Neurotrophins in the Neuropathophysiology, Course, and Complications of Obstructive Sleep Apnea—A Narrative Review
Abstract
1. Introduction
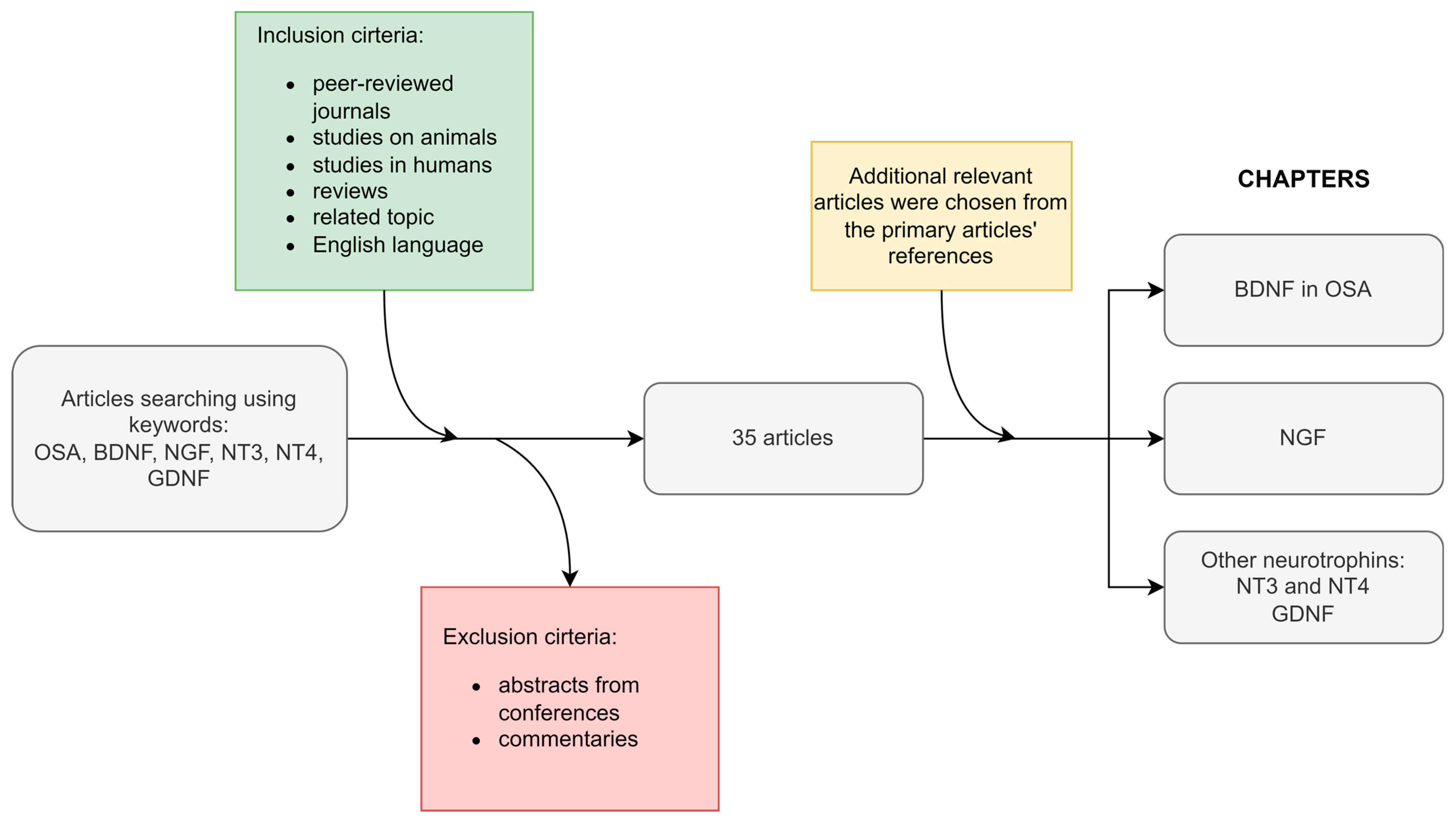
2. Methodology and the Article Scheme
3. BDNF in OSA
3.1. Arousals in OSA and Their Influence on BDNF
3.2. Intermittent Hypoxia in OSA and Its Influence on BDNF
3.3. The Association between Sleep, Pain, OSA Therapy, and BDNF
3.4. Summary—BDNF in OSA
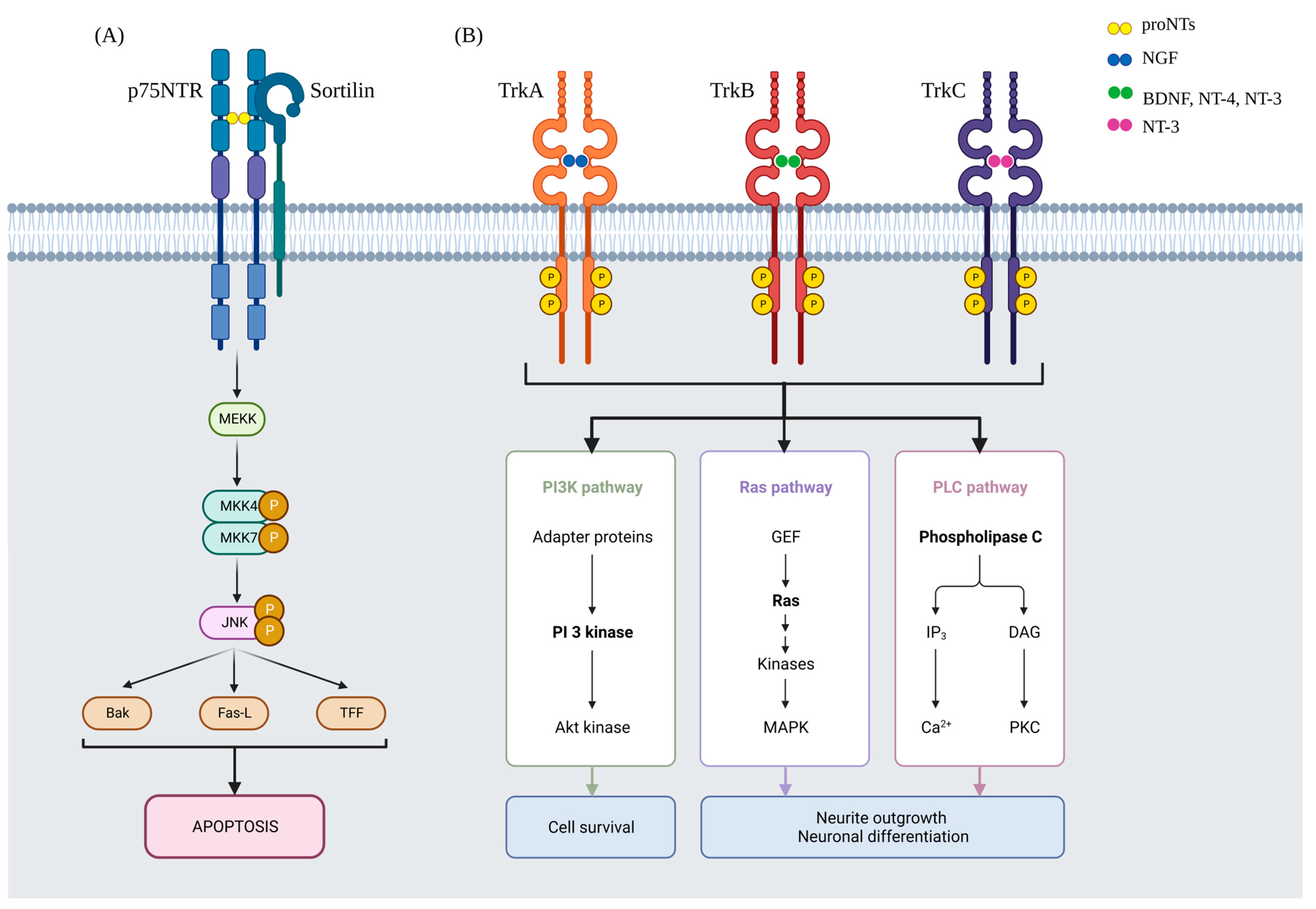
4. NGF
5. Other Neurotrophins
5.1. NT3 and NT4
5.2. GDNF
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tong, Q.; Wang, F.; Zhou, H.Z.; Sun, H.L.; Song, H.; Shu, Y.Y.; Gong, Y.; Zhang, W.T.; Cai, T.X.; Yang, F.Q.; et al. Structural and functional insights into lipid-bound nerve growth factors. FASEB J. 2012, 26, 3811–3821. [Google Scholar] [CrossRef] [PubMed]
- Werner, P.; Paluru, P.; Simpson, A.M.; Latney, B.; Iyer, R.; Brodeur, G.M.; Goldmuntz, E. Mutations in NTRK3 suggest a novel signaling pathway in human congenital heart disease. Hum. Mutat. 2014, 35, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Omar, N.A.; Kumar, J.; Teoh, S.L. Neurotrophin-3 and neurotrophin-4: The unsung heroes that lies behind the meninges. Neuropeptides 2022, 92, 102226. [Google Scholar] [CrossRef]
- Yeo, G.S.; Connie Hung, C.C.; Rochford, J.; Keogh, J.; Gray, J.; Sivaramakrishnan, S.; O’Rahilly, S.; Farooqi, I.S. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat. Neurosci. 2004, 7, 1187–1189. [Google Scholar] [CrossRef]
- Nordvall, G.; Forsell, P.; Sandin, J. Neurotrophin-targeted therapeutics: A gateway to cognition and more? Drug Discov. Today 2022, 10, 103318. [Google Scholar] [CrossRef] [PubMed]
- Doyle, K.M.; Kennedy, D.; Gorman, A.M.; Gupta, S.; Healy, S.J.; Samali, A. Unfolded proteins and endoplasmic reticulum stress in neurodegenerative disorders. J. Cell. Mol. Med. 2011, 15, 2025–2039. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef]
- Lu, P.; Jones, L.L.; Snyder, E.Y.; Tuszynski, M.H. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp. Neurol. 2003, 181, 115–129. [Google Scholar] [CrossRef]
- Gulati, A. Understanding neurogenesis in the adult human brain. Indian J. Pharmacol. 2015, 47, 583–584. [Google Scholar] [CrossRef]
- Shohayeb, B.; Diab, M.; Ahmed, M.; Ng, D.C.H. Factors that influence adult neurogenesis as potential therapy. Transl. Neurodegener. 2018, 7, 4. [Google Scholar] [CrossRef]
- Arai, K.; Lo, E.H. Chapter 18—Gliogenesis. In Primer on Cerebrovascular Diseases, 2nd ed.; Caplan, L.R., Biller, J., Leary, M.C., Lo, E.H., Thomas, A.J., Yenari, M., Zhang, J.H., Eds.; Academic Press: San Diego, MA, USA, 2017; pp. 91–95. [Google Scholar]
- Berg, D.A.; Belnoue, L.; Song, H.; Simon, A. Neurotransmitter-mediated control of neurogenesis in the adult vertebrate brain. Development 2013, 140, 2548–2561. [Google Scholar] [CrossRef] [PubMed]
- Ota, Y.; Zanetti, A.T.; Hallock, R.M. The role of astrocytes in the regulation of synaptic plasticity and memory formation. Neural Plast. 2013, 2013, 185463. [Google Scholar] [CrossRef] [PubMed]
- Risher, W.C.; Eroglu, C. Chapter 3—Astrocytes and synaptogenesis. In Synapse Development and Maturation; Rubenstein, J., Rakic, P., Chen, B., Kwan, K.Y., Cline, H.T., Cardin, J., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 55–75. [Google Scholar]
- Satarker, S.; Bojja, S.L.; Gurram, P.C.; Mudgal, J.; Arora, D.; Nampoothiri, M. Astrocytic Glutamatergic Transmission and Its Implications in Neurodegenerative Disorders. Cells 2022, 11, 1139. [Google Scholar] [CrossRef] [PubMed]
- Rajkowska, G.; Miguel-Hidalgo, J.J. Gliogenesis and glial pathology in depression. CNS Neurol. Disord. Drug Targets 2007, 6, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Martins-Macedo, J.; Salgado, A.J.; Gomes, E.D.; Pinto, L. Adult brain cytogenesis in the context of mood disorders: From neurogenesis to the emergent role of gliogenesis. Neurosci. Biobehav. Rev. 2021, 131, 411–428. [Google Scholar] [CrossRef]
- Ditmer, M.; Gabryelska, A.; Turkiewicz, S.; Bialasiewicz, P.; Malecka-Wojciesko, E.; Sochal, M. Sleep Problems in Chronic Inflammatory Diseases: Prevalence, Treatment, and New Perspectives: A Narrative Review. J. Clin. Med. 2021, 11, 67. [Google Scholar] [CrossRef]
- Turnbull, C.D. Intermittent hypoxia, cardiovascular disease and obstructive sleep apnoea. J. Thorac. Dis. 2018, 10, S33–S39. [Google Scholar] [CrossRef]
- Ota, H.; Fujita, Y.; Yamauchi, M.; Muro, S.; Kimura, H.; Takasawa, S. Relationship Between Intermittent Hypoxia and Type 2 Diabetes in Sleep Apnea Syndrome. Int. J. Mol. Sci. 2019, 20, 4756. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Yi, S.; Chen, B.B.; Cui, P.P.; Wang, Y.; Li, Y.Z. mTOR/NF-κB signaling pathway protects hippocampal neurons from injury induced by intermittent hypoxia in rats. Int. J. Neurosci. 2021, 131, 994–1003. [Google Scholar] [CrossRef]
- Turkiewicz, S.; Ditmer, M.; Sochal, M.; Białasiewicz, P.; Strzelecki, D.; Gabryelska, A. Obstructive Sleep Apnea as an Acceleration Trigger of Cellular Senescence Processes through Telomere Shortening. Int. J. Mol. Sci. 2021, 22, 12536. [Google Scholar] [CrossRef]
- Khalaji, A.; Behnoush, A.H.; Shobeiri, P.; Saeedian, B.; Teixeira, A.L.; Rezaei, N. Association between brain-derived neurotrophic factor levels and obstructive sleep apnea: A systematic review and meta-analysis. Sleep Breath. 2022. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Yung, W.H. Chronic intermittent hypoxia-induced deficits in synaptic plasticity and neurocognitive functions: A role for brain-derived neurotrophic factor. Acta Pharmacol. Sin. 2012, 33, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Brzecka, A. Brain preconditioning and obstructive sleep apnea syndrome. Acta Neurobiol. Exp. 2005, 65, 213–220. [Google Scholar]
- Ventriglia, M.; Zanardini, R.; Bonomini, C.; Zanetti, O.; Volpe, D.; Pasqualetti, P.; Gennarelli, M.; Bocchio-Chiavetto, L. Serum brain-derived neurotrophic factor levels in different neurological diseases. BioMed Res. Int. 2013, 2013, 901082. [Google Scholar] [CrossRef]
- El Ghamry, R.; El-Sheikh, M.; Abdel Meguid, M.; Nagib, S.; Aly El Gabry, D. Plasma brain-derived neurotrophic factor (BDNF) in Egyptian children with attention deficit hyperactivity disorder. Middle East Curr. Psychiatry 2021, 28, 22. [Google Scholar] [CrossRef]
- Goren, J.L. Brain-derived neurotrophic factor and schizophrenia. Ment. Health Clin. 2016, 6, 285–288. [Google Scholar] [CrossRef]
- Liang, F.Q.; Allen, G.; Earnest, D. Role of brain-derived neurotrophic factor in the circadian regulation of the suprachiasmatic pacemaker by light. J. Neurosci. 2000, 20, 2978–2987. [Google Scholar] [CrossRef]
- Sochal, M.; Ditmer, M.; Gabryelska, A.; Białasiewicz, P. The Role of Brain-Derived Neurotrophic Factor in Immune-Related Diseases: A Narrative Review. J. Clin. Med. 2022, 11, 6023. [Google Scholar] [CrossRef]
- Wu, C.-H.; Chen, C.-C.; Hung, T.-H.; Chuang, Y.-C.; Chao, M.; Shyue, S.-K.; Chen, S.-F. Activation of TrkB/Akt signaling by a TrkB receptor agonist improves long-term histological and functional outcomes in experimental intracerebral hemorrhage. J. Biomed. Sci. 2019, 26, 53. [Google Scholar] [CrossRef]
- Kermani, P.; Hempstead, B. BDNF Actions in the Cardiovascular System: Roles in Development, Adulthood and Response to Injury. Front. Physiol. 2019, 10, 455. [Google Scholar] [CrossRef]
- Shah, F.; Forsgren, S.; Holmlund, T.; Levring Jäghagen, E.; Berggren, D.; Franklin, K.A.; Stål, P. Neurotrophic factor BDNF is upregulated in soft palate muscles of snorers and sleep apnea patients. Laryngoscope Investig. Otolaryngol. 2019, 4, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lu, H.; Hu, L.; Gong, W.; Wang, J.; Fu, C.; Liu, Z.; Li, S. Chronic intermittent hypoxia affects endogenous serotonergic inputs and expression of synaptic proteins in rat hypoglossal nucleus. Am. J. Transl. Res. 2017, 9, 546–557. [Google Scholar] [PubMed]
- Erickson, J.T.; Conover, J.C.; Borday, V.; Champagnat, J.; Barbacid, M.; Yancopoulos, G.; Katz, D.M. Mice lacking brain-derived neurotrophic factor exhibit visceral sensory neuron losses distinct from mice lacking NT4 and display a severe developmental deficit in control of breathing. J. Neurosci. 1996, 16, 5361–5371. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, M.; O’Sullivan, M.; Mery, V.P.; Lafontaine, A.L.; Robinson, A.; Gros, P.; Martin, J.G.; Benedetti, A.; Kimoff, R.J. Inflammatory markers and BDNF in obstructive sleep apnea (OSA) in Parkinson’s disease (PD). Sleep Med. 2022, 90, 258–261. [Google Scholar] [CrossRef]
- More, C.E.; Papp, C.; Harsanyi, S.; Gesztelyi, R.; Mikaczo, A.; Tajti, G.; Kardos, L.; Seres, I.; Lorincz, H.; Csapo, K.; et al. Altered irisin/BDNF axis parallels excessive daytime sleepiness in obstructive sleep apnea patients. Respir. Res. 2019, 20, 67. [Google Scholar] [CrossRef] [PubMed]
- Flores, K.R.; Viccaro, F.; Aquilini, M.; Scarpino, S.; Ronchetti, F.; Mancini, R.; Di Napoli, A.; Scozzi, D.; Ricci, A. Protective role of brain derived neurotrophic factor (BDNF) in obstructive sleep apnea syndrome (OSAS) patients. PLoS ONE 2020, 15, e0227834. [Google Scholar] [CrossRef]
- Wang, W.H.; He, G.P.; Xiao, X.P.; Gu, C.; Chen, H.Y. Relationship between brain-derived neurotrophic factor and cognitive function of obstructive sleep apnea/hypopnea syndrome patients. Asian Pac. J. Trop. Med. 2012, 5, 906–910. [Google Scholar] [CrossRef]
- Zhu, Y.; Fenik, P.; Zhan, G.; Xin, R.; Veasey, S.C. Degeneration in Arousal Neurons in Chronic Sleep Disruption Modeling Sleep Apnea. Front. Neurol. 2015, 6, 109. [Google Scholar] [CrossRef]
- Szelenberger, W. Neurobiologia Snu. Adv. Respir. Med. 2007, 75, 3–8. [Google Scholar] [CrossRef]
- Torrisi, S.A.; Lavanco, G.; Maurel, O.M.; Gulisano, W.; Laudani, S.; Geraci, F.; Grasso, M.; Barbagallo, C.; Caraci, F.; Bucolo, C.; et al. A novel arousal-based individual screening reveals susceptibility and resilience to PTSD-like phenotypes in mice. Neurobiol. Stress 2021, 14, 100286. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Hao, J.W.; Li, G.L.; Ji, X.J.; Zhou, M.; Yao, Y.M. Long-lasting neurobehavioral alterations in burn-injured mice resembling post-traumatic stress disorder in humans. Exp. Neurol. 2020, 323, 113084. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.L.; Hardy, N.F.; Jimenez, D.V.; Maynard, K.R.; Kardian, A.S.; Pollock, C.J.; Schloesser, R.J.; Martinowich, K. Loss of promoter IV-driven BDNF expression impacts oscillatory activity during sleep, sensory information processing and fear regulation. Transl. Psychiatry 2016, 6, e873. [Google Scholar] [CrossRef] [PubMed]
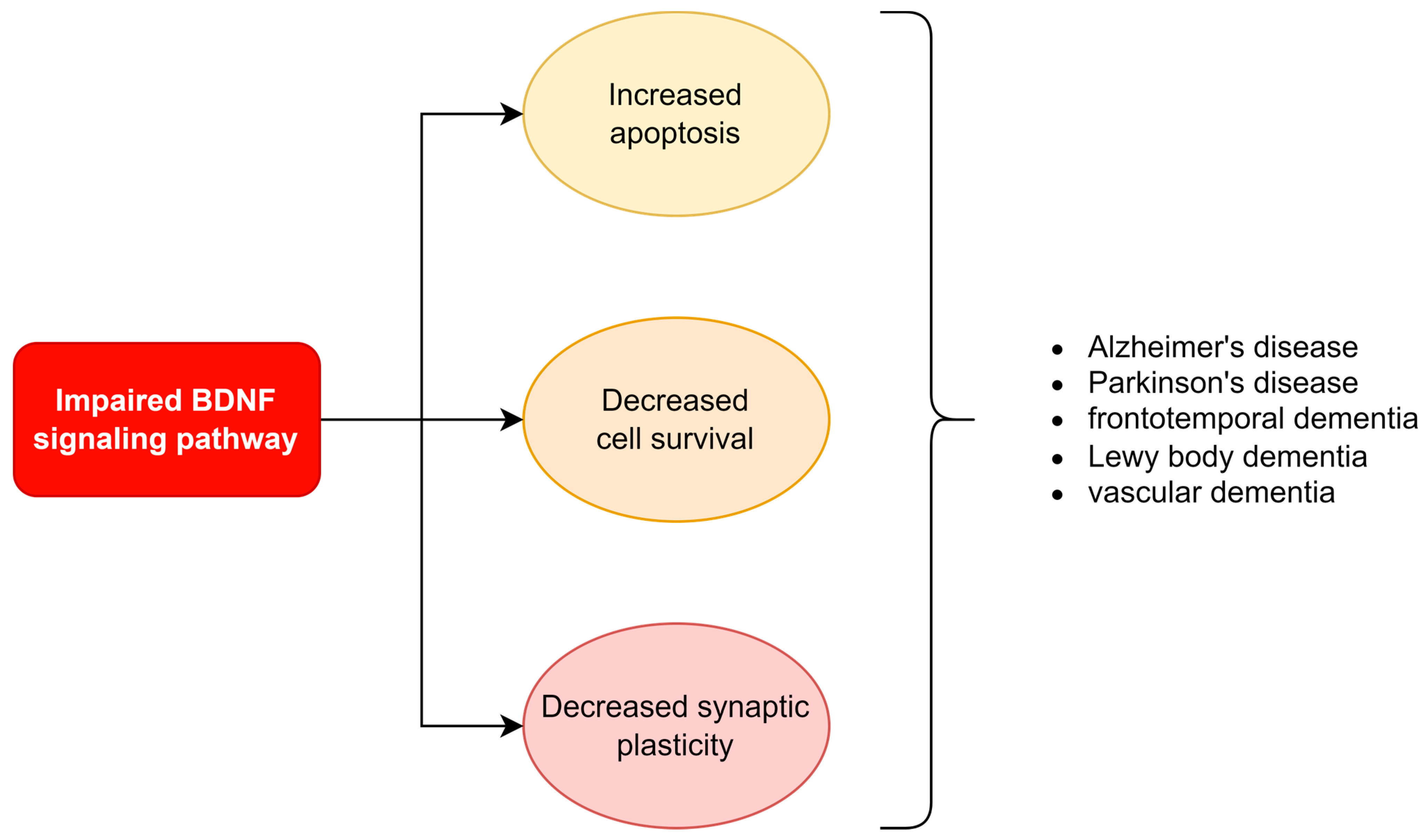
- Andrade, A.G.; Bubu, O.M.; Varga, A.W.; Osorio, R.S. The Relationship between Obstructive Sleep Apnea and Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 64, S255–S270. [Google Scholar] [CrossRef]
- Castrén, E.; Monteggia, L.M. Brain-Derived Neurotrophic Factor Signaling in Depression and Antidepressant Action. Biol. Psychiatry 2021, 90, 128–136. [Google Scholar] [CrossRef]
- Campos-Rodriguez, F.; Cordero-Guevara, J.; Asensio-Cruz, M.I.; Sanchez-Armengol, A.; Sanchez-Lopez, V.; Arellano-Orden, E.; Gozal, D.; Martinez-Garcia, M.A. Interleukin 6 as a marker of depression in women with sleep apnea. J. Sleep Res. 2021, 30, e13035. [Google Scholar] [CrossRef] [PubMed]
- Gabryelska, A.; Turkiewicz, S.; Ditmer, M.; Karuga, F.F.; Strzelecki, D.; Białasiewicz, P.; Sochal, M. BDNF and proBDNF Serum Protein Levels in Obstructive Sleep Apnea Patients and Their Involvement in Insomnia and Depression Symptoms. J. Clin. Med. 2022, 11, 7135. [Google Scholar] [CrossRef] [PubMed]
- Germain, A.; Kupfer, D.J. Circadian rhythm disturbances in depression. Hum. Psychopharmacol. 2008, 23, 571–585. [Google Scholar] [CrossRef]
- Xie, H.; Leung, K.L.; Chen, L.; Chan, Y.S.; Ng, P.C.; Fok, T.F.; Wing, Y.K.; Ke, Y.; Li, A.M.; Yung, W.H. Brain-derived neurotrophic factor rescues and prevents chronic intermittent hypoxia-induced impairment of hippocampal long-term synaptic plasticity. Neurobiol. Dis. 2010, 40, 155–162. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, J.; Qu, Y.; Sun, Q.; Li, T.T.; Yan, M.L.; Duan, M.J.; Li, K.X.; Wang, Y.R.; Huang, S.Y.; et al. Metoprolol prevents neuronal dendrite remodeling in a canine model of chronic obstructive sleep apnea. Acta Pharmacol. Sin. 2020, 41, 620–628. [Google Scholar] [CrossRef]
- An, J.R.; Zhao, Y.S.; Luo, L.F.; Guan, P.; Tan, M.; Ji, E.S. Huperzine A, reduces brain iron overload and alleviates cognitive deficit in mice exposed to chronic intermittent hypoxia. Life Sci. 2020, 250, 117573. [Google Scholar] [CrossRef]
- Fang, Y.Y.; Luo, M.; Yue, S.; Han, Y.; Zhang, H.J.; Zhou, Y.H.; Liu, K.; Liu, H.G. 7,8-Dihydroxyflavone protects retinal ganglion cells against chronic intermittent hypoxia-induced oxidative stress damage via activation of the BDNF/TrkB signaling pathway. Sleep Breath. 2022, 26, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guo, X.; Shi, Y.; Ma, J.; Wang, G. Intermittent hypoxia with or without hypercapnia is associated with tumorigenesis by decreasing the expression of brain derived neurotrophic factor and miR-34a in rats. Chin. Med. J. 2014, 127, 43–47. [Google Scholar]
- Ling, J.; Yu, Q.; Li, Y.; Yuan, X.; Wang, X.; Liu, W.; Guo, T.; Duan, Y.; Li, L. Edaravone Improves Intermittent Hypoxia-Induced Cognitive Impairment and Hippocampal Damage in Rats. Biol. Pharm. Bull. 2020, 43, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Vermehren-Schmaedick, A.; Jenkins, V.K.; Knopp, S.J.; Balkowiec, A.; Bissonnette, J.M. Acute intermittent hypoxia-induced expression of brain-derived neurotrophic factor is disrupted in the brainstem of methyl-CpG-binding protein 2 null mice. Neuroscience 2012, 206, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Baker-Herman, T.L.; Fuller, D.D.; Bavis, R.W.; Zabka, A.G.; Golder, F.J.; Doperalski, N.J.; Johnson, R.A.; Watters, J.J.; Mitchell, G.S. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat. Neurosci. 2004, 7, 48–55. [Google Scholar] [CrossRef]
- Lovett-Barr, M.R.; Satriotomo, I.; Muir, G.D.; Wilkerson, J.E.; Hoffman, M.S.; Vinit, S.; Mitchell, G.S. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. J. Neurosci. 2012, 32, 3591–3600. [Google Scholar] [CrossRef]
- Dong, Z.; Han, H.; Li, H.; Bai, Y.; Wang, W.; Tu, M.; Peng, Y.; Zhou, L.; He, W.; Wu, X.; et al. Long-term potentiation decay and memory loss are mediated by AMPAR endocytosis. J. Clin. Investig. 2015, 125, 234–247. [Google Scholar] [CrossRef]
- Cui, F.; Guo, J.; Hu, H.-F.; Zhang, Y.; Shi, M. Chronic intermittent hypobaric hypoxia improves markers of iron metabolism in a model of dietary-induced obesity. J. Inflamm. 2020, 17, 36. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Tan, M.; Song, J.X.; An, J.R.; Yang, X.Y.; Li, W.Y.; Guo, Y.J.; Ji, E.S. Involvement of Hepcidin in Cognitive Damage Induced by Chronic Intermittent Hypoxia in Mice. Oxidative Med. Cell. Longev. 2021, 2021, 8520967. [Google Scholar] [CrossRef]
- Panaree, B.; Chantana, M.; Wasana, S.; Chairat, N. Effects of obstructive sleep apnea on serum brain-derived neurotrophic factor protein, cortisol, and lipid levels. Sleep Breath. 2011, 15, 649–656. [Google Scholar] [CrossRef]
- Campos-Rodriguez, F.; Asensio-Cruz, M.I.; Cordero-Guevara, J.; Jurado-Gamez, B.; Carmona-Bernal, C.; Gonzalez-Martinez, M.; Troncoso, M.F.; Sanchez-Lopez, V.; Arellano-Orden, E.; Garcia-Sanchez, M.I.; et al. Effect of continuous positive airway pressure on inflammatory, antioxidant, and depression biomarkers in women with obstructive sleep apnea: A randomized controlled trial. Sleep 2019, 42, zsz145. [Google Scholar] [CrossRef] [PubMed]
- Makhout, S.; Vermeiren, E.; Van De Maele, K.; Bruyndonckx, L.; De Winter, B.Y.; Van Hoorenbeeck, K.; Verhulst, S.L.; Van Eyck, A. The Role of Brain-Derived Neurotrophic Factor in Obstructive Sleep Apnea and Endothelial Function in a Pediatric Population with Obesity. Front. Med. 2022, 8, 3086. [Google Scholar] [CrossRef] [PubMed]
- Gabryelska, A.; Sochal, M. Evaluation of HIF-1 Involvement in the BDNF and ProBDNF Signaling Pathways among Obstructive Sleep Apnea Patients. Int. J. Mol. Sci. 2022, 23, 14876. [Google Scholar] [CrossRef]
- Arosio, B.; Guerini, F.R.; Voshaar, R.C.O.; Aprahamian, I. Blood Brain-Derived Neurotrophic Factor (BDNF) and Major Depression: Do We Have a Translational Perspective? Front. Behav. Neurosci. 2021, 15, 626906. [Google Scholar] [CrossRef] [PubMed]
- Gabryelska, A.; Szmyd, B.; Szemraj, J.; Stawski, R.; Sochal, M.; Białasiewicz, P. Patients with obstructive sleep apnea present with chronic upregulation of serum HIF-1α protein. J. Clin. Sleep Med. 2020, 16, 1761–1768. [Google Scholar] [CrossRef]
- Lu, D.; Li, N.; Yao, X.; Zhou, L. Potential inflammatory markers in obstructive sleep apnea-hypopnea syndrome. Bosn. J. Basic Med. Sci. 2017, 17, 47–53. [Google Scholar] [CrossRef]
- Semenza, G.L. Expression of hypoxia-inducible factor 1: Mechanisms and consequences. Biochem. Pharmacol. 2000, 59, 47–53. [Google Scholar] [CrossRef]
- Helan, M.; Aravamudan, B.; Hartman, W.R.; Thompson, M.A.; Johnson, B.D.; Pabelick, C.M.; Prakash, Y.S. BDNF secretion by human pulmonary artery endothelial cells in response to hypoxia. J. Mol. Cell. Cardiol. 2014, 68, 89–97. [Google Scholar] [CrossRef]
- Gao, Y.; Jing, M.; Ge, R.; Lang, L. Induction of hypoxia-inducible factor-1α by BDNF protects retinoblastoma cells against chemotherapy-induced apoptosis. Mol. Cell. Biochem. 2016, 414, 77–84. [Google Scholar] [CrossRef]
- Jin, H.; Zhu, Y.; Li, Y.; Ding, X.; Ma, W.; Han, X.; Wang, B. BDNF-mediated mitophagy alleviates high-glucose-induced brain microvascular endothelial cell injury. Apoptosis 2019, 24, 511–528. [Google Scholar] [CrossRef]
- Chen, H.; Xiong, T.; Qu, Y.; Zhao, F.; Ferriero, D.; Mu, D. mTOR activates hypoxia-inducible factor-1α and inhibits neuronal apoptosis in the developing rat brain during the early phase after hypoxia-ischemia. Neurosci. Lett. 2012, 507, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Abdo Qaid, E.Y.; Zulkipli, N.N.; Zakaria, R.; Ahmad, A.H.; Othman, Z.; Muthuraju, S.; Sasongko, T.H. The role of mTOR signalling pathway in hypoxia-induced cognitive impairment. Int. J. Neurosci. 2021, 131, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Datta, S.R.; Greenberg, M.E. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr. Opin. Neurobiol. 2001, 11, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Fung, S.J.; Xi, M.C.; Zhang, J.H.; Sampogna, S.; Yamuy, J.; Morales, F.R.; Chase, M.H. Apnea promotes glutamate-induced excitotoxicity in hippocampal neurons. Brain Res. 2007, 1179, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.D.; Manadas, B.J.; Melo, C.V.; Gomes, J.R.; Mendes, C.S.; Grãos, M.M.; Carvalho, R.F.; Carvalho, A.P.; Duarte, C.B. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005, 12, 1329–1343. [Google Scholar] [CrossRef]
- Gejl, A.K.; Enevold, C.; Bugge, A.; Andersen, M.S.; Nielsen, C.H.; Andersen, L.B. Associations between serum and plasma brain-derived neurotrophic factor and influence of storage time and centrifugation strategy. Sci. Rep. 2019, 9, 9655. [Google Scholar] [CrossRef]
- Lommatzsch, M.; Zingler, D.; Schuhbaeck, K.; Schloetcke, K.; Zingler, C.; Schuff-Werner, P.; Virchow, J.C. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol. Aging 2005, 26, 115–123. [Google Scholar] [CrossRef]
- Gavrilova, S.I.; Alvarez, A. Cerebrolysin in the therapy of mild cognitive impairment and dementia due to Alzheimer’s disease: 30 years of clinical use. Med. Res. Rev. 2021, 41, 2775–2803. [Google Scholar] [CrossRef]
- Alvarez, X.A.; Alvarez, I.; Iglesias, O.; Crespo, I.; Figueroa, J.; Aleixandre, M.; Linares, C.; Granizo, E.; Garcia-Fantini, M.; Marey, J.; et al. Synergistic Increase of Serum BDNF in Alzheimer Patients Treated with Cerebrolysin and Donepezil: Association with Cognitive Improvement in ApoE4 Cases. Int. J. Neuropsychopharmacol. 2016, 19. [Google Scholar] [CrossRef]
- Watson, A.J.; Henson, K.; Dorsey, S.G.; Frank, M.G. The truncated TrkB receptor influences mammalian sleep. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2015, 308, R199–R207. [Google Scholar] [CrossRef]
- Garner, J.M.; Chambers, J.; Barnes, A.K.; Datta, S. Changes in Brain-Derived Neurotrophic Factor Expression Influence Sleep-Wake Activity and Homeostatic Regulation of Rapid Eye Movement Sleep. Sleep 2018, 41, zsx194. [Google Scholar] [CrossRef]
- Mongrain, V.; Warby, S.C. Determinants of cortical synchrony. Sleep 2012, 35, 309–310. [Google Scholar] [CrossRef] [PubMed]
- Kerner, N.A.; Roose, S.P. Obstructive Sleep Apnea is Linked to Depression and Cognitive Impairment: Evidence and Potential Mechanisms. Am. J. Geriatr. Psychiatry 2016, 24, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Athar, W.; Card, M.E.; Charokopos, A.; Akgün, K.M.; DeRycke, E.C.; Haskell, S.G.; Yaggi, H.K.; Bastian, L.A. Obstructive Sleep Apnea and Pain Intensity in Young Adults. Ann. Am. Thorac. Soc. 2020, 17, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarski, P.; Karuga, F.F.; Szmyd, B.; Sochal, M.; Bialasiewicz, P.; Strzelecki, D.; Gabryelska, A. The Role of Inflammation, Hypoxia, and Opioid Receptor Expression in Pain Modulation in Patients Suffering from Obstructive Sleep Apnea. Int. J. Mol. Sci. 2022, 23, 9080. [Google Scholar] [CrossRef]
- Cappoli, N.; Tabolacci, E.; Aceto, P.; Dello Russo, C. The emerging role of the BDNF-TrkB signaling pathway in the modulation of pain perception. J. Neuroimmunol. 2020, 349, 577406. [Google Scholar] [CrossRef] [PubMed]
- Sochal, M.; Małecka-Panas, E.; Gabryelska, A.; Fichna, J.; Talar-Wojnarowska, R.; Szmyd, B.; Białasiewicz, P. Brain-derived neurotrophic factor is elevated in the blood serum of Crohn’s disease patients, but is not influenced by anti-TNF-α treatment—A pilot study. Neurogastroenterol. Motil. 2021, 33, e13978. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, J.; Ren, Y.; Zhou, W.; Yin, G. The release of glutamate from cortical neurons regulated by BDNF via the TrkB/Src/PLC-gamma1 pathway. J. Cell. Biochem. 2013, 114, 144–151. [Google Scholar] [CrossRef]
- Merighi, A.; Salio, C.; Ghirri, A.; Lossi, L.; Ferrini, F.; Betelli, C.; Bardoni, R. BDNF as a pain modulator. Prog. Neurobiol. 2008, 85, 297–317. [Google Scholar] [CrossRef]
- Sikandar, S.; Minett, M.S.; Millet, Q.; Santana-Varela, S.; Lau, J.; Wood, J.N.; Zhao, J. Brain-derived neurotrophic factor derived from sensory neurons plays a critical role in chronic pain. Brain 2018, 141, 1028–1039. [Google Scholar] [CrossRef]
- Retamal, J.; Reyes, A.; Ramirez, P.; Bravo, D.; Hernandez, A.; Pelissier, T.; Villanueva, L.; Constandil, L. Burst-Like Subcutaneous Electrical Stimulation Induces BDNF-Mediated, Cyclotraxin B-Sensitive Central Sensitization in Rat Spinal Cord. Front. Pharmacol. 2018, 9, 1143. [Google Scholar] [CrossRef] [PubMed]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef] [PubMed]
- Haas, L.; Portela, L.V.; Bohmer, A.E.; Oses, J.P.; Lara, D.R. Increased plasma levels of brain derived neurotrophic factor (BDNF) in patients with fibromyalgia. Neurochem. Res. 2010, 35, 830–834. [Google Scholar] [CrossRef]
- Zanette, S.A.; Dussan-Sarria, J.A.; Souza, A.; Deitos, A.; Torres, I.L.; Caumo, W. Higher serum S100B and BDNF levels are correlated with a lower pressure-pain threshold in fibromyalgia. Mol. Pain 2014, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Giese, M.; Unternahrer, E.; Huttig, H.; Beck, J.; Brand, S.; Calabrese, P.; Holsboer-Trachsler, E.; Eckert, A. BDNF: An indicator of insomnia? Mol. Psychiatry 2014, 19, 151–152. [Google Scholar] [CrossRef]
- Tang, N.K. Insomnia Co-Occurring with Chronic Pain: Clinical Features, Interaction, Assessments and Possible Interventions. Rev. Pain 2008, 2, 2–7. [Google Scholar] [CrossRef]
- Staats, R.; Stoll, P.; Zingler, D.; Virchow, J.C.; Lommatzsch, M. Regulation of brain-derived neurotrophic factor (BDNF) during sleep apnoea treatment. Thorax 2005, 60, 688–692. [Google Scholar] [CrossRef]
- Dechant, G.; Barde, Y.-A. The neurotrophin receptor p75NTR: Novel functions and implications for diseases of the nervous system. Nat. Neurosci. 2002, 5, 1131–1136. [Google Scholar] [CrossRef]
- Skeldal, S.; Sykes, A.M.; Glerup, S.; Matusica, D.; Palstra, N.; Autio, H.; Boskovic, Z.; Madsen, P.; Castrén, E.; Nykjaer, A.; et al. Mapping of the interaction site between sortilin and the p75 neurotrophin receptor reveals a regulatory role for the sortilin intracellular domain in p75 neurotrophin receptor shedding and apoptosis. J. Biol. Chem. 2012, 287, 43798–43809. [Google Scholar] [CrossRef]
- Tietjens, J.R.; Claman, D.; Kezirian, E.J.; De Marco, T.; Mirzayan, A.; Sadroonri, B.; Goldberg, A.N.; Long, C.; Gerstenfeld, E.P.; Yeghiazarians, Y. Obstructive Sleep Apnea in Cardiovascular Disease: A Review of the Literature and Proposed Multidisciplinary Clinical Management Strategy. J. Am. Heart Assoc. 2019, 8, e010440. [Google Scholar] [CrossRef]
- Yu, L.; Li, X.; Huang, B.; Zhou, X.; Wang, M.; Zhou, L.; Meng, G.; Wang, Y.; Wang, Z.; Deng, J.; et al. Atrial Fibrillation in Acute Obstructive Sleep Apnea: Autonomic Nervous Mechanism and Modulation. J. Am. Heart Assoc. 2017, 6, e006264. [Google Scholar] [CrossRef] [PubMed]
- Xiaokereti, J.; Guo, Y.-K.; Dong, Z.-Y.; Ma, M.; Lu, Y.-M.; Li, Y.-D.; Zhou, X.-H.; Zhang, L.; Tang, B.-P. Enhanced atrial internal-external neural remodeling facilitates atrial fibrillation in the chronic obstructive sleep apnea model. PLoS ONE 2021, 16, e0247308. [Google Scholar] [CrossRef] [PubMed]
- Huan, H.B.; Wen, X.D.; Chen, X.J.; Wu, L.; Wu, L.L.; Zhang, L.; Yang, D.P.; Zhang, X.; Bie, P.; Qian, C.; et al. Sympathetic nervous system promotes hepatocarcinogenesis by modulating inflammation through activation of alpha1-adrenergic receptors of Kupffer cells. Brain Behav. Immun. 2017, 59, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Chen, W.T.; Chen, S.Y.; Lee, T.M. Taurine Alleviates Sympathetic Innervation by Inhibiting NLRP3 Inflammasome in Postinfarcted Rats. J. Cardiovasc. Pharmacol. 2021, 77, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guo, Y.; Xiaokereti, J.; Cao, G.; Li, H.; Sun, H.; Li, K.; Zhou, X.; Tang, B. Ganglionated Plexi Ablation Suppresses Chronic Obstructive Sleep Apnea-Related Atrial Fibrillation by Inhibiting Cardiac Autonomic Hyperactivation. Front. Physiol. 2021, 12, 640295. [Google Scholar] [CrossRef]
- Ding, X.; Yu, C.; Liu, Y.; Yan, S.; Li, W.; Wang, D.; Sun, L.; Han, Y.; Li, M.; Zhang, S.; et al. Chronic obstructive sleep apnea accelerates pulmonary remodeling via TGF-β/miR-185/CoLA1 signaling in a canine model. Oncotarget 2016, 7, 57545–57555. [Google Scholar] [CrossRef]
- Goldbart, A.D.; Mager, E.; Veling, M.C.; Goldman, J.L.; Kheirandish-Gozal, L.; Serpero, L.D.; Piedimonte, G.; Gozal, D. Neurotrophins and tonsillar hypertrophy in children with obstructive sleep apnea. Pediatr. Res. 2007, 62, 489–494. [Google Scholar] [CrossRef]
- Li, J.; Xing, J.; Lu, J. Nerve Growth Factor, Muscle Afferent Receptors and Autonomic Responsiveness with Femoral Artery Occlusion. J. Mod. Physiol. Res. 2014, 1, 1–18. [Google Scholar]
- Gabryelska, A.; Sochal, M.; Turkiewicz, S.; Białasiewicz, P. Relationship between HIF-1 and Circadian Clock Proteins in Obstructive Sleep Apnea Patients—Preliminary Study. J. Clin. Med. 2020, 9, 1599. [Google Scholar] [CrossRef]
- Gabryelska, A.; Stawski, R.; Sochal, M.; Szmyd, B.; Bialasiewicz, P. Influence of one-night CPAP therapy on the changes of HIF-1alpha protein in OSA patients: A pilot study. J. Sleep Res. 2020, 29, e12995. [Google Scholar] [CrossRef]
- Sakamoto, K.; Higo-Yamamoto, S.; Egi, Y.; Miyazaki, K.; Oishi, K. Memory dysfunction and anxiety-like behavior in a mouse model of chronic sleep disorders. Biochem. Biophys. Res. Commun. 2020, 529, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Silva Junior, J.F.; Eckeli, A.L.; Ribeiro, C.C.C.; Batista, R.F.L.; da Silva, A.A.M.; Alves, C.M.C. Influence of excessive daily sleeping and sleep quality on BDNF and NGF serum levels in adolescents. Sleep Med. 2021, 84, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, V.; Nair, D.; Zhang, S.X.L.; Hakim, F.; Kaushal, N.; Kayali, F.; Wang, Y.; Li, R.C.; Carreras, A.; Gozal, D. Disrupted sleep without sleep curtailment induces sleepiness and cognitive dysfunction via the tumor necrosis factor-α pathway. J. Neuroinflamm. 2012, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Bothwell, M. NGF, BDNF, NT3, and NT4. Handb. Exp. Pharmacol. 2014, 220, 3–15. [Google Scholar] [CrossRef]
- Kushikata, T.; Kubota, T.; Fang, J.; Krueger, J.M. Neurotrophins 3 and 4 enhance non-rapid eye movement sleep in rabbits. Neurosci. Lett. 2003, 346, 161–164. [Google Scholar] [CrossRef]
- Huang, L.; Guo, H.; Hellard, D.T.; Katz, D.M. Glial cell line-derived neurotrophic factor (GDNF) is required for differentiation of pontine noradrenergic neurons and patterning of central respiratory output. Neuroscience 2005, 130, 95–105. [Google Scholar] [CrossRef]
- Sasaki, A.; Kanai, M.; Kijima, K.; Akaba, K.; Hashimoto, M.; Hasegawa, H.; Otaki, S.; Koizumi, T.; Kusuda, S.; Ogawa, Y.; et al. Molecular analysis of congenital central hypoventilation syndrome. Hum. Genet. 2003, 114, 22–26. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Bisgard, G.E. Postnatal growth of the carotid body. Respir. Physiol. Neurobiol. 2005, 149, 181–190. [Google Scholar] [CrossRef]
- Szily, M.; Tarnoki, A.D.; Tarnoki, D.L.; Kovacs, D.T.; Forgo, B.; Lee, J.; Kim, E.; Sung, J.; Kunos, L.; Meszaros, M.; et al. Genetic influences on the onset of obstructive sleep apnoea and daytime sleepiness: A twin study. Respir. Res. 2019, 20, 125. [Google Scholar] [CrossRef]
- Cao, Y.; Zhu, Q.; Cai, X.; Wu, T.; Aierken, X.; Ahmat, A.; Liu, S.; Li, N. Glial Cell-Derived Neurotrophic Factor Functions as a Potential Candidate Gene in Obstructive Sleep Apnea Based on a Combination of Bioinformatics and Targeted Capture Sequencing Analyses. BioMed Res. Int. 2021, 2021, 6656943. [Google Scholar] [CrossRef]
- Cao, Y.; Cai, X.; Zhu, Q.; Li, N. Screening and identification of potential biomarkers for obstructive sleep apnea via microarray analysis. Medicine 2021, 100, e24435. [Google Scholar] [CrossRef] [PubMed]
- Larkin, E.K.; Patel, S.R.; Goodloe, R.J.; Li, Y.; Zhu, X.; Gray-McGuire, C.; Adams, M.D.; Redline, S. A candidate gene study of obstructive sleep apnea in European Americans and African Americans. Am. J. Respir. Crit. Care Med. 2010, 182, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Gislason, T.; Pack, A.I.; Helgadottir, H.T.; Stefansson, K.; Besenbacher, S.; Jonsdottir, I. The CRP and GDNF genes do not contribute to apnea-hypopnea index or risk of obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2011, 184, 143–144. [Google Scholar] [CrossRef] [PubMed]
| Summary of Main Findings |
|---|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabryelska, A.; Turkiewicz, S.; Ditmer, M.; Sochal, M. Neurotrophins in the Neuropathophysiology, Course, and Complications of Obstructive Sleep Apnea—A Narrative Review. Int. J. Mol. Sci. 2023, 24, 1808. https://doi.org/10.3390/ijms24031808
Gabryelska A, Turkiewicz S, Ditmer M, Sochal M. Neurotrophins in the Neuropathophysiology, Course, and Complications of Obstructive Sleep Apnea—A Narrative Review. International Journal of Molecular Sciences. 2023; 24(3):1808. https://doi.org/10.3390/ijms24031808
Chicago/Turabian StyleGabryelska, Agata, Szymon Turkiewicz, Marta Ditmer, and Marcin Sochal. 2023. "Neurotrophins in the Neuropathophysiology, Course, and Complications of Obstructive Sleep Apnea—A Narrative Review" International Journal of Molecular Sciences 24, no. 3: 1808. https://doi.org/10.3390/ijms24031808
APA StyleGabryelska, A., Turkiewicz, S., Ditmer, M., & Sochal, M. (2023). Neurotrophins in the Neuropathophysiology, Course, and Complications of Obstructive Sleep Apnea—A Narrative Review. International Journal of Molecular Sciences, 24(3), 1808. https://doi.org/10.3390/ijms24031808






