Inflammation in Hypervolemic Hemodialysis Patients: The Roles of RelB and Caspase-4
Abstract
:1. Introduction
2. Results
2.1. Overhydration Is Assoiated with Older Age, Diabetes and Inflammation
2.2. Granulocyte Numbers Are Elevated in Hypervolemia
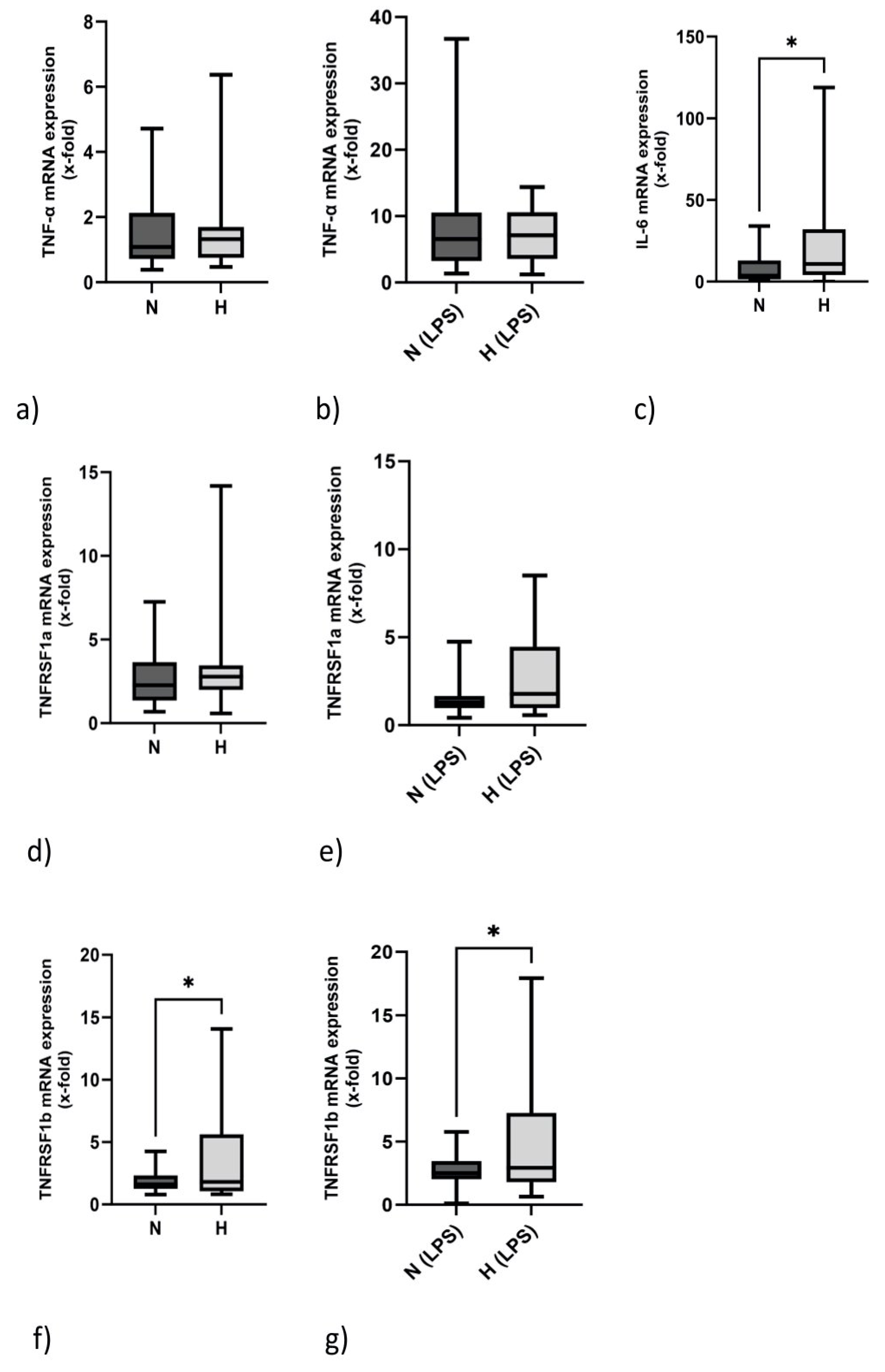
2.3. IL-6 but Not TNF-α mRNA Expression Is Elevated in Hypervolemia
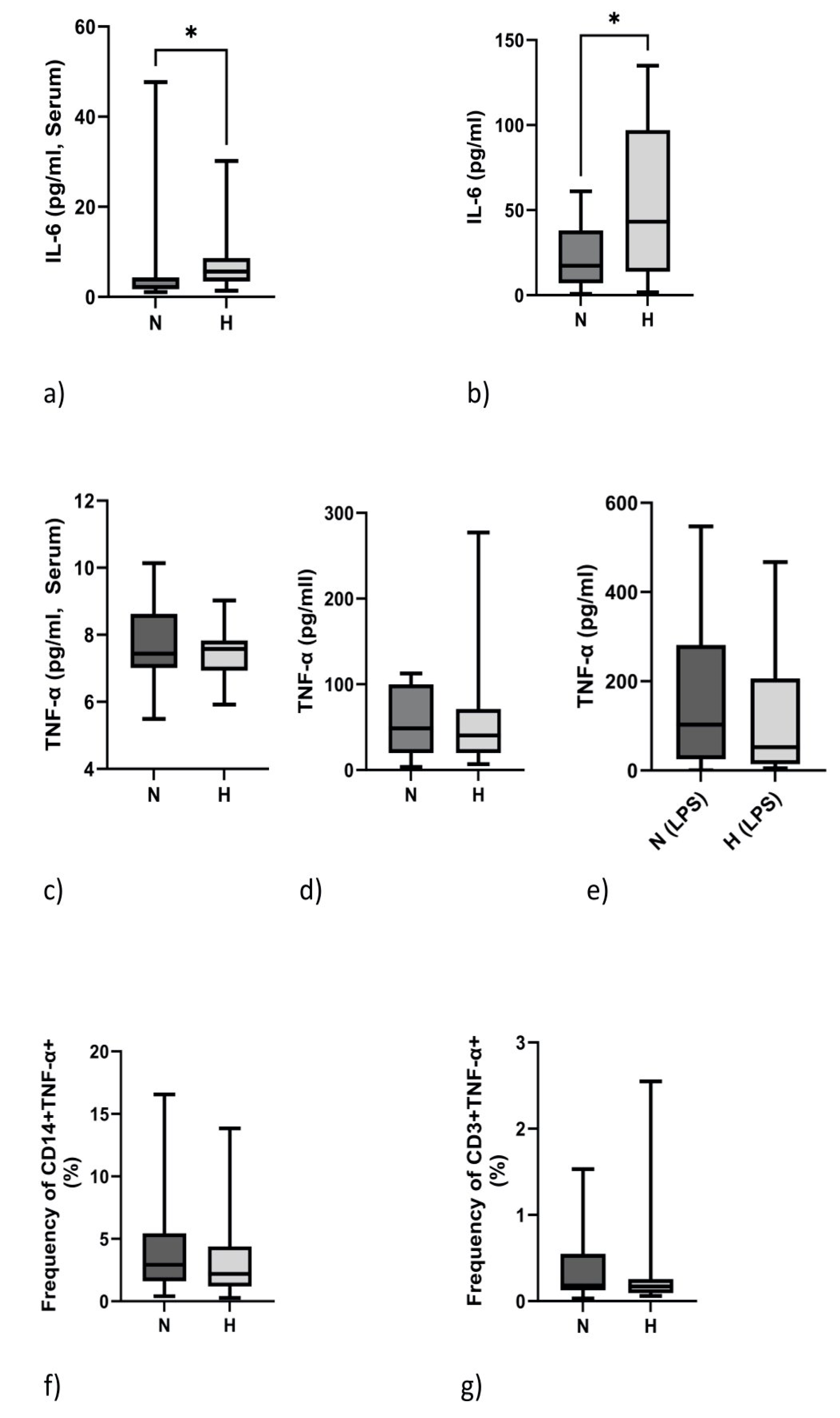
2.4. IL-6 but Not TNF-α Protein Expression Is Elevated in Hypervolemia
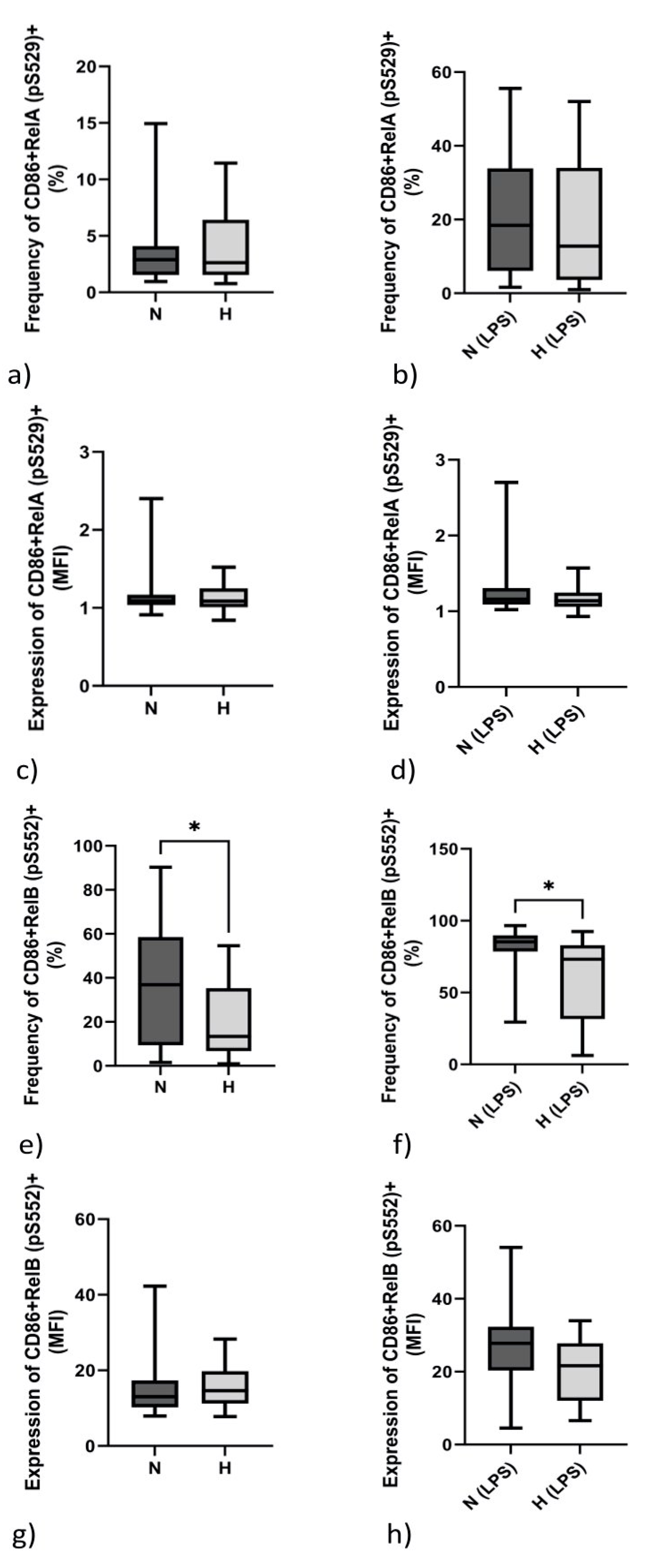
2.5. Monocytic Transcription Factor RelB (pS552) but Not RelA (pS529) Differs between Normo- and Hypervolemic Patients
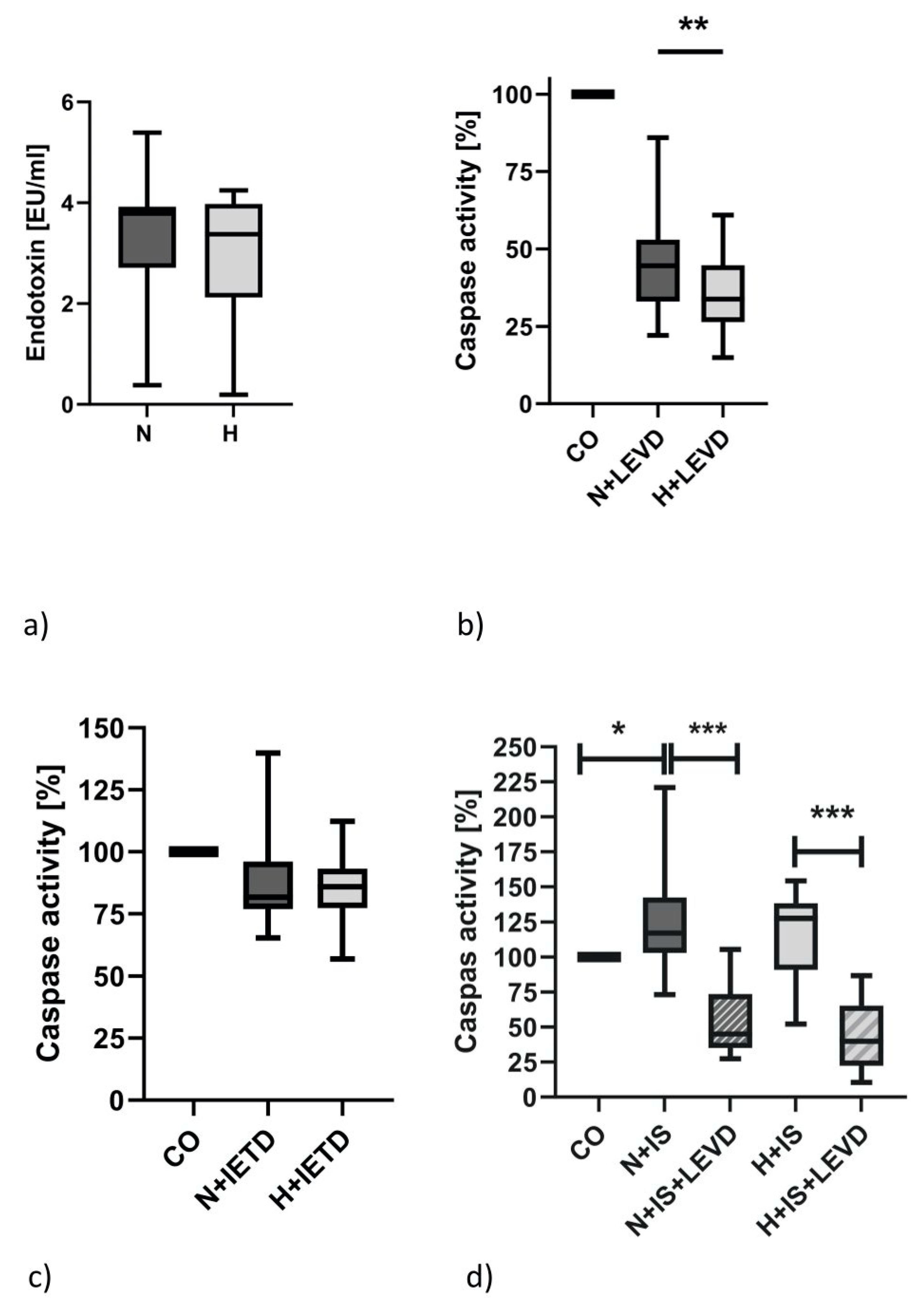
2.6. Caspase-4 Activity but Not the Serum Endotoxin Level Is Elevated in Hypervolemia
3. Discussion
Limitations of the Study
4. Materials and Methods
4.1. Study Population
4.2. PBMC Isolation
4.3. RNA/cDNA/qPCR
4.4. Analysis of TNF-α Secretion by Flow Cytometry
4.5. Analysis of RelA and RelB by Phospho-Flow Cytometry
4.6. Caspase-4 Assay
4.7. Endotoxin Detection
4.8. Cytokine Analysis
4.9. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, Y.H.; Sarriff, A.; Adnan, A.S.; Khan, A.H.; Mallhi, T.H. Chronic Kidney Disease, Fluid Overload and Diuretics: A Complicated Triangle. PLoS ONE 2016, 11, e0159335. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.-C.; Kuo, K.-L.; Peng, C.-H.; Wu, C.H.; Lien, Y.C.; Wang, Y.C.; Tarng, D.C. Volume overload correlates with cardiovascular risk factors in patients with chronic kidney disease. Kidney Int. 2014, 85, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Vega, A.; Abad, S.; Macías, N.; Aragoncillo, I.; García-Prieto, A.; Linares, T.; Luño, J. Any grade of relative overhydration is associated with long-term mortality in patients with Stages 4 and 5 non-dialysis chronic kidney disease. Clin. Kidney J. 2018, 11, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Dekker, M.J.E.; Marcelli, D.; Canaud, B.J.; Carioni, P.; Wang, Y.; Grassmann, A. Impact of fluid status and inflammation and their interaction on survival: A study in an international hemodialysis patient cohort. Kidney Int. 2017, 91, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Damman, K.; van Deursen, V.M.; Navis, G.; Voors, A.A.; van Veldhuisen, D.J.; Hillege, H.L. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J. Am. Coll. Cardiol. 2009, 53, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, L. Inflammation and Cardiovascular Disease Associated With Hemodialysis for End-Stage Renal Disease. Front. Pharmacol. 2022, 13, 800950. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D. CKD impairs barrier function and alters microbial flora of the intestine: A major link to inflammation and uremic toxicity. Curr. Opin. Nephrol. Hypertens. 2012, 21, 587–592. [Google Scholar] [CrossRef]
- McIntyre, C.W.; Harrison, L.E.A.; Eldehni, M.T.; Jefferies, H.J.; Szeto, C.C.; John, S.G.; Li, P.K. Circulating endotoxemia: A novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 133–141. [Google Scholar] [CrossRef]
- Terpstra, M.L.; Singh, R.; Geerlings, S.E.; Bemelman, F.J. Measurement of the intestinal permeability in chronic kidney disease. World J. Nephrol. 2016, 5, 378–388. [Google Scholar] [CrossRef]
- Beberashvili, I.; Azar, A.; Sinuani, I.; Shapiro, G.; Feldman, L.; Stav, K.; Averbukh, Z. Bioimpedance phase angle predicts muscle function, quality of life and clinical outcome in maintenance hemodialysis patients. Eur. J. Clin. Nutr. 2014, 68, 683–689. [Google Scholar] [CrossRef]
- Zelová, H.; Hošek, J. TNF-α signalling and inflammation: Interactions between old acquaintances. Inflamm. Res. 2013, 62, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, S.R.; Oral, H.; Lee, J.; Nakano, M.; Taffet, G.E.; Mann, D.L. Hemodynamic regulation of tumor necrosis factor-alpha gene and protein expression in adult feline myocardium. Circ. Res. 1997, 81, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.R. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Baldwin, A.S. Activation of nuclear factor-kappaB-dependent transcription by tumor necrosis factor-alpha is mediated through phosphorylation of RelA/p65 on serine 529. J. Biol. Chem. 1998, 273, 29411–29416. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Westerheide, S.D.; Hanson, J.L.; Baldwin, A.S. Tumor necrosis factor alpha-induced phosphorylation of RelA/p65 on Ser529 is controlled by casein kinase II. J. Biol. Chem. 2000, 275, 32592–32597. [Google Scholar] [CrossRef]
- Senftleben, U.; Cao, Y.; Xiao, G.; Greten, F.R.; Krahn, G.; Bonizzi, G.; Karin, M. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science 2001, 293, 1495–1499. [Google Scholar] [CrossRef]
- Marienfeld, R.; Berberich-Siebelt, F.; Berberich, I.; Denk, A.; Serfling, E.; Neumann, M. Signal-specific and phosphorylation-dependent RelB degradation: A potential mechanism of NF-kappaB control. Oncogene 2001, 20, 8142–8147. [Google Scholar] [CrossRef]
- Ulrich, C.; Wilke, A.; Schleicher, N.; Girndt, M.; Fiedler, R. Hypervolemia-Induced Immune Disturbances Do Not Involve IL-1ß but IL-6 and IL-10 Activation in Haemodialysis Patients. Toxins 2020, 12, 159. [Google Scholar] [CrossRef]
- Zamyatina, A.; Heine, H. Lipopolysaccharide Recognition in the Crossroads of TLR4 and Caspase-4/11 Mediated Inflammatory Pathways. Front. Immunol. 2020, 11, 585146. [Google Scholar] [CrossRef]
- Piccoli, A. Estimation of fluid volumes in hemodialysis patients: Comparing bioimpedance with isotopic and dilution methods. Kidney Int. 2014, 85, 738–741. [Google Scholar] [CrossRef]
- Piccoli, A.; Codognotto, M.; Piasentin, P.; Naso, A. Combined evaluation of nutrition and hydration in dialysis patients with bioelectrical impedance vector analysis (BIVA). Clin. Nutr. 2014, 33, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-C.; Tsai, J.-C.; Chen, S.-C.; Chiu, Y.W.; Hwang, S.J.; Hung, C.C.; Chen, H.C. Association of fluid overload with kidney disease progression in advanced CKD: A prospective cohort study. Am. J. Kidney Dis. 2014, 63, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Levin, N.W.; Kotanko, P.; Eckardt, K.-U.; Kasiske, B.L.; Chazot, C.; Cheung, A.K.; London, G.M. Blood pressure in chronic kidney disease stage 5D-report from a Kidney Disease: Improving Global Outcomes controversies conference. Kidney Int. 2010, 77, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Canaud, B.; Stephens, M.P.; Nikam, M.; Etter, M.; Collins, A. Multitargeted interventions to reduce dialysis-induced systemic stress. Clin. Kidney J. 2021, 14, i72–i84. [Google Scholar] [CrossRef] [PubMed]
- Zsom, L.; Faludi, M.; Fülöp, T.; Dossabhoy, N.R.; Rosivall, L.; Tapolyai, M.B. The association of overhydration with chronic inflammation in chronic maintenance hemodiafiltration patients. Hemodial. Int. 2019, 23, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.-I.; Lee, A.-H.; Shin, H.-Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Yang, S.H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Parameswaran, N.; Patial, S. Tumor necrosis factor-α signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef]
- Caldwell, A.B.; Cheng, Z.; Vargas, J.D.; Birnbaum, H.A.; Hoffmann, A. Network dynamics determine the autocrine and paracrine signaling functions of TNF. Genes Dev. 2014, 28, 2120–2133. [Google Scholar] [CrossRef]
- Baud, V.; Collares, D. Post-Translational Modifications of RelB NF-κB Subunit and Associated Functions. Cells 2016, 5, 22. [Google Scholar] [CrossRef]
- Saccani, S.; Pantano, S.; Natoli, G. Modulation of NF-kappaB activity by exchange of dimers. Mol. Cell 2003, 11, 1563–1574. [Google Scholar] [CrossRef]
- McMillan, D.H.; Woeller, C.F.; Thatcher, T.H.; Spinelli, S.L.; Maggirwar, S.B.; Sime, P.J.; Phipps, R.P. Attenuation of inflammatory mediator production by the NF-κB member RelB is mediated by microRNA-146a in lung fibroblasts. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 304, L774–L781. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, C.; Kneser, L.; Fiedler, R.; Beckert, J.; Wildgrube, S.; Seibert, E.; Girndt, M. Pyroptosis: A Common Feature of Immune Cells of Haemodialysis Patients. Toxins 2021, 13, 839. [Google Scholar] [CrossRef]
- Kim, H.Y.; Yoo, T.-H.; Hwang, Y.; Lee, G.H.; Kim, B.; Jang, J.; Lee, W.W. Indoxyl sulfate (IS)-mediated immune dysfunction provokes endothelial damage in patients with end-stage renal disease (ESRD). Sci. Rep. 2017, 7, 3057. [Google Scholar] [CrossRef] [PubMed]
| N (n = 23) | H (n = 17) | Statistics | |
|---|---|---|---|
| Age (years) | 57.6 ± 14.3 | 67.5 ± 10.2 | 0.020 |
| Sex (f, %) | 60.9 | 52.9 | 0.749 |
| Diabetes (%) | 17.4 | 52.9 | 0.038 |
| BMI (kg/m2) | 26.7 ± 8.1 | 29.4 ± 6.9 | 0.184 |
| Dialysis vintage (years) | 7.6 ± 5.7 | 7.1 ± 10.9 | 0.073 |
| Kt/V | 1.6 ± 0.4 | 1.2 ± 0.3 | 0.002 |
| CRP (mg/dL) | 5.2 ± 6.3 | 14.2 ± 16.1 | 0.020 |
| Albumin (g/L) | 40.1 ± 3.2 | 38.6 ± 4.7 | 0.256 |
| Hemoglobin (mmol/L) | 6.9 ± 0.7 | 7.0 ± 0.9 | 0.948 |
| Creatinine (µmol/L) | 930 ± 210 | 679 ± 246 | 0.001 |
| ECW (L) | 14.9 ± 6.2 | 20.4 ± 4.9 | 0.001 |
| ICW (L) | 22.5 ± 3.8 | 24.8 ± 4.6 | 0.084 |
| TBW (L) | 37.3 ± 9.4 | 45.2 ± 9.2 | 0.009 |
| Interdialytic weight gain (kg) | 1.9 ± 2.0 | 3.0 ± 1.2 | 0.062 |
| Hypertensive drugs (n) | 2.4 ± 1.3 | 2.4 ± 1.1 | 0.836 |
| Diuretic drugs (n) | 0.7 ± 0.9 | 1.2 ± 1.0 | 0.126 |
| Systolic BP (mm Hg) | 127 ± 27 | 143 ± 31 | 0.093 |
| Diastolic BP (mm Hg) | 69 ± 13 | 70 ± 20 | 0.806 |
| N (n = 23) | H (n = 17) | Statistics | |
|---|---|---|---|
| CD45+ (×106/mL) | 9.1 ± 3.0 | 10.8 ± 4.3 | 0.079 |
| Granulocytes (×106/mL) | 5.7 ± 1.9 | 7.3 ± 3.2 | 0.065 |
| CD14+ Monocytes (×106/mL) | 0.7 ± 0.4 | 0.8 ± 0.3 | 0.381 |
| CD3+ Lymphocytes (×106/mL) | 1.3 ± 0.6 | 1.2 ± 0.5 | 0.728 |
| CD4+ (×106/mL) | 0.7 ± 0.4 | 0.6 ± 0.3 | 0.636 |
| CD8+ (×106/mL) | 0.4 ± 0.2 | 0.5 ± 0.3 | 0.216 |
| Treg+ (CD25+CD127low, ×106/mL) | 0.02 ± 0.02 | 0.02 ± 0.02 | 0.120 |
| %CD4+RelA (pS529)+ (Basal) | %CD4+RelA (pS529)+ (LPS) | %CD4+RelB (pS552)+ (Basal) | %CD4+RelB (pS552)+ (LPS) | |
|---|---|---|---|---|
| N | 10.8 ± 10.4 | 16.2 ± 13.4 | 57.0 ± 24.2 | 78.9 ± 19.7 |
| H | 16.1 ± 13.3 | 18.8 ± 15.5 | 61.2 ± 28.5 | 61.5 ± 28.0 |
| p-value | 0.112 | 0.568 | 0.656 | 0.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulrich, C.; Canim, Z.; Herberger, E.; Girndt, M.; Fiedler, R. Inflammation in Hypervolemic Hemodialysis Patients: The Roles of RelB and Caspase-4. Int. J. Mol. Sci. 2023, 24, 17550. https://doi.org/10.3390/ijms242417550
Ulrich C, Canim Z, Herberger E, Girndt M, Fiedler R. Inflammation in Hypervolemic Hemodialysis Patients: The Roles of RelB and Caspase-4. International Journal of Molecular Sciences. 2023; 24(24):17550. https://doi.org/10.3390/ijms242417550
Chicago/Turabian StyleUlrich, Christof, Zeynep Canim, Eva Herberger, Matthias Girndt, and Roman Fiedler. 2023. "Inflammation in Hypervolemic Hemodialysis Patients: The Roles of RelB and Caspase-4" International Journal of Molecular Sciences 24, no. 24: 17550. https://doi.org/10.3390/ijms242417550
APA StyleUlrich, C., Canim, Z., Herberger, E., Girndt, M., & Fiedler, R. (2023). Inflammation in Hypervolemic Hemodialysis Patients: The Roles of RelB and Caspase-4. International Journal of Molecular Sciences, 24(24), 17550. https://doi.org/10.3390/ijms242417550





