|---|---|---|
| BGN | Biglycan | TNBC cells | MS analysis of CM | [11] |
| CD44 | Cluster of differentiation 44 | TNBC cells | MS analysis of CM; CM and patient serum in mouse model | [11,22] |
| CD109 | Cluster of differentiation 109 | TNBC cells | MS analysis of CM | [11] |
| DAG1 | Dystroglycan | TNBC cells | MS analysis of CM | [11] |
| DCN | Decorin | TNBC cells | MS analysis of CM | [11] |
| ECM1 | Extracellular matrix protein 1 | TNBC cells | MS analysis of CM; TNBC cell line depleted of LRP-1 | [11,26] |
| EFEMP1/FBLN3 | EGF-containing fibulin-like extracellular matrix protein 1, Fibulin 3 | TNBC cells | MS analysis of CM | [11] |
| FBLN1 | Fibulin 1 | CAFs | TNBC cells treated with CAF CM | [15] |
| FMOD | Fibromodulin | TNBC cells | MS analysis of CM | [11] |
| IGFBP4 | Insulin-like growth factor-binding protein 4 | TNBC cells | MS analysis of CM | [11] |
| IGFBP7 | Insulin-like growth factor-binding protein 7 | TNBC cells | MS analysis of CM | [11] |
| IGFBP10/ Cyr61/CCN1 | Insulin-like growth factor-binding protein 10, Cysteine-rich angiogenic inducer 61, CCN1 | TNBC cells (exosomes) | Migration of TNBC cells decreased by neutralizing antibodies | [36] |
| L1CAM | L1 cell adhesion molecule | TNBC cells | MS analysis of CM | [11] |
| LGALS1 | Galectin 1 | TNBC cells | MS analysis of CM | [11] |
| LGALS3BP | Galectin 3-binding protein | TNBC cells | MS analysis of CM | [11] |
| LOXL2 | Lysyl oxidase-like 2 | TNBC cells | MS analysis of CM | [11] |
| LTBP1 | Latent TGFβ-binding protein 1 | TNBC cells | MS analysis of CM | [11] |
| NRCAM | Neuronal cell adhesion molecule | TNBC cells | MS analysis of CM | [11] |
| P4HB | Protein disulfide-isomerase, prolyl 4-hydroxylase beta | TNBC cells | MS analysis of CM | [11] |
| PLOD1 | Procollagen-lysine, 2-oxoglutarate 5-dioxygenase 1 | TNBC cells | MS analysis of CM | [11] |
| PPIB | Peptidyl-prolyl cis-trans isomerase B | TNBC cells | MS analysis of CM | [11] |
| RELN | Reelin | TNBC cells | TNBC cells (knockdown of integrin alpha3) | [37] |
| TF | Tissue factor | TNBC cells | CM from TNBC cell lines depleted of Syndecan-1 | [16] |
| TLN1 | Talin 1 | TNBC cells | MS analysis of CM | [11] |
| TNC | Tenascin C | TNBC cells | MS analysis of CM | [11] |
| Protein | Aliases | Source | Evidence | Refs. |
|---|---|---|---|---|
| CTSD | Cathepsin D | TNBC cells | TNBC cells treated with tocotrienols (vitamin E) | [60] |
| CTSZ | Cathepsin Z | TNBC cells | MS analysis of CM | [11] |
| GGH | Gamma-glutamyl hydrolase | TNBC cells | MS analysis of CM | [11] |
| PCSK9 | Proprotein convertase subtilisin/kexin type 9 | TNBC cells | MS analysis of CM | [11] |
| PLAT | Tissue plasminogen activator | TNBC cells | TNBC cell line depleted of LRP-1 | [26] |
| PLAU | uPA, urokinase plasminogen activators | TNBC cells; xenograft tumors | TNBC cells (inducible silencing of HMGA1); interstitial fluid of xenograft tumors | [25,28] |
| PLG | Plasminogen | TNBC cells | TNBC cell line depleted of LRP-1 | [26] |
| APLP2 | Amyloid-beta precursor-like protein 2 | TNBC cells | MS analysis of CM | [11] |
| APP | Amyloid-beta precursor protein | TNBC cells | MS analysis of CM | [11] |
| PI3 | Elafin, peptidase inhibitor 3 | TNBC cells | MS analysis of CM | [11] |
| SERPINE1 | Serine protease inhibitor E1, plasminogen activator inhibitor 1 (PAI1) | TNBC cells; xenograft tumors | MS analysis of CM; interstitial fluid of xenograft tumors; TNBC cells (inducible silencing of HMGA1); TNBC cells treated with tocotrienols (vitamin E); TNBC cell line depleted of LRP-1 | [11,25,26,28,60] |
| TIMP1 | Tissue inhibitor of metalloproteinases 1 | TNBC cells; xenograft tumors | MS analysis of CM; interstitial fluid of xenograft tumors | [11,28] |
| TIMP2 | Tissue inhibitor of metalloproteinases 2 | TNBC cells | MS analysis of CM | [11] |
| TIMP1, -2, -3 | Tissue inhibitor of metalloproteinases | TNBC cells | TNBC cell line depleted of LRP-1 | [26] |
| Membrane and Extracellular Vesicle Proteins | ||||
|---|---|---|---|---|
| Protein | Aliases | Source | Evidence | Refs. |
| ANXA2 | Annexin II, annexin A2 | TNBC cells | TNBC cell lines | [116] |
| BCAP31 | B cell receptor-associated protein 31 | TNBC cells | TNBC cell line treated with Palbociclib | [117] |
| CD151 | Cluster of differentiation 151 | TNBC tumors | Exosomes from TNBC patient serum | [118] |
| IL-3Rα | Interleukin-3 receptor subunit α | TNBC tumors and cells | TNBC tumor expression and modulation of IL-3R-containing EVs | [119] |
| ITGB4 | Integrin β4 | TNBC cells | TNBC cells in coculture with CAFs; exosomes from TNBC cells | [120,121] |
| SPANXB1 | Sperm protein associated with the nucleus on the X chromosome B1 | TNBC tumors | Circulating EVs from TNBC patients | [122] |
| TSPAN11 | Tetraspanin 11 | TNBC cells | TNBC cell line treated with Palbociclib | [117] |
| UCHL1 | Ubiquitin carboxyl-terminal hydrolase isozyme L1 | TNBC tumors and cells | Exosomes, serum, and CM | [123] |
| Peptide Hormones | ||||
| FST | Follistatin | TNBC cells | TNBC cell line depleted of LRP-1 | [26] |
| PENK | Proenkephalin | CAFs | TNBC cells treated with CAF CM | [15] |
| Metabolic Proteins | ||||
| ENO1 | Alpha-enolase | TNBC cells | MS analysis of CM | [11] |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | TNBC cells | MS analysis of CM | [11] |
| LDHA | Lactate dehydrogenase A | TNBC cells | MS analysis of CM | [11] |
| LDHB | Lactate dehydrogenase B | TNBC cells | MS analysis of CM | [11] |
| LPA | Lipoprotein A | TNBC cells | MS analysis of CM | [11] |
| TXNIP | Thioredoxin-interacting protein | TNBC cells | TNBC cells cocultured with hBMECS | [7] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McHenry, P.R.; Prosperi, J.R. Proteins Found in the Triple-Negative Breast Cancer Secretome and Their Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 2100. https://doi.org/10.3390/ijms24032100
McHenry PR, Prosperi JR. Proteins Found in the Triple-Negative Breast Cancer Secretome and Their Therapeutic Potential. International Journal of Molecular Sciences. 2023; 24(3):2100. https://doi.org/10.3390/ijms24032100
Chicago/Turabian StyleMcHenry, Peter R., and Jenifer R. Prosperi. 2023. "Proteins Found in the Triple-Negative Breast Cancer Secretome and Their Therapeutic Potential" International Journal of Molecular Sciences 24, no. 3: 2100. https://doi.org/10.3390/ijms24032100
APA StyleMcHenry, P. R., & Prosperi, J. R. (2023). Proteins Found in the Triple-Negative Breast Cancer Secretome and Their Therapeutic Potential. International Journal of Molecular Sciences, 24(3), 2100. https://doi.org/10.3390/ijms24032100





