Bioprospecting for Rhizobacteria with the Ability to Enhance Drought Tolerance in Lessertia frutescens
Abstract
:1. Introduction
2. Results
2.1. Plant Materials, Soil Preparation, Inoculation, and Planting
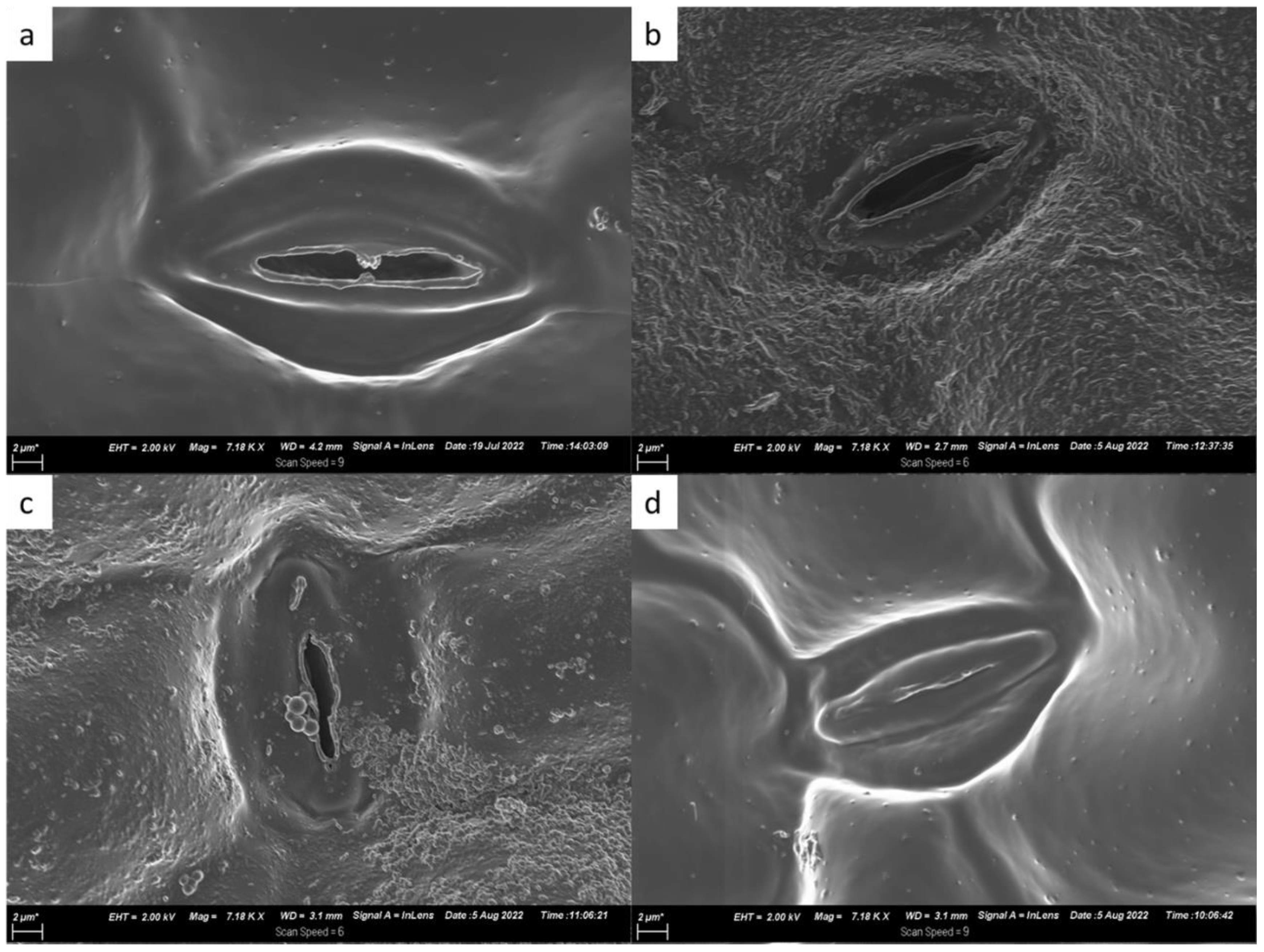
2.2. Morphological Characteristics
2.3. Osmolyte Proline Content Assay
2.4. Total Flavonoid Compounds
2.5. Total Phenolic Compounds
2.6. Total Triterpenes
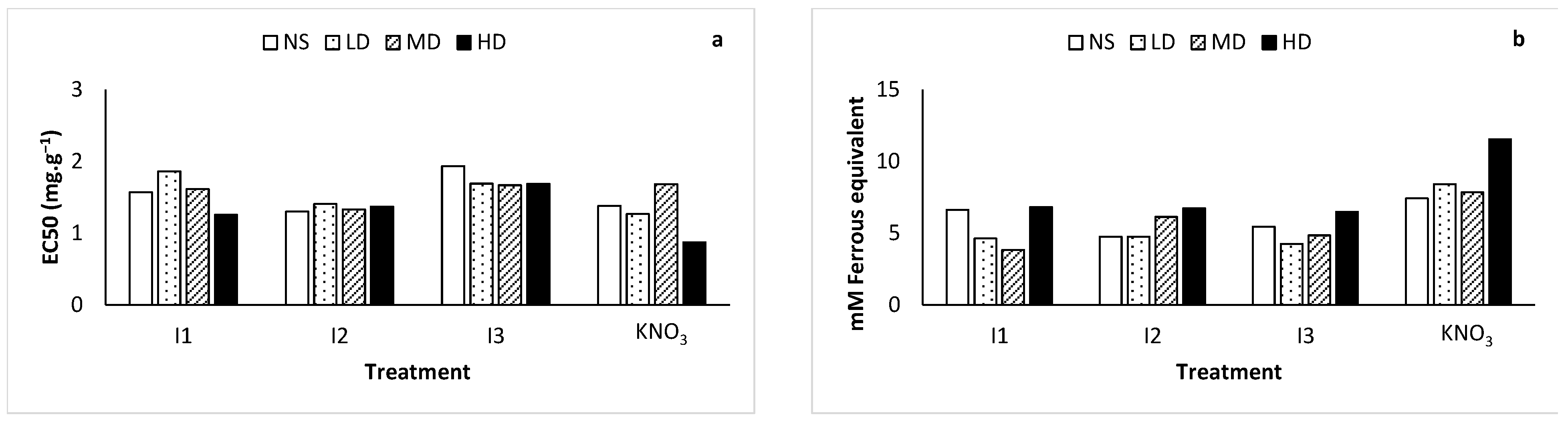
2.7. Antioxidant Activities
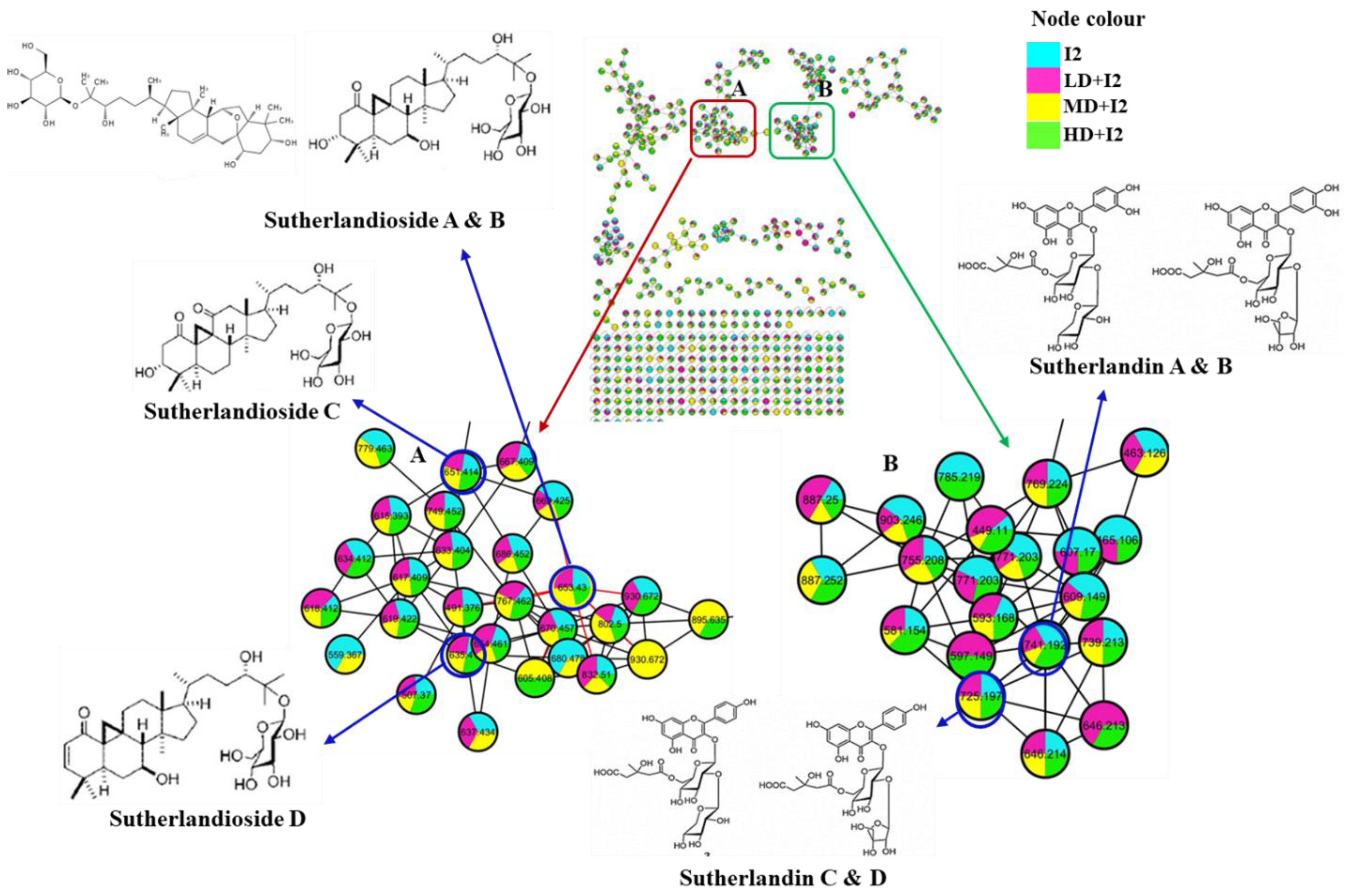
2.8. Metabolomic Composition of Drought Stressed Lessertia frutescens Treated with I2
| Molecular Formula | m/z Value | Retention Time (min) | Observed Fragmentation Ions | Compound Name | Samples |
|---|---|---|---|---|---|
| C36H60O10 | 652.4186 | 9.56 | 653.4315; 491.3750; 473.3657; 455.3556; 437.3448; 419.3339 | Sutherlandioside A and B | I1, I2, I3, KNO3, LD + I1, LD + I2, LD + I3, LD + KNO3, MD + I1, MD + I2, MD + I3, MD + KNO3, HD + I1, HD + I2, HD + I3, HD + KNO3 |
| C36H58O10 | 650.4030 | 8.85 | 651.4130; 633.4041; 489.3596; 471.3511 | Sutherlandioside C | I1, I2, I3, KNO3, LD + I1, LD + I2, LD + I3, LD + KNO3, MD + I1, MD + I2, MD + I3, MD + KNO3, HD + I1, HD + I2, HD + I3, HD + KNO3 |
| C36H58O9 | 634.4081 | 10.11 | 635.3539; 617.4042; 473.3637; 455.3539; 437.3432; 419.3326 | Sutherlandioside D | I1, I2, I3, KNO3, LD + I1, LD + I2, LD + I3, LD + KNO3, MD + I1, MD + I2, MD + I3, MD + KNO3, HD + I1, HD + I2, HD + I3, HD + KNO3 |
| C32H36O20 | 740.1800 | 6.91 | 763.1735; 741.1909; 609.1471; 302.9976 | Sutherlandin A and B | I1, I2, I3, KNO3, LD + I1, LD + I2, LD + I3, LD + KNO3, MD + I1, MD + I2, MD + I3, MD + KNO3, HD + I1, HD + I2, HD + I3, HD + KNO3 |
| C32H36O19 | 724.1851 | 7.20 | 747.1782; 725.1962; 593.1530; 287.0561 | Sutherlandin C and D | I1, I2, I3, KNO3, LD + I1, LD + I2, LD + I3, LD + KNO3, MD + I1, MD + I2, MD + I3, MD + KNO3, HD + I1, HD + I2, HD + I3, HD + KNO3 |
3. Discussion
4. Materials and Methods
4.1. Source of Bacterial Isolates and Commercial Inoculant
4.2. Plant Materials, Soil Preparation, Inoculation, and Planting
4.3. Morphological Characteristics
4.4. Osmolyte Proline Content
4.5. Total Flavonoid Compounds
4.6. Total Phenolic Compounds
4.7. Total Triterpenes
4.8. Antioxidant Activities
4.8.1. Radical Scavenging Activity
4.8.2. Ferric Reducing Antioxidant Potency
4.9. Untargeted Metabolomics of Lessertia frutescens under Drought from Ultra-High Performance Liquid Chromatography Quadruple Time of Flight Mass Spectrometry
4.9.1. Molecular Networking and Metabolite Annotation
4.9.2. Metabolomics Identification of Sutherlandiosides A–D and Sutherlandins A–D in Drought Stricken Lessertia frutescens
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rastogi, S.; Shah, S.; Kumar, R.; Vashisth, D.; Akhtar, M.Q.; Kumar, A.; Dwivedi, U.N.; Shasany, A.K. Ocimum metabolomics in response to abiotic stresses: Cold, flood, drought and salinity. PLoS ONE 2019, 14, e0210903. [Google Scholar] [CrossRef] [PubMed]
- Baher, Z.F.; Mirza, M.; Ghorbanli, M.; Bagher Rezaii, M. The influence of water stress on plant height, herbal and essential oil yield and composition in Satureja hortensis L. Flavour Fragr. J. 2002, 17, 275–277. [Google Scholar] [CrossRef]
- Alishah, H.M.; Heidari, R.; Hassani, A.; Dizaji, A.A. Effect of Water Stress on Some Morphological and Biochemical Characteristics of Purple Basil (Ocimum basilicum). J. Biol. Sci. 2006, 6, 763–767. [Google Scholar]
- Chiappero, J.; Cappellari, L.d.R.; Palermo, T.B.; Giordano, W.; Khan, N.; Banchio, E. Antioxidant status of medicinal and aromatic plants under the influence of growth-promoting rhizobacteria and osmotic stress. Ind. Crops Prod. 2021, 167, 113541. [Google Scholar] [CrossRef]
- Gorgi, O.E.; Fallah, H.; Niknejad, Y.; Tari, D.B. Effect of Plant growth promoting rhizobacteria (PGPR) and mycorrhizal fungi inoculations on essential oil in Melissa officinalis L. under drought stress. Biologia 2022, 77, 11–20. [Google Scholar] [CrossRef]
- Asghari, B.; Khademian, R.; Sedaghati, B. Plant growth promoting rhizobacteria (PGPR) confer drought resistance and stimulate biosynthesis of secondary metabolites in pennyroyal (Mentha pulegium L.) under water shortage condition. Sci. Hortic. 2020, 263, 109132. [Google Scholar] [CrossRef]
- Manukyan, A. Effect of Growing Factor on Productivity and Quality of Lemon Catmint, Lemon Balm and Sage under Soilless Greenhouse Condition: I Drought Stress. Med. Aromat. Plant Sci. Biotechnol. 2011, 5, 119–125. [Google Scholar]
- Cappellari, L.d.R.; Chiappero, J.; Santoro, M.V.; Giordano, W.; Banchio, E. Inducing phenolic production and volatile organic compounds emission by inoculating Mentha piperita with plant growth-promoting rhizobacteria. Sci. Hortic. 2017, 220, 193–198. [Google Scholar] [CrossRef]
- Vafadar, F.; Amooaghaie, R.; Ehsanzadeh, P.; Ghanadian, M. Salinity stress alters ion homeostasis, antioxidant activities and the production of rosmarinic acid, luteolin and apigenin in Dracocephalum kotschyi Boiss. Biologia 2020, 75, 2147–2158. [Google Scholar] [CrossRef]
- Bandurska, H.; Niedziela, J.; Pietrowska-Borek, M.; Nuc, K.; Chadzinikolau, T.; Radzikowska, D. Regulation of proline biosynthesis and resistance to drought stress in two barley (Hordeum vulgare L.) genotypes of different origin. Plant Physiol. Biochem. 2017, 118, 427–437. [Google Scholar] [CrossRef]
- Kumar, R.; Swapnil, P.; Meena, M.; Selpair, S.; Yadav, B.G. Plant Growth-Promoting Rhizobacteria (PGPR): Approaches to Alleviate Abiotic Stresses for Enhancement of Growth and Development of Medicinal Plants. Sustainability 2022, 14, 15514. [Google Scholar] [CrossRef]
- Pandey, S.; Gupta, S. Evaluation of Pseudomonas sp. for its multifarious plant growth promoting potential and its ability to alleviate biotic and abiotic stress in tomato (Solanum lycopersicum) plants. Sci. Rep. 2020, 10, 20951. [Google Scholar] [CrossRef] [PubMed]
- Mncwangi, N.P.; Viljoen, A.M. Quantitative variation of amino acids in Sutherlandia frutescens (Cancer bush)—Towards setting parameters for quality control. S. Afr. J. Bot. 2012, 82, 46–52. [Google Scholar] [CrossRef]
- Colling, J.; Stander, M.A.; Makunga, N.P. Nitrogen supply and abiotic stress influence canavanine synthesis and the productivity of in vitro regenerated Sutherlandia frutescens microshoots. J. Plant Physiol. 2010, 167, 1521–1524. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, A.; Ahmed, B.; Khan, M.S.; El-Beltagi, H.S.; Umar, S.; Lee, J. Bioprospecting Plant Growth Promoting Rhizobacteria for Enhancing the Biological Properties and Phytochemical Composition of Medicinally Important Crops. Molecules 2022, 27, 1407. [Google Scholar] [CrossRef]
- Mamta, G.; Rahi, P.; Pathania, V.; Gulati, A.; Singh, B.; Bhanwra, R.K.; Tewari, R. Comparative efficiency of phosphate-solubilizing bacteria under greenhouse conditions for promoting growth and aloin-A content of Aloe barbadensis. Arch. Agron. Soil Sci. 2012, 58, 437–449. [Google Scholar] [CrossRef]
- Lin, S.; Li, J.; Wu, J.; Yang, F.; Pei, L.; Shang, X. Interactive effects of maternal exposure to chemical fertilizer and socio-economic status on the risk of low birth weight. BMC Public Health 2022, 22, 1206. [Google Scholar] [CrossRef]
- Jjagwe, J.; Chelimo, K.; Karungi, J.; Komakech, A.J.; Lederer, J. Comparative Performance of Organic Fertilizers in Maize (Zea mays L.) Growth, Yield, and Economic Results. Agronomy 2020, 10, 69. [Google Scholar] [CrossRef]
- Ramabulana, A.-T.; Petras, D.; Madala, N.E.; Tugizimana, F. Metabolomics and Molecular Networking to Characterize the Chemical Space of Four Momordica Plant Species. Metabolites 2021, 11, 763. [Google Scholar] [CrossRef]
- Albrecht, C.; Stander, M.; Grobbelaar, M.; Colling, J.; Kossmann, J.; Hills, P.; Makunga, N. LC–MS-based metabolomics assists with quality assessment and traceability of wild and cultivated plants of Sutherlandia frutescens (Fabaceae). S. Afr. J. Bot. 2012, 82, 33–45. [Google Scholar] [CrossRef]
- Avula, B.; Wang, Y.-H.; Smillie, T.J.; Fu, X.; Li, X.C.; Mabusela, W.; Syce, J.; Johnson, Q.; Folk, W.; Khan, I.A. Quantitative determination of flavonoids and cycloartanol glycosides from aerial parts of Sutherlandia frutescens (L.) R. BR. by using LC-UV/ELSD methods and confirmation by using LC–MS method. J. Pharm. Biomed. Anal. 2010, 52, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Gamalero, E.; Glick, B.R. Recent Advances in Bacterial Amelioration of Plant Drought and Salt Stress. Biology 2022, 11, 437. [Google Scholar] [CrossRef]
- Mohammed, M.; Jaiswal, S.K.; Dakora, F.D. Distribution and correlation between phylogeny and functional traits of cowpea (Vigna unguiculata L. Walp.)-nodulating microsymbionts from Ghana and South Africa. Sci. Rep. 2018, 8, 18006. [Google Scholar] [CrossRef]
- Masenya, T.A.; Mashela, P.W.; Pofu, K.M. Efficacy of rhizobia strains on growth and chemical composition of cancer bush (Sutherlandia frutescens). Acta Agric. Scand. Sect. B Soil Plant Sci. 2022, 72, 358–363. [Google Scholar] [CrossRef]
- Makgato, M.J.; Araya, H.T.; du Plooy, C.P.; Mokgehle, S.N.; Mudau, F.N. Effects of rhizobium inoculation on N2 fixation, phytochemical profiles and rhizosphere soil microbes of cancer bush (Lessertia frutescens (L.). Agronomy 2020, 10, 1675. [Google Scholar] [CrossRef]
- Qu, Q.; Zhang, Z.; Peijnenburg, W.J.G.M.; Liu, W.; Lu, T.; Hu, B.; Chen, J.; Chen, J.; Lin, Z.; Qian, H. Rhizosphere Microbiome Assembly and Its Impact on Plant Growth. J. Agric. Food Chem. 2020, 68, 5024–5038. [Google Scholar] [CrossRef] [PubMed]
- Gerding, M.; O’Hara, G.W.; Howieson, J.G.; Bräu, L. Overcoming non-selective nodulation of Lessertia by soil-borne rhizobium in the presence of inoculant Mesorhizobium. Plant Soil 2014, 380, 117–132. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Sankar, B.; Kishorekumar, A.; Gopi, R.; Somasundaram, R.; Panneerselvam, R. Pseudomonas fluorescens enhances biomass yield and ajmalicine production in Catharanthus roseus under water deficit stress. Colloids Surf. B Biointerfaces 2007, 60, 7–11. [Google Scholar] [CrossRef]
- Chiappero, J.; Cappellari, L.d.R.; Sosa Alderete, L.G.; Palermo, T.B.; Banchio, E. Plant growth promoting rhizobacteria improve the antioxidant status in Mentha piperita grown under drought stress leading to an enhancement of plant growth and total phenolic content. Ind. Crops Prod. 2019, 139, 111553. [Google Scholar] [CrossRef]
- Ma, Y.; Dias, M.C.; Freitas, H. Drought and Salinity Stress Responses and Microbe-Induced Tolerance in Plants. Front. Plant Sci. 2020, 11, 591911. [Google Scholar] [CrossRef]
- Gericke, N. Muthi to medicine. S. Afr. J. Bot. 2011, 77, 850–856. [Google Scholar] [CrossRef]
- Irshad, A.; Rehman, R.N.U.; Abrar, M.M.; Saeed, Q.; Sharif, R.; Hu, T. Contribution of Rhizobium–Legume Symbiosis in Salt Stress Tolerance in Medicago truncatula Evaluated through Photosynthesis, Antioxidant Enzymes, and Compatible Solutes Accumulation. Sustainability 2021, 13, 3369. [Google Scholar] [CrossRef]
- Ayuso-Calles, M.; Flores-Félix, J.D.; Rivas, R. Overview of the Role of Rhizobacteria in Plant Salt Stress Tolerance. Agronomy 2021, 11, 1759. [Google Scholar] [CrossRef]
- Bharti, N.; Barnawal, D. Chapter Five—Amelioration of Salinity Stress by PGPR: ACC Deaminase and ROS Scavenging Enzymes Activity. In PGPR Amelioration in Sustainable Agriculture; Singh, A.K., Kumar, A., Singh, P.K., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 85–106. [Google Scholar]
- Sukweenadhi, J.; Balusamy, S.R.; Kim, Y.-J.; Lee, C.H.; Kim, Y.-J.; Koh, S.C.; Yang, D.C. A Growth-Promoting Bacteria, Paenibacillus yonginensis DCY84T Enhanced Salt Stress Tolerance by Activating Defense-Related Systems in Panax ginseng. Front. Plant Sci. 2018, 9, 813. [Google Scholar] [CrossRef]
- Cisse, E.-H.M.; Zhang, L.-J.; Pu, Y.-J.; Miao, L.-F.; Li, D.-D.; Zhang, J.; Yang, F. Exogenous Ca2+ Associated with Melatonin Alleviates Drought-Induced Damage in the Woody Tree Dalbergia odorifera. J. Plant Growth Regul. 2021, 41, 2359–2374. [Google Scholar] [CrossRef]
- Zonyane, S.; Fawole, O.A.; Grange, C.L.; Stander, M.A.; Opara, U.L.; Makunga, N.P. The implication of chemotypic variation on the anti-oxidant and anti-cancer activities of Sutherlandia frutescens (L.) R.Br. (Fabaceae) from different geographic locations. Antioxidants 2020, 9, 152. [Google Scholar] [CrossRef]
- van Wyk, A.S.; Prinsloo, G. Health, safety and quality concerns of plant-based traditional medicines and herbal remedies. S. Afr. J. Bot. 2020, 133, 54–62. [Google Scholar] [CrossRef]
- Dirar, A.I.; Alsaadi, D.H.M.; Wada, M.; Mohamed, M.A.; Watanabe, T.; Devkota, H.P. Effects of extraction solvents on total phenolic and flavonoid contents and biological activities of extracts from Sudanese medicinal plants. S. Afr. J. Bot. 2019, 120, 261–267. [Google Scholar] [CrossRef]
- Muffler, K.; Leipold, D.; Scheller, M.-C.; Haas, C.; Steingroewer, J.; Bley, T.; Neuhaus, H.E.; Mirata, M.A.; Schrader, J.; Ulber, R. Biotransformation of triterpenes. Process Biochem. 2011, 46, 1–15. [Google Scholar] [CrossRef]
- Parmar, S.K.; Sharma, T.P.; Airao, V.B.; Bhatt, R.; Aghara, R.; Chavda, S.; Rabadiya, S.O.; Gangwal, A.P. Neuropharmacological effects of triterpenoids. Phytopharmacology 2013, 4, 354–372. [Google Scholar]
- Fu, X.; Li, X.-C.; Smillie, T.J.; Carvalho, P.; Mabusela, W.; Syce, J.; Johnson, Q.; Folk, W.; Avery, M.A.; Khan, I.A. Cycloartane glycosides from Sutherlandia frutescens. J. Nat. Prod. 2008, 71, 1749–1753. [Google Scholar] [CrossRef]
- Aminin, D.L.; Menchinskaya, E.S.; Pisliagin, E.A.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I. Anticancer Activity of Sea Cucumber Triterpene Glycosides. Mar. Drugs 2015, 13, 1202–1223. [Google Scholar] [CrossRef]
- Wen, B.; Xiao, W.; Mu, Q.; Li, D.; Chen, X.; Wu, H.; Li, L.; Peng, F. How does nitrate regulate plant senescence? Plant Physiol. Biochem. 2020, 157, 60–69. [Google Scholar] [CrossRef]
- Xu, X.; Du, X.; Wang, F.; Sha, J.; Chen, Q.; Tian, G.; Zhu, Z.; Ge, S.; Jiang, Y. Effects of Potassium Levels on Plant Growth, Accumulation and Distribution of Carbon, and Nitrate Metabolism in Apple Dwarf Rootstock Seedlings. Front. Plant Sci. 2020, 11, 904. [Google Scholar] [CrossRef]
- Wilkens, R.T.; Spoerke, J.M.; Stamp, N.E. Differential Responses of Growth and Two Soluble Phenolics of Tomato to Resource Availability. Ecology 1996, 77, 247–258. [Google Scholar] [CrossRef]
- Acharya, D.; Enslin, G.; Chen, W.; Sandasi, M.; Mavimbela, T.; Viljoen, A. A chemometric approach to the quality control of Sutherlandia (cancer bush). Biochem. Syst. Ecol. 2014, 56, 221–230. [Google Scholar] [CrossRef]
- Tinte, M.M.; Masike, K.; Steenkamp, P.A.; Huyser, J.; van der Hooft, J.J.J.; Tugizimana, F. Computational Metabolomics Tools Reveal Metabolic Reconfigurations Underlying the Effects of Biostimulant Seaweed Extracts on Maize Plants under Drought Stress Conditions. Metabolites 2022, 12, 487. [Google Scholar] [CrossRef]
- Broughton, W.; Dilworth, M.J. Control of leghaemoglobin synthesis in snake beans. Biochem. J. 1971, 125, 1075–1080. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Sulaiman, C.T.; Balachandran, I. Total phenolics and total flavonoids in selected Indian medicinal plants. Indian J. Pharm. Sci. 2012, 74, 258–260. [Google Scholar] [CrossRef]
- Ahmed, M.; Ji, M.; Qin, P.; Gu, Z.; Liu, Y.; Sikandar, A.; Iqbal, M.; Javeed, A. Phytochemical screening, total phenolic and flavonoids contents and antioxidant activities of Citrullus colocynthis L. and Cannabis sativa L. J. Appl. Ecol. Environ. Res. 2019, 17, 6961–6979. [Google Scholar] [CrossRef]
- Pedrosa, A.M.; de Castro, W.V.; Castro, A.H.F.; Duarte-Almeida, J.M. Validated spectrophotometric method for quantification of total triterpenes in plant matrices. DARU J. Fac. Pharm. 2020, 28, 281–286. [Google Scholar] [CrossRef]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.M. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A rapid tool for turning tandem mass spectra into metabolite structure information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hlongwane, M.M.; Dakora, F.D.; Mohammed, M.; Mokgalaka-Fleischmann, N.S. Bioprospecting for Rhizobacteria with the Ability to Enhance Drought Tolerance in Lessertia frutescens. Int. J. Mol. Sci. 2023, 24, 17585. https://doi.org/10.3390/ijms242417585
Hlongwane MM, Dakora FD, Mohammed M, Mokgalaka-Fleischmann NS. Bioprospecting for Rhizobacteria with the Ability to Enhance Drought Tolerance in Lessertia frutescens. International Journal of Molecular Sciences. 2023; 24(24):17585. https://doi.org/10.3390/ijms242417585
Chicago/Turabian StyleHlongwane, Mokgadi M., Felix D. Dakora, Mustapha Mohammed, and Ntebogeng S. Mokgalaka-Fleischmann. 2023. "Bioprospecting for Rhizobacteria with the Ability to Enhance Drought Tolerance in Lessertia frutescens" International Journal of Molecular Sciences 24, no. 24: 17585. https://doi.org/10.3390/ijms242417585
APA StyleHlongwane, M. M., Dakora, F. D., Mohammed, M., & Mokgalaka-Fleischmann, N. S. (2023). Bioprospecting for Rhizobacteria with the Ability to Enhance Drought Tolerance in Lessertia frutescens. International Journal of Molecular Sciences, 24(24), 17585. https://doi.org/10.3390/ijms242417585





