Real-Time Impedance-Based Monitoring of the Growth and Inhibition of Osteomyelitis Biofilm Pathogen Staphylococcus aureus Treated with Novel Bisphosphonate-Fluoroquinolone Antimicrobial Conjugates
Abstract
1. Introduction
2. Results
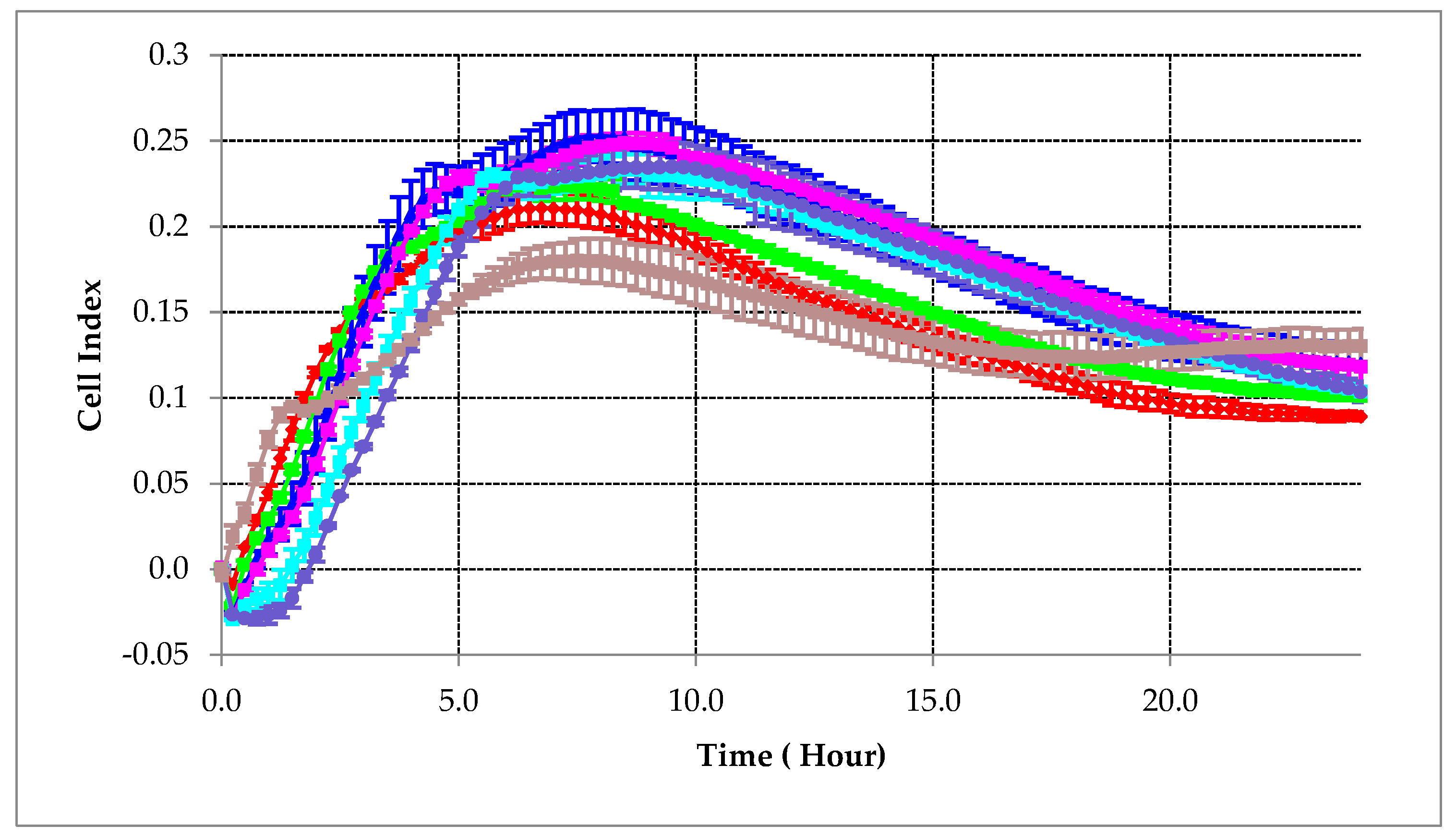
2.1. Cell Suspension Concentration Optimization
2.2. Evaluating the Effectiveness of Testing Compounds on Biofilm Growth Inhibition
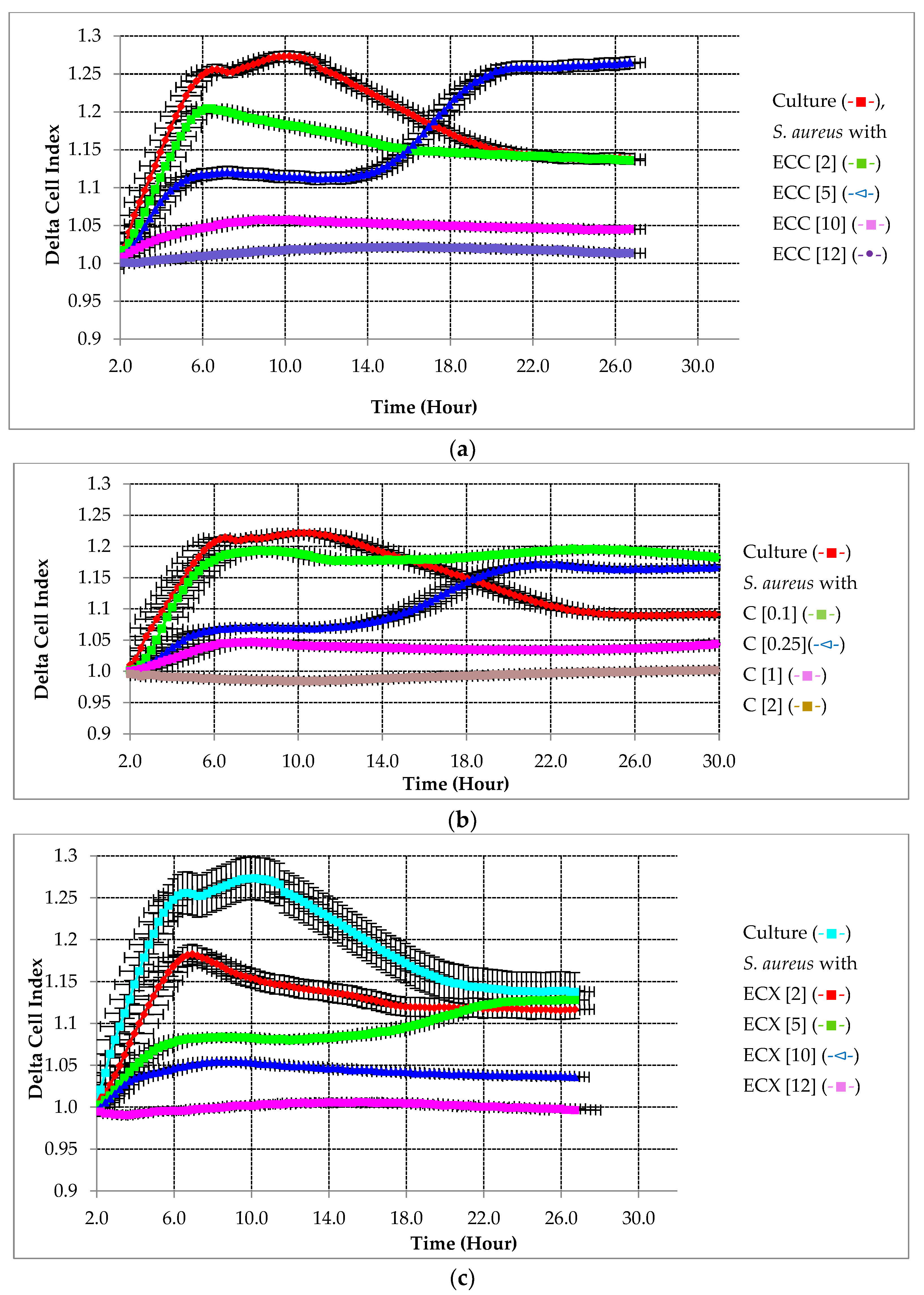
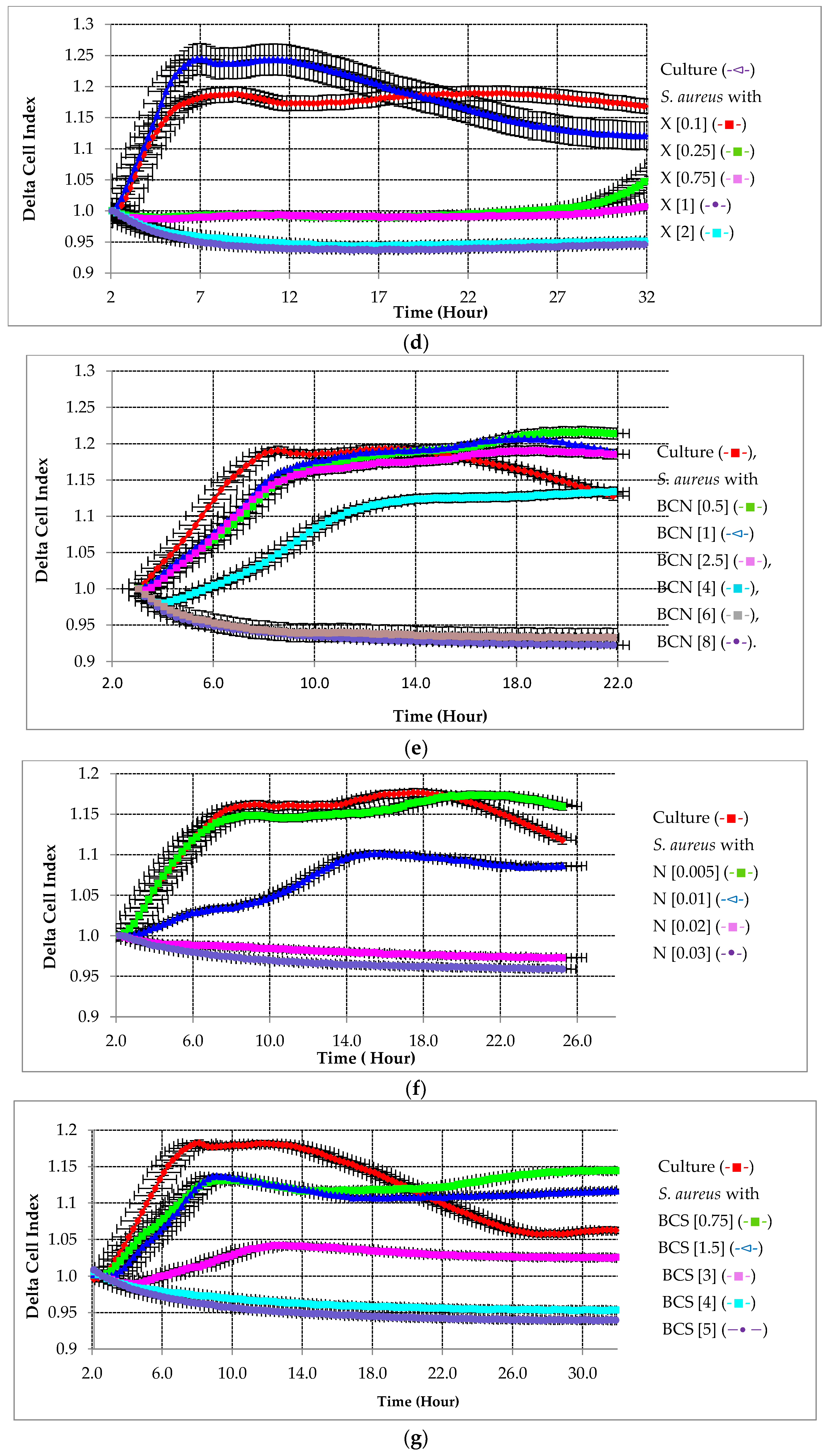
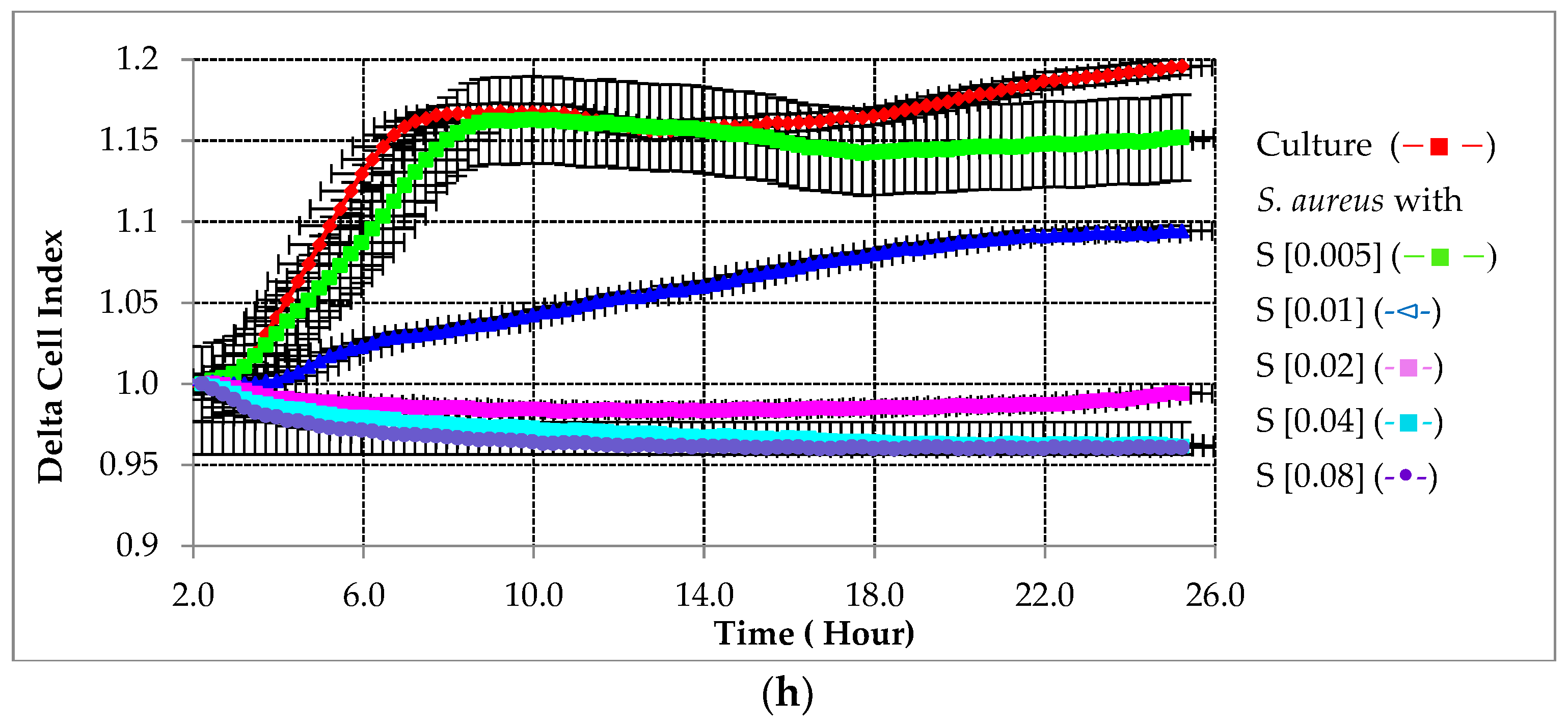
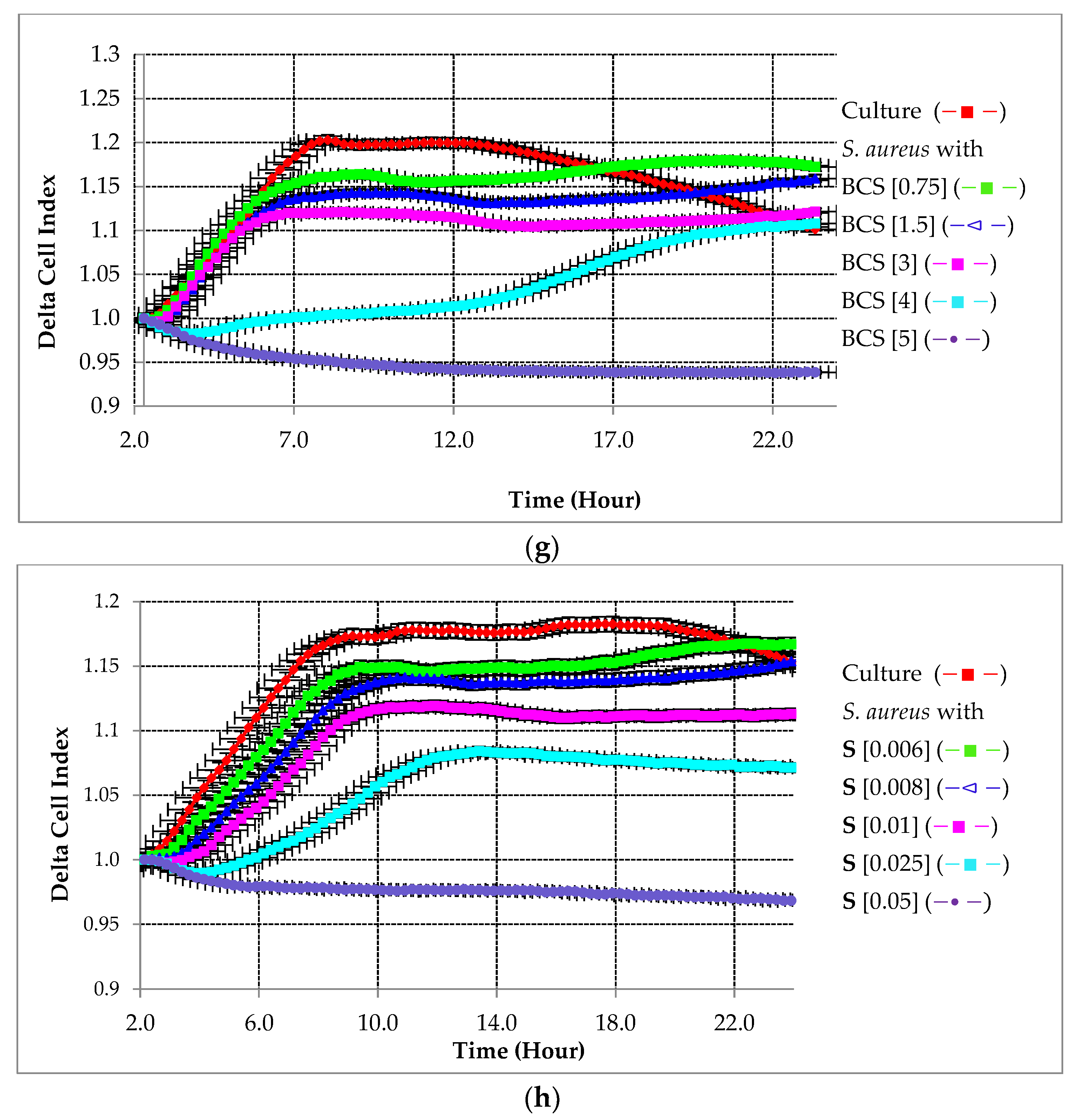
2.2.1. Preventative Effect of Testing Compounds on Biofilm Growth in the Absence of HA
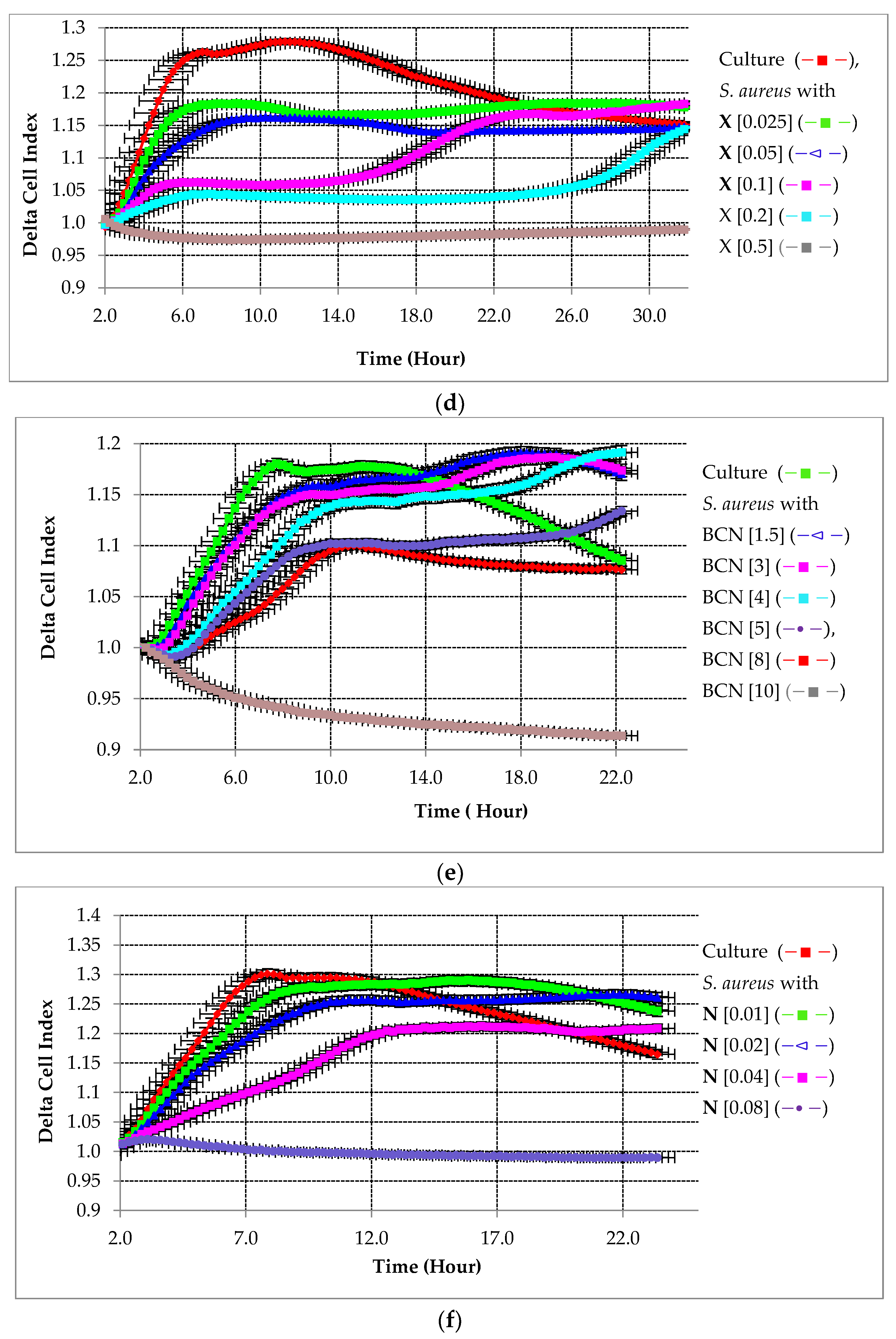
2.2.2. Preventative Effect of Testing Compounds on Biofilm Growth in the Presence of HA
2.3. Calculation of the MIC50
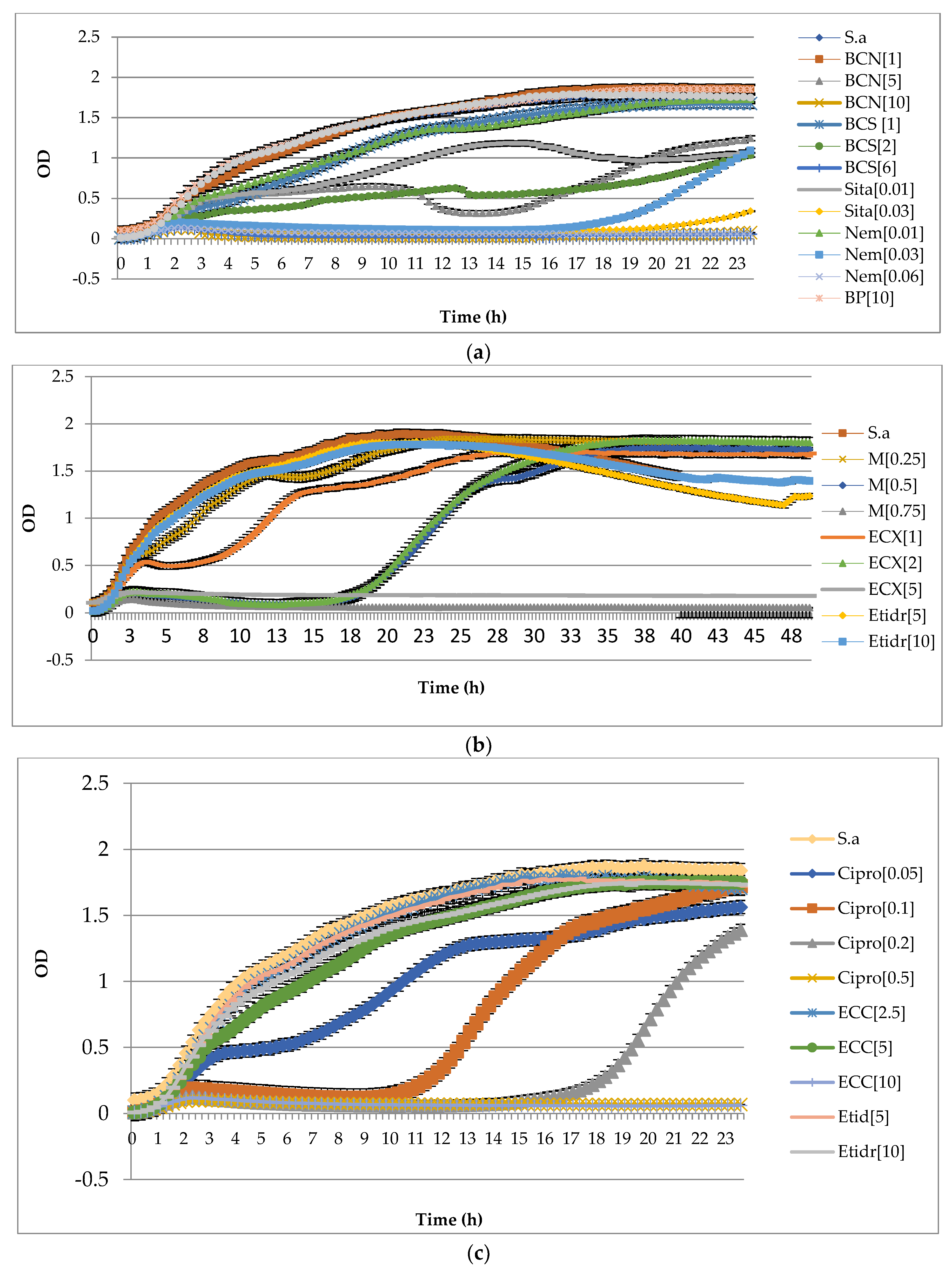
2.4. Bio-Screen Study of Biofilm Growth
2.5. Affinity of Tested Compounds to HA
2.6. Preventative Antimicrobial Assays through Spectroscopic Analysis
3. Discussion
4. Materials and Methods
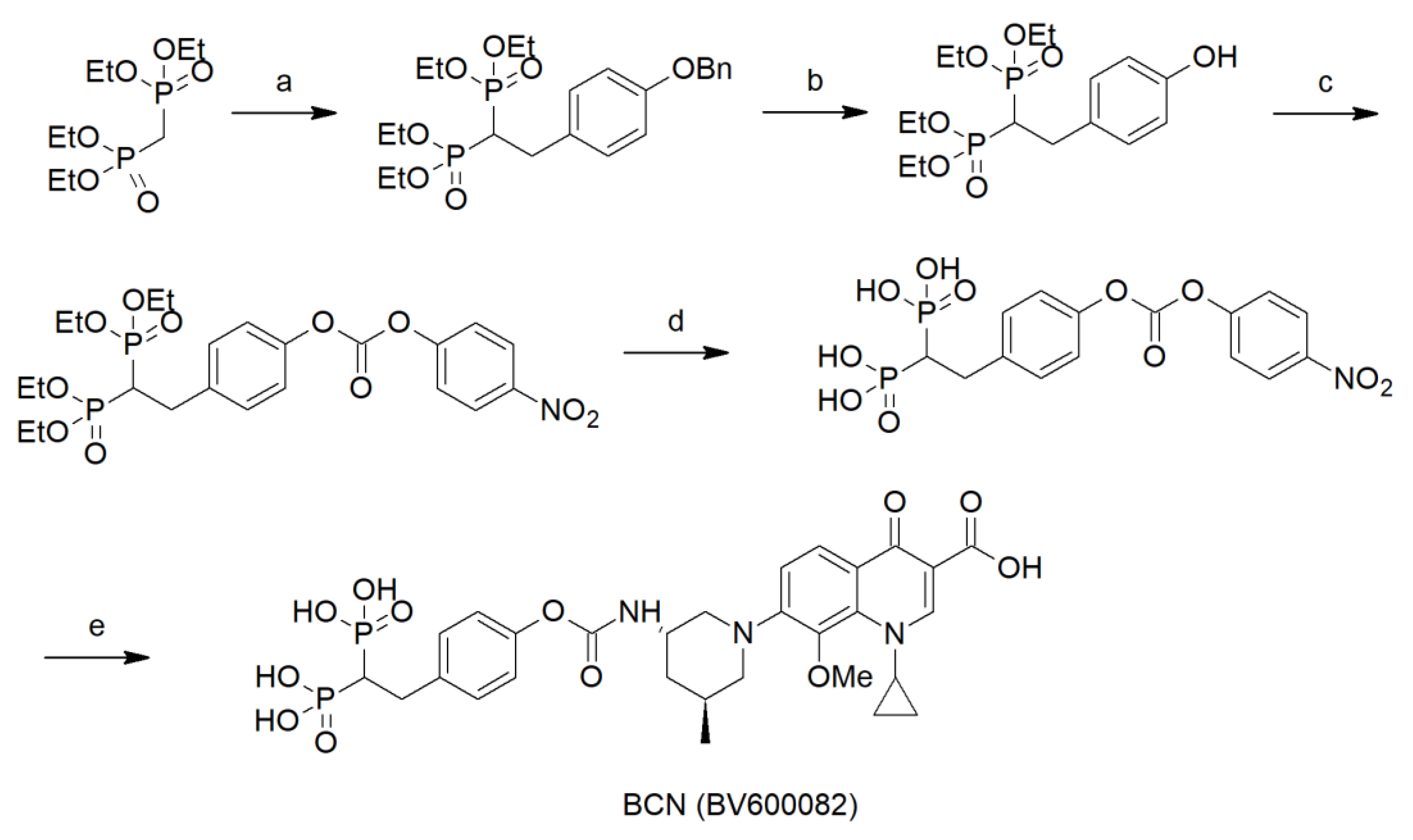
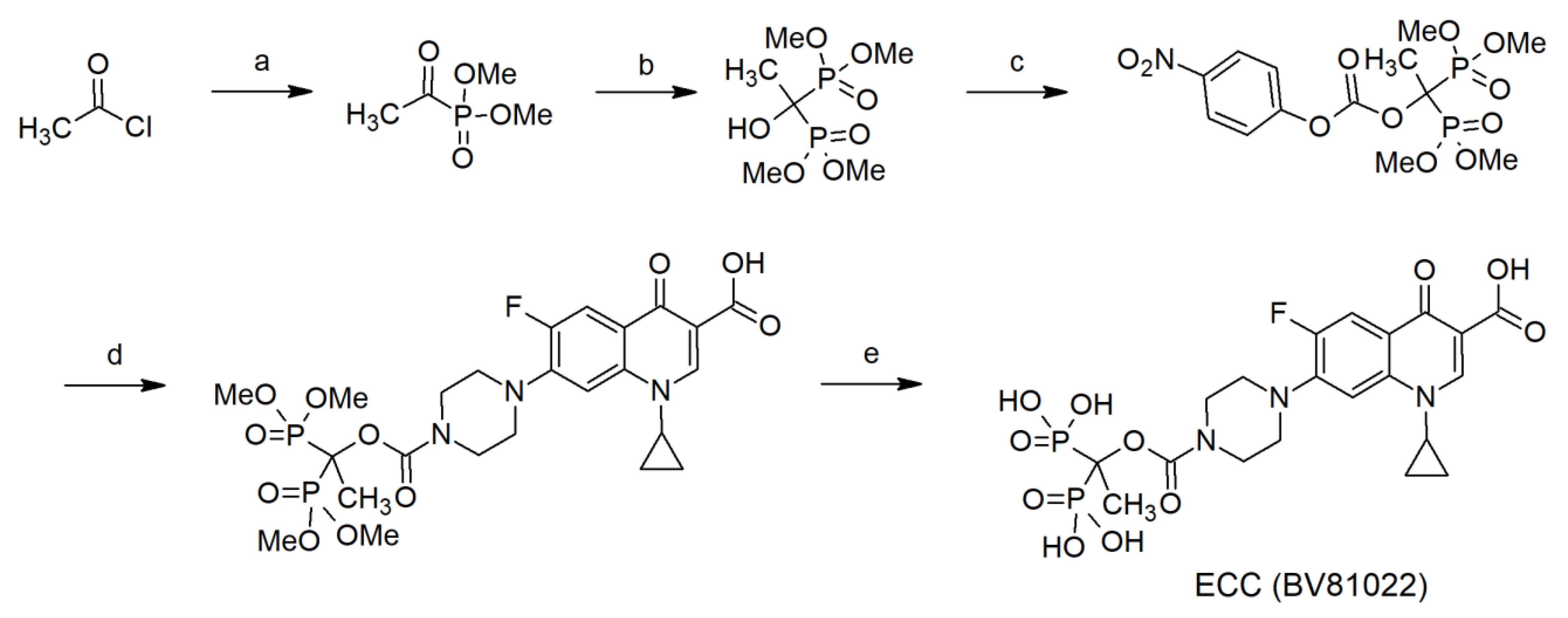
4.1. Synthesis
4.2. Experimental Strain and Culture Conditions
4.3. Antimicrobials Tested
4.4. Monitoring of Biofilm Formation in Real Time
4.5. Optimizing the Cell Suspension Concentration
4.6. Monitoring the Biofilm Inhibition Effect of BP-Antibiotic Conjugates and the Parent Antibiotics
4.7. Minimum Inhibitory Concentration Measurement
4.8. Affinity of BP-Antibiotic Conjugates to Hydroxyapatite
4.9. Infection Preventative Experiment in the Presence of HA via Real-Time Analysis
4.10. Growth Analysis Using Bio-Screen C Automated Microbiology Growth Curve Analysis System
4.11. Preventative Antimicrobial Assays through Spectroscopic Analysis
4.12. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Bridier, A.; Sánchez-Vizuete, P.; Guilbaud, M.; Piard, J.C.; Naitali, M.; Briandet, R. Biofilm-associated persistence of food-borne pathogens. Food Microbiol. 2015, 45, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. APMIS Suppl. 2013, 121, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Sochocka, M.; Zwolińska, K.; Leszek, J. The infectious etiology of Alzheimer’s disease. Curr Neuropharmacol 2017, 15, 996–1009. [Google Scholar] [CrossRef]
- Brady, R.A.; Leid, J.G.; Calhoun, J.H.; Costerton, J.W.; Shirtliff, M.E. Osteomyelitis and the role of biofilms in chronic infection. FEMS Immunol. Med. Microbiol. 2008, 52, 13–22. [Google Scholar] [CrossRef]
- Lew, D.P.; Waldvogel, F.A. Osteomyelitis. Lancet 2004, 364, 369–379. [Google Scholar] [CrossRef]
- Junka, A.; Szymczyk, P.; Ziółkowski, G.; Karuga-Kuzniewska, E.; Smutnicka, D.; Bil-Lula, I.; Bartoszewicz, M.; Mahabady, S.; Sedghizadeh, P.P. Bad to the Bone: On in vitro and ex vivo microbial biofilm ability to directly destroy colonized bone surfaces without participation of host immunity or osteoclastogenesis. PLoS ONE 2017, 11, 12. [Google Scholar] [CrossRef]
- Adjei-Sowah, E.; Peng, Y.; Weeks, J.; Jonason, J.H.; de Mesy Bentley, K.; Masters, E.; Morita, Y.; Muthukrishnan, G.; Cherian, P.; Hu, X.E.; et al. Development of bisphosphonate-conjugated antibiotics to overcome pharmacodynamic limitations of local therapy: Initial results with carbamate linked sitafloxacin and tedizolid. Antibiotics 2021, 10, 732. [Google Scholar] [CrossRef]
- Stoodley, P.; Ehrlich, G.D.; Sedghizadeh, P.P.; Hall-Stoodley, L.; Baratz, M.E.; Altman, D.T.; Sotereanos, N.G.; Costerton, J.W.; Demeo, P. Orthopaedic biofilm infections. Curr. Orthop. Pract. 2011, 22, 558–563. [Google Scholar] [CrossRef]
- Hoiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial bio-films. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef]
- Kim, B.N.; Kim, E.S.; Oh, M.D. Oral antibiotic treatment of staphylococcal bone and joint infections in adults. J. Antimicrob. Chemother. 2014, 69, 309–322. [Google Scholar] [CrossRef]
- Sedghizadeh, P.P.; Sun, S.; Junka, A.F.; Richard, E.; Sadrerafi, K.; Mahabady, S.; Bakhshalian, N.; Tjokro, N.; Bartoszewicz, M.; Oleksy, M.; et al. Design, Synthesis, and Antimicrobial Evaluation of a Novel Bone-Targeting Bisphosphonate-Ciprofloxacin Conjugate for the Treatment of Osteomyelitis Biofilms. J. Med. Chem. 2017, 60, 2326–2343. [Google Scholar] [CrossRef]
- Russell, R.; Graham, G. BPs: The first 40 years. Bone 2011, 49, 2–19. [Google Scholar] [CrossRef]
- Hengst, V.C.; Kissel, O.T.; Storm, G. Bone targeting potential of BP-targeted liposomes: Preparation, characterization and hydroxyapatite binding in vitro. Int. J. Pharmacol. 2007, 331, 224–227. [Google Scholar] [CrossRef]
- Coenye, T.; Nelis, H.J. In Vitro and in Vivo model systems to study microbial biofilm formation. J. Microbiol. Methods 2010, 83, 89–105. [Google Scholar] [CrossRef]
- Ferrer, M.D.; Rodriguez, J.C.; Alvarez, L.; Artacho, A.; Royo, G.; Mira, A. Effect of antibiotics on biofilm inhibition and induction measured by real-time cell analysis. J. Appl. Microbiol. 2016, 122, 640–650. [Google Scholar] [CrossRef]
- Nicholson, D.A.; Vaughn, H.A. General method of preparation of tetramethyl alkyl-1-hydroxy-1, l-diphosphonates. J. Org. Chem. 1971, 36, 3843–3845. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, L.; Tao, J.; Srinivasan, V.; Boyce, B.F.; Ebetino, F.H.; Oyajobi, B.O.; Boeckman, R.K., Jr.; Xing, L. Synthesis of a Bone-Targeted Bortezomib with In Vivo Anti-Myeloma Effects in Mice. Pharmaceutics 2018, 10, 154. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Hidalgo-Cantabrana, C.; Rodríguez, A.; García, P.; Ruas-Madiedo, P. Monitoring in real time the formation and removal of biofilms from clinical related pathogens using an impedance-based technology. PLoS ONE 2016, 11, e0163966. [Google Scholar] [CrossRef]
- Ren, Y.; Xue, T.; Rainbolt, J.; de Mesy Bentley, K.L.; Galloway, C.A.; Liu, Y.; Cherian, P.; Neighbors, J.; Hofstee, M.I.; Ebetino, F.H.; et al. Efficacy of Bisphosphonate-Conjugated Sitafloxacin in a Murine Model of S. aureus Osteomyelitis: Evidence of “Target & Release” Kinetics and Killing of Bacteria within Canaliculi. Front. Cell. Infect. Microbiol. 2022, 12, 910970. [Google Scholar]
- Zimmerli, W.; Sendi, P. Orthopaedic biofilm infections. APMIS 2017, 125, 353–364. [Google Scholar] [CrossRef]
- Lamkhao, S.; Phaya, M.; Jansakun, C.; Chanadet, N.; Thongkorn, K.; Rujijanagul, G.; Bangrak, P.; Randorn, C. Synthesis of hydroxyapatite with antibacterial properties using microwave-assisted combustion method. Sci. Rep. 2019, 9, 4015. [Google Scholar] [CrossRef]


| Antimicrobial MIC50 [µg/mL] | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X | X + HA | C | C + HA | ECC | ECC + HA | ECX | ECX + HA | S | S + HA | N | N + HA | BCN | BCN + HA | BCS | BCS + HA |
| 0.11 | 0.09 | 0.09 | 0.24 | 4.88 | 28 | 5.10 | 9.6 | 0.01 | 0.02 | 0.01 | 0.04 | 1.48 | 3.11 | 4.21 | 7.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedghizadeh, P.P.; Cherian, P.; Roshandel, S.; Tjokro, N.; Chen, C.; Junka, A.F.; Hu, E.; Neighbors, J.; Pawlak, J.; Russell, R.G.G.; et al. Real-Time Impedance-Based Monitoring of the Growth and Inhibition of Osteomyelitis Biofilm Pathogen Staphylococcus aureus Treated with Novel Bisphosphonate-Fluoroquinolone Antimicrobial Conjugates. Int. J. Mol. Sci. 2023, 24, 1985. https://doi.org/10.3390/ijms24031985
Sedghizadeh PP, Cherian P, Roshandel S, Tjokro N, Chen C, Junka AF, Hu E, Neighbors J, Pawlak J, Russell RGG, et al. Real-Time Impedance-Based Monitoring of the Growth and Inhibition of Osteomyelitis Biofilm Pathogen Staphylococcus aureus Treated with Novel Bisphosphonate-Fluoroquinolone Antimicrobial Conjugates. International Journal of Molecular Sciences. 2023; 24(3):1985. https://doi.org/10.3390/ijms24031985
Chicago/Turabian StyleSedghizadeh, Parish P., Philip Cherian, Sahar Roshandel, Natalia Tjokro, Casey Chen, Adam F. Junka, Eric Hu, Jeffrey Neighbors, Jacek Pawlak, R. Graham G. Russell, and et al. 2023. "Real-Time Impedance-Based Monitoring of the Growth and Inhibition of Osteomyelitis Biofilm Pathogen Staphylococcus aureus Treated with Novel Bisphosphonate-Fluoroquinolone Antimicrobial Conjugates" International Journal of Molecular Sciences 24, no. 3: 1985. https://doi.org/10.3390/ijms24031985
APA StyleSedghizadeh, P. P., Cherian, P., Roshandel, S., Tjokro, N., Chen, C., Junka, A. F., Hu, E., Neighbors, J., Pawlak, J., Russell, R. G. G., McKenna, C. E., Ebetino, F. H., Sun, S., & Sodagar, E. (2023). Real-Time Impedance-Based Monitoring of the Growth and Inhibition of Osteomyelitis Biofilm Pathogen Staphylococcus aureus Treated with Novel Bisphosphonate-Fluoroquinolone Antimicrobial Conjugates. International Journal of Molecular Sciences, 24(3), 1985. https://doi.org/10.3390/ijms24031985










