Comparing the Toxicological Responses of Pulmonary Air–Liquid Interface Models upon Exposure to Differentially Treated Carbon Fibers
Abstract
1. Introduction
2. Results
2.1. Physico-Chemical Properties of Deposited Carbon Fibers
2.2. Cytotoxicity upon Exposure to Pre-Treated Carbon Fibers
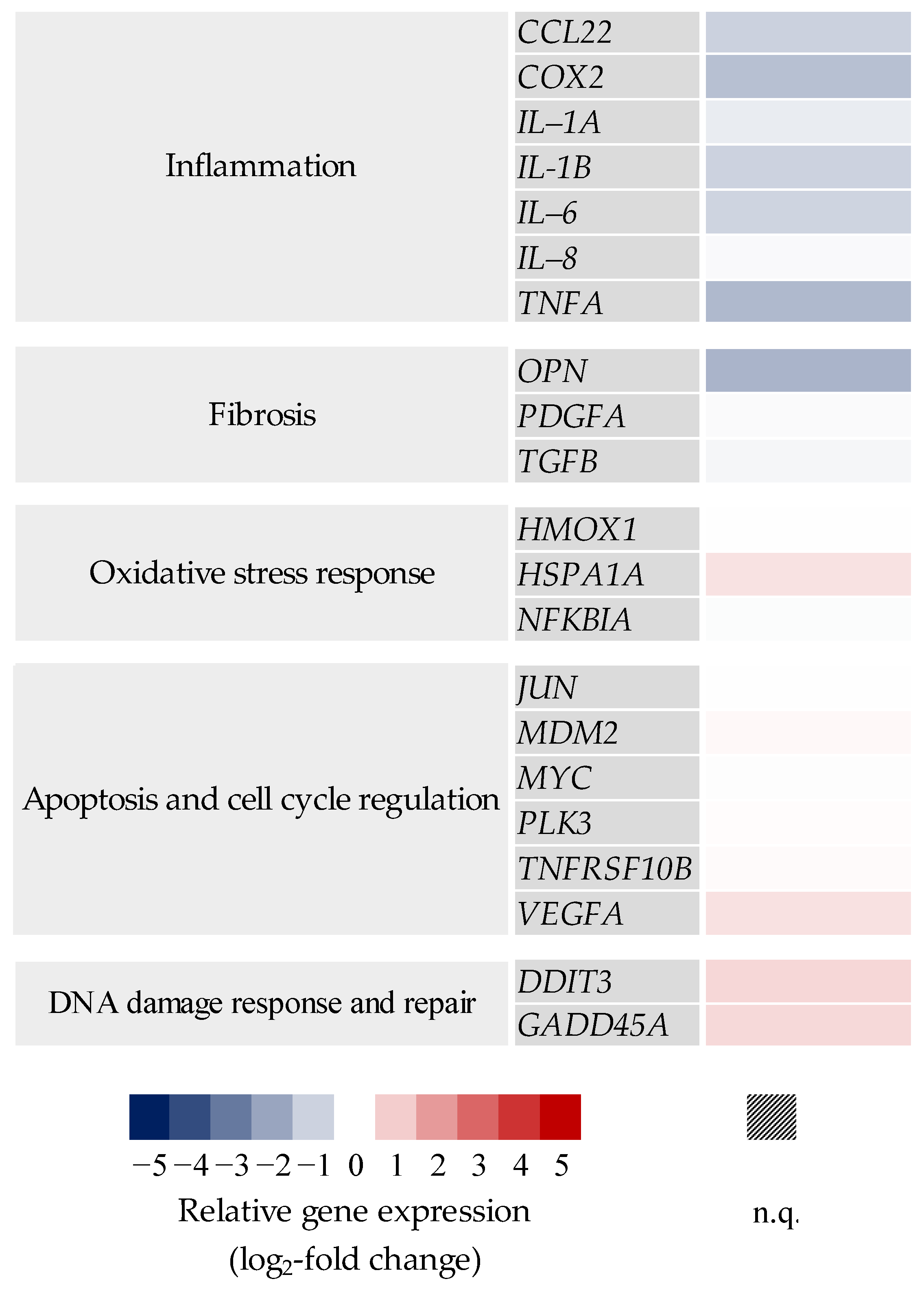
2.3. Transcriptional Toxicity Profiles upon Exposure to Pre-Treated Carbon Fibers
2.3.1. BEAS-2B Mono- and BEAS-2B/dTHP-1 Co-Cultures
2.3.2. BEAS-2B/dTHP-1/CCD-33Lu Triple-Cultures
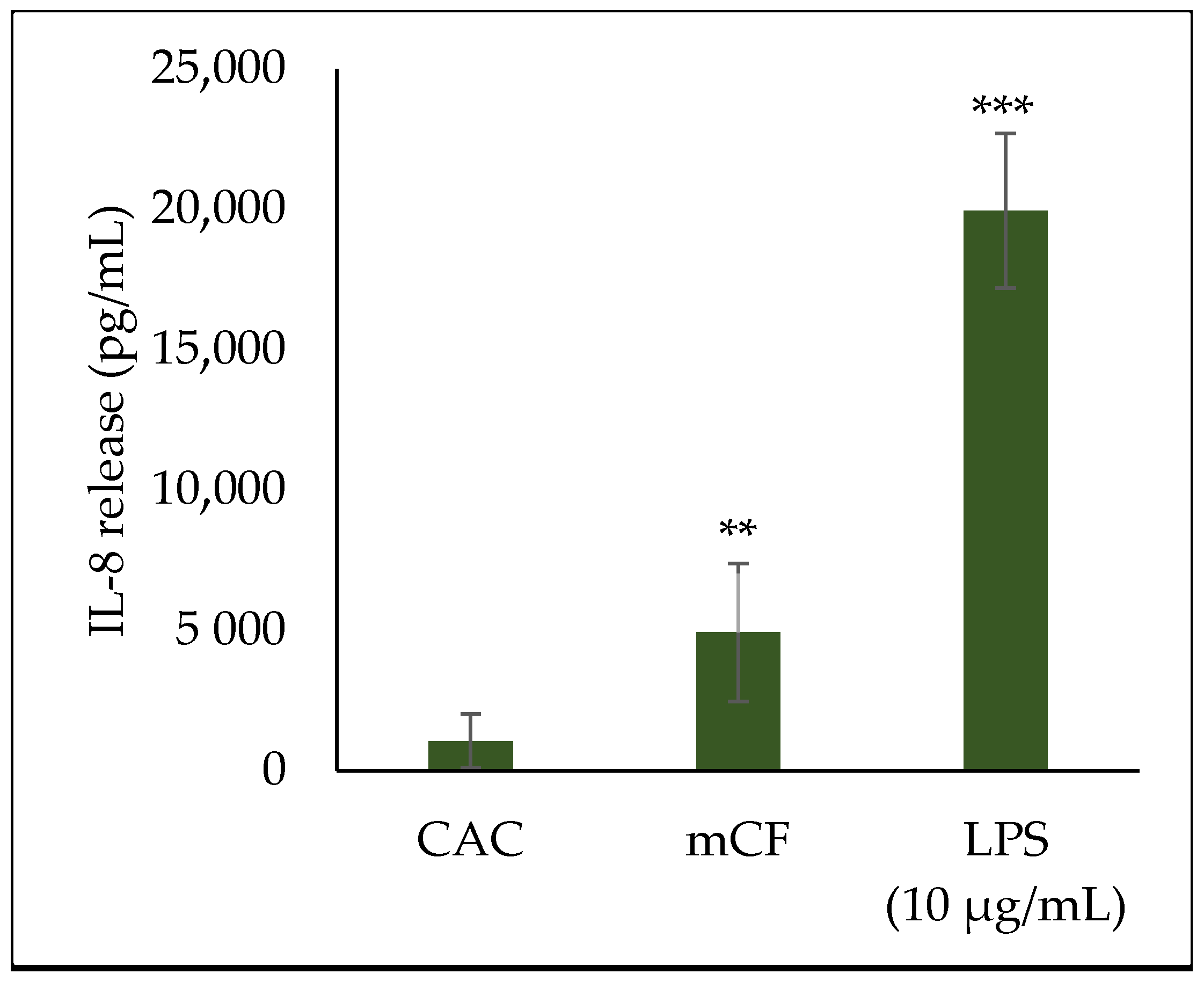
2.4. Cytokine Release upon Exposure to Pre-Treated Carbon Fibers
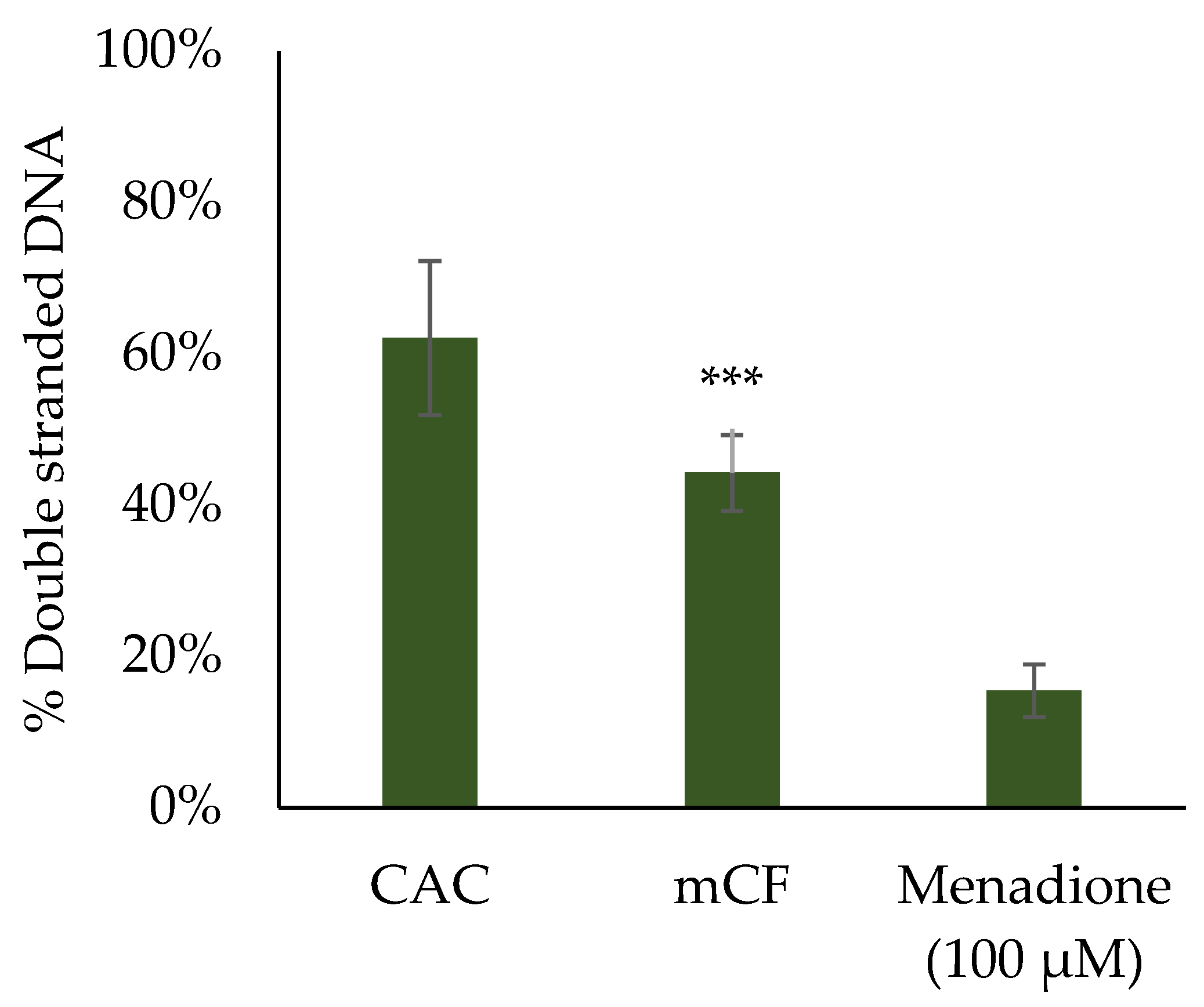
2.5. Induction of DNA Strand Breaks upon Exposure to Pre-Treated Carbon Fibers
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Fiber Preparation and Characterization
4.3. Cell Culture
4.4. Exposure via the Vitrocell® Automated Exposure Station (AES)
4.4.1. Preparation of Cell Culture Models for ALI Exposure
4.4.2. Exposure via Vitrocell® AES
4.4.3. Harvesting of Cells
4.5. Cytotoxicity Assessment via Lactate Dehydrogenase (LDH) Assay
4.6. Gene Expression Analysis via High-Throughput RT-qPCR
4.7. Interleukin-8 Enzyme-Linked Immunosorbent Assay (IL-8 ELISA)
4.8. Evaluation of DNA Strand Breaks by Alkaline Unwinding
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AES | Automated Exposure Station |
| ALI | Air–liquid interface |
| AP-1 | Activator protein 1 |
| AU | Alkaline unwinding |
| CAC | Clean air control |
| CB | Carbon black particles |
| CF | Carbon fiber |
| CFRP | Carbon fiber-reinforced polymer |
| dTHP-1 | differentiated THP-1 cells |
| ELISA | Enzyme-Linked immunosorbent assay |
| FBS | Fetal bovine serum |
| HT RT qPCR | High-throughput RT qPCR |
| IL | Interleukin |
| JNK | c-Jun N-terminal kinases |
| LDH | Lactate Dehydrogenase |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinases |
| mCF | Mechanically treated CF |
| MWCNT | Multi-walled carbon nanotubes |
| NF-κB | Nuclear factor κB |
| NLRP3 | NOD-, LRR-, and pyrin domain-containing protein 3 |
| PAN | Polyacrylonitrile |
| PBS | Phosphate-buffered saline |
| PMA | Phorbol 12-myristate 13-acetate |
| PM2.5 | Particulate matter 2.5 µm |
| PM10 | Particulate matter 10 µm |
| QCM | Quartz crystal microbalance |
| ROS | Reactive oxygen species |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| tmCF | Thermo-mechanically treated CF |
| vCF | Virgin carbon fibers |
| WHO | World Health Organization |
References
- Park, S.-J. Carbon Fibers; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Frank, E.; Hermanutz, F.; Buchmeiser, M.R. Carbon fibers: Precursors, manufacturing, and properties. Macromol. Mater. Eng. 2012, 297, 493–501. [Google Scholar] [CrossRef]
- Lengsfeld, H.; Mainka, H.; Altstädt, V. Carbon Fibers: Production, Applications, Processing; Carl Hanser Verlag GmbH Co KG: Munich, Germany, 2020. [Google Scholar]
- Forintos, N.; Czigany, T. Multifunctional application of carbon fiber reinforced polymer composites: Electrical properties of the reinforcing carbon fibers–A short review. Compos. Part B Eng. 2019, 162, 331–343. [Google Scholar] [CrossRef]
- Bäger, D.; Simonow, B.; Kehren, D.; Dziurowitz, N.; Wenzlaff, D.; Thim, C.; Meyer-Plath, A.; Plitzko, S. Pechbasierte Carbonfasern als Quelle alveolengängiger Fasern bei mechanischer Bearbeitung von carbonfaserverstärkten Kunststoffen (CFK). Gefahrst. Reinhalt. Luft 2019, 79, 13–16. [Google Scholar] [CrossRef]
- Kehren, D.; Simonow, B.; Bäger, D.; Dziurowitz, N.; Wenzlaff, D.; Thim, C.; Neuhoff, J.; Meyer-Plath, A.; Plitzko, S. Release of respirable fibrous dust from carbon fibers due to splitting along the fiber axis. Aerosol Air Qual. Res. 2019, 19, 2185–2195. [Google Scholar] [CrossRef]
- Große, A.; Naumann, R.; Hofmann, M.; Kehren, D.; Bäger, D.; Plitzko, S. CarboBreak–Conditions and Mechanisms for Releasing Alveolar Fibrous Carbon Fibre Fragments. Available online: https://nanopartikel.info/wp-content/uploads/2021/11/CarboBreak_Poster_STFI_Final_web2.pdf (accessed on 30 November 2022).
- Giżyński, M.; Romelczyk-Baishya, B. Investigation of carbon fiber–reinforced thermoplastic polymers using thermogravimetric analysis. J. Thermoplast. Compos. Mater. 2021, 34, 126–140. [Google Scholar] [CrossRef]
- Quicker, P.; Stockschläder, J.; Stapf, D.; Baumann, W.; Wexler, M.; Beckmann, M.; Thiel, C.; Teipel, U.; Seiler, E.; Hoppe, H.; et al. Möglichkeiten und Grenzen der Entsorgung Carbonfaserverstärkter Kunststoffabfälle in Thermischen Prozessen. Available online: https://www.umweltbundesamt.de/publikationen/moeglichkeiten-grenzen-der-entsorgung (accessed on 30 November 2022).
- Donaldson, K.; Poland, C.A.; Murphy, F.A.; MacFarlane, M.; Chernova, T.; Schinwald, A. Pulmonary toxicity of carbon nanotubes and asbestos—Similarities and differences. Adv. Drug Deliv. Rev. 2013, 65, 2078–2086. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Schlagenhauf, L.; Setyan, A. Transformation of the released asbestos, carbon fibers and carbon nanotubes from composite materials and the changes of their potential health impacts. J. Nanobiotechnol. 2017, 15, 15. [Google Scholar] [CrossRef]
- Amtsblatt der Europäischen Union. Verordnung (EG) Nr. 761/2009 der Kommission vom 23. Juli 2009 zur Änderung der Verordnung (EG) Nr. 440/2008 zur Festlegung von Prüfmethoden gemäß der Verordnung (EG) Nr. 1907/2006 des Europäischen Parlaments und des Rates zur Registrierung, Bewertung, Zulassung und Beschränkung chemischer Stoffe (REACH) zwecks Anpassung an den technischen Fortschritt. Off. J. Eur. Union 2009, 52, L220. [Google Scholar]
- WHO. Determination of Airborne Fibre Number Concentrations. A Recommended Method, by Phase- Contrast Optical Microscopy (Membrane Filter Method). Available online: https://www.who.int/occupational_health/publications/en/oehairbornefibre.pdf?ua=1 (accessed on 5 July 2019).
- Donaldson, K.; Murphy, F.A.; Duffin, R.; Poland, C.A. Asbestos, carbon nanotubes and the pleural mesothelium: A review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part. Fibre Toxicol. 2010, 7, 5. [Google Scholar] [CrossRef]
- Bernstein, D.M. Synthetic vitreous fibers: A review toxicology, epidemiology and regulations. Crit. Rev. Toxicol. 2007, 37, 839–886. [Google Scholar] [CrossRef]
- Gibbs, H.H.; Wendt, R.C.; Wilson, F.C. Carbon fiber structure and stability studies. Polym. Eng. Sci. 1979, 19, 342–349. [Google Scholar] [CrossRef]
- Zhang, J.; Chevali, V.S.; Wang, H.; Wang, C.-H. Current status of carbon fibre and carbon fibre composites recycling. Compos. Part B Eng. 2020, 193, 108053. [Google Scholar] [CrossRef]
- Kumoi, J.; Ikegami, A.; Fujitani, Y.; Morikawa, K.; Ichihara, G.; Yano, T.; Ichihara, S. Factory site analysis of respirable fibers generated during the process of cutting and grinding of carbon fibers-reinforced plastics. Int. Arch. Occup. Environ. Health 2022, 95, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Owen, P.E.; Glaister, J.R.; Ballantyne, B.; Clary, J.J. Subchronic inhalation toxicology of carbon fibers. J. Occup. Med. 1986, 28, 373–376. [Google Scholar] [PubMed]
- Warheit, D.B.; Hansen, J.F.; Hartsky, M.A.; Carakostas, M.C. Acute Inhalation Toxicity Studies in Rats with a Respirable-Sized Experimental Carbon Fibre: Pulmonary Biochemical and Cellular Effects. Ann. Occup. Hyg. 1994, 38, 769–776. [Google Scholar]
- Holt, P.F.; Horne, M. Dust from carbon fibre. Environ. Res. 1978, 17, 276–283. [Google Scholar] [CrossRef]
- Moriyama, A.; Hasegawa, T.; Nagaya, C.; Hamada, K.; Himaki, T.; Murakami, M.; Horie, M.; Takahashi, J.; Iwahashi, H.; Moritomi, H. Assessment of harmfulness and biological effect of carbon fiber dust generated during new carbon fiber recycling method. J. Hazard. Mater. 2019, 378, 120777. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, X.F.; Wang, Z.H.; Ding, J.; Shi, W.B. Toxicological mechanisms of Carbon-based nanomaterials. Adv. Mater. Res. 2012, 345, 12–17. [Google Scholar] [CrossRef]
- Krug, H.F.; Wick, P. Nanotoxicology: An interdisciplinary challenge. Angew. Chem. Int. Ed. 2011, 50, 1260–1278. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, X.; Sun, L.; Wei, Y.; Wei, X. Cellular Toxicity and Immunological Effects of Carbon-based Nanomaterials. Part. Fibre Toxicol. 2019, 16, 18. [Google Scholar] [CrossRef]
- Fatkhutdinova, L.M.; Khaliullin, T.O.; Vasil’yeva, O.L.; Zalyalov, R.R.; Mustafin, I.G.; Kisin, E.R.; Birch, M.E.; Yanamala, N.; Shvedova, A.A. Fibrosis biomarkers in workers exposed to MWCNTs. Toxicol. Appl. Pharmacol. 2016, 299, 125–131. [Google Scholar] [CrossRef]
- Shvedova, A.A.; Yanamala, N.; Kisin, E.R.; Khailullin, T.O.; Birch, M.E.; Fatkhutdinova, L.M. Integrated Analysis of Dysregulated ncRNA and mRNA Expression Profiles in Humans Exposed to Carbon Nanotubes. PLoS ONE 2016, 11, e0150628. [Google Scholar] [CrossRef] [PubMed]
- Borm, P.J.A.; Driscoll, K.E. The hazards and risks of inhaled poorly soluble particles-where do we stand after 30 years of research? Part. Fibre Toxicol. 2019, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Valberg, P.A.; Long, C.M.; Sax, S.N. Integrating studies on carcinogenic risk of carbon black: Epidemiology, animal exposures, and mechanism of action. J. Occup. Environ. Med. 2006, 48, 1291–1307. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, R.; Thakur, A.K. The toxicological mechanisms of environmental soot (black carbon) and carbon black: Focus on oxidative stress and inflammatory pathways. Front. Immunol. 2017, 8, 763. [Google Scholar] [CrossRef]
- Diabaté, S.; Bergfeldt, B.; Plaumann, D.; Übel, C.; Weiss, C. Anti-oxidative and inflammatory responses induced by fly ash particles and carbon black in lung epithelial cells. Anal. Bioanal. Chem. 2011, 401, 3197–3212. [Google Scholar] [CrossRef]
- Schins, R.P.; Knaapen, A.M. Genotoxicity of poorly soluble particles. Inhal. Toxicol. 2007, 19, 189–198. [Google Scholar] [CrossRef]
- Guhad, F. Introduction to the 3Rs (refinement, reduction and replacement). J. Am. Assoc. Lab. Anim. Sci. 2005, 44, 58–59. [Google Scholar]
- Lacroix, G.; Koch, W.; Ritter, D.; Gutleb, A.C.; Larsen, S.T.; Loret, T.; Zanetti, F.; Constant, S.; Chortarea, S.; Rothen-Rutishauser, B. Air–liquid Interface in vitro models for respiratory toxicology research: Consensus workshop and recommendations. Appl. Vitr. Toxicol. 2018, 4, 91–106. [Google Scholar] [CrossRef]
- Paur, H.-R.; Cassee, F.R.; Teeguarden, J.; Fissan, H.; Diabate, S.; Aufderheide, M.; Kreyling, W.G.; Hänninen, O.; Kasper, G.; Riediker, M.; et al. In-vitro cell exposure studies for the assessment of nanoparticle toxicity in the lung—A dialog between aerosol science and biology. J. Aerosol Sci. 2011, 42, 668–692. [Google Scholar] [CrossRef]
- Mülhopt, S.; Dilger, M.; Diabaté, S.; Schlager, C.; Krebs, T.; Zimmermann, R.; Buters, J.; Oeder, S.; Wäscher, T.; Weiss, C.; et al. Toxicity testing of combustion aerosols at the air–liquid interface with a self-contained and easy-to-use exposure system. J. Aerosol Sci. 2016, 96, 38–55. [Google Scholar] [CrossRef]
- Ihantola, T.; Di Bucchianico, S.; Happo, M.; Ihalainen, M.; Uski, O.; Bauer, S.; Kuuspalo, K.; Sippula, O.; Tissari, J.; Oeder, S. Influence of wood species on toxicity of log-wood stove combustion aerosols: A parallel animal and air-liquid interface cell exposure study on spruce and pine smoke. Part. Fibre Toxicol. 2020, 17, 27. [Google Scholar] [CrossRef] [PubMed]
- Li, X. In vitro toxicity testing of cigarette smoke based on the air-liquid interface exposure: A review. Toxicol. Vitr. 2016, 36, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.; Bonstingl, G.; Höfler, A.; Meindl, C.; Leitinger, G.; Pieber, T.R.; Roblegg, E. Comparison of two in vitro systems to assess cellular effects of nanoparticles-containing aerosols. Toxicol. Vitr. 2013, 27, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Friesen, A.; Fritsch-Decker, S.; Hufnagel, M.; Mülhopt, S.; Stapf, D.; Hartwig, A.; Weiss, C. Comparing a-Quartz-Induced Cytotoxicity and Interleukin-8 Release in Pulmonary Mono- and Co-Cultures Exposed under Submerged and Air-Liquid Interface Conditions. Int. J. Mol. Sci. 2022, 23, 6412. [Google Scholar] [CrossRef] [PubMed]
- Friesen, A.; Fritsch-Decker, S.; Hufnagel, M.; Mülhopt, S.; Stapf, D.; Weiss, C.; Hartwig, A. Gene Expression Profiling of Mono-and Co-Culture Models of the Respiratory Tract Exposed to Crystalline Quartz under Submerged and Air-Liquid Interface Conditions. Int. J. Mol. Sci. 2022, 23, 7773. [Google Scholar] [CrossRef] [PubMed]
- Kletting, S.; Barthold, S.; Repnik, U.; Griffiths, G.; Loretz, B.; Schneider-Daum, N.; de Souza Carvalho-Wodarz, C.; Lehr, C.-M. Co-culture of human alveolar epithelial (hAELVi) and macrophage (THP-1) cell lines. ALTEX 2018, 35, 211–222. [Google Scholar] [CrossRef]
- Loret, T.; Peyret, E.; Dubreuil, M.; Aguerre-Chariol, O.; Bressot, C.; le Bihan, O.; Amodeo, T.; Trouiller, B.; Braun, A.; Egles, C.; et al. Air-liquid interface exposure to aerosols of poorly soluble nanomaterials induces different biological activation levels compared to exposure to suspensions. Part. Fibre Toxicol. 2016, 13, 58. [Google Scholar] [CrossRef]
- Barosova, H.; Karakocak, B.B.; Septiadi, D.; Petri-Fink, A.; Stone, V.; Rothen-Rutishauser, B. An In Vitro Lung System to Assess the Proinflammatory Hazard of Carbon Nanotube Aerosols. Int. J. Mol. Sci. 2020, 21, 5335. [Google Scholar] [CrossRef]
- Hilton, G.; Barosova, H.; Petri-Fink, A.; Rothen-Rutishauser, B.; Bereman, M. Leveraging proteomics to compare submerged versus air-liquid interface carbon nanotube exposure to a 3D lung cell model. Toxicol. Vitr. 2019, 54, 58–66. [Google Scholar] [CrossRef]
- Skuland, T.; Låg, M.; Gutleb, A.C.; Brinchmann, B.C.; Serchi, T.; Øvrevik, J.; Holme, J.A.; Refsnes, M. Pro-inflammatory effects of crystalline- and nano-sized non-crystalline silica particles in a 3D alveolar model. Part. Fibre Toxicol. 2020, 17, 13. [Google Scholar] [CrossRef] [PubMed]
- Hufnagel, M.; Neuberger, R.; Wall, J.; Link, M.; Friesen, A.; Hartwig, A. Impact of Differentiated Macrophage-Like Cells on the Transcriptional Toxicity Profile of CuO Nanoparticles in Co-Cultured Lung Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 5044. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, S.; Hartwig, A. PARP1 Is Required for ATM-Mediated p53 Activation and p53-Mediated Gene Expression after Ionizing Radiation. Chem. Res. Toxicol. 2020, 33, 1933–1940. [Google Scholar] [CrossRef]
- Izzotti, A.; Cartiglia, C.; Balansky, R.; D’Agostini, F.; Longobardi, M.; De Flora, S. Selective induction of gene expression in rat lung by hexavalent chromium. Mol. Carcinog. 2002, 35, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, P.; Fischer, F.; Sann, J.; Walter, D.; Hartwig, A. Impact of Nano-and Micro-Sized Chromium (III) Particles on Cytotoxicity and Gene Expression Profiles Related to Genomic Stability in Human Keratinocytes and Alveolar Epithelial Cells. Nanomaterials 2022, 12, 1294. [Google Scholar] [CrossRef]
- Ye, J.; Shi, X.; Jones, W.; Rojanasakul, Y.; Cheng, N.; Schwegler-Berry, D.; Baron, P.; Deye, G.J.; Li, C.; Castranova, V. Critical role of glass fiber length in TNF-alpha production and transcription factor activation in macrophages. Am. J. Physiol. 1999, 276, L426–L434. [Google Scholar]
- Dilger, M.; Orasche, J.; Zimmermann, R.; Paur, H.-R.; Diabaté, S.; Weiss, C. Toxicity of wood smoke particles in human A549 lung epithelial cells: The role of PAHs, soot and zinc. Arch. Toxicol. 2016, 90, 3029–3044. [Google Scholar] [CrossRef]
- Kang, M.; Lim, C.-H.; Han, J.-H. Comparison of toxicity and deposition of nano-sized carbon black aerosol prepared with or without dispersing sonication. Toxicol. Res. 2013, 29, 121–127. [Google Scholar] [CrossRef]
- Guzmán, E.; Santini, E.; Zabiegaj, D.; Ferrari, M.; Liggieri, L.; Ravera, F. Interaction of carbon black particles and dipalmitoylphosphatidylcholine at the water/air interface: Thermodynamics and rheology. J. Phys. Chem. C 2015, 119, 26937–26947. [Google Scholar] [CrossRef]
- Martin, T.R.; Meyer, S.W.; Luchtel, D.R. An evaluation of the toxicity of carbon fiber composites for lung cells in vitro and in vivo. Environ. Res. 1989, 49, 246–261. [Google Scholar] [CrossRef] [PubMed]
- Chapple, R.; Chivas-Joly, C.; Kose, O.; Erskine, E.L.; Ferry, L.; Lopez-Cuesta, J.-M.; Kandola, B.K.; Forest, V. Graphene oxide incorporating carbon fibre-reinforced composites submitted to simultaneous impact and fire: Physicochemical characterisation and toxicology of the by-products. J. Hazard. Mater. 2022, 424, 127544. [Google Scholar] [CrossRef]
- Westphal Götz, A.; Nina, R.; Alexander, B.; Weber Daniel, G.; Isabell, F.; Christian, M.; Nina, K.; Bryan, H.; Mattenklott, M.; Thomas, B.; et al. Multi-walled carbon nanotubes induce stronger migration of inflammatory cells in vitro than asbestos or granular particles but a similar pattern of inflammatory mediators. Toxicol. Vitr. 2019, 58, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gunter, M.E.; Fukagawa, N.K. Differential activation of the inflammasome in THP-1 cells exposed to chrysotile asbestos and Libby “six-mix” amphiboles and subsequent activation of BEAS-2B cells. Cytokine 2012, 60, 718–730. [Google Scholar] [CrossRef] [PubMed]
- Di Ianni, E.; Erdem, J.S.; Møller, P.; Sahlgren, N.M.; Poulsen, S.S.; Knudsen, K.B.; Zienolddiny, S.; Saber, A.T.; Wallin, H.; Vogel, U. In vitro-in vivo correlations of pulmonary inflammogenicity and genotoxicity of MWCNT. Part. Fibre Toxicol. 2021, 18, 25. [Google Scholar] [CrossRef] [PubMed]
- Ventura, C.; Pereira, J.F.S.; Matos, P.; Marques, B.; Jordan, P.; Sousa-Uva, A.; Silva, M.J. Cytotoxicity and genotoxicity of MWCNT-7 and crocidolite: Assessment in alveolar epithelial cells versus their coculture with monocyte-derived macrophages. Nanotoxicology 2020, 14, 479–503. [Google Scholar] [CrossRef]
- Vuong, N.Q.; Goegan, P.; Mohottalage, S.; Breznan, D.; Ariganello, M.; Williams, A.; Elisma, F.; Karthikeyan, S.; Vincent, R.; Kumarathasan, P. Proteomic changes in human lung epithelial cells (A549) in response to carbon black and titanium dioxide exposures. J. Proteom. 2016, 149, 53–63. [Google Scholar] [CrossRef]
- Godiska, R.; Chantry, D.; Raport, C.J.; Sozzani, S.; Allavena, P.; Leviten, D.; Mantovani, A.; Gray, P.W. Human macrophage–derived chemokine (MDC), a novel chemoattractant for monocytes, monocyte-derived dendritic cells, and natural killer cells. J. Exp. Med. 1997, 185, 1595–1604. [Google Scholar] [CrossRef]
- Sethi, J.K.; Hotamisligil, G.S. Metabolic Messengers: Tumour necrosis factor. Nat. Metab. 2021, 3, 1302–1312. [Google Scholar] [CrossRef]
- Tripathi, P.; Aggarwal, A. NF-kB transcription factor: A key player in the generation of immune response. Curr. Sci. 2006, 90, 519–531. [Google Scholar]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.; Angel, P.; Schorpp-Kistner, M. AP-1 subunits: Quarrel and harmony among siblings. J. Cell Sci. 2004, 117, 5965–5973. [Google Scholar] [CrossRef] [PubMed]
- Schreck, I.; Al-Rawi, M.; Mingot, J.-M.; Scholl, C.; Diefenbacher, M.E.; O’Donnell, P.; Bohmann, D.; Weiss, C. c-Jun localizes to the nucleus independent of its phosphorylation by and interaction with JNK and vice versa promotes nuclear accumulation of JNK. Biochem. Biophys. Res. Commun. 2011, 407, 735–740. [Google Scholar] [CrossRef]
- Shi, J.-H.; Sun, S.-C. Tumor necrosis factor receptor-associated factor regulation of nuclear factor κB and mitogen-activated protein kinase pathways. Front. Immunol. 2018, 9, 1849. [Google Scholar] [CrossRef] [PubMed]
- Iurlaro, R.; Muñoz-Pinedo, C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016, 283, 2640–2652. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Meng, Y.; Sheng, X.; Guan, Y.; Zhang, F.; Han, Z.; Kang, Y.; Tai, G.; Zhou, Y.; Cheng, H. Tunicamycin enhances human colon cancer cells to TRAIL-induced apoptosis by JNK-CHOP-mediated DR5 upregulation and the inhibition of the EGFR pathway. Anti Cancer Drugs 2017, 28, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.V.; Resar, L.M.; Emison, E.; Kim, S.; Li, Q.; Prescott, J.E.; Wonsey, D.; Zeller, K. Function of the c-Myc oncogenic transcription factor. Exp. Cell Res. 1999, 253, 63–77. [Google Scholar] [CrossRef]
- Wu, L.-W.; Mayo, L.D.; Dunbar, J.D.; Kessler, K.M.; Ozes, O.N.; Warren, R.S.; Donner, D.B. VRAP is an adaptor protein that binds KDR, a receptor for vascular endothelial cell growth factor. J. Biol. Chem. 2000, 275, 6059–6062. [Google Scholar] [CrossRef]
- Xie, S.; Wu, H.; Wang, Q.; Cogswell, J.P.; Husain, I.; Conn, C.; Stambrook, P.; Jhanwar-Uniyal, M.; Dai, W. Plk3 functionally links DNA damage to cell cycle arrest and apoptosis at least in part via the p53 pathway. J. Biol. Chem. 2001, 276, 43305–43312. [Google Scholar] [CrossRef]
- Oda, E.; Ohki, R.; Murasawa, H.; Nemoto, J.; Shibue, T.; Yamashita, T.; Tokino, T.; Taniguchi, T.; Tanaka, N. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 2000, 288, 1053–1058. [Google Scholar] [CrossRef]
- Shi, D.; Gu, W. Dual roles of MDM2 in the regulation of p53: Ubiquitination dependent and ubiquitination independent mechanisms of MDM2 repression of p53 activity. Genes Cancer 2012, 3, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, P.H.; Bendtsen, K.M.; Knudsen, K.B.; Poulsen, S.S.; Stoeger, T.; Vogel, U. Nanomaterial-and shape-dependency of TLR2 and TLR4 mediated signaling following pulmonary exposure to carbonaceous nanomaterials in mice. Part. Fibre Toxicol. 2021, 18, 40. [Google Scholar] [CrossRef] [PubMed]
- Clift, M.J.; Endes, C.; Vanhecke, D.; Wick, P.; Gehr, P.; Schins, R.P.; Petri-Fink, A.; Rothen-Rutishauser, B. A comparative study of different in vitro lung cell culture systems to assess the most beneficial tool for screening the potential adverse effects of carbon nanotubes. Toxicol. Sci. 2014, 137, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Figarol, A.; Pourchez, J.; Boudard, D.; Forest, V.; Akono, C.; Tulliani, J.-M.; Lecompte, J.-P.; Cottier, M.; Bernache-Assollant, D.; Grosseau, P. In vitro toxicity of carbon nanotubes, nano-graphite and carbon black, similar impacts of acid functionalization. Toxicol. Vitr. 2015, 30, 476–485. [Google Scholar] [CrossRef]
- Ursini, C.L.; Maiello, R.; Ciervo, A.; Fresegna, A.M.; Buresti, G.; Superti, F.; Marchetti, M.; Iavicoli, S.; Cavallo, D. Evaluation of uptake, cytotoxicity and inflammatory effects in respiratory cells exposed to pristine and-OH and-COOH functionalized multi-wall carbon nanotubes. J. Appl. Toxicol. 2016, 36, 394–403. [Google Scholar] [CrossRef]
- Vales, G.; Rubio, L.; Marcos, R. Genotoxic and cell-transformation effects of multi-walled carbon nanotubes (MWCNT) following in vitro sub-chronic exposures. J. Hazard. Mater. 2016, 306, 193–202. [Google Scholar] [CrossRef]
- Ye, S.-F.; Wu, Y.-H.; Hou, Z.-Q.; Zhang, Q.-Q. ROS and NF-κB are involved in upregulation of IL-8 in A549 cells exposed to multi-walled carbon nanotubes. Biochem. Biophys. Res. Commun. 2009, 379, 643–648. [Google Scholar] [CrossRef]
- Stermann, T.; Nguyen, T.; Stahlmecke, B.; Todea, A.M.; Woeste, S.; Hacheney, I.; Krutmann, J.; Unfried, K.; Schins, R.P.; Rossi, A. Carbon nanoparticles adversely affect CFTR expression and toxicologically relevant pathways. Sci. Rep. 2022, 12, 14255. [Google Scholar] [CrossRef]
- Mostovenko, E.; Young, T.; Muldoon, P.P.; Bishop, L.; Canal, C.G.; Vucetic, A.; Zeidler-Erdely, P.C.; Erdely, A.; Campen, M.J.; Ottens, A.K. Nanoparticle exposure driven circulating bioactive peptidome causes systemic inflammation and vascular dysfunction. Part. Fibre Toxicol. 2019, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- Totlandsdal, A.I.; Refsnes, M.; Låg, M. Mechanisms involved in ultrafine carbon black-induced release of IL-6 from primary rat epithelial lung cells. Toxicol. Vitr. 2010, 24, 10–20. [Google Scholar] [CrossRef]
- Reisetter, A.C.; Stebounova, L.V.; Baltrusaitis, J.; Powers, L.; Gupta, A.; Grassian, V.H.; Monick, M.M. Induction of inflammasome-dependent pyroptosis by carbon black nanoparticles. J. Biol. Chem. 2011, 286, 21844–21852. [Google Scholar] [CrossRef] [PubMed]
- Kesavardhana, S.; Malireddi, R.S.; Kanneganti, T.-D. Caspases in cell death, inflammation, and pyroptosis. Annu. Rev. Immunol. 2020, 38, 567–595. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Ma, Q. Integration of inflammation, fibrosis, and cancer induced by carbon nanotubes. Nanotoxicology 2019, 13, 1244–1274. [Google Scholar] [CrossRef] [PubMed]
- Hindman, B.; Ma, Q. Carbon nanotubes and crystalline silica induce matrix remodeling and contraction by stimulating myofibroblast transformation in a three-dimensional culture of human pulmonary fibroblasts: Role of dimension and rigidity. Arch. Toxicol. 2018, 92, 3291–3305. [Google Scholar] [CrossRef] [PubMed]
- Chortarea, S.; Zerimariam, F.; Barosova, H.; Septiadi, D.; Clift, M.J.; Petri-Fink, A.; Rothen-Rutishauser, B. Profibrotic activity of Multiwalled carbon nanotubes upon prolonged exposures in different human lung cell types. Appl. Vitr. Toxicol. 2019, 5, 47–61. [Google Scholar] [CrossRef]
- Barosova, H.; Maione, A.G.; Septiadi, D.; Sharma, M.; Haeni, L.; Balog, S.; O’Connell, O.; Jackson, G.R.; Brown, D.; Clippinger, A.J.; et al. Use of EpiAlveolar Lung Model to Predict Fibrotic Potential of Multiwalled Carbon Nanotubes. ACS Nano 2020, 14, 3941–3956. [Google Scholar] [CrossRef] [PubMed]
- Jauhiainen, A.; Thomsen, C.; Strömbom, L.; Grundevik, P.; Andersson, C.; Danielsson, A.; Andersson, M.K.; Nerman, O.; Rörkvist, L.; Ståhlberg, A. Distinct cytoplasmic and nuclear functions of the stress induced protein DDIT3/CHOP/GADD153. PLoS ONE 2012, 7, e33208. [Google Scholar] [CrossRef]
- Zhan, Q. Gadd45a, a p53-and BRCA1-regulated stress protein, in cellular response to DNA damage. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2005, 569, 133–143. [Google Scholar] [CrossRef]
- Yamashita, K.; Yoshioka, Y.; Higashisaka, K.; Morishita, Y.; Yoshida, T.; Fujimura, M.; Kayamuro, H.; Nabeshi, H.; Yamashita, T.; Nagano, K.; et al. Carbon nanotubes elicit DNA damage and inflammatory response relative to their size and shape. Inflammation 2010, 33, 276–280. [Google Scholar] [CrossRef]
- Siegrist, K.J.; Reynolds, S.H.; Porter, D.W.; Mercer, R.R.; Bauer, A.K.; Lowry, D.; Cena, L.; Stueckle, T.A.; Kashon, M.L.; Wiley, J. Mitsui-7, heat-treated, and nitrogen-doped multi-walled carbon nanotubes elicit genotoxicity in human lung epithelial cells. Part. Fibre Toxicol. 2019, 16, 36. [Google Scholar] [CrossRef]
- Toyokuni, S. Genotoxicity and carcinogenicity risk of carbon nanotubes. Adv. Drug Deliv. Rev. 2013, 65, 2098–2110. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, I.; Fruijtier-Pölloth, C.; Ngiewih, Y.; Levy, L. Evaluating the evidence on genotoxicity and reproductive toxicity of carbon black: A critical review. Crit. Rev. Toxicol. 2018, 48, 143–169. [Google Scholar] [CrossRef] [PubMed]
- Bhabra, G.; Sood, A.; Fisher, B.; Cartwright, L.; Saunders, M.; Evans, W.H.; Surprenant, A.; Lopez-Castejon, G.; Mann, S.; Davis, S.A. Nanoparticles can cause DNA damage across a cellular barrier. Nat. Nanotechnol. 2009, 4, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Mülhopt, S.; Diabaté, S.; Krebs, T.; Weiss, C.; Paur, H. Lung Toxicity Determination by In Vitro Exposure at the Air Liquid Interface with an Integrated Online Dose Measurement. J. Phys. Conf. Ser. 2009, 170, 012008. [Google Scholar] [CrossRef]
- Reddel, R.R.; Ke, Y.; Gerwin, B.I.; McMenamin, M.G.; Lechner, J.F.; Su, R.T.; Brash, D.E.; Park, J.-B.; Rhim, J.S.; Harris, C.C. Transformation of Human Bronchial Epithelial Cells by Infection with SV40 or Adenovirus-12 SV40 Hybrid Virus, or Transfection via Strontium Phosphate Coprecipitation with a Plasmid Containing SV40 Early Region Genes. Cancer Res. 1988, 48, 1904–1909. [Google Scholar]
- Tsuchiya, S.; Yamabe, M.; Yamaguchi, Y.; Kobayashi, Y.; Konno, T.; Tada, K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 1980, 26, 171–176. [Google Scholar] [CrossRef]
- Tsuchiya, S.; Kobayashi, Y.; Goto, Y.; Okumura, H.; Nakae, S.; Konno, T.; Tada, K. Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res. 1982, 42, 1530–1536. [Google Scholar]
- Fritsch-Decker, S.; Marquardt, C.; Stoeger, T.; Diabaté, S.; Weiss, C. Revisiting the stress paradigm for silica nanoparticles: Decoupling of the anti-oxidative defense, pro-inflammatory response and cytotoxicity. Arch. Toxicol. 2018, 92, 2163–2174. [Google Scholar] [CrossRef]
- Diabaté, S.; Armand, L.; Murugadoss, S.; Dilger, M.; Fritsch-Decker, S.; Schlager, C.; Béal, D.; Arnal, M.-E.; Biola-Clier, M.; Ambrose, S. Air–liquid interface exposure of lung epithelial cells to low doses of nanoparticles to assess pulmonary adverse effects. Nanomaterials 2021, 11, 65. [Google Scholar] [CrossRef]
- Hufnagel, M.; May, N.; Wall, J.; Wingert, N.; Garcia-Käufer, M.; Arif, A.; Hübner, C.; Berger, M.; Mülhopt, S.; Baumann, W.; et al. Impact of Nanocomposite Combustion Aerosols on A549 Cells and a 3D Airway Model. Nanomaterials 2021, 11, 1685. [Google Scholar] [CrossRef]
- Murugadoss, S.; Mülhopt, S.; Diabaté, S.; Ghosh, M.; Paur, H.-R.; Stapf, D.; Weiss, C.; Hoet, P.H. Agglomeration State of Titanium-Dioxide (TiO2) Nanomaterials Influences the Dose Deposition and Cytotoxic Responses in Human Bronchial Epithelial Cells at the Air-Liquid Interface. Nanomaterials 2021, 11, 3226. [Google Scholar] [CrossRef]
- Liu, B.; Pui, D.; Wang, X.; Lewis, C. Sampling of carbon fiber aerosols. Aerosol Sci. Technol. 1983, 2, 499–511. [Google Scholar] [CrossRef]
- Hartwig, A.; Dally, H.; Schlepegrell, R. Sensitive analysis of oxidative DNA damage in mammalian cells: Use of the bacterial Fpg protein in combination with alkaline unwinding. Toxicol. Lett. 1996, 88, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Hufnagel, M.; Schoch, S.; Wall, J.; Strauch, B.M.; Hartwig, A. Toxicity and Gene Expression Profiling of Copper-and Titanium-Based Nanoparticles Using Air–Liquid Interface Exposure. Chem. Res. Toxicol. 2020, 33, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
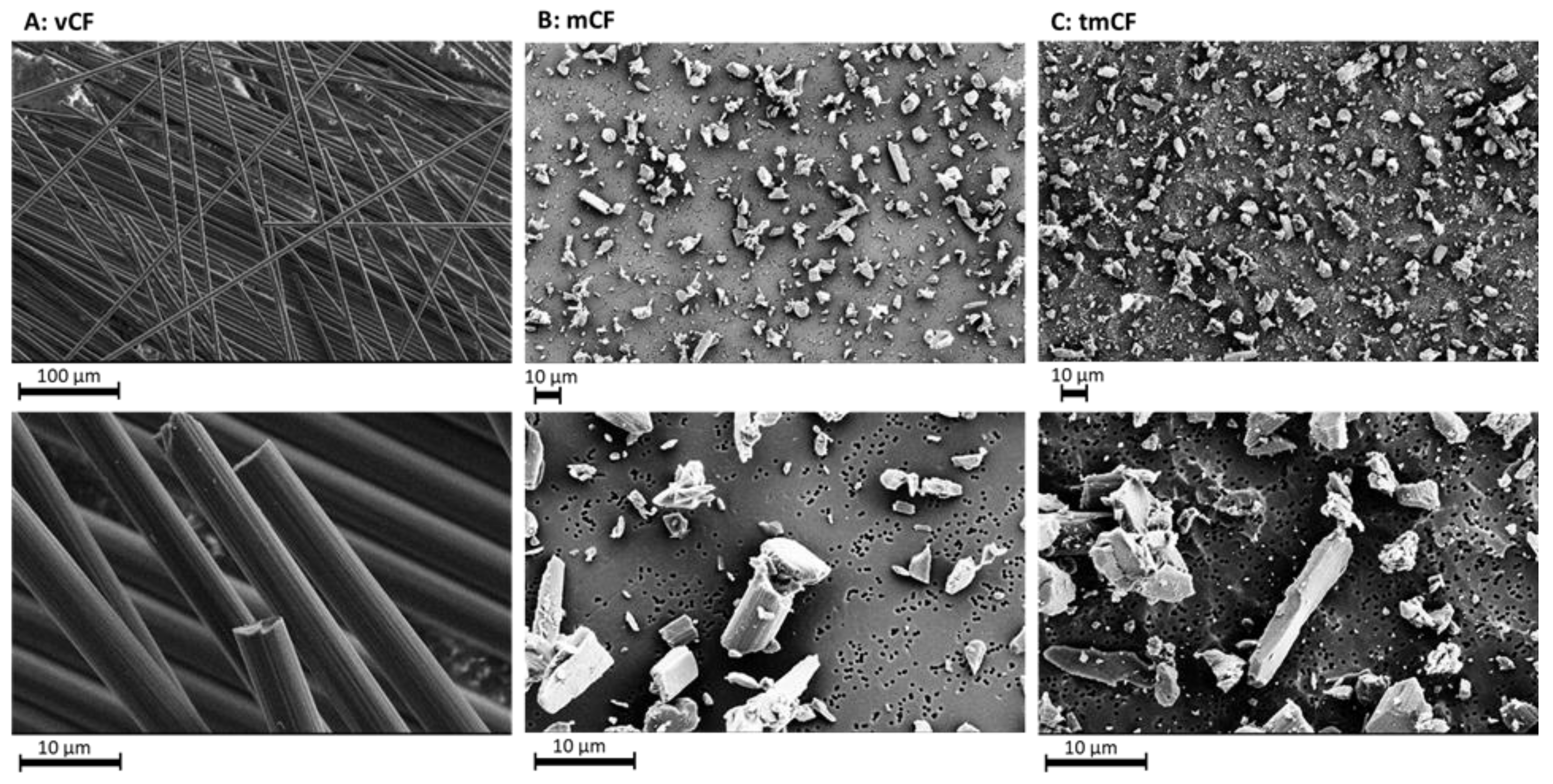
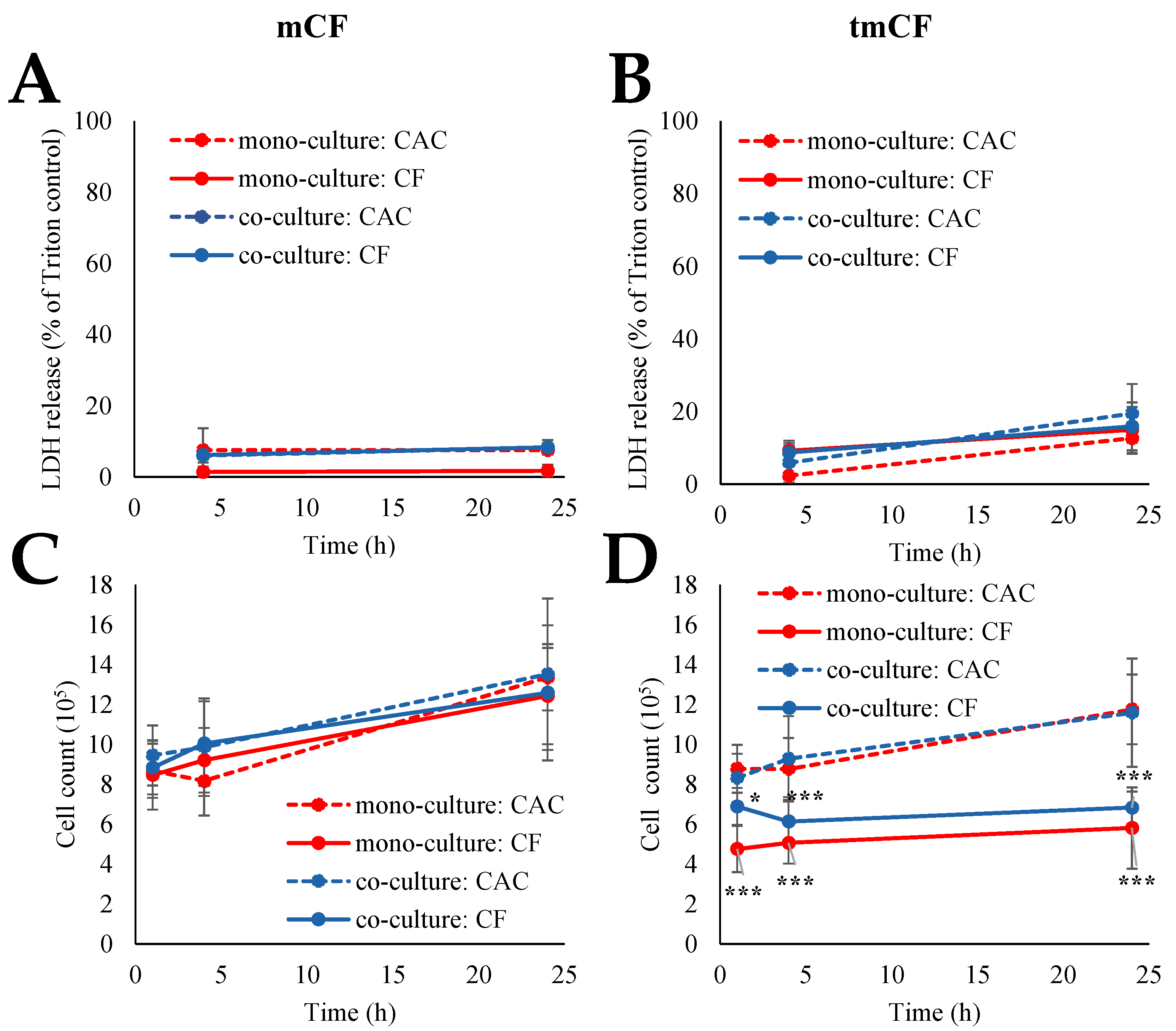
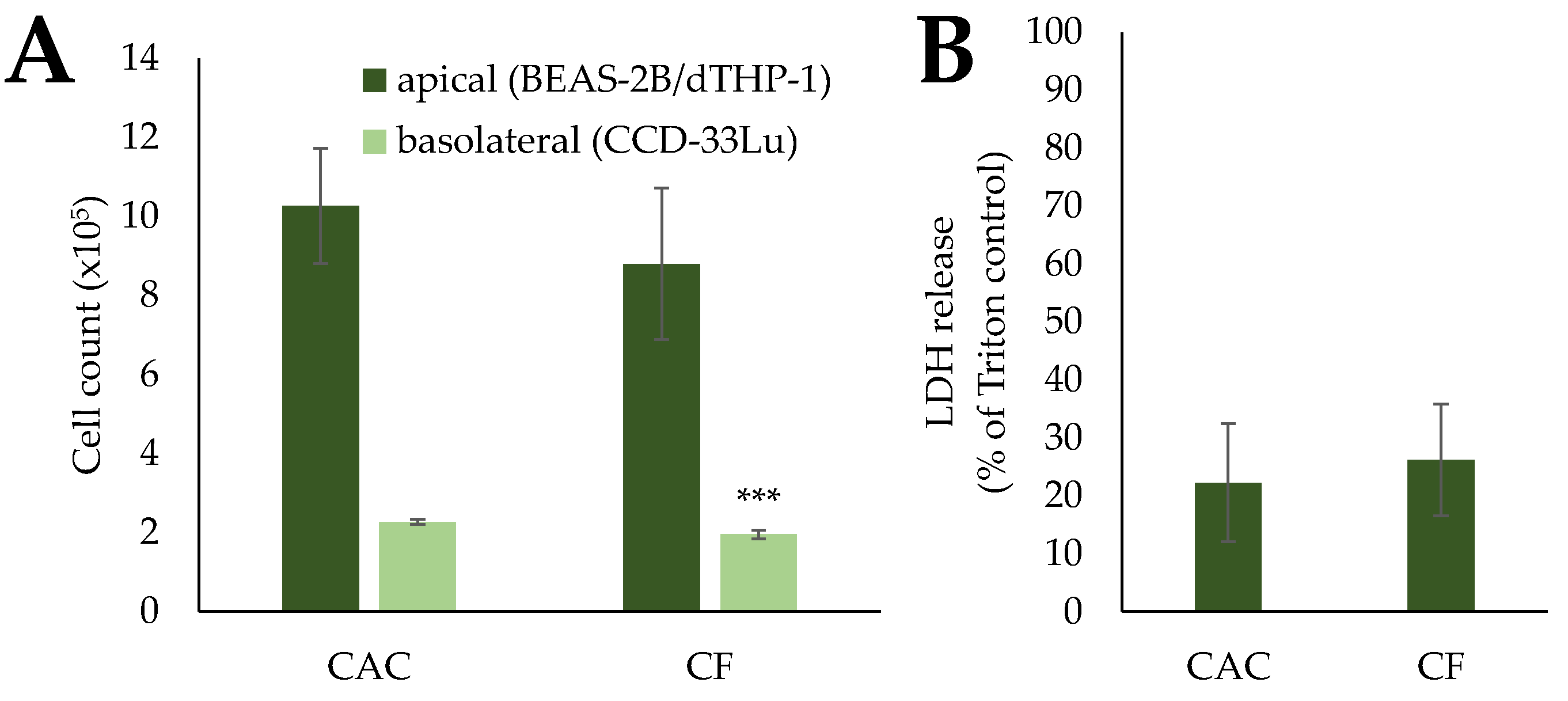
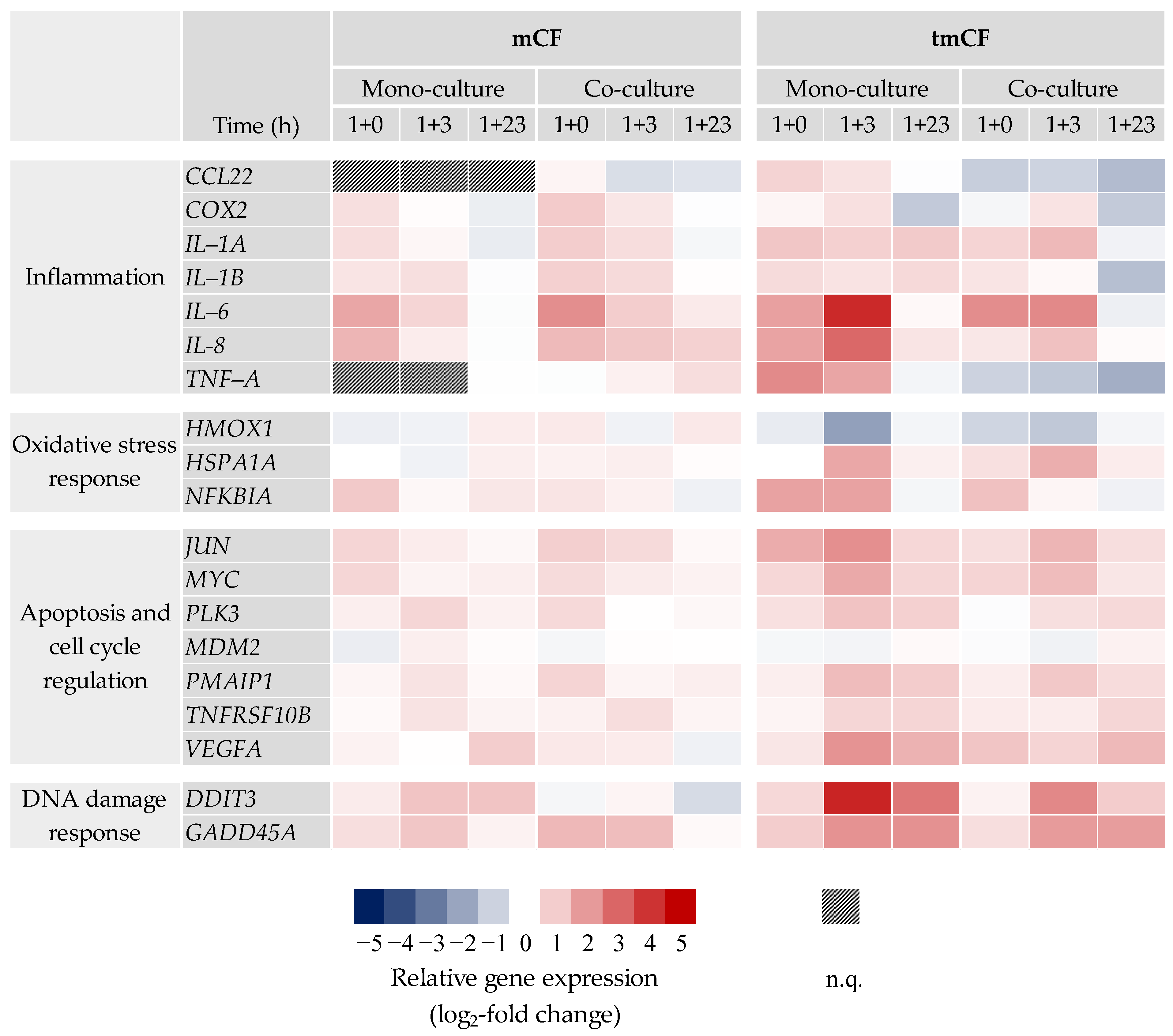


| mCF | tmCF | |
|---|---|---|
| Exposure Dose in AES | ||
| Number concentration (welas) cN a | 2.7 × 106 ± 8.9 × 105 cm3 | 8.8 × 106 ± 3.8 × 106 cm3 |
| Aerodynamic equivalent diameter dae a | 3.74 ± 0.89 µm | 4.90 ± 0.45 µm |
| Relevant in vitro dose on membranes | ||
| Online mass surface dose (QCM) | 7.00 ± 1.25 µg/cm2 | 6.55 ± 1.96 µg/cm2 |
| Mass surface dose b | 2.46 ± 2.88 µg/cm2 | 4.38 ± 4.58 µg/cm2 |
| Number surface dose cN b | 4934 ± 2795/cm2 | 3391 ± 3895/cm2 |
| Particle fraction | 53.7 ± 22.6% | 76.5 ± 25.5% |
| Fiber fraction | 26.2 ± 12.1% | 14.2 ± 15.1% |
| WHO fiber fraction | 20.1 ± 10.4% | 9.4 ± 10.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friesen, A.; Fritsch-Decker, S.; Mülhopt, S.; Quarz, C.; Mahl, J.; Baumann, W.; Hauser, M.; Wexler, M.; Schlager, C.; Gutmann, B.; et al. Comparing the Toxicological Responses of Pulmonary Air–Liquid Interface Models upon Exposure to Differentially Treated Carbon Fibers. Int. J. Mol. Sci. 2023, 24, 1927. https://doi.org/10.3390/ijms24031927
Friesen A, Fritsch-Decker S, Mülhopt S, Quarz C, Mahl J, Baumann W, Hauser M, Wexler M, Schlager C, Gutmann B, et al. Comparing the Toxicological Responses of Pulmonary Air–Liquid Interface Models upon Exposure to Differentially Treated Carbon Fibers. International Journal of Molecular Sciences. 2023; 24(3):1927. https://doi.org/10.3390/ijms24031927
Chicago/Turabian StyleFriesen, Alexandra, Susanne Fritsch-Decker, Sonja Mülhopt, Caroline Quarz, Jonathan Mahl, Werner Baumann, Manuela Hauser, Manuela Wexler, Christoph Schlager, Bastian Gutmann, and et al. 2023. "Comparing the Toxicological Responses of Pulmonary Air–Liquid Interface Models upon Exposure to Differentially Treated Carbon Fibers" International Journal of Molecular Sciences 24, no. 3: 1927. https://doi.org/10.3390/ijms24031927
APA StyleFriesen, A., Fritsch-Decker, S., Mülhopt, S., Quarz, C., Mahl, J., Baumann, W., Hauser, M., Wexler, M., Schlager, C., Gutmann, B., Krebs, T., Goßmann, A.-K., Weis, F., Hufnagel, M., Stapf, D., Hartwig, A., & Weiss, C. (2023). Comparing the Toxicological Responses of Pulmonary Air–Liquid Interface Models upon Exposure to Differentially Treated Carbon Fibers. International Journal of Molecular Sciences, 24(3), 1927. https://doi.org/10.3390/ijms24031927






