Cannabidiol Modulates Alterations in PFC microRNAs in a Rat Model of Depression
Abstract
1. Introduction
2. Results
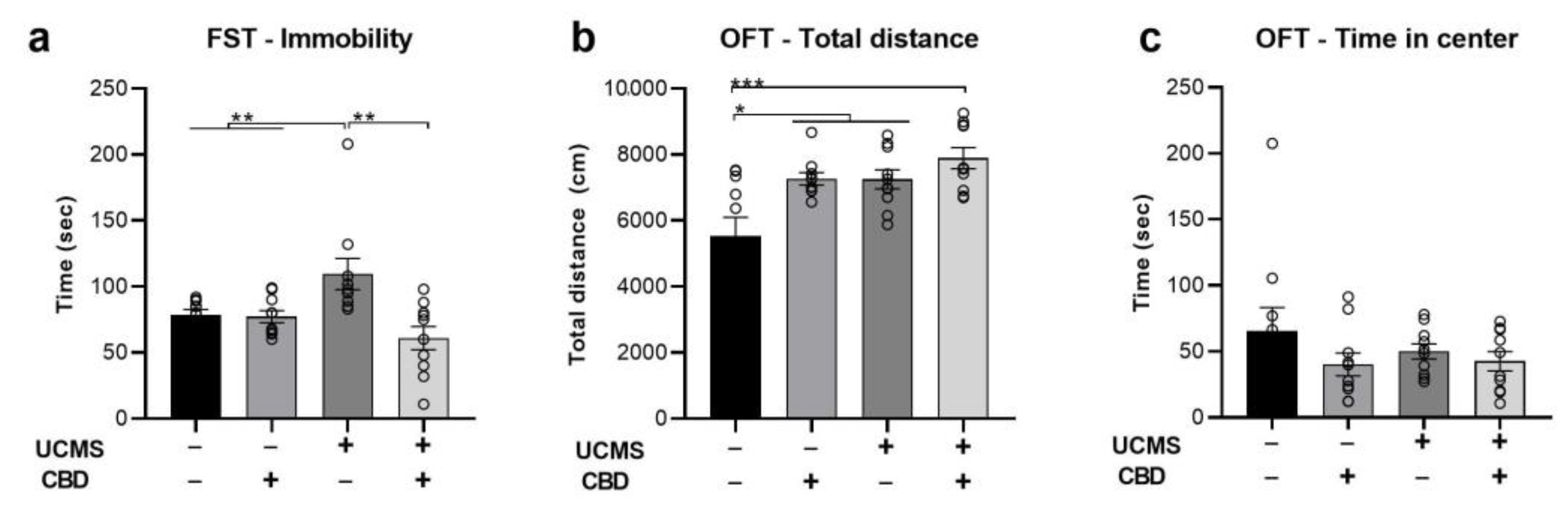
2.1. The Effects of Chronic CBD Administration during UCMS on Behavior
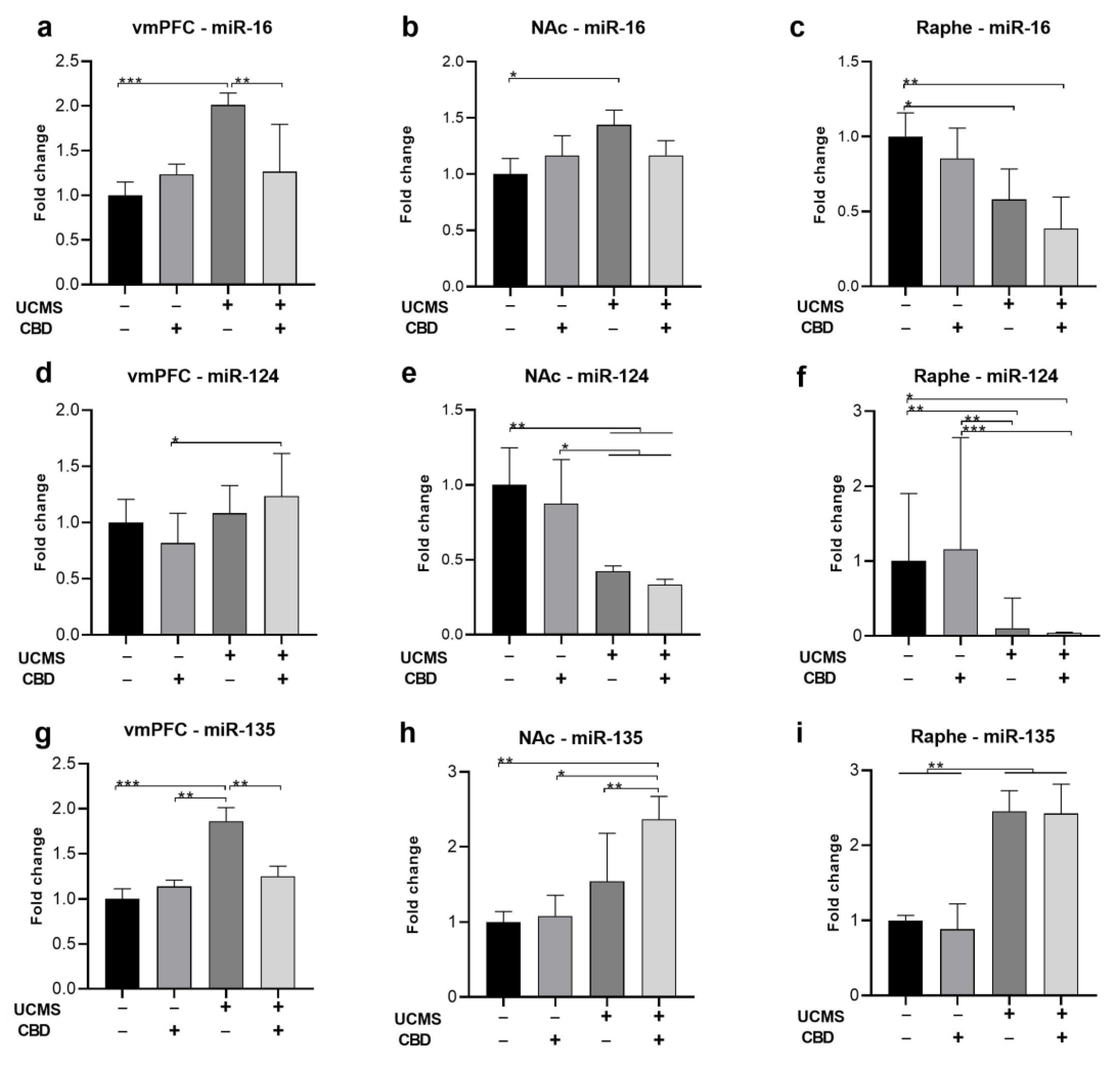
2.2. The Effects of Chronic CBD Administration during UCMS on miRNA Expression
2.2.1. miR-16
2.2.2. miR-124
2.2.3. miR-135
2.3. The Effects of Chronic CBD Administration during UCMS on Possible Target Genes
2.3.1. htr1a
2.3.2. slc6a4
2.3.3. ctnnb1
2.3.4. cnr1
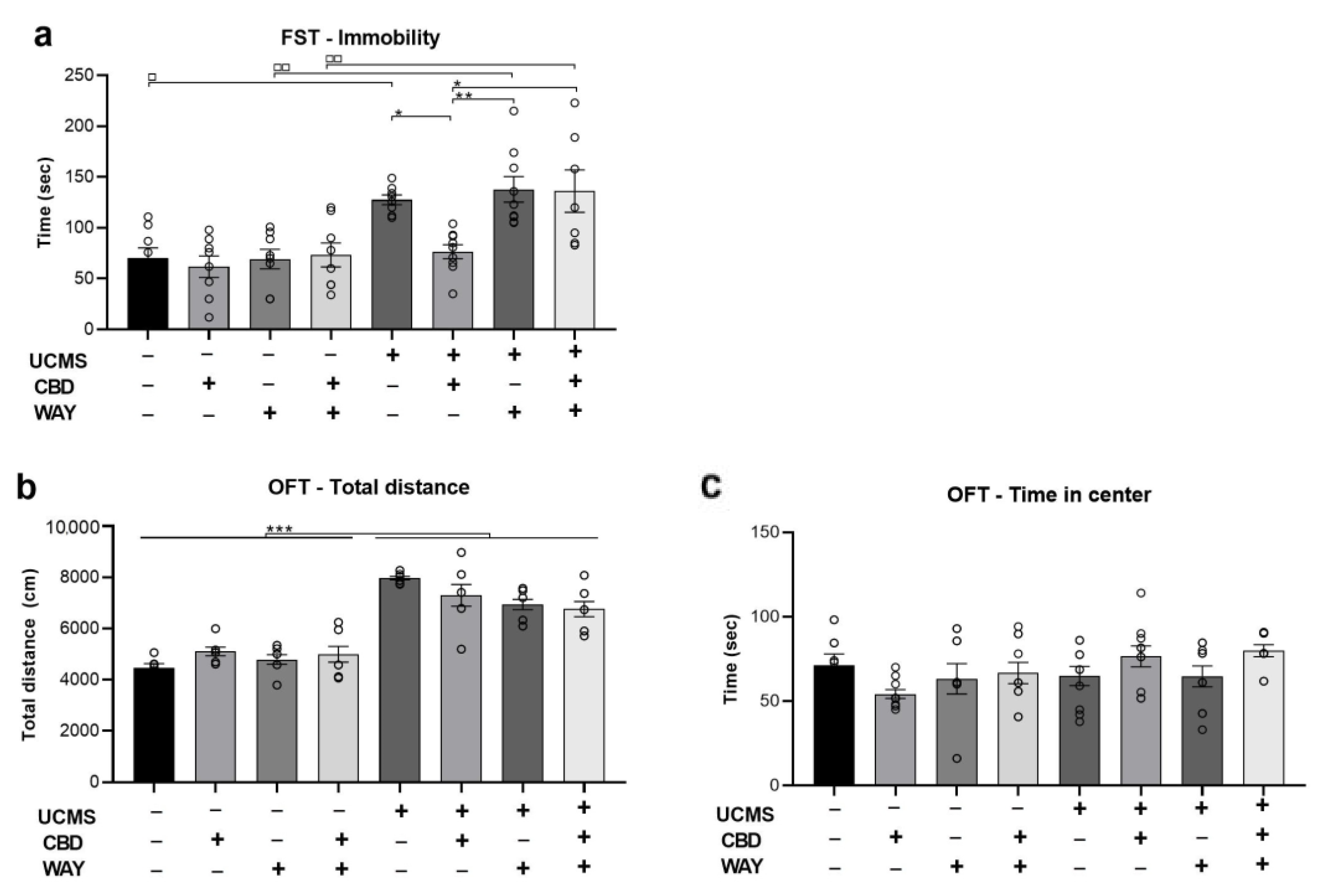
2.4. Does the 5-HT1a Antagonist WAY100635 Block the Effects of Chronic CBD Administration during UCMS on Behavior
3. Discussion
3.1. Alterations in miRNAs
3.1.1. miR-16
3.1.2. miR-124
3.1.3. miR-135
3.2. Alterations in Serotonergic Targets, β-catenin, and CB1
3.2.1. htr1a (5HT1a Gene)
3.2.2. slc6a4 (SERT Gene)
3.2.3. ctnnb1 (β-catenin Gene)
3.2.4. cnr1 (CB1 Gene)
4. Materials and Methods
4.1. Subjects
4.2. UCMS Protocol
4.3. Pharmacological Agents
4.4. Behavioral Tests
4.4.1. Locomotor Activity and Anxiety-like Behavior
4.4.2. Forced Swim Test (FST)
4.5. Quantitative Real-Time PCR (qRT-PCR)
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maletic, V.; Robinson, M.; Oakes, T.; Iyengar, S.; Ball, S.G.; Russell, J. Neurobiology of depression: An integrated view of key findings. Int. J. Clin. Pract. 2007, 61, 2030–2040. [Google Scholar] [CrossRef]
- Pigott, H.E.; Leventhal, A.M.; Alter, G.S.; Boren, J.J. Efficacy and effectiveness of antidepressants: Current status of research. Psychother. Psychosom. 2010, 79, 267–279. [Google Scholar] [CrossRef]
- Shbiro, L.; Hen-Shoval, D.; Hazut, N.; Rapps, K.; Dar, S.; Zalsman, G.; Mechoulam, R.; Weller, A.; Shoval, G. Effects of cannabidiol in males and females in two different rat models of depression. Physiol. Behav. 2019, 201, 59–63. [Google Scholar] [CrossRef]
- Zanelati, T.V.; Biojone, C.; Moreira, F.A.; Guimarães, F.S.; Joca, S.R.L. Antidepressant-like effects of cannabidiol in mice: Possible involvement of 5-HT1A receptors. Br. J. Pharmacol. 2010, 159, 122–128. [Google Scholar] [CrossRef]
- Réus, G.Z.; Stringari, R.B.; Ribeiro, K.F.; Luft, T.; Abelaira, H.M.; Fries, G.R.; Aguiar, B.W.; Kapczinski, F.; Hallak, J.E.; Zuardi, A.W.; et al. Administration of cannabidiol and imipramine induces antidepressant-like effects in the forced swimming test and increases brain-derived neurotrophic factor levels in the rat amygdala. Acta Neuropsychiatr. 2011, 23, 241–248. [Google Scholar] [CrossRef]
- Shoval, G.; Shbiro, L.; Hershkovitz, L.; Hazut, N.; Zalsman, G.; Mechoulam, R.; Weller, A. Prohedonic effect of cannabidiol in a rat model of depression. Neuropsychobiology 2016, 73, 123–129. [Google Scholar] [CrossRef]
- Linge, R.; Jiménez-Sánchez, L.; Campa, L.; Pilar-Cuéllar, F.; Vidal, R.; Pazos, A.; Adell, A.; Díaz, Á. Cannabidiol induces rapid-acting antidepressant-like effects and enhances cortical 5-HT/glutamate neurotransmission: Role of 5-HT1A receptors. Neuropharmacology 2016, 103, 16–26. [Google Scholar] [CrossRef]
- Ferber, S.G.; Hazani, R.; Shoval, G.; Weller, A. Targeting the Endocannabinoid System in Borderline Personality Disorder: Corticolimbic and Hypothalamic Perspectives. Curr. Neuropharmacol. 2021, 19, 360–371. [Google Scholar]
- Zhornitsky, S.; Potvin, S. Cannabidiol in humans—The quest for therapeutic targets. Pharmaceuticals 2012, 5, 529–552. [Google Scholar] [CrossRef]
- McPartland, J.M.; Glass, M.; Pertwee, R.G. Meta-analysis of cannabinoid ligand binding affinity and receptor distribution: Interspecies differences. Br. J. Pharmacol. 2007, 152, 583–593. [Google Scholar] [CrossRef]
- Campos, A.C.; Moreira, F.A.; Gomes, F.V.; Del Bel, E.A.; Guimaraes, F.S. Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3364–3378. [Google Scholar] [CrossRef]
- Resstel, L.B.; Tavares, R.F.; Lisboa, S.F.; Joca, S.R.; Corrêa, F.M.; Guimarães, F.S. 5-HT1A receptors are involved in the cannabidiol-induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. Br. J. Pharmacol. 2009, 156, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Sartim, A.G.; Guimarães, F.S.; Joca, S.R.L. Antidepressant-like effect of cannabidiol injection into the ventral medial prefrontal cortex—Possible involvement of 5-HT1A and CB1 receptors. Behav. Brain Res. 2016, 303, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Fogaça, M.V.; Campos, A.C.; Guimarães, F.S. Cannabidiol and 5-HT1A receptors. In Neuropathology of Drug Addictions and Substance Misuse; Academic Press: Cambridge, MA, USA, 2016; pp. 749–759. [Google Scholar]
- Aberle, H.; Bauer, A.; Stappert, J.; Kispert, A.; Kemler, R. β-catenin is a target for the ubiquitin–proteasome pathway. EMBO J. 1977, 16, 3797–3804. [Google Scholar] [CrossRef] [PubMed]
- Teo, C.H.; Soga, T.; Parhar, I.S. Brain beta-catenin signalling during stress and depression. Neurosignals 2018, 26, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Mizrachi Zer-Aviv, T.; Islami, L.; Hamilton, P.J.; Parise, E.M.; Nestler, E.J.; Sbarski, B.; Akirav, I. Enhancing Endocannabinoid Signaling via β-Catenin in the Nucleus Accumbens Attenuates PTSD-and Depression-like Behavior of Male Rats. Biomedicines 2022, 10, 1789. [Google Scholar] [CrossRef]
- Dias, C.; Feng, J.; Sun, H.; Mazei-Robison, M.S.; Damez-Werno, D.; Scobie, K.; Bagot, R.; LaBonté, B.; Ribeiro, E.; Liu, X.; et al. β-catenin mediates stress resilience through Dicer1/microRNA regulation. Nature 2014, 516, 51–55. [Google Scholar] [CrossRef]
- Hansen, K.F.; Obrietan, K. MicroRNA as therapeutic targets for treatment of depression. Neuropsychiatr. Dis. Treat. 2013, 9, 1011. [Google Scholar]
- O’connor, R.M.; Dinan, T.G.; Cryan, J.F. Little things on which happiness depends: microRNAs as novel therapeutic targets for the treatment of anxiety and depression. Mol. Psychiatry 2012, 17, 359–376. [Google Scholar] [CrossRef]
- Serafini, G.; Pompili, M.; Hansen, K.F.; Obrietan, K.; Dwivedi, Y.; Shomron, N.; Girardi, P. The involvement of microRNAs in major depression, suicidal behavior, and related disorders: A focus on miR-185 and miR-491-3p. Cell. Mol. Neurobiol. 2014, 34, 17–30. [Google Scholar] [CrossRef]
- Ferrúa, C.P.; Giorgi, R.; da Rosa, L.C.; do Amaral, C.C.; Ghisleni, G.C.; Pinheiro, R.T.; Nedel, F. MicroRNAs expressed in depression and their associated pathways: A systematic review and a bioinformatics analysis. J. Chem. Neuroanat. 2019, 100, 101650. [Google Scholar] [CrossRef]
- Allen, L.; Dwivedi, Y. MicroRNA mediators of early life stress vulnerability to depression and suicidal behavior. Mol. Psychiatry 2020, 25, 308–320. [Google Scholar] [CrossRef]
- Muiños-Gimeno, M.; Espinosa-Parrilla, Y.; Guidi, M.; Kagerbauer, B.; Sipilä, T.; Maron, E.; Pettai, K.; Kananen, L.; Navinés, R.; Martín-Santos, R.; et al. Human microRNAs miR-22, miR-138-2, miR-148a, and miR-488 are associated with panic disorder and regulate several anxiety candidate genes and related pathways. Biol. Psychiatry 2011, 69, 526–533. [Google Scholar] [CrossRef]
- Haramati, S.; Navon, I.; Issler, O.; Ezra-Nevo, G.; Gil, S.; Zwang, R.; Hornstein, E.; Chen, A. MicroRNA as repressors of stress-induced anxiety: The case of amygdalar miR-34. J. Neurosci. 2011, 31, 14191–14203. [Google Scholar] [CrossRef]
- Bai, M.; Zhu, X.; Zhang, Y.; Zhang, S.; Zhang, L.; Xue, L.; Yi, J.; Yao, S.; Zhang, X. Abnormal hippocampal BDNF and miR-16 expression is associated with depression-like behaviors induced by stress during early life. PLoS ONE 2012, e46921. [Google Scholar] [CrossRef]
- Issler, O.; Haramati, S.; Paul, E.D.; Maeno, H.; Navon, I.; Zwang, R.; Gil, S.; Mayberg, H.S.; Dunlop, B.W.; Menke, A.; et al. MicroRNA 135 is essential for chronic stress resiliency, antidepressant efficacy, and intact serotonergic activity. Neuron 2014, 83, 344–360. [Google Scholar] [CrossRef]
- Higuchi, F.; Uchida, S.; Yamagata, H.; Abe-Higuchi, N.; Hobara, T.; Hara, K.; Kobayashi, A.; Shintaku, T.; Itoh, Y.; Suzuki, T.; et al. Hippocampal microRNA-124 enhances chronic stress resilience in mice. J. Neurosci. 2016, 36, 7253–7267. [Google Scholar] [CrossRef]
- Volk, N.; Pape, J.C.; Engel, M.; Zannas, A.S.; Cattane, N.; Cattaneo, A.; Binder, E.B.; Chen, A. Amygdalar microRNA-15a is essential for coping with chronic stress. Cell Rep. 2016, 17, 1882–1891. [Google Scholar] [CrossRef]
- Maurel, O.M.; Torrisi, S.A.; Barbagallo, C.; Purrello, M.; Salomone, S.; Drago, F.; Leggio, G.M. Dysregulation of miR-15a-5p, miR-497a-5p and miR-511-5p is associated with modulation of BDNF and FKBP5 in brain areas of PTSD-related susceptible and resilient mice. Int. J. Mol. Sci. 2021, 22, 5157. [Google Scholar] [CrossRef]
- Song, M.F.; Dong, J.Z.; Wang, Y.W.; He, J.; Ju, X.; Zhang, L.; Zhang, Y.H.; Shi, J.F.; Lv, Y.Y. CSF miR-16 is decreased in major depression patients and its neutralization in rats induces depression-like behaviors via a serotonin transmitter system. J. Affect. Disord. 2015, 178, 25–31. [Google Scholar] [CrossRef]
- Baudry, A.; Mouillet-Richard, S.; Schneider, B.; Launay, J.M.; Kellermann, O. miR-16 targets the serotonin transporter: A new facet for adaptive responses to antidepressants. Science 2010, 329, 1537–1541. [Google Scholar] [CrossRef]
- Portugalov, A.; Zaidan, H.; Gaisler-Salomon, I.; Hillard, C.J.; Akirav, I. FAAH inhibition restores early life stress-induced alterations in PFC microRNAs associated with depressive-like behavior in male and female rats. IJMS 2022, 23, 16101. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, D.; Xu, J.; Jiang, H.; Pan, F. Early adolescent stress-induced changes in prefrontal cortex miRNA-135a and hippocampal miRNA-16 in male rats. Dev. Psychobiol. 2017, 59, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Adlakha, Y.K.; Saini, N. Brain microRNAs and insights into biological functions and therapeutic potential of brain enriched miRNA-128. Mol. Cancer 2014, 13, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Xia, W.; Liu, J.C.; Yang, J.Y.; Lee, D.F.; Xia, J.; Bartholomeusz, G.; Li, Y.; Pan, Y.; Li, Z.; et al. Erk associates with and primes GSK-3β for its inactivation resulting in upregulation of β-catenin. Mol. Cell 2005, 19, 159–170. [Google Scholar] [CrossRef]
- Mendoza, M.C.; Er, E.E.; Blenis, J. The Ras-ERK and PI3K-mTOR pathways: Cross-talk and compensation. Trends Biochem. Sci. 2011, 36, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Bower, K.A.; Chen, G.; Shi, X.; Ke, Z.J.; Luo, J. Interaction between ERK and GSK3β mediates basic fibroblast growth factor-induced apoptosis in SK-N-MC neuroblastoma cells. J. Biol. Chem. 2008, 283, 9248–9256. [Google Scholar] [CrossRef] [PubMed]
- Bewernick, B.H.; Hurlemann, R.; Matusch, A.; Kayser, S.; Grubert, C.; Hadrysiewicz, B.; Axmacher, N.; Lemke, M.; Cooper-Mahkorn, D.; Cohen, M.X.; et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol. Psychiatry 2010, 67, 110–116. [Google Scholar] [CrossRef]
- Campbell, S.; MacQueen, G. The role of the hippocampus in the pathophysiology of major depression. J. Psychiatry Neurosci. 2004, 29, 417–426. [Google Scholar]
- Koenigs, M.; Huey, E.D.; Calamia, M.; Raymont, V.; Tranel, D.; Grafman, J. Distinct regions of prefrontal cortex mediate resistance and vulnerability to depression. J. Neurosci. 2008, 28, 12341–12348. [Google Scholar] [CrossRef]
- Zhu, S.; Shi, R.; Wang, J.; Wang, J.F.; Li, X.M. Unpredictable chronic mild stress not chronic restraint stress induces depressive behaviours in mice. Neuroreport 2014, 25, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.D.; An, S.C.; Zhang, X. Involvement of hippocampal serotonin and neuropeptide Y in depression induced by chronic unpredicted mild stress. Brain Res. Bull. 2008, 77, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, M.K.; Bishnoi, M.; Kulkarni, S.K. Anti-depressant like effect of curcumin and its combination with piperine in unpredictable chronic stress-induced behavioral, biochemical and neurochemical changes. Pharmacol. Biochem. Behav. 2009, 92, 39–43. [Google Scholar] [CrossRef]
- Bangasser, D.A.; Cuarenta, A. Sex differences in anxiety and depression: Circuits and mechanisms. Nat. Rev. Neurosci. 2021, 22, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Eid, R.S.; Gobinath, A.R.; Galea, L.A. Sex differences in depression: Insights from clinical and preclinical studies. Prog. Neurobiol. 2019, 176, 86–102. [Google Scholar] [CrossRef] [PubMed]
- Zurawek, D.; Kusmider, M.; Faron-Gorecka, A.; Gruca, P.; Pabian, P.; Kolasa, M.; Solich, J.; Szafran-Pilch, K.; Papp, M.; Dziedzicka-Wasylewska, M. Time-dependent miR-16 serum fluctuations together with reciprocal changes in the expression level of miR-16 in mesocortical circuit contribute to stress resilient phenotype in chronic mild stress–an animal model of depression. Eur. Neuropsychopharmacol. 2016, 26, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Gheysarzadeh, A.; Sadeghifard, N.; Afraidooni, L.; Pooyan, F.; Mofid, M.R.; Valadbeigi, H.; Bakhtiari, H.; Keikhavani, S. Serum-based microRNA biomarkers for major depression: MiR-16, miR-135a, and miR-1202. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2018, 23, 39. [Google Scholar] [CrossRef]
- Dwivedi, Y.; Roy, B.; Lugli, G.; Rizavi, H.; Zhang, H.; Smalheiser, N.R. Chronic corticosterone-mediated dysregulation of microRNA network in prefrontal cortex of rats: Relevance to depression pathophysiology. Transl. Psychiatry 2015, 5, e682. [Google Scholar] [CrossRef]
- Gu, Z.; Pan, J.; Chen, L. MiR-124 suppression in the prefrontal cortex reduces depression-like behavior in mice. Biosci. Rep. 2019, 39, BSR20190186. [Google Scholar] [CrossRef]
- Lou, D.; Wang, J.; Wang, X. miR-124 ameliorates depressive-like behavior by targeting STAT3 to regulate microglial activation. Mol. Cell. Probes 2019, 48, 101470. [Google Scholar] [CrossRef]
- Periyasamy, P.; Liao, K.; Kook, Y.H.; Niu, F.; Callen, S.E.; Guo, M.L.; Buch, S. Cocaine-mediated downregulation of miR-124 activates microglia by targeting KLF4 and TLR4 signaling. Mol. Neurobiol. 2018, 55, 3196–3210. [Google Scholar] [CrossRef]
- Cabana-Domínguez, J.; Arenas, C.; Cormand, B.; Fernàndez-Castillo, N. MiR-9, miR-153 and miR-124 are down-regulated by acute exposure to cocaine in a dopaminergic cell model and may contribute to cocaine dependence. Transl. Psychiatry 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Dash, S.; Balasubramaniam, M.; Martínez-Rivera, F.J.; Godino, A.; Peck, E.G.; Patnaik, S.; Suar, M.; Calipari, E.S.; Nestler, E.J.; Villalta, F.; et al. Cocaine-regulated microRNA miR-124 controls poly (ADP-ribose) polymerase-1 expression in neuronal cells. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Yang, W.; Liu, M.; Zhang, Q.; Zhang, J.; Chen, J.; Chen, Q.; Suo, L. Knockdown of miR-124 reduces depression-like behavior by targeting CREB1 and BDNF. Curr. Neurovascular Res. 2020, 17, 196–203. [Google Scholar] [CrossRef]
- Fakhoury, M. Revisiting the serotonin hypothesis: Implications for major depressive disorders. Mol. Neurobiol. 2016, 53, 2778–2786. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Olivier, J.D.A.; Nonkes, L.J.P.; Homberg, J.R. Conserved role for the serotonin transporter gene in rat and mouse neurobehavioral endophenotypes. Neurosci. Biobehav. Rev. 2010, 34, 373–386. [Google Scholar] [CrossRef]
- Moya, P.R.; Wendland, J.R.; Salemme, J.; Fried, R.L.; Murphy, D.L. miR-15a and miR-16 regulate serotonin transporter expression in human placental and rat brain raphe cells. Int. J. Neuropsychopharmacol. 2013, 16, 621–629. [Google Scholar] [CrossRef]
- Dahlhoff, M.; Siegmund, A.; Golub, Y.; Wolf, E.; Holsboer, F.; Wotjak, C.T. AKT/GSK-3β/β-catenin signalling within hippocampus and amygdala reflects genetically determined differences in posttraumatic stress disorder like symptoms. Neuroscience 2010, 169, 1216–1226. [Google Scholar] [CrossRef]
- Chen, Y.C.; Tan, Q.R.; Dang, W.; Wang, H.N.; Zhang, R.B.; Li, Z.Y.; Lin, H.; Liu, R. The effect of citalopram on chronic stress-induced depressive-like behavior in rats through GSK3β/β-catenin activation in the medial prefrontal cortex. Brain Res. Bull. 2012, 88, 338–344. [Google Scholar] [CrossRef]
- Pilar-Cuéllar, F.; Vidal, R.; Pazos, A. Subchronic treatment with fluoxetine and ketanserin increases hippocampal brain-derived neurotrophic factor, β-catenin and antidepressant-like effects. Br. J. Pharmacol. 2012, 165, 1046–1057. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y.; Vallée, J.N. Possible actions of cannabidiol in obsessive-compulsive disorder by targeting the WNT/β-catenin pathway. Mol. Psychiatry 2022, 27, 230–248. [Google Scholar] [CrossRef] [PubMed]
- Sbarski, B.; Akirav, I. Cannabinoids as therapeutics for PTSD. Pharmacol. Ther. 2020, 211, 107551. [Google Scholar] [CrossRef] [PubMed]
- Hillard, C.J.; Beatka, M.; Sarvaideo, J. Endocannabinoid signaling and the hypothalamic-pituitary-adrenal axis. Compr. Physiol. 2016, 7, 1. [Google Scholar] [PubMed]
- Hill, M.N.; McLaughlin, R.J.; Bingham, B.; Shrestha, L.; Lee, T.T.; Gray, J.M.; Hillard, C.J.; Gorzalka, B.B.; Viau, V. Endogenous cannabinoid signaling is essential for stress adaptation. Proc. Natl. Acad. Sci. USA 2010, 107, 9406–9411. [Google Scholar] [CrossRef]
- Hill, M.N.; Karatsoreos, I.N.; Hillard, C.J.; McEwen, B.S. Rapid elevations in limbic endocannabinoid content by glucocorticoid hormones in vivo. Psychoneuroendocrinology 2010, 35, 1333–1338. [Google Scholar] [CrossRef]
- Alteba, S.; Zer-Aviv, T.M.; Tenenhaus, A.; David, G.B.; Adelman, J.; Hillard, C.J.; Doron, R.; Akirav, I. Antidepressant-like effects of URB597 and JZL184 in male and female rats exposed to early life stress. Eur. Neuropsychopharmacol. 2020, 39, 70–86. [Google Scholar] [CrossRef]
- Alteba, S.; Portugalov, A.; Hillard, C.J.; Akirav, I. Inhibition of fatty acid amide hydrolase (FAAH) during adolescence and exposure to early life stress may exacerbate depression-like behaviors in male and female rats. Neuroscience 2021, 455, 89–106. [Google Scholar] [CrossRef]
- Burstein, O.; Shoshan, N.; Doron, R.; Akirav, I. Cannabinoids prevent depressive-like symptoms and alterations in BDNF expression in a rat model of PTSD. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 84, 129–139. [Google Scholar] [CrossRef]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.M.; Denovan-Wright, E. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef]
- Chung, H.; Fierro, A.; Pessoa-Mahana, C.D. Cannabidiol binding and negative allosteric modulation at the cannabinoid type 1 receptor in the presence of delta-9-tetrahydrocannabinol: An In Silico study. PLoS ONE 2019, 14, e0220025. [Google Scholar] [CrossRef]
- Willner, P.; Muscat, R.; Papp, M. Chronic mild stress-induced anhedonia: A realistic animal model of depression. Neurosci. Biobehav. Rev. 1992, 16, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Grønli, J.; Murison, R.; Bjorvatn, B.; Sørensen, E.; Portas, C.M.; Ursin, R. Chronic mild stress affects sucrose intake and sleep in rats. Behav. Brain Res. 2004, 150, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Sales, A.J.; Fogaça, M.V.; Sartim, A.G.; Pereira, V.S.; Wegener, G.; Guimarães, F.S.; Joca, S.R. Cannabidiol induces rapid and sustained antidepressant-like effects through increased BDNF signaling and synaptogenesis in the prefrontal cortex. Mol. Neurobiol. 2019, 56, 1070–1081. [Google Scholar] [CrossRef] [PubMed]
- Rogóż, Z.; Kabziński, M.; Sadaj, W.; Rachwalska, P.; Gądek-Michalska, A. Effect of co-treatment with fluoxetine or mirtazapine and risperidone on the active behaviors and plasma corticosterone concentration in rats subjected to the forced swim test. Pharmacol. Rep. 2012, 64, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Zaidan, H.; Ramaswami, G.; Barak, M.; Li, J.B.; Gaisler-Salomon, I. Pre-reproductive stress and fluoxetine treatment in rats affect offspring A-to-I RNA editing, gene expression and social behavior. Environ. Epigenetics 2018, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Iffland, K.; Grotenhermen, F. An update on safety and side effects of cannabidiol: A review of clinical data and relevant animal studies. Cannabis Cannabinoid Res. 2017, 2, 139–154. [Google Scholar] [CrossRef]

| miR-16 vmPFC | miR-16 NAC | miR-16 Raphe | miR-124 vmPFC | miR-124 NAC | miR-124 Raphe | miR-135 vmPFC | miR-135 NAC | miR-135 Raphe | |
|---|---|---|---|---|---|---|---|---|---|
| FST—immobility | r = 0.277 p = 0.102 | r = 0.250 p = 0.147 | r = 0.069 p = 716 | r = 0.087 p = 0.613 | r = −0.040 p = 0.816 | r = 0.054 p = 0.749 | r = 0.428 * p = 0.012 | r = −0.339 * p = 0.046 | r = 0.187 p = 0.340 |
| OFT—total distance | r = 0.176 p = 0.305 | r = 0.322 p = 0.060 | r = −0.473 ** p = 0.008 | r = −0.159 p = 0.355 | r = 0.455 ** p = 0.005 | r = 0.060 p = 0.718 | r = 0.012 p = 0.944 | r = 0.192 p = 0.268 | r = 0.474 * p = 0.011 |
| OFT—time in center | r = 0.0.14 p = 0.933 | r = −0.2.33 p = 0.198 | r = 0.276 p = 0.140 | r = −0.184 p = 0.282 | r = −0.142 p = 0.403 | r = 0.157 p = 0.347 | r = −0.75 p = 0.675 | r = −0.229 p = 0.186 | r = −0.70 p = 0.723 |
| hrt1a | slc6a4 | ctnnb1 | cnr1 | |
|---|---|---|---|---|
| miR-16 | r = 0.591 *** p = 0.000 | r = −0.432 * p = 0.031 | r = 0.140 p = 0.436 | r = 0.250 p = 0.160 |
| miR-124 | r = −0.131 p = 0.469 | r = 0.281 p = 0.183 | r = 0.027 p = 0.880 | r = −0.075 p = 0.682 |
| miR-135 | r = 0.478 ** p = 0.008 | r = −0.346 p = 0.091 | r = 0.001 p = 0.997 | r = 0.258 p = 0.154 |
| hrt1a | slc6a4 | ctnnb1 | cnr1 | |
|---|---|---|---|---|
| miR-16 | r = −0.404 p = 0.051 | r = −0.33 p = 0.878 | r = 0.479 ** p = 0.006 | r = 0.229 p = 0.215 |
| miR-124 | r = −0.272 p = 0.198 | r = −0.56 p = 0.794 | r = 0.463 ** p = 0.008 | r = 0.224 p = 0.234 |
| miR-135 | r = −0.324 p = 0.114 | r = −0.080 p = 0.724 | r = 0.222 p = 0.222 | r = 0.226 p = 0.231 |
| hrt1a | slc6a4 | ctnnb1 | cnr1 | |
|---|---|---|---|---|
| miR-16 | r = 0.125 p = 0.579 | r = 0.064 p = 0.795 | r = −0.205 p = 0.325 | r = 0.012 p = 0.956 |
| miR-124 | r = −0.358 p = 0.056 | r = 0.408 * p = 0.035 | r = −0.175 p = 0.330 | r = 0.147 p = 0.438 |
| miR-135 | r = 0.191 p = 0.382 | r = 0.232 p = 0.298 | r = 0.050 p = 0.815 | r = 0.324 p = 0.142 |
| Name | Description | GeneBankID (NM) | Protein Name | Primer Sequence | Efficacy | Description |
|---|---|---|---|---|---|---|
| Hprt | Housekeeping gene; used as a reference gene | NM_012583.2 | HPRT | F: 5′CGCCAGCTTCCTCCTCAG3′ | NM_012583.2 | HPRT |
| Htr1a | Serotonergic auto-receptor | R: 5′ATAACCTGGTTCATCATCACTAATCAC3′ | 99.83 | R: 5′ATAACCTGGTTCATCATCACTAATCAC3′ | 99.83 | |
| Slc6a4 | The serotonergic transporter | NM_012585.1 | 5HT1a | F: 5′CCACGGCTACACCATCTACTC3′ | NM_012585.1 | 5HT1a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bright, U.; Akirav, I. Cannabidiol Modulates Alterations in PFC microRNAs in a Rat Model of Depression. Int. J. Mol. Sci. 2023, 24, 2052. https://doi.org/10.3390/ijms24032052
Bright U, Akirav I. Cannabidiol Modulates Alterations in PFC microRNAs in a Rat Model of Depression. International Journal of Molecular Sciences. 2023; 24(3):2052. https://doi.org/10.3390/ijms24032052
Chicago/Turabian StyleBright, Uri, and Irit Akirav. 2023. "Cannabidiol Modulates Alterations in PFC microRNAs in a Rat Model of Depression" International Journal of Molecular Sciences 24, no. 3: 2052. https://doi.org/10.3390/ijms24032052
APA StyleBright, U., & Akirav, I. (2023). Cannabidiol Modulates Alterations in PFC microRNAs in a Rat Model of Depression. International Journal of Molecular Sciences, 24(3), 2052. https://doi.org/10.3390/ijms24032052





