Sigma-1 Receptor Signaling: In Search of New Therapeutic Alternatives for Cardiovascular and Renal Diseases
Abstract
1. Introduction
2. Sig-1R Structure and Intracellular Localization
3. Sig-1R Ligands
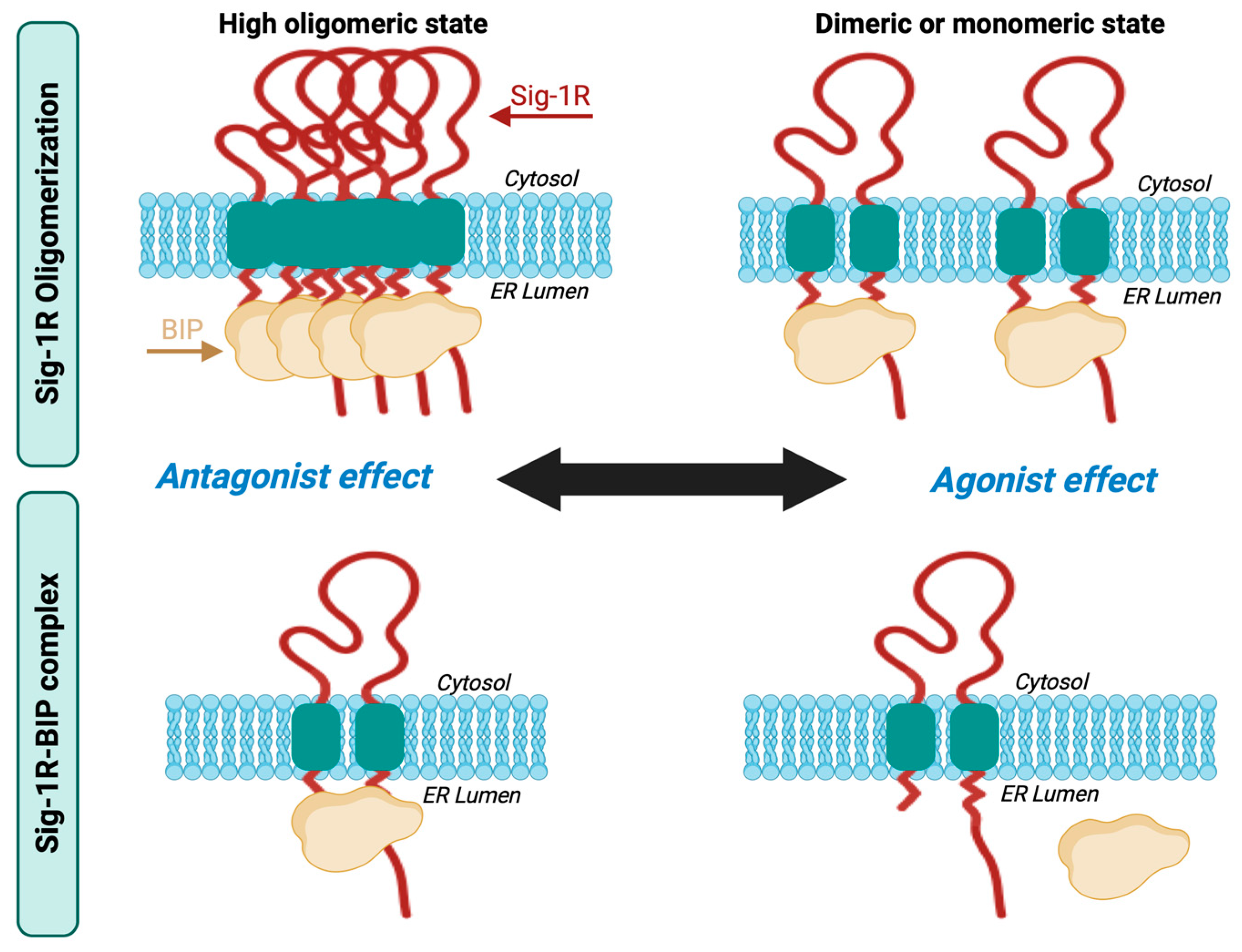
4. Receptor Oligomerization and the Sig-1R-BiP Complex
5. Sig-1R Signaling Pathways
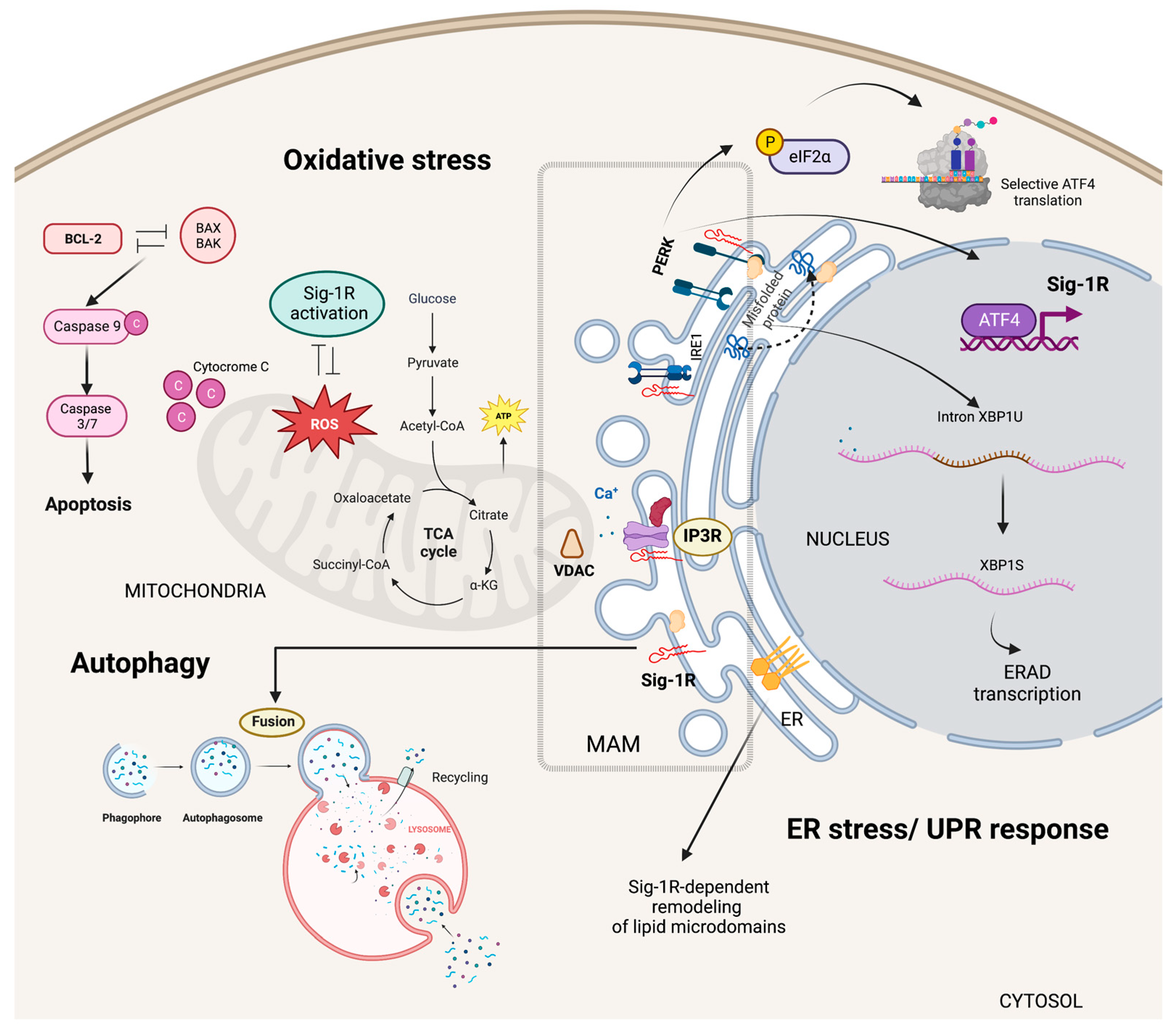
5.1. Cytoplasmic Organelles
5.1.1. ER-Mitochondria Interface
5.1.2. Specialized ER Lipid Microdomains
5.1.3. ER Stress Responses
5.1.4. Autophagy and Mitophagy
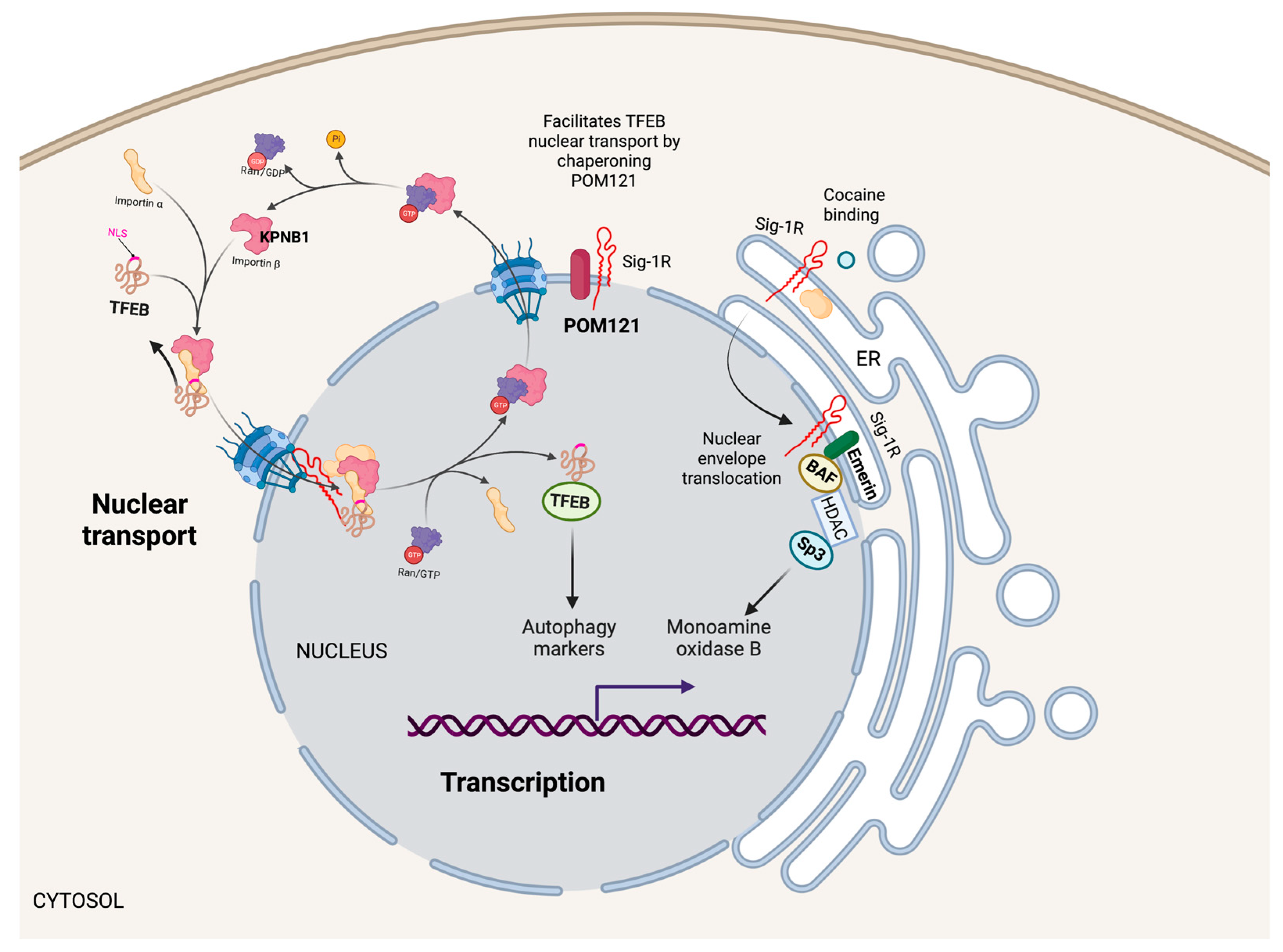
5.2. Nucleus
5.2.1. Transcription
5.2.2. Nuclear Transport
5.3. Plasma Membrane
5.3.1. Ion Channels
5.3.2. RTK Signaling
5.3.3. GPCR Signaling
5.3.4. Cell-Matrix Interactions
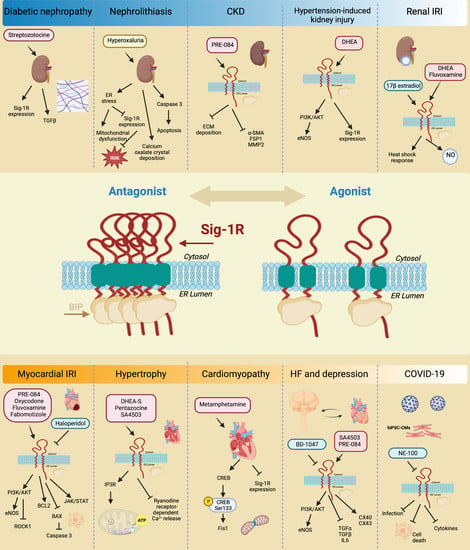
6. Sig-1R Signaling Regulates Organ Function and Disease
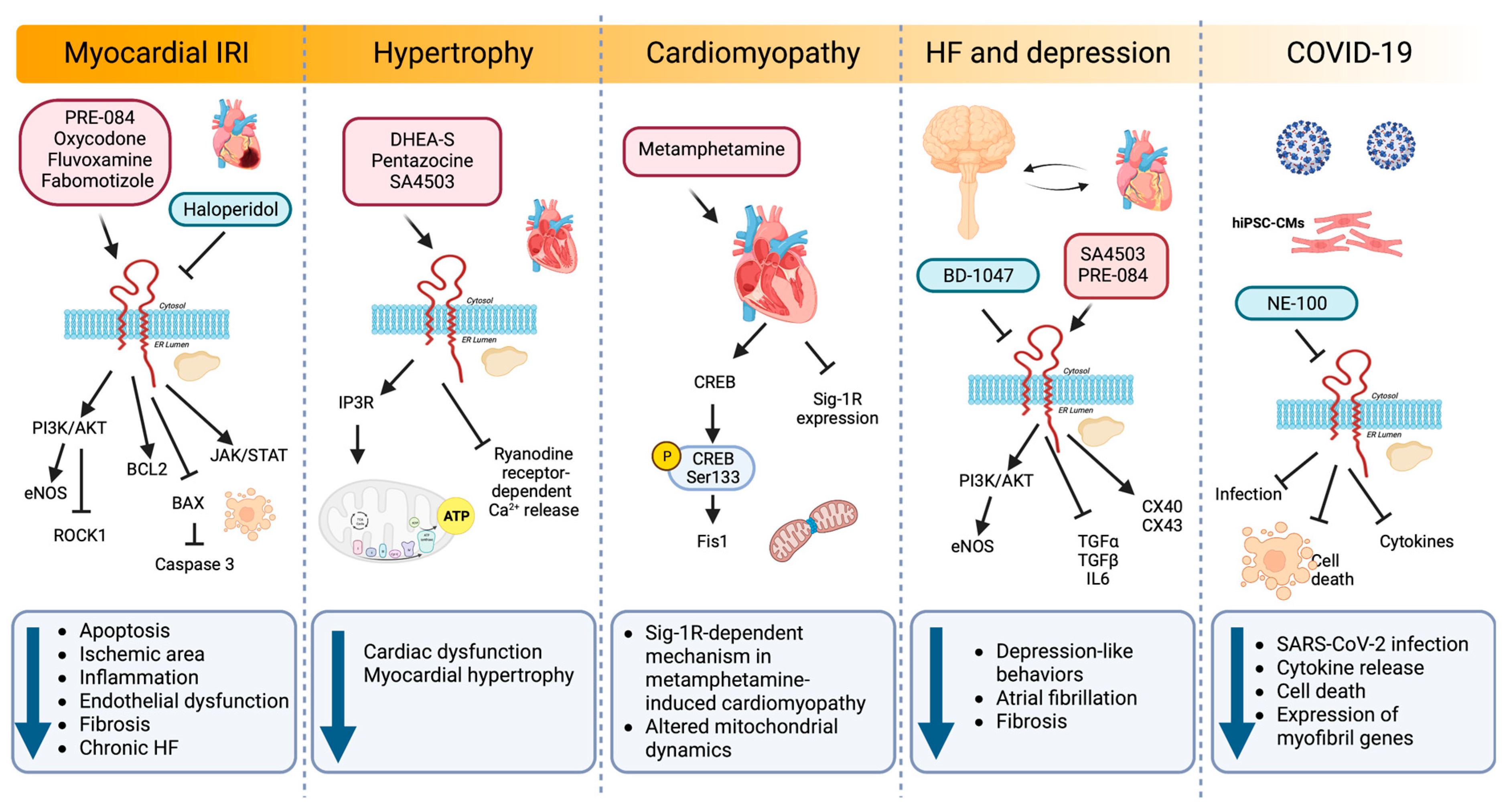
6.1. Heart
6.1.1. Myocardial IRI and HF
6.1.2. Hypertrophy
6.1.3. Cardiac Fibrosis
6.1.4. Cardiomyopathy
6.1.5. Relationship between Sig-1R, HF and Depression
6.1.6. ER Stress
6.1.7. SARS-CoV-2
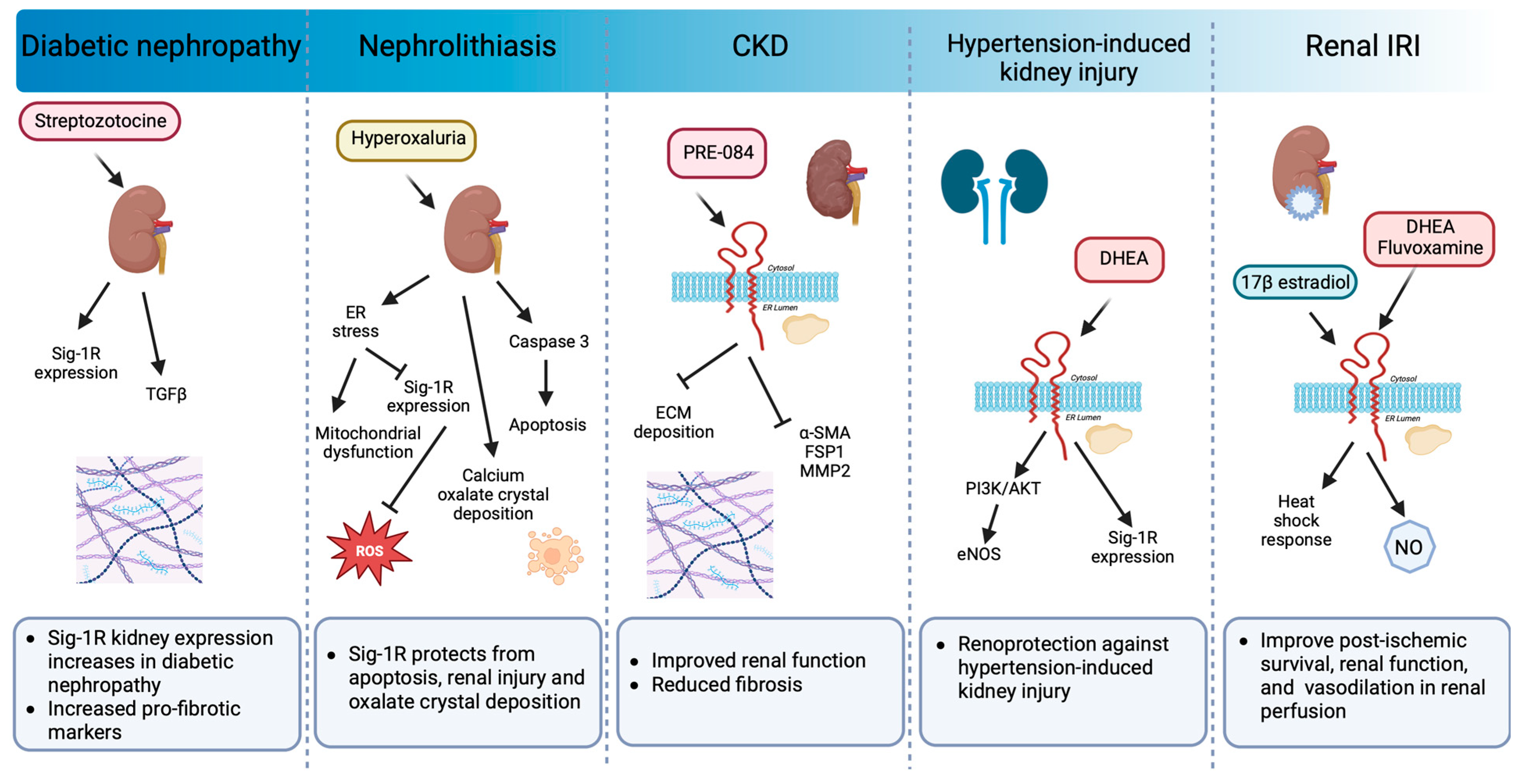
6.2. Kidney
7. Expectations of the Therapeutic Use of Sig-1R Agonists and Antagonists
8. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paronetto, M.P.; Passacantilli, I.; Sette, C. Alternative splicing and cell survival: From tissue homeostasis to disease. Cell Death Differ. 2016, 23, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.R.; Eades, C.G.; Thompson, J.A.; Huppler, R.E.; Gilbert, P.E. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther. 1976, 197, 517–532. [Google Scholar] [PubMed]
- Su, T.P. Evidence for sigma opioid receptor: Binding of [3H]SKF-10047 to etorphine-inaccessible sites in guinea-pig brain. J. Pharmacol. Exp. Ther. 1982, 223, 284–290. [Google Scholar] [PubMed]
- Su, T.P.; Hayashi, T. Understanding the molecular mechanism of sigma-1 receptors: Towards a hypothesis that sigma-1 receptors are intracellular amplifiers for signal transduction. Curr. Med. Chem. 2003, 10, 2073–2080. [Google Scholar] [CrossRef] [PubMed]
- Aishwarya, R.; Abdullah, C.S.; Morshed, M.; Remex, N.S.; Bhuiyan, M.S. Sigmar1’s Molecular, Cellular, and Biological Functions in Regulating Cellular Pathophysiology. Front. Physiol. 2021, 12, 705575. [Google Scholar] [CrossRef]
- Hayashi, T. The Sigma-1 Receptor in Cellular Stress Signaling. Front. Neurosci. 2019, 13, 733. [Google Scholar] [CrossRef]
- Mavlyutov, T.A.; Epstein, M.L.; Andersen, K.A.; Ziskind-Conhaim, L.; Ruoho, A.E. The sigma-1 receptor is enriched in postsynaptic sites of C-terminals in mouse motoneurons. An anatomical and behavioral study. Neuroscience 2010, 167, 247–255. [Google Scholar] [CrossRef]
- Bernard-Marissal, N.; Medard, J.J.; Azzedine, H.; Chrast, R. Dysfunction in endoplasmic reticulum-mitochondria crosstalk underlies SIGMAR1 loss of function mediated motor neuron degeneration. Brain 2015, 138, 875–890. [Google Scholar] [CrossRef]
- Langa, F.; Codony, X.; Tovar, V.; Lavado, A.; Gimenez, E.; Cozar, P.; Cantero, M.; Dordal, A.; Hernandez, E.; Perez, R.; et al. Generation and phenotypic analysis of sigma receptor type I (sigma 1) knockout mice. Eur. J. Neurosci. 2003, 18, 2188–2196. [Google Scholar] [CrossRef]
- Sha, S.; Hong, J.; Qu, W.J.; Lu, Z.H.; Li, L.; Yu, W.F.; Chen, L. Sex-related neurogenesis decrease in hippocampal dentate gyrus with depressive-like behaviors in sigma-1 receptor knockout mice. Eur. Neuropsychopharmacol. 2015, 25, 1275–1286. [Google Scholar] [CrossRef]
- Sabino, V.; Cottone, P.; Parylak, S.L.; Steardo, L.; Zorrilla, E.P. Sigma-1 receptor knockout mice display a depressive-like phenotype. Behav. Brain Res. 2009, 198, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, C.S.; Alam, S.; Aishwarya, R.; Miriyala, S.; Panchatcharam, M.; Bhuiyan, M.A.N.; Peretik, J.M.; Orr, A.W.; James, J.; Osinska, H.; et al. Cardiac Dysfunction in the Sigma 1 Receptor Knockout Mouse Associated With Impaired Mitochondrial Dynamics and Bioenergetics. J. Am. Heart Assoc. 2018, 7, e009775. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Su, T. The sigma receptor: Evolution of the concept in neuropsychopharmacology. Curr. Neuropharmacol. 2005, 3, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Penke, B.; Fulop, L.; Szucs, M.; Frecska, E. The Role of Sigma-1 Receptor, an Intracellular Chaperone in Neurodegenerative Diseases. Curr. Neuropharmacol. 2018, 16, 97–116. [Google Scholar] [CrossRef]
- Ryskamp, D.A.; Korban, S.; Zhemkov, V.; Kraskovskaya, N.; Bezprozvanny, I. Neuronal Sigma-1 Receptors: Signaling Functions and Protective Roles in Neurodegenerative Diseases. Front. Neurosci. 2019, 13, 862. [Google Scholar] [CrossRef]
- Gao, Q.J.; Yang, B.; Chen, J.; Shi, S.B.; Yang, H.J.; Liu, X. Sigma-1 Receptor Stimulation with PRE-084 Ameliorates Myocardial Ischemia-Reperfusion Injury in Rats. Chin. Med. J. 2018, 131, 539–543. [Google Scholar] [CrossRef]
- Ji, M.; Cheng, J.; Zhang, D. Oxycodone protects cardiac microvascular endothelial cells against ischemia/reperfusion injury by binding to Sigma-1 Receptor. Bioengineered 2022, 13, 9628–9644. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, C.; Pi, Z.; Li, R.; Han, P.; Guo, L. Oxycodone protects cardiomyocytes from ischemia-reperfusion-induced apoptosis via PI3K/Akt pathway. Pharmazie 2020, 75, 430–435. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, X.; Chen, X.; Han, X.; Sun, Y.; Fo, Y.; Wang, X.; Qu, C.; Yang, B. Activation of the sigma-1 receptor exerts cardioprotection in a rodent model of chronic heart failure by stimulation of angiogenesis. Mol. Med. 2022, 28, 87. [Google Scholar] [CrossRef]
- Xie, Y.; Ge, C.L.; Zhang, Z.Y.; Fei, G.X. Oxycodone inhibits myocardial cell apoptosis after myocardial ischemia-reperfusion injury in rats via RhoA/ROCK1 signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 6371–6379. [Google Scholar] [CrossRef]
- Kryzhanovskii, S.A.; Tsorin, I.B.; Stolyaruk, V.N.; Vititnova, M.B.; Ionova, E.O.; Barchukov, V.V.; Kozhevnikova, L.M.; Seredenin, S.B. Examination of Cardioprotective Effects of Fabomotizole Hydrochloride in Translational Rat Model of Chronic Heart Failure. Bull. Exp. Biol. Med. 2019, 168, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.S.; Fukunaga, K. Stimulation of sigma-1 receptor signaling by dehydroepiandrosterone ameliorates pressure overload-induced hypertrophy and dysfunctions in ovariectomized rats. Expert Opin. Ther. Targets 2009, 13, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- Tagashira, H.; Bhuiyan, M.S.; Fukunaga, K. Diverse regulation of IP3 and ryanodine receptors by pentazocine through sigma1-receptor in cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1201–H1212. [Google Scholar] [CrossRef] [PubMed]
- Tagashira, H.; Bhuiyan, S.; Shioda, N.; Hasegawa, H.; Kanai, H.; Fukunaga, K. Sigma1-receptor stimulation with fluvoxamine ameliorates transverse aortic constriction-induced myocardial hypertrophy and dysfunction in mice. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1535–H1545. [Google Scholar] [CrossRef]
- Abdullah, C.S.; Aishwarya, R.; Alam, S.; Morshed, M.; Remex, N.S.; Nitu, S.; Kolluru, G.K.; Traylor, J.; Miriyala, S.; Panchatcharam, M.; et al. Methamphetamine induces cardiomyopathy by Sigmar1 inhibition-dependent impairment of mitochondrial dynamics and function. Commun. Biol. 2020, 3, 682. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, C.; Guo, Y.; Liu, X.; Ye, T.; Fo, Y.; Qu, C.; Liang, J.; Shi, S.; Yang, B. Chronic stimulation of the sigma-1 receptor ameliorates ventricular ionic and structural remodeling in a rodent model of depression. Life Sci. 2020, 257, 118047. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, C.; Chen, X.; Liu, X.; Ye, T.; Fo, Y.; Shi, S.; Qu, C.; Liang, J.; Shen, B.; et al. Sigma-1 receptor ligands improves ventricular repolarization-related ion remodeling in rats with major depression disorder. Psychopharmacology 2021, 238, 487–499. [Google Scholar] [CrossRef]
- Ito, K.; Hirooka, Y.; Sunagawa, K. Brain sigma-1 receptor stimulation improves mental disorder and cardiac function in mice with myocardial infarction. J. Cardiovasc. Pharmacol. 2013, 62, 222–228. [Google Scholar] [CrossRef]
- Salerno, J.A.; Torquato, T.; Temerozo, J.R.; Goto-Silva, L.; Karmirian, K.; Mendes, M.A.; Sacramento, C.Q.; Fintelman-Rodrigues, N.; Souza, L.R.Q.; Ornelas, I.M.; et al. Inhibition of SARS-CoV-2 infection in human iPSC-derived cardiomyocytes by targeting the Sigma-1 receptor disrupts cytoarchitecture and beating. PeerJ 2021, 9, e12595. [Google Scholar] [CrossRef]
- Lewis, R.; Li, J.; McCormick, P.J.; Huang, C.L.-H.; Jeevaratnam, K. Is the sigma-1 receptor a potential pharmacological target for cardiac pathologies? A systematic review. Int. J. Cardiol. Heart Vasc. 2020, 26, 100449. [Google Scholar] [CrossRef]
- Hosszu, A.; Antal, Z.; Lenart, L.; Hodrea, J.; Koszegi, S.; Balogh, D.B.; Banki, N.F.; Wagner, L.; Denes, A.; Hamar, P.; et al. sigma1-Receptor Agonism Protects against Renal Ischemia-Reperfusion Injury. J. Am. Soc. Nephrol. 2017, 28, 152–165. [Google Scholar] [CrossRef]
- Haritha, C.V.; Lingaraju, M.C.; Mathesh, K.; Jadhav, S.E.; Shyamkumar, T.S.; Aneesha, V.A.; Parida, S.; Singh, T.U.; Kumar, D. PRE-084 ameliorates adenine-induced renal fibrosis in rats. Tissue Cell 2022, 79, 101905. [Google Scholar] [CrossRef] [PubMed]
- Hosszu, A.; Antal, Z.; Veres-Szekely, A.; Lenart, L.; Balogh, D.B.; Szkibinszkij, E.; Illesy, L.; Hodrea, J.; Banki, N.F.; Wagner, L.; et al. The role of Sigma-1 receptor in sex-specific heat shock response in an experimental rat model of renal ischaemia/reperfusion injury. Transpl. Int. 2018, 31, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef]
- Rangaswami, J.; Bhalla, V.; Blair, J.E.A.; Chang, T.I.; Costa, S.; Lentine, K.L.; Lerma, E.V.; Mezue, K.; Molitch, M.; Mullens, W.; et al. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement From the American Heart Association. Circulation 2019, 139, e840–e878. [Google Scholar] [CrossRef] [PubMed]
- Su, T.P.; Hayashi, T.; Maurice, T.; Buch, S.; Ruoho, A.E. The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends Pharmacol. Sci. 2010, 31, 557–566. [Google Scholar] [CrossRef]
- Hanner, M.; Moebius, F.F.; Flandorfer, A.; Knaus, H.G.; Striessnig, J.; Kempner, E.; Glossmann, H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc. Natl. Acad. Sci. USA 1996, 93, 8072–8077. [Google Scholar] [CrossRef]
- Rousseaux, C.G.; Greene, S.F. Sigma receptors [sigmaRs]: Biology in normal and diseased states. J. Recept. Signal Transduct. Res. 2016, 36, 327–388. [Google Scholar] [CrossRef]
- Aydar, E.; Palmer, C.P.; Klyachko, V.A.; Jackson, M.B. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron 2002, 34, 399–410. [Google Scholar] [CrossRef]
- Hayashi, T.; Su, T.P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 2007, 131, 596–610. [Google Scholar] [CrossRef]
- Ortega-Roldan, J.L.; Ossa, F.; Amin, N.T.; Schnell, J.R. Solution NMR studies reveal the location of the second transmembrane domain of the human sigma-1 receptor. FEBS Lett. 2015, 589, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.R.; Zheng, S.; Gurpinar, E.; Koehl, A.; Manglik, A.; Kruse, A.C. Crystal structure of the human sigma1 receptor. Nature 2016, 532, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Mavlyutov, T.A.; Yang, H.; Epstein, M.L.; Ruoho, A.E.; Yang, J.; Guo, L.W. APEX2-enhanced electron microscopy distinguishes sigma-1 receptor localization in the nucleoplasmic reticulum. Oncotarget 2017, 8, 51317–51330. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, M.E.; Prasad, P.D.; Huang, W.; Seth, P.; Leibach, F.H.; Ganapathy, V. Molecular and ligand-binding characterization of the sigma-receptor in the Jurkat human T lymphocyte cell line. J. Pharmacol. Exp. Ther. 1999, 289, 251–260. [Google Scholar] [PubMed]
- Seth, P.; Ganapathy, M.E.; Conway, S.J.; Bridges, C.D.; Smith, S.B.; Casellas, P.; Ganapathy, V. Expression pattern of the type 1 sigma receptor in the brain and identity of critical anionic amino acid residues in the ligand-binding domain of the receptor. Biochim. Biophys. Acta 2001, 1540, 59–67. [Google Scholar] [CrossRef]
- Pal, A.; Chu, U.B.; Ramachandran, S.; Grawoig, D.; Guo, L.W.; Hajipour, A.R.; Ruoho, A.E. Juxtaposition of the steroid binding domain-like I and II regions constitutes a ligand binding site in the sigma-1 receptor. J. Biol. Chem. 2008, 283, 19646–19656. [Google Scholar] [CrossRef]
- Kopanchuk, S.; Vavers, E.; Veiksina, S.; Ligi, K.; Zvejniece, L.; Dambrova, M.; Rinken, A. Intracellular dynamics of the Sigma-1 receptor observed with super-resolution imaging microscopy. PLoS ONE 2022, 17, e0268563. [Google Scholar] [CrossRef]
- Mavlyutov, T.A.; Ruoho, A.E. Ligand-dependent localization and intracellular stability of sigma-1 receptors in CHO-K1 cells. J. Mol. Signal. 2007, 2, 8. [Google Scholar] [CrossRef][Green Version]
- Abdullah, C.S.; Aishwarya, R.; Alam, S.; Remex, N.S.; Morshed, M.; Nitu, S.; Miriyala, S.; Panchatcharam, M.; Hartman, B.; King, J.; et al. The molecular role of Sigmar1 in regulating mitochondrial function through mitochondrial localization in cardiomyocytes. Mitochondrion 2022, 62, 159–175. [Google Scholar] [CrossRef]
- Watanabe, S.; Ilieva, H.; Tamada, H.; Nomura, H.; Komine, O.; Endo, F.; Jin, S.; Mancias, P.; Kiyama, H.; Yamanaka, K. Mitochondria-associated membrane collapse is a common pathomechanism in SIGMAR1- and SOD1-linked ALS. EMBO Mol. Med. 2016, 8, 1421–1437. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Su, T.P. Intracellular dynamics of sigma-1 receptors (sigma(1) binding sites) in NG108-15 cells. J. Pharmacol. Exp. Ther. 2003, 306, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.Y.; Chuang, J.Y.; Tsai, M.S.; Wang, X.F.; Xi, Z.X.; Hung, J.J.; Chang, W.C.; Bonci, A.; Su, T.P. Sigma-1 receptor mediates cocaine-induced transcriptional regulation by recruiting chromatin-remodeling factors at the nuclear envelope. Proc. Natl. Acad. Sci. USA 2015, 112, E6562–E6570. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Patel, C.; Shenkman, M.; Kessel, A.; Ben-Tal, N.; Lederkremer, G.Z. The Sigma-1 receptor is an ER-localized type II membrane protein. J. Biol. Chem. 2021, 297, 101299. [Google Scholar] [CrossRef] [PubMed]
- Maurice, T.; Roman, F.J.; Privat, A. Modulation by neurosteroids of the in vivo (+)-[3H]SKF-10,047 binding to sigma 1 receptors in the mouse forebrain. J. Neurosci. Res. 1996, 46, 734–743. [Google Scholar] [CrossRef]
- Su, T.P.; London, E.D.; Jaffe, J.H. Steroid binding at sigma receptors suggests a link between endocrine, nervous, and immune systems. Science 1988, 240, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.P.; Mahen, R.; Schnell, E.; Djamgoz, M.B.; Aydar, E. Sigma-1 receptors bind cholesterol and remodel lipid rafts in breast cancer cell lines. Cancer Res. 2007, 67, 11166–11175. [Google Scholar] [CrossRef]
- Ramachandran, S.; Chu, U.B.; Mavlyutov, T.A.; Pal, A.; Pyne, S.; Ruoho, A.E. The sigma1 receptor interacts with N-alkyl amines and endogenous sphingolipids. Eur. J. Pharmacol. 2009, 609, 19–26. [Google Scholar] [CrossRef]
- Gomez-Soler, M.; Fernandez-Duenas, V.; Portillo-Salido, E.; Perez, P.; Zamanillo, D.; Vela, J.M.; Burgueno, J.; Ciruela, F. Predicting the antinociceptive efficacy of sigma(1) receptor ligands by a novel receptor fluorescence resonance energy transfer (FRET) based biosensor. J. Med. Chem. 2014, 57, 238–242. [Google Scholar] [CrossRef]
- Mishra, A.K.; Mavlyutov, T.; Singh, D.R.; Biener, G.; Yang, J.; Oliver, J.A.; Ruoho, A.; Raicu, V. The sigma-1 receptors are present in monomeric and oligomeric forms in living cells in the presence and absence of ligands. Biochem. J. 2015, 466, 263–271. [Google Scholar] [CrossRef]
- Schmidt, H.R.; Betz, R.M.; Dror, R.O.; Kruse, A.C. Structural basis for sigma(1) receptor ligand recognition. Nat. Struct. Mol. Biol. 2018, 25, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Kourrich, S.; Hayashi, T.; Chuang, J.Y.; Tsai, S.Y.; Su, T.P.; Bonci, A. Dynamic interaction between sigma-1 receptor and Kv1.2 shapes neuronal and behavioral responses to cocaine. Cell 2013, 152, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Li, H.; Kim, H.W.; Shin, S.E.; Seo, M.S.; An, J.R.; Ha, K.S.; Han, E.T.; Hong, S.H.; Choi, I.W.; et al. Escitalopram, a selective serotonin reuptake inhibitor, inhibits voltage-dependent K(+) channels in coronary arterial smooth muscle cells. Korean J. Physiol. Pharmacol. 2017, 21, 415–421. [Google Scholar] [CrossRef]
- Hernandez, L.L.; Appel, J.B. Dopaminergic involvement in the mechanism of action of pentazocine. Behav. Neural Biol. 1979, 26, 384–400. [Google Scholar] [CrossRef] [PubMed]
- Chertkow, Y.; Weinreb, O.; Youdim, M.B.; Silver, H. The effect of chronic co-administration of fluvoxamine and haloperidol compared to clozapine on the GABA system in the rat frontal cortex. Int. J. Neuropsychopharmacol. 2006, 9, 287–296. [Google Scholar] [CrossRef]
- Niitsu, T.; Iyo, M.; Hashimoto, K. Sigma-1 receptor agonists as therapeutic drugs for cognitive impairment in neuropsychiatric diseases. Curr. Pharm. Des. 2012, 18, 875–883. [Google Scholar] [CrossRef]
- Hayashi, T.; Tsai, S.Y.; Mori, T.; Fujimoto, M.; Su, T.P. Targeting ligand-operated chaperone sigma-1 receptors in the treatment of neuropsychiatric disorders. Expert Opin. Ther. Targets 2011, 15, 557–577. [Google Scholar] [CrossRef]
- Glennon, R.A.; Ablordeppey, S.Y.; Ismaiel, A.M.; el-Ashmawy, M.B.; Fischer, J.B.; Howie, K.B. Structural features important for sigma 1 receptor binding. J. Med. Chem. 1994, 37, 1214–1219. [Google Scholar] [CrossRef]
- Pascual, R.; Almansa, C.; Plata-Salaman, C.; Vela, J.M. A New Pharmacophore Model for the Design of Sigma-1 Ligands Validated on a Large Experimental Dataset. Front. Pharmacol. 2019, 10, 519. [Google Scholar] [CrossRef]
- Gromek, K.A.; Suchy, F.P.; Meddaugh, H.R.; Wrobel, R.L.; LaPointe, L.M.; Chu, U.B.; Primm, J.G.; Ruoho, A.E.; Senes, A.; Fox, B.G. The oligomeric states of the purified sigma-1 receptor are stabilized by ligands. J. Biol. Chem. 2014, 289, 20333–20344. [Google Scholar] [CrossRef]
- Pobre, K.F.R.; Poet, G.J.; Hendershot, L.M. The endoplasmic reticulum (ER) chaperone BiP is a master regulator of ER functions: Getting by with a little help from ERdj friends. J. Biol. Chem. 2019, 294, 2098–2108. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Roldan, J.L.; Ossa, F.; Schnell, J.R. Characterization of the human sigma-1 receptor chaperone domain structure and binding immunoglobulin protein (BiP) interactions. J. Biol. Chem. 2013, 288, 21448–21457. [Google Scholar] [CrossRef] [PubMed]
- Shioda, N.; Ishikawa, K.; Tagashira, H.; Ishizuka, T.; Yawo, H.; Fukunaga, K. Expression of a truncated form of the endoplasmic reticulum chaperone protein, sigma1 receptor, promotes mitochondrial energy depletion and apoptosis. J. Biol. Chem. 2012, 287, 23318–23331. [Google Scholar] [CrossRef] [PubMed]
- Tagashira, H.; Shinoda, Y.; Shioda, N.; Fukunaga, K. Methyl pyruvate rescues mitochondrial damage caused by SIGMAR1 mutation related to amyotrophic lateral sclerosis. Biochim. Biophys. Acta 2014, 1840, 3320–3334. [Google Scholar] [CrossRef] [PubMed]
- Natsvlishvili, N.; Goguadze, N.; Zhuravliova, E.; Mikeladze, D. Sigma-1 receptor directly interacts with Rac1-GTPase in the brain mitochondria. BMC Biochem. 2015, 16, 11. [Google Scholar] [CrossRef]
- Meunier, J.; Ieni, J.; Maurice, T. The anti-amnesic and neuroprotective effects of donepezil against amyloid beta25-35 peptide-induced toxicity in mice involve an interaction with the sigma1 receptor. Br. J. Pharmacol. 2006, 149, 998–1012. [Google Scholar] [CrossRef]
- Dong, H.; Ma, Y.; Ren, Z.; Xu, B.; Zhang, Y.; Chen, J.; Yang, B. Sigma-1 Receptor Modulates Neuroinflammation After Traumatic Brain Injury. Cell. Mol. Neurobiol. 2016, 36, 639–645. [Google Scholar] [CrossRef]
- Wang, L.; Eldred, J.A.; Sidaway, P.; Sanderson, J.; Smith, A.J.; Bowater, R.P.; Reddan, J.R.; Wormstone, I.M. Sigma 1 receptor stimulation protects against oxidative damage through suppression of the ER stress responses in the human lens. Mech. Ageing Dev. 2012, 133, 665–674. [Google Scholar] [CrossRef]
- Pal, A.; Fontanilla, D.; Gopalakrishnan, A.; Chae, Y.K.; Markley, J.L.; Ruoho, A.E. The sigma-1 receptor protects against cellular oxidative stress and activates antioxidant response elements. Eur. J. Pharmacol. 2012, 682, 12–20. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Rothman, R.K.; Su, T.P. Insights into the Sigma-1 receptor chaperone’s cellular functions: A microarray report. Synapse 2012, 66, 42–51. [Google Scholar] [CrossRef]
- Wang, J.; Shanmugam, A.; Markand, S.; Zorrilla, E.; Ganapathy, V.; Smith, S.B. Sigma 1 receptor regulates the oxidative stress response in primary retinal Muller glial cells via NRF2 signaling and system xc(-), the Na(+)-independent glutamate-cystine exchanger. Free. Radic. Biol. Med. 2015, 86, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Su, T.C.; Lin, S.H.; Lee, P.T.; Yeh, S.H.; Hsieh, T.H.; Chou, S.Y.; Su, T.P.; Hung, J.J.; Chang, W.C.; Lee, Y.C.; et al. The sigma-1 receptor-zinc finger protein 179 pathway protects against hydrogen peroxide-induced cell injury. Neuropharmacology 2016, 105, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Weng, T.Y.; Tsai, S.A.; Su, T.P. Roles of sigma-1 receptors on mitochondrial functions relevant to neurodegenerative diseases. J. Biomed. Sci. 2017, 24, 74. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Bhardwaj, A.; Cheng, J.; Alkayed, N.J.; Hurn, P.D.; Kirsch, J.R. Sigma receptor agonists provide neuroprotection in vitro by preserving bcl-2. Anesth. Analg. 2007, 104, 1179–1184, tables of contents. [Google Scholar] [CrossRef] [PubMed]
- Meunier, J.; Hayashi, T. Sigma-1 receptors regulate Bcl-2 expression by reactive oxygen species-dependent transcriptional regulation of nuclear factor kappaB. J. Pharmacol. Exp. Ther. 2010, 332, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Tchedre, K.T.; Yorio, T. sigma-1 receptors protect RGC-5 cells from apoptosis by regulating intracellular calcium, Bax levels, and caspase-3 activation. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2577–2588. [Google Scholar] [CrossRef] [PubMed]
- Spruce, B.A.; Campbell, L.A.; McTavish, N.; Cooper, M.A.; Appleyard, M.V.; O’Neill, M.; Howie, J.; Samson, J.; Watt, S.; Murray, K.; et al. Small molecule antagonists of the sigma-1 receptor cause selective release of the death program in tumor and self-reliant cells and inhibit tumor growth in vitro and in vivo. Cancer Res. 2004, 64, 4875–4886. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Zhang, Y.; Lv, X.; Chen, X.; Zhou, R.; Nguyen, L.K.; Wu, X.; Yao, H. Molecular Mechanisms Involving Sigma-1 Receptor in Cell Apoptosis of BV-2 Microglial Cells Induced by Methamphetamine. CNS Neurol. Disord. Drug Targets 2016, 15, 857–865. [Google Scholar] [CrossRef]
- Achison, M.; Boylan, M.T.; Hupp, T.R.; Spruce, B.A. HIF-1alpha contributes to tumour-selective killing by the sigma receptor antagonist rimcazole. Oncogene 2007, 26, 1137–1146. [Google Scholar] [CrossRef]
- Hayashi, T.; Rizzuto, R.; Hajnoczky, G.; Su, T.P. MAM: More than just a housekeeper. Trends Cell Biol. 2009, 19, 81–88. [Google Scholar] [CrossRef]
- Hayashi, T.; Fujimoto, M. Detergent-resistant microdomains determine the localization of sigma-1 receptors to the endoplasmic reticulum-mitochondria junction. Mol. Pharmacol. 2010, 77, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Zhemkov, V.; Ditlev, J.A.; Lee, W.R.; Wilson, M.; Liou, J.; Rosen, M.K.; Bezprozvanny, I. The role of sigma 1 receptor in organization of endoplasmic reticulum signaling microdomains. Elife 2021, 10, e65192. [Google Scholar] [CrossRef] [PubMed]
- Vollrath, J.T.; Sechi, A.; Dreser, A.; Katona, I.; Wiemuth, D.; Vervoorts, J.; Dohmen, M.; Chandrasekar, A.; Prause, J.; Brauers, E.; et al. Loss of function of the ALS protein SigR1 leads to ER pathology associated with defective autophagy and lipid raft disturbances. Cell Death Dis. 2014, 5, e1290. [Google Scholar] [CrossRef] [PubMed]
- Delprat, B.; Crouzier, L.; Su, T.P.; Maurice, T. At the Crossing of ER Stress and MAMs: A Key Role of Sigma-1 Receptor? Adv. Exp. Med. Biol. 2020, 1131, 699–718. [Google Scholar] [CrossRef]
- Kourrich, S.; Su, T.P.; Fujimoto, M.; Bonci, A. The sigma-1 receptor: Roles in neuronal plasticity and disease. Trends Neurosci. 2012, 35, 762–771. [Google Scholar] [CrossRef]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Bravo, R.; Vicencio, J.M.; Parra, V.; Troncoso, R.; Munoz, J.P.; Bui, M.; Quiroga, C.; Rodriguez, A.E.; Verdejo, H.E.; Ferreira, J.; et al. Increased ER-mitochondrial coupling promotes mitochondrial respiration and bioenergetics during early phases of ER stress. J. Cell Sci. 2011, 124, 2143–2152. [Google Scholar] [CrossRef]
- Ma, Y.; Hendershot, L.M. ER chaperone functions during normal and stress conditions. J. Chem. Neuroanat. 2004, 28, 51–65. [Google Scholar] [CrossRef]
- Li, J.; Zhang, D.; Brundel, B.; Wiersma, M. Imbalance of ER and Mitochondria Interactions: Prelude to Cardiac Ageing and Disease? Cells 2019, 8, 1617. [Google Scholar] [CrossRef]
- Koshenov, Z.; Oflaz, F.E.; Hirtl, M.; Pilic, J.; Bachkoenig, O.A.; Gottschalk, B.; Madreiter-Sokolowski, C.T.; Rost, R.; Malli, R.; Graier, W.F. Sigma-1 Receptor Promotes Mitochondrial Bioenergetics by Orchestrating ER Ca(2+) Leak during Early ER Stress. Metabolites 2021, 11, 422. [Google Scholar] [CrossRef]
- Ha, Y.; Dun, Y.; Thangaraju, M.; Duplantier, J.; Dong, Z.; Liu, K.; Ganapathy, V.; Smith, S.B. Sigma receptor 1 modulates endoplasmic reticulum stress in retinal neurons. Investig. Ophthalmol. Vis. Sci. 2011, 52, 527–540. [Google Scholar] [CrossRef]
- Ha, Y.; Shanmugam, A.K.; Markand, S.; Zorrilla, E.; Ganapathy, V.; Smith, S.B. Sigma receptor 1 modulates ER stress and Bcl2 in murine retina. Cell Tissue Res. 2014, 356, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Mitsuda, T.; Omi, T.; Tanimukai, H.; Sakagami, Y.; Tagami, S.; Okochi, M.; Kudo, T.; Takeda, M. Sigma-1Rs are upregulated via PERK/eIF2alpha/ATF4 pathway and execute protective function in ER stress. Biochem. Biophys. Res. Commun. 2011, 415, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Omi, T.; Tanimukai, H.; Kanayama, D.; Sakagami, Y.; Tagami, S.; Okochi, M.; Morihara, T.; Sato, M.; Yanagida, K.; Kitasyoji, A.; et al. Fluvoxamine alleviates ER stress via induction of Sigma-1 receptor. Cell Death Dis. 2014, 5, e1332. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Li, H.; Yasumura, D.; Cohen, H.R.; Zhang, C.; Panning, B.; Shokat, K.M.; Lavail, M.M.; Walter, P. IRE1 signaling affects cell fate during the unfolded protein response. Science 2007, 318, 944–949. [Google Scholar] [CrossRef]
- Mori, T.; Hayashi, T.; Hayashi, E.; Su, T.P. Sigma-1 receptor chaperone at the ER-mitochondrion interface mediates the mitochondrion-ER-nucleus signaling for cellular survival. PLoS ONE 2013, 8, e76941. [Google Scholar] [CrossRef]
- Hamasaki, M.; Furuta, N.; Matsuda, A.; Nezu, A.; Yamamoto, A.; Fujita, N.; Oomori, H.; Noda, T.; Haraguchi, T.; Hiraoka, Y.; et al. Autophagosomes form at ER-mitochondria contact sites. Nature 2013, 495, 389–393. [Google Scholar] [CrossRef]
- Garofalo, T.; Matarrese, P.; Manganelli, V.; Marconi, M.; Tinari, A.; Gambardella, L.; Faggioni, A.; Misasi, R.; Sorice, M.; Malorni, W. Evidence for the involvement of lipid rafts localized at the ER-mitochondria associated membranes in autophagosome formation. Autophagy 2016, 12, 917–935. [Google Scholar] [CrossRef]
- Dreser, A.; Vollrath, J.T.; Sechi, A.; Johann, S.; Roos, A.; Yamoah, A.; Katona, I.; Bohlega, S.; Wiemuth, D.; Tian, Y.; et al. The ALS-linked E102Q mutation in Sigma receptor-1 leads to ER stress-mediated defects in protein homeostasis and dysregulation of RNA-binding proteins. Cell Death Differ. 2017, 24, 1655–1671. [Google Scholar] [CrossRef]
- Kasahara, R.; Yamamoto, N.; Suzuki, K.; Sobue, K. The sigma1 receptor regulates accumulation of GM1 ganglioside-enriched autophagosomes in astrocytes. Neuroscience 2017, 340, 176–187. [Google Scholar] [CrossRef]
- Cao, L.; Walker, M.P.; Vaidya, N.K.; Fu, M.; Kumar, S.; Kumar, A. Cocaine-Mediated Autophagy in Astrocytes Involves Sigma 1 Receptor, PI3K, mTOR, Atg5/7, Beclin-1 and Induces Type II Programed Cell Death. Mol. Neurobiol. 2016, 53, 4417–4430. [Google Scholar] [CrossRef] [PubMed]
- MacVicar, T.D.; Mannack, L.V.; Lees, R.M.; Lane, J.D. Targeted siRNA Screens Identify ER-to-Mitochondrial Calcium Exchange in Autophagy and Mitophagy Responses in RPE1 Cells. Int. J. Mol. Sci. 2015, 16, 13356–13380. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Liu, Y.; Li, S.; Li, X.J.; Yang, W. PINK1-PRKN mediated mitophagy: Differences between in vitro and in vivo models. Autophagy 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Shen, H.; Li, J.; Guo, L.W. SIGMAR1/Sigma-1 receptor ablation impairs autophagosome clearance. Autophagy 2019, 15, 1539–1557. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Mysona, B.; Dun, Y.; Gnana-Prakasam, J.P.; Pabla, N.; Li, W.; Dong, Z.; Ganapathy, V.; Smith, S.B. Expression, subcellular localization, and regulation of sigma receptor in retinal muller cells. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5576–5582. [Google Scholar] [CrossRef]
- Mavlyutov, T.A.; Epstein, M.; Guo, L.W. Subcellular localization of the sigma-1 receptor in retinal neurons—An electron microscopy study. Sci. Rep. 2015, 5, 10689. [Google Scholar] [CrossRef]
- Miki, Y.; Mori, F.; Kon, T.; Tanji, K.; Toyoshima, Y.; Yoshida, M.; Sasaki, H.; Kakita, A.; Takahashi, H.; Wakabayashi, K. Accumulation of the sigma-1 receptor is common to neuronal nuclear inclusions in various neurodegenerative diseases. Neuropathology 2014, 34, 148–158. [Google Scholar] [CrossRef]
- DeJesus-Hernandez, M.; Mackenzie, I.R.; Boeve, B.F.; Boxer, A.L.; Baker, M.; Rutherford, N.J.; Nicholson, A.M.; Finch, N.A.; Flynn, H.; Adamson, J.; et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 2011, 72, 245–256. [Google Scholar] [CrossRef]
- Lee, P.T.; Lievens, J.C.; Wang, S.M.; Chuang, J.Y.; Khalil, B.; Wu, H.E.; Chang, W.C.; Maurice, T.; Su, T.P. Sigma-1 receptor chaperones rescue nucleocytoplasmic transport deficit seen in cellular and Drosophila ALS/FTD models. Nat. Commun. 2020, 11, 5580. [Google Scholar] [CrossRef]
- Wang, S.M.; Wu, H.E.; Yasui, Y.; Geva, M.; Hayden, M.; Maurice, T.; Cozzolino, M.; Su, T.P. Nucleoporin POM121 signals TFEB-mediated autophagy via activation of SIGMAR1/sigma-1 receptor chaperone by pridopidine. Autophagy 2022, 19, 126–151. [Google Scholar] [CrossRef]
- Su, T.P.; Su, T.C.; Nakamura, Y.; Tsai, S.Y. The Sigma-1 Receptor as a Pluripotent Modulator in Living Systems. Trends Pharmacol. Sci. 2016, 37, 262–278. [Google Scholar] [CrossRef] [PubMed]
- Le Guennec, J.Y.; Ouadid-Ahidouch, H.; Soriani, O.; Besson, P.; Ahidouch, A.; Vandier, C. Voltage-gated ion channels, new targets in anti-cancer research. Recent Pat. Anti Cancer Drug Discov. 2007, 2, 189–202. [Google Scholar] [CrossRef]
- Carnally, S.M.; Johannessen, M.; Henderson, R.M.; Jackson, M.B.; Edwardson, J.M. Demonstration of a direct interaction between sigma-1 receptors and acid-sensing ion channels. Biophys. J. 2010, 98, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.G.; Roh, D.H.; Yoon, S.Y.; Choi, S.R.; Choi, H.S.; Moon, J.Y.; Kang, S.Y.; Kim, H.W.; Han, H.J.; Beitz, A.J.; et al. Role of peripheral sigma-1 receptors in ischaemic pain: Potential interactions with ASIC and P2X receptors. Eur. J. Pain 2016, 20, 594–606. [Google Scholar] [CrossRef]
- Sanguinetti, M.C.; Tristani-Firouzi, M. hERG potassium channels and cardiac arrhythmia. Nature 2006, 440, 463–469. [Google Scholar] [CrossRef]
- Crottes, D.; Martial, S.; Rapetti-Mauss, R.; Pisani, D.F.; Loriol, C.; Pellissier, B.; Martin, P.; Chevet, E.; Borgese, F.; Soriani, O. Sig1R protein regulates hERG channel expression through a post-translational mechanism in leukemic cells. J. Biol. Chem. 2011, 286, 27947–27958. [Google Scholar] [CrossRef]
- Ganapathi, S.B.; Fox, T.E.; Kester, M.; Elmslie, K.S. Ceramide modulates HERG potassium channel gating by translocation into lipid rafts. Am. J. Physiol. Cell Physiol. 2010, 299, C74–C86. [Google Scholar] [CrossRef]
- Kinoshita, M.; Matsuoka, Y.; Suzuki, T.; Mirrielees, J.; Yang, J. Sigma-1 receptor alters the kinetics of Kv1.3 voltage gated potassium channels but not the sensitivity to receptor ligands. Brain Res. 2012, 1452, 1–9. [Google Scholar] [CrossRef]
- Balasuriya, D.; Stewart, A.P.; Crottes, D.; Borgese, F.; Soriani, O.; Edwardson, J.M. The sigma-1 receptor binds to the Nav1.5 voltage-gated Na+ channel with 4-fold symmetry. J. Biol. Chem. 2012, 287, 37021–37029. [Google Scholar] [CrossRef]
- Gao, X.F.; Yao, J.J.; He, Y.L.; Hu, C.; Mei, Y.A. Sigma-1 receptor agonists directly inhibit Nav1.2/1.4 channels. PLoS ONE 2012, 7, e49384. [Google Scholar] [CrossRef]
- Johannessen, M.; Ramachandran, S.; Riemer, L.; Ramos-Serrano, A.; Ruoho, A.E.; Jackson, M.B. Voltage-gated sodium channel modulation by sigma-receptors in cardiac myocytes and heterologous systems. Am. J. Physiol. Cell Physiol. 2009, 296, C1049–C1057. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, M.; Fontanilla, D.; Mavlyutov, T.; Ruoho, A.E.; Jackson, M.B. Antagonist action of progesterone at sigma-receptors in the modulation of voltage-gated sodium channels. Am. J. Physiol. Cell Physiol. 2011, 300, C328–C337. [Google Scholar] [CrossRef] [PubMed]
- Yermolaieva, O.; Leonard, A.S.; Schnizler, M.K.; Abboud, F.M.; Welsh, M.J. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc. Natl. Acad. Sci. USA 2004, 101, 6752–6757. [Google Scholar] [CrossRef] [PubMed]
- Herrera, Y.; Katnik, C.; Rodriguez, J.D.; Hall, A.A.; Willing, A.; Pennypacker, K.R.; Cuevas, J. sigma-1 receptor modulation of acid-sensing ion channel a (ASIC1a) and ASIC1a-induced Ca2+ influx in rat cortical neurons. J. Pharmacol. Exp. Ther. 2008, 327, 491–502. [Google Scholar] [CrossRef]
- Gibbons, D.D.; Kutschke, W.J.; Weiss, R.M.; Benson, C.J. Heart failure induces changes in acid-sensing ion channels in sensory neurons innervating skeletal muscle. J. Physiol. 2015, 593, 4575–4587. [Google Scholar] [CrossRef]
- Redd, M.A.; Scheuer, S.E.; Saez, N.J.; Yoshikawa, Y.; Chiu, H.S.; Gao, L.; Hicks, M.; Villanueva, J.E.; Joshi, Y.; Chow, C.Y.; et al. Therapeutic Inhibition of Acid-Sensing Ion Channel 1a Recovers Heart Function After Ischemia-Reperfusion Injury. Circulation 2021, 144, 947–960. [Google Scholar] [CrossRef]
- Sudhesh Dev, S.; Zainal Abidin, S.A.; Farghadani, R.; Othman, I.; Naidu, R. Receptor Tyrosine Kinases and Their Signaling Pathways as Therapeutic Targets of Curcumin in Cancer. Front. Pharmacol. 2021, 12, 772510. [Google Scholar] [CrossRef]
- Hashimoto, K. Sigma-1 receptor chaperone and brain-derived neurotrophic factor: Emerging links between cardiovascular disease and depression. Prog. Neurobiol. 2013, 100, 15–29. [Google Scholar] [CrossRef]
- Fujimoto, M.; Hayashi, T.; Urfer, R.; Mita, S.; Su, T.P. Sigma-1 receptor chaperones regulate the secretion of brain-derived neurotrophic factor. Synapse 2012, 66, 630–639. [Google Scholar] [CrossRef]
- Kimura, Y.; Fujita, Y.; Shibata, K.; Mori, M.; Yamashita, T. Sigma-1 receptor enhances neurite elongation of cerebellar granule neurons via TrkB signaling. PLoS ONE 2013, 8, e75760. [Google Scholar] [CrossRef]
- Hang, P.Z.; Zhu, H.; Li, P.F.; Liu, J.; Ge, F.Q.; Zhao, J.; Du, Z.M. The Emerging Role of BDNF/TrkB Signaling in Cardiovascular Diseases. Life 2021, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Yokoyama, M.; Toko, H.; Tateno, K.; Moriya, J.; Shimizu, I.; Nojima, A.; Ito, T.; Yoshida, Y.; Kobayashi, Y.; et al. Brain-derived neurotrophic factor protects against cardiac dysfunction after myocardial infarction via a central nervous system-mediated pathway. Arter. Thromb. Vasc. Biol. 2012, 32, 1902–1909. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Su, T.P. Sigma-1 receptors (sigma(1) binding sites) form raft-like microdomains and target lipid droplets on the endoplasmic reticulum: Roles in endoplasmic reticulum lipid compartmentalization and export. J. Pharmacol. Exp. Ther. 2003, 306, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Makki, N.; Thiel, K.W.; Miller, F.J., Jr. The epidermal growth factor receptor and its ligands in cardiovascular disease. Int. J. Mol. Sci. 2013, 14, 20597–20613. [Google Scholar] [CrossRef]
- Melenhorst, W.B.; Mulder, G.M.; Xi, Q.; Hoenderop, J.G.; Kimura, K.; Eguchi, S.; van Goor, H. Epidermal growth factor receptor signaling in the kidney: Key roles in physiology and disease. Hypertension 2008, 52, 987–993. [Google Scholar] [CrossRef]
- Tang, J.; Liu, N.; Zhuang, S. Role of epidermal growth factor receptor in acute and chronic kidney injury. Kidney Int. 2013, 83, 804–810. [Google Scholar] [CrossRef]
- Zeng, F.; Singh, A.B.; Harris, R.C. The role of the EGF family of ligands and receptors in renal development, physiology and pathophysiology. Exp. Cell Res. 2009, 315, 602–610. [Google Scholar] [CrossRef]
- Takebayashi, M.; Hayashi, T.; Su, T.P. Sigma-1 receptors potentiate epidermal growth factor signaling towards neuritogenesis in PC12 cells: Potential relation to lipid raft reconstitution. Synapse 2004, 53, 90–103. [Google Scholar] [CrossRef]
- Yao, H.; Kim, K.; Duan, M.; Hayashi, T.; Guo, M.; Morgello, S.; Prat, A.; Wang, J.; Su, T.P.; Buch, S. Cocaine hijacks sigma1 receptor to initiate induction of activated leukocyte cell adhesion molecule: Implication for increased monocyte adhesion and migration in the CNS. J. Neurosci. 2011, 31, 5942–5955. [Google Scholar] [CrossRef]
- Hussain, T.; Lokhandwala, M.F. Renal dopamine receptor function in hypertension. Hypertension 1998, 32, 187–197. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Sumida, T.S.; Nomura, S.; Satoh, M.; Higo, T.; Ito, M.; Ko, T.; Fujita, K.; Sweet, M.E.; Sanbe, A.; et al. Cardiac dopamine D1 receptor triggers ventricular arrhythmia in chronic heart failure. Nat. Commun. 2020, 11, 4364. [Google Scholar] [CrossRef] [PubMed]
- Hryciw, D.H.; McAinch, A.J. Cannabinoid receptors in the kidney. Curr. Opin. Nephrol. Hypertens. 2016, 25, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Alfulaij, N.; Meiners, F.; Michalek, J.; Small-Howard, A.L.; Turner, H.C.; Stokes, A.J. Cannabinoids, the Heart of the Matter. J. Am. Heart Assoc. 2018, 7, e009099. [Google Scholar] [CrossRef] [PubMed]
- Kim, F.J.; Kovalyshyn, I.; Burgman, M.; Neilan, C.; Chien, C.C.; Pasternak, G.W. Sigma 1 receptor modulation of G-protein-coupled receptor signaling: Potentiation of opioid transduction independent from receptor binding. Mol. Pharmacol. 2010, 77, 695–703. [Google Scholar] [CrossRef]
- Rodriguez-Munoz, M.; Sanchez-Blazquez, P.; Herrero-Labrador, R.; Martinez-Murillo, R.; Merlos, M.; Vela, J.M.; Garzon, J. The sigma1 receptor engages the redox-regulated HINT1 protein to bring opioid analgesia under NMDA receptor negative control. Antioxid. Redox Signal. 2015, 22, 799–818. [Google Scholar] [CrossRef]
- Bergeron, R.; de Montigny, C.; Debonnel, G. Potentiation of neuronal NMDA response induced by dehydroepiandrosterone and its suppression by progesterone: Effects mediated via sigma receptors. J. Neurosci. 1996, 16, 1193–1202. [Google Scholar] [CrossRef]
- Martina, M.; Turcotte, M.E.; Halman, S.; Bergeron, R. The sigma-1 receptor modulates NMDA receptor synaptic transmission and plasticity via SK channels in rat hippocampus. J. Physiol. 2007, 578, 143–157. [Google Scholar] [CrossRef]
- Balasuriya, D.; Stewart, A.P.; Edwardson, J.M. The sigma-1 receptor interacts directly with GluN1 but not GluN2A in the GluN1/GluN2A NMDA receptor. J. Neurosci. 2013, 33, 18219–18224. [Google Scholar] [CrossRef]
- Pabba, M.; Wong, A.Y.; Ahlskog, N.; Hristova, E.; Biscaro, D.; Nassrallah, W.; Ngsee, J.K.; Snyder, M.; Beique, J.C.; Bergeron, R. NMDA receptors are upregulated and trafficked to the plasma membrane after sigma-1 receptor activation in the rat hippocampus. J. Neurosci. 2014, 34, 11325–11338. [Google Scholar] [CrossRef]
- Sanchez-Blazquez, P.; Rodriguez-Munoz, M.; Herrero-Labrador, R.; Burgueno, J.; Zamanillo, D.; Garzon, J. The calcium-sensitive Sigma-1 receptor prevents cannabinoids from provoking glutamate NMDA receptor hypofunction: Implications in antinociception and psychotic diseases. Int. J. Neuropsychopharmacol. 2014, 17, 1943–1955. [Google Scholar] [CrossRef]
- Cheng, M.H.; Block, E.; Hu, F.; Cobanoglu, M.C.; Sorkin, A.; Bahar, I. Insights into the Modulation of Dopamine Transporter Function by Amphetamine, Orphenadrine, and Cocaine Binding. Front. Neurol. 2015, 6, 134. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Moreno, E.; Aymerich, M.; Marcellino, D.; McCormick, P.J.; Mallol, J.; Cortes, A.; Casado, V.; Canela, E.I.; Ortiz, J.; et al. Direct involvement of sigma-1 receptors in the dopamine D1 receptor-mediated effects of cocaine. Proc. Natl. Acad. Sci. USA 2010, 107, 18676–18681. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.; Moreno-Delgado, D.; Navarro, G.; Hoffmann, H.M.; Fuentes, S.; Rosell-Vilar, S.; Gasperini, P.; Rodriguez-Ruiz, M.; Medrano, M.; Mallol, J.; et al. Cocaine disrupts histamine H3 receptor modulation of dopamine D1 receptor signaling: Sigma1-D1-H3 receptor complexes as key targets for reducing cocaine’s effects. J. Neurosci. 2014, 34, 3545–3558. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Moreno, E.; Bonaventura, J.; Brugarolas, M.; Farre, D.; Aguinaga, D.; Mallol, J.; Cortes, A.; Casado, V.; Lluis, C.; et al. Cocaine inhibits dopamine D2 receptor signaling via sigma-1-D2 receptor heteromers. PLoS ONE 2013, 8, e61245. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Sonoda, S.; Agui, S.; Yoshida, M.; Ohinata, K.; Yoshikawa, M. Rubiscolin-6, a delta opioid peptide derived from spinach Rubisco, has anxiolytic effect via activating sigma1 and dopamine D1 receptors. Peptides 2007, 28, 1998–2003. [Google Scholar] [CrossRef]
- Schwartz, M.A. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb. Perspect. Biol. 2010, 2, a005066. [Google Scholar] [CrossRef]
- Zhang, H.; Cuevas, J. sigma Receptor activation blocks potassium channels and depresses neuroexcitability in rat intracardiac neurons. J. Pharmacol. Exp. Ther. 2005, 313, 1387–1396. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.; Tagashira, H.; Fukunaga, K. Crucial interactions between selective serotonin uptake inhibitors and sigma-1 receptor in heart failure. J. Pharmacol. Sci. 2013, 121, 177–184. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.; Tagashira, H.; Shioda, N.; Fukunaga, K. Targeting sigma-1 receptor with fluvoxamine ameliorates pressure-overload-induced hypertrophy and dysfunctions. Expert Opin. Ther. Targets 2010, 14, 1009–1022. [Google Scholar] [CrossRef]
- Ela, C.; Barg, J.; Vogel, Z.; Hasin, Y.; Eilam, Y. Sigma receptor ligands modulate contractility, Ca++ influx and beating rate in cultured cardiac myocytes. J. Pharmacol. Exp. Ther. 1994, 269, 1300–1309. [Google Scholar]
- Dumont, M.; Lemaire, S. Interaction of 1,3-di(2-[5-3H]tolyl) guanidine with sigma 2 binding sites in rat heart membrane preparations. Eur. J. Pharmacol. 1991, 209, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Novakova, M.; Bruderova, V.; Sulova, Z.; Kopacek, J.; Lacinova, L.; Kvetnansky, R.; Vasku, A.; Kaplan, P.; Krizanova, O.; Jurkovicova, D. Modulation of expression of the sigma receptors in the heart of rat and mouse in normal and pathological conditions. Gen. Physiol. Biophys. 2007, 26, 110–117. [Google Scholar] [PubMed]
- Monassier, L.; Bousquet, P. Sigma receptors: From discovery to highlights of their implications in the cardiovascular system. Fundam. Clin. Pharmacol. 2002, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Novakova, M.; Ela, C.; Barg, J.; Vogel, Z.; Hasin, Y.; Eilam, Y. Inotropic action of sigma receptor ligands in isolated cardiac myocytes from adult rats. Eur. J. Pharmacol. 1995, 286, 19–30. [Google Scholar] [CrossRef]
- Stracina, T.; Slaninova, I.; Polanska, H.; Axmanova, M.; Olejnickova, V.; Konecny, P.; Masarik, M.; Krizanova, O.; Novakova, M. Long-Term Haloperidol Treatment Prolongs QT Interval and Increases Expression of Sigma 1 and IP3 Receptors in Guinea Pig Hearts. Tohoku J. Exp. Med. 2015, 236, 199–207. [Google Scholar] [CrossRef][Green Version]
- Novakova, M.; Sedlakova, B.; Sirova, M.; Fialova, K.; Krizanova, O. Haloperidol increases expression of the inositol 1,4,5-trisphosphate receptors in rat cardiac atria, but not in ventricles. Gen. Physiol. Biophys. 2010, 29, 381–389. [Google Scholar] [CrossRef][Green Version]
- Bhuiyan, M.S.; Fukunaga, K. Targeting sigma-1 receptor signaling by endogenous ligands for cardioprotection. Expert Opin. Ther. Targets 2011, 15, 145–155. [Google Scholar] [CrossRef]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac Fibrosis: The Fibroblast Awakens. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef]
- Sala, V.; Gallo, S.; Leo, C.; Gatti, S.; Gelb, B.D.; Crepaldi, T. Signaling to cardiac hypertrophy: Insights from human and mouse RASopathies. Mol. Med. 2012, 18, 938–947. [Google Scholar] [CrossRef]
- Ling, H.; Zhang, T.; Pereira, L.; Means, C.K.; Cheng, H.; Gu, Y.; Dalton, N.D.; Peterson, K.L.; Chen, J.; Bers, D.; et al. Requirement for Ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. J. Clin. Investig. 2009, 119, 1230–1240. [Google Scholar] [CrossRef]
- Liang, Y.; Sheikh, F. Scaffold Proteins Regulating Extracellular Regulated Kinase Function in Cardiac Hypertrophy and Disease. Front. Pharmacol. 2016, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Minamino, T.; Tsukamoto, Y.; Liao, Y.; Tsukamoto, O.; Takashima, S.; Hirata, A.; Fujita, M.; Nagamachi, Y.; Nakatani, T.; et al. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: Possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation 2004, 110, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Zhao, M.; Chen, L.; Wang, Y.; Wu, S.; Wu, W.; Liu, X. MicroRNA-297 promotes cardiomyocyte hypertrophy via targeting sigma-1 receptor. Life Sci. 2017, 175, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tagashira, H.; Zhang, C.; Lu, Y.M.; Hasegawa, H.; Kanai, H.; Han, F.; Fukunaga, K. Stimulation of sigma1-receptor restores abnormal mitochondrial Ca2+ mobilization and ATP production following cardiac hypertrophy. Biochim. Biophys. Acta 2013, 1830, 3082–3094. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Cardiac fibrosis. Cardiovasc. Res. 2021, 117, 1450–1488. [Google Scholar] [CrossRef]
- Rehman, M.; Vodret, S.; Braga, L.; Guarnaccia, C.; Celsi, F.; Rossetti, G.; Martinelli, V.; Battini, T.; Long, C.; Vukusic, K.; et al. High-throughput screening discovers antifibrotic properties of haloperidol by hindering myofibroblast activation. JCI Insight 2019, 4, e123987. [Google Scholar] [CrossRef]
- Kevil, C.G.; Goeders, N.E.; Woolard, M.D.; Bhuiyan, M.S.; Dominic, P.; Kolluru, G.K.; Arnold, C.L.; Traylor, J.G.; Orr, A.W. Methamphetamine Use and Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1739–1746. [Google Scholar] [CrossRef]
- Nguyen, E.C.; McCracken, K.A.; Liu, Y.; Pouw, B.; Matsumoto, R.R. Involvement of sigma (sigma) receptors in the acute actions of methamphetamine: Receptor binding and behavioral studies. Neuropharmacology 2005, 49, 638–645. [Google Scholar] [CrossRef]
- Sambo, D.O.; Lin, M.; Owens, A.; Lebowitz, J.J.; Richardson, B.; Jagnarine, D.A.; Shetty, M.; Rodriquez, M.; Alonge, T.; Ali, M.; et al. The sigma-1 receptor modulates methamphetamine dysregulation of dopamine neurotransmission. Nat. Commun. 2017, 8, 2228. [Google Scholar] [CrossRef]
- Naqvi, S.; Martin, K.J.; Arthur, J.S. CREB phosphorylation at Ser133 regulates transcription via distinct mechanisms downstream of cAMP and MAPK signalling. Biochem. J. 2014, 458, 469–479. [Google Scholar] [CrossRef]
- Nguyen, L.; Lucke-Wold, B.P.; Mookerjee, S.; Kaushal, N.; Matsumoto, R.R. Sigma-1 Receptors and Neurodegenerative Diseases: Towards a Hypothesis of Sigma-1 Receptors as Amplifiers of Neurodegeneration and Neuroprotection. Adv. Exp. Med. Biol. 2017, 964, 133–152. [Google Scholar] [CrossRef]
- Harshfield, E.L.; Pennells, L.; Schwartz, J.E.; Willeit, P.; Kaptoge, S.; Bell, S.; Shaffer, J.A.; Bolton, T.; Spackman, S.; Wassertheil-Smoller, S.; et al. Association Between Depressive Symptoms and Incident Cardiovascular Diseases. JAMA 2020, 324, 2396–2405. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Liu, X.; Qu, C.; Zhang, C.; Fo, Y.; Guo, Y.; Chen, X.; Shi, S.; Yang, B. Chronic inhibition of the sigma-1 receptor exacerbates atrial fibrillation susceptibility in rats by promoting atrial remodeling. Life Sci. 2019, 235, 116837. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qu, C.; Yang, H.; Shi, S.; Zhang, C.; Zhang, Y.; Liang, J.; Yang, B. Chronic stimulation of the sigma-1 receptor ameliorates autonomic nerve dysfunction and atrial fibrillation susceptibility in a rat model of depression. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1521–H1531. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qu, C.; Shi, S.; Ye, T.; Wang, L.; Liu, S.; Zhang, C.; Liang, J.; Hu, D.; Yang, B. The Reversal Effect of Sigma-1 Receptor (S1R) Agonist, SA4503, on Atrial Fibrillation After Depression and Its Underlying Mechanism. Front. Physiol. 2019, 10, 1346. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Hirooka, Y.; Matsukawa, R.; Nakano, M.; Sunagawa, K. Decreased brain sigma-1 receptor contributes to the relationship between heart failure and depression. Cardiovasc. Res. 2012, 93, 33–40. [Google Scholar] [CrossRef]
- Kaufman, R.J. Orchestrating the unfolded protein response in health and disease. J. Clin. Investig. 2002, 110, 1389–1398. [Google Scholar] [CrossRef]
- Alam, S.; Abdullah, C.S.; Aishwarya, R.; Orr, A.W.; Traylor, J.; Miriyala, S.; Panchatcharam, M.; Pattillo, C.B.; Bhuiyan, M.S. Sigmar1 regulates endoplasmic reticulum stress-induced C/EBP-homologous protein expression in cardiomyocytes. BioSci. Rep. 2017, 37, BSR20170898. [Google Scholar] [CrossRef]
- Magadum, A.; Kishore, R. Cardiovascular Manifestations of COVID-19 Infection. Cells 2020, 9, 2508. [Google Scholar] [CrossRef]
- Vela, J.M. Repurposing Sigma-1 Receptor Ligands for COVID-19 Therapy? Front. Pharmacol. 2020, 11, 582310. [Google Scholar] [CrossRef]
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; White, K.M.; O’Meara, M.J.; Rezelj, V.V.; Guo, J.Z.; Swaney, D.L.; et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020, 583, 459–468. [Google Scholar] [CrossRef]
- Hellewell, S.B.; Bruce, A.; Feinstein, G.; Orringer, J.; Williams, W.; Bowen, W.D. Rat liver and kidney contain high densities of sigma 1 and sigma 2 receptors: Characterization by ligand binding and photoaffinity labeling. Eur. J. Pharmacol. 1994, 268, 9–18. [Google Scholar] [CrossRef]
- Milardovic, I.; Vitlov Uljevic, M.; Vukojevic, K.; Kostic, S.; Filipovic, N. Renal expression of sigma 1 receptors in diabetic rats. Acta Histochem. 2020, 122, 151580. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Naura, A.S.; Singla, S.K. A deleterious interplay between endoplasmic reticulum stress and its functional linkage to mitochondria in nephrolithiasis. Free. Radic. Biol. Med. 2021, 168, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, S.; Fukunaga, K. Stimulation of Sigma-1 receptor by dehydroepiandrosterone ameliorates hypertension-induced kidney hypertrophy in ovariectomized rats. Exp. Biol. Med. 2010, 235, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Melo, Z.; Gutierrez-Mercado, Y.K.; Garcia-Martinez, D.; Portilla-de-Buen, E.; Canales-Aguirre, A.A.; Gonzalez-Gonzalez, R.; Franco-Acevedo, A.; Palomino, J.; Echavarria, R. Sex-dependent mechanisms involved in renal tolerance to ischemia-reperfusion: Role of inflammation and histone H3 citrullination. Transpl. Immunol. 2020, 63, 101331. [Google Scholar] [CrossRef]
- Ye, N.; Qin, W.; Tian, S.; Xu, Q.; Wold, E.A.; Zhou, J.; Zhen, X.C. Small Molecules Selectively Targeting Sigma-1 Receptor for the Treatment of Neurological Diseases. J. Med. Chem. 2020, 63, 15187–15217. [Google Scholar] [CrossRef]
- Jiang, W.; Krishnan, R.; Kuchibhatla, M.; Cuffe, M.S.; Martsberger, C.; Arias, R.M.; O’Connor, C.M.; SADHART-CHF Investigators. Characteristics of depression remission and its relation with cardiovascular outcome among patients with chronic heart failure (from the SADHART-CHF Study). Am. J. Cardiol. 2011, 107, 545–551. [Google Scholar] [CrossRef]
- Jiang, W.; O’Connor, C.; Silva, S.G.; Kuchibhatla, M.; Cuffe, M.S.; Callwood, D.D.; Zakhary, B.; Henke, E.; Arias, R.M.; Krishnan, R.; et al. Safety and efficacy of sertraline for depression in patients with CHF (SADHART-CHF): A randomized, double-blind, placebo-controlled trial of sertraline for major depression with congestive heart failure. Am. Heart J. 2008, 156, 437–444. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Jiang, W.; Kuchibhatla, M.; Silva, S.G.; Cuffe, M.S.; Callwood, D.D.; Zakhary, B.; Stough, W.G.; Arias, R.M.; Rivelli, S.K.; et al. Safety and efficacy of sertraline for depression in patients with heart failure: Results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J. Am. Coll. Cardiol. 2010, 56, 692–699. [Google Scholar] [CrossRef]
- Serebruany, V.L.; Glassman, A.H.; Malinin, A.I.; Nemeroff, C.B.; Musselman, D.L.; van Zyl, L.T.; Finkel, M.S.; Krishnan, K.R.; Gaffney, M.; Harrison, W.; et al. Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acute coronary events: The Sertraline AntiDepressant Heart Attack Randomized Trial (SADHART) Platelet Substudy. Circulation 2003, 108, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Serebruany, V.L.; Suckow, R.F.; Cooper, T.B.; O’Connor, C.M.; Malinin, A.I.; Krishnan, K.R.; van Zyl, L.T.; Lekht, V.; Glassman, A.H.; Sertraline Antidepressant Heart Attack Randomized Trial. Relationship between release of platelet/endothelial biomarkers and plasma levels of sertraline and N-desmethylsertraline in acute coronary syndrome patients receiving SSRI treatment for depression. Am. J. Psychiatry 2005, 162, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Swenson, J.R.; O’Connor, C.M.; Barton, D.; Van Zyl, L.T.; Swedberg, K.; Forman, L.M.; Gaffney, M.; Glassman, A.H.; Sertraline Antidepressant Heart Attack Randomized Trial (SADHART) Group. Influence of depression and effect of treatment with sertraline on quality of life after hospitalization for acute coronary syndrome. Am. J. Cardiol. 2003, 92, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.L.; Fiuzat, M.; Kuchibhatla, M.; Krishnan, R.; O’Connor, C.M.; Jiang, W.; SADHART-CHF Investigators. Health status and depression remission in patients with chronic heart failure: Patient-reported outcomes from the SADHART-CHF trial. Circ. Heart Fail. 2012, 5, 688–692. [Google Scholar] [CrossRef]
- Xiong, G.L.; Prybol, K.; Boyle, S.H.; Hall, R.; Streilein, R.D.; Steffens, D.C.; Krishnan, R.; Rogers, J.G.; O’Connor, C.M.; Jiang, W.; et al. Inflammation Markers and Major Depressive Disorder in Patients With Chronic Heart Failure: Results From the Sertraline Against Depression and Heart Disease in Chronic Heart Failure Study. Psychosom. Med. 2015, 77, 808–815. [Google Scholar] [CrossRef]
- Urfer, R.; Moebius, H.J.; Skoloudik, D.; Santamarina, E.; Sato, W.; Mita, S.; Muir, K.W.; Cutamesine Stroke Recovery Study Group. Phase II trial of the Sigma-1 receptor agonist cutamesine (SA4503) for recovery enhancement after acute ischemic stroke. Stroke 2014, 45, 3304–3310. [Google Scholar] [CrossRef]
- Rossini, D.; Serretti, A.; Franchini, L.; Mandelli, L.; Smeraldi, E.; De Ronchi, D.; Zanardi, R. Sertraline versus fluvoxamine in the treatment of elderly patients with major depression: A double-blind, randomized trial. J. Clin. Psychopharmacol. 2005, 25, 471–475. [Google Scholar] [CrossRef]
- Strik, J.J.; Honig, A.; Lousberg, R.; Cheriex, E.C.; Van Praag, H.M. Cardiac side-effects of two selective serotonin reuptake inhibitors in middle-aged and elderly depressed patients. Int. Clin. Psychopharmacol. 1998, 13, 263–267. [Google Scholar] [CrossRef]
- Lekakis, J.; Ikonomidis, I.; Papoutsi, Z.; Moutsatsou, P.; Nikolaou, M.; Parissis, J.; Kremastinos, D.T. Selective serotonin re-uptake inhibitors decrease the cytokine-induced endothelial adhesion molecule expression, the endothelial adhesiveness to monocytes and the circulating levels of vascular adhesion molecules. Int. J. Cardiol. 2010, 139, 150–158. [Google Scholar] [CrossRef]
- Orlando, R.; De Martin, S.; Andrighetto, L.; Floreani, M.; Palatini, P. Fluvoxamine pharmacokinetics in healthy elderly subjects and elderly patients with chronic heart failure. Br. J. Clin. Pharmacol. 2010, 69, 279–286. [Google Scholar] [CrossRef]
- Lenze, E.J.; Mattar, C.; Zorumski, C.F.; Stevens, A.; Schweiger, J.; Nicol, G.E.; Miller, J.P.; Yang, L.; Yingling, M.; Avidan, M.S.; et al. Fluvoxamine vs Placebo and Clinical Deterioration in Outpatients With Symptomatic COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 2292–2300. [Google Scholar] [CrossRef] [PubMed]
- Kohler, C.A.; Freitas, T.H.; Stubbs, B.; Maes, M.; Solmi, M.; Veronese, N.; de Andrade, N.Q.; Morris, G.; Fernandes, B.S.; Brunoni, A.R.; et al. Peripheral Alterations in Cytokine and Chemokine Levels After Antidepressant Drug Treatment for Major Depressive Disorder: Systematic Review and Meta-Analysis. Mol. Neurobiol. 2018, 55, 4195–4206. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K. Repurposing of CNS drugs to treat COVID-19 infection: Targeting the sigma-1 receptor. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 249–258. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Suzuki, T.; Hashimoto, K. Mechanisms of action of fluvoxamine for COVID-19: A historical review. Mol. Psychiatry 2022, 27, 1898–1907. [Google Scholar] [CrossRef]
- Seftel, D.; Boulware, D.R. Prospective Cohort of Fluvoxamine for Early Treatment of Coronavirus Disease 19. Open Forum. Infect. Dis. 2021, 8, ofab050. [Google Scholar] [CrossRef] [PubMed]
- Reis, G.; Dos Santos Moreira-Silva, E.A.; Silva, D.C.M.; Thabane, L.; Milagres, A.C.; Ferreira, T.S.; Dos Santos, C.V.Q.; de Souza Campos, V.H.; Nogueira, A.M.R.; de Almeida, A.; et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: The TOGETHER randomised, platform clinical trial. Lancet Glob. Health 2022, 10, e42–e51. [Google Scholar] [CrossRef]
- Calusic, M.; Marcec, R.; Luksa, L.; Jurkovic, I.; Kovac, N.; Mihaljevic, S.; Likic, R. Safety and efficacy of fluvoxamine in COVID-19 ICU patients: An open label, prospective cohort trial with matched controls. Br. J. Clin. Pharmacol. 2022, 88, 2065–2073. [Google Scholar] [CrossRef]
- Oskotsky, T.; Maric, I.; Tang, A.; Oskotsky, B.; Wong, R.J.; Aghaeepour, N.; Sirota, M.; Stevenson, D.K. Mortality Risk Among Patients With COVID-19 Prescribed Selective Serotonin Reuptake Inhibitor Antidepressants. JAMA Netw. Open 2021, 4, e2133090. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, X.; Gillette, T.G.; Deng, Y.; Wang, Z.V. Unfolded Protein Response as a Therapeutic Target in Cardiovascular Disease. Curr. Top. Med. Chem. 2019, 19, 1902–1917. [Google Scholar] [CrossRef]
- Shu, S.; Zhu, J.; Liu, Z.; Tang, C.; Cai, J.; Dong, Z. Endoplasmic reticulum stress is activated in post-ischemic kidneys to promote chronic kidney disease. EBioMedicine 2018, 37, 269–280. [Google Scholar] [CrossRef]
- Wang, Z.V.; Deng, Y.; Gao, N.; Pedrozo, Z.; Li, D.L.; Morales, C.R.; Criollo, A.; Luo, X.; Tan, W.; Jiang, N.; et al. Spliced X-box binding protein 1 couples the unfolded protein response to hexosamine biosynthetic pathway. Cell 2014, 156, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.; Hossain, G.S.; Kocerha, J. The Potential for microRNA Therapeutics and Clinical Research. Front. Genet. 2019, 10, 478. [Google Scholar] [CrossRef] [PubMed]
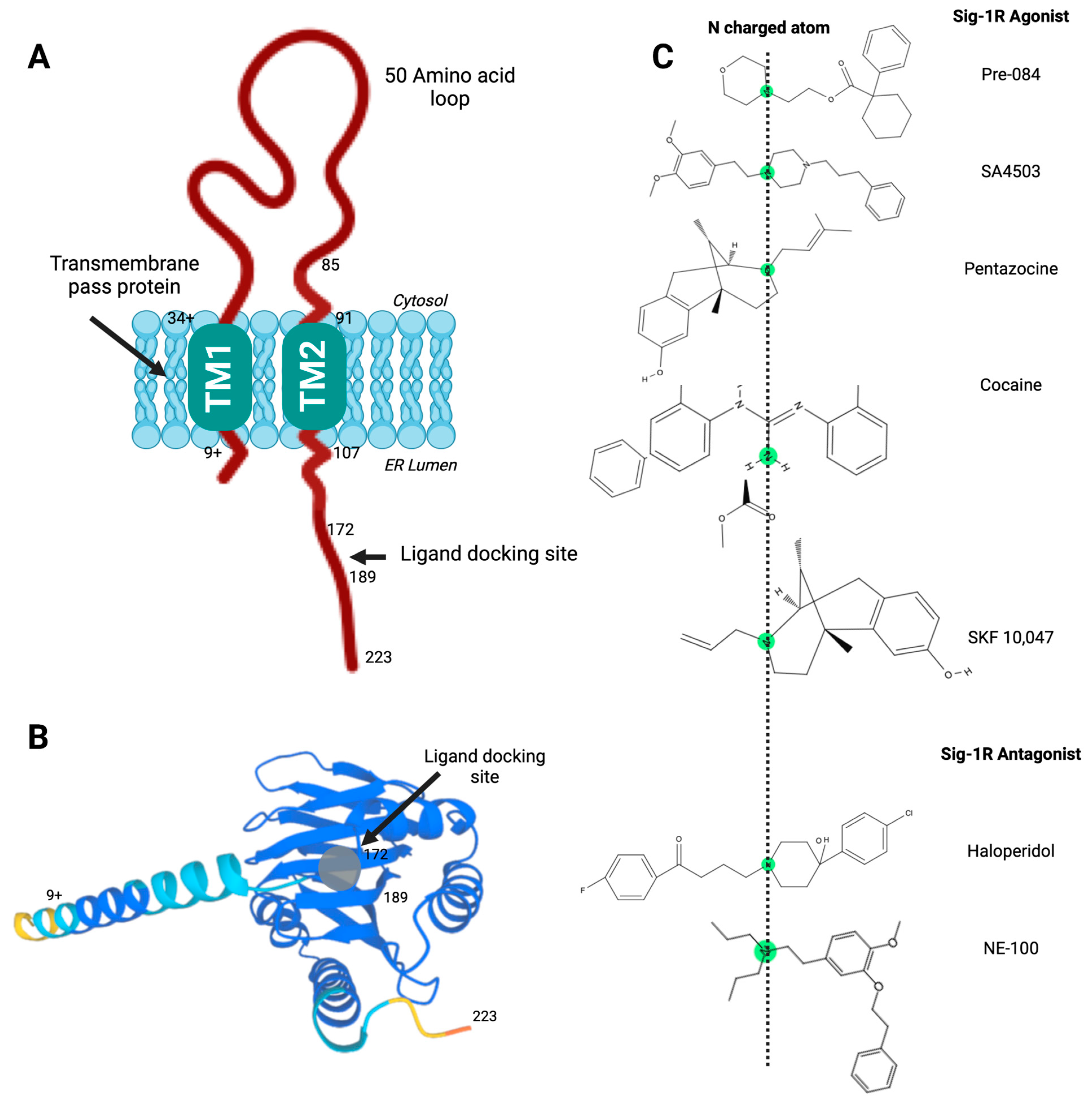

| Ligand Type | IUPAC Name | Name | Classification | CAS |
|---|---|---|---|---|
| Natural Agonist | [(3S,8S,9S,10R,13S,14S,17S)-17-acetyl-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl] hydrogen sulfate | PREG-S | Steroid sulfate | 1247-64-9 |
| (3S,8R,9S,10R,13S,14S)-3-hydroxy-10,13-dimethyl-1,2,3,4,7,8,9,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-17-one | DHEA | Androstanoid | 53-43-0 | |
| (8S,9S,10R,13S,14S,17S)-17-acetyl-10,13-dimethyl-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-one | Progesterone | C21-steroid hormone | 57-83-0 | |
| Synthetic Agonist | 1-[2-(3,4-dimethoxyphenyl)ethyl]-4-(3-phenylpropyl)piperazine;dihydrochloride | SA4503 * | Piperazines derivative | 165377-44-6 |
| 2-morpholin-4-ylethyl 1-phenylcyclohexane-1-carboxylate | PRE-084 | Phencyclidine derivative | 138847-85-5 | |
| 1,2-bis(2-methylphenyl)guanidine | DTG | Guanidine derivative | 97-39-2 | |
| (1R,9R,13R)-1,13-dimethyl-10-(3-methylbut-2-enyl)-10-azatricyclo [7.3.1.02,7]trideca-2(7),3,5-trien-4-ol | Pentazocine * | Synthetic opioid | 359-83-1 | |
| (1R,9R,13R)-1,13-dimethyl-10-prop-2-enyl-10-azatricyclo [7.3.1.02,7]trideca-2(7),3,5-trien-4-ol | Alazocine, SKF-10047 | Synthetic opioid | 14198-28-8 | |
| 4-(3-methylsulfonylphenyl)-1-propylpiperidine | Pridopidine * | Phenylpiperidine | 346688-38-8 | |
| 1-(2,2-diphenyloxolan-3-yl)-N,N-dimethylmethanamine;hydrochloride | ANAVEX 2-73 *, AE-37 hydrochloride | Blarcamesine hydrochloride | 195615-84-0 | |
| 1-[3-[2-(1-benzothiophen-5-yl)ethoxy]propyl]azetidin-3-ol;(Z)-but-2-enedioic acid | T-817MA * | Edonerpic maleate | 519187-97-4 | |
| methyl (1R,2R,3S,5S)-3-benzoyloxy-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate | Cocaine | Tropane alkaloid | 50-36-2 | |
| Synthetic Antagonist | 4-[4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl]-1-(4-fluorophenyl)butan-1-one | Haloperidol * | Phenylbutylpiperadine derivative | 52-86-8 |
| 4-Methoxy-3-(2-phenylethoxy)-N,N-dipropylbenzeneethanamine hydrochloride | NE-100 | Hydrochloride | 149409-57-4 | |
| 4-[2-(5-methyl-1-naphthalen-2-ylpyrazol-3-yl)oxyethyl]morpholine | S1RA *, E-52862 | Hydrochloride polymorph and solvate | 878141-96-9 | |
| N-(2-(3,4-Dichlorophenyl)ethyl)-N-methyl-2-(dimethylamino)ethylamine | BD-1047 | Primary amine | 138356-20-4 | |
| 1-[2-(3,4-Dichlorophenyl)ethyl]-4-methylpiperazine | BD-1063 | Primary amine | 150208-28-9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munguia-Galaviz, F.J.; Miranda-Diaz, A.G.; Cardenas-Sosa, M.A.; Echavarria, R. Sigma-1 Receptor Signaling: In Search of New Therapeutic Alternatives for Cardiovascular and Renal Diseases. Int. J. Mol. Sci. 2023, 24, 1997. https://doi.org/10.3390/ijms24031997
Munguia-Galaviz FJ, Miranda-Diaz AG, Cardenas-Sosa MA, Echavarria R. Sigma-1 Receptor Signaling: In Search of New Therapeutic Alternatives for Cardiovascular and Renal Diseases. International Journal of Molecular Sciences. 2023; 24(3):1997. https://doi.org/10.3390/ijms24031997
Chicago/Turabian StyleMunguia-Galaviz, Francisco Javier, Alejandra Guillermina Miranda-Diaz, Miguel Alejandro Cardenas-Sosa, and Raquel Echavarria. 2023. "Sigma-1 Receptor Signaling: In Search of New Therapeutic Alternatives for Cardiovascular and Renal Diseases" International Journal of Molecular Sciences 24, no. 3: 1997. https://doi.org/10.3390/ijms24031997
APA StyleMunguia-Galaviz, F. J., Miranda-Diaz, A. G., Cardenas-Sosa, M. A., & Echavarria, R. (2023). Sigma-1 Receptor Signaling: In Search of New Therapeutic Alternatives for Cardiovascular and Renal Diseases. International Journal of Molecular Sciences, 24(3), 1997. https://doi.org/10.3390/ijms24031997