Antitumor Effect of Korean Red Ginseng through Blockade of PD-1/PD-L1 Interaction in a Humanized PD-L1 Knock-In MC38 Cancer Mouse Model
Abstract
1. Introduction
2. Results
2.1. RGE Blockade of Targeting Human PD-1/PD-L1
2.2. RGE Enhances Tumor-Infiltrating CD8+ Cell-Mediated CRC Cell Killing
2.3. Antitumor Effect of RGE in a Humanized PD-1/PD-L1 Knock-In Tumor Model
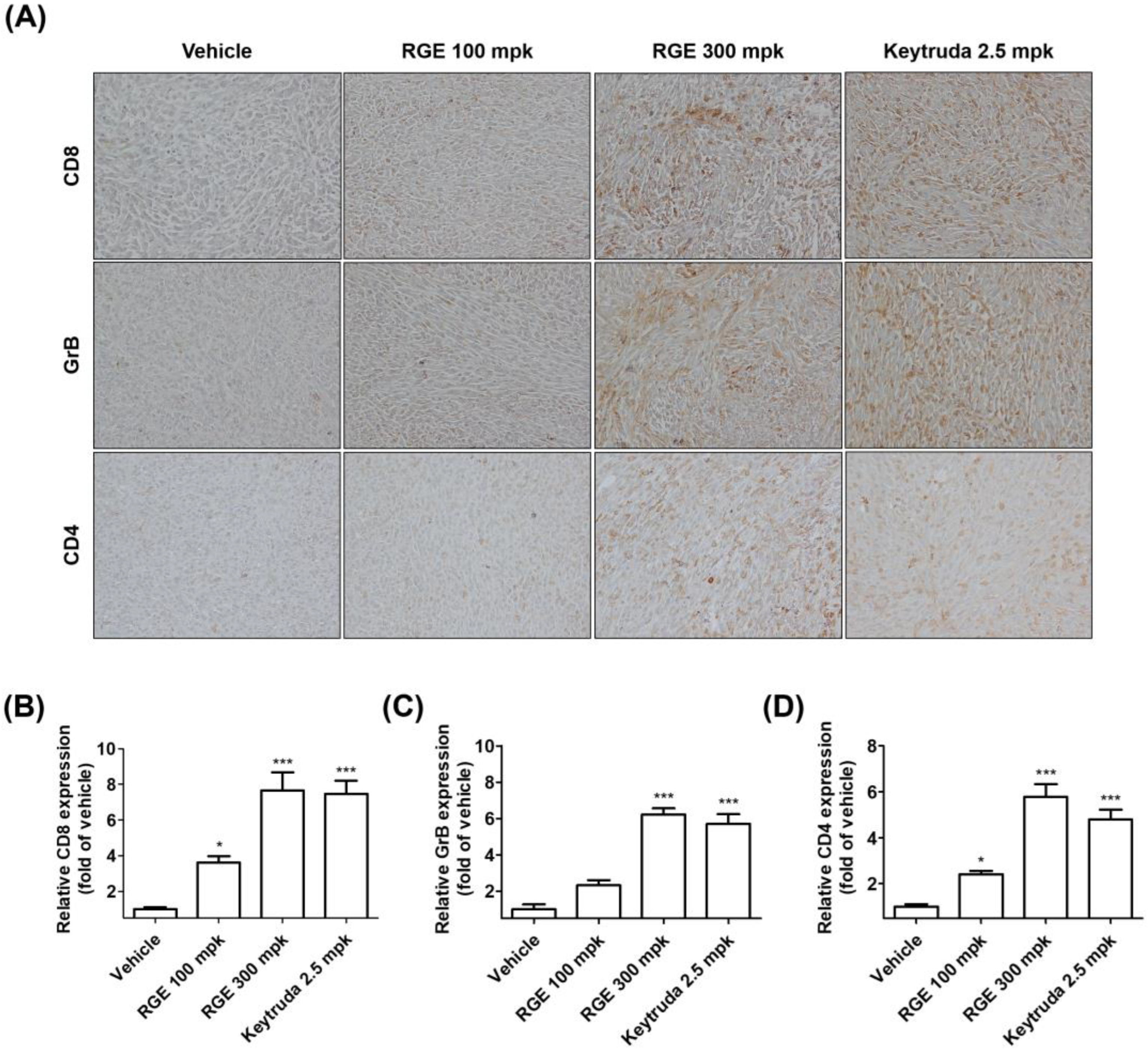
2.4. RGE Activates Tumor-Infiltrating CD8+ Cells in hPD-1/hPD-L1 MC38 Tumor Tissues
2.5. Identification of Ginsenoside Rg3 of RGE by HPLC-DAD Analysis
2.6. Validation of the Analytical HPLC Method
3. Discussion
4. Materials and Methods
4.1. Preparation of RGE
4.2. PD-1/PD-L1 Protein Interaction Assay
4.3. Human PD-L1 MC38 Cell Line
4.4. Humanized PD-1/PD-L1 Knock-In Tumor Model
4.5. Tumor-Infiltrating CD8+ T Cell Isolation and Stimulation
4.6. Cell Viability Assay
4.7. IL-2 Measurement Assay
4.8. Co-Culture Experiments with Tumor-Infiltrated CD8+ T Cells and MC38 Cells
4.9. Granzyme B Measurement Assay
4.10. In Vivo RGE Treatment
4.11. Immunohistochemistry
4.12. Sample Preparation for HPLC Analysis
4.13. Optimization of Chromatographic Conditions
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morad, G.; Helmink, B.A.; Sharma, P.; Wargo, J.A. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 2021, 184, 5309–5337. [Google Scholar] [CrossRef] [PubMed]
- Jalalvand, M.; Darbeheshti, F.; Rezaei, N. Immune checkpoint inhibitors: Review of the existing evidence and challenges in breast cancer. Immunotherapy 2021, 13, 587–603. [Google Scholar] [CrossRef] [PubMed]
- Doroshow, D.B.; Bhalla, S.; Beasley, M.B.; Sholl, L.M.; Kerr, K.M.; Gnjatic, S.; Wistuba, I.I.; Rimm, D.L.; Tsao, M.S.; Hirsch, F.R. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2021, 18, 345–362. [Google Scholar] [CrossRef]
- Gong, J.; Chehrazi-Raffle, A.; Reddi, S.; Salgia, R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: A comprehensive review of registration trials and future considerations. J. Immunother. Cancer 2018, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. Mech. Dis. 2021, 16, 223–249. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.H.; Kim, S.W.; Seo, H.W.; Youn, S.H.; Kyung, J.S.; Lee, Y.Y.; In, G.; Park, C.-K.; Han, C.-K. Physiological and pharmacological features of the non-saponin components in Korean Red Ginseng. J. Ginseng Res. 2020, 44, 527–537. [Google Scholar] [CrossRef]
- Shin, S.-H.; Ye, M.-K.; Lee, D.-W.; Chae, M.-H.; Hwang, Y.-J. Korean Red Ginseng and Ginsenoside Rg3 Suppress Asian Sand Dust-Induced Epithelial–Mesenchymal Transition in Nasal Epithelial Cells. Molecules 2022, 27, 2642. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Irfan, M.; Quah, Y.; Saba, E.; Kim, S.-D.; Park, S.-C.; Jeong, M.-G.; Kwak, Y.-S.; Rhee, M.H. The increasing hematopoietic effect of the combined treatment of Korean Red Ginseng and Colla corii asini on cyclophosphamide-induced immunosuppression in mice. J. Ginseng Res. 2021, 45, 591–598. [Google Scholar] [CrossRef]
- Xu, M.L.; Kim, H.-J.; Choi, Y.-R. Intake of Korean Red Ginseng Extract and Saponin Enhances the Protection Conferred by Vaccination with Inactivated Influenza a Virus. J. Ginseng Res. 2012, 36, 396–402. [Google Scholar] [CrossRef]
- Ham, S.W.; Kim, J.-K.; Jeon, H.-Y.; Kim, E.-J.; Jin, X.; Eun, K.; Park, C.G.; Lee, S.Y.; Seo, S.; Kim, J.Y.; et al. Korean Red ginseng extract inhibits glioblastoma propagation by blocking the Wnt signaling pathway. J. Ethnopharmacol. 2019, 236, 393–400. [Google Scholar] [CrossRef]
- Jeon, H.; Kim, H.Y.; Bae, C.H.; Lee, Y.; Kim, S. Korean Red Ginseng Regulates Intestinal Tight Junction and Inflammation in the Colon of a Parkinson’s Disease Mouse Model. J. Med. Food 2020, 23, 1231–1237. [Google Scholar] [CrossRef]
- Bae, C.H.; Kim, J.; Nam, W.; Kim, H.; Kim, J.; Nam, B.; Park, S.; Lee, J.; Sim, J. Fermented Red Ginseng Alleviates Ovalbumin-Induced Inflammation in Mice by Suppressing Interleukin-4 and Immunoglobulin E Expression. J. Med. Food 2021, 24, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.J.; Kim, S.K.; Lee, N.Y.; Choi, Y.R.; Kim, H.S.; Gupta, H.; Youn, G.S.; Sung, H.; Shin, M.J.; Suk, K.T. Effect of Korean Red Ginseng on metabolic syndrome. J. Ginseng Res. 2021, 45, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.-S.; Kang, H.-R.; Jung, C.-Y.; Park, S.-S.; Lee, S.-H.; Kim, E.-J. Efficacy of Korean red ginseng (Panax ginseng) for middle-aged and moderate level of chronic fatigue patients: A randomized, double-blind, placebo-controlled trial. Complement. Ther. Med. 2019, 48, 102246. [Google Scholar] [CrossRef]
- Kang, J.-Y.; Kim, D.-Y.; Lee, J.-S.; Hwang, S.-J.; Kim, G.-H.; Hyun, S.-H.; Son, C.-G. Korean Red Ginseng Ameliorates Fatigue via Modulation of 5-HT and Corticosterone in a Sleep-Deprived Mouse Model. Nutrients 2021, 13, 3121. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-S.; Lee, J.-H.; Oh, M.; Choi, K.-M.; Jeong, M.R.; Park, J.-D.; Kwon, D.Y.; Ha, K.-C.; Park, E.-O.; Lee, N.; et al. Preventive Effect of Korean Red Ginseng for Acute Respiratory Illness: A Randomized and Double-Blind Clinical Trial. J. Korean Med. Sci. 2012, 27, 1472–1478. [Google Scholar] [CrossRef]
- Zheng, Y.; Lee, E.-H.; Lee, J.-H.; In, G.; Kim, J.; Lee, M.-H.; Lee, O.-H.; Kang, I.-J. Preclinical Research on a Mixture of Red Ginseng and Licorice Extracts in the Treatment and Prevention of Obesity. Nutrients 2020, 12, 2744. [Google Scholar] [CrossRef]
- Park, N.Y.; Rico, C.W.; Lee, S.C.; Kang, M.Y. Comparative effects of doenjang prepared from soybean and brown rice on the body weight and lipid metabolism in high fat-fed mice. J. Clin. Biochem. Nutr. 2012, 51, 235–240. [Google Scholar] [CrossRef]
- Shin, K.K.; Yi, Y.-S.; Kim, J.K.; Kim, H.; Hossain, M.A.; Kim, J.-H.; Cho, J.Y. Korean Red Ginseng Plays an Anti-Aging Role by Modulating Expression of Aging-Related Genes and Immune Cell Subsets. Molecules 2020, 25, 1492. [Google Scholar] [CrossRef]
- Ahn, H.; Han, B.-C.; Hong, E.-J.; An, B.-S.; Lee, E.; Lee, S.-H.; Lee, G.-S. Korean Red Ginseng attenuates ultraviolet-mediated inflammasome activation in keratinocytes. J. Ginseng Res. 2021, 45, 456–463. [Google Scholar] [CrossRef]
- Chung, Y.S.; Lee, I.O.; Lee, J.-Y.; Nam, E.J.; Kim, S.W.; Kim, Y.T.; Kim, S. Effects of Korean Red Ginseng (Panax ginseng C.A. Meyer) on Menopausal Symptoms in Premenopausal Women After Gynecologic Cancer Surgery: A Double-Blind, Randomized Controlled Trial. J. Altern. Complement. Med. 2021, 27, 66–72. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, W.S.; Yu, T.; Sung, G.-H.; Park, K.W.; Yoon, K.; Son, Y.-J.; Hwang, H.; Kwak, Y.-S.; Lee, C.-M.; et al. ATF-2/CREB/IRF-3-targeted anti-inflammatory activity of Korean red ginseng water extract. J. Ethnopharmacol. 2014, 154, 218–228. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.; Cho, J. Korean Red Ginseng Extract Exhibits Neuroprotective Effects through Inhibition of Apoptotic Cell Death. Biol. Pharm. Bull. 2014, 37, 938–946. [Google Scholar] [CrossRef]
- Kim, S.J.; Kwak, H.J.; Kim, D.S.; Choi, H.M.; Sim, J.E.; Kim, S.H.; Um, J.Y.; Hong, S.H. Protective mechanism of Korean Red Ginseng in cisplatin-induced ototoxicity through attenuation of nuclear factor-κB and caspase-1 activation. Mol. Med. Rep. 2015, 12, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Won, J.E.; Lee, J.; Han, H.D.; Lee, Y. Korean Red Ginseng attenuates Di-(2-ethylhexyl) phthalate-induced inflammatory response in endometrial cancer cells and an endometriosis mouse model. J. Ginseng Res. 2022, 46, 592–600. [Google Scholar] [CrossRef]
- Kim, H.I.; Lee, I.; Jung, Y.S.; Chon, S.J.; Yun, B.H.; Seo, S.K. Korean red ginseng induces extrinsic and intrinsic apoptotic pathways in MCF-7 breast cancer cells and MCF-10A non-malignant breast cells. J. Obstet. Gynaecol. Res. 2021, 47, 2758–2766. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.-J.; Jin, H.; Lee, B.-Y. The non-saponin fraction of Korean Red Ginseng ameliorates sarcopenia by regulating immune homeostasis in 22–26-month-old C57BL/6J mice. J. Ginseng Res. 2022, 46, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.S.; Yoon, M. Korean red ginseng (Panax ginseng) inhibits obesity and improves lipid metabolism in high fat diet-fed castrated mice. J. Ethnopharmacol. 2018, 210, 80–87. [Google Scholar] [CrossRef]
- Lee, S.H.; Yu, J.; Cho, S.H. Effect of a combination of Korean red ginseng extract and probiotics on the prevention of atopic dermatitis in a murine model. J. Ethnopharmacol. 2021, 283, 114687. [Google Scholar] [CrossRef]
- Yim, N.H.; Kim, Y.S.; Chung, H.S. Inhibition of Programmed Death Receptor-1/Programmed Death Ligand-1 Interactions by Ginsenoside Metabolites. Molecules 2020, 25, 2068. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Yao, H.; Li, C.; Fang, J.-Y.; Xu, J. Regulation of PD-L1: Emerging Routes for Targeting Tumor Immune Evasion. Front. Pharmacol. 2018, 9, 536. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Huang, Q.; Xie, Y.; Wu, X.; Ma, H.; Zhang, Y.; Xia, Y. Improvement of the anticancer efficacy of PD-1/PD-L1 blockade via combination therapy and PD-L1 regulation. J. Hematol. Oncol. 2022, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Ge, J.; Xiang, B.; Wu, X.; Ma, J.; Zhou, M.; Li, X.; et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol. Cancer 2019, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Pagès, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.-S.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet 2018, 391, 2128–2139. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.; Stadler, Z.K.; Cercek, A.; Mendelsohn, R.B.; Shia, J.; Segal, N.H.; Diaz, L.A., Jr. Immunotherapy in colorectal cancer: Rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 361–375. [Google Scholar] [CrossRef]
- Casak, S.J.; Marcus, L.; Fashoyin-Aje, L.; Mushti, S.L.; Cheng, J.; Shen, Y.L.; Pierce, W.F.; Her, L.; Goldberg, K.B.; Theoret, M.R.; et al. FDA Approval Summary: Pembrolizumab for the First-line Treatment of Patients with MSI-H/dMMR Advanced Unresectable or Metastatic Colorectal Carcinoma. Clin. Cancer Res. 2021, 27, 4680–4684. [Google Scholar] [CrossRef]
- Keizer, R.J.; Huitema, A.D.R.; Schellens, J.H.M.; Beijnen, J.H. Clinical Pharmacokinetics of Therapeutic Monoclonal Antibodies. Clin. Pharmacokinet. 2010, 49, 493–507. [Google Scholar] [CrossRef]
- Subbotin, V.; Fiksel, G. Modeling multi-needle injection into solid tumor. Am. J. Cancer Res. 2019, 9, 2209–2215. [Google Scholar] [PubMed]
- Zhong, Z.; Vong, C.T.; Chen, F.; Tan, H.; Zhang, C.; Wang, N.; Cui, L.; Wang, Y.; Feng, Y. Immunomodulatory potential of natural products from herbal medicines as immune checkpoints inhibitors: Helping to fight against cancer via multiple targets. Med. Res. Rev. 2022, 42, 1246–1279. [Google Scholar] [CrossRef]
- Im, E.; Sim, D.Y.; Lee, H.-J.; Park, J.E.; Park, W.Y.; Ko, S.; Kim, B.; Shim, B.S.; Kim, S.-H. Immune functions as a ligand or a receptor, cancer prognosis potential, clinical implication of VISTA in cancer immunotherapy. Semin. Cancer Biol. 2021, 86, 1066–1075. [Google Scholar] [CrossRef]
- Kim, E.J.; Kwon, K.A.; Lee, Y.E.; Kim, J.H.; Kim, S.H.; Kim, J.H. Korean Red Ginseng extract reduces hypoxia-induced epithelial-mesenchymal transition by repressing NF-κB and ERK1/2 pathways in colon cancer. J. Ginseng Res. 2018, 42, 288–297. [Google Scholar] [CrossRef]
- Jeong, Y.A.; Kim, B.R.; Kim, D.Y.; Jeong, S.; Na, Y.J.; Kim, J.L.; Yun, H.K.; Kim, B.G.; Park, S.H.; Jo, M.J.; et al. Korean Red Ginseng Extract Increases Apoptosis by Activation of the Noxa Pathway in Colorectal Cancer. Nutrients 2019, 11, 2026. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Yang, Y.; Yang, Y.; Zhang, Y.; Yue, Z.; Pan, Z.; Ren, X. Ginsenoside Rg3 attenuates cisplatin resistance in lung cancer by downregulating PD-L1 and resuming immune. Biomed. Pharmacother. 2017, 96, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhao, J.; Qu, L.; Duan, Z.; Fu, R.; Zhu, C.; Fan, D. Ginsenoside Rh4 suppresses aerobic glycolysis and the expression of PD-L1 via targeting AKT in esophageal cancer. Biochem. Pharmacol. 2020, 178, 114038. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Yang, J.; Qu, L.; Deng, X.; Duan, Z.; Fu, R.; Liang, L.; Fan, D. Ginsenoside Rk1 induces apoptosis and downregulates the expression of PD-L1 by targeting the NF-κB pathway in lung adenocarcinoma. Food Funct. 2020, 11, 456–471. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yan, S.-J.; Zhang, H.-T.; Li, N.; Liu, T.; Zhang, Y.-L.; Li, X.-X.; Ma, Q.; Qiu, X.-C.; Fan, Q.-Y.; et al. Ginsenoside Rh2 enhances the antitumor immunological response of a melanoma mice model. Oncol. Lett. 2016, 13, 681–685. [Google Scholar] [CrossRef] [PubMed]



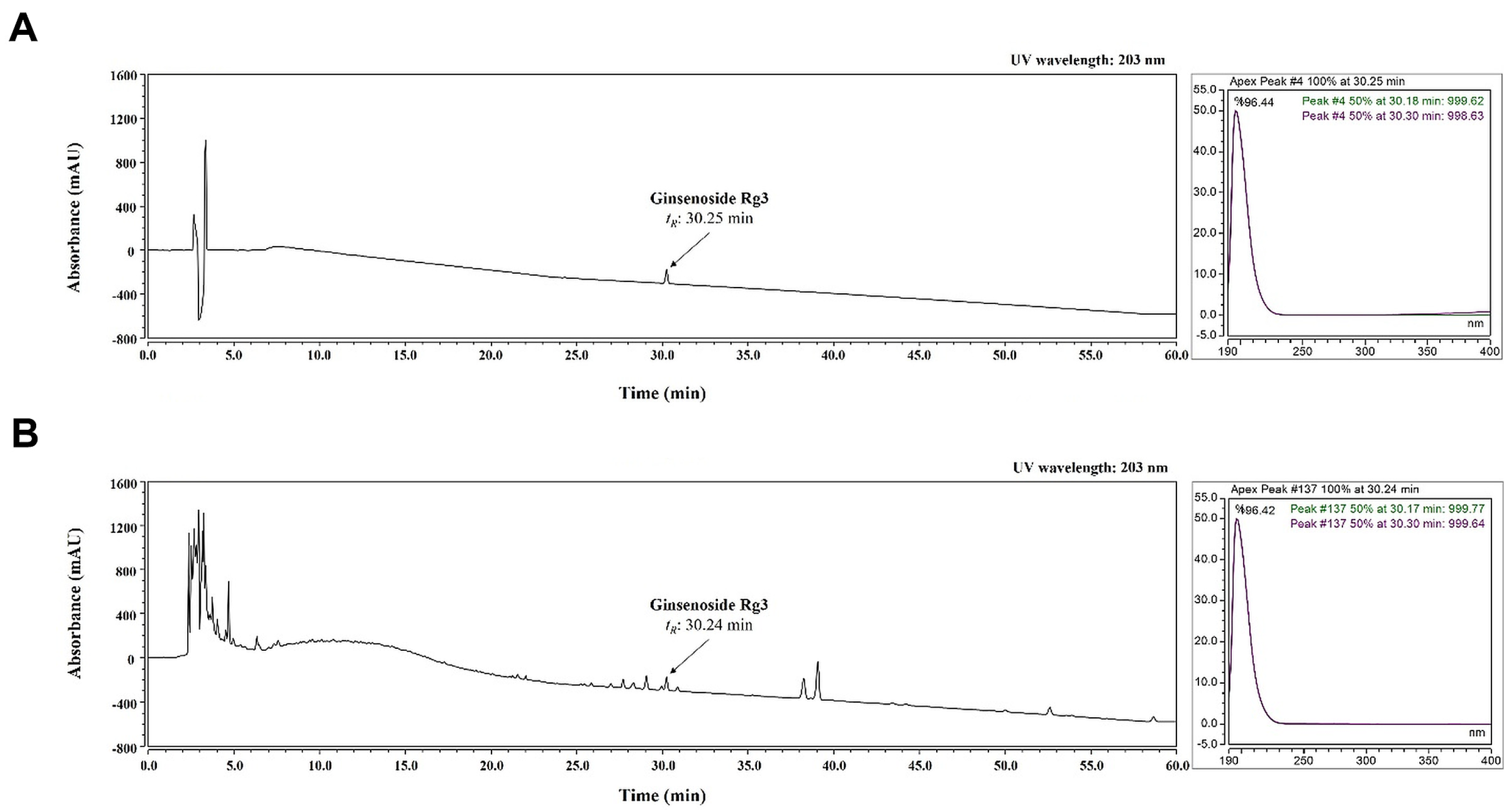
| Analytes | Regression Equation | R2 | Content (mg/g) |
|---|---|---|---|
| ginsenoside Rg3 | y = 0.0702x + 0.5987 | 0.9999 | 6.0423 ± 0.0228 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.-J.; Yang, J.-H.; Yang, H.J.; Cho, C.-K.; Choi, J.-G.; Chung, H.-S. Antitumor Effect of Korean Red Ginseng through Blockade of PD-1/PD-L1 Interaction in a Humanized PD-L1 Knock-In MC38 Cancer Mouse Model. Int. J. Mol. Sci. 2023, 24, 1894. https://doi.org/10.3390/ijms24031894
Lee E-J, Yang J-H, Yang HJ, Cho C-K, Choi J-G, Chung H-S. Antitumor Effect of Korean Red Ginseng through Blockade of PD-1/PD-L1 Interaction in a Humanized PD-L1 Knock-In MC38 Cancer Mouse Model. International Journal of Molecular Sciences. 2023; 24(3):1894. https://doi.org/10.3390/ijms24031894
Chicago/Turabian StyleLee, Eun-Ji, Ju-Hye Yang, Hye Jin Yang, Chong-Kwan Cho, Jang-Gi Choi, and Hwan-Suck Chung. 2023. "Antitumor Effect of Korean Red Ginseng through Blockade of PD-1/PD-L1 Interaction in a Humanized PD-L1 Knock-In MC38 Cancer Mouse Model" International Journal of Molecular Sciences 24, no. 3: 1894. https://doi.org/10.3390/ijms24031894
APA StyleLee, E.-J., Yang, J.-H., Yang, H. J., Cho, C.-K., Choi, J.-G., & Chung, H.-S. (2023). Antitumor Effect of Korean Red Ginseng through Blockade of PD-1/PD-L1 Interaction in a Humanized PD-L1 Knock-In MC38 Cancer Mouse Model. International Journal of Molecular Sciences, 24(3), 1894. https://doi.org/10.3390/ijms24031894







