Efficacy of Polydeoxyribonucleotide in Promoting the Healing of Diabetic Wounds in a Murine Model of Streptozotocin-Induced Diabetes: A Pilot Experiment
Abstract
1. Introduction
2. Results
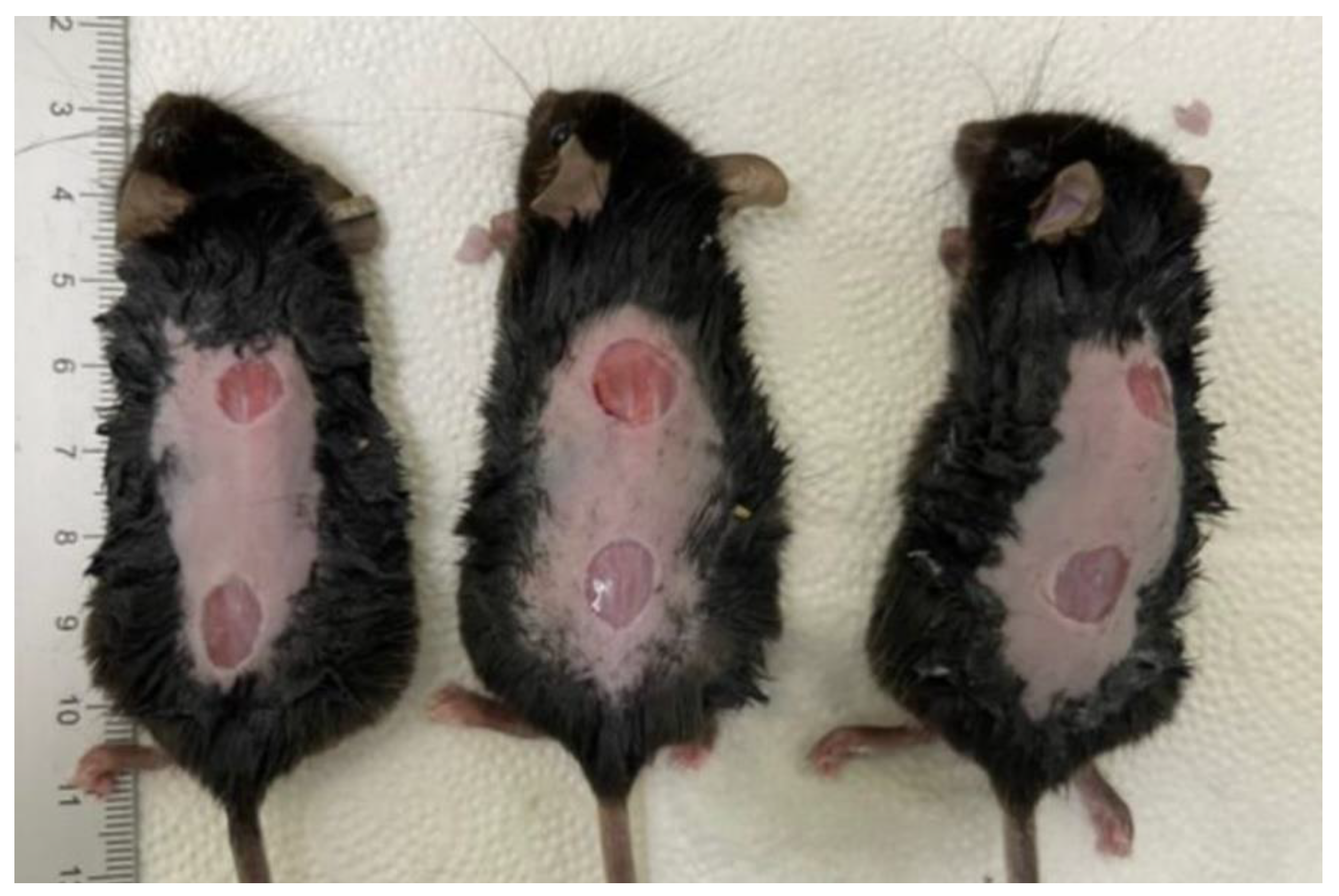
2.1. Gross Examinations of the Diabetic Wounds at 3, 7, 10 and 14 Days
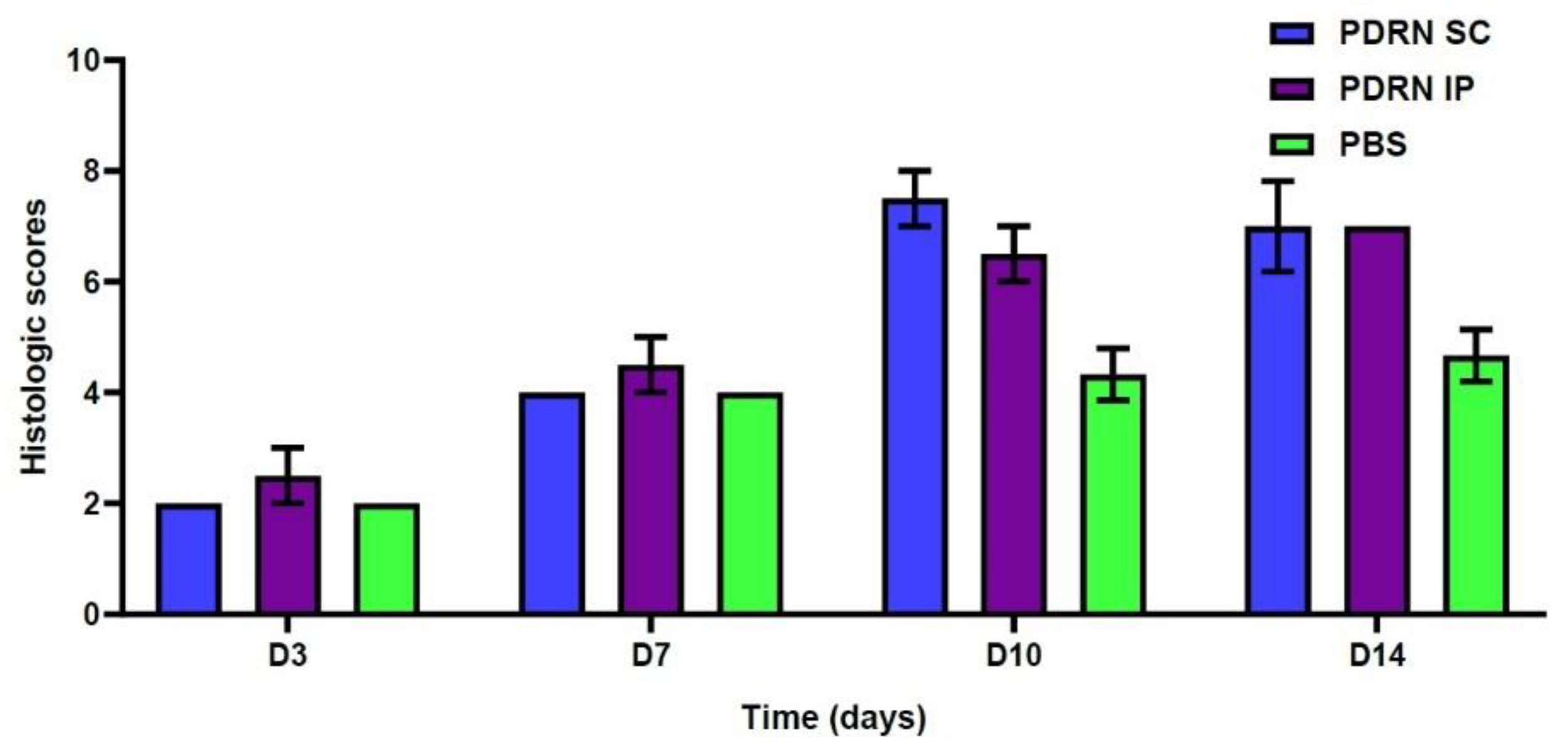
2.2. Histologic Examinations
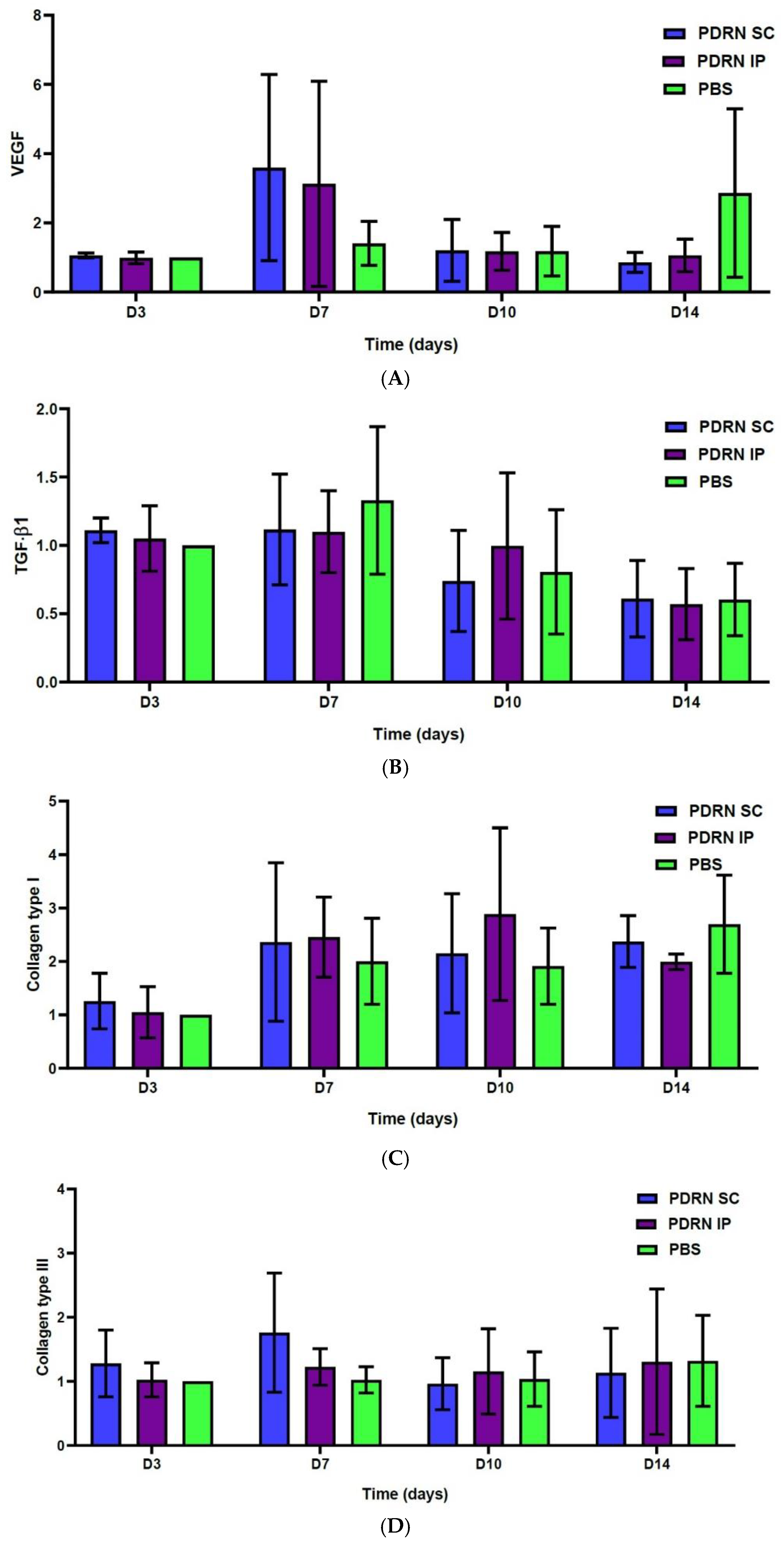
2.3. Results of the Western Blotting Analysis
3. Discussion
- At 10 days, the PDRN SC group showed a significantly smaller diameter of diabetic wounds as compared with the PBS group (2.1 ± 0.1 versus 4.87 ± 0.38 mm, respectively; p = 0.0024). Likewise, the PDRN IP group also showed a significantly smaller diameter of diabetic wounds as compared with the PBS group (2.5 ± 0.3 versus 4.87 ± 0.38 mm, respectively; p = 0.0053).
- At 14 days, the PDRN SC group showed a significantly smaller diameter of diabetic wounds as compared with the PBS group (1.3 ± 0.2 versus 5.2 ± 0.50 mm, respectively; p = 0.0002). Likewise, the PDRN IP group also showed a significantly smaller diameter of diabetic wounds as compared with the PBS group (1.95 ± 0.21 versus 5.2 ± 0.50 mm, respectively; p = 0.0036).
- At 10 days, the PDRN SC group had significantly higher histologic scores as compared with the PBS group (7.5 ± 0.5 versus 4.33 ± 0.47, respectively; p = 0.0055). Likewise, the PDRN IP group had significantly higher histologic scores as compared with the PBS group (6.5 ± 0.5 versus 4.33 ± 0.47, respectively; p = 0.0158).
- At 14 days, the PDRN SC group had significantly higher histologic scores as compared with the PBS group (7.0 ± 0.82 versus 4.67 ± 0.47, respectively; p = 0.0248). Likewise, the PDRN IP group had significantly higher histologic scores as compared with the PBS group (7.0 ± 0.0 versus 4.67 ± 0.47, respectively; p = 0.0069).
- At 7 days, the PDRN SC group showed a significantly greater expression of VEGF as compared with the PBS group (p < 0.0001). Of note, at 7 days, the PDRN SC group showed a significantly greater expression of VEGF as compared with the PDRN IP group (p < 0.0001).
- At 10 days, the PDRN SC group showed a significantly lower expression of TGF-β1 as compared with the PBS group (p < 0.0001). Of note, at 10 days, the PDRN SC group showed a significantly lower expression of TGF-β1 as compared with the PDRN IP group (p < 0.0001).
- At 7 and 10 days, the PDRN SC and PDRN IP groups showed a significantly greater expression of collagen type I as compared with the PBS group (p < 0.0001).
- At 7 days, the PDRN SC group showed a significantly greater expression of collagen type III as compared with the PBS group (p < 0.0001).
4. Materials and Methods
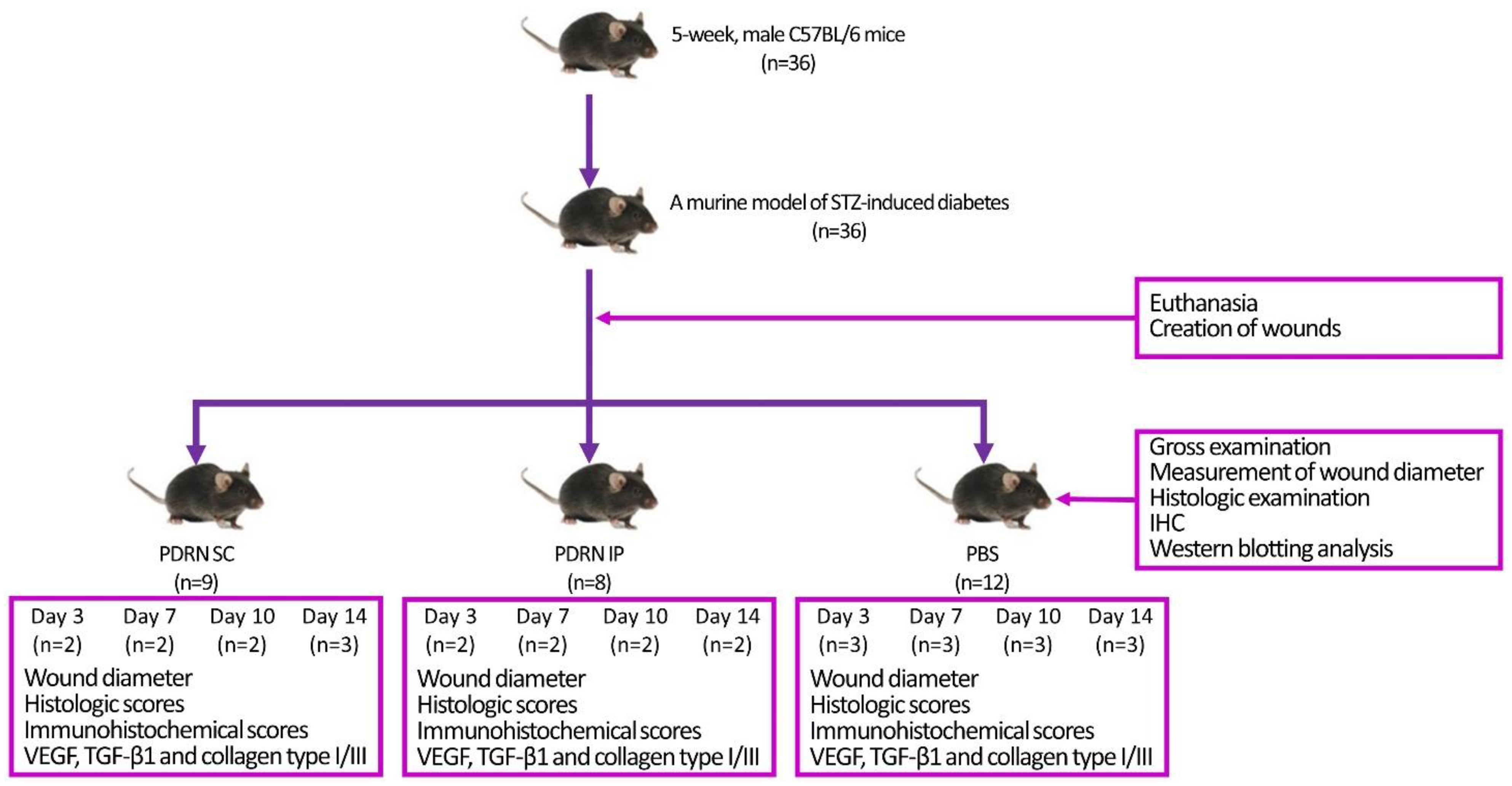
4.1. Experimental Animals and Setting
4.2. Establishment of a Murine Model of STZ-Induced Diabetes
4.3. Rationale of the Estimation of the Number of Experimental Animals
4.4. Dosing Rationale
4.5. Rationale of Route of Administration
4.6. Creation of Wounds
4.7. Experimental Procedures
4.8. Evaluation and Criteria
4.9. Statistical Analysis of the Experimental Data
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maffi, P.; Secchi, A. The Burden of Diabetes: Emerging Data. Dev. Ophthalmol. 2017, 60, 1–5. [Google Scholar]
- Corriere, M.; Rooparinesingh, N.; Kalyani, R.R. Epidemiology of diabetes and diabetes complications in the elderly: An emerging public health burden. Curr. Diab. Rep. 2013, 13, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Noh, J. The Diabetes Epidemic in Korea. Endocrinol. Metab. 2016, 31, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, M.A.; Komatsu, W.R.; de Sa, J.R.; Chacra, A.R.; Dib, S.A. Clinical inertia on insulin treatment intensification in type 2 diabetes mellitus patients of a tertiary public diabetes center with limited pharmacologic armamentarium from an upper-middle income country. Diabetol. Metab. Syndr. 2018, 10, 77. [Google Scholar] [CrossRef]
- Megallaa, M.H.; Ismail, A.A.; Zeitoun, M.H.; Khalifa, M.S. Association of diabetic foot ulcers with chronic vascular diabetic complications in patients with type 2 diabetes. Diabetes Metab. Syndr. 2019, 13, 1287–1292. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Deng, J.; Li, W.; Nie, X. Fibroblast Growth Factor in Diabetic Foot Ulcer: Progress and Therapeutic Prospects. Front. Endocrinol. 2021, 12, 744868. [Google Scholar] [CrossRef]
- Lee, K.M.; Kim, W.H.; Lee, J.H.; Choi, M.S. Risk factors of treatment failure in diabetic foot ulcer patients. Arch. Plast. Surg. 2013, 40, 123–128. [Google Scholar] [CrossRef]
- Brem, H.; Sheehan, P.; Rosenberg, H.J.; Schneider, J.S.; Boulton, A.J. Evidence-based protocol for diabetic foot ulcers. Plast. Reconstr. Surg. 2006, 117, 193S–209S; discussion 210S–211S. [Google Scholar] [CrossRef]
- Yazdanpanah, L.; Nasiri, M.; Adarvishi, S. Literature review on the management of diabetic foot ulcer. World J. Diabetes 2015, 6, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Alexiadou, K.; Doupis, J. Management of diabetic foot ulcers. Diabetes Ther. 2012, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Golinko, M.S.; Margolis, D.J.; Tal, A.; Hoffstad, O.; Boulton, A.J.; Brem, H. Preliminary development of a diabetic foot ulcer database from a wound electronic medical record: A tool to decrease limb amputations. Wound Repair Regen. 2009, 17, 657–665. [Google Scholar] [CrossRef]
- Xiao, J.; Li, J.; Cai, L.; Chakrabarti, S.; Li, X. Cytokines and diabetes research. J. Diabetes Res. 2014, 2014, 920613. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.C.; Liu, I.H.; Fang, A.H.; Wen, C.H.; Wu, C.S. Hyperglycaemic conditions decrease cultured keratinocyte mobility: Implications for impaired wound healing in patients with diabetes. Br. J. Dermatol. 2008, 159, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Maione, A.G.; Smith, A.; Kashpur, O.; Yanez, V.; Knight, E.; Mooney, D.J.; Veves, A.; Tomic-Canic, M.; Garlick, J.A. Altered ECM deposition by diabetic foot ulcer-derived fibroblasts implicates fibronectin in chronic wound repair. Wound Repair Regen. 2016, 24, 630–643. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.J. The pathway to foot ulceration in diabetes. Med. Clin. North Am. 2013, 97, 775–790. [Google Scholar] [CrossRef]
- Dinh, T.L.; Veves, A. A review of the mechanisms implicated in the pathogenesis of the diabetic foot. Int. J. Low. Extrem. Wounds 2005, 4, 154–159. [Google Scholar] [CrossRef]
- Dufrane, D.; van Steenberghe, M.; Guiot, Y.; Goebbels, R.M.; Saliez, A.; Gianello, P. Streptozotocin-induced diabetes in large animals (pigs/primates): Role of GLUT2 transporter and beta-cell plasticity. Transplantation 2006, 81, 36–45. [Google Scholar] [CrossRef]
- Kleinert, M.; Clemmensen, C.; Hofmann, S.M.; Moore, M.C.; Renner, S.; Woods, S.C.; Huypens, P.; Beckers, J.; de Angelis, M.H.; Schürmann, A.; et al. Animal models of obesity and diabetes mellitus. Nat. Rev. Endocrinol. 2018, 14, 140–162. [Google Scholar] [CrossRef]
- Roep, B.O.; Atkinson, M. Animal models have little to teach us about type 1 diabetes: 1. In support of this proposal. Diabetologia 2004, 47, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Arndt, T.; Jörns, A.; Weiss, H.; Tiedge, M.; Hedrich, H.J.; Lenzen, S.; Wedekind, D. A variable CD3⁺ T-cell frequency in peripheral blood lymphocytes associated with type 1 diabetes mellitus development in the LEW.1AR1-iddm rat. PLoS ONE 2013, 8, e64305. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.L.; Schuurman, H.J. Validity of animal models of type 1 diabetes, and strategies to enhance their utility in translational research. Eur. J. Pharmacol. 2015, 759, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Kottaisamy, C.P.D.; Raj, D.S.; Prasanth Kumar, V.; Sankaran, U. Experimental animal models for diabetes and its related complications—A review. Lab. Anim. Res. 2021, 37, 23. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yan, L.J. Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic β cell glucotoxicity. Diabetes Metab. Syndr. Obes. 2015, 8, 181–188. [Google Scholar] [PubMed]
- Szkudelski, T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 2001, 50, 537–546. [Google Scholar]
- Lenzen, S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 2008, 51, 216–226. [Google Scholar] [CrossRef]
- Ghasemi, A.; Khalifi, S.; Jedi, S. Streptozotocin-nicotinamide-induced rat model of type 2 diabetes (review). Acta. Physiol. Hung. 2014, 101, 408–420. [Google Scholar] [CrossRef]
- Squadrito, F.; Bitto, A.; Irrera, N.; Pizzino, G.; Pallio, G.; Minutoli, L.; Altavilla, D. Pharmacological Activity and Clinical Use of PDRN. Front. Pharmacol. 2017, 8, 224. [Google Scholar] [CrossRef]
- Guizzardi, S.; Galli, C.; Govoni, P.; Boratto, R.; Cattarini, G.; Martini, D.; Belletti, S.; Scandroglio, R. Polydeoxyribonucleotide (PDRN) promotes human osteoblast proliferation: A new proposal for bone tissue repair. Life Sci. 2003, 73, 1973–1983. [Google Scholar] [CrossRef]
- Sini, P.; Denti, A.; Cattarini, G.; Daglio, M.; Tira, M.E.; Balduini, C. Effect of polydeoxyribonucleotides on human fibroblasts in primary culture. Cell Biochem. Funct. 1999, 17, 107–114. [Google Scholar] [CrossRef]
- Galeano, M.; Bitto, A.; Altavilla, D.; Minutoli, L.; Polito, F.; Calo, M.; Cascio, P.L.; d’Alcontres, F.S.; Squadrito, F. Polydeoxyribonucleotide stimulates angiogenesis and wound healing in the genetically diabetic mouse. Wound Repair Regen. 2008, 16, 208–217. [Google Scholar] [CrossRef]
- Bitto, A.; Galeano, M.; Squadrito, F.; Minutoli, L.; Polito, F.; Dye, J.F.; Clayton, E.A.; Calò, M.; Venuti, F.S.; Vaccaro, M.; et al. Polydeoxyribonucleotide improves angiogenesis and wound healing in experimental thermal injury. Crit. Care Med. 2008, 36, 1594–1602. [Google Scholar] [CrossRef]
- Polito, F.; Bitto, A.; Galeano, M.; Irrera, N.; Marini, H.; Calò, M.; Squadrito, F.; Altavilla, D. Polydeoxyribonucleotide restores blood flow in an experimental model of ischemic skin flaps. J. Vasc. Surg. 2012, 55, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Bitto, A.; Polito, F.; Altavilla, D.; Minutoli, L.; Migliorato, A.; Squadrito, F. Polydeoxyribonucleotide (PDRN) restores blood flow in an experimental model of peripheral artery occlusive disease. J. Vasc. Surg. 2008, 48, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.Y.; Park, J.U.; Choi, M.H.; Kim, S.; Kim, H.E.; Jeong, S.H. Polydeoxyribonucleotide-delivering therapeutic hydrogel for diabetic wound healing. Sci. Rep. 2020, 10, 16811. [Google Scholar] [CrossRef]
- Kwon, T.R.; Han, S.W.; Kim, J.H.; Lee, B.C.; Kim, J.M.; Hong, J.Y.; Kim, B.J. Polydeoxyribonucleotides Improve Diabetic Wound Healing in Mouse Animal Model for Experimental Validation. Ann. Dermatol. 2019, 31, 403–413. [Google Scholar] [CrossRef]
- Colangelo, M.T.; Galli, C.; Guizzardi, S. The effects of polydeoxyribonucleotide on wound healing and tissue regeneration: A systematic review of the literature. Regen. Med. 2020, 15, 1801–1821. [Google Scholar] [CrossRef]
- Chavan, A.J.; Haley, B.E.; Volkin, D.B.; Marfia, K.E.; Verticelli, A.M.; Bruner, M.W.; Draper, J.P.; Burke, C.J.; Middaugh, C.R. Interaction of nucleotides with acidic fibroblast growth factor (FGF-1). Biochemestry 1994, 33, 7193–7202. [Google Scholar] [CrossRef]
- Middlemiss, P.J.; Gysbers, J.W.; Rathbone, M.P. Extracellular guanosine and guanosine-5’-trisphoshate increase NGF synthesis and release from cultured mouse neopallial astrocytes. Brain Res. 1995, 677, 152–156. [Google Scholar] [CrossRef]
- Irrera, N.; D’Ascola, A.; Pallio, G.; Bitto, A.; Mannino, F.; Arcoraci, V.; Rottura, M.; Ieni, A.; Minutoli, L.; Metro, D.; et al. β-Caryophyllene Inhibits Cell Proliferation through a Direct Modulation of CB2 Receptors in Glioblastoma Cells. Cancers 2020, 12, 1038. [Google Scholar] [CrossRef] [PubMed]
- Thellung, S.; Florio, T.; Maragliano, A.; Cattarini, G.; Schettini, G. Polydeoxyribonucleotides enhance the proliferation of human skin fibroblasts: Involvement of A2 purinergic receptor subtypes. Life Sci. 1999, 64, 1661–1674. [Google Scholar] [CrossRef] [PubMed]
- Muratore, O.; Schito, A.P.; Cattarini, G.; Tonoli, E.L.; Gianoglio, S.; Schiappacasse, S.; Felli, L.; Picchetta, F.; Schito, G.C. Evaluation of the trophic effect of human placental polydeoxyribonucleotide on human knee skin fibroblasts in primary culture. Cell Mol. Life Sci. 1997, 53, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Gennero, L.; Denysenko, T.; Calisti, G.F.; Vercelli, A.; Vercelli, C.M.; Amedeo, S.; Mioletti, S.; Parino, E.; Montanaro, M.; Melcarne, A.; et al. Protective effects of polydeoxyribonucleotides on cartilage degradation in experimental cultures. Cell Biochem. Funct. 2013, 31, 214–227. [Google Scholar] [CrossRef]
- Raposio, E.; Guida, C.; Coradeghini, R.; Scanarotti, C.; Parodi, A.; Baldelli, I.; Fiocca, R.; Santi, P.L. In vitro polydeoxyribonucleotide effects on human pre-adipocytes. Cell Prolif. 2008, 41, 739–754. [Google Scholar] [CrossRef]
- Guizzardi, S.; Martini, D.; Bacchelli, B.; Valdatta, L.; Thione, A.; Scamoni, S.; Uggeri, J.; Ruggeri, A. Effect of heat deproteinate bone and polynucleotides on bone regeneration: An experimental study on rat. Micron 2007, 38, 722–728. [Google Scholar] [CrossRef]
- Sheth, S.; Brito, R.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Adenosine Receptors: Expression, Function and Regulation. Int. J. Mol. Sci. 2014, 15, 2024–2052. [Google Scholar] [CrossRef]
- Cronstein, B.N.; Sitkovsky, M. Adenosine and adenosine receptors in the pathogenesis and treatment of rheumatic diseases. Nat. Rev. Rheumatol. 2017, 13, 41–51. [Google Scholar] [CrossRef]
- Effendi, W.I.; Nagano, T.; Kobayashi, K.; Nishimura, Y. Focusing on Adenosine Receptors as a Potential Targeted Therapy in Human Diseases. Cells 2020, 9, 785. [Google Scholar] [CrossRef]
- Chhabra, P.; Linden, J.; Lobo, P.; Okusa, M.D.; Brayman, K.L. The immunosuppressive role of adenosine A2A receptors in ischemia reperfusion injury and islet transplantation. Curr. Diabetes Rev. 2012, 8, 419–433. [Google Scholar] [CrossRef]
- Haskó, G.; Pacher, P. A2A receptors in inflammation and injury: Lessons learned from transgenic animals. J. Leukoc. Biol. 2008, 83, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Altavilla, D.; Squadrito, F.; Polito, F.; Irrera, N.; Calò, M.; Lo Cascio, P.; Galeano, M.; La Cava, L.; Minutoli, L.; Marini, H.; et al. Activation of adenosine A2A receptors restores the altered cell-cycle machinery during impaired wound healing in genetically diabetic mice. Surgery 2011, 149, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Bitto, A.; Polito, F.; Irrera, N.; D’Ascola, A.; Avenoso, A.; Nastasi, G.; Campo, G.; Micali, A.; Bagnato, G.; Minutoli, L.; et al. Polydeoxyribonucleotide reduces cytokine production and the severity of collagen-induced arthritis by stimulation of adenosine A2A receptor. Arthritis Rheum. 2011, 63, 3364–3371. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Park, G.; Lee, J.; Bae, H. The Effect of Polydeoxyribonucleotide on Chronic Non-healing Wound of an Amputee: A Case Report. Ann. Rehabil. Med. 2018, 42, 630–633. [Google Scholar] [CrossRef]
- Chung, K.I.; Kim, H.K.; Kim, W.S.; Bae, T.H. The effects of polydeoxyribonucleotide on the survival of random pattern skin flaps in rats. Arch. Plast. Surg. 2013, 40, 181–186. [Google Scholar] [CrossRef]
- Jeong, W.; Yang, C.E.; Roh, T.S.; Kim, J.H.; Lee, J.H.; Lee, W.J. Scar Prevention and Enhanced Wound Healing Induced by Polydeoxyribonucleotide in a Rat Incisional Wound-Healing Model. Int. J. Mol. Sci. 2017, 18, 1698. [Google Scholar] [CrossRef]
- Lee, W.Y.; Park, K.D.; Park, Y. The effect of polydeoxyribonucleotide on the treatment of radiating leg pain due to cystic mass lesion in inner aspect of right sciatic foramen: A CARE compliant case report. Medicine 2018, 97, e12794. [Google Scholar] [CrossRef]
- Koo, Y.; Yun, Y. Effects of polydeoxyribonucleotides (PDRN) on wound healing: Electric cell-substrate impedance sensing (ECIS). Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 554–560. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.; Choi, J.; Jeong, W.; Kwon, S. Polydeoxyribonucleotide Improves Peripheral Tissue Oxygenation and Accelerates Angiogenesis in Diabetic Foot Ulcers. Arch. Plast. Surg. 2017, 44, 482–489. [Google Scholar] [CrossRef]
- Peplow, P.V.; Baxter, G.D. Gene expression and release of growth factors during delayed wound healing: A review of studies in diabetic animals and possible combined laser phototherapy and growth factor treatment to enhance healing. Photomed. Laser Surg. 2012, 30, 617–636. [Google Scholar] [CrossRef]
- Reynolds, L.E.; Conti, F.J.; Lucas, M.; Grose, R.; Robinson, S.; Stone, M.; Saunders, G.; Dickson, C.; Hynes, R.O.; Lacy-Hulbert, A.; et al. Accelerated re-epithelialization in beta3-integrin-deficient- mice is associated with enhanced TGF-beta1 signaling. Nat. Med. 2005, 11, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Wolfensohn, S.E.; Lloyd, M.H. Aleutian disease in laboratory ferrets. Vet. Rec. 1994, 134, 100. [Google Scholar] [CrossRef] [PubMed]
- Ahn, T.H.; Cho, S.B. Adjuvant Therapy for Revision Rhinoplasty of Contracted Nose Using Polydeoxyribonucleotide and Invasive Bipolar Radiofrequency. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1645. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.J.; Kim, D.Y.; Cheon, G.-W.; Park, H.J.; Ahn, T.H. Polydeoxyribonucleotide and Microlens Array-type, Nanosecond-domain Neodymium:Yttrium-aluminum-garnet Laser Treatment for Scars from Costal Cartilage Harvest Surgery: Case Series of 9 Patients. Med. Lasers 2021, 10, 90–95. [Google Scholar] [CrossRef]
- Bitto, A.; Oteri, G.; Pisano, M.; Polito, F.; Irrera, N.; Minutoli, L.; Squadrito, F.; Altavilla, D. Adenosine receptor stimulation by polynucleotides (PDRN) reduces inflammation in experimental periodontitis. J. Clin. Periodontol. 2013, 40, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Belmontesi, M. Polydeoxyribonucleotide for the improvement of a hypertrophic retracting scar-An interesting case report. J. Cosmet. Dermatol. 2020, 19, 2982–2986. [Google Scholar] [CrossRef]
- Kim, B.R.; Kwon, S.H.; Kim, J.W.; Jeong, W.J.; Cha, W.; Jung, Y.H.; Na, J.I.; Huh, C.H.; Shin, J.W. Early Postoperative Injections of Polydeoxyribonucleotide Prevent Hypertrophic Scarring after Thyroidectomy: A Randomized Controlled Trial. Adv. Wound Care, 2022; ahead of print. [Google Scholar] [CrossRef]
- Edirisinghe, S.L.; Nikapitiya, C.; Dananjaya, S.H.S.; Park, J.; Kim, D.; Choi, D.; De Zoysa, M. Effect of Polydeoxyribonucleotide (PDRN) Treatment on Corneal Wound Healing in Zebrafish (Danio rerio). Int. J. Mol. Sci. 2022, 23, 13525. [Google Scholar] [CrossRef]
- Antonuccio, P.; Pallio, G.; Marini, H.R.; Irrera, N.; Romeo, C.; Puzzolo, D.; Freni, J.; Santoro, G.; Pirrotta, I.; Squadrito, F.; et al. Involvement of Hypoxia-Inducible Factor 1-α in Experimental Testicular Ischemia and Reperfusion: Effects of Polydeoxyribonucleotide and Selenium. Int. J. Mol. Sci. 2022, 23, 13144. [Google Scholar] [CrossRef]
- Noh, T.K.; Chung, B.Y.; Kim, S.Y.; Lee, M.H.; Kim, M.J.; Youn, C.S.; Lee, M.W.; Chang, S.E. Novel Anti-Melanogenesis Properties of Polydeoxyribonucleotide, a Popular Wound Healing Booster. Int. J. Mol. Sci. 2016, 17, 1448. [Google Scholar] [CrossRef]
- Irrera, N.; Bitto, A.; Vaccaro, M.; Mannino, F.; Squadrito, V.; Pallio, G.; Arcoraci, V.; Minutoli, L.; Ieni, A.; Lentini, M.; et al. PDRN, a Bioactive Natural Compound, Ameliorates Imiquimod-Induced Psoriasis through NF-κB Pathway Inhibition and Wnt/β-Catenin Signaling Modulation. Int. J. Mol. Sci. 2020, 21, 1215. [Google Scholar] [CrossRef]
- An, J.; Park, S.H.; Ko, I.-G.; Jin, J.-J.; Hwang, L.; Ji, E.-S.; Kim, S.-H.; Kim, C.-J.; Park, S.Y.; Hwang, J.-J.; et al. Polydeoxyribonucleotide Ameliorates Lipopolysaccharide-Induced Lung Injury by Inhibiting Apoptotic Cell Death in Rats. Int. J. Mol. Sci. 2017, 18, 1847. [Google Scholar] [CrossRef] [PubMed]
- Ko, I.-G.; Jin, J.-J.; Hwang, L.; Kim, S.-H.; Kim, C.-J.; Han, J.H.; Lee, S.; Kim, H.I.; Shin, H.P.; Jeon, J.W. Polydeoxyribonucleotide Exerts Protective Effect Against CCl4-Induced Acute Liver Injury Through Inactivation of NF-κB/MAPK Signaling Pathway in Mice. Int. J. Mol. Sci. 2020, 21, 7894. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.L.; Janecek, J.L.; Kittredge, J.A.; Hering, B.J.; Schuurman, H.J. The streptozotocin-induced diabetic nude mouse model: Differences between animals from different sources. Comp. Med. 2011, 61, 356–360. [Google Scholar] [PubMed]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015, 70, 5–47. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.H.; Roh, K.H.; Lim, N.Y.; Park, S.J.; Park, S.; Kim, H.W. Role of the JAK/STAT pathway in a streptozotocin-induced diabetic retinopathy mouse model. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 3553–3563. [Google Scholar] [CrossRef]
- Rubegni, P.; De Aloe, G.; Mazzatenta, C.; Cattarini, L.; Fimiani, M. Clinical evaluation of the trophic effect of polydeoxyribonucleotide (PDRN) in patients undergoing skin explants. A Pilot Study. Curr. Med. Res. Opin. 2001, 17, 128–131. [Google Scholar] [CrossRef]
- Daniels Gatward, L.F.; Kennard, M.R.; Smith, L.I.F.; King, A.J.F. The use of mice in diabetes research: The impact of physiological characteristics, choice of model and husbandry practices. Diabet. Med. 2021, 38, e14711. [Google Scholar] [CrossRef]
- Gvazava, I.G.; Kosykh, A.V.; Rogovaya, O.S.; Popova, O.P.; Sobyanin, K.A.; Khrushchev, A.C.; Timofeev, A.V.; Vorotelyak, E.A. A Simplified Streptozotocin-Induced Diabetes Model in Nude Mice. Acta Nat. 2020, 12, 98–104. [Google Scholar] [CrossRef]
- Saadane, A.; Lessieur, E.M.; Du, Y.; Liu, H.; Kern, T.S. Successful induction of diabetes in mice demonstrates no gender difference in development of early diabetic retinopathy. PLoS ONE 2020, 15, e0238727. [Google Scholar] [CrossRef]
- Kang, S.H.; Choi, M.S.; Kim, H.K.; Kim, W.S.; Bae, T.H.; Kim, M.K.; Chang, S.H. Polydeoxyribonucleotide improves tendon healing following achilles tendon injury in rats. J. Orthop. Res. 2018, 36, 1767–1776. [Google Scholar] [CrossRef]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar] [PubMed]
- Levin-Arama, M.; Abraham, L.; Waner, T.; Harmelin, A.; Steinberg, D.M.; Lahav, T.; Harlev, M. Subcutaneous Compared with Intraperitoneal Ketamine-Xylazine for Anesthesia of Mice. J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 794–800. [Google Scholar] [PubMed]
- Kick, B.L.; Gumber, S.; Wang, H.; Moore, R.H.; Taylor, D.K. Evaluation of 4 Presurgical Skin Preparation Methods in Mice. J. Am. Assoc. Lab. Anim. Sci. 2019, 58, 71–77. [Google Scholar] [CrossRef]
- Dai, T.; Kharkwal, G.B.; Tanaka, M.; Huang, Y.Y.; Bil de Arce, V.J.; Hamblin, M.R. Animal models of external traumatic wound infections. Virulence 2011, 2, 296–315. [Google Scholar] [CrossRef]
- Mendes, J.J.; Leandro, C.I.; Bonaparte, D.P.; Pinto, A.L. A rat model of diabetic wound infection for the evaluation of topical antimicrobial therapies. Comp. Med. 2012, 62, 37–48. [Google Scholar] [PubMed]
- Schoell, A.R.; Heyde, B.R.; Weir, D.E.; Chiang, P.C.; Hu, Y.; Tung, D.K. Euthanasia method for mice in rapid time-course pulmonary pharmacokinetic studies. J. Am. Assoc. Lab. Anim. Sci. 2009, 48, 506–511. [Google Scholar]
- Ferrari, G.; Terushkin, V.; Wolff, M.J.; Zhang, X.; Valacca, C.; Poggio, P.; Pintucci, G.; Mignatti, P. TGF-β1 induces endothelial cell apoptosis by shifting VEGF activation of p38(MAPK) from the prosurvival p38β to proapoptotic p38α. Mol. Cancer Res. 2012, 10, 605–614. [Google Scholar] [CrossRef]
- Chellini, F.; Tani, A.; Vallone, L.; Nosi, D.; Pavan, P.; Bambi, F.; ZecchiOrlandini, S.; Sassoli, C. Platelet-Rich Plasma Prevents In Vitro Transforming Growth Factor-β1-Induced Fibroblast to Myofibroblast Transition: Involvement of Vascular Endothelial Growth Factor (VEGF)-A/VEGF Receptor-1-Mediated Signaling. Cells 2018, 7, 142. [Google Scholar] [CrossRef]



| Variables | Values | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PDRN SC (n = 9) | PDRN IP (n = 8) | PBS (n = 12) | ||||||||||
| 3 Days (n = 2) | 7 Days (n = 2) | 10 Days (n = 2) | 14 Days (n = 3) | 3 Days (n = 2) | 7 Days (n = 2) | 10 Days (n = 2) | 14 Days (n = 2) | 3 Days (n = 3) | 7 Days (n = 3) | 10 Days (n = 3) | 14 Days (n = 3) | |
| Diameter (mm) | 8.0 ± 0.2 | 6.3 ± 0.30 | 2.1 ± 0.1 | 1.3 ± 0.2 | 7.95 ± 0.75 | 6.2 ± 0.50 | 2.5 ± 0.3 | 1.95 ± 0.21 | 7.63 ± 0.92 | 6.67 ± 0.17 | 4.87 ± 0.38 | 5.2 ± 0.50 |
| Variables | Values | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PDRN SC (n = 9) | PDRN IP (n = 8) | PBS (n = 12) | ||||||||||
| 3 Days (n = 2) | 7 Days (n = 2) | 10 Days (n = 2) | 14 Days (n = 3) | 3 Days (n = 2) | 7 Days (n = 2) | 10 Days (n = 2) | 14 Days (n = 2) | 3 Days (n = 3) | 7 Days (n = 3) | 10 Days (n = 3) | 14 Days (n = 3) | |
| Histologic scores | 2.0 ± 0.0 | 4.0 ± 0.0 | 7.5 ± 0.5 | 7.0 ± 0.82 | 2.5 ± 0.5 | 4.5 ± 0.5 | 6.5 ± 0.5 | 7.0 ± 0.0 | 2.0 ± 0.0 | 4.0 ± 0.0 | 4.33 ± 0.47 | 4.67 ± 0.47 |
| Criteria | ||
|---|---|---|
| Epidermal and Dermal Regeneration | Granulation Tissue Thickness | |
| 1 | Little epidermal and dermal organization | Thin granulation layer |
| 2 | Moderate epidermal and dermal organization | Moderate granulation layer |
| 3 | Complete remodeling of epidermis and dermis | Thick granulation layer |
| 4 | Very thick granulation layer | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, J.; Park, S.; Park, H.Y.; Lee, K.A. Efficacy of Polydeoxyribonucleotide in Promoting the Healing of Diabetic Wounds in a Murine Model of Streptozotocin-Induced Diabetes: A Pilot Experiment. Int. J. Mol. Sci. 2023, 24, 1932. https://doi.org/10.3390/ijms24031932
Yun J, Park S, Park HY, Lee KA. Efficacy of Polydeoxyribonucleotide in Promoting the Healing of Diabetic Wounds in a Murine Model of Streptozotocin-Induced Diabetes: A Pilot Experiment. International Journal of Molecular Sciences. 2023; 24(3):1932. https://doi.org/10.3390/ijms24031932
Chicago/Turabian StyleYun, Jiyoung, SaeGwang Park, Ha Young Park, and Kyung Ah Lee. 2023. "Efficacy of Polydeoxyribonucleotide in Promoting the Healing of Diabetic Wounds in a Murine Model of Streptozotocin-Induced Diabetes: A Pilot Experiment" International Journal of Molecular Sciences 24, no. 3: 1932. https://doi.org/10.3390/ijms24031932
APA StyleYun, J., Park, S., Park, H. Y., & Lee, K. A. (2023). Efficacy of Polydeoxyribonucleotide in Promoting the Healing of Diabetic Wounds in a Murine Model of Streptozotocin-Induced Diabetes: A Pilot Experiment. International Journal of Molecular Sciences, 24(3), 1932. https://doi.org/10.3390/ijms24031932






