Epigenetic Factors Related to Low Back Pain: A Systematic Review of the Current Literature
Abstract
1. Introduction
2. Materials and Methods
2.1. Review Design
2.2. Search Strategy
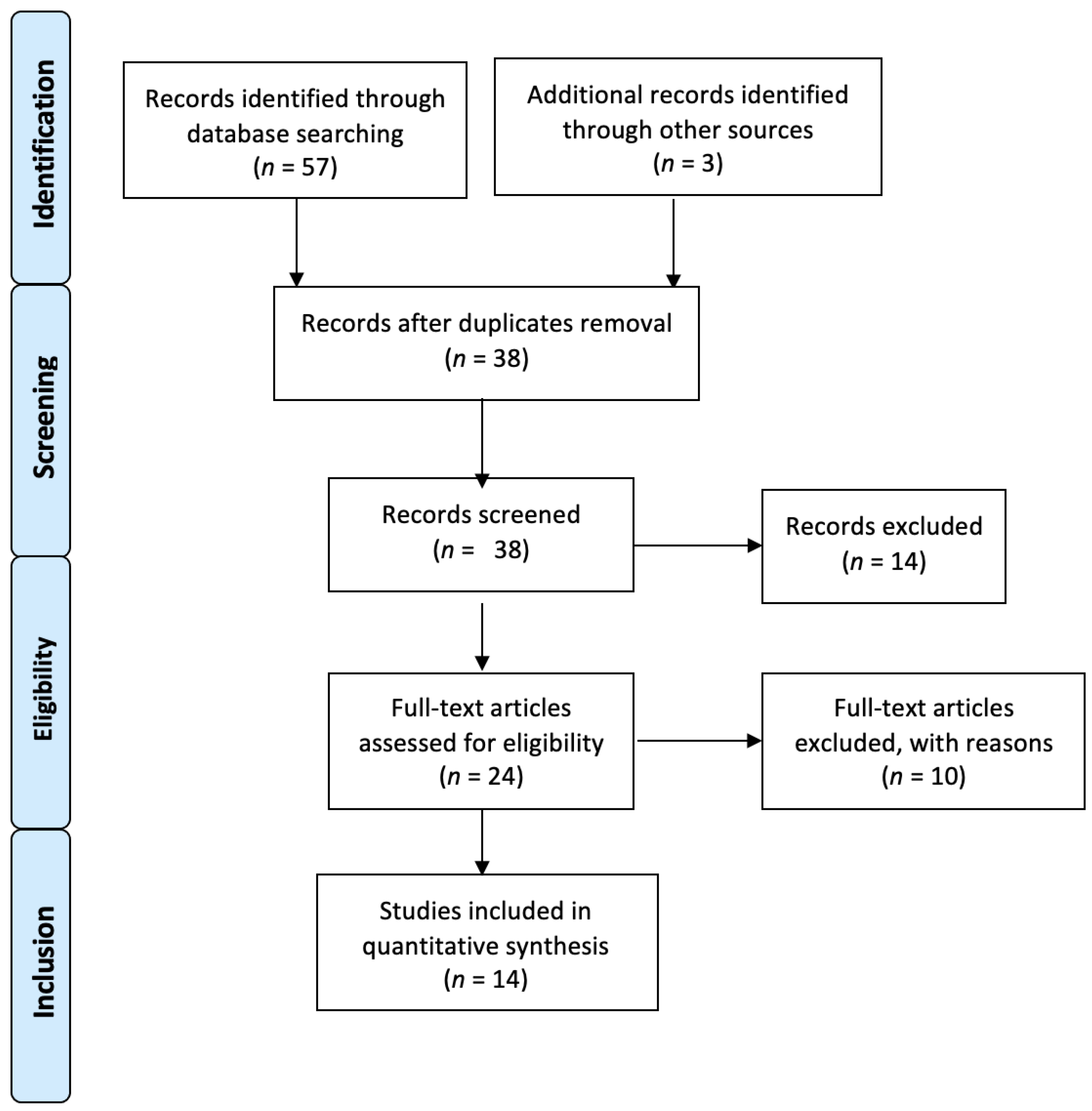
2.3. Study Selection
2.4. Data Extraction
2.5. Methodological Quality Assessment of Included Studies
3. Results
3.1. Included Studies
3.2. Cohort Characteristics
3.3. Pain and Spine Disease Evaluation
3.4. Epigenetic Factors Associated with Low Back Pain
| Study Design (Level of Evidence) | Study Population | Age (Mean/Range) Gender Ethnicity | Pain Assessment/Spine Disease | Type of Biological Sample and Technique Used | Gene(s) Involved | Results | Authors |
|---|---|---|---|---|---|---|---|
| Retrospective case–control (III) | Low back pain group: 10 Healthy pain-free patients: 23 Non-degenerative IVDs: 5 | Low back pain group: 45.6 ± 2.8 - 3 males - 5 females - 2 unknown Healthy pain-free: 41.2 ± 2.3 - 14 females - 9 males Non-degenerative IVDs: 58.2 ± 4.4 5 males Canadian | Scale from 0 to 100 and ODI questionnaire \ Degenerative disc disease | Intervertebral disc DNA bisulphite treatment followed by pyrosequencing | SPARC | SPARC promoter was significantly hypermethylated in patients with low back pain measured with ODI score (p < 0.01), in surgical patients (p < 0.05), and in lumbar disc degeneration based on MRI image scoring (p < 0.001) compared to IVD controls. | M. Tajerian (2011) [30] |
| Retrospective case–control study (III) | Low back pain group: 14 Control group: 4 | NS Chinese | NS \ Control group: vertebral fractures Case: degenerative disc disease | Vertebral Cartilagenous Endplate Quantitative RT-PCR | EZH2 | It was found that the expression of EZH2 increased in degenerated human cartilaginous endplates compared to controls (p < 0.01). This downregulated SOX9 and upregulated the levels of MMP13 and ADTAMTS4, which resulted in the activation of NF-κb or Wnt/β-catenin signaling. | C. Jiang (2019) [31] |
| Genome-wide association study (GWAS) (II) | Low back pain spine surgery group: 16 IVD early-stage degeneration: (1) Pfirmann I: 3 (2) Pfirmann II: 3 (3) Pfirmann III: 2 IVD advanced-stage degeneration: (1) Pfirmann IV: 8 | Low back pain spine surgery group: 55.6 years Males: 6 (37.5%) Females: 10 (62.5%) Japanese | NS \ Degenerative disc disease: 1 Spinal trauma: 6 Lumbar spine stenosis: 3 Lumbar degenerative spondylolisthesis: 4 Lumbar degenerative scoliosi: 1 | Intervertebral disc (nucleous pulposus) bisulfite treatment followed by array-based genome wide methylation analysis | CpGs of the whole genome | A total of 220 differently methylated loci (DML) were identified between early and advanced disc degeneration. Four of these were hypomethylated and 216 hypermethylated in the advanced disc degeneration group. | A. Ikuno (2019) [36] |
| Retrospective case–control study (III) | Degenerative disc group: 52 Control group: 43 | Degenerative disc group: 55.5 ± 3.55 Males: 23 (44%) Females: 29 (56%) Control group: 15.5 ± 1.68 Males: 17 (40%) Females: 26 (60%) Chinese | NS \ Lumbar disc degeneration | Intervertebral disc RNA-seq, RNA scope, and RT-PCR | ALKBH5 | The author found an increased expression of ALKBH5 during IVD degeneration and NPC senescence, due to decreased KDM4A-mediated H3K9me3 modification, compared to normal IVD (p < 0.05). | G. Li (2022) [34] |
| Study Design (Level of Evidence) | Study Population | Age (Mean/Range) Gender Ethnicity | Pain Assessment/Spine Disease | Type of Biological Sample and Technique Used | Gene(s) Involved | Results | Authors |
|---|---|---|---|---|---|---|---|
| Genome-wide association study (GWAS) (II) | 4863 individuals from five different study groups with low back pain due to lumbar disc degeneration 38 individuals in one of the cohorts were tested for DNA methylation levels (four monozygotic twin pairs, eight dizygotic twin pairs, and fourteen unrelated indivduals | All individuals: 57.7 years Males: 1605 (33%) Females: 3258 (67%) Caucasian | NS \ Lumbar disc degeneration | Peripheral blood 4863 analyzed in five genotyping studies and collected in one GWAS meta-analysis. Of these, 38 involved analysis for PARK2 methylation by array-based technology. | PARK2 | The authors tested for an association between lumbar disc degeneration and DNA methylation variants at three CpG sites in the PARK2 promoter. A significant association between DNA methylation at CpG site cg15832436 and LDD (β = 8.74 × 10−4, SE = 2.49 × 10−4, p = 0.006) was observed. A positive trend was also observed for the other two loci, though without reaching significance. | M.K. Williams (2012) [35] |
| Prospective cohort study (II) | 12 patients suffering from chronic back pain or postherpetic nevralgia | 69.3 ± 11.3 (44–81) Males: 5 (41%) Female: 7 (59%) Japanese | Douleur Neuropathique 4 Questionnaire (DN4) or Short-Form McGill Pain Questionnaire (SF-MPQ) \ NS | Peripheral blood whole-blood array-based global DNA methylation analysis (Illumia) | TRPA1 | A significant correlation between an increase in the DNA methylation level at the CpG island of the TRPA1 gene and an increase in the DN4 score (p = 0.001; r = 0.82), which represents the diversity of the neuropathic pain symptoms. There was also a significant inverse correlation between TRPA1 expression and DN4 (p = 0.04; r = −0.65). | N. Sukenaga (2016) [38] |
| Retrospective case–control study (III) | Chronic low back pain group: 50 Pain-free controls: 48 | Chronic low back pain group: 44.5 ± 12.7; (19–85) Males: 22 (44%) Females: 28 (56%) Pain-free controls: 39.9 ± 14.7; (19–85) Males: 25 (52.1%) Females: 23 (47.9%) Non-Hispanic White: 50 Non-Hispanic Black: 48 | NS \ NS | Peripheral blood reduced representation bisulfite sequencing | CpGs of the whole genome | The authors identified 28,325 hypermethylated and 36,936 hypomethylated CpG sites (p < 0.05). After correcting for multiple testing, the authors identified 159 DMRs (q < 0.01 and methylation difference >10%), the majority of which were in the CpG island (50%) and promoter regions (48%) on the associated genes. The genes associated with the differentially methylated regions were highly enriched in biological processes that have previously been implicated in immune signaling, endochondral ossification, and G-protein-coupled transmissions. | E. N. Aroke (2020) [32] |
| Genome-wide association study (GWAS) (II) | Discovery cohort: 32 - Control group: 16 - Low back pain group: 16 Validation cohort: 63 - Control group: 16 - Low back pain group: 37 | Discovery cohort: - Control females: 43.8 ± 4.6 - Low back pain females: 41.3 ± 3.8 - Control males: 43.8 ± 4.0 - Low back pain males: 42.6 ± 3.6 Males: 16 (50%) Females: 16 (50%) Validation cohort: - Control females: 38.5 ± 3.5 - Low back pain females: 46.1 ± 2.7 - Control males: 43.1 ± 3.2 - Low back pain males: 48.4 ± 2.6 Males: 31 (49%) Females: 32 (51%) Caucasian | Canadian adaptation of NIH low back pain taskforce, DN4 and ODI \ NS | T cells isolated from peripheral blood Array-based methylation analyis (Illumina) after bisulfite treatment, followed by validation by pyrosequencing | 850,000 CpG sites | Of the 736,414 CpGs identified in men, 179 were hypermethylated and 240 were hypomethylated in LBP patients compared to controls. Of the 735,863 CpGs identified in women, 601 were hypermethylated and 1895 were hypomethylated (p-value < 0.05). The generation of a polygenic methylation score for LBP in men and women with three surrogate CpG loci: cg07420274 for women; cg21149944 and cg22831726 for men. In women, the percentage of methylation at position cg07420274 was 39.5 ± 6 2.7% and 49.7 ± 6 3.2% in the control (n = 21) and LBP groups (n = 25), respectively (p < 0.05). A statistically significant association between methylation at cg07420274 and LBP was observed (OR = 1.05, 95% CI: 1.01–1.11, p < 0.03). In men, a statistically significant association was found between LBP and cg21149944 methylation (OR = 0.89, 95% CI: 0.82–0.95, p < 0.0015) as well as cg22831726 methylation (OR = 0.9, 95% CI: 0.84–0.96, p < 0.0036). | S. Grègoire (2021) [6] |
| Prospective case–control study (II) | Chronic low back pain group: 15 Acute low back pain: 14 Healthy controls: 16 | Chronic low back pain group: 39.4 (8.6) Males: 8 (50%) Females: 8 (50%) Acute low back pain: 33.5 (9.2) Males: 8 (57.1%) Females: 6 (42.9%) Healthy controls: 36.2 (14.3) Males: 6 (37.5%) Females: 10 (62.5%) Black, Asian, White | Brief Pain Inventory (BPI), Short-Form McGill Pain Questionnaire (SF-MPQ), and quantitative sensory testing (QST) \ NS | Peripheral blood ELISA-based DNA methylation and H4 histone acetylation levels quantification | NS | Global histone H4 histone acetylation was higher in participants with pain compared to healthy controls (p < 0.05, t = 2.261). The mechanical pain threshold, windup ratio measurement 1, and warm detection threshold at the site of pain were positively correlated with H4 acetylation (all rp = −0.315, p < 0.05). Global DNA methylation in cLBP participants was significantly lower than aLBP participants and healthy controls (p < 0.05). cLBP participants showed highervL2 mRNA expression than aLBP participants and healthy controls (p < 0.05). | C. Eller (2021) [40] |
| Epigenome-wide association study (EWAS) (II) | Chronic low back pain cohort: 48 Pain-free control cohort: 50 | Chronic low back pain cohort: 44.2 ± 12.95 Males: 21 (43.7%) Females: 27 (56.3%) Pain-free control cohort: 39.66 ± 14.51 Males: 26 (52%) Females: 24 (48%) Black, White | NS \ Brief Pain Inventory (BPI), quantitative sensory testing (QST), and measurement of conditioned pain modulation (CPM) | Peripheral blood Array-based DNA methylation analysis | Whole-genome CpGs | Based on CPM efficiency (deficient versus efficient CPM participants), the authors identified 6006 differently methylated CpGs (DMCs) in chronic LBP patients and 18,305 in controls. Most of the DMCs were hypomethylated and annotated to genes of relevance to pain: OPRM1, CACNA2D3, GNA12, LPL, NAXD, and ASHD1 in both groups. Conversely, MAPK-Ras signaling pathways were enriched only in the chronic LBP group (p = 0.004). | B.R. Goodin (2022) [45] |
| Genome-wide association study (GWAS) (II) | Chronic low back pain cohort: 49 - Non-Hispanic Blacks: 25 - Non-Hispanic White: 24 Pain-free control cohort: 49 - Non-hispanic blacks: 24 - Non-Hispanic whites: 25 | Chronic low-back pain cohort: - Non-Hispanic Black: 43.5 (10.6) - Non-Hispanic White: 45.8 (14.9) Males: 21 (43%) Females: 28 (57%) Pain-free control cohort: - Non-Hispanic Black: 40.7 (16.5) - Non-Hispanic White: 39.3 (12.6) Males: 25 (51%) Females: 24 (49%) Non-Hispanic Black and White | NS \ NS | Peripheral blood RRBS (reduced representation bisulfite sequencing) | Global DNA methylation | Among participants with chronic low back pain, the authors identified 2873 differently methylated loci (DML) with a difference at least of 10% and p < 0.0001, many of those related to pain/nociception processing. | E.N. Aroke (2022) [42] |
| Genome-wide association study (GWAS) (II) | Discovery cohort: from UK Biobank cohort - Chronic low back pain: 70,633 (304,525 controls) - Acute low back pain: 32,209 (304,525 controls) Replication cohort: from HUNT study - Chronic low back pain: 19,760 - Acute low back pain: 4379 - Controls: 39,983 | NS Anglo-American | Pain for more than 3 months, and HUNT2&3 survey \ NS | Peripheral blood DNA genotyping | Global DNA | The authors found 13 genomic loci that reached genome-wide significance in back pain analyses and hypothesized that epigenetic markers would act in brain tissues. The authors found 9 of 13 loci colocalized with epigenetic markers in multiple brain tisseus (p < 0.05). | A. Bortsov (2022) [37] |
| Study Design (Level of Evidence) | Study Population | Age (Mean/Range) Gender Ethnicity | Pain Assessment/Spine Disease | Type of Biological Sample and Technique Used | Gene(s) Involved | Results | Authors |
|---|---|---|---|---|---|---|---|
| Prospective cohort study (II) | Chronic low back pain group: 44 (1) Therapy responders: 14 (2) Non-responders: 20 Healthy volunteer group: 20 | Chronic low back pain group: 44 (1) Therapy responders: 43 ± 13 Males: 7 (50%) Females: 7 (50%) (2) Non-responders: 47 ± 11 Males: 7 (35%) Females: 13 (65%) Healthy volunteer group: 41 ± 10 Males: 11 (55%) Females: 9 (45%) Caucasian | Pain Numerical Rating Scale (NRS) \ NS | CD4+ T cells harvested from peripheral blood Semi-quantitative RNA seq RT PCR | MiRNA-124a MiRNA-150 MiRNA-155 | MiRNA-124a (patients: 0.79 ± 0.63 vs. healthy volunteers: 0.30 ± 0.16; p < 0.001); miRNA-150 (patients: 0.75 ± 0.21 vs. healthy volunteers: 0.56 ± 0.20; p = 0.025); and miRNA-155 (patients: 0.55 ± 0.14 vs. healthy volunteers: 0.38 ± 0.16; p = 0.017) were significantly upregulated in CLBP patients when compared with healthy volunteers. After the multidisciplinary treatment program, patients who respond to the treatment showed only an increase in miRNA-124a expression (before treatment: 0.54 ± 0.26 vs. after treatment: 1.05 ± 0.56, p = 0.007). | B. Luchting (2016) [39] |
| Retrospective case–control study (III) | 12 subjects affected by degenerative spine disease or thoracolumbar fracture or scoliosis | 41.1 (11–68) Males: 4 (33%) Females: 8 (67%) Chinese | NS \ Lumbar disc herniation Lumbar stenosis Thoracolumbar fracture | Intervertebral disc RT-qPCR | FBXO6 | RT-qPCR showed that expression of FBXO6 mRNA was significantly higher in nucleus pulposus than in anulus fibrosus tissues (p < 0.001). Moreover, FBXO6 was highly expressed in non-degenerated discs and decreased with the severity of degeneration (p < 0.001) in relation to miR-133a-5p upregulation, suggesting a role in the mir133a-5p/FBXO6 axis in IVD degeneration. The silencing of FBXO6 cause inhibited proliferation, enhanced apoptosis, suppressed ECM synthesis, and accelerated ECM degradation. | X-F. Du (2021) [33] |
3.4.1. Methylation-Regulated Epigenetic Markers Investigated on Intervertebral Disc Tissue (Table 1)
3.4.2. Methylation-Regulated Epigenetic Markers Investigated on Peripheral Blood (Table 2)
3.4.3. Epigenetic Regulation through microRNA Signaling (Table 3)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoy, D.; Bain, C.; Williams, G.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Vos, T.; Buchbinder, R. A Systematic Review of the Global Prevalence of Low Back Pain. Arthritis Rheum. 2012, 64, 2028–2037. [Google Scholar] [CrossRef] [PubMed]
- Hartvigsen, J.; Hancock, M.J.; Kongsted, A.; Louw, Q.; Ferreira, M.L.; Genevay, S.; Hoy, D.; Karppinen, J.; Pransky, G.; Sieper, J.; et al. What Low Back Pain Is and Why We Need to Pay Attention. Lancet 2018, 391, 2356–2367. [Google Scholar] [CrossRef] [PubMed]
- Manchikanti, L.; Singh, V.; Falco, F.J.E.; Benyamin, R.M.; Hirsch, J.A. Epidemiology of Low Back Pain in Adults. Neuromodulation 2014, 17, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Allen, C.; Arora, M.; Barber, R.M.; Brown, A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coggeshall, M.; et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 310 Diseases and Injuries, 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed]
- Grégoire, S.; Cheishvili, D.; Salmon-Divon, M.; Dymov, S.; Topham, L.; Calderon, V.; Shir, Y.; Szyf, M.; Stone, L.S. Epigenetic Signature of Chronic Low Back Pain in Human T Cells. Pain Rep. 2021, 6, e960. [Google Scholar] [CrossRef]
- Urits, I.; Burshtein, A.; Sharma, M.; Testa, L.; Gold, P.A.; Orhurhu, V.; Viswanath, O.; Jones, M.R.; Sidransky, M.A.; Spektor, B.; et al. Low Back Pain, a Comprehensive Review: Pathophysiology, Diagnosis, and Treatment. Curr. Pain Headache Rep. 2019, 23, 23. [Google Scholar] [CrossRef]
- Heuch, I.; Foss, I.S. Acute Low Back Usually Resolves Quickly but Persistent Low Back Pain Often Persists. J. Physiother. 2013, 59, 127. [Google Scholar] [CrossRef]
- Atlas, S.J.; Deyo, R.A. Evaluating and Managing Acute Low Back Pain in the Primary Care Setting. J. Gen. Intern. Med. 2001, 16, 120–131. [Google Scholar] [CrossRef]
- Knezevic, N.N.; Candido, K.D.; Vlaeyen, J.W.S.; Van Zundert, J.; Cohen, S.P. Low Back Pain. Lancet 2021, 398, 78–92. [Google Scholar] [CrossRef]
- Kraft, D.E. Low Back Pain in the Adolescent Athlete. Pediatr. Clin. N. Am. 2002, 49, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Santoro, D.; Satta, E. Pain in Renal Disease. J. Pain Palliat. Care Pharmacother. 2014, 28, 409–411. [Google Scholar] [CrossRef] [PubMed]
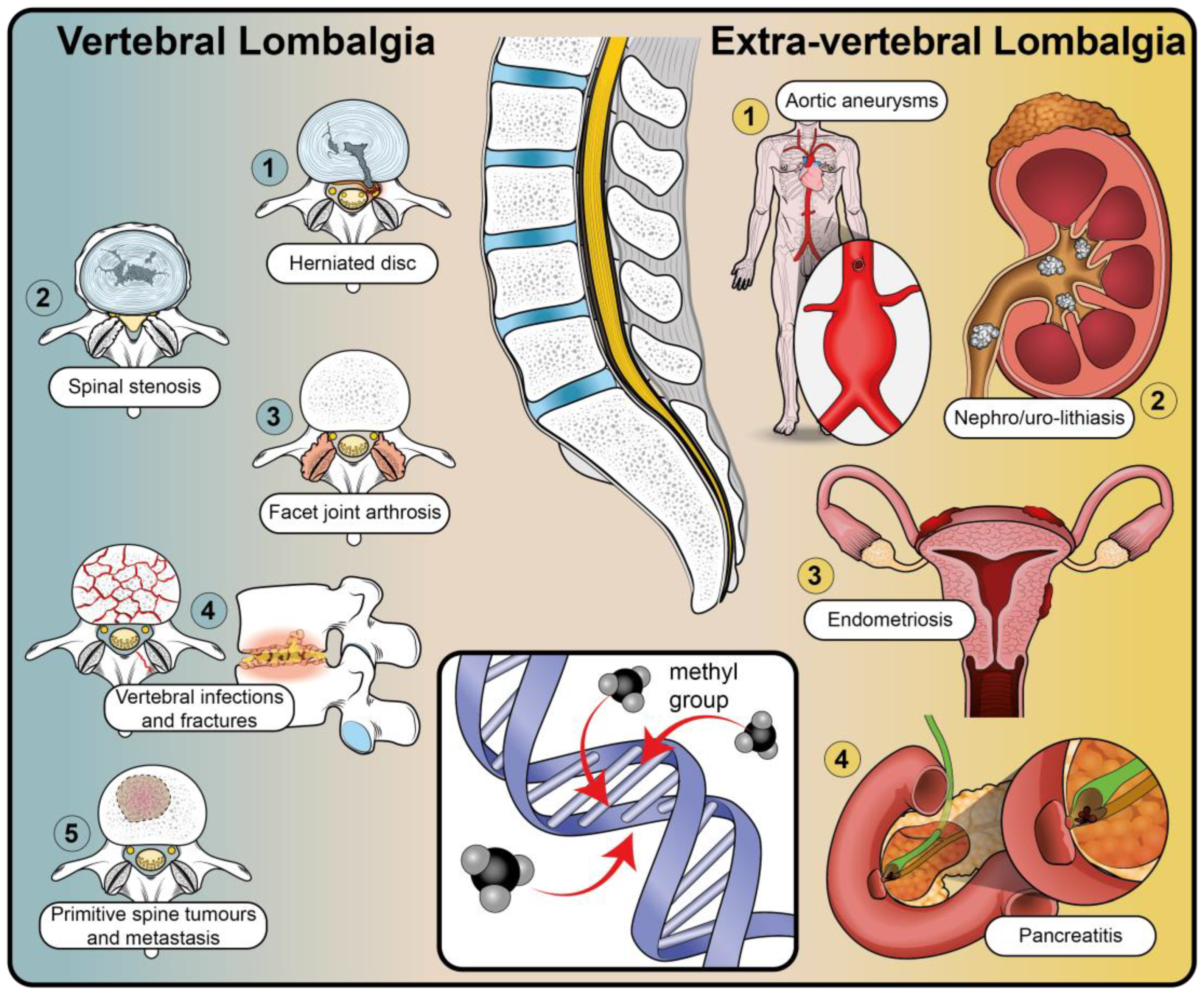
- Verhagen, A.P.; Downie, A.; Popal, N.; Maher, C.; Koes, B.W. Red Flags Presented in Current Low Back Pain Guidelines: A Review. Eur. Spine J. 2016, 25, 2788–2802. [Google Scholar] [CrossRef] [PubMed]
- Troyer, M.R. Differential Diagnosis of Endometriosis in a Young Adult Woman with Nonspecific Low Back Pain. Phys. Ther. 2007, 87, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Malik, T.F.; Aurelio, D.M. Extraintestinal Manifestations of Inflammatory Bowel Disease; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- de Oliveira Sá, M.V.B.; Pacheco, F.J.S.; Barretto, F.J.T. Elderly Patient with Prostate Cancer and Back Pain. Eur. J. Intern. Med. 2020, 72, 88–89. [Google Scholar] [CrossRef] [PubMed]
- Foster, N.E.; Anema, J.R.; Cherkin, D.; Chou, R.; Cohen, S.P.; Gross, D.P.; Ferreira, P.H.; Fritz, J.M.; Koes, B.W.; Peul, W.; et al. Prevention and Treatment of Low Back Pain: Evidence, Challenges, and Promising Directions. Lancet 2018, 391, 2368–2383. [Google Scholar] [CrossRef]
- Dario, A.B.; Ferreira, M.L.; Refshauge, K.M.; Lima, T.S.; Ordoñana, J.R.; Ferreira, P.H. The Relationship between Obesity, Low Back Pain, and Lumbar Disc Degeneration When Genetics and the Environment Are Considered: A Systematic Review of Twin Studies. Spine J. 2015, 15, 1106–1117. [Google Scholar] [CrossRef]
- Pinheiro, M.B.; Ferreira, M.L.; Refshauge, K.; Colodro-Conde, L.; Carrillo, E.; Hopper, J.L.; Ordoñana, J.R.; Ferreira, P.H. Genetics and the Environment Affect the Relationship between Depression and Low Back Pain: A Co-Twin Control Study of Spanish Twins. Pain 2015, 156, 496–503. [Google Scholar] [CrossRef]
- Patterson, T.G.; Carvalho-e-Silva, A.P.; Aquino, D.; Ferreira, M.; Ferreira, P. Factors Associated with Care-Seeking for Low Back Pain When Genetics and the Familial Environment Are Considered. Musculoskelet. Sci. Pract. 2021, 53, 102365. [Google Scholar] [CrossRef]
- Fitz-James, M.H.; Cavalli, G. Molecular Mechanisms of Transgenerational Epigenetic Inheritance. Nat. Rev. Genet. 2022, 23, 325–341. [Google Scholar] [CrossRef]
- Klemm, S.L.; Shipony, Z.; Greenleaf, W.J. Chromatin Accessibility and the Regulatory Epigenome. Nat. Rev. Genet. 2019, 20, 207–220. [Google Scholar] [CrossRef]
- Ciampi De Andrade, D.; Maschietto, M.; Galhardoni, R.; Gouveia, G.; Chile, T.; Victorino Krepischi, A.C.; Dale, C.S.; Brunoni, A.R.; Parravano, D.C.; Cueva Moscoso, A.S.; et al. Epigenetics Insights into Chronic Pain: DNA Hypomethylation in Fibromyalgia—A Controlled Pilot-Study. Pain 2017, 158, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- D’Agnelli, S.; Arendt-Nielsen, L.; Gerra, M.C.; Zatorri, K.; Boggiani, L.; Baciarello, M.; Bignami, E. Fibromyalgia: Genetics and Epigenetics Insights May Provide the Basis for the Development of Diagnostic Biomarkers. Mol. Pain 2019, 15. [Google Scholar] [CrossRef]
- Mauck, M.; Van De Ven, T.; Shaw, A.D. Epigenetics of Chronic Pain after Thoracic Surgery. Curr. Opin. Anaesthesiol. 2014, 27, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- OCEBM Levels of Evidence Working Group*. The Oxford 2011 Levels of Evidence. Oxford Centre for Evidence-Based Medicine. Available online: http://www.cebm.net/index.aspx?o=5653 (accessed on 3 October 2022).
- Sideri, S.; Papageorgiou, S.N.; Eliades, T. Registration in the International Prospective Register of Systematic Reviews (PROSPERO) of Systematic Review Protocols Was Associated with Increased Review Quality. J. Clin. Epidemiol. 2018, 100, 103–110. [Google Scholar] [CrossRef]
- NIH National Heart, Lung and Blood Institute. Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/assessing-cardiovascular-risk (accessed on 3 October 2022).
- Tajerian, M.; Alvarado, S.; Millecamps, M.; Dashwood, T.; Anderson, K.M.; Haglund, L.; Ouellet, J.; Szyf, M.; Stone, L.S. DNA Methylation of SPARC and Chronic Low Back Pain. Mol. Pain 2011, 7, 65. [Google Scholar] [CrossRef]
- Williams, F.M.K.; Bansal, A.T.; Van Meurs, J.B.; Bell, J.T.; Meulenbelt, I.; Suri, P.; Rivadeneira, F.; Sambrook, P.N.; Hofman, A.; Bierma-Zeinstra, S.; et al. Novel Genetic Variants Associated with Lumbar Disc Degeneration in Northern Europeans: A Meta-Analysis of 4600 Subjects. Ann. Rheum. Dis. 2013, 72, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Sukenaga, N.; Ikeda-Miyagawa, Y.; Tanada, D.; Tunetoh, T.; Nakano, S.; Inui, T.; Satoh, K.; Okutani, H.; Noguchi, K.; Hirose, M. Correlation between DNA Methylation of TRPA1 and Chronic Pain States in Human Whole Blood Cells. Pain Med. 2016, 17, 1906–1910. [Google Scholar] [CrossRef]
- Luchting, B.; Heyn, J.; Hinske, L.C.; Azad, S.C. Expression of MiRNA-124a in CD4 Cells Reflects Response to a Multidisciplinary Treatment Program in Patients with Chronic Low Back Pain. Spine 2017, 42, E226–E233. [Google Scholar] [CrossRef]
- Jiang, C.; Guo, Q.; Jin, Y.; Xu, J.J.; Sun, Z.M.; Zhu, D.C.; Lin, J.H.; Tian, N.F.; Sun, L.J.; Zhang, X.L.; et al. Inhibition of EZH2 Ameliorates Cartilage Endplate Degeneration and Attenuates the Progression of Intervertebral Disc Degeneration via Demethylation of Sox-9. EBioMedicine 2019, 48, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Ikuno, A.; Akeda, K.; Takebayashi, S.I.; Shimaoka, M.; Okumura, K.; Sudo, A. Genome-Wide Analysis of DNA Methylation Profile Identifies Differentially Methylated Loci Associated with Human Intervertebral Disc Degeneration. PLoS ONE 2019, 14, e0222188. [Google Scholar] [CrossRef] [PubMed]
- Aroke, E.N.; Joseph, P.V.; Roy, A.; Overstreet, D.S.; Tollefsbol, T.O.; Vance, D.E.; Goodin, B.R. Could Epigenetics Help Explain Racial Disparities in Chronic Pain? J. Pain Res. 2019, 12, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Du, X.F.; Cui, H.T.; Pan, H.H.; Long, J.; Cui, H.W.; Chen, S.L.; Wang, J.R.; Li, Z.M.; Liu, H.; Huang, Y.C.; et al. Role of the MiR-133a-5p/FBXO6 Axis in the Regulation of Intervertebral Disc Degeneration. J. Orthop. Transl. 2021, 29, 123–133. [Google Scholar] [CrossRef]
- Eller, O.C.; Glidden, N.; Knight, B.; McKearney, N.; Perry, M.; Bernier Carney, K.M.; Starkweather, A.; Young, E.E.; Baumbauer, K.M. A Role for Global DNA Methylation Level and IL2 Expression in the Transition from Acute to Chronic Low Back Pain. Front. Pain Res. 2021, 2, 744148. [Google Scholar] [CrossRef]
- Goodin, B.R.; Overstreet, D.S.; Penn, T.M.; Bakshi, R.; Quinn, T.L.; Sims, A.; Ptacek, T.; Jackson, P.; Long, D.L.; Aroke, E.N. Epigenome-Wide DNA Methylation Profiling of Conditioned Pain Modulation in Individuals with Non-Specific Chronic Low Back Pain. Clin. Epigenetics 2022, 14, 45. [Google Scholar] [CrossRef]
- Aroke, E.N.; Jackson, P.; Meng, L.; Huo, Z.; Overstreet, D.S.; Penn, T.M.; Quinn, T.L.; Cruz-Almeida, Y.; Goodin, B.R. Differential DNA Methylation in Black and White Individuals with Chronic Low Back Pain Enrich Different Genomic Pathways. Neurobiol. Pain 2022, 11, 100086. [Google Scholar] [CrossRef]
- Li, G.; Luo, R.; Zhang, W.; He, S.; Wang, B.; Liang, H.; Song, Y.; Ke, W.; Shi, Y.; Feng, X.; et al. M6A Hypomethylation of DNMT3B Regulated by ALKBH5 Promotes Intervertebral Disc Degeneration via E4F1 Deficiency. Clin. Transl. Med. 2022, 12, e765. [Google Scholar] [CrossRef]
- Aroke, E.N.; Overstreet, D.S.; Penn, T.M.; Crossman, D.K.; Jackson, P.; Tollefsbol, T.O.; Quinn, T.L.; Yi, N.; Goodin, B.R. Identification of DNA Methylation Associated Enrichment Pathways in Adults with Non-Specific Chronic Low Back Pain. Mol. Pain 2020, 16, 1744806920972889. [Google Scholar] [CrossRef]
- Bortsov, A.V.; Parisien, M.; Khoury, S.; Martinsen, A.E.; Lie, M.U.; Heuch, I.; Hveem, K.; Zwart, J.A.; Winsvold, B.S.; Diatchenko, L. Brain-Specific Genes Contribute to Chronic but Not to Acute Back Pain. Pain Rep. 2022, 7, E1018. [Google Scholar] [CrossRef]
- Oh, E.S.; Petronis, A. Origins of Human Disease: The Chrono-Epigenetic Perspective. Nat. Rev. Genet. 2021, 22, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Chmielewska, M.; Janusz, P.; Andrusiewicz, M.; Kotwicki, T.; Kotwicka, M. Methylation of Estrogen Receptor 2 (ESR2) in Deep Paravertebral Muscles and Its Association with Idiopathic Scoliosis. Sci. Rep. 2020, 10, 22331. [Google Scholar] [CrossRef] [PubMed]
- Brekken, R.A.; Sage, E.H. SPARC, a Matricellular Protein: At the Crossroads of Cell-Matrix Communication. Matrix Biol. 2001, 19, 816–827. [Google Scholar] [CrossRef]
- Gruber, H.E.; Ingram, J.A.; Leslie, K.; Hanley, E.N. Cellular, but Not Matrix, Immunolocalization of SPARC in the Human Intervertebral Disc: Decreasing Localization with Aging and Disc Degeneration. Spine 2004, 29, 2223–2228. [Google Scholar] [CrossRef]
- Gruber, H.E.; Sage, E.H.; Norton, H.J.; Funk, S.; Ingram, J.; Hanley, E.N. Targeted Deletion of the SPARC Gene Accelerates Disc Degeneration in the Aging Mouse. J. Histochem. Cytochem. 2005, 53, 1131–1138. [Google Scholar] [CrossRef]
- Millecamps, I.; Tajerian, M.; Sage, E.H.; Stone, L.S. Behavioral Signs of Chronic Back Pain in the SPARC-Null Mouse. Spine 2011, 36, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.T.; Loomis, A.K.; Butcher, L.M.; Gao, F.; Zhang, B.; Hyde, C.L.; Sun, J.; Wu, H.; Ward, K.; Harris, J.; et al. Differential Methylation of the TRPA1 Promoter in Pain Sensitivity. Nat. Commun. 2014, 5, 2978. [Google Scholar] [CrossRef]
- Dorsey, S.G.; Renn, C.L.; Griffioen, M.; Lassiter, C.B.; Zhu, S.; Huot-Creasy, H.; McCracken, C.; Mahurkar, A.; Shetty, A.C.; Jackson-Cook, C.K.; et al. Whole Blood Transcriptomic Profiles Can Differentiate Vulnerability to Chronic Low Back Pain. PLoS ONE 2019, 14, e0216539. [Google Scholar] [CrossRef]
- Li, D.; Liu, J.; Guo, B.; Liang, C.; Dang, L.; Lu, C.; He, X.; Cheung, H.Y.S.; Xu, L.; Lu, C.; et al. Osteoclast-Derived Exosomal MiR-214-3p Inhibits Osteoblastic Bone Formation. Nat. Commun. 2016, 7, 10872. [Google Scholar] [CrossRef]
- Nemoda, Z.; Massart, R.; Suderman, M.; Hallett, M.; Li, T.; Coote, M.; Cody, N.; Sun, Z.S.; Soares, C.N.; Turecki, G.; et al. Maternal Depression Is Associated with DNA Methylation Changes in Cord Blood T Lymphocytes and Adult Hippocampi. Transl. Psychiatry 2015, 5, e545. [Google Scholar] [CrossRef]
- Borghol, N.; Suderman, M.; Mcardle, W.; Racine, A.; Hallett, M.; Pembrey, M.; Hertzman, C.; Power, C.; Szyf, M. Associations with Early-Life Socio-Economic Position in Adult DNA Methylation. Int. J. Epidemiol. 2012, 41, 62–74. [Google Scholar] [CrossRef]
- Cao-Lei, L.; Massart, R.; Suderman, M.J.; Machnes, Z.; Elgbeili, G.; Laplante, D.P.; Szyf, M.; King, S. DNA Methylation Signatures Triggered by Prenatal Maternal Stress Exposure to a Natural Disaster: Project Ice Storm. PLoS ONE 2014, 9, e107653. [Google Scholar] [CrossRef] [PubMed]
- Capitani, N.; Patrussi, L.; Baldari, C.T. Nature vs. Nurture: The Two Opposing Behaviors of Cytotoxic T Lymphocytes in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 11221. [Google Scholar] [CrossRef] [PubMed]
- Faldini, C.; Manzetti, M.; Neri, S.; Barile, F.; Viroli, G.; Geraci, G.; Ursini, F.; Ruffilli, A. Epigenetic and Genetic Factors Related to Curve Progression in Adolescent Idiopathic Scoliosis: A Systematic Scoping Review of the Current Literature. Int. J. Mol. Sci. 2022, 23, 5914. [Google Scholar] [CrossRef]
- Soffer, D.; Stoekenbroek, R.; Plakogiannis, R. Small Interfering Ribonucleic Acid for Cholesterol Lowering—Inclisiran: Inclisiran for Cholesterol Lowering. J. Clin. Lipidol. 2022, 16, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Italiano, A.; Soria, J.C.; Toulmonde, M.; Michot, J.M.; Lucchesi, C.; Varga, A.; Coindre, J.M.; Blakemore, S.J.; Clawson, A.; Suttle, B.; et al. Tazemetostat, an EZH2 Inhibitor, in Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma and Advanced Solid Tumours: A First-in-Human, Open-Label, Phase 1 Study. Lancet. Oncol. 2018, 19, 649–659. [Google Scholar] [CrossRef]
- Souza Monteiro de Araujo, D.; Nassini, R.; Geppetti, P.; De Logu, F. TRPA1 as a Therapeutic Target for Nociceptive Pain. Expert Opin. Ther. Targets 2020, 24, 997–1008. [Google Scholar] [CrossRef]
- Giorgi, S.; Nikolaeva-Koleva, M.; Alarcón-Alarcón, D.; Butrón, L.; González-Rodríguez, S. Is TRPA1 Burning Down TRPV1 as Druggable Target for the Treatment of Chronic Pain? Int. J. Mol. Sci. 2019, 20, 2906. [Google Scholar] [CrossRef]
- Mizuno, Y. More than 20 Years of the Discovery of Park2. Neurosci. Res. 2020, 159, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Gadd, D.A.; Hillary, R.F.; McCartney, D.L.; Zaghlool, S.B.; Stevenson, A.J.; Cheng, Y.; Fawns-Ritchie, C.; Nangle, C.; Campbell, A.; Flaig, R.; et al. Epigenetic Scores for the Circulating Proteome as Tools for Disease Prediction. eLife 2022, 11, e71802. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruffilli, A.; Neri, S.; Manzetti, M.; Barile, F.; Viroli, G.; Traversari, M.; Assirelli, E.; Vita, F.; Geraci, G.; Faldini, C. Epigenetic Factors Related to Low Back Pain: A Systematic Review of the Current Literature. Int. J. Mol. Sci. 2023, 24, 1854. https://doi.org/10.3390/ijms24031854
Ruffilli A, Neri S, Manzetti M, Barile F, Viroli G, Traversari M, Assirelli E, Vita F, Geraci G, Faldini C. Epigenetic Factors Related to Low Back Pain: A Systematic Review of the Current Literature. International Journal of Molecular Sciences. 2023; 24(3):1854. https://doi.org/10.3390/ijms24031854
Chicago/Turabian StyleRuffilli, Alberto, Simona Neri, Marco Manzetti, Francesca Barile, Giovanni Viroli, Matteo Traversari, Elisa Assirelli, Fabio Vita, Giuseppe Geraci, and Cesare Faldini. 2023. "Epigenetic Factors Related to Low Back Pain: A Systematic Review of the Current Literature" International Journal of Molecular Sciences 24, no. 3: 1854. https://doi.org/10.3390/ijms24031854
APA StyleRuffilli, A., Neri, S., Manzetti, M., Barile, F., Viroli, G., Traversari, M., Assirelli, E., Vita, F., Geraci, G., & Faldini, C. (2023). Epigenetic Factors Related to Low Back Pain: A Systematic Review of the Current Literature. International Journal of Molecular Sciences, 24(3), 1854. https://doi.org/10.3390/ijms24031854