CRK12: A Key Player in Regulating the Phaseolus vulgaris-Rhizobium tropici Symbiotic Interaction
Abstract
1. Introduction
2. Results
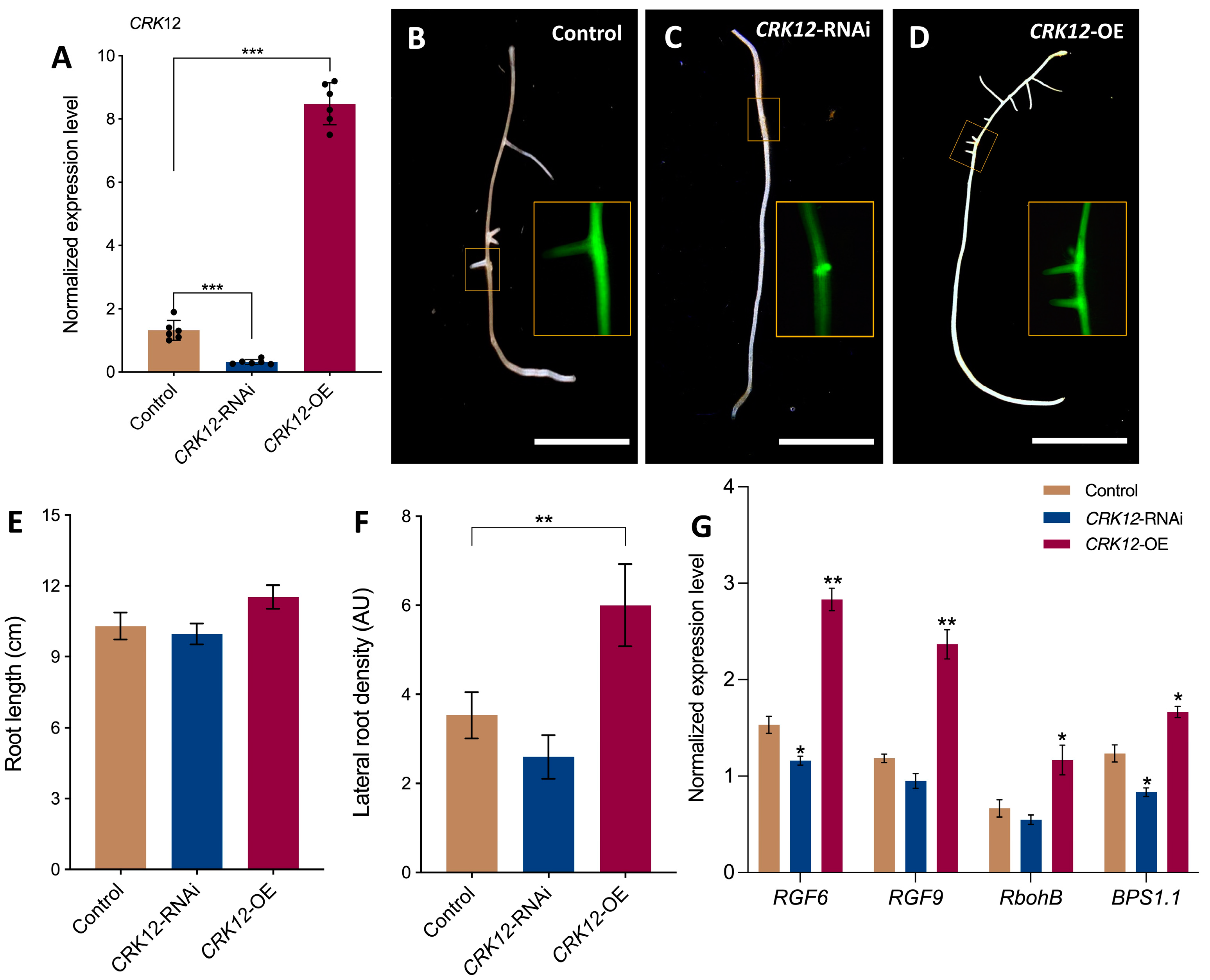
2.1. Structure of CRK12 Gene and Its Expression
2.2. Spatiotemporal Promoter Expression Analysis and Protein Subcellular Localization CRK12
2.3. CRK12 Alter Root and Root Hair Morphology
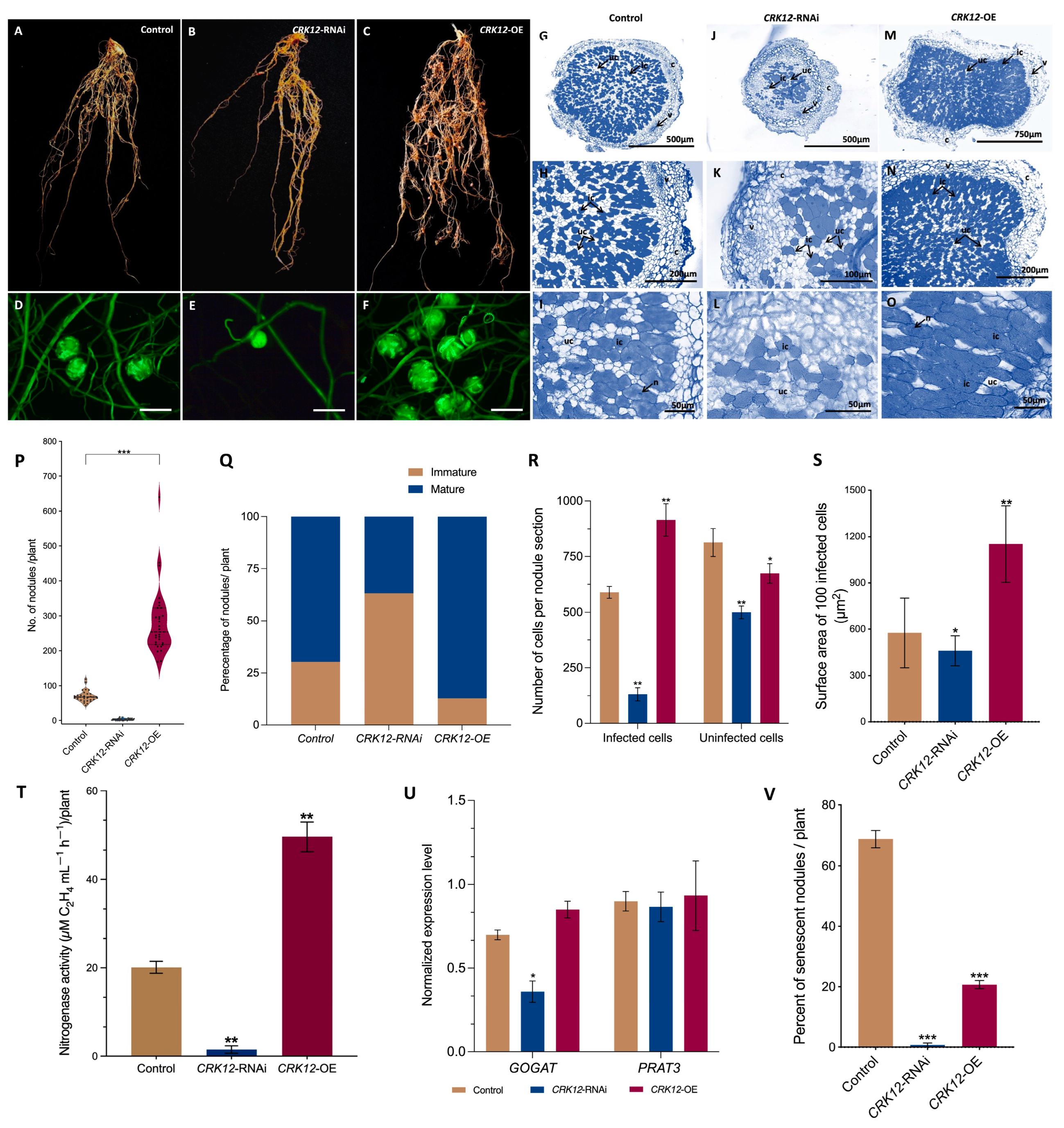
2.4. CRK12 Regulates Nodule Numbers and Infection Units in P. vulgaris
2.5. CRK12 Overexpression Results in Hypernodulation in P. vulgaris Transgenic Roots
3. Discussion
4. Materials and Methods
4.1. Plant Material and Rhizobium Inoculation
4.2. Gene and Protein Structure
4.3. Promoter Construction and Composite Plant Production
4.4. Subcellular Localization Analysis of CRK12
4.5. Cloning of CRK12 Overexpression and Silencing Constructs
4.6. Expression Analysis
4.7. Root Hair Measurements
4.8. Nodule Phenotype and Microscopy
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santoni, V.; Vinh, J.; Pflieger, D.; Sommerer, N.; Maurel, C. A proteomic study reveals novel insights into the diversity of aquaporin forms expressed in the plasma membrane of plant roots. Biochem. J. 2003, 373, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Alexandersson, E.; Saalbach, G.; Larsson, C.; Kjellbom, P. Arabidopsis Plasma Membrane Proteomics Identifies Components of Transport, Signal Transduction and Membrane Trafficking. Plant Cell Physiol. 2004, 45, 1543–1556. [Google Scholar] [CrossRef]
- Marmagne, A.; Rouet, M.-A.; Ferro, M.; Rolland, N.; Alcon, C.; Joyard, J.; Garin, J.; Barbier-Brygoo, H.; Ephritikhine, G. Identification of New Intrinsic Proteins in Arabidopsis Plasma Membrane Proteome. Mol. Cell. Proteom. 2004, 3, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z. A Superfamily of Proteins with Novel Cysteine-Rich Repeats. Plant Physiol. 2001, 126, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, T.; Hatano, K.-I.; Miyauchi, Y.; Suwa, Y.-I.; Sawano, Y.; Tanokura, M. A Secreted Protein with Plant-Specific Cysteine-Rich Motif Functions as a Mannose-Binding Lectin That Exhibits Antifungal Activity. Plant Physiol. 2014, 166, 766–778. [Google Scholar] [CrossRef]
- Bourdais, G.; Burdiak, P.; Gauthier, A.; Nitsch, L.; Salojärvi, J.; Rayapuram, C.; Idänheimo, N.; Hunter, K.; Kimura, S.; Merilo, E.; et al. Large-Scale Phenomics Identifies Primary and Fine-Tuning Roles for CRKs in Responses Related to Oxidative Stress. PLOS Genet. 2015, 11, e1005373. [Google Scholar] [CrossRef]
- Li, T.-G.; Zhang, D.-D.; Zhou, L.; Kong, Z.-Q.; Hussaini, A.S.; Wang, D.; Li, J.-J.; Short, D.P.G.; Dhar, N.; Klosterman, S.J.; et al. Genome-Wide Identification and Functional Analyses of the CRK Gene Family in Cotton Reveals GbCRK18 Confers Verticillium Wilt Resistance in Gossypium barbadense. Front. Plant Sci. 2018, 9, 1266. [Google Scholar] [CrossRef]
- Chern, M.; Xu, Q.; Bart, R.S.; Bai, W.; Ruan, D.; Sze-To, W.H.; Canlas, P.E.; Jain, R.; Chen, X.; Ronald, P.C. A Genetic Screen Identifies a Requirement for Cysteine-Rich–Receptor-Like Kinases in Rice NH1 (OsNPR1)-Mediated Immunity. PLoS Genet. 2016, 12, e1006049. [Google Scholar] [CrossRef]
- Burdiak, P.; Rusaczonek, A.; Witoń, D.; Głów, D.; Karpiński, S. Cysteine-rich receptor-like kinase CRK5 as a regulator of growth, development, and ultraviolet radiation responses in Arabidopsis thaliana. J. Exp. Bot. 2015, 66, 3325–3337. [Google Scholar] [CrossRef]
- Quezada, E.-H.; García, G.-X.; Arthikala, M.-K.; Melappa, G.; Lara, M.; Nanjareddy, K. Cysteine-Rich Receptor-Like Kinase Gene Family Identification in the Phaseolus Genome and Comparative Analysis of Their Expression Profiles Specific to Mycorrhizal and Rhizobial Symbiosis. Genes 2019, 10, 59. [Google Scholar] [CrossRef]
- Delgado-Cerrone, L.; Alvarez, A.; Mena, E.; De León, I.P.; Montesano, M. Genome-wide analysis of the soybean CRK-family and transcriptional regulation by biotic stress signals triggering plant immunity. PLoS ONE 2018, 13, e0207438. [Google Scholar] [CrossRef]
- Yadeta, K.A.; Elmore, J.M.; Creer, A.Y.; Feng, B.; Franco, J.Y.; Rufian, J.S.; He, P.; Phinney, B.; Coaker, G. A Cysteine-Rich Protein Kinase Associates with a Membrane Immune Complex and the Cysteine Residues Are Required for Cell Death. Plant Physiol. 2016, 173, 771–787. [Google Scholar] [CrossRef]
- Pelagio-Flores, R.; Muñoz-Parra, E.; Barrera-Ortiz, S.; Ortiz-Castro, R.; Saenz-Mata, J.; Ortega-Amaro, M.A.; Jiménez-Bremont, J.F.; López-Bucio, J. The cysteine-rich receptor-like protein kinase CRK28 modulates Arabidopsis growth and development and influences abscisic acid responses. Planta 2019, 251, 2. [Google Scholar] [CrossRef] [PubMed]
- Arellano-Villagómez, F.C.; Guevara-Olvera, L.; Zuñiga-Mayo, V.M.; Cerbantez-Bueno, V.E.; Verdugo-Perales, M.; Medina, H.R.; De Folter, S.; Acosta-García, G. Arabidopsis cysteine-rich receptor-like protein kinase CRK33 affects stomatal density and drought tolerance. Plant Signal. Behav. 2021, 16, 1905335. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Kim, Y.C.; Kwon, S.J.; Ryu, C.-M.; Park, O.K. The Arabidopsis Cysteine-Rich Receptor-Like Kinase CRK36 Regulates Immunity through Interaction with the Cytoplasmic Kinase BIK1. Front. Plant Sci. 2017, 8, 1856. [Google Scholar] [CrossRef]
- Mou, S.; Meng, Q.; Gao, F.; Zhang, T.; He, W.; Guan, D.; He, S. A cysteine-rich receptor-like protein kinase CaCKR5 modulates immune response against Ralstonia solanacearum infection in pepper. BMC Plant Biol. 2021, 21, 382. [Google Scholar] [CrossRef]
- Chen, K.; Du, L.; Chen, Z. Sensitization of defense responses and activation of programmed cell death by a pathogen-induced receptor-like protein kinase in Arabidopsis. Plant Mol. Biol. 2003, 53, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Han, X.; Shi, R.; Yang, G.; Qi, L.; Wang, R.; Li, G. Arabidopsis cysteine-rich receptor-like kinase 45 positively regulates disease resistance to Pseudomonas syringae. Plant Physiol. Biochem. 2013, 73, 383–391. [Google Scholar] [CrossRef]
- Yeh, Y.-H.; Chang, Y.-H.; Huang, P.-Y.; Huang, J.-B.; Zimmerli, L. Enhanced Arabidopsis pattern-triggered immunity by overexpression of cysteine-rich receptor-like kinases. Front. Plant Sci. 2015, 6, 322. [Google Scholar] [CrossRef]
- Saintenac, C.; Cambon, F.; Aouini, L.; Verstappen, E.; Ghaffary, S.M.T.; Poucet, T.; Marande, W.; Berges, H.; Xu, S.; Jaouannet, M.; et al. A wheat cysteine-rich receptor-like kinase confers broad-spectrum resistance against Septoria tritici blotch. Nat. Commun. 2021, 12, 433. [Google Scholar] [CrossRef]
- Gu, J.; Sun, J.; Liu, N.; Sun, X.; Liu, C.; Wu, L.; Liu, G.; Zeng, F.; Hou, C.; Han, S.; et al. A novel cysteine-rich receptor-like kinase gene, TaCRK2, contributes to leaf rust resistance in wheat. Mol. Plant Pathol. 2020, 21, 732–746. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Guo, F.; Xu, G.; Yu, J.; Zhang, L.; Wei, X.; Zhu, X.; Zhang, Z. The Receptor-like Kinase TaCRK-7A Inhibits Fusarium pseudograminearum Growth and Mediates Resistance to Fusarium Crown Rot in Wheat. Biology 2021, 10, 1122. [Google Scholar] [CrossRef] [PubMed]
- Peters, N.K.; Frost, J.W.; Long, S.R. A Plant Flavone, Luteolin, Induces Expression of Rhizobium meliloti Nodulation Genes. Science 1986, 233, 977–980. [Google Scholar] [CrossRef]
- Redmond, J.W.; Batley, M.; Djordjevic, M.A.; Innes, R.W.; Kuempel, P.L.; Rolfe, B.G. Flavones induce expression of nodulation genes in Rhizobium. Nature 1986, 323, 632–635. [Google Scholar] [CrossRef]
- Bourcy, M.; Brocard, L.; Pislariu, C.I.; Cosson, V.; Mergaert, P.; Tadege, M.; Mysore, K.S.; Udvardi, M.K.; Gourion, B.; Ratet, P. Medicago truncatulaDNF2 is aPI-PLC-XD-containing protein required for bacteroid persistence and prevention of nodule early senescence and defense-like reactions. New Phytol. 2012, 197, 1250–1261. [Google Scholar] [CrossRef]
- Berrabah, F.; Bourcy, M.; Eschstruth, A.; Cayrel, A.; Guefrachi, I.; Mergaert, P.; Wen, J.; Jean, V.; Mysore, K.; Gourion, B.; et al. A non RD receptor-like kinase prevents nodule early senescence and defense-like reactions during symbiosis. New Phytol. 2014, 203, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yu, H.; Luo, L.; Duan, L.; Cai, L.; He, X.; Wen, J.; Mysore, K.S.; Li, G.; Xiao, A.; et al. NODULES WITH ACTIVATED DEFENSE 1 is required for maintenance of rhizobial endosymbiosis in Medicago truncatula. New Phytol. 2016, 212, 176–191. [Google Scholar] [CrossRef]
- Domonkos, Á.; Kovács, S.; Gombár, A.; Kiss, E.; Horváth, B.; Kováts, G.Z.; Farkas, A.; Tóth, M.T.; Ayaydin, F.; Bóka, K.; et al. NAD1 Controls Defense-Like Responses in Medicago truncatula Symbiotic Nitrogen Fixing Nodules Following Rhizobial Colonization in a BacA-Independent Manner. Genes 2017, 8, 387. [Google Scholar] [CrossRef]
- Yu, H.; Xiao, A.; Dong, R.; Fan, Y.; Zhang, X.; Liu, C.; Wang, C.; Zhu, H.; Duanmu, D.; Cao, Y.; et al. Suppression of innate immunity mediated by the CDPK-Rboh complex is required for rhizobial colonization in Medicago truncatula nodules. New Phytol. 2018, 220, 425–434. [Google Scholar] [CrossRef]
- Quilbé, J.; Lamy, L.; Brottier, L.; Leleux, P.; Fardoux, J.; Rivallan, R.; Benichou, T.; Guyonnet, R.; Becana, M.; Villar, I.; et al. Genetics of nodulation in Aeschynomene evenia uncovers mechanisms of the rhizobium–legume symbiosis. Nat. Commun. 2021, 12, 829. [Google Scholar] [CrossRef]
- Miyakawa, T.; Miyazono, K.-I.; Sawano, Y.; Hatano, K.-I.; Tanokura, M. Crystal structure of ginkbilobin-2 with homology to the extracellular domain of plant cysteine-rich receptor-like kinases. Proteins: Struct. Funct. Bioinform. 2009, 77, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Inzé, D.; Depicker, A. GATEWAY™ vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 2002, 7, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Vinuesa, P.; Neumann-Silkow, F.; Pacios-Bras, C.; Spaink, H.P.; Martínez-Romero, E.; Werner, D. Genetic Analysis of a pH-Regulated Operon from Rhizobium tropici CIAT899 Involved in Acid Tolerance and Nodulation Competitiveness. Mol. Plant-Microbe Interactions 2003, 16, 159–168. [Google Scholar] [CrossRef]
- Cermola, M.; Fedorova, E.; Riccio, A.; Favre, R.; Patriarca, E.J.; Cermola, E.F.M.; Sujkowska-Rybkowska, M.; Ważny, R.; Via, V.D.; Traubenik, S.; et al. Nodule Invasion and Symbiosome Differentiation During Rhizobium etli-Phaseolus vulgaris Symbiosis. Mol. Plant-Microbe Interact. 2000, 13, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Considine, M.J.; Foyer, C.H.; Zaffagnini, M.; Fermani, S.; Marchand, C.H.; Costa, A.; Sparla, F.; Rouhier, N.; Geigenberger, P.; Lemaire, S.D.; et al. Redox regulation of plant development. Antioxidants Redox Signal. 2014, 21, 1305–1326. [Google Scholar] [CrossRef]
- He, Y.; Zhou, J.; Shan, L.; Meng, X. Plant cell surface receptor-mediated signaling—A common theme amid diversity. J. Cell Sci. 2018, 131, jcs209353. [Google Scholar] [CrossRef]
- Mulligan, J.T.; Long, S.R. Induction of Rhizobium meliloti nodC expression by plant exudate requires nodD. Proc. Natl. Acad. Sci. USA 1985, 82, 6609–6613. [Google Scholar] [CrossRef]
- Madsen, E.B.; Madsen, L.H.; Radutoiu, S.; Olbryt, M.; Rakwalska, M.; Szczyglowski, K.; Sato, S.; Kaneko, T.; Tabata, S.; Sandal, N.; et al. A receptor kinase gene of the LysM type is involved in legumeperception of rhizobial signals. Nature 2003, 425, 637–640. [Google Scholar] [CrossRef]
- Radutoiu, S.; Madsen, L.H.; Madsen, E.B.; Felle, H.H.; Umehara, Y.; Grønlund, M.; Sato, S.; Nakamura, Y.; Tabata, S.; Sandal, N.; et al. Plant recognition of symbiotic bacteria requires two LysM receptor-like kinases. Nature 2003, 425, 585–592. [Google Scholar] [CrossRef]
- Murakami, E.; Cheng, J.; Gysel, K.; Bozsoki, Z.; Kawaharada, Y.; Hjuler, C.T.; Sørensen, K.K.; Tao, K.; Kelly, S.; Venice, F.; et al. Epidermal LysM receptor ensures robust symbiotic signalling in Lotus japonicus. Elife 2018, 7, e33506. [Google Scholar] [CrossRef]
- Roy, S.; Liu, W.; Nandety, R.S.; Crook, A.D.; Mysore, K.S.; Pislariu, C.I.; Frugoli, J.A.; Dickstein, R.; Udvardi, M.K. Celebrating 20 Years of Genetic Discoveries in Legume Nodulation and Symbiotic Nitrogen Fixation. Plant Cell 2019, 32, 15–41. [Google Scholar] [CrossRef] [PubMed]
- Berrabah, F.; Ratet, P.; Gourion, B. Multiple steps control immunity during the intracellular accommodation of rhizobia. J. Exp. Bot. 2015, 66, 1977–1985. [Google Scholar] [CrossRef] [PubMed]
- Berrabah, F.; Balliau, T.; Aït-Salem, E.H.; George, J.; Zivy, M.; Ratet, P.; Gourion, B. Control of the ethylene signaling pathway prevents plant defenses during intracellular accommodation of the rhizobia. New Phytol. 2018, 219, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Fan, B.; Du, L.; Chen, Z. Activation of hypersensitive cell death by pathogen-induced receptor-like protein kinases from Arabidopsis. Plant Mol. Biol. 2004, 56, 271–283. [Google Scholar] [CrossRef]
- Wrzaczek, M.; Brosché, M.; Kangasjärvi, J. ROS signaling loops—Production, perception, regulation. Curr. Opin. Plant Biol. 2013, 16, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Frank, M.; Reid, D. No Home without Hormones: How Plant Hormones Control Legume Nodule Organogenesis. Plant Commun. 2020, 1, 100104. [Google Scholar] [CrossRef]
- Li, M.; Zhu, Y.; Li, S.; Zhang, W.; Yin, C.; Lin, Y. Regulation of Phytohormones on the Growth and Development of Plant Root Hair. Front. Plant Sci. 2022, 13, 865302. [Google Scholar] [CrossRef]
- Mangano, S.; Denita-Juarez, S.P.; Choi, H.-S.; Marzol, E.; Hwang, Y.; Ranocha, P.; Velasquez, S.M.; Borassi, C.; Barberini, M.L.; Aptekmann, A.A.; et al. Molecular link between auxin and ROS-mediated polar growth. Proc. Natl. Acad. Sci. USA 2017, 114, 5289–5294. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kamiya, N.; Morinaka, Y.; Matsuoka, M.; Sazuka, T. Auxin Biosynthesis by the YUCCA Genes in Rice. Plant Physiol. 2007, 143, 1362–1371. [Google Scholar] [CrossRef]
- Delay, C.; Imin, N.; Djordjevic, M.A. Regulation of Arabidopsis root development by small signaling peptides. Front Plant Sci. 2013, 6, 352. [Google Scholar] [CrossRef]
- Montiel, J.; Nava, N.; Cárdenas, L.; Sánchez-López, R.; Arthikala, M.-K.; Santana, O.; Sánchez, F.; Quinto, C. A Phaseolus vulgaris NADPH Oxidase Gene is Required for Root Infection by Rhizobia. Plant Cell Physiol. 2012, 53, 1751–1767. [Google Scholar] [CrossRef] [PubMed]
- Arthikala, M.-K.; Nanjareddy, K.; Lara, M. In BPS1 Downregulated Roots, the BYPASS1 Signal Disrupts the Induction of Cortical Cell Divisions in Bean-Rhizobium Symbiosis. Genes 2018, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Li, S.; Deng, Z.; Wang, X.; Chen, T.; Zhang, J.; Chen, S.; Ling, H.; Zhang, A.; Wang, D.; et al. Molecular analysis of three new receptor-like kinase genes from hexaploid wheat and evidence for their participation in the wheat hypersensitive response to stripe rust fungus infection. Plant J. 2007, 52, 420–434. [Google Scholar] [CrossRef] [PubMed]
- Smakowska-Luzan, E.; Mott, G.A.; Parys, K.; Stegmann, M.; Howton, T.C.; Layeghifard, M.; Neuhold, J.; Lehner, A.; Kong, J.; Grünwald, K.; et al. An extracellular network of Arabidopsis leucine-rich repeat receptor kinases. Nature 2018, 553, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Gochicoa, M.-T.; Camut, S.; Timmers, A.C.; Niebel, A.; Hervé, C.; Boutet, E.; Bono, J.-J.; Imberty, A.; Cullimore, J.V. Characterization of Four Lectin-Like Receptor Kinases Expressed in Roots of Medicago truncatula. Structure, Location, Regulation of Expression, and Potential Role in the Symbiosis with Sinorhizobium meliloti. Plant Physiol. 2003, 133, 1893–1910. [Google Scholar] [CrossRef] [PubMed]
- Arthikala, M.; Sánchez-López, R.; Nava, N.; Santana, O.; Cárdenas, L.; Quinto, C. RbohB, a Phaseolus vulgaris NADPH oxidase gene, enhances symbiosome number, bacteroid size, and nitrogen fixation in nodules and impairs mycorrhizal colonization. New Phytol. 2014, 202, 886–900. [Google Scholar] [CrossRef]
- Nanjareddy, K.; Arthikala, M.-K.; Aguirre, A.-L.; Gómez, B.-M.; Lara, M. Plant Promoter Analysis: Identification and Characterization of Root Nodule Specific Promoter in the Common Bean. J. Vis. Exp. 2017, 130, e56140. [Google Scholar] [CrossRef]
- Broughton, W.J.; Dilworth, M.J. Control of leghaemoglobin synthesis in snake beans. Biochem. J. 1971, 125, 1075–1080. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef]
- Ma, Z.; Bielenberg, D.G.; Brown, K.M.; Lynch, J.P. Regulation of root hair density by phosphorus availability in Arabidopsis thaliana. Plant Cell Environ. 2001, 24, 459–467. [Google Scholar] [CrossRef]
- Mercante, F.M.; Franco, A.A. Expression of nod genes in Rhizobium tropici, R. etli, R. leguminosarum bv. phaseoli and bean nodulation in the presence of Mimosa flocculosa and Leucaena leucocephala seed exudates. Rev. Bras. Ciênc. Solo 2000, 24, 301–310. [Google Scholar] [CrossRef]
- Jefferson, R.A. Assaying chimeric genes in plants: The GUS gene fusion system. Plant Mol. Biol. Rep. 1987, 5, 387–405. [Google Scholar] [CrossRef]
- Chen, T.-K.; Yang, H.-T.; Fang, S.-C.; Lien, Y.-C.; Yang, T.-T.; Ko, S.-S. Hybrid-Cut: An Improved Sectioning Method for Recalcitrant Plant Tissue Samples. J. Vis. Exp. 2016, 117, e54754. [Google Scholar] [CrossRef]
- Burris, R.H. Methodology. In Biology of Nitrogen Fixation; Quispel, A., Ed.; North-Holland Publishing Co.: Amsterdam, The Netherlands, 1974; pp. 3–42. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lecona, A.M.; Nanjareddy, K.; Blanco, L.; Piazza, V.; Vera-Núñez, J.A.; Lara, M.; Arthikala, M.-K. CRK12: A Key Player in Regulating the Phaseolus vulgaris-Rhizobium tropici Symbiotic Interaction. Int. J. Mol. Sci. 2023, 24, 11720. https://doi.org/10.3390/ijms241411720
Lecona AM, Nanjareddy K, Blanco L, Piazza V, Vera-Núñez JA, Lara M, Arthikala M-K. CRK12: A Key Player in Regulating the Phaseolus vulgaris-Rhizobium tropici Symbiotic Interaction. International Journal of Molecular Sciences. 2023; 24(14):11720. https://doi.org/10.3390/ijms241411720
Chicago/Turabian StyleLecona, Antonino M., Kalpana Nanjareddy, Lourdes Blanco, Valeria Piazza, José Antonio Vera-Núñez, Miguel Lara, and Manoj-Kumar Arthikala. 2023. "CRK12: A Key Player in Regulating the Phaseolus vulgaris-Rhizobium tropici Symbiotic Interaction" International Journal of Molecular Sciences 24, no. 14: 11720. https://doi.org/10.3390/ijms241411720
APA StyleLecona, A. M., Nanjareddy, K., Blanco, L., Piazza, V., Vera-Núñez, J. A., Lara, M., & Arthikala, M.-K. (2023). CRK12: A Key Player in Regulating the Phaseolus vulgaris-Rhizobium tropici Symbiotic Interaction. International Journal of Molecular Sciences, 24(14), 11720. https://doi.org/10.3390/ijms241411720






