Off-Target Effects of P2Y12 Receptor Inhibitors: Focus on Early Myocardial Fibrosis Modulation
Abstract
:1. Introduction
2. Off-Target Effects of P2Y12 Receptor Inhibitors
2.1. P2Y12 Platelet Receptors and Inhibitors
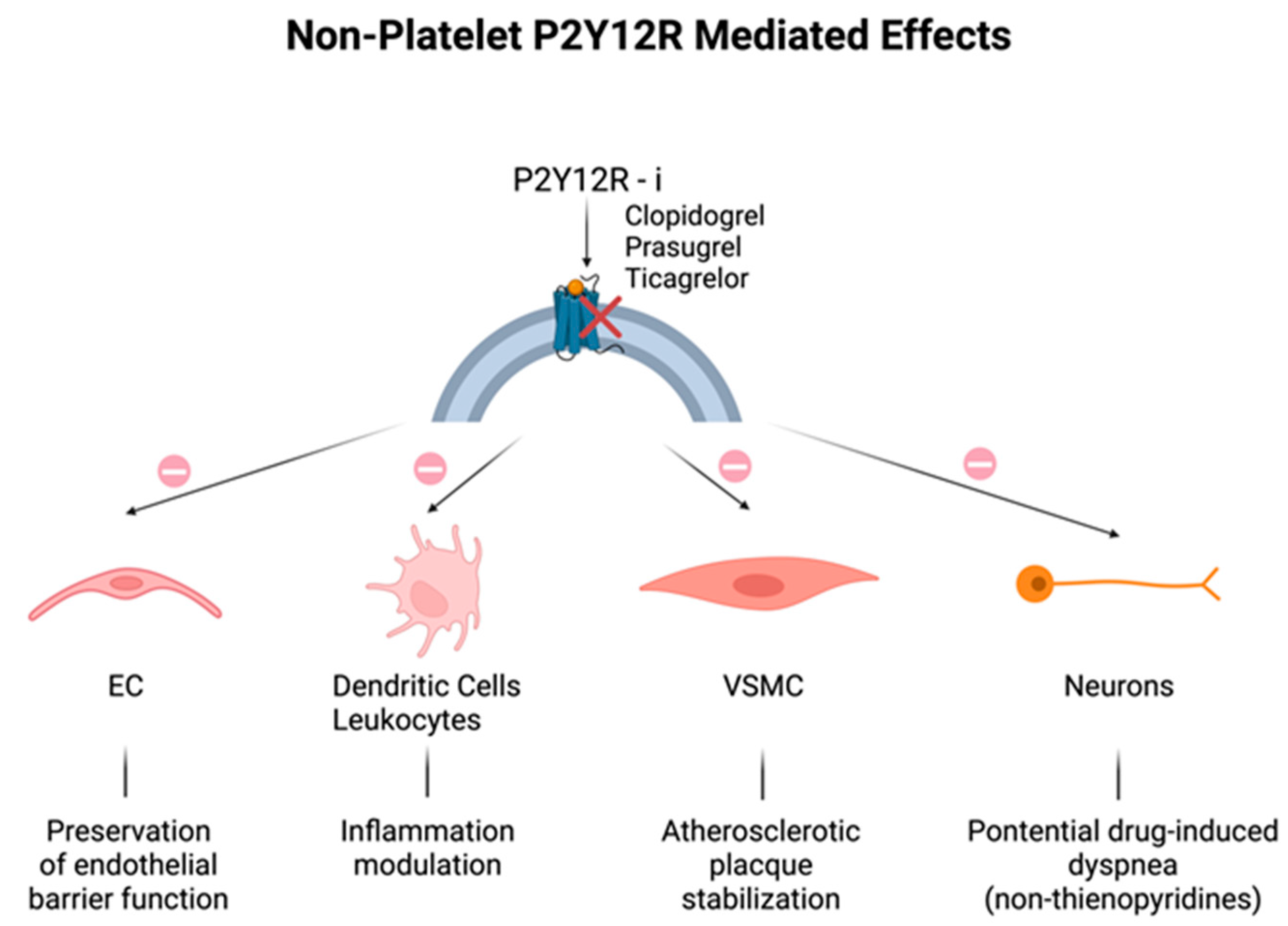
2.2. Non-Platelet P2Y12 Receptors
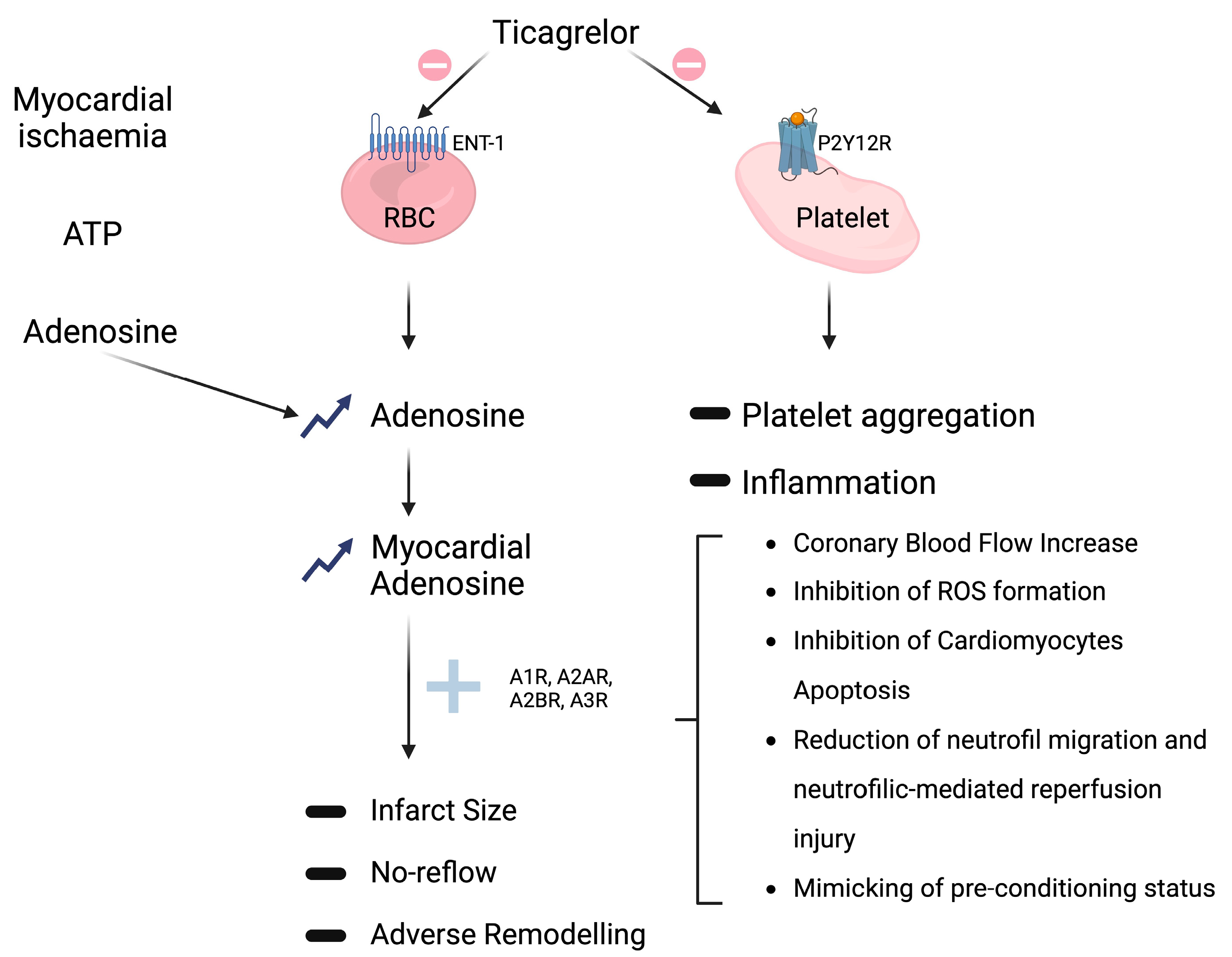
2.3. Non-P2Y12-Mediated Effects
Adenosine-Mediated Effects
3. Myocardial Fibrosis
3.1. Molecular Signaling Pathways
Wnt/β-Catenin Pathway
4. Potential Role of P2Y12 Receptor Inhibitors on Myocardial Fibrosis Modulation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Timmis, A.; Vardas, P.; Townsend, N.; Torbica, A.; Katus, H.; De Smedt, D.; Gale, C.P.; Maggioni, A.P.; Petersen, S.E.; Huculeci, R.; et al. European Society of Cardiology: Cardiovascular disease statistics 2021. Eur. Heart J. 2022, 43, 716–799, Corrigendum in Eur. Heart J. 2022, 43, 799. [Google Scholar] [CrossRef]
- Roger, V.L. Epidemiology of Heart Failure. Circ. Res. 2013, 113, 646–659. [Google Scholar] [CrossRef]
- Talman, V.; Ruskoaho, H. Cardiac fibrosis in myocardial infarction—From repair and remodeling to regeneration. Cell Tissue Res. 2016, 365, 563–581. [Google Scholar] [CrossRef]
- Spoladore, R.; Falasconi, G.; Fiore, G.; Di Maio, S.; Preda, A.; Slavich, M.; Margonato, A.; Fragasso, G. Cardiac fibrosis: Emerging agents in preclinical and clinical development. Expert Opin. Investig. Drugs 2021, 30, 153–166. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Wallentin, L. P2Y12 inhibitors: Differences in properties and mechanisms of action and potential consequences for clinical use. Eur. Heart J. 2009, 30, 1964–1977. [Google Scholar] [CrossRef]
- Valgimigli, M.; Bueno, H.; Byrne, R.; Collet, J.-P.; Costa, F.; Jeppsson, A.; Jüni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur. Heart J. 2018, 39, 213–260, Corrigendum in Eur. Heart J. 2018, 39, 2089. [Google Scholar] [CrossRef]
- Adamski, P.; Koziński, M.; Ostrowska, M.; Fabiszak, T.; Navarese, E.P.; Paciorek, P.; Grześk, G.; Kubica, J. Overview of pleiotropic effects of platelet P2Y12 receptor inhibitors. Thromb. Haemost. 2014, 112, 224–242. [Google Scholar] [CrossRef]
- Nylander, S.; Schulz, R. Effects of P2Y12 receptor antagonists beyond platelet inhibition–comparison of ticagrelor with thienopyridines. Br. J. Pharmacol. 2016, 173, 1163–1178. [Google Scholar] [CrossRef]
- Aslam, M.; Tanislav, C.; Troidl, C.; Schulz, R.; Hamm, C.; Gündüz, D. cAMP controls the restoration of endothelial barrier function after thrombin-induced hyperpermeability via Rac1 activation. Physiol. Rep. 2014, 2, e12175. [Google Scholar] [CrossRef]
- Cattaneo, M.; Schulz, R.; Nylander, S. Adenosine-Mediated Effects of Ticagrelor. J. Am. Coll. Cardiol. 2014, 63, 2503–2509. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Xie, N.; Illes, P.; Di Virgilio, F.; Ulrich, H.; Semyanov, A.; Verkhratsky, A.; Sperlagh, B.; Yu, S.-G.; Huang, C.; et al. From purines to purinergic signalling: Molecular functions and human diseases. Signal Transduct. Target. Ther. 2021, 6, 162. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.; Bachelot-Loza, C.; Nesseler, N.; Gaussem, P.; Gouin-Thibault, I. P2Y12 Inhibition beyond Thrombosis: Effects on Inflammation. Int. J. Mol. Sci. 2020, 21, 1391. [Google Scholar] [CrossRef]
- Procopio, M.C.; Lauro, R.; Nasso, C.; Carerj, S.; Squadrito, F.; Bitto, A.; Di Bella, G.; Micari, A.; Irrera, N.; Costa, F. Role of Adenosine and Purinergic Receptors in Myocardial Infarction: Focus on Different Signal Transduction Pathways. Biomedicines 2021, 9, 204. [Google Scholar] [CrossRef]
- Baqi, Y.; Müller, C.E. Antithrombotic P2Y12 receptor antagonists: Recent developments in drug discovery. Drug Discov. Today 2019, 24, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Teng, R.; Oliver, S.; Hayes, M.A.; Butler, K. Absorption, Distribution, Metabolism, and Excretion of Ticagrelor in Healthy Subjects. Drug Metab. Dispos. 2010, 38, 1514–1521. [Google Scholar] [CrossRef] [PubMed]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.-J.; Ardissino, D.; De Servi, S.; Murphy, S.A.; et al. Prasugrel versus Clopidogrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef]
- Aradi, D.; Kirtane, A.; Bonello, L.; Gurbel, P.A.; Tantry, U.S.; Huber, K.; Freynhofer, M.K.; ten Berg, J.; Janssen, P.; Angiolillo, D.J.; et al. Bleeding and stent thrombosis on P2Y12-inhibitors: Collaborative analysis on the role of platelet reactivity for risk stratification after percutaneous coronary intervention. Eur. Heart J. 2015, 36, 1762–1771. [Google Scholar] [CrossRef]
- Husted, S.; James, S.; Becker, R.C.; Horrow, J.; Katus, H.; Storey, R.F.; Cannon, C.P.; Heras, M.; Lopes, R.D.; Morais, J.; et al. Ticagrelor Versus Clopidogrel in Elderly Patients with Acute Coronary Syndromes. Circ. Cardiovasc. Qual. Outcomes 2012, 5, 680–688. [Google Scholar] [CrossRef]
- Hechler, B.; Gachet, C. Purinergic Receptors in Thrombosis and Inflammation. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2307–2315. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.R.; Storey, R.F. The role of platelets in inflammation. Thromb. Haemost. 2015, 114, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Rohrbach, S.; Rafiq, A.; Nazli, S.; Piper, H.M.; Noll, T.; Schulz, R.; Gündüz, D.; Schluter, K.-D. Hypoxia-reoxygenation-induced endothelial barrier failure: Role of RhoA, Rac1 and myosin light chain kinase. J. Physiol. 2013, 591, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Satonaka, H.; Nagata, D.; Takahashi, M.; Kiyosue, A.; Myojo, M.; Fujita, D.; Ishimitsu, T.; Nagano, T.; Nagai, R.; Hirata, Y. Involvement of P2Y12 receptor in vascular smooth muscle inflammatory changes via MCP-1 upregulation and monocyte adhesion. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H853–H861. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, M.; Faioni, E.M. Why does ticagrelor induce dyspnea? Thromb. Haemost. 2012, 108, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- van Giezen, J.J.J.; Sidaway, J.; Glaves, P.; Kirk, I.; Björkman, J.-A. Ticagrelor Inhibits Adenosine Uptake In Vitro and Enhances Adenosine-Mediated Hyperemia Responses in a Canine Model. J. Cardiovasc. Pharmacol. Ther. 2012, 17, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Nanhwan, M.K.; Ling, S.; Kodakandla, M.; Nylander, S.; Ye, Y.; Birnbaum, Y. Chronic Treatment with Ticagrelor Limits Myocardial Infarct Size. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2078–2085. [Google Scholar] [CrossRef]
- Gurbel, P.A.; Jeong, Y.-H.; Tantry, U.S. The Dogged Search for Cryptic Effects of Ticagrelor. Circulation 2016, 134, 1720–1723. [Google Scholar] [CrossRef]
- Vilahur, G.; Gutiérrez, M.; Casani, L.; Varela, L.; Capdevila, A.; Pons-Lladó, G.; Carreras, F.; Carlsson, L.; Hidalgo, A.; Badimon, L.; et al. Protective Effects of Ticagrelor on Myocardial Injury After Infarction. Circulation 2016, 134, 1708–1719. [Google Scholar] [CrossRef]
- Alexopoulos, D.; Moulias, A.; Koutsogiannis, N.; Xanthopoulou, I.; Kakkavas, A.; Mavronasiou, E.; Davlouros, P.; Hahalis, G.; R, O.; W, P.; et al. Differential Effect of Ticagrelor Versus Prasugrel on Coronary Blood Flow Velocity in Patients with Non–ST-Elevation Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. Circ. Cardiovasc. Interv. 2013, 6, 277–283. [Google Scholar] [CrossRef]
- Bonello, L.; Laine, M.; Kipson, N.; Mancini, J.; Helal, O.; Fromonot, J.; Gariboldi, V.; Condo, J.; Thuny, F.; Frere, C.; et al. Ticagrelor Increases Adenosine Plasma Concentration in Patients with an Acute Coronary Syndrome. J. Am. Coll. Cardiol. 2014, 63, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Wittfeldt, A.; Emanuelsson, H.; Brandrup-Wognsen, G.; van Giezen, J.; Jonasson, J.; Nylander, S.; Gan, L.-M. Ticagrelor Enhances Adenosine-Induced Coronary Vasodilatory Responses in Humans. J. Am. Coll. Cardiol. 2013, 61, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.S.; Hong, S.J.; Cho, S.-A.; Kim, J.-H.; Cho, J.Y.; Lee, S.H.; Joo, H.J.; Park, J.H.; Yu, C.W.; Lim, D.-S. Comparison of Ticagrelor Versus Prasugrel for Inflammation, Vascular Function, and Circulating Endothelial Progenitor Cells in Diabetic Patients with Non–ST-Segment Elevation Acute Coronary Syndrome Requiring Coronary Stenting. JACC Cardiovasc. Interv. 2017, 10, 1646–1658. [Google Scholar] [CrossRef] [PubMed]
- Ariotti, S.; Ortega-Paz, L.; van Leeuwen, M.; Brugaletta, S.; Leonardi, S.; Akkerhuis, K.M.; Rimoldi, S.F.; Janssens, G.; Gianni, U.; Berge, J.C.v.D.; et al. Effects of Ticagrelor, Prasugrel, or Clopidogrel on Endothelial Function and Other Vascular Biomarkers. JACC Cardiovasc. Interv. 2018, 11, 1576–1586. [Google Scholar] [CrossRef] [PubMed]
- Storey, R.F.; James, S.K.; Siegbahn, A.; Varenhorst, C.; Held, C.; Ycas, J.; Husted, S.E.; Cannon, C.P.; Becker, R.C.; Steg, P.G.; et al. Lower mortality following pulmonary adverse events and sepsis with ticagrelor compared to clopidogrel in the PLATO study. Platelets 2014, 25, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Alsharif, K.F.; Thomas, M.R.; Judge, H.M.; Khan, H.; Prince, L.R.; Sabroe, I.; Ridger, V.C.; Storey, R.F. Ticagrelor potentiates adenosine-induced stimulation of neutrophil chemotaxis and phagocytosis. Vasc. Pharmacol. 2015, 71, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Bonaca, M.P.; Bhatt, D.L.; Cohen, M.; Steg, P.G.; Storey, R.F.; Jensen, E.C.; Magnani, G.; Bansilal, S.; Fish, M.P.; Im, K.; et al. Long-Term Use of Ticagrelor in Patients with Prior Myocardial Infarction. N. Engl. J. Med. 2015, 372, 1791–1800. [Google Scholar] [CrossRef]
- Sexton, T.R.; Zhang, G.; Macaulay, T.E.; Callahan, L.A.; Charnigo, R.; Vsevolozhskaya, O.A.; Li, Z.; Smyth, S. Ticagrelor Reduces Thromboinflammatory Markers in Patients with Pneumonia. JACC Basic. Transl. Sci. 2018, 3, 435–449. [Google Scholar] [CrossRef]
- Lancellotti, P.; Musumeci, L.; Jacques, N.; Servais, L.; Goffin, E.; Pirotte, B.; Oury, C. Antibacterial Activity of Ticagrelor in Conventional Antiplatelet Dosages Against Antibiotic-Resistant Gram-Positive Bacteria. JAMA Cardiol. 2019, 4, 596–599. [Google Scholar] [CrossRef]
- Armstrong, D.; Summers, C.; Ewart, L.; Nylander, S.; Sidaway, J.E.; van Giezen, J.J.J. Characterization of the Adenosine Pharmacology of Ticagrelor Reveals Therapeutically Relevant Inhibition of Equilibrative Nucleoside Transporter 1. J. Cardiovasc. Pharmacol. Ther. 2014, 19, 209–219. [Google Scholar] [CrossRef]
- Babbitt, D.G.; Virmani, R.; Forman, M.B. Intracoronary adenosine administered after reperfusion limits vascular injury after prolonged ischemia in the canine model. Circulation 1989, 80, 1388–1399. [Google Scholar] [CrossRef] [PubMed]
- Thornton, J.D.; Liu, G.S.; Olsson, R.A.; Downey, J.M. Intravenous pretreatment with A1-selective adenosine analogues protects the heart against infarction. Circulation 1992, 85, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Tantry, U.S.; Jeong, Y.-H.; Gurbel, P.A. More Evidence for Non-P2Y12-Mediated Effects of Ticagrelor. JACC Cardiovasc. Interv. 2017, 10, 1659–1661. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Ren, S.; Zhang, L.; Liu, W.; Zhao, Y.; Chen, C.; Mao, X.; Chen, Z.; Gu, X. A Review of the Role of the Antiplatelet Drug Ticagrelor in the Management of Acute Coronary Syndrome, Acute Thrombotic Disease, and Other Diseases. Med. Sci. Monit. 2022, 28, e935664. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.S.; Gabriel-Costa, D.; Sudo, R.T.; Wang, H.; Groban, L.; Ferraz, E.B.; Nascimento, J.H.M.; Fraga, C.A.M.; Barreiro, E.J.; Zapata-Sudo, G. Adenosine A2A receptor agonist prevents cardiac remodeling and dysfunction in spontaneously hypertensive male rats after myocardial infarction. Drug Des. Devel Ther. 2017, 11, 553–562. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.S.; Pereira, S.L.; Maia, R.D.C.; Landgraf, S.S.; Caruso-Neves, C.; Kümmerle, A.E.; Fraga, C.A.M.; Barreiro, E.J.; Sudo, R.T.; Zapata-Sudo, G. N-acylhydrazone improves exercise intolerance in rats submitted to myocardial infarction by the recovery of calcium homeostasis in skeletal muscle. Life Sci. 2014, 94, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, F.; Epperson, S.A.; Ramirez-Sanchez, I.; Yamazaki, K.G.; Brunton, L.L. Regulation of cardiac fibroblast collagen synthesis by adenosine: Roles for Epac and PI3K. Am. J. Physiol. Cell Physiol. 2009, 296, C1178–C1184. [Google Scholar] [CrossRef]
- Guo, F.; Wang, X.; Guo, Y.; Wan, W.; Cui, Y.; Wang, J.; Liu, W. Shenfu Administration Improves Cardiac Fibrosis in Rats with Myocardial Ischemia-Reperfusion Through Adenosine A2a Receptor Activation. Hum. Exp. Toxicol. 2022, 41, 096032712210776. [Google Scholar] [CrossRef]
- Tamargo, J.; López-Sendón, J. Novel therapeutic targets for the treatment of heart failure. Nat. Rev. Drug Discov. 2011, 10, 536–555. [Google Scholar] [CrossRef]
- Amulic, B.; Cazalet, C.; Hayes, G.L.; Metzler, K.D.; Zychlinsky, A. Neutrophil Function: From Mechanisms to Disease. Annu. Rev. Immunol. 2012, 30, 459–489. [Google Scholar] [CrossRef]
- Prabhu, S.D.; Frangogiannis, N.G. The Biological Basis for Cardiac Repair After Myocardial Infarction. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef] [PubMed]
- Scalise, R.F.M.; De Sarro, R.; Caracciolo, A.; Lauro, R.; Squadrito, F.; Carerj, S.; Bitto, A.; Micari, A.; Di Bella, G.; Costa, F.; et al. Fibrosis after Myocardial Infarction: An Overview on Cellular Processes, Molecular Pathways, Clinical Evaluation and Prognostic Value. Med. Sci. 2021, 9, 16. [Google Scholar] [CrossRef]
- Stuart, S.D.F.; De Jesus, N.M.; Lindsey, M.L.; Ripplinger, C.M. The crossroads of inflammation, fibrosis, and arrhythmia following myocardial infarction. J. Mol. Cell Cardiol. 2016, 91, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, Y.; Watabe, T. Roles of TGF- β Signals in Endothelial-Mesenchymal Transition during Cardiac Fibrosis. Int. J. Inflam. 2011, 2011, 724080. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Fu, W.; Li, L.; Xia, X.; Liao, Q.; Yue, R.; Chen, H.; Chen, X.; An, S.; Zeng, C.; et al. Therapeutic effect of a novel Wnt pathway inhibitor on cardiac regeneration after myocardial infarction. Clin. Sci. (Lon.) 2017, 131, 2919–2932, Correction in Clin. Sci. (Lon.) 2019, 133, 149. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, G.; Hu, M.C.-T.; Yao, Z.; Tan, T.-H. Activation of the Hematopoietic Progenitor Kinase-1 (HPK1)-dependent, Stress-activated c-Jun N-terminal Kinase (JNK) Pathway by Transforming Growth Factor β (TGF-β)-activated Kinase (TAK1), a Kinase Mediator of TGF β Signal Transduction. J. Biol. Chem. 1997, 272, 22771–22775. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Fu, W.; Wang, W.E.; Zeng, C. Wnt signaling pathways in myocardial infarction and the therapeutic effects of Wnt pathway inhibitors. Acta Pharmacol. Sin. 2019, 40, 9–12. [Google Scholar] [CrossRef]
- Shi, L.; Wu, Z.; Miao, J.; Du, S.; Ai, S.; Xu, E.; Feng, M.; Song, J.; Guan, W. Adenosine interaction with adenosine receptor A2a promotes gastric cancer metastasis by enhancing PI3K–AKT–mTOR signaling. Mol. Biol. Cell 2019, 30, 2527–2534. [Google Scholar] [CrossRef]
- Hemmings, B.A.; Restuccia, D.F. PI3K-PKB/Akt Pathway. Cold Spring Harb. Perspect. Biol. 2012, 4, a011189. [Google Scholar] [CrossRef]
- Guo, Y.; Gupte, M.; Umbarkar, P.; Singh, A.P.; Sui, J.Y.; Force, T.; Lal, H. Entanglement of GSK-3β, β-catenin and TGF-β1 signaling network to regulate myocardial fibrosis. J. Mol. Cell Cardiol. 2017, 110, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ramirez, A.; Waddell, D.S.; Li, Z.; Liu, X.; Wang, X.-F. Axin and GSK3-β control Smad3 protein stability and modulate TGF-β signaling. Genes Dev. 2008, 22, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Lal, H.; Ahmad, F.; Zhou, J.; Yu, J.E.; Vagnozzi, R.J.; Guo, Y.; Yu, D.; Tsai, E.J.; Woodgett, J.; Gao, E.; et al. Cardiac Fibroblast Glycogen Synthase Kinase-3β Regulates Ventricular Remodeling and Dysfunction in Ischemic Heart. Circulation 2014, 130, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Blyszczuk, P.; Müller-Edenborn, B.; Valenta, T.; Osto, E.; Stellato, M.; Behnke, S.; Glatz, K.; Basler, K.; Lüscher, T.F.; Distler, O.; et al. Transforming growth factor-β-dependent Wnt secretion controls myofibroblast formation and myocardial fibrosis progression in experimental autoimmune myocarditis. Eur. Heart J. 2017, 38, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhang, L.; Ni, A.; Zhang, Z.; Mirotsou, M.; Mao, L.; Pratt, R.E.; Dzau, V.J. Exogenously administered secreted frizzled related protein 2 (Sfrp2) reduces fibrosis and improves cardiac function in a rat model of myocardial infarction. Proc. Natl. Acad. Sci. USA 2010, 107, 21110–21115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Deb, A.; Zhang, Z.; Pachori, A.; He, W.; Guo, J.; Pratt, R.; Dzau, V.J. Secreted frizzled related protein 2 protects cells from apoptosis by blocking the effect of canonical Wnt3a. J. Mol. Cell Cardiol. 2009, 46, 370–377. [Google Scholar] [CrossRef]
- Aisagbonhi, O.; Rai, M.; Ryzhov, S.; Atria, N.; Feoktistov, I.; Hatzopoulos, A.K. Experimental myocardial infarction triggers canonical Wnt signaling and endothelial-to-mesenchymal transition. Dis. Model. Mech. 2011, 4, 469–483. [Google Scholar] [CrossRef]
- Duan, J.; Gherghe, C.; Liu, D.; Hamlett, E.; Srikantha, L.; Rodgers, L.; Regan, J.N.; Rojas, M.; Willis, M.; Leask, A.; et al. Wnt1/βcatenin injury response activates the epicardium and cardiac fibroblasts to promote cardiac repair. EMBO J. 2012, 31, 429–442. [Google Scholar] [CrossRef]
- Park, Y.; Koh, J.S.; Lee, J.-H.; Park, J.-H.; Oh, J.H.; Chun, W.; Bae, J.-W.; Kim, J.S.; Kim, W.; Suh, J.-W.; et al. Effect of Ticagrelor on Left Ventricular Remodeling in Patients with ST-Segment Elevation Myocardial Infarction (HEALING-AMI). JACC Cardiovasc. Interv. 2020, 13, 2220–2234. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lofrumento, F.; Irrera, N.; Licordari, R.; Perfetti, S.; Nasso, E.; Liotta, P.; Isgrò, G.; Garcia-Ruiz, V.; Squadrito, F.; Carerj, S.; et al. Off-Target Effects of P2Y12 Receptor Inhibitors: Focus on Early Myocardial Fibrosis Modulation. Int. J. Mol. Sci. 2023, 24, 17546. https://doi.org/10.3390/ijms242417546
Lofrumento F, Irrera N, Licordari R, Perfetti S, Nasso E, Liotta P, Isgrò G, Garcia-Ruiz V, Squadrito F, Carerj S, et al. Off-Target Effects of P2Y12 Receptor Inhibitors: Focus on Early Myocardial Fibrosis Modulation. International Journal of Molecular Sciences. 2023; 24(24):17546. https://doi.org/10.3390/ijms242417546
Chicago/Turabian StyleLofrumento, Francesca, Natasha Irrera, Roberto Licordari, Silvia Perfetti, Enrica Nasso, Paolo Liotta, Giovanni Isgrò, Victoria Garcia-Ruiz, Francesco Squadrito, Scipione Carerj, and et al. 2023. "Off-Target Effects of P2Y12 Receptor Inhibitors: Focus on Early Myocardial Fibrosis Modulation" International Journal of Molecular Sciences 24, no. 24: 17546. https://doi.org/10.3390/ijms242417546
APA StyleLofrumento, F., Irrera, N., Licordari, R., Perfetti, S., Nasso, E., Liotta, P., Isgrò, G., Garcia-Ruiz, V., Squadrito, F., Carerj, S., Di Bella, G., Micari, A., & Costa, F. (2023). Off-Target Effects of P2Y12 Receptor Inhibitors: Focus on Early Myocardial Fibrosis Modulation. International Journal of Molecular Sciences, 24(24), 17546. https://doi.org/10.3390/ijms242417546





