Moringa oleifera: An Updated Comprehensive Review of Its Pharmacological Activities, Ethnomedicinal, Phytopharmaceutical Formulation, Clinical, Phytochemical, and Toxicological Aspects
Abstract
1. Introduction
2. Material Method
2.1. Article Eligibility Criteria
2.2. Software and Techniques Used
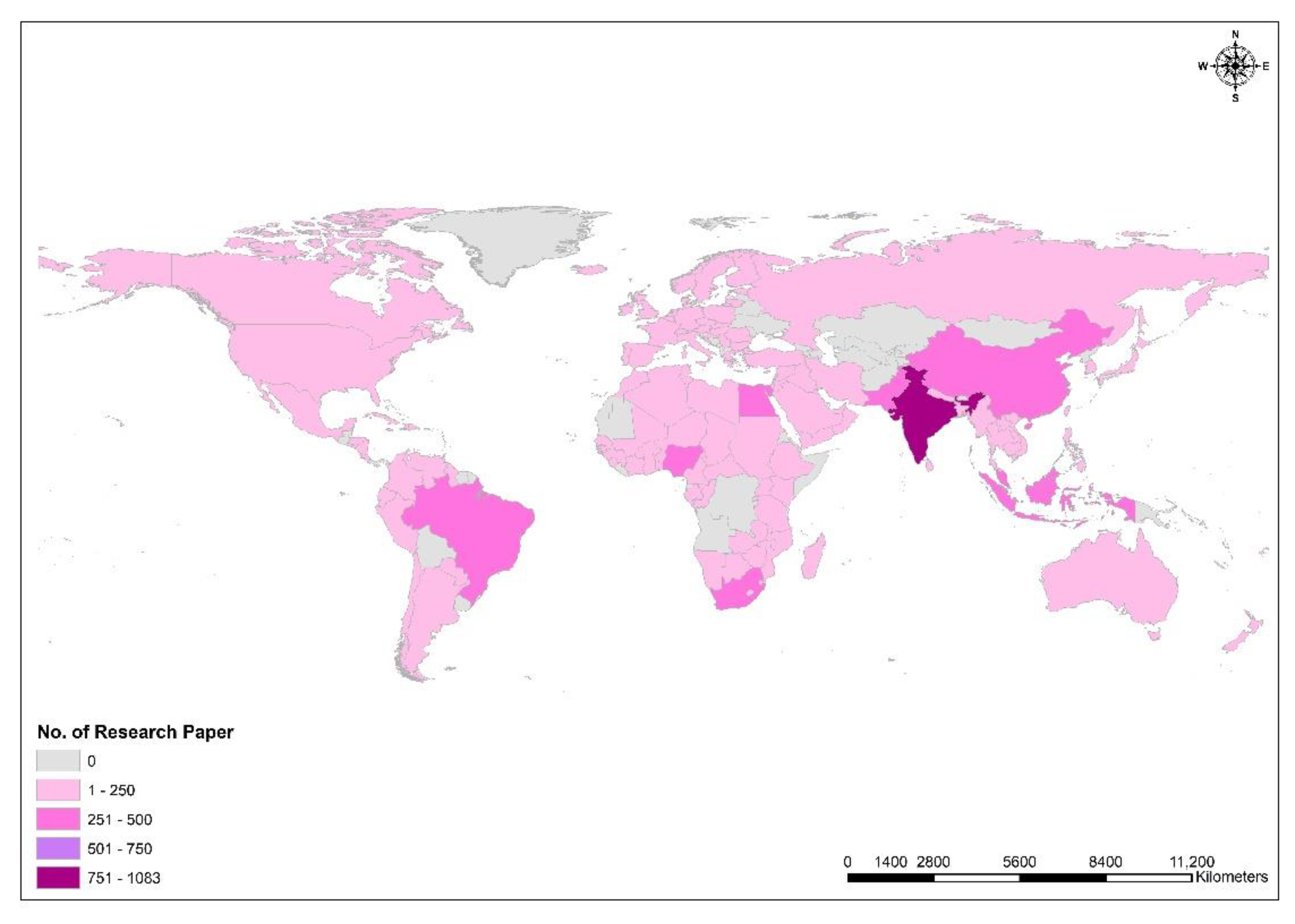
3. Worldwide Research and Collaboration
4. Taxonomical Classification
5. Morphology
6. Botanical and Geographical Distribution
7. Ethnomedicinal/Traditional Properties
8. Pharmacological Uses
8.1. Antimicrobial and Antifungal Activity
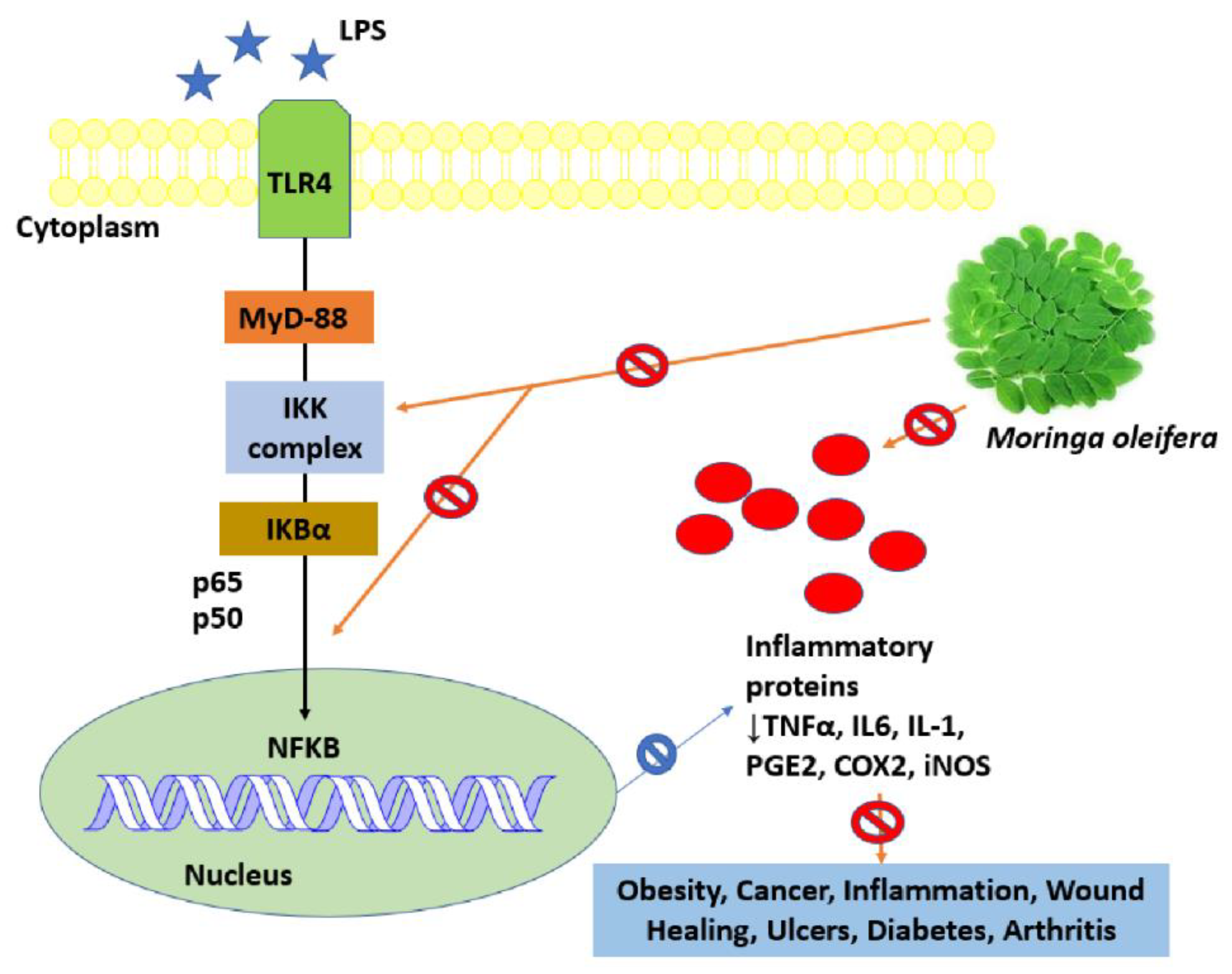
8.2. Anti-Inflammatory Activity
8.3. Oxidative Stress
8.4. Anti-Oxidant Activity
8.5. Anti-Cancer Activity
8.6. Fertility and Anti-Fertility Activity
8.7. Hepatoprotective Activity
8.8. Cardiovascular Activity
8.9. Anti-Ulcer/Gastroprotective Activity
8.10. Analgesic/Antipyretic Activity
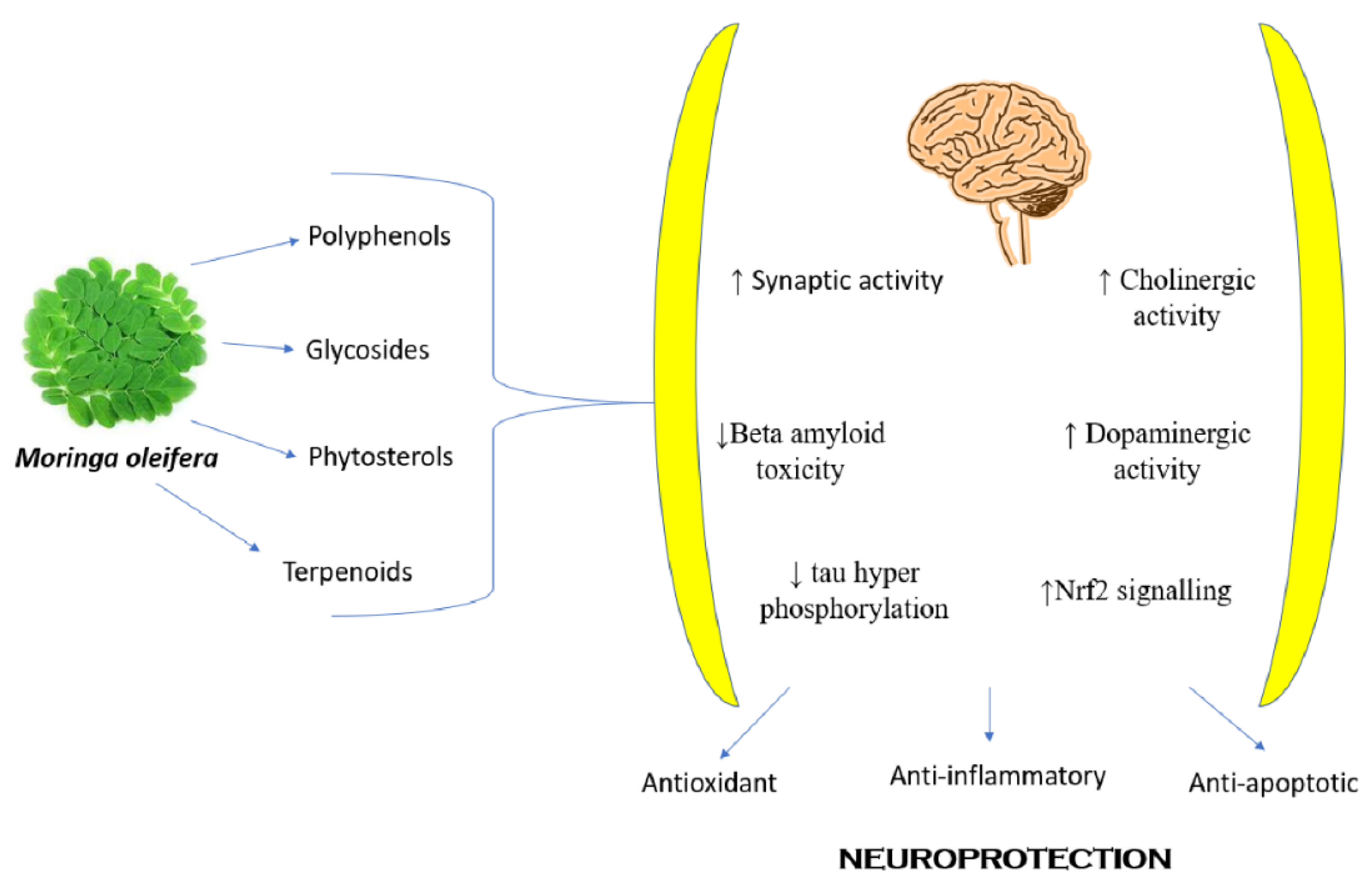
8.11. Neuropharmacological Activity
8.12. Neuropathic Pain
8.13. Wound Healing Effect
8.14. Immunomodulatory Activity
8.15. Hematological Activity
8.16. Anti-Obesity Activity
8.17. Anti-Allergic Activity
8.18. Anti-Diabetic Activity
8.19. Diuretic Activity
8.20. Angiotensin Converting Enzyme (ACE) Activity
8.21. Anti-Venom Effect
8.22. Cytotoxicity Effect
9. Toxicity
10. Clinical Trials
11. Phytopharmaceutical Formulations
12. Miscellaneous Uses
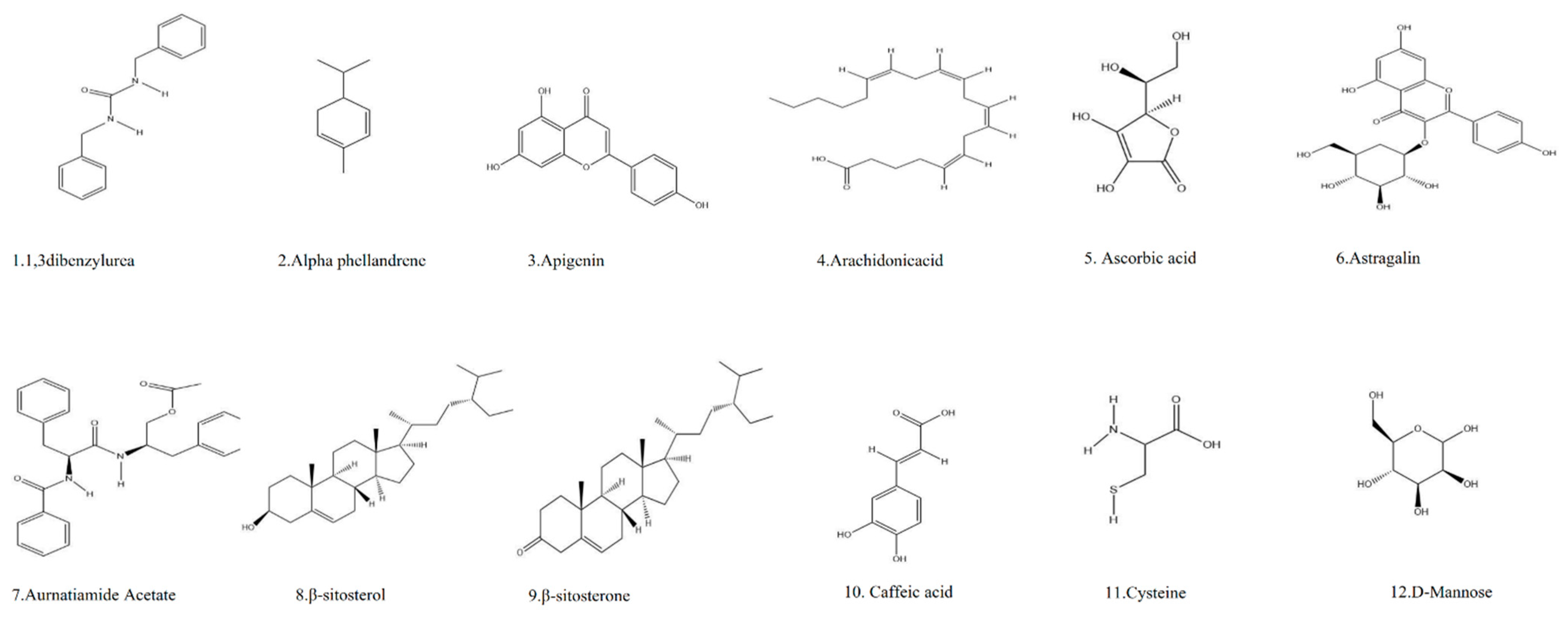
13. Phytochemistry
14. Current Status
15. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuglie, L.J. Producing Food without Pesticides: Local Solutions to Crop Pest Control in West Africa, 1st ed.; Church World Service: Dakar, Senegal, 1998; pp. 1–158. [Google Scholar]
- Gandji, K.; Chadare, F.J.; Idohou, R.; Salako, V.K.; Assogbadjo, A.E.; Glèlè, R.L.K. Status and utilisation of Moringa oleifera Lam: A review. Afr. Crop Sci. J. 2018, 26, 137–156. [Google Scholar] [CrossRef]
- Chaudhary, K.; Chourasia, S. Nutraceutical properties of Moringa oleifera: A review. EJPMR 2017, 4, 646–655. [Google Scholar]
- Gopinath, L.R.; Jeevitha, S.; Gokiladevi, T.; Archaya, S. Isolation and Identification of therapeutic compounds from Moringa oleifera and its antimicrobial activity. IOSR-JPBS 2017, 12, 1–10. [Google Scholar]
- Kasolo, J.N.; Bimenya, G.S.; Ojok, L.; Ochieng, J.; Ogwal-Okeng, J.W. Phytochemicals and uses of Moringa oleifera leaves in Ugandan rural communities. J. Med. Plant Res. 2010, 20104, 753–757. [Google Scholar]
- Anwar, F.; Ashraf, M.; Bhanger, M.I. Interprovenance variation in the composition of Moringa oleifera oilseeds from pakistan. J. Am. Oil Chem. Soc. 2005, 82, 45–51. [Google Scholar] [CrossRef]
- Ansari, M.M.; Kumar, S.D. Fortification of Food and Beverages with Phytonutrients. Public Health Nutr. 2012, 2, 241–253. [Google Scholar]
- Choudhary, M.K.; Bodakhe, S.H.; Gupta, S.K. Assessment of the antiulcer potential of Moringa oleifera root-bark extract in rats. J. Acupunct. Meridian Stud. 2013, 6, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Posmontier, B. The medicinal qualities of Moringa oleifera. Holist. Nurs. Pr. 2011, 25, 80–87. [Google Scholar] [CrossRef]
- Aekthammarat, D.; Pannangpetch, P.; Tangsucharit, P. Moringa oleifera leaf extract lowers high blood pressure by alleviating vascular dysfunction and decreasing oxidative stress in L-NAME hypertensive rats. Phytomedicine 2019, 54, 9–16. [Google Scholar] [CrossRef]
- Tayo, G.M.; Pone, G.W.; Komtangi, M.C.; Yondo, G.; Ngangout, A.M.; Mbida, M. Anthelminthic Activity of Moringa oleifera Leaf Extracts Evaluated in Vitro on Four Developmental Stages of Haemonchus contortus from Goats. AJPS 2014, 5, 1702–1710. [Google Scholar] [CrossRef]
- Hannan, M.A.; Kang, J.Y.; Mohibbullah, M.; Hong, Y.K.; Lee, H.; Choi, J.S.; Choi, I.S.; Moon, I.S. Moringa oleifera with promising neuronal survival and neurite outgrowth promoting potentials. J. Ethnopharmacol. 2014, 152, 142–150. [Google Scholar] [CrossRef]
- Paikra, B.K.; Dhongade, H.K.J.; Gidwani, B. Phytochemistry and Pharmacology of Moringa oleifera Lam. J Pharmacopunct. 2017, 20, 194–200. [Google Scholar]
- Mallenakuppe, R.; Homabalegowda, H.; Gouri, M.D.; Basavaraju, P.S.; Chandrashekharaiah, U.B. History, Taxonomy and Propagation of Moringa oleifera-A Review. Int. J. Life Sci. 2019, 5, 2322–2327. [Google Scholar] [CrossRef]
- Guevara, A.P.; Vargas, C.; Sakurai, H.; Fujiwara, Y.; Hashimoto, K.; Maoka, T.; Kozuka, M.; Ito, Y.; Tokuda, H.; Nishino, H. An antitumor promoter from Moringa oleifera Lam. Mutat. Res. 1999, 440, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Vimala, G.; Gricilda Shoba, F. A review on Antiulcer Activity of Few Indian Medicinal Plants. Int. J. Microbiol. 2014, 2014, 519590. [Google Scholar] [CrossRef]
- Popoola, J.O.; Obembe, O.O. Local knowledge, use pattern and geographical distribution of Moringa oleifera Lam. (Moringaceae) in Nigeria. J. Ethnopharmacol. 2013, 150, 682–691. [Google Scholar] [CrossRef]
- Swatią, A.; Virk, A.K.; Kumari, C.; Ali, A.; Garg, P.; Thakur, P.; Attri, C.; Kulshrestha, S. Moringa oleifera—A never die tree: An overview. Asian J. Pharm. Clin. Res. 2018, 11, 57–65. [Google Scholar] [CrossRef]
- Gopalakrishnan, L.; Doriya, K.; Kumar, D.S. Moringa oleifera: A review on nutritive importance and its medicinal application. Food Sci. Hum. Wellness 2016, 5, 49–56. [Google Scholar] [CrossRef]
- Stohs, S.J.; Hartman, M.J. Review of the Safety and Efficacy of Moringa oleifera. Phytother. Res. 2015, 29, 796–804. [Google Scholar] [CrossRef]
- Bhat, S.K.; Joy, A.E. Antianxiety effect of ethanolic extract of leaves of Moringa oleifera in Swiss albino mice. Arch. Med. Health Sci. 2014, 2, 5–7. [Google Scholar]
- Misra, A.; Srivastava, S.; Srivastava, M. Evaluation of antidiarrheal potential of Moringa oleifera (Lam.) leaves. J. Pharmacogn. Phytochem. 2014, 2, 43–46. [Google Scholar]
- Tahkur, R.S.; Soren, G.; Pathapati, R.M.; Buchineni, M. Diuretic activity of Moringa oleifera leaves extract in swiss albino rats. J. Pharm. Innov. 2016, 5, 8–10. [Google Scholar]
- Woldeyohannes, M.G.; Eshete, G.T.; Abiye, A.A.; Hailu, A.E.; Huluka, S.A.; Tadesse, W.T. Antidiarrheal and Antisecretory Effect of 80% Hydromethanolic Leaf Extract of Moringa stenopetala Baker f. in Mice. Biochem. Res. Int. 2022, 2022, 2090–2247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Peng, L.; Li, W.; Dai, T.; Nie, L.; Xie, J.; Ai, Y.; Li, L.; Tian, Y.; Sheng, J. Polyphenol Extract of Moringa oleifera Leaves Alleviates Colonic Inflammation in Dextran Sulfate Sodium-Treated Mice. Evid. Based Complement. Altern. Med. 2020, 2020, 6295402. [Google Scholar] [CrossRef] [PubMed]
- Karadi, R.V.; Gadge, N.B.; Alagawadi, K.R.; Savadi, R.V. Effect of Moringa oleifera Lam. root-wood on ethylene glycol induced urolithiasis in rats. J. Ethnopharmacol. 2006, 105, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Ghasi, S.; Nwobodo, E.; Ofili, J.O. Hypocholesterolemic effects of crude extract of leaf of Moringa oleifera Lam in high-fat diet fed wistar rats. J. Ethnopharmacol. 2000, 69, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, R.; Sharma, V.; Pracheta. A Review on Horse Radish Tree (Moringa oleifera): A Multipurpose Tree with High Economic and Commercial Importance. Asian J. Biotechnol. 2011, 3, 317–328. [Google Scholar] [CrossRef]
- Debnath, S.; Guha, D. Role of Moringa oleifera on enterochromaffin cell count and serotonin content of experimental ulcer model. Indian J. Exp. Biol. 2007, 45, 726–731. [Google Scholar]
- Mahajan, S.G.; Mali, R.G.; Mehta, A.A. Protective Effect of Ethanolic Extract of Seeds of Moringa oleifera Lam. Against Inflammation Associated with Development of Arthritis in Rats. J. Immunotoxicol. 2007, 4, 39–47. [Google Scholar]
- Rathi, B.S.; Bodhankar, S.L.; Baheti, A.M. Evaluation of aqueous leaves extract of Moringa oleifera Linn for wound healing in albino rats. Indian J. Exp. Biol. 2006, 44, 898–901. [Google Scholar]
- Bhattacharya, A.; Tiwari, P.; Sahu, P.K.; Kumar, S. A review of the phytochemical and pharmacological characteristics of Moringa oleifera. J. Pharm. Bioallied Sci. 2018, 10, 181–191. [Google Scholar] [PubMed]
- Pandey, A.; Pandey, R.D.; Tripathi, P.; Gupta, P.P.; Haider, J.; Bhatt, S.; Singh, A.V. Moringa oleifera Lam. (Sahijan)—A plant with a plethora of diverse therapeutic benefits: An Updated Retrospection. Int. J. Med. Aromat. 2012, 1, 1–8. [Google Scholar] [CrossRef]
- Meireles, D.; Gomes, J.; Lopes, L. A review of properties, nutritional and pharmaceutical applications of Moringa oleifera: Integrative approach on conventional and traditional Asian medicine. Adv. Tradit. Med. 2020, 20, 495–515. [Google Scholar] [CrossRef]
- Liu, W.L.; Wu, B.F.; Shang, J.H.; Wang, X.F.; Zhao, Y.L.; Huang, A.X. Moringa oleifera seed ethanol extract and its active component kaempferol potentiate pentobarbital-induced sleeping behaviours in mice via a GABAergic mechanism. Pharm. Biol. 2022, 60, 810–824. [Google Scholar] [CrossRef] [PubMed]
- Gothai, S.; Arulselvan, P.; Tan, W.S.; Fakurazi, S. Wound healing properties of ethyl acetate fraction of Moringa oleifera in normal human dermal fibroblasts. J. Intercult. Ethnopharmacol. 2016, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Toma, A.; Deyno, S. Phytochemistry and pharmacological activities of Moringa oleifera. Indian, J. Pharmacol. 2014, 4, 222–231. [Google Scholar]
- Tripathi, B. Charaka Samhita, of Agnivesa, 3rd ed.; Chaukambha Surbharati Prakashan: Varanasi, India, 1994; pp. 15–20. [Google Scholar]
- Gupta, K.A. Astanga Hridaya, 13th ed.; Chaukambha Sanskrit Sansthan: Varanasi, India, 2000; pp. 5–17. [Google Scholar]
- Sharma, H.R. Kashyap Samhita, 3rd ed.; Chaukhambha Sanskrit Sansthan: Varanasi, India, 2010; pp. 2–4. [Google Scholar]
- Srikanthmurthy, K.R. SharangadharaSamhita, 12th ed.; Master Kheladi Lal and Sons: Benaras City, India, 1933; pp. 14–20. [Google Scholar]
- Laksmipati, V.S. Yogaratnakara, 7th ed.; Chaukambha Sanskrit Sansthan: Varanasi, India, 1999; pp. 1–5. [Google Scholar]
- Mishra, G.; Singh, P.; Verma, R.; Kumar, S.; Srivastav, S.; Jha, K.K.; Khosa, R.L. Traditional uses, phytochemistry and pharmacological properties of Moringa oleifera plant: An overview. Der Pharmacia Lett. 2011, 3, 141–164. [Google Scholar]
- Upadhyay, P.; Yadav, M.K.; Mishra, S.; Sharma, P.; Purohit, S. Moringa oleifera: A review of the medical evidence for its nutritional and pharmacological properties. Int. J. Res. Pharm. Sci. 2015, 5, 12–16. [Google Scholar]
- Singh, A.; Navneet. Ethnomedicinal, pharmacological and antimicrobial aspects of Moringa oleifera lam.: A review. J. Phytopharmacol. 2018, 7, 45–50. [Google Scholar] [CrossRef]
- Banik, S.; Biswas, S.; Karmakar, S. Extraction, purification, and activity of protease from the leaves of Moringa oleifera. F1000Research 2018, 7, 1151. [Google Scholar] [CrossRef]
- Parvathy, M.V.S.; Umamaheshwari, A. Cytotoxic Effect of Moringa oleifera Leaf Extracts on Human Multiple Myeloma Cell Lines. Trends Medical Res. 2007, 2, 44–50. [Google Scholar]
- Venkatesh, N.; Devi, K.G.; Venkateswarlu, G.; Sudhakar, A.M.S. effect of hydroalcoholic extract of Moringa oleifera leaves on fertility hormone and sperm quality of male albino rats. Int. J. Curr. Med. Pharm. Res. 2019, 1, 83–87. [Google Scholar]
- Balogun, T.A.; Buliamimu, K.D.; Chukwudozie, O.S.; Tiamiyu, Z.A.; Idowu, T.J. Anticancer Potential of Moringa oleifera on BRCA-1 Gene: Systems Biology. Bioinform. Biol. Insights. 2021, 15, 11779322211010703. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Elkholy, S.F.; Mahfouz, S.A.; Alam, P.; Sharaf-Eldin, M.A. HPLC based estimation and extraction of rutin, quercetin and gallic acid in Moringa oleifera plants grown in Saudi Arabia. J. Chem. Pharm. 2016, 8, 1243–1246. [Google Scholar]
- Singh, B.N.; Singh, B.R.; Singh, R.L. Oxidative DNA damage protective activity, antioxidant and anti-quorum sensing potentials of Moringa oleifera. Food Chem. Toxicol. 2009, 47, 1109–1116. [Google Scholar] [CrossRef]
- Ndong, M.; Uehara, M.; Katsumata, S.; Suzuki, K. Effects of oral administration of Moringa oleifera Lam on glucose tol-erance in Goto-Kakizaki and Wistar rats. J. Clin. Biochem. Nutri. 2007, 40, 229–233. [Google Scholar] [CrossRef]
- Zhang, M.; Hettiarachchy, S.N.; Horax, R.; Kannan, A.; Praisoody, M.D.A.; Muhundan, A.; Mallangi, C.R. Phytochemicals, antioxidant and antimicrobial activity of Hibiscus sabdariffa, Centella asiatica, Moringa oleifera and Murraya koenigii leaves. J. Med. Plants Res. 2011, 5, 6672–6680. [Google Scholar]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: An overview. Int. J. Mol. Sci. 2015, 16, 12791–12835. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Castillo, C.M.S.; Caroca, R.; Lazo-Vélez, M.A.; Antonyak, H.; Polishchuk, A.; Lysiuk, R.; Oliinyk, P.; Masi, L.D.; et al. Phytochemicals for the Prevention and Treatment of Oxidative Stress-Induced Diseases. World J. Gastrointest. Endosc. 2022, 22, 1–9. [Google Scholar]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Skin Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Chung, T.W.; Moon, S.K.; Chang, Y.C.; Ko, J.H.; Lee, Y.C.; Cho, G.; Kim, S.H.; Kim, J.G.; Kim, C.H. Novel and therapeutic effect of caffeic acid and caffeic acid phenyl ester on hepatocarcinoma cells: Complete regression of hepatoma growth and metastasis by dual mechanism. FASEB J. 2004, 18, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- Pandi, A.; Kalappan, V.M. Pharmacological and therapeutic applications of Sinapic acid—An updated review. Mol. Biol. Rep. 2021, 48, 3733–3745. [Google Scholar] [CrossRef] [PubMed]
- Alhakmani, F.; Kumar, S.; Khan, S.A. Estimation of total phenolic content, in-vitro antioxidant and anti-inflammatory activity of flowers of Moringa oleifera. Asian Pac. J. Trop. Biomed. 2013, 3, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Bennour, N.; Mighri, H.; Eljani, H.; Zammouri, T.; Akrout, A. Effect of Solvent Evaporation Method on Phenolic Compounds and the Antioxidant Activity of Moringa oleifera Cultivated in Southern Tunisia. S. Afr. J. Bot. 2020, 129, 181–190. [Google Scholar] [CrossRef]
- Riaz, A.; Rasul, A.; Hussain, G.; Zahoor, M.K.; Jabeen, F.; Subhani, Z.; Younis, T.; Ali, M.; Sarfraz, I.; Selamoglu, Z. Astragalin: A Bioactive Phytochemical with Potential Therapeutic Activities. Adv. Pharmacol. Sci. 2018, 2018, 9794625. [Google Scholar] [CrossRef]
- Borgonovo, G.; De Petrocellis, L.; Schiano Moriello, A.; Bertoli, S.; Leone, A.; Battezzati, A.; Mazzini, S.; Bassoli, A. Moringin, A Stable Isothiocyanate from Moringa oleifera, activates the Somatosensory and Pain Receptor TRPA1 Channel In Vitro. Molecules 2020, 25, 976. [Google Scholar] [CrossRef]
- Liao, P.-C.; Lai, M.-H.; Hsu, K.-P.; Kuo, Y.-H.; Chen, J.; Tsai, M.-C.; Li, C.-X.; Yin, X.-J.; Jeyashoke, N.; Chao, L.K.-P. Identification of β-Sitosterol as in Vitro Anti-Inflammatory Constituent in Moringa oleifera. J. Agric. Food Chem. 2018, 66, 10748–10759. [Google Scholar] [CrossRef]
- Sari, K.; Sirajuddin, S.; Maddeppungeng, M.; Hadju, V.; Saleh, A.; Hastuti, H. Moringa oleifera Intake during Pregnancy and Breastfeeding toward Docosahexaenoic Acid and Arachidonic Acid Levels in Breast Milk. Open Access Maced. J. Med. Sci. 2020, 8, 757–761. [Google Scholar] [CrossRef]
- Snehal, M.; Parag, G.; Jyotsna, W.; Satyanarayan, N.N. Intensified synthesis of structured lipids from oleic acid rich moringa oil in the presence of supercritical CO2. Food Bioprod. Process. 2020, 112, 86–95. [Google Scholar]
- Kaur, G.; Invally, M.; Sanzagiri, R.; Buttar, H.S. Evaluation of the antidepressant activity of Moringa oleifera alone and in combination with fluoxetine. J. Ayurveda Integr. Med. 2015, 6, 273–279. [Google Scholar] [CrossRef]
- Korbecki, J.; Bajdak-Rusinek, K. The effect of palmitic acid on inflammatory response in macrophages: An overview of molecular mechanisms. Inflamm. Res. 2019, 68, 915–932. [Google Scholar] [CrossRef] [PubMed]
- Rue, E.A.; Rush, M.D.; Van Breemen, R.B. Procyanidins: A comprehensive review encompassing structure elucidation via mass spectrometry. Phytochem. Rev. 2018, 17, 1–16. [Google Scholar] [CrossRef]
- Maurya, S.K.; Singh, A.K. Clinical Efficacy of Moringa oleifera Lam. Stems Bark in Urinary Tract Infections. Int. Sch. Res. Not. 2014, 2014, 906843. [Google Scholar]
- Padla, E.P.; Solis, L.T.; Levida, R.M.; Shen, C.C.; Ragasa, C.Y. Antimicrobial isothiocyanates from the seeds of Moringa oleifera Lam. Z. Für Nat. C 2012, 67, 557–564. [Google Scholar] [CrossRef]
- Ahmadua, T.; Ahmad, K.; Ismail, S.I.; Rashed, O.; Asib, N.; Omar, D. Antifungal efficacy of moringa oliefera leaves and seed extract against Botrytis cinerea causing graymold disease of tomato. Braz. J. Biol. 2020, 81, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Moodley, J.S.; Krishna, S.B.N.; Pillay, K. Green synthesis of silver nanoparticles from Moringa oleifera leaf extracts and its antimicrobial potential. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Dinesha, B.L.; Nidoni, U.; Ramachandra., C.T.; Naik, N.; Sankalpa, K.B. Effect of extraction methods on physicochemical, nutritional, antinutritional, antioxidant and antimicrobial activity of Moringa (Moringa oleifera Lam.) seed kernel oil. J. Appl. Nat. Sci. 2018, 10, 287–295. [Google Scholar] [CrossRef]
- Anzano, A.; De Falco, B.; Ammar, M.; Ricciardelli, A.; Grauso, L.; Sabbah, M.; Capparelli, R.; Lanzotti, V. Chemical Analysis and Antimicrobial Activity of Moringa oleifera Lam. Leaves and Seeds. Molecules 2022, 27, 8920. [Google Scholar]
- Tan, W.S.; Arulselvan, P.; Karthivashan, G.; Fakurazi, S. Moringa oleifera Flower Extract Suppresses the Activation of Inflammatory Mediators in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages via NF-κB Pathway. Mediators Inflamm. 2015, 2015, 720171. [Google Scholar] [CrossRef]
- Cuellar-Núñez, M.L.; Gonzalez de Mejia, E.; Loarca-Piña, G. Moringa oleifera leaves alleviated inflammation through downregulation of IL- 2, IL- 6, and TNF- α in a colitis associated colorectal cancer model. Int. Food Res. J. 2021, 144, 110318. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Khalil, S.R.; Awad, A.; Abu Zeid, E.H.; El-Aziz, R.A.; El-Serehy, H.A. Ethanolic Extract of Moringa oleifera Leaves Influences NF-κB Signaling Pathway to Restore Kidney Tissue from Cobalt-Mediated Oxidative Injury and Inflammation in Rats. Nutrients 2020, 12, 1031. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Debnath, T.; Tang, Y.; Ryu, Y.B.; Moon, S.H.; Kim, E.K. Topical application of Moringa oleifera leaf extract ameliorates experimentally induced atopic dermatitis by the regulation of Th1/Th2/Th17 balance. Biomed. Pharmacother. 2016, 84, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.M.; Al-Osaimi, S.H.; Hassan Mohamed, E.; Aldharani, A.; Alkhedaide, A.; Althobaiti, F.; Mohamed, W.A. Protective effects of Moringa oleifera Leaf Extract against Methotrexate-Induced Oxidative Stress and Apoptosis on Mouse Spleen. Evid. -Based Complement. Altern. Med. 2020, 2020, 6738474. [Google Scholar]
- Zhou, Y.; Yang, W.; Li, Z.; Luo, D.; Li, W.; Zhang, Y.; Wang, X.; Fang, M.; Chen, Q.; Jin, X. Moringa oleifera stem extract protect skin keratinocytes against oxidative stress injury by enhancement of antioxidant defense systems and activation of PPARα. Biomed. Pharmacother. 2018, 107, 44–53. [Google Scholar] [CrossRef]
- El-Hadary, A.E.; Ramadan, M.F. Antioxidant traits and protective impact of Moringa oleifera leaf extract against diclofenac sodium-induced liver toxicity in rats. J. Food Biochem. 2019, 43, e12704. [Google Scholar] [CrossRef]
- Nunthanawanich, P.; Sompong, W.; Sirikwanpong, S.; Mäkynen, K.; Adisakwattana, S.; Dahlan, W.; Ngamukote, S. Moringa oleifera aqueous leaf extract inhibits reducing monosaccharide-induced protein glycation and oxidation of bovine serum albumin. Springer Plus. 2016, 5, 1098. [Google Scholar] [CrossRef]
- Albuquerque Costa, R.; de Sousa, O.V.; Hofer, E.; Mafezoli, J.; Barbosa, F.G.; Vieira, R. Thiocarbamates from Moringa oleifera Seeds Bioactive against Virulent and Multidrug-Resistant Vibrio Species. BioMed Res. Int. 2017, 2017, 7963747. [Google Scholar] [CrossRef]
- Vergara-Jimenez, M.; Almatrafi, M.M.; Fernandez, M.L. Bioactive Components in Moringa oleifera Leaves Protect against Chronic Disease. Antioxidant 2017, 6, 91. [Google Scholar] [CrossRef]
- Agrawal, N.D.; Nirala, S.K.; Shukla, S.; Mathur, R. Co-administration of adjuvants along with Moringa oleifera attenuates beryllium-induced oxidative stress and histopathological alterations in rats. Pharm. Biol. 2015, 53, 1465–1473. [Google Scholar] [CrossRef]
- Sasikala, V.; Rooban, B.N.; Priya, S.G.; Sahasranamam, V.; Abraham, A. Moringa oleifera prevents selenite-induced cataractogenesis in rat pups. J. Ocul. Pharmacol. Ther. 2010, 26, 441–447. [Google Scholar] [CrossRef]
- Vongsak, B.; Mangmool, S.; Gritsanapan, W. Antioxidant Activity and Induction of mRNA Expressions of Antioxidant Enzymes in HEK-293 Cells of Moringa oleifera Leaf Extract. Planta Med. 2015, 81, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Mohd Fisall, U.F.; Ismail, N.Z.; Adebayo, I.A.; Arsad, H. Dichloromethane fraction of Moringa oleifera leaf methanolic extract selectively inhibits breast cancer cells (MCF7) by induction of apoptosis via upregulation of Bax, p53 and caspase 8 expressions. Mol. Biol. Rep. 2021, 48, 4465–4475. [Google Scholar] [CrossRef] [PubMed]
- Attah, A.F.; Moody, J.O.; Sonibare, M.A.; Salahdeen, H.H.; Akindele, O.O.; Nnamani, P.O.; Diyaolu, O.A.; Raji, Y. Aqueous extract of Moringa oleifera leaf used in Nigerian ethnomedicine alters conception and some pregnancy outcomes in Wistar rat. S. Afr. J. Bot. 2020, 129, 255–262. [Google Scholar] [CrossRef]
- Sharifudin, S.A.; Fakurazi, S.; Hidayat, M.T.; Hairuszah, I.; Aris, M.; Moklas, M.; Arulselvan, P. Therapeutic potential of Moringa oleifera extracts against acetaminophen-induced hepatotoxicity in rats. Pharm. Biol. 2013, 51, 279–288. [Google Scholar] [CrossRef]
- Hamza, A.A. Ameliorative effects of Moringa oleifera Lam seed extract on liver fibrosis in rats. Food Chem. Toxicol. 2010, 48, 345–355. [Google Scholar] [CrossRef]
- Nandave, M.; Ojha, S.K.; Joshi, S.; Kumari, S.; Arya, D.S. Moringa oleifera leaf extract prevents isoproterenol-induced myocardial damage in rats: Evidence for an antioxidant, antiperoxidative, and cardioprotective intervention. J. Med. Food. 2009, 12, 47–55. [Google Scholar] [CrossRef]
- Panda, S.; Kar, A.; Sharma, P.; Sharma, A. Cardioprotective potential of N,alpha-L-rhamnopyranosyl vincosamide, an indole alkaloid, isolated from the leaves of Moringa oleifera in isoproterenol induced cardiotoxic rats: In vivo and in vitro studies. Bioorganic Med. Chem. Lett. 2012, 23, 959–962. [Google Scholar] [CrossRef]
- Ijioma, S.N.; Nwaogazi, E.N.; Nwankwo, A.A.; Oshilonya, H.; Ekeleme, C.M.; Oshilonya, L.U. Histological exhibition of the gastroprotective effect of Moringa oleifera leaf extract. Comp. Clin. Pathol. 2018, 27, 327–332. [Google Scholar] [CrossRef]
- Mallya, R.; Chatterjee, P.K.; Vinodini, N.A.; Chatterjee, P.; Mithra, P. Moringa oleifera Leaf Extract: Beneficial Effects on Cadmium Induced Toxicities—A review. J. Clin. Diagn. Res. 2017, 11, CE01. [Google Scholar] [CrossRef]
- Martínez-Gonzálezb, C.L.; Martíneza, L.; Martínez-Ortizb, E.J.; González-Trujanoa, M.E.; Déciga-Camposc, M.; Ventura-Martínezd, R.; Díaz-Revale, I. Moringa oleifera, a species with potential analgesic and anti-inflammatory activities. Biomed. Pharmacother. 2017, 87, 482–488. [Google Scholar] [CrossRef]
- Mohan, M.; Kaul, N.; Punekar, A.; Girnar, R.; Junnare, P.; Patil, L. Nootropic activity of Moringa oleifera leaves. J. Nat. Remedies 2005, 5, 59–62. [Google Scholar]
- Amrutia, J.N.; Lala, M.; Srinivasa, U.; Shabaraya, A.R.; Semuel, M.R. Anticonvulsant activity of Moringa oleifera leaf. Int. Res. J. Pharm. 2011, 2, 160–162. [Google Scholar]
- Ray, K.; Guha, D. Effect of Moringa oleifera root extract on penicillin-induced epileptic rats. Biog. Amines 2005, 19, 223–231. [Google Scholar] [CrossRef]
- Khongrum, J.; Wattanathorn, J.; Muchimapura, S.; Thukhum-mee, W.; Thipkaew, C.; Wannanon, P.; Tong-Un, T. Moringa oleifera Leaves Extract Attenuates Neuropathic Pain Induced by Chronic Constriction Injury. Am. J. Appl. Sci. 2012, 9, 1182–1187. [Google Scholar]
- Raafat, K.; Hdaib, F. Neuroprotective Effects of Moringa oleifera: Bio-guided GC-MS Identification of Active Compounds in Diabetic Neuropathic Pain Model. Chin. J. Integr. Med. 2017. Online ahead of print. [Google Scholar] [CrossRef]
- Muhammad, A.A.; Arulselvan, P.; Cheah, P.S.; Abas, F.; Fakurazi, S. Evaluation of wound healing properties of bioactive aqueous fraction from Moringa oleifera Lam on experimentally induced diabetic animal model. Drug Des. Devel. Ther. 2016, 10, 1715–1730. [Google Scholar] [CrossRef]
- Awodele, O.; Oreagba, I.A.; Odoma, S.; Jaime, A.; Da Silva, T.; Osunkalu, V.O. Toxicological evaluation of the aqueous leaf extract of Moringa oleifera Lam. (Moringaceae). J. Ethnopharmacol. 2012, 139, 330–336. [Google Scholar] [CrossRef]
- Mehwish, H.M.; Rajoka, M.S.; Xiong, Y.; Zheng, K.; Xiao, H.; Liu, Z.; Zhu, Q.; He, Z. Moringa oleifera—A Functional Food and Its Potential Immunomodulatory Effects. Food Rev. Int. 2020, 38, 1533–1552. [Google Scholar] [CrossRef]
- Suzana, D.; Suyatna, F.D.; Andrajati, R.; Sari, S.P.; Mun’im, A. Effect of Moringa oleifera leaves extract against hematology and blood biochemical value of patients with iron deficiency anaemia. J. Young Pharm. 2017, 9, 79–84. [Google Scholar] [CrossRef]
- Bais, S.; Singh, G.S.; Sharma, R. Anti-obesity and hypolipidemic activity of Moringa oleifera leaves against high fat diet-induced obesity in rats. Adv Biol. 2014, 2014, 162914. [Google Scholar] [CrossRef]
- Redha, A.A.; Perna, S.; Riva, A.; Petrangolini, G.; Peroni, G.; Nichetti, M.; Iannello, G.; Naso, M.; Faliva, M.A.; Rondanelli, M. Novel insights on anti-obesity potential of the miracle tree, Moringa oleifera: A systematic review. J. Funct. Foods 2021, 84, 104600. [Google Scholar] [CrossRef]
- Al-maliki, A.; El Rabey, H.A. The Antidiabetic Effect of Low Doses of Moringa oleifera Lam. Seeds on Streptozotocin Induced Diabetes and Diabetic Nephropathy in Male Rats. Biomed Res. Int. 2015, 2015, 381040. [Google Scholar]
- Villarruel-López, A.; López-de la Mora, D.A.; Vázquez-Paulino, O.D.; Puebla-Mora, A.G.; Torres-Vitela, M.R.; Guerrero-Quiroz, L.A.; Nuño, K. Effect of Moringa oleifera consumption on diabetic rats. BMC Complement. Altern. Med. 2018, 18, 120–127. [Google Scholar] [CrossRef]
- Azad, S.B.; Ansari, P.; Azam, S.; Hossain, S.M.; Shahid, M.I.; Hasan, M.; Hannan, J. Anti-hyperglycaemic activity of Moringa oleifera is partly mediated by carbohydrase inhibition and glucose-fibre binding. Biosci. Rep. 2017, 37, BSR20170059. [Google Scholar] [CrossRef]
- Khan, H.; Jaiswal, V.; Kulshreshtha, S.; Khan, A. Potential Angiotensin Converting Enzyme Inhibitors from Moringa oleifera. Recent Patents Biotechnol. 2019, 13, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Hussain, T.; Kataria, M.; Seth, A.; Malik, M.; Dash, A.; Chand, S.; Khan, M. Role of selective Bioactive Compounds as an Angiotensin Converting Enzyme Inhibitor. Res. Sq. 2020, 7, 1–25. [Google Scholar]
- Adeyi, A.O.; Ajisebiola, S.B.; Adeyi, E.O.; Alimba, C.G.; Okorie, U.G. Antivenom activity of Moringa oleifera leave against pathophysiological alterations, somatic mutation and biological activities of Naja nigricollis venom. Sci. Afr. 2020, 8, 68–76. [Google Scholar] [CrossRef]
- Nayak, G.; Rao, A.; Mullick, P.; Mutalik, S.; Kalthur, S.G.; Adiga, S.K.; Kalthur, G. Ethanolic extract of Moringa oleifera leaves alleviate cyclophosphamide induced testicular toxicity by improving endocrine function and modulating cell specific gene expression in mouse testis. J Ethnopharmacol. 2020, 259, 112922. [Google Scholar] [CrossRef]
- Adedapo, A.A.; Mogbojuri, O.M.; Emikpe, B.O. Safety evaluations of the aqueous extract of the leaves of Moringa oleifera in rats. J. Med. Plant Res. 2009, 3, 586–591. [Google Scholar]
- Ajibade, T.O.; Arowolo, R.; Olayemi, F.O. Phytochemical screening and toxicity studies on the methanol extract of the seeds of Moringa oleifera. J. Complement. Integr. Med. 2013, 10, 11–16. [Google Scholar] [CrossRef]
- Reddy, Y.R.R.; Lokanatha, O.; Ratnam, K.S.V.P.; Reddy, C.S.; Raju, I.S.; Reddy, C.D. Acute and Sub Acute Toxicity of Moringa oleifera Stem Bark Extract in Swiss Albino Mice. IJLBPR 2013, 2, 73–82. [Google Scholar]
- Asiedu-Gyekye, I.J.; Frimpong-Manso, S.; Awortwe, C.; Antwi, D.A.; Nyarko, A.K. Micro- and Macroelemental Composition and Safety Evaluation of the Nutraceutical Moringa oleifera Leaves. J Toxicol. 2014, 2014, 786979. [Google Scholar] [CrossRef] [PubMed]
- US National Library of Medicine. Clinicaltrial.gov. Available online: https://clinicaltrials.gov/ct2/results?term=moringa+oleifera&Search=Search (accessed on 4 November 2022).
- Sathyavathi, R.; Krishna, M.B.M.; Rao, D.N. Biosynthesis of Silver Nanoparticles Using Moringa oleifera Leaf Extract and Its Application to Optical Limiting. J. Nanosci. Nanotechnol. 2011, 11, 2031–2035. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, V.C.; Krishna, B. Antiulcer activity of a polyherbal formulation (PHF) from indian medicinal plants. Chin. J. Nat. Med. 2013, 11, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.S.; Shah, B.N.; Gupta, A.; Shah, D.P. Development and evaluation of polyherbal ointments for anti-inflammatory therapy. Int. J. Pharm. Res. 2014, 6, 114–119. [Google Scholar]
- Vibhute, S.; Kasture, V.; Kasture, S.; Kendre, P.; Rupnar, S.; Pande, V. Design and characterization of Moringa oleifera seed oil impregnated anti-inflammatory topical micro-dispersion. Der Pharmacia Lett. 2015, 7, 7–16. [Google Scholar]
- Panya, T.; Chansri, N.; Daodee, S. Development and evaluation of lozenge from Moringa oleifera leaf extract. Res. J. Pharm. Technol. 2016, 9, 805–809. [Google Scholar] [CrossRef]
- Abd-Rabou, A.A.; Zoheir, K.M.A.; Kishta, M.S.; Shalby, A.B.; Ezzo, M.I. Nano-micelle of Moringa oleifera seed oil triggers mitochondrial cancer cell apoptosis. Asian Pac. J. Cancer Prev. 2016, 17, 4929–4933. [Google Scholar] [PubMed]
- Srivastava, R.; Srivastava, S.; Singh, S.P. Thermoreversible in-situ nasal gel formulations and their pharmaceutical evaluation for the treatment of allergic rhinitis containing extracts of moringa olifera and embelia ribes. Int. J. Pharm. 2017, 9, 16–20. [Google Scholar] [CrossRef]
- Chin, C.; Jalil, J.; Ng, P.Y.; Ng, S. Development and formulation of Moringa oleifera standardised leaf extract film dressing for wound healing application. J. Ethnopharmacol. 2018, 212, 188–199. [Google Scholar] [CrossRef]
- Murdiana, H.E.; Revika, E.; Rahmawati, D.; Puspitasari, T.R.; Putri, A.D.; Murti, B.T. Moringa oleifera lam. based effervescent tablets: Design, formulation and physicochemical evaluation. Int. J. Drug Deliv. Technol. 2018, 8, 222–228. [Google Scholar]
- Suryadevara, V.; Doppalapudi, S.; Sasidhar Reddivallam, L.; Anne, R.; Mudda, M. Formulation and evaluation of anti-inflammatory cream by using Moringa oleifera seed oil. Pharmacogn. Res. 2018, 10, 195–204. [Google Scholar] [CrossRef]
- Komal Labh, A.; Rajeshkumar, S.; Roy, A.; Santhoshkumar, J.; Lakshmi, T. Herbal formulation mediated synthesis of silver nanoparticles and its antifungal activity against candida albicans. Indian J. Public Health Res. Dev. 2019, 10, 3454–3458. [Google Scholar] [CrossRef]
- Chin, C.; Ng, P.; Ng, S. Moringa oleifera standardised aqueous leaf extract-loaded hydrocolloid film dressing: In vivo dermal safety and wound healing evaluation in STZ/HFD diabetic rat model. Drug Deliv. Transl. Res. 2019, 9, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Srivastava, S.; Singh, S.P. Comparative study of anti-allergic activity of two poly-herbal formulations in ova-challenged allergic rhinitis mice model. Orient. Pharm. Exp. Med. 2019, 19, 37–47. [Google Scholar] [CrossRef]
- Chin, C.; Ng, S. Development of Moringa oleifera standardized leaf extract nanofibers impregnated onto hydrocolloid film as A potential chronic wound dressing. Fibers Polym. 2020, 21, 2462–2472. [Google Scholar] [CrossRef]
- Hassabo, A.G.; Shaarawy, S.; Mohamed, A.L.; Hebiesh, A. Multifarious cellulosic through innovation of highly sustainable composites based on moringa and other natural precursors. Int. J. Biol. Macromol. 2020, 165, 141–155. [Google Scholar] [CrossRef]
- Pagano, C.; Perioli, L.; Baiocchi, C.; Bartoccini, A.; Beccari, T.; Blasi, F.; Ricci, M. Preparation and characterization of polymeric microparticles loaded with Moringa oleifera leaf extract for exuding wound treatment. Int. J. Pharm. 2020, 587, 119700. [Google Scholar] [CrossRef]
- Aisida, S.O.; Madubuonu, N.; Alnasir, M.H.; Ahmad, I.; Botha, S.; Maaza, M.; Ezema, F.I. Biogenic synthesis of iron oxide nanorods using Moringa oleifera leaf extract for antibacterial applications. Appl. Nanosci. 2020, 10, 305–315. [Google Scholar] [CrossRef]
- Isimi, C.Y.; John-Africa, L.B.; Ekere, K.E.; Olayemi, O.J.; Aremu, O.I.; Emeje, M.O. Formulation, evaluation and anti-hemorroidal activity of suppositories containing Moringa oleifera lam. seed oil. Acta Pharm. Sci. 2021, 59, 647–665. [Google Scholar] [CrossRef]
- Aristianti, A.; Nurkhaeri, N.; Tandiarrang, V.Y.; Awaluddin, A.; Muslimin, L. Formulation and pharmacological studies of leaves of moringa (Moringa oleifera), a novel hepatoprotection in oral drug formulations. Open Access Maced. J. Med. Sci. 2021, 9, 151–156. [Google Scholar] [CrossRef]
- Alsammarraie, H.J.M.; Khan, N.A.K.; Mahmud, R. Formulation, evaluation, and in vivo anti-inflammatory and anti-arthritic activities of moringa granules. Int. J. Appl. Pharm. 2021, 13, 112–120. [Google Scholar] [CrossRef]
- Rani, K.C.; Hasanah, T.U.; Ilmiah, B.; Jayani, N.I.E. Formulation of moringa extract chewable gummy tablet with na-alginate and pectin as carriers. Res. J. Pharm. Technol. 2022, 15, 2513–2520. [Google Scholar] [CrossRef]
- Ali, A.; Garg, P.; Goyal, R.; Kaur, G.; Li, X.; Negi, P.; Valis, M.; Kuca, K.; Kulshrestha, S. A Novel Herbal Hydrogel Formulation of Moringa oleifera for Wound Healing. Plants 2020, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Wanjiru, J.; Gathirwa, J.; Sauli, E.; Swai, H.S. Formulation, optimization, and evaluation of Moringa oleifera leaf polyphenol-loaded phytosome delivery system against breast cancer cell lines. Molecules 2022, 27, 4430. [Google Scholar] [CrossRef]
- Iskandar, B.; Novita, G.; Annisa, F.F.; Leny Hafiz, I.; Surboyo, M.D.C.; Lee, C. Evaluation of physical quality and antioxidant activity of ethanol extract of moringa leaves (Moringa oleifera LAM) formulated in emulgel preparation. Res. J. Pharm. Technol. 2022, 15, 2703–2708. [Google Scholar] [CrossRef]
- Engsuwana, J.; Waranuch, N.; Limpeanchob, N.; Ingkaninan, K. HPLC methods for quality control of Moringa oleifera extract using isothiocyanates and astragalin as bioactive markers. Scienceasia 2017, 43, 169–174. [Google Scholar] [CrossRef]
- Yadav, V.; Ahmad, S.; Zahra, K. Assessment of the protective effects of Moringa oleifera leaf extract against Neem- Oil induced toxicity in zebra fish, Danio rerio. J. Pharmacogn. Phytochem. 2019, 8, 4263–4270. [Google Scholar]
- Kanchani, A.; Harris, K.D. Effect of Foliar Application of Moringa (Moringa oleifera) Leaf Extract with Recommended Fertilizer on Growth and Yield of Okra (Abelmoschus Esculentus). J. Agric. Sci. 2019, 13, 38–54. [Google Scholar] [CrossRef]
- Manzoor, M.; Ali, H.; Muhammad, A.; Alam, I.; Khalid, S.H.; Idrees, A.; Arif, M. Potential of Moringa (Moringa oleifera: Moringaceae) as plant growth regulator and bio-Pesticide against wheat aphids on wheat crop (Triticum aestivum; Poaceae). J. Biopestic. 2015, 8, 120–127. [Google Scholar]
- Arif, Y.; Bajguz, A.; Hayat, S. Moringa oleifera Extract as a Natural Plant Biostimulant. J. Plant Growth Regul. 2022. [Google Scholar] [CrossRef]
- Hassarajani, S.; Souza, T.D.; Mengi, S.A. Efficacy study of the bioactive fraction (F-3) of Acorus calamus in hyperlipidemia. Indian J. Pharmacol. 2007, 39, 196–200. [Google Scholar]
- Randriamboavonjy, J.I.; Rio, M.; Pacaud, P.; Loirand, G.; Tesse, A. Moringa oleifera seeds attenuate vascular oxidative and nitrosative stresses in spontaneously hypertensive rats. Oxid. Med. Cell. Longev. 2017, 2017, 4129459. [Google Scholar] [CrossRef] [PubMed]
- Halaby, M.S.; Metwally, E.M.; Omar, A.A. Effect of Moringa oleifera on serum lipids and kidney function of hyperlipidaemic rats. Res. J. Appl. Sci. 2013, 9, 5189–5198. [Google Scholar]
- Karim, N.A.; Ibrahim, M.D.; Kntayya, S.B.; Rukayadi, Y.; Hamid, H.A.; Razis, A.F. Moringa oleifera Lam: Targeting Chemoprevention. Asian Pac. J. Cancer Prev. 2016, 17, 3675–3686. [Google Scholar]
- Ganguly, R.; Hazra, R.; Ray, K.; Guha, D. Effect of Moringa oleifera in experimental model of Alzheimer’s disease: Role of antioxidants. Ann. Neurosci. 2005, 12, 36–39. [Google Scholar] [CrossRef]
- Giacoipo, S.; Rajan, T.S.; De Nicola, G.R.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. The Isothiocyanate Isolated from Moringa oleifera Shows Potent Anti-Inflammatory Activity in the Treatment of Murine Subacute Parkinson’s Disease. Rejuvenation Res. 2017, 20, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Palomares-Alonso, F.; Jung, H.; Vidal-Cantú, G.C.; Tomé, R.; Susana, I.; Esquivel, G.; Dinora, F.; Cruz, D.L.; Pérez, V.; González, I.; et al. Moringa oleifera Extracts and Praziquantel Combination: Bioavailability in Rats and Cysticidal Activity in a Murine Model. Rev. Bras. Farmacogn. 2020, 30, 251–256. [Google Scholar] [CrossRef]
- Kawada, N.; Seki, S.; Inoue, M.; Kuroki, T. Effect of antioxidants, resveratrol, quercetin, and N-acetylcysteine, on the functions of cultured rat hepatic stellate cells and Kupfer cells. Hepatology 1998, 27, 1265–1274. [Google Scholar] [CrossRef]
- Ferreira, P.M.P.; Farias, D.F.; de Abreu Oliveira, J.T.; de Fátima Carvalho, A. Moringa oleifera: Bioactive compounds and nutritional potential. Rev. Nutr. 2008, 21, 1–10. [Google Scholar] [CrossRef]
- Paul, S.; Basak, P.; Majumder, R.; Mukherjee, A.; Ghosh, J.; Patra, S.; Jana, N.K. Biochemical estimation of Moringa oleifera leaf extract for synthesis of silver nanoparticle mediated drug delivery system. J. Plant Biochem. Biotechnol. 2019, 29, 86–93. [Google Scholar] [CrossRef]
- Luetragoon, T.; Pankla Sranujit, R.; Noysang, C.; Thongsri, Y.; Potup, P.; Suphrom, N.; Nuengchamnong, N.; Usuwanthim, K. Bioactive Compounds in Moringa oleifera Lam. Leaves Inhibit the Pro-Inflammatory Mediators in Lipopolysaccharide-Induced Human Monocyte-Derived Macrophages. Molecules 2020, 25, 191. [Google Scholar] [CrossRef] [PubMed]
- Tolba, H.; Elmaaty, A.; Farag, G.; Mansour, D.; El-akkad, H. Immunological effect of Moringa oleifera leaf extract on vaccinated and non-vaccinated Hubbard chickens experimentally infected with Newcastle virus. Saudi J. Biol. Sci. 2021, 29, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Basra, S.; Afzal, I.; Nawaz, M.; Rehman, H.U. Growth promoting potential of fresh and stored Moringa oleifera leaf extracts in improving seedling vigor, growth and productivity of wheat crop. Environ. Sci. Pollut. Res. 1999, 24, 27601–27612. [Google Scholar] [CrossRef]
- Damilola, A.M.; Temitope, M.F.O. Assessment of Moringa oleifera as Bio-Pesticide against Podagricaspp on the growth and yield of Okra (Abelmoschus esculentus L. Moench). J. Horttic. 2020, 7, 1–10. [Google Scholar]
- Abd El-Hack, M.E.; Alagawany, M.; Elrys, A.S.; Desoky, E.S.M.; Tolba, H.M.N.; Elnahal, A.S.M.; Elnesr, S.S.; Swelum, A.A. Effect of Forage Moringa oleifera L. (moringa) on Animal Health and Nutrition and Its Beneficial Applications in Soil, Plants and Water Purification. Agriculture 2018, 8, 145. [Google Scholar] [CrossRef]
- Iqbal, M.A. Response of Canola (Brassica napus L.) to Foliar Application of Moringa (Moringa olifera L.) and Brassica (Bras-sica napus L.) Water Extracts. Master’s Thesis, Department of Agronomy, University of Agriculture, Faisalabad, Pakistan, 2014. [Google Scholar]
- Nagar, P.K.; Iyer, R.I.; Sircar, P.K. Cytokinins in developing fruits of Moringa pterigosperma Gaertn. Physiol. Plant 1982, 55, 45–50. [Google Scholar] [CrossRef]
- Clasen, C.; Mclaughlin, N.; Nayaar, S.; Boisson, R.; Gupta, D.; Shah, N. Microbiological effectiveness and cost of disinfecting water by boiling in semi-urban India Am. Soc. Trop. Med. Hyg. 2008, 2008, 407–413. [Google Scholar] [CrossRef]
- Clasen, T.; Cairncross, S.; Haller, L.; Bartram, J.; Walker, D. Cost-effectiveness of water quality interventions for preventing diarrhoeal disease in developing countries. J. Water Health 2007, 5, 599–608. [Google Scholar]
- Pruss-Ustun, A.; Bartram, J.; Clasen, O.; Cumming, V. Curtis Burden of disease from inadequate water, sanitation and hygiene in low- and middle-income settings: A retrospective analysis of data from 145 countries. Trop. Med. Int. Health. 2014, 19, 894–905. [Google Scholar] [CrossRef]
- Akinyeye, J.; Solanke, E.O.; Adebiyi, I.O. Phytochemical and antimicrobial evaluation of leaf and seed of moringa olifera extracts. IJRMHS 2014, 4, 2083–2307. [Google Scholar]
- Mahmood, K.T.; Mugal, T.; Haq, I.U. Moringa oleifera: A natural gift—A review. J. Pharm. Sci. Res. 2010, 2, 775–781. [Google Scholar]
- López-Salazar, H.; Camacho-Díaz, B.H.; Ávila-Reyes, S.V.; Pérez-García, M.D.; Cortazar, M.G.; Ocampo, M.L.A.; Jiménez-Aparicio, A.R. Identification and Quantification of β-Sitosterol β-d-Glucoside of an Ethanolic Extract Obtained by Microwave-Assisted Extraction from Agave angustifolia Haw. Molecules 2019, 24, 3926. [Google Scholar] [CrossRef]
- Saini, R.K.; Shetty, N.P.; Giridhar, P. Carotenoid content in vegetative and reproductive parts of commercially grown Moringa oleifera Lam. cultivars from India by LC–APCI–MS. Eur. Food Res. Technol. 2014, 238, 971–978. [Google Scholar] [CrossRef]
- Sahakitpichan, P.; Mahidol, C.; Disadee, W.; Ruchirawat, S.; Kanchanapoom, T. Unusual glycosides of pyrrole alkaloid and 4′-hydroxyphenylethanamide from leaves of Moringa oleifera. Phytochemistry 2011, 72, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Abd Rani, N.Z.; Husain, K.; Kumolosasi, E. Moringa Genus: A Review of Phytochemistry and Pharmacology. Front. Pharmacol. 2018, 9, 1–26. [Google Scholar] [CrossRef]
- AbdullRazis, A.F.; Ibrahim, M.D.; Kntayya, S.B. Health benefits of Moringa oleifera. Asian Pac. J. Cancer Prev. 2014, 15, 8571–8576. [Google Scholar] [CrossRef] [PubMed]
- Anzano, A.; Ammar, M.; Papaianni, M.; Grauso, L.; Sabbah, M.; Capparelli, R.; Lanzotti, V. Moringa oleifera Lam.: A Phytochemical and Pharmacological Overview. Horticulturae 2021, 7, 409. [Google Scholar] [CrossRef]
- Matic, I.; Guidi, A.; Kenzo, M.; Mattei, M.; Galgani, A. Investigation of medicinal plants traditionally used as dietary supplements: A review on Moringa oleifera. J. Public Health Afr. 2018, 9, 841. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Ghosh, G.; Agrawal, D.; Sahu, P.K.; Kumar, S.; Mishra, S.S. GC-MS profiling of ethanolic extract of Moringa oleifera leaf. Int. J. Pharm. Bio. Sci. 2014, 5, 263–275. [Google Scholar]
- Faizi, S.; Siddiqui, B.S.; Saleem, R.; Siddiqui, S.; Aftab, K. Isolation and structure elucidation of new nitrile and mustard oil glycosides from Moringa oleifera and their effect on blood pressure. J. Nat. Prod. 1994, 57, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.B.; Das, A.K.; Banerji, N. Chemical investigations on the gum exudate from sajna (Moringa oleifera). Carbohydr. Res. 1982, 102, 253–262. [Google Scholar] [CrossRef]
- Zhu, Y.; Yin, Q.; Yang, Y. Comprehensive investigation of Moringa oleifera from different regions by simultaneous determination of 11 polyphenols using UPLC-ESI-MS/MS. Molecules 2020, 25, 676. [Google Scholar] [CrossRef] [PubMed]
- Vongsak, B.; Sithisarn, P.; Gritsanapan, W. Simultaneous HPLC quantitative analysis of active compounds in leaves of Moringa oleifera Lam. J. Chromatogr. Sci. 2014, 52, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.Y.; Lin, S.; Kuo, G. Content and distribution of flavonoids among 91 edible plant species. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. S1), 275–279. [Google Scholar]
- Bajpai, M.; Pande, A.; Tewari, S.K.; Prakash, D. Phenolic contents and antioxidant activity of some food and medicinal plants. Int. J. Food Sci. Nutr. 2005, 56, 287–291. [Google Scholar] [CrossRef]
- Prakash, D.; Suri, S.; Upadhyay, G.; Singh, B.N. Total phenol, antioxidant and free radical scavenging activities of some medicinal plants. Int. J. Food Sci. Nutr. 2007, 58, 18–28. [Google Scholar] [CrossRef]
- Leone, A.; Fiorillo, G.; Criscuoli, F.; Ravasenghi, S.; Santagostini, L.; Fico, G.; Spadafranca, A.; Battezzati, A.; Schiraldi, A.; Pozzi, F.; et al. Nutritional Characterization and Phenolic Profiling of Moringa oleifera Leaves Grown in Chad, Sahrawi Refugee Camps, and Haiti. Int. J. Mol. Sci. 2015, 16, 18923–18937. [Google Scholar] [CrossRef]
- Girme, A.; Bhoj, P.; Saste, G. Development and Validation of RP-HPLC Method for Vicenin-2, Orientin, Cynaroside, Betulinic Acid, Genistein, and Major Eight Bioactive Constituents with LC-ESI-MS/MS Profiling in Ocimum Genus. J. AOAC Int. 2021, 104, 1634–1651. [Google Scholar] [CrossRef]
- Desai, S.; Tatke, P.; Gabhe, S. Enhanced HPLC-DAD Method for Fast Determination of Quercetin-3-O-β-d-Glucoside in Extracts and Polyherbal Formulations Containing Azadirachta indica-Optimization and Validation. JOC 2017, 55, 706–711. [Google Scholar] [CrossRef]
- Faizi, S.; Sumbul, S.; Versiani, M.A.; Saleem, R.; Sana, A.; Siddiqui, H. GC/GCMS analysis of the petroleum ether and dichloromethane extract of Moringa oleifera roots. Asian Pac. J. Trop. Biomed. 2014, 4, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Förster, N.; Ulrichs, C.; Schreiner, M.; Müller, C.T.; Mewis, I. Development of a reliable extraction and quantification method for glucosinolates in Moringa oleifera. Food Chem. 2015, 166, 456–464. [Google Scholar] [CrossRef] [PubMed]



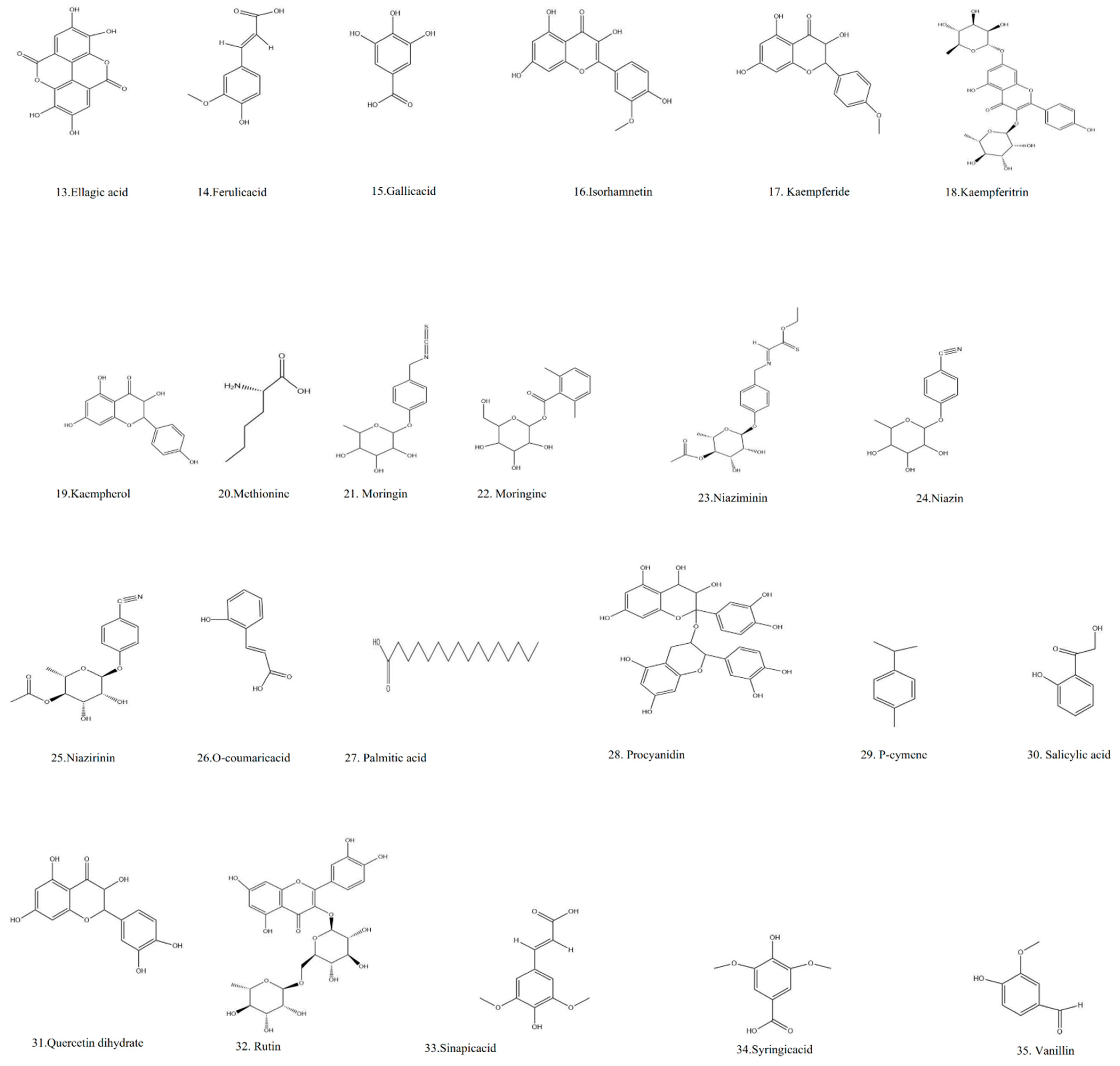
| Name of Ayurvedic Text | Form of Plant Used | Treatment | References |
|---|---|---|---|
| Charaka Samhita (1000 BC- 4th Cent. AD) | Powder Decoction | Used for treatment of worms and headache, Ascites, edema Hiccough and asthma, deafness, tinnitus in the ear, worm’s manifestation. | [38] |
| Ashtanga Hridaya (7th Cent. AD) | Oil | Ear ache, deafness, and tinnitus in the ear | [39] |
| Kashyapa Samhita (6–7th Cent AD) | Decoction Oil | Puerperal disorder, sleeplessness Edema | [40] |
| Sharangadhara Samhita (13 Cent. AD) | Decoction | Conjunctivitis | [41] |
| Yogaratnakara (17th Cent. A.D.) | Decoction | Enlargement of spleen, worm edema, Ascites, fever, abscess. | [42] |
| Plant Part | Compound | Class | Structure | Therapeutic Activity | References |
| Leaves | Rutin (555.6 µg/g) | Flavonoid |  | Found to have maximum affinity and interaction towards BRAC-1 gene. | [49,50] |
| Leaves | Kaempferol (197.6 µg/g) | Flavonoid | 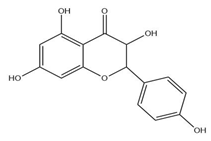 | Oxidative damage protective activity. | [51] |
| Leaves | Quercetin (2030.9 µmol/100 g) | Flavonoid | 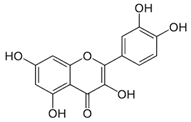 | Exerts an excellent effect as anti-diabetic agent. | [52] |
| Leaves | O coumaric acid (0.536 mg/g) | Phenolic acid | 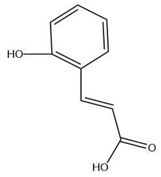 | Antioxidant and anti-microbial | [53,54] |
| Leaves | Myricetin (5.804 mg/g) | Flavonoid | 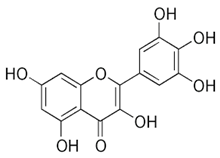 | Potential prevention of diabetes mellitus and other diabetic complications | [54] |
| Leaves | Ellagic acid (0.078 to 0.128 mg/g) | Polyphenol | 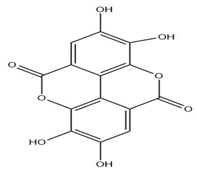 | Prevents viral and bacterial infections, potential antioxidant | [54,55] |
| Leaves | Ferulic acid (0.078 to 0.128 mg/g) | Phenol |  | Promising results as anti- cancer, antioxidant, antithrombotic, anti-arrhythmic, and anti-inflammatory. | [54,56] |
| Leaves | Caffeic acid (0.409 mg/g) | Phenol | 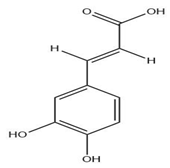 | Boosts athletic performance, reduces fatigue, helps weight loss, protects against herpes, HIV, cancer. | [54,57] |
| Leaves | Sinapic acid (trace amount) | Phenol |  | Cardioprotective, renoprotective, anxiolytic, neuroprotective. | [54,58] |
| Leaves | Gallic acid (1.034 mg/g) | Phenol | 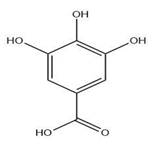 | Anti-inflammatory, anti-neoplastic, anti-oxidant | [54,59] |
| Leaves | Syringic acid (trace amount) | Phenol |  | Anti-oxidant, antimicrobial. | [54,60] |
| Leaves | Isorhamnetin (0.118 mg/g) | Flavonoid |  | Anti-oxidant | [54,61] |
| Seeds | Myricetin (5.804 mg/g) | Flavonoid | 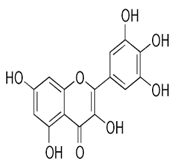 | Potential prevention of diabetes mellitus and other diabetic complications | [54] |
| Seeds | Glucomoringin | Glucosinolates | 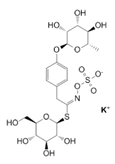 | Anti-inflammatory, pain relieving, anti-oxidant, antihypertensive. | [62] |
| Seeds | β-sitosterol | Phytosterol | 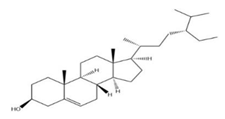 | Anti-inflammatory | [63] |
| Seeds | Arachidic acid | Fatty acid | 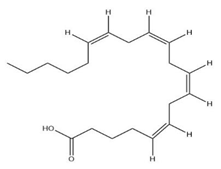 | Increased breast milk production | [64] |
| Seeds | Oleic acid (70% w/w) | Fatty acid |  | Reduces blood pressure and reduces free radical damage to the cell. | [65] |
| Seeds | Myristic acid | Fatty acid | 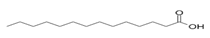 | Anxiolytic effect, used in membrane localization of the enzyme. | [66] |
| Seeds | Palmitic acid | Fatty acid |  | Trypanocidal and anti-leukemic effect | [67] |
| Seeds | Procyaniadin | Flavonoid | 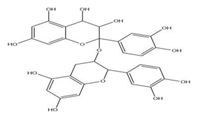 | Cardioprotective | [68] |
| Flower | D-mannose | Carbohydrate |  | Treatment of deficiency caused by genetic defects, and acute urinary tract infections. | [69] |
| Stem | β-sitosterol | Phytosterol | 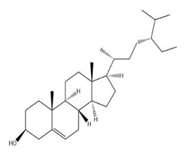 | Anti-oxidant, cardiovascular, immunomodulatory | [63] |
| Plant Part Used | Nature of Extract | Formulation | Method of Preparation and Polymers/Excipients Used | Application | Inference | References |
|---|---|---|---|---|---|---|
| Leaves | Ethyl acetate | Polyherbal formulation | Suspending method (carboxy methyl cellulose) | Anti-ulcer |
| [121] |
| Leaves | Aqueous/methanolic | Polyherbal ointments | Water in oil mixing (wool fat, hard paraffin, cetostearyl alcohol, PEG4000, PEG400, sorbitol mono-oleate, liquid paraffin, white beeswax, span 60, tween 60) | Edema |
| [122] |
| Seed | Oil | Micro-dispersion | Vortexing (Span 80, tween 80) | Anti-inflammatory |
| [123] |
| Leaves | Ethanolic | Lozenges | Wet granulation (Polyvinyl-pyrrolidone, magnesium stearate, menthol, vanillin) | Anti-microbial activity |
| [124] |
| Seed | Oil | Nano-micelle | Microemulsion method (Tween 80, Ethanol) | Mitochondrial cancer cell apoptosis |
| [125] |
| Leaves + fruits (Embelia ribes) | Hydro-alcoholic | Thermo-reversible in-situ nasal gel | Cold method (poly (ethylene glycol) (PEG400), Pluronic F127, xanthum gum, carbopol 934), hydroxypropyl methylcellulose (HPMC K4M). | Allergic rhinitis |
| [126] |
| Leaves | Aqueous, ethanolic | Film dressing | Solvent casting method (Alginate, pectin) | Wound healing |
| [127] |
| Leaves | Ethanolic | Effervescent tablets | Wet granulation (70% ethanol, lactose, citric acid, tartaric acid, sodium bicarbonate, aspartame, PEG600) | Anti-anemia |
| [128] |
| Seed | Oil | Anti-inflammatory cream | Triturating process (Oleic acid, sodium hydroxide, potassium hydroxide, aluminum hydroxide, liquid ammonia, sodium benzoate). | Anti-inflammatory |
| [129] |
| Leaves | Silver NPs (AgNPs) | Shaking method (Silver nitrate) | Anti-fungal activity |
| [130] | |
| Leaves | Aqueous | Hydrocolloid film dressing | Solvent casting method (sodium alginate, pectin) | Wound healing in diabetic condition |
| [131] |
| Leaves | Hydro-alcoholic | In-situ gel | Cold technique (Pluronic F127, gellan gum, glycerine, Carbopol 934) | Allergic rhinitis |
| [132] |
| Leaves | Aqueous | Nanofibers impregnated onto Hydrocolloid film | Electrospinning (poly-(ethylene oxide) (PEO), sodium alginate, pectin, glycerol) | Chronic Wound dressing |
| [133] |
| Leaves | Aqueous/Ethanolic | Silver nanoparticle loaded Composites | Sodium hypophosphite, silver nitrate, citric acid, Kaolin (clay), Chitosan (LMW), sodium carbonate. | Anti-oxidant |
| [134] |
| Leaves | Hydro-alcoholic | Polymeric microparticles (MPs) | Spray dried method (Chitosan) | Exuding wound treatment |
| [135] |
| Leaves | Aqueous | Iron oxide nanorods | Mixing method (Iron (III) chloride hexahydrate) | Anti-bacterial property |
| [136] |
| Seed | Oil | Suppositories | Pour molding method (Macrogol, dika fat, liquid paraffin, Polyethylene glycol 1000 & 4000, petroleum ether) | Hemorrhoids |
| [137] |
| Leaves | Ethanolic | Oral suspension | Stirring method (Sodium carboxymethyl cellulose, propylene glycol, benzoate, sorbitol) | Hepato-protection against Isoniazid |
| [138] |
| Leaves | Ethanolic | Granules | Wet granulation method (Gum Arabic, HPMC, Methocel K100M CR, magnesium stearate, Avicel PH200, tween 20, 40, 80, span 20, 40, Poloxamer 407, sodium lauryl sulphate) | Anti-inflammatory and anti-arthritic |
| [139] |
| Leaves | Powder | Chewable gummy tablets (CGT) | Heating and Congealing (Gelatin, high methoxyl pectin, mannitol, sucrose, propylene glycol, citric acid, corn oil, sodium benzoate) | Evaluation of Chewable gummy tablets |
| [140] |
| Seeds | n-hexane | Herbal hydrogel | Mixing method (Carbopol, propylparaben sodium, methylparaben sodium, propylene glycol, triethanolamine) | Wound healing |
| [141] |
| Leaves | Aqueous | Phytosome | Thin-layer hydration method (soy phosphatidylcholine, TrizolTM) | Breast cancer |
| [142] |
| Leaves | Ethanolic | Emulgel | Dissolving method (Carbopol 940, triethanolamine Tween 80) | Anti-oxidant activity |
| [143] |
| S. No | Extract | Methanolic Roots | Ethanolic Roots | Methanolic Leaves | Ethanolic Leaves | Aqueous Leaves | Methanolic Seeds | Ethanolic Seeds | Aqueous Seeds | |
|---|---|---|---|---|---|---|---|---|---|---|
| Chemical | ||||||||||
| 1. | Alkaloid | + | + | + | + | + | + | + | + | |
| 2. | Tannins | + | + | + | + | + | + | - | + | |
| 3. | Flavonoids | + | + | + | + | + | - | + | - | |
| 4. | Saponins | + | + | + | + | + | - | + | - | |
| 5. | Terpenoids | + | + | + | + | + | - | - | - | |
| 6. | Glycosides | + | + | + | + | + | - | - | - | |
| 7. | Steroids | + | + | + | + | + | - | - | - | |
| 8. | Coumarins | - | - | + | + | - | - | - | - | |
| 9. | Proteins | - | - | - | - | - | + | + | - | |
| 10. | Starch | + | + | - | - | - | - | - | - | |
| Constituents | Concentrations # | Category | Technique Used | Reference |
|---|---|---|---|---|
| LEAVES | ||||
| Isoquercetin | 1575.28 μg/g (w/w) | Flavonoids | UPLC-ESI-MS/MS | [181] |
| Astragalin | 0.153 μg/g (w/w) | Flavonoids | HPLC | [182] |
| Isorhamnetin | 2.9 mg/g (w/w) | Flavonoids | HPLC | [183] |
| Daidzein | ND * | Flavonoid | HPLC | [183] |
| Apigenin | ND * | Aglycone | HPLC | [184] |
| Luteolin | ND * | Flavonoid | HPLC | [184] |
| Genistein | ND * | Flavonoid | HPLC | [184] |
| 4-(α-L-rhamnopyranosyloxy) benzyl glucosinolate | 33.9 mg/g | Glucosinolates | LC/MS | [184] |
| 4-[(2′ -O-acetyl-α-L-rhamnosyloxy) benzyl] Glucosinolate | 21.84 to 59.4 mg/g | Glucosinolates | HPLC | [184] |
| Epicatechin | 5.68 mg/g | Flavonoid | HPLC | [184] |
| Ferulic acid | 0.078 mg/g | Phenolic acid | HPLC | [184] |
| Caffeic acid | 0.409 mg/g | Phenolic acid | HPLC | [184] |
| Ellagic acid | 0.018 mg/g | Phenolic acid | HPLC & MS/MS | [185] |
| Sinalbin | 2.36 mg/g | Glucosinolates | HPLC | [185] |
| Sinapic acid | ND | Phenolic acid | HPLC &MS/MS | [185] |
| Chlorogenic acid | 0.018 mg/g | Phenolic acid | HPLC &MS/MS | [185] |
| Gallic acid | 1.034 mg/g | Phenolic acid | HPLC &MS/MS | [186] |
| Salicylic acid | 0.14 μg/g | Phenolic acid | HPLC GC MS | [186] |
| Vicenin-2 | 193.43 ng/mg | Flavonoid | HPLC PDA | [187] |
| Quercetin-3-O-(6′′-malonyl) glucoside | ND * | Flavonoid | HPLC DAD | [188] |
| Pyrrolemarumine-4′′ -O-α-Lrhamnopyranoside | NA ^ | Pyrole alkaloid | NMR | [189] |
| 4-[(4′ -O-Acetyl-α-L-rhamnosyloxy)benzyl] | 2.16 to 5.0 mg/g | Glucosinolates | HPLC | [190] |
| SEEDS | ||||
| Niazimicin | NA ^ | Isothiocyanates | HPLC | [173] |
| Niazirin | NA ^ | Isothiocyanates | HPLC | [173] |
| ROOTS | ||||
| Arachidic acid | ND * | Fatty acid | GC-MS | [190] |
| BARK | ||||
| β-Sitosterol-3-O-β-D-galactopyranoside | 26.67 mg/g | Glucoside | HPTLC | [190] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pareek, A.; Pant, M.; Gupta, M.M.; Kashania, P.; Ratan, Y.; Jain, V.; Pareek, A.; Chuturgoon, A.A. Moringa oleifera: An Updated Comprehensive Review of Its Pharmacological Activities, Ethnomedicinal, Phytopharmaceutical Formulation, Clinical, Phytochemical, and Toxicological Aspects. Int. J. Mol. Sci. 2023, 24, 2098. https://doi.org/10.3390/ijms24032098
Pareek A, Pant M, Gupta MM, Kashania P, Ratan Y, Jain V, Pareek A, Chuturgoon AA. Moringa oleifera: An Updated Comprehensive Review of Its Pharmacological Activities, Ethnomedicinal, Phytopharmaceutical Formulation, Clinical, Phytochemical, and Toxicological Aspects. International Journal of Molecular Sciences. 2023; 24(3):2098. https://doi.org/10.3390/ijms24032098
Chicago/Turabian StylePareek, Ashutosh, Malvika Pant, Madan Mohan Gupta, Pushpa Kashania, Yashumati Ratan, Vivek Jain, Aaushi Pareek, and Anil A. Chuturgoon. 2023. "Moringa oleifera: An Updated Comprehensive Review of Its Pharmacological Activities, Ethnomedicinal, Phytopharmaceutical Formulation, Clinical, Phytochemical, and Toxicological Aspects" International Journal of Molecular Sciences 24, no. 3: 2098. https://doi.org/10.3390/ijms24032098
APA StylePareek, A., Pant, M., Gupta, M. M., Kashania, P., Ratan, Y., Jain, V., Pareek, A., & Chuturgoon, A. A. (2023). Moringa oleifera: An Updated Comprehensive Review of Its Pharmacological Activities, Ethnomedicinal, Phytopharmaceutical Formulation, Clinical, Phytochemical, and Toxicological Aspects. International Journal of Molecular Sciences, 24(3), 2098. https://doi.org/10.3390/ijms24032098






