Cell Therapy of Vascular and Neuropathic Complications of Diabetes: Can We Avoid Limb Amputation?
Abstract
:1. Introduction
1.1. The Global Burden of Diabetes Mellitus
Types of Diabetes
- Type 1 diabetes (previously known as insulin-dependent, juvenile or childhood-onset) is characterized by deficient insulin production and requires daily administration of insulin. Prevalence of type 1 Diabetes Mellitus in 2017 was approximately 9 million people; the majority of them live in high-income countries.
- Type 2 diabetes may be due either by insufficient insulin production by the beta cells of the pancreatic islets or by defective action in peripheral tissues (muscle, liver) also called insulin resistance. Combination of both leads to high levels of blood sugar if not treated. Factors that contribute to developing type 2 diabetes include being overweight, not getting enough exercise, and genetics [3].
- Gestational diabetes, in which there is dysregulated glucose levels is at an increased risk of complications during pregnancy and at delivery. These women and possibly their children are also at increased risk of type 2 diabetes in the future.
- Monogenic diabetes, directly linked to the mutation of a gene related with the pancreatic beta cell physiology.
1.2. Economic Burden of Diabetes
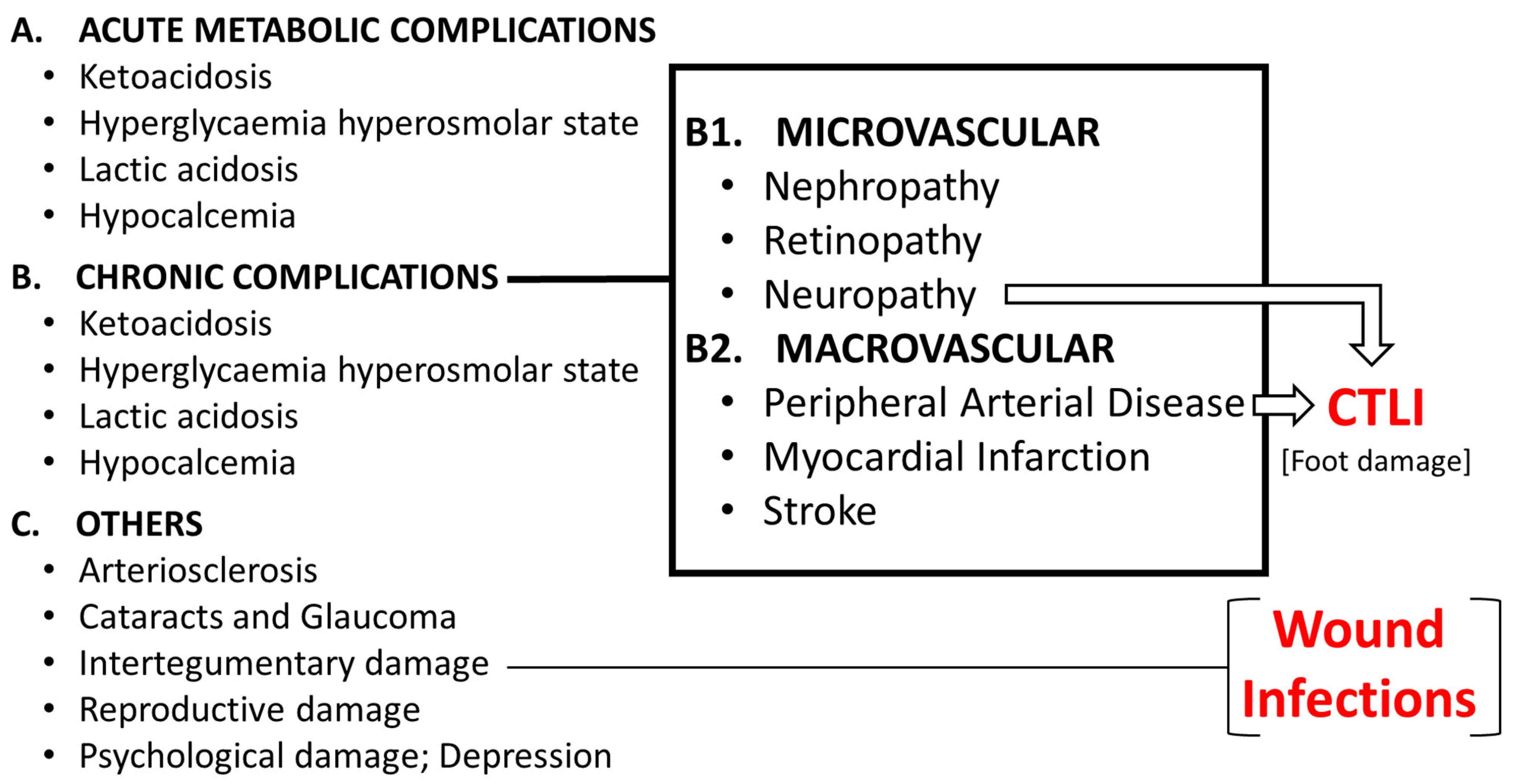
1.3. Complications of Diabetes
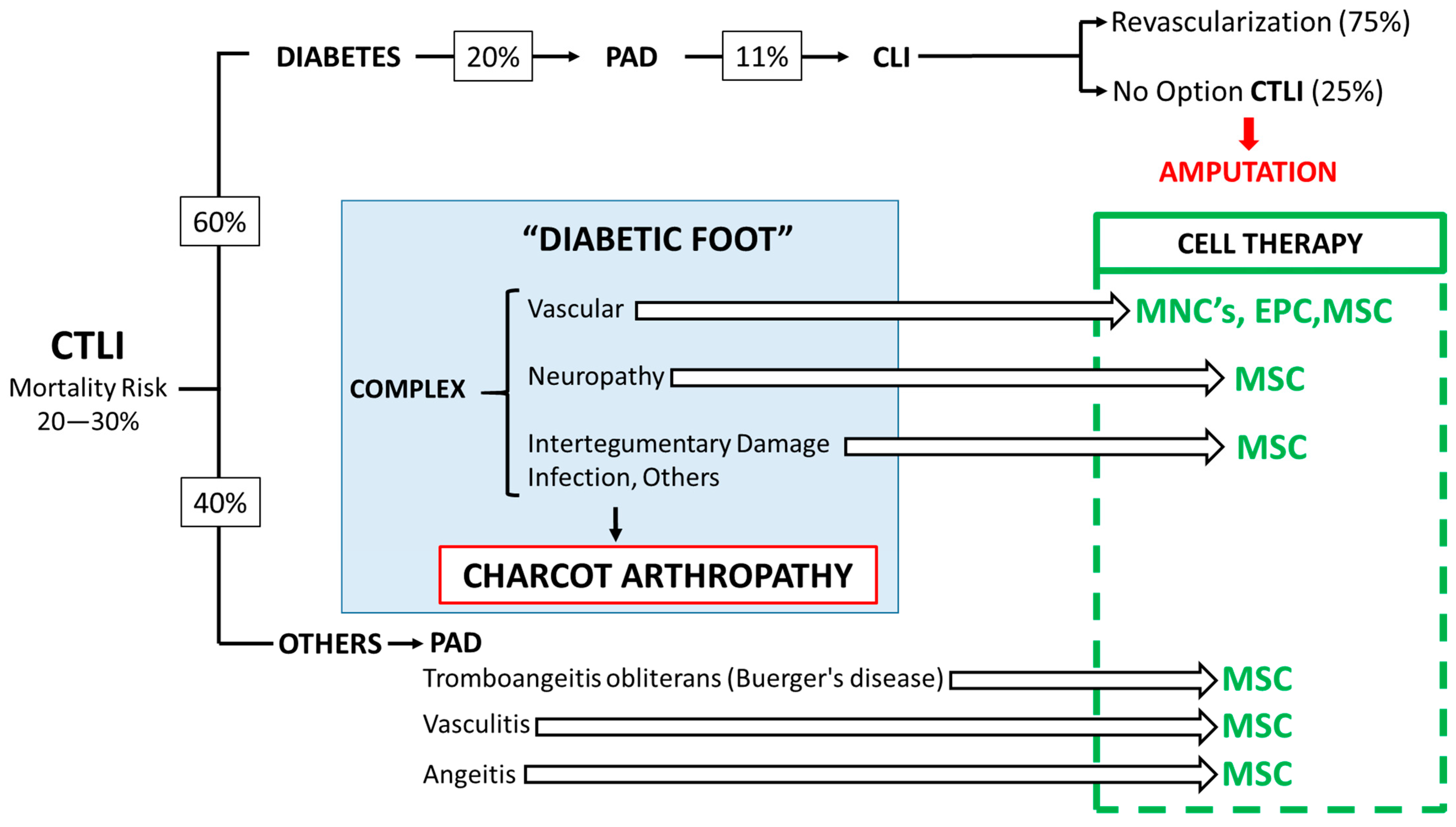
1.3.1. The Diabetic Foot
1.3.2. The Complex Nature of the Diabetic Foot
1.3.3. Arthropathy of Charcot
1.3.4. Treatment of CTLI
Conventional Medical Care
Advanced Therapies: Clinical Trials with ATMP to Treat the Diabetic Foot
Photodynamic Therapies
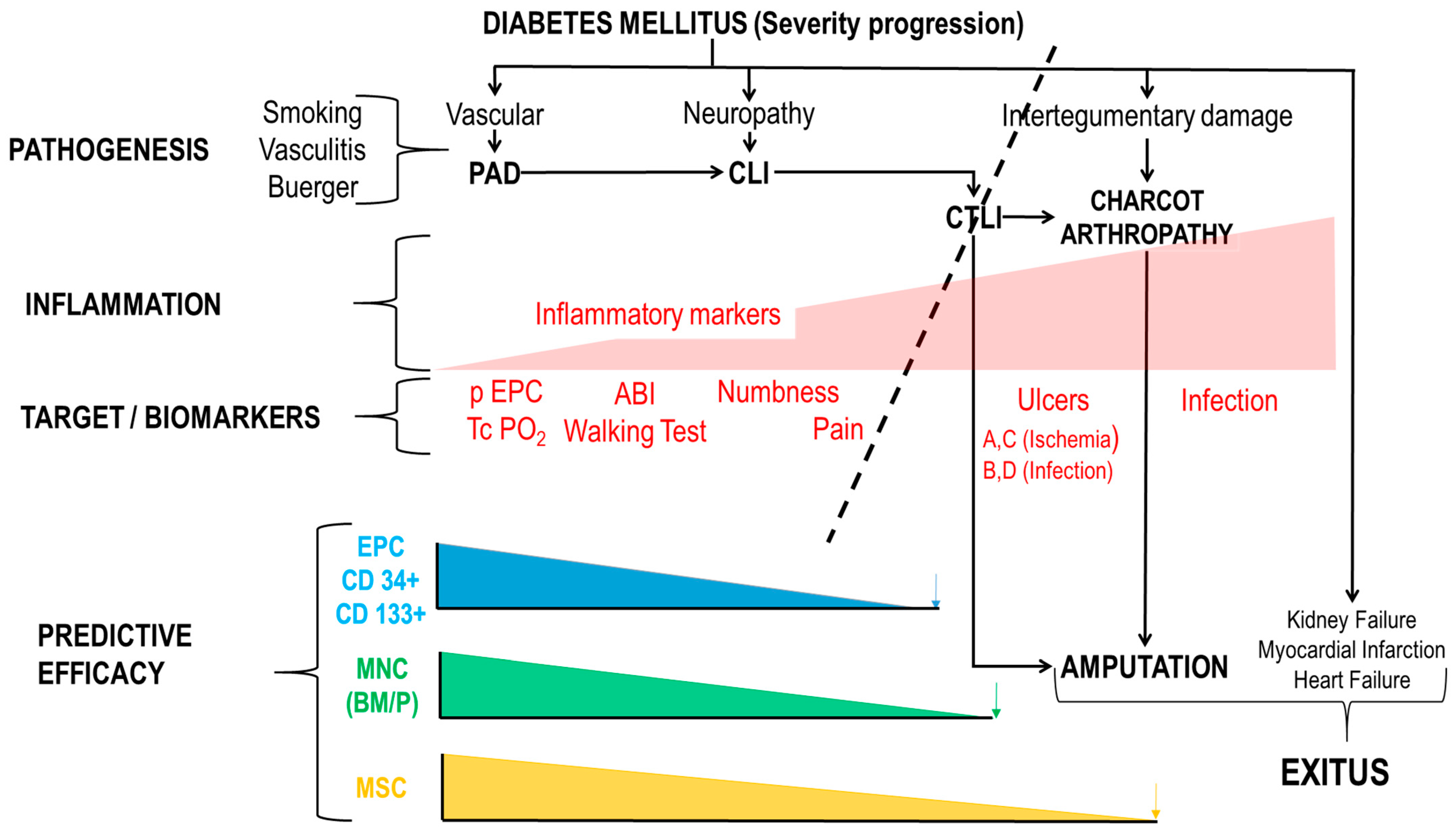
1.4. End-Points with ATMP Clinical Trials and Mechanism of Action (MoA)
- Stage I: Origin-diabetes mellitus, vasculitis, smoking.
- Stage II: PAD (20% of people with diabetes mellitus). Intermittent claudication.
- Stage III: The combination of PAD and diabetic neuropathy generates chronic life-threatening limb ischemia (CTLI)—intense ischemic pain.
- Stage IV: 25% of CLI cannot be revascularized. “No Option” CLI or CTLI.
- Stage V: Appearance of ulcers.
- Stage VI: CA-PAD. Neuropathy and intertegumentary damage led to CA and the need for Amputation.
1.4.1. Mechanism of Action
- Mobilization of endothelial progenitors
- Regeneration of tissues
- Anti-inflammatory effects
Mobilization of Endothelial Progenitors
Regeneration
Anti-Inflammatory Effects
1.4.2. Primary and Secondary End-Points in Clinical Trials with ATMP
- TcPO2: Primary
- Peripheral EPC: Primary
- Inflammatory markers: Primary and secondary
- ◦
- C-reactive protein
- ◦
- Proinflammatory cytokines: TNF-α, IL-6, IL-1B
- ◦
- Anti-inflammatory cytokines: IL-4, IL-10
- Inflammasome: Secondary
- Pain-walking test: Primary
- Ulcer healing: Primary and Secondary
- i.
- Ulcer Infections
- ii.
- Increase in collateral vessels indicating vascular remodeling Revascularization with TcPO2 > 40 mm Hg
- iii.
- Keeping kidney function with glomerular filtration rate > 30 mL/min
2. Results: Clinical Trials with ATMPs
2.1. Clinical Trials with ATMP of CTLI in Diabetic Patients
| Product | ClinicalTrails.gov (NCT, First Posted-IATA FPS) | Mechanism of Action | References |
|---|---|---|---|
| BM-MNC (autologous) | Phase I NCT00872326 First Posted IATA-FPS: 30 March 2009 | 1—Mobilization of EPC DM 2—Regenerative Cytokines 3—Inflammation and Immune | Completed [30,31] Soria et al., 2023 (this work) |
| BM-MNC (autologous) | Phase I NCT00987363 First Posted IATA-FPS: 30 September 2009 | 1—Mobilization of EPC DM 2—Regenerative Cytokines 3—Inflammation and Immune | Completed [40] |
| BM-MNC (autologous) | Phase I-II, Multicentric NCT014008381 First Posted IATA-FPS: 30 August 2011 | 1—Mobilization of EPC DM 2—Regenerative Cytokines 3—Inflammation and Immune | Completed No publications |
| Adipose-derived MSC-Diabetes (autologous) | Phase I-IIa NCT01257776 First Posted IATA-FPS: 10 December 2015 | 2—Regenerative Cytokines 3—Inflammation and Immune | Completed [35,41,42] Soria et al., 2023 (this work) |
| Adipose-derived MSC-No Diabetes (autologous) | Phase I-IIa NCT01745744 First Posted IATA-FPS: 10 December 2012 | 2—Regenerative Cytokines 3—Inflammation and Immune | [35,41,42] Soria et al., 2023 (this work) |
| Endothelial Progenitor Cells (autologous) | NCT02287974 First posted IATA-FPS: 10 November 2014 Last: 19 December 2018 | 1—Mobilization of EPC DM 2—Regenerative Cytokines 3—Inflammation and Immune | Recruitment closed by sponsor No publications |
| Adipose-derived MSC-(allogenic) | Phase II, Multicentric NCT04466007 First Posted IATA-FPS: 11 January 2021 | 2—Regenerative Cytokines 3—Inflammation and Immune | Recruitment completed [43] No data yet, CRD to be opened in 2024. |
2.1.1. Regulatory Requirements
2.1.2. Autologous ATMPs
Bone Marrow Mononuclear Cells (BM-MNCs) and Peripheral Blood Mononuclear Cells (PB-MNCs)
Allogeneic-MSC: Mesenchymal Stromal Cells
Neovascularization
Ulcer Healing
Pain and Walking Capabilities
Transcutaneous Oxygen-TcPO2
Ankle-Arm Index (ABI)
Rutherford-Becker Scale
Amputations
Endothelial Progenitor Cells (EPC)
3. Discussion
3.1. Autologous vs. Allogenic ATMPs
- (a)
- A new source of healthy allogeneic donors. In the NOMA Project we used healthy young female altruistic donors of adipose tissue [69].
- (b)
- A cost-effective mass production under GMP conditions (to be developed).
- (c)
- A safe, friendly and less costly procedure for administration, for example, via the intramuscular route. Described in the NOMA Project [69].
- (d)
- A new xeno-free culture medium. In fact, a xeno-free and human-component free has been developed by B. Soria (patent pending).
3.2. Adverse Effects of Cell Therapy with ATMP
3.2.1. Bone-Marrow Mononuclear Cells (BM-MNC)
3.2.2. Autologous MSC
Ulcers, Amputations and QALY: Cost-Effectiveness of Treatments
Responders and Non-Responders
MSC Origin and Effect
4. Material and Methods
4.1. Neovascularization
- i.
- Flow cytometry.
4.2. Ulcer Healing
4.3. Pain and Walking Capabilities
4.4. Transcutaneous Oxygen-TcPO2
4.5. Ankle-Arm Index (ABI)
4.6. Rutherford-Becker Scale
4.7. Amputations
5. Conclusions
6. Patents
- Bernat Soria, Abdelkrim Hmadcha, Lourdes Acosta, Natalia Escacena (2012) “Method for predicting treatment response and test for safe use of mesenchymal stem cells on inflammatory diseases” PCT/EP2014/066600
- Soria Escoms, B. (2020) “Safe and Effective Pharmaceutical Products for COVID-19 and other Inflammatory, Autoimmune and Degenerative Diseases”. EP20382405 (14 May 2020).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IHME. I.f.H.M.a.E. In Global Burden of Disease Study 2019; Global Burden of Disease Collaborative Network: Washington, DC, USA, 2019. [Google Scholar]
- Magliano, D.J.; Boyko, E.J. IDF Diabetes Atlas; IDF Diabetes Atlas 10th Edition Scientific Committee: Brussels, Belgium, 2021. [Google Scholar]
- Romero-Gómez, M.; Zelber-Sagi, S.; Martín, F.; Bugianesi, E.; Soria, B. Nutrition could prevent or promote non-alcoholic fatty liver disease: An opportunity for intervention. BMJ (Clin. Res. Ed.) 2023, 383, e075179. [Google Scholar] [CrossRef] [PubMed]
- Crespo, C.; Brosa, M.; Soria-Juan, A.; Lopez-Alba, A.; López-Martínez, N.; Soria, B. Costes directos de la diabetes mellitus y de sus complicaciones en España (Estudio SECCAID: Spain estimated cost Ciberdem-Cabimer in Diabetes). Av. Diabetol. 2013, 29, 182–189. [Google Scholar] [CrossRef]
- Barnes, J.A.; Eid, M.A.; Creager, M.A.; Goodney, P.P. Epidemiology and Risk of Amputation in Patients With Diabetes Mellitus and Peripheral Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1808–1817. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Chen, Y.; Jiang, N.; Li, Z.; Xu, S. Burden of Peripheral Artery Disease and Its Attributable Risk Factors in 204 Countries and Territories from 1990 to 2019. Front. Cardiovasc. Med. 2022, 9, 868370. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.B.; Suresh, K.R.; Murad, M.H.; et al. Global Vascular Guidelines on the Management of Chronic Limb-Threatening Ischemia. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2019, 58, S1–S109. [Google Scholar] [CrossRef] [PubMed]
- Prompers, L.; Huijberts, M.; Apelqvist, J.; Jude, E.; Piaggesi, A.; Bakker, K.; Edmonds, M.; Holstein, P.; Jirkovska, A.; Mauricio, D.; et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia 2007, 50, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lu, J.; Jing, Y.; Tang, S.; Zhu, D.; Bi, Y. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis (†). Ann. Med. 2017, 49, 106–116. [Google Scholar] [CrossRef]
- Hart, O.; Xue, N.; Davis-Havill, B.; Pottier, M.; Prakash, M.; Reimann, S.A.; King, J.; Xu, W.; Khashram, M. The Incidence of Chronic Limb-Threatening Ischemia in the Midland Region of New Zealand over a 12-Year Period. J. Clin. Med. 2022, 11, 3303. [Google Scholar] [CrossRef]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.; Bell, K.; Caporusso, J.; Durand-Zaleski, I.; Komori, K.; et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2007, 33 (Suppl. S1), S5–S67. [Google Scholar] [CrossRef]
- Boulton, A.J. The diabetic foot: Grand overview, epidemiology and pathogenesis. Diabetes/Metab. Res. Rev. 2008, 24 (Suppl. S1), S3–S6. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Berendt, A.R.; Deery, H.G.; Embil, J.M.; Joseph, W.S.; Karchmer, A.W.; LeFrock, J.L.; Lew, D.P.; Mader, J.T.; Norden, C.; et al. Diagnosis and treatment of diabetic foot infections. Plast. Reconstr. Surg. 2006, 117, 212s–238s. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Agostini, C.; Avogaro, A. Autologous stem cell therapy for peripheral arterial disease meta-analysis and systematic review of the literature. Atherosclerosis 2010, 209, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Nehler, M.R.; Peyton, B.D. Is revascularization and limb salvage always the treatment for critical limb ischemia? J. Cardiovasc. Surg. 2004, 45, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Sprengers, R.W.; Moll, F.L.; Verhaar, M.C. Stem cell therapy in PAD. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2010, 39 (Suppl. S1), S38–S43. [Google Scholar] [CrossRef]
- Watelet, J.; Soury, P.; Menard, J.F.; Plissonnier, D.; Peillon, C.; Lestrat, J.P.; Testart, J. Femoropopliteal bypass: In situ or reversed vein grafts? Ten-year results of a randomized prospective study. Ann. Vasc. Surg. 1997, 11, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Alavi, A.; Sibbald, R.G.; Mayer, D.; Goodman, L.; Botros, M.; Armstrong, D.G.; Woo, K.; Boeni, T.; Ayello, E.A.; Kirsner, R.S. Diabetic foot ulcers: Part I. Pathophysiology and prevention. J. Am. Acad. Dermatol. 2014, 70, 1-e1. [Google Scholar] [CrossRef] [PubMed]
- Galkowska, H.; Wojewodzka, U.; Olszewski, W.L. Chemokines, cytokines, and growth factors in keratinocytes and dermal endothelial cells in the margin of chronic diabetic foot ulcers. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2006, 14, 558–565. [Google Scholar] [CrossRef]
- Faglia, E.; Clerici, G.; Clerissi, J.; Gabrielli, L.; Losa, S.; Mantero, M.; Caminiti, M.; Curci, V.; Quarantiello, A.; Lupattelli, T.; et al. Long-term prognosis of diabetic patients with critical limb ischemia: A population-based cohort study. Diabetes Care 2009, 32, 822–827. [Google Scholar] [CrossRef]
- Ferraresi, R.; Mauri, G.; Losurdo, F.; Troisi, N.; Brancaccio, D.; Caravaggi, C.; Neri, L. BAD transmission and SAD distribution: A new scenario for critical limb ischemia. J. Cardiovasc. Surg. 2018, 59, 655–664. [Google Scholar] [CrossRef]
- Hata, Y.; Iida, O.; Takahara, M.; Asai, M.; Masuda, M.; Okamoto, S.; Ishihara, T.; Nanto, K.; Kanda, T.; Tsujimura, T.; et al. Infrapopliteal Anatomic Severity and Delayed Wound Healing in Patients With Chronic Limb-Threatening Ischemia in the Era of the Global Limb Anatomic Staging System. J. Endovasc. Ther. Off. J. Int. Soc. Endovasc. Spec. 2020, 27, 641–646. [Google Scholar] [CrossRef]
- Uccioli, L.; Meloni, M.; Izzo, V.; Giurato, L.; Merolla, S.; Gandini, R. Critical limb ischemia: Current challenges and future prospects. Vasc. Health Risk Manag. 2018, 14, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Ferraresi, R.; Centola, M.; Ferlini, M.; Da Ros, R.; Caravaggi, C.; Assaloni, R.; Sganzaroli, A.; Pomidossi, G.; Bonanomi, C.; Danzi, G.B. Long-term outcomes after angioplasty of isolated, below-the-knee arteries in diabetic patients with critical limb ischaemia. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2009, 37, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, P.; Clares, B.; Hmadcha, A.; Ruiz, A.; Soria, B. Development of a cell-based medicinal product: Regulatory structures in the European Union. Br. Med. Bull. 2013, 105, 85–105. [Google Scholar] [CrossRef] [PubMed]
- Everett, E.; Mathioudakis, N. Update on management of diabetic foot ulcers. Ann. N. Y. Acad. Sci. 2018, 1411, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Pantò, F.; Adamo, L.; Giordano, C.; Licciardello, C. Efficacy and safety of photodynamic therapy with RLP068 for diabetic foot ulcers: A review of the literature and clinical experience. Drugs Context 2020, 9. [Google Scholar] [CrossRef]
- Fadini, G.P.; Miorin, M.; Facco, M.; Bonamico, S.; Baesso, I.; Grego, F.; Menegolo, M.; de Kreutzenberg, S.V.; Tiengo, A.; Agostini, C.; et al. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J. Am. Coll. Cardiol. 2005, 45, 1449–1457. [Google Scholar] [CrossRef]
- Loomans, C.J.; de Koning, E.J.; Staal, F.J.; Rookmaaker, M.B.; Verseyden, C.; de Boer, H.C.; Verhaar, M.C.; Braam, B.; Rabelink, T.J.; van Zonneveld, A.J. Endothelial progenitor cell dysfunction: A novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes 2004, 53, 195–199. [Google Scholar] [CrossRef]
- Ruiz-Salmeron, R.; de la Cuesta-Diaz, A.; Constantino-Bermejo, M.; Pérez-Camacho, I.; Marcos-Sánchez, F.; Hmadcha, A.; Soria, B. Angiographic demonstration of neoangiogenesis after intra-arterial infusion of autologous bone marrow mononuclear cells in diabetic patients with critical limb ischemia. Cell Transplant. 2011, 20, 1629–1639. [Google Scholar] [CrossRef]
- Soria, B.; Bedoya, F.J.; Tejedo, J.R.; Hmadcha, A.; Ruiz-Salmerón, R.; Lim, S.; Martin, F. Cell therapy for diabetes mellitus: An opportunity for stem cells? Cells Tissues Organs 2008, 188, 70–77. [Google Scholar] [CrossRef]
- Ferraro, F.; Lymperi, S.; Méndez-Ferrer, S.; Saez, B.; Spencer, J.A.; Yeap, B.Y.; Masselli, E.; Graiani, G.; Prezioso, L.; Rizzini, E.L.; et al. Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Sci. Transl. Med. 2011, 3, 104ra101. [Google Scholar] [CrossRef]
- del Toro, R.; Méndez-Ferrer, S. Autonomic regulation of hematopoiesis and cancer. Haematologica 2013, 98, 1663–1666. [Google Scholar] [CrossRef]
- Saito, F.; Nakatani, T.; Iwase, M.; Maeda, Y.; Murao, Y.; Suzuki, Y.; Fukushima, M.; Ide, C. Administration of cultured autologous bone marrow stromal cells into cerebrospinal fluid in spinal injury patients: A pilot study. Restor. Neurol. Neurosci. 2012, 30, 127–136. [Google Scholar] [CrossRef]
- Escacena, N. Medicamento Celular Como Alternativa Terapéutica en la Isquemia Crónica Crítica de Miembros Inferiores en Pacientes Diabéticos sin Posibilidades de Revascularización; CSIC-JA-UPO-USE—Centro Andaluz de Biología Molecular y Medicina Regenerativa (CABIMER): Andalusia, Spain, 2016. [Google Scholar]
- Beltrán-Camacho, L.; Rojas-Torres, M.; Durán-Ruiz, M.C. Current Status of Angiogenic Cell Therapy and Related Strategies Applied in Critical Limb Ischemia. Int. J. Mol. Sci. 2021, 22, 2335. [Google Scholar] [CrossRef]
- Gupta, P.K.; Shivashankar, P.; Rajkumar, M.; Mahapatra, S.S.; Desai, S.C.; Dhar, A.; Krishna, V.; Raviraja, N.S.; Bhat, S.; Viswanathan, P.; et al. Label extension, single-arm, phase III study shows efficacy and safety of stempeucel® in patients with critical limb ischemia due to atherosclerotic peripheral arterial disease. Stem Cell Res. Ther. 2023, 14, 60. [Google Scholar] [CrossRef]
- Procházka, V.; Gumulec, J.; Jalůvka, F.; Salounová, D.; Jonszta, T.; Czerný, D.; Krajča, J.; Urbanec, R.; Klement, P.; Martinek, J.; et al. Cell therapy, a new standard in management of chronic critical limb ischemia and foot ulcer. Cell Transplant. 2010, 19, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Jaluvka, F.; Ihnat, P.; Madaric, J.; Vrtkova, A.; Janosek, J.; Prochazka, V. Current Status of Cell-Based Therapy in Patients with Critical Limb Ischemia. Int. J. Mol. Sci. 2020, 21, 8999. [Google Scholar] [CrossRef]
- Chacon Quevedo, A.; Garcia-Revillo Garcia, J.; Sagrario Lombardo Galera, M.; Canis Lopez, M.; Munoz Carvajal, I.; Martin-Palanco, V.; De Alcala Martinez Gomez, D.; Garcia Rospide, V.; Patricio Linares Palomino, J.; Capel Aleman, A.; et al. Autologous bone marrow mononuclear cells in the treatment of chronic limb-threatening ischaemia: A proof-of-concept trial. Cardiovasc. Res. 2022, 118, cvac066-236. [Google Scholar] [CrossRef]
- Acosta, L.; Hmadcha, A.; Escacena, N.; Pérez-Camacho, I.; de la Cuesta, A.; Ruiz-Salmeron, R.; Gauthier, B.R.; Soria, B. Adipose mesenchymal stromal cells isolated from type 2 diabetic patients display reduced fibrinolytic activity. Diabetes 2013, 62, 4266–4269. [Google Scholar] [CrossRef]
- Soria, B.; Hmadcha, A.; Ruiz-Salmeron, R.; de la Cuesta-Diaz, A.; Pérez-Camacho, I.; Marcos-Sánchez, F. Avoiding lower extremity amputation: Cell therapy of critical ischaemia of the limbs (CIL). Design and interim results of four clinical trials. Int. Soc. Stem Cell Res. 2015, W1029, 16–17. [Google Scholar]
- Soria-Juan, B.; Garcia-Arranz, M.; Llanos Jiménez, L.; Aparicio, C.; Gonzalez, A.; Mahillo Fernandez, I.; Riera Del Moral, L.; Grochowicz, L.; Andreu, E.J.; Marin, P.; et al. Efficacy and safety of intramuscular administration of allogeneic adipose tissue derived and expanded mesenchymal stromal cells in diabetic patients with critical limb ischemia with no possibility of revascularization: Study protocol for a randomized controlled double-blind phase II clinical trial (The NOMA Trial). Trials 2021, 22, 595. [Google Scholar] [CrossRef]
- Tateishi-Yuyama, E.; Matsubara, H.; Murohara, T.; Ikeda, U.; Shintani, S.; Masaki, H.; Amano, K.; Kishimoto, Y.; Yoshimoto, K.; Akashi, H.; et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: A pilot study and a randomised controlled trial. Lancet 2002, 360, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Tepper, O.M.; Galiano, R.D.; Capla, J.M.; Kalka, C.; Gagne, P.J.; Jacobowitz, G.R.; Levine, J.P.; Gurtner, G.C. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 2002, 106, 2781–2786. [Google Scholar] [CrossRef]
- Rutherford, R.B.; Baker, J.D.; Ernst, C.; Johnston, K.W.; Porter, J.M.; Ahn, S.; Jones, D.N. Recommended standards for reports dealing with lower extremity ischemia: Revised version. J. Vasc. Surg. 1997, 26, 517–538. [Google Scholar] [CrossRef]
- Rutherford, R.B.; Flanigan, D.P.; Gupta, S.K. Suggested standards for reports dealing with lower extremity ischemia. Prepared by the Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery/North American Chapter, International Society for Cardiovascular Surgery. J. Vasc. Surg. 1986, 4, 80–94. [Google Scholar] [CrossRef]
- Bobis, S.; Jarocha, D.; Majka, M. Mesenchymal stem cells: Characteristics and clinical applications. Folia Histochem. Et Cytobiol. 2006, 44, 215–230. [Google Scholar]
- Jayasinghe, M.; Prathiraja, O.; Perera, P.B.; Jena, R.; Silva, M.S.; Weerawarna, P.S.H.; Singhal, M.; Kayani, A.M.A.; Karnakoti, S.; Jain, S. The Role of Mesenchymal Stem Cells in the Treatment of Type 1 Diabetes. Cureus 2022, 14, e27337. [Google Scholar] [CrossRef] [PubMed]
- Negi, N.; Griffin, M.D. Effects of mesenchymal stromal cells on regulatory T cells: Current understanding and clinical relevance. Stem Cells 2020, 38, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. The Alchemist’s Nightmare: Might Mesenchymal Stem Cells That Are Recruited to Repair the Injured Heart Be Transformed Into Fibroblasts Rather Than Cardiomyocytes? Circulation 2018, 137, 2068–2073. [Google Scholar] [CrossRef]
- Kinnaird, T.; Stabile, E.; Burnett, M.S.; Lee, C.W.; Barr, S.; Fuchs, S.; Epstein, S.E. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ. Res. 2004, 94, 678–685. [Google Scholar] [CrossRef]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant. 2016, 25, 829–848. [Google Scholar] [CrossRef]
- Wei, X.; Yang, X.; Han, Z.P.; Qu, F.F.; Shao, L.; Shi, Y.F. Mesenchymal stem cells: A new trend for cell therapy. Acta Pharmacol. Sin. 2013, 34, 747–754. [Google Scholar] [CrossRef]
- Mildmay-White, A.; Khan, W. Cell Surface Markers on Adipose-Derived Stem Cells: A Systematic Review. Curr. Stem Cell Res. Ther. 2017, 12, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Vasanthan, J.; Gurusamy, N.; Rajasingh, S.; Sigamani, V.; Kirankumar, S.; Thomas, E.L.; Rajasingh, J. Role of Human Mesenchymal Stem Cells in Regenerative Therapy. Cells 2020, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Rautiainen, S.; Laaksonen, T.; Koivuniemi, R. Angiogenic Effects and Crosstalk of Adipose-Derived Mesenchymal Stem/Stromal Cells and Their Extracellular Vesicles with Endothelial Cells. Int. J. Mol. Sci. 2021, 22, 10890. [Google Scholar] [CrossRef] [PubMed]
- Geevarghese, A.; Herman, I.M. Pericyte-endothelial crosstalk: Implications and opportunities for advanced cellular therapies. Transl. Res. J. Lab. Clin. Med. 2014, 163, 296–306. [Google Scholar] [CrossRef]
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; van der Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997, 275, 964–967. [Google Scholar] [CrossRef]
- Tongers, J.; Roncalli, J.G.; Losordo, D.W. Role of endothelial progenitor cells during ischemia-induced vasculogenesis and collateral formation. Microvasc. Res. 2010, 79, 200–206. [Google Scholar] [CrossRef]
- Urbich, C.; Dimmeler, S. Endothelial progenitor cells functional characterization. Trends Cardiovasc. Med. 2004, 14, 318–322. [Google Scholar] [CrossRef]
- Hristov, M.; Erl, W.; Weber, P.C. Endothelial progenitor cells: Mobilization, differentiation, and homing. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1185–1189. [Google Scholar] [CrossRef]
- Cheng, M.; Guan, X.; Li, H.; Cui, X.; Zhang, X.; Li, X.; Jing, X.; Wu, H.; Avsar, E. Shear stress regulates late EPC differentiation via mechanosensitive molecule-mediated cytoskeletal rearrangement. PLoS ONE 2013, 8, e67675. [Google Scholar] [CrossRef]
- Saigawa, T.; Kato, K.; Ozawa, T.; Toba, K.; Makiyama, Y.; Minagawa, S.; Hashimoto, S.; Furukawa, T.; Nakamura, Y.; Hanawa, H.; et al. Clinical application of bone marrow implantation in patients with arteriosclerosis obliterans, and the association between efficacy and the number of implanted bone marrow cells. Circ. J. Off. J. Jpn. Circ. Soc. 2004, 68, 1189–1193. [Google Scholar] [CrossRef]
- Klepanec, A.; Mistrik, M.; Altaner, C.; Valachovicova, M.; Olejarova, I.; Slysko, R.; Balazs, T.; Urlandova, T.; Hladikova, D.; Liska, B.; et al. No difference in intra-arterial and intramuscular delivery of autologous bone marrow cells in patients with advanced critical limb ischemia. Cell Transplant. 2012, 21, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Walter, D.H.; Krankenberg, H.; Balzer, J.O.; Kalka, C.; Baumgartner, I.; Schlüter, M.; Tonn, T.; Seeger, F.; Dimmeler, S.; Lindhoff-Last, E.; et al. Intraarterial administration of bone marrow mononuclear cells in patients with critical limb ischemia: A randomized-start, placebo-controlled pilot trial (PROVASA). Circulation. Cardiovasc. Interv. 2011, 4, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Lara-Hernández, R. Análisis y Evaluación del Tratamiento de la Isquemia Crítica de Miembros Inferiores No Revascularizable Mediante Trasplante Autólogo de Progenitores Hematopoyéticos; Universitat de Valencia: Valencia, Spain, 2015. [Google Scholar]
- Soria-Juan, B.; Escacena, N.; Capilla-González, V.; Aguilera, Y.; Llanos, L.; Tejedo, J.R.; Bedoya, F.J.; Juan, V.; De la Cuesta, A.; Ruiz-Salmerón, R.; et al. Cost-Effective, Safe, and Personalized Cell Therapy for Critical Limb Ischemia in Type 2 Diabetes Mellitus. Front. Immunol. 2019, 10, 1151. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.S.; Alves, L.; Bogalho, I.; Cabral, J.M.S.; da Silva, C.L. Impact of Donor Age on the Osteogenic Supportive Capacity of Mesenchymal Stromal Cell-Derived Extracellular Matrix. Front. Cell Dev. Biol. 2021, 9, 747521. [Google Scholar] [CrossRef]
- Fafián-Labora, J.A.; Morente-López, M.; Arufe, M.C. Effect of aging on behaviour of mesenchymal stem cells. World J. Stem Cells 2019, 11, 337–346. [Google Scholar] [CrossRef]
- Ganguly, P.; El-Jawhari, J.J.; Giannoudis, P.V.; Burska, A.N.; Ponchel, F.; Jones, E.A. Age-related Changes in Bone Marrow Mesenchymal Stromal Cells: A Potential Impact on Osteoporosis and Osteoarthritis Development. Cell Transplant. 2017, 26, 1520–1529. [Google Scholar] [CrossRef]
- Campisi, J.; Kapahi, P.; Lithgow, G.J.; Melov, S.; Newman, J.C.; Verdin, E. From discoveries in ageing research to therapeutics for healthy ageing. Nature 2019, 571, 183–192. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Q.; Wang, Y.; Li, L.; Bu, H.; Bao, J. Senescence of mesenchymal stem cells (Review). Int. J. Mol. Med. 2017, 39, 775–782. [Google Scholar] [CrossRef]
- Barć, P.; Skóra, J.; Pupka, A.; Turkiewicz, D.; Dorobisz, A.T.; Garcarek, J.; Tomasiewicz, B.; Szyber, P. Bone-marrow cells in therapy of critical limb ischaemia of lower extremities—Own experience. Acta Angiol. 2006, 12, 155–166. [Google Scholar]
- Pignon, B.; Sevestre, M.A.; Kanagaratnam, L.; Pernod, G.; Stephan, D.; Emmerich, J.; Clement, C.; Sarlon, G.; Boulon, C.; Tournois, C.; et al. Autologous Bone Marrow Mononuclear Cell Implantation and Its Impact on the Outcome of Patients With Critical Limb Ischemia—Results of a Randomized, Double-Blind, Placebo-Controlled Trial. Circ. J. Off. J. Jpn. Circ. Soc. 2017, 81, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Nolan, D.; McDonnell, K.; Vahdat, L.; Benezra, R.; Altorki, N.; Mittal, V. Bone marrow-derived endothelial progenitor cells contribute to the angiogenic switch in tumor growth and metastatic progression. Biochim. Et Biophys. Acta 2009, 1796, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Wickersheim, A.; Kerber, M.; de Miguel, L.S.; Plate, K.H.; Machein, M.R. Endothelial progenitor cells do not contribute to tumor endothelium in primary and metastatic tumors. Int. J. Cancer 2009, 125, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Hashi, C.K.; Zhu, Y.; Yang, G.Y.; Young, W.L.; Hsiao, B.S.; Wang, K.; Chu, B.; Li, S. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc. Natl. Acad. Sci. USA 2007, 104, 11915–11920. [Google Scholar] [CrossRef] [PubMed]
- Soria, B.; Hmadcha, A.; Acosta, L.; Escacena, N. Method For Predicting Treatment Response And Test For Safe Use Of Mesenchymal Stem Cells On Inflammatory Diseases. U.S. Patent Application 14/909,190, 17 November 2015. [Google Scholar]
- Capilla-González, V.; López-Beas, J.; Escacena, N.; Aguilera, Y.; de la Cuesta, A.; Ruiz-Salmerón, R.; Martín, F.; Hmadcha, A.; Soria, B. PDGF Restores the Defective Phenotype of Adipose-Derived Mesenchymal Stromal Cells from Diabetic Patients. Mol. Ther. J. Am. Soc. Gene Ther. 2018, 26, 2696–2709. [Google Scholar] [CrossRef] [PubMed]
- Madaric, J.; Klepanec, A.; Valachovicova, M.; Mistrik, M.; Bucova, M.; Olejarova, I.; Necpal, R.; Madaricova, T.; Paulis, L.; Vulev, I. Characteristics of responders to autologous bone marrow cell therapy for no-option critical limb ischemia. Stem Cell Res. Ther. 2016, 7, 116. [Google Scholar] [CrossRef]
- Salybekov, A.A.; Wolfien, M.; Kobayashi, S.; Steinhoff, G.; Asahara, T. Personalized Cell Therapy for Patients with Peripheral Arterial Diseases in the Context of Genetic Alterations: Artificial Intelligence-Based Responder and Non-Responder Prediction. Cells 2021, 10, 3266. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, Y.; Li, N.; Wang, H.; Chen, B.; Liang, Z.; Ren, R.; Lu, D.; Boey, J.; Armstrong, D.G.; et al. Efficacy and long-term longitudinal follow-up of bone marrow mesenchymal cell transplantation therapy in a diabetic patient with recurrent lower limb bullosis diabeticorum. Stem Cell Res. Ther. 2018, 9, 99. [Google Scholar] [CrossRef]
- Iwase, T.; Nagaya, N.; Fujii, T.; Itoh, T.; Murakami, S.; Matsumoto, T.; Kangawa, K.; Kitamura, S. Comparison of angiogenic potency between mesenchymal stem cells and mononuclear cells in a rat model of hindlimb ischemia. Cardiovasc Res 2005, 66, 543–551. [Google Scholar] [CrossRef]
- Lu, D.; Chen, B.; Liang, Z.; Deng, W.; Jiang, Y.; Li, S.; Xu, J.; Wu, Q.; Zhang, Z.; Xie, B.; et al. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: A double-blind, randomized, controlled trial. Diabetes Res. Clin. Pract. 2011, 92, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Jiang, Y.; Deng, W.; Zhang, Y.; Liang, Z.; Wu, Q.; Jiang, X.; Zhang, L.; Gao, F.; Cao, Y.; et al. Long-Term Outcomes of BMMSC Compared with BMMNC for Treatment of Critical Limb Ischemia and Foot Ulcer in Patients with Diabetes. Cell Transplant. 2019, 28, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.; Hughes, C.M.; Lagan, K.M.; Bell, P.M.; Stevenson, M.R. An evaluation of three wound measurement techniques in diabetic foot wounds. Diabetes Care 2007, 30, 2641–2642. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Lavery, L.A.; Harkless, L.B. Treatment-based classification system for assessment and care of diabetic feet. J. Am. Podiatr. Med. Assoc. 1996, 86, 311–316. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Lavery, L.A.; Harkless, L.B. Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care 1998, 21, 855–859. [Google Scholar] [CrossRef]
- Caplan, A.I.; Correa, D. The MSC: An injury drugstore. Cell Stem Cell 2011, 9, 11–15. [Google Scholar] [CrossRef] [PubMed]








| 2021 | 2030 | 2045 | Increase (%) | |
|---|---|---|---|---|
| Global | 537 | 643 | 783 | 46 |
| Europe | 61 | 67 | 69 | 13 |
| North-America & Caribbean | 51 | 57 | 63 | 24 |
| Western Pacific | 206 | 238 | 260 | 27 |
| South & Central America | 32 | 40 | 49 | 50 |
| Africa | 24 | 33 | 55 | 134 |
| Middle East & North Africa | 73 | 95 | 136 | 87 |
| South East Asia | 90 | 113 | 152 | 68 |
| Country | Cost (in USD Billion) |
|---|---|
| United States of America | 379.5 |
| China | 165.3 |
| Brazil | 42.9 |
| Germany | 41.3 |
| Japan | 35.6 |
| United Kingdom | 23.4 |
| France | 22.7 |
| Mexico | 19.9 |
| Spain | 15.5 |
| Italy | 14.7 |
| Bone Marrow (%) | Cord Blood (%) | Peripheral Blood (%) | |
|---|---|---|---|
| Mononuclear Cells | 24.6 to 87.4 of white blood cells | ND | ND |
| Monocytes | 6.3 | 6.5 | |
| Stem Cells CD34+ (Hematopoietic Stem Cells-HSC) | 1.8 to 11.5 of mononuclear cells | 0.3 of mononuclear cells | |
| -Early non-committed HSC (CD38−) | 8.5 to 50.4% of CD34+ cells | ND | ND |
| % of Mononuclear Cells | |||
| Monocytes (CD45+CD14+) | 6.3 | 14.1 | 16.4 |
| Lymphocytes | 23.1 to 47.5 | 38.3 | 33.2 |
| 15.7 | 11.7 | 8.2 |
| 51.6 | 46.8 | 62.8 |
| 27.4 | 32.2 | 37 |
| 19 | 19.6 | 24.1 |
| 2.5 | 0.8 | 3.6 |
| 6.3 | 18.2 | 0.86 |
| Dendritic cells | 1 | 0.88 | 1.4 |
| VEGFR2 expressing cells | 0.5 to 20.3 of White blood cells | ND | ND |
| CXCR4 (Proangiogenic) | 0.4 to 9.7 | ND | ND |
| Rutherford Classification | ||
|---|---|---|
| Grade | Category | Symptoms |
| Asymptomatic, hemodynamically unstable | ||
| I | 1 2 3 | Mild Claudication Moderate Claudication Severe Claudication |
| II | 4 5 | Ischemic pain at rest Ulcers, gangrene |
| III | 6 | Loss of tissue No function Amputation need |
| Baseline Cases (%) | 3 Months Cases (%) | 12 Months Cases (%) | |||
|---|---|---|---|---|---|
| Rutherford- Becker | Categories | Cat 0 | 0 | 4 (25%) | |
| Cat 1 | 5 (26.4%) | 9 (56.25%) | |||
| Cat 2 | 12 (63.1%) | 3 (18.75%) | |||
| Cat 3 | |||||
| Cat 4 | 3 (15%) | ||||
| Cat 5 | 11 (55%) | 2 (10.5%) | |||
| Cat 6 | 6 (30%) | ||||
| ULCERS University of Texas | Stage A | No Ulcer | 1 (5%) | - | - |
| A0 | 3 (15%) | 15 (79%) | 14 (87.5%) | ||
| A1 | 2 (10.5%) | 1(6.25%) | |||
| A2 | 1 (5%) | 1 (5.3%) | |||
| A3 | |||||
| Stage C | C0 | 3 (15%) | |||
| C1 | 3 (15%) | ||||
| C2 | 3 (15%) | ||||
| C3 | 6 (30%) | ||||
| RB Grade | Basal Cases (%) | 1 Month Cases (%) | 3 Months Cases (%) | 6 Months Cases (%) | 9 Months Cases (%) | 12 Months Cases (%) | |
|---|---|---|---|---|---|---|---|
| Control | 0 | 1 (10%) | 1 (10%) | ||||
| I | 1 (10%) | 2 (20%) | 2 (20%) | 2 (20%) | |||
| II | 8 (80%) | 7 (70%) | 7 (70%) | 5 (50%) | 2 (20%) | 6 (60%) | |
| III | 2 (20%) | 3 (30%) | - | 1 (10%) | 5 (50%) | 1 (10%) | |
| Total | 10 | 10 | 8 | 8 | 10 | 10 | |
| Exp. Group 1 | 0 | 3 (30%) | 3 (30%) | 4 (40%) | |||
| I | 4 (40%) | 4 (40%) | 1 (10%) | 1 (10%) | 1 (10%) | ||
| II | 5 (50%) | 1 (10%) | 2 (20%) | 2 (20%) | 2 (20%) | 2 (20%) | |
| III | 5 (50%) | 5 (50%) | 4 (40%) | 4 (40%) | 4 (40%) | 3 (30%) | |
| Total | 10 | 10 | 10 | 10 | 10 | 10 | |
| Exp. Group 2 | 0 | 1 (10%) | 5 (50%) | 4 (40%) | 6 (60%) | 6 (60%) | |
| I | 3 (30%) | 3 (30%) | 3 (30%) | 2 (20%) | 3 (30%) | ||
| II | 6 (60%) | 4 (40%) | - | - | - | - | |
| III | 4 (40%) | 1 (10%) | 1 (10%) | 2 (20%) | 2 (20%) | 1 (10%) | |
| Total | 10 | 9 | 9 | 9 | 10 | 10 |
| Control | Exp. Group 1 | Exp. Group 2 | |
|---|---|---|---|
| 6 Months | Patients (%) | ||
| Patients with Amputation | 10% (1) | 20% (2) | 10% (1) |
| Patients with major amputation | 0% | 0% | 0% |
| Total Amputations | 2 | 3 | 4 |
| 12 Months | Patients (%) | ||
| Patients with Amputation | 10% (1) | 10% (1) | 10% (1) |
| Patients with major amputation | 0% | 0% | 0% |
| Total Amputations | 2 | 1 | 1 |
| 1 Year | 2 Years | 5 Years | 10 Years | 20 Years | |
|---|---|---|---|---|---|
| SoC | 81.6 | 92.1 | 100 | 100 | 100 |
| SoC + CD34+ | 23.2 | 36.4 | 41.9 | NA | NA |
| SoC + BM-MSC | 8 | 10 | 25 | 26 | 40 |
| Source | HLA Matching | GMP Manufacture | Off-the-Self Pharmaceutical Product |
|---|---|---|---|
| Autologous | High | Expensive | No |
| Allogeneic | Low | Affordable | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soria, B.; Escacena, N.; Gonzaga, A.; Soria-Juan, B.; Andreu, E.; Hmadcha, A.; Gutierrez-Vilchez, A.M.; Cahuana, G.; Tejedo, J.R.; De la Cuesta, A.; et al. Cell Therapy of Vascular and Neuropathic Complications of Diabetes: Can We Avoid Limb Amputation? Int. J. Mol. Sci. 2023, 24, 17512. https://doi.org/10.3390/ijms242417512
Soria B, Escacena N, Gonzaga A, Soria-Juan B, Andreu E, Hmadcha A, Gutierrez-Vilchez AM, Cahuana G, Tejedo JR, De la Cuesta A, et al. Cell Therapy of Vascular and Neuropathic Complications of Diabetes: Can We Avoid Limb Amputation? International Journal of Molecular Sciences. 2023; 24(24):17512. https://doi.org/10.3390/ijms242417512
Chicago/Turabian StyleSoria, Bernat, Natalia Escacena, Aitor Gonzaga, Barbara Soria-Juan, Etelvina Andreu, Abdelkrim Hmadcha, Ana Maria Gutierrez-Vilchez, Gladys Cahuana, Juan R. Tejedo, Antonio De la Cuesta, and et al. 2023. "Cell Therapy of Vascular and Neuropathic Complications of Diabetes: Can We Avoid Limb Amputation?" International Journal of Molecular Sciences 24, no. 24: 17512. https://doi.org/10.3390/ijms242417512
APA StyleSoria, B., Escacena, N., Gonzaga, A., Soria-Juan, B., Andreu, E., Hmadcha, A., Gutierrez-Vilchez, A. M., Cahuana, G., Tejedo, J. R., De la Cuesta, A., Miralles, M., García-Gómez, S., & Hernández-Blasco, L. (2023). Cell Therapy of Vascular and Neuropathic Complications of Diabetes: Can We Avoid Limb Amputation? International Journal of Molecular Sciences, 24(24), 17512. https://doi.org/10.3390/ijms242417512









