Macromolecular Crowding and DNA: Bridging the Gap between In Vitro and In Vivo
Abstract
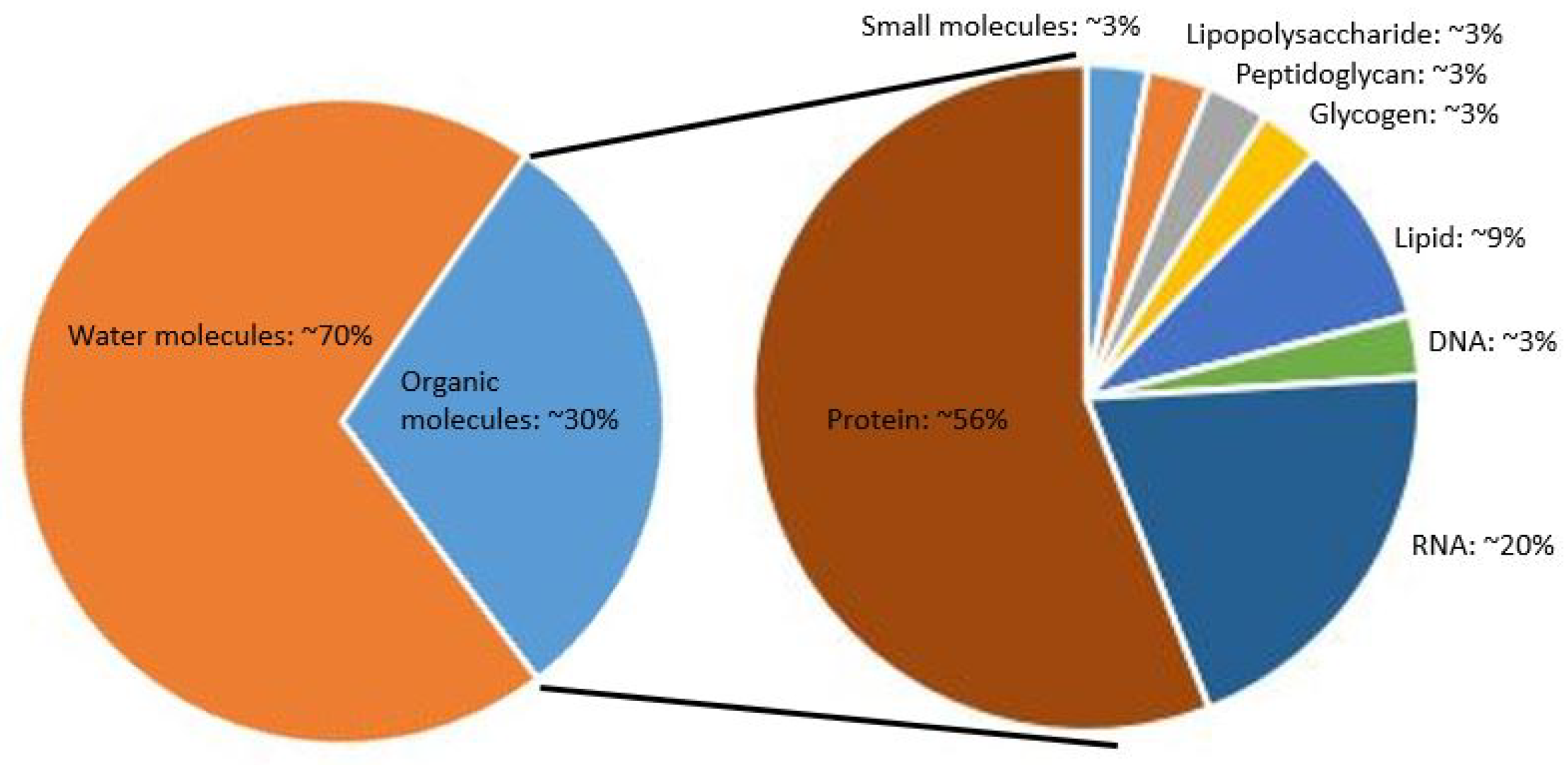
1. Introduction
2. Compaction and Extension of DNA
2.1. Polyethylene Glycol
2.2. Dextran
2.3. Bovine Serum Albumin
2.4. Outlook
3. Kinetics
3.1. Crowders vs. Viscogens
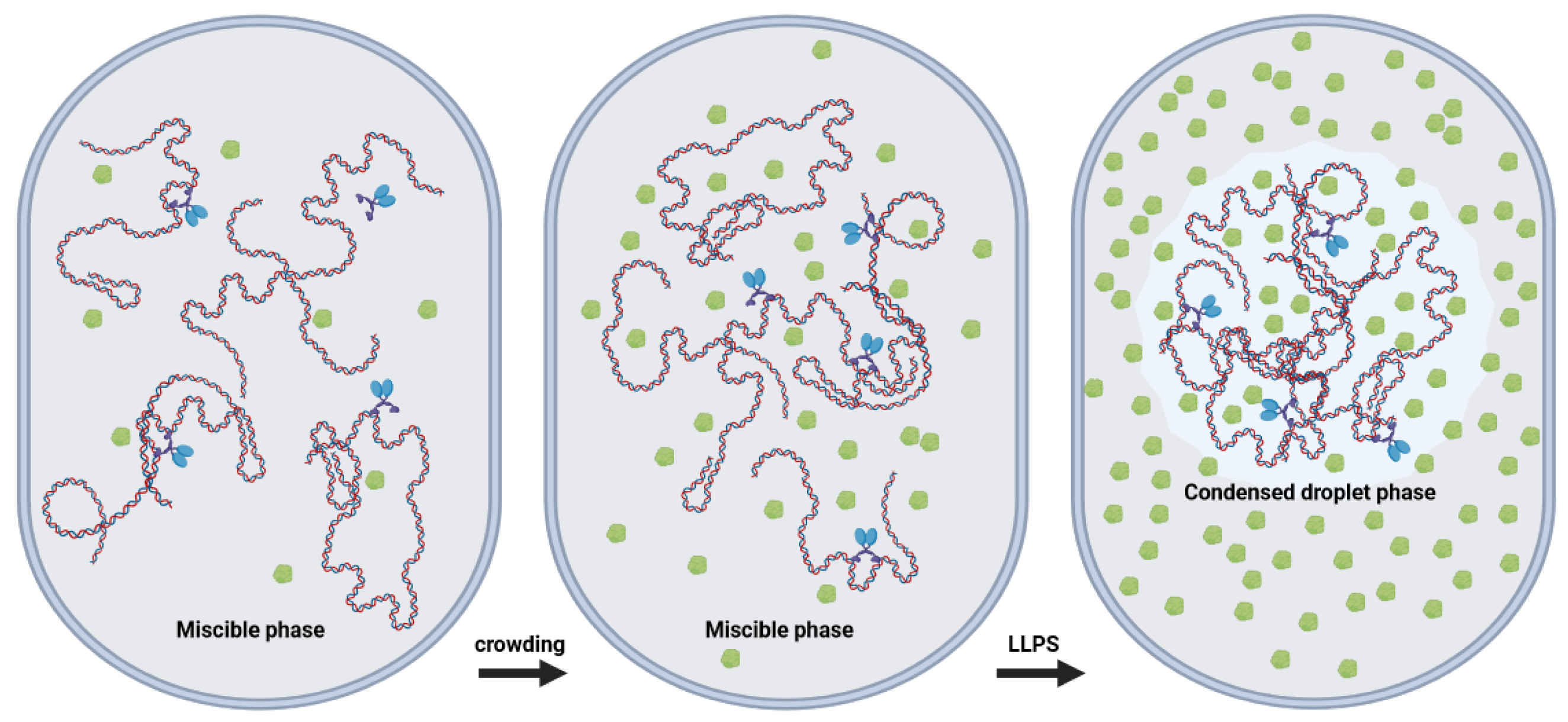
3.2. Phase Separation
4. Protein–DNA Interactions
5. Theoretical Models
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kohata, K.; Miyoshi, D. RNA phase separation–mediated direction of molecular trafficking under conditions of molecular crowding. Biophys. Rev. 2020, 12, 669–676. [Google Scholar] [CrossRef]
- Mardoum, W.M.; Gorczyca, S.M.; Regan, K.E.; Wu, T.C.; Robertson-Anderson, R.M. Crowding Induces Entropically-Driven Changes to DNA Dynamics That Depend on Crowder Structure and Ionic Conditions. Front. Phys. 2018, 6, 53. [Google Scholar] [CrossRef]
- Scott, S.; Shaheen, C.; McGuinness, B.; Metera, K.; Kouzine, F.; Levens, D.; Benham, C.J.; Leslie, S. Single-molecule visualization of the effects of ionic strength and crowding on structure-mediated interactions in supercoiled DNA molecules. Nucleic Acids Res. 2019, 47, 6360–6368. [Google Scholar] [CrossRef]
- Ellis, R.J.; Minton, A.P. Join the crowd. Nature 2003, 425, 27–28. [Google Scholar] [CrossRef]
- Nakano, S.I.; Miyoshi, D.; Sugimoto, N. Effects of molecular crowding on the structures, interactions, and functions of nucleic acids. Chem. Rev. 2014, 114, 2733–2758. [Google Scholar] [CrossRef]
- Miyoshi, D.; Sugimoto, N. Molecular crowding effects on structure and stability of DNA. Biochimie 2008, 90, 1040–1051. [Google Scholar] [CrossRef]
- Zimmerman, S.B.; Trach, S.O. Estimation of macromolecule concentrations and excluded volume effects for the cytoplasm of Escherichia coli. J. Mol. Biol. 1991, 222, 599–620. [Google Scholar] [CrossRef]
- Bohrmann, B.; Haider, M.; Kellenberger, E. Concentration evaluation of chromatin in unstained resin-embedded sections by means of low-dose ratio-contrast imaging in STEM. Ultramicroscopy 1993, 49, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Brangwynne, C.P. Liquid phase condensation in cell physiology and disease. Science 2017, 357, eaaf4382. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, I.M.; Zaslavsky, B.Y.; Breydo, L.; Turoverov, K.K.; Uversky, V.N. Beyond the excluded volume effects: Mechanistic complexity of the crowded milieu. Molecules 2015, 20, 1377–1409. [Google Scholar] [CrossRef] [PubMed]
- Berezhkovskii, A.M.; Szabo, A. Theory of crowding effects on bimolecular reaction rates. J. Phys. Chem. B 2016, 120, 5998–6002. [Google Scholar] [CrossRef]
- Liebherr, R.B.; Hutterer, A.; Mickert, M.J.; Vogl, F.C.; Beutner, A.; Lechner, A.; Hummel, H.; Gorris, H.H. Three-in-one enzyme assay based on single molecule detection in femtoliter arrays. Anal. Bioanal. Chem. 2015, 407, 7443–7452. [Google Scholar] [CrossRef]
- Sozański, K.; Ruhnow, F.; Wiśniewska, A.; Tabaka, M.; Diez, S.; Hołyst, R. Small Crowders Slow Down Kinesin-1 Stepping by Hindering Motor Domain Diffusion. Phys. Rev. Lett. 2015, 115, 218102. [Google Scholar] [CrossRef]
- Walter, H.; Brooks, D.E. Phase separation in cytoplasm, due to macromolecular crowding, is the basis for microcompartmentation. FEBS Lett. 1995, 361, 135–139. [Google Scholar] [CrossRef]
- Levone, B.R.; Lenzken, S.C.; Antonaci, M.; Maiser, A.; Rapp, A.; Conte, F.; Reber, S.; Mechtersheimer, J.; Ronchi, A.E.; Mühlemann, O.; et al. FUS-dependent liquid–liquid phase separation is important for DNA repair initiation. J. Cell Biol. 2021, 220, e202008030. [Google Scholar] [CrossRef] [PubMed]
- Poon, J.; Bailey, M.; Winzor, D.J.; Davidson, B.E.; Sawyer, W.H. Effects of molecular crowding on the interaction between DNA and the Escherichia coli regulatory protein TyrR. Biophys. J. 1997, 73, 3257–3264. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheng, C.; Jia, J.L.; Ran, S.Y. Polyethylene glycol and divalent salt-induced DNA reentrant condensation revealed by single molecule measurements. Soft Matter 2015, 11, 3927–3935. [Google Scholar] [CrossRef]
- Chapman, C.D.; Gorczyca, S.; Robertson-Anderson, R.M. Crowding induces complex ergodic diffusion and dynamic elongation of large DNA molecules. Biophys. J. 2015, 108, 1220–1228. [Google Scholar] [CrossRef]
- Gorczyca, S.M.; Chapman, C.D.; Robertson-Anderson, R.M. Universal scaling of crowding-induced DNA mobility is coupled with topology-dependent molecular compaction and elongation. Soft Matter 2015, 11, 7762–7768. [Google Scholar] [CrossRef]
- Krotova, M.; Vasilevskaya, V.; Makita, N.; Yoshikawa, K.; Khokhlov, A. DNA compaction in a crowded environment with negatively charged proteins. Phys. Rev. Lett. 2010, 105, 128302. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Hirota, S.; Makita, N.; Yoshikawa, Y. Compaction of DNA induced by like-charge protein: Opposite salt-effect against the polymer-salt-induced condensation with neutral polymer. J. Phys. Chem. Lett. 2010, 1, 1763–1766. [Google Scholar] [CrossRef]
- Stiehl, O.; Weidner-Hertrampf, K.; Weiss, M. Kinetics of conformational fluctuations in DNA hairpin-loops in crowded fluids. New J. Phys. 2013, 15, 113010. [Google Scholar] [CrossRef]
- Stiehl, O.; Weidner-Hertrampf, K.; Weiss, M. Corrigendum: Kinetics of conformational fluctuations in DNA hairpin-loops in crowded fluids (2013 New J. Phys. 15 113010). New J. Phys. 2016, 18, 099501. [Google Scholar] [CrossRef]
- Gupta, A.N.; van der Maarel, J.R. Compaction of plasmid DNA by macromolecular crowding. Macromolecules 2017, 50, 1666–1671. [Google Scholar] [CrossRef]
- Khatun, S.; Singh, A.; Shikha, K.; Ganguly, A.; Gupta, A.N. Plasmid DNA Undergoes Two Compaction Regimes under Macromolecular Crowding. ACS Macro Lett. 2022, 11, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ng, S.Y.; Gupta, A.N.; Feng, Y.P.; Ho, B.; Lapp, A.; Egelhaaf, S.U.; Forsyth, V.T.; Haertlein, M.; Moulin, M.; et al. Effect of crowding on the conformation of interwound DNA strands from neutron scattering measurements and Monte Carlo simulations. Phys. Rev. E 2010, 81, 061905. [Google Scholar] [CrossRef] [PubMed]
- Koga, S.; Williams, D.S.; Perriman, A.W.; Mann, S. Peptide–nucleotide microdroplets as a step towards a membrane-free protocell model. Nat. Chem. 2011, 3, 720–724. [Google Scholar] [CrossRef]
- Hristov, P.; Mitkov, I.; Sirakova, D.; Mehandgiiski, I.; Radoslavov, G. Measurement of casein micelle size in raw dairy cattle milk by dynamic light scattering. In Milk Proteins–From Structure to Biological Properties and Health Aspects; InTech: Rijeka, Croatia, 2016. [Google Scholar]
- Arold, S.T.; Leonard, P.G.; Parkinson, G.N.; Ladbury, J.E. H-NS forms a superhelical protein scaffold for DNA condensation. Proc. Natl. Acad. Sci. USA 2010, 107, 15728–15732. [Google Scholar] [CrossRef]
- Smith, A.E.; Zhou, L.Z.; Gorensek, A.H.; Senske, M.; Pielak, G.J. In-cell thermodynamics and a new role for protein surfaces. Proc. Natl. Acad. Sci. USA 2016, 113, 1725–1730. [Google Scholar] [CrossRef]
- Park, S.; Barnes, R.; Lin, Y.; Jeon, B.j.; Najafi, S.; Delaney, K.T.; Fredrickson, G.H.; Shea, J.E.; Hwang, D.S.; Han, S. Dehydration entropy drives liquid-liquid phase separation by molecular crowding. Commun. Chem. 2020, 3, 83. [Google Scholar] [CrossRef]
- Köhn, B.; Schwarz, P.; Wittung-Stafshede, P.; Kovermann, M. Impact of crowded environments on binding between protein and single-stranded DNA. Sci. Rep. 2021, 11, 17682. [Google Scholar] [CrossRef] [PubMed]
- Rickgauer, J.P.; Grigorieff, N.; Denk, W. Single-protein detection in crowded molecular environments in cryo-EM images. eLife 2017, 6, e25648. [Google Scholar] [CrossRef] [PubMed]
- Rivas, G.; Fernández, J.A.; Minton, A.P. Direct observation of the enhancement of noncooperative protein self-assembly by macromolecular crowding: Indefinite linear self-association of bacterial cell division protein FtsZ. Proc. Natl. Acad. Sci. USA 2001, 98, 3150–3155. [Google Scholar] [CrossRef]
- Rivas, G.; Stafford, W.; Minton, A.P. Characterization of Heterologous Protein–Protein Interactions Using Analytical Ultracentrifugation. Methods 1999, 19, 194–212. [Google Scholar] [CrossRef][Green Version]
- Wallace, M.I.; Ying, L.; Balasubramanian, S.; Klenerman, D. FRET fluctuation spectroscopy: Exploring the conformational dynamics of a DNA hairpin loop. J. Phys. Chem. B 2000, 104, 11551–11555. [Google Scholar] [CrossRef]
- Chung, S.; Lerner, E.; Jin, Y.; Kim, S.; Alhadid, Y.; Grimaud, L.W.; Zhang, I.X.; Knobler, C.M.; Gelbart, W.M.; Weiss, S. The effect of macromolecular crowding on single-round transcription by Escherichia coli RNA polymerase. Nucleic Acids Res. 2019, 47, 1440–1450. [Google Scholar] [CrossRef]
- Louie, D.; Serwer, P. Quantification of the effect of excluded volume on double-stranded DNA. J. Mol. Biol. 1994, 242, 547–558. [Google Scholar] [CrossRef]
- Murphy, L.D.; Zimmerman, S.B. Condensation and cohesion of λ DNA in cell extracts and other media: Implications for the structure and function of DNA in prokaryotes. Biophys. Chem. 1995, 57, 71–92. [Google Scholar] [CrossRef]
- Ernst, D.; Hellmann, M.; Köhler, J.; Weiss, M. Fractional Brownian motion in crowded fluids. Soft Matter 2012, 8, 4886–4889. [Google Scholar] [CrossRef]
- Lin, S.N.; Wuite, G.J.; Dame, R.T. Effect of different crowding agents on the architectural properties of the bacterial nucleoid-associated protein HU. Int. J. Mol. Sci. 2020, 21, 9553. [Google Scholar] [CrossRef]
- Cristofalo, M.; Marrano, C.; Salerno, D.; Corti, R.; Cassina, V.; Mammola, A.; Gherardi, M.; Sclavi, B.; Lagomarsino, M.C.; Mantegazza, F. Cooperative effects on the compaction of DNA fragments by the nucleoid protein H-NS and the crowding agent PEG probed by Magnetic Tweezers. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2020, 1864, 129725. [Google Scholar] [CrossRef]
- Leicher, R.; Osunsade, A.; Chua, G.N.L.; Faulkner, S.C.; Latham, A.P.; Watters, J.W.; Nguyen, T.; Beckwitt, E.C.; Christodoulou-Rubalcava, S.; Young, P.G.; et al. Single-stranded nucleic acid binding and coacervation by linker histone H1. Nat. Struct. Mol. Biol. 2022, 29, 463–471. [Google Scholar] [CrossRef]
- Wiggins, P.A.; Dame, R.T.; Noom, M.C.; Wuite, G.J. Protein-mediated molecular bridging: A key mechanism in biopolymer organization. Biophys. J. 2009, 97, 1997–2003. [Google Scholar] [CrossRef]
- Pastré, D.; Hamon, L.; Mechulam, A.; Sorel, I.; Baconnais, S.; Curmi, P.A.; Le Cam, E.; Piétrement, O. Atomic force microscopy imaging of DNA under macromolecular crowding conditions. Biomacromolecules 2007, 8, 3712–3717. [Google Scholar] [CrossRef]
- Goobes, R.; Kahana, N.; Cohen, O.; Minsky, A. Metabolic buffering exerted by macromolecular crowding on DNA–DNA interactions: Origin and physiological significance. Biochemistry 2003, 42, 2431–2440. [Google Scholar] [CrossRef]
- Ramos, J.É.B.; de Vries, R.; Ruggiero Neto, J. DNA ψ-condensation and reentrant decondensation: Effect of the PEG degree of polymerization. J. Phys. Chem. B 2005, 109, 23661–23665. [Google Scholar] [CrossRef]
- Keller, D.; Bustamante, C. Theory of the interaction of light with large inhomogeneous molecular aggregates. II. Psi-type circular dichroism. J. Chem. Phys. 1986, 84, 2972–2980. [Google Scholar] [CrossRef]
- Ojala, H.; Ziedaite, G.; Wallin, A.E.; Bamford, D.H.; Hæggström, E. Optical tweezers reveal force plateau and internal friction in PEG-induced DNA condensation. Eur. Biophys. J. 2014, 43, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Muller, S.J. Polymer-monovalent salt-induced DNA compaction studied via single-molecule microfluidic trapping. Lab Chip 2012, 12, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Ichikawa, M.; Ishido, T.; Ishikawa, M.; Baba, Y.; Yoshikawa, K. How environmental solution conditions determine the compaction velocity of single DNA molecules. Nucleic Acids Res. 2012, 40, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Froehlich, E.; Mandeville, J.; Arnold, D.; Kreplak, L.; Tajmir-Riahi, H. PEG and mPEG–anthracene induce DNA condensation and particle formation. J. Phys. Chem. B 2011, 115, 9873–9879. [Google Scholar] [CrossRef]
- Kawakita, H.; Uneyama, T.; Kojima, M.; Morishima, K.; Masubuchi, Y.; Watanabe, H. Formation of globules and aggregates of DNA chains in DNA/polyethylene glycol/monovalent salt aqueous solutions. J. Chem. Phys. 2009, 131, 094901. [Google Scholar] [CrossRef]
- Saito, T.; Iwaki, T.; Yoshikawa, K. DNA compaction induced by neutral polymer is retarded more effectively by divalent anion than monovalent anion. Chem. Phys. Lett. 2008, 465, 40–44. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Yoshikawa, Y.; Koyama, Y.; Kanbe, T. Highly effective compaction of long duplex DNA induced by polyethylene glycol with pendant amino groups. J. Am. Chem. Soc. 1997, 119, 6473–6477. [Google Scholar] [CrossRef]
- Vasilevskaya, V.; Khokhlov, A.; Matsuzawa, Y.; Yoshikawa, K. Collapse of single DNA molecule in poly (ethylene glycol) solutions. J. Chem. Phys. 1995, 102, 6595–6602. [Google Scholar] [CrossRef]
- Frisch, H.; Fesciyan, S. DNA phase transitions: The ψ transition of single coils. J. Polym. Sci. Polym. Lett. Ed. 1979, 17, 309–315. [Google Scholar] [CrossRef]
- Maniatis, T.; Venable, J.H., Jr.; Lerman, L.S. The structure of Ψ DNA. J. Mol. Biol. 1974, 84, 37–64. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, K.; Matsuzawa, Y.; Yoshikawa, K.; Khokhlov, A.; Doi, M. Direct observation of the coil-globule transition in dna molecules. Biopolym. Orig. Res. Biomol. 1994, 34, 555–558. [Google Scholar] [CrossRef]
- Pelta, J.; Livolant, F.; Sikorav, J.L. DNA Aggregation Induced by Polyamines and Cobalthexamine (*). J. Biol. Chem. 1996, 271, 5656–5662. [Google Scholar] [CrossRef]
- Woolley, P.; Wills, P.R. Excluded-volume effect of inert macromolecules on the melting of nucleic acids. Biophys. Chem. 1985, 22, 89–94. [Google Scholar] [CrossRef]
- Spink, C.; Chaires, J. Selective stabilization of triplex DNA by poly (ethylene glycols). J. Am. Chem. Soc. 1995, 117, 12887–12888. [Google Scholar] [CrossRef]
- Spink, C.H.; Chaires, J.B. Effects of hydration, ion release, and excluded volume on the melting of triplex and duplex DNA. Biochemistry 1999, 38, 496–508. [Google Scholar] [CrossRef]
- Scott, S.; Xu, Z.M.; Kouzine, F.; Berard, D.J.; Shaheen, C.; Gravel, B.; Saunders, L.; Hofkirchner, A.; Leroux, C.; Laurin, J.; et al. Visualizing structure-mediated interactions in supercoiled DNA molecules. Nucleic Acids Res. 2018, 46, 4622–4631. [Google Scholar] [CrossRef]
- Sokolova, E.; Spruijt, E.; Hansen, M.M.; Dubuc, E.; Groen, J.; Chokkalingam, V.; Piruska, A.; Heus, H.A.; Huck, W.T. Enhanced transcription rates in membrane-free protocells formed by coacervation of cell lysate. Proc. Natl. Acad. Sci. USA 2013, 110, 11692–11697. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Size Limits of Very Small Microorganisms: Proceedings of a Workshop; National Academies Press: Washington, DC, USA, 1999. [Google Scholar]
- Baltierra-Jasso, L.E.; Morten, M.J.; Linda Laflör Quinn, S.D.; Magennis, S.W. Crowding-Induced Hybridization of Single DNA Hairpins. J. Am. Chem. Soc. 2015, 137, 16020–16023. [Google Scholar] [CrossRef] [PubMed]
- Teif, V.B.; Bohinc, K. Condensed DNA: Condensing the concepts. Prog. Biophys. Mol. Biol. 2011, 105, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Finzi, L.; Olson, W.K. The emerging role of DNA supercoiling as a dynamic player in genomic structure and function. Biophys. Rev. 2016, 8, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Dorman, C.J. H-NS: A universal regulator for a dynamic genome. Nat. Rev. Microbiol. 2004, 2, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Lucchini, S.; Rowley, G.; Goldberg, M.D.; Hurd, D.; Harrison, M.; Hinton, J.C.D. H-NS mediates the silencing of laterally acquired genes in bacteria. PLoS Pathog. 2006, 2, e81. [Google Scholar] [CrossRef] [PubMed]
- Dillon, S.C.; Dorman, C.J. Bacterial nucleoid-associated proteins, nucleoid structure and gene expression. Nat. Rev. Microbiol. 2010, 8, 185–195. [Google Scholar] [CrossRef]
- Laurens, N.; Driessen, R.P.; Heller, I.; Vorselen, D.; Noom, M.C.; Hol, F.J.; White, M.F.; Dame, R.T.; Wuite, G.J. Alba shapes the archaeal genome using a delicate balance of bridging and stiffening the DNA. Nat. Commun. 2012, 3, 1328. [Google Scholar] [CrossRef] [PubMed]
- Driessen, R.P.; Lin, S.N.; Waterreus, W.J.; van der Meulen, A.L.; van der Valk, R.A.; Laurens, N.; Moolenaar, G.F.; Pannu, N.S.; Wuite, G.J.; Goosen, N.; et al. Diverse architectural properties of Sso10a proteins: Evidence for a role in chromatin compaction and organization. Sci. Rep. 2016, 6, 29422. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.B.; Wang, X.L.; Zhang, X.H.; Ran, S.Y.; Yan, J.; Li, M. Compaction dynamics of single (DNA) molecules under tension. J. Am. Chem. Soc. 2006, 128, 15040–15041. [Google Scholar] [CrossRef]
- Widom, J.; Baldwin, R. Monomolecular condensation of lambda-DNA induced by cobalt hexammine. Biopolymers 1983, 22, 1595–1620. [Google Scholar] [CrossRef] [PubMed]
- Arscott, P.G.; Ma, C.; Wenner, J.R.; Bloomfield, V.A. DNA condensation by cobalt hexaammine III in alcohol-water mixtures: Dielectric constant and other solvent effects. Biopolymers 1995, 36, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.D. Cationic liposomes for gene therapy. Angew. Chem. Int. Ed. 1998, 37, 1768–1785. [Google Scholar] [CrossRef]
- Krishnamoorthy, G.; Duportail, G.; Mély, Y. Structure and dynamics of condensed DNA probed by 1,1′-(4,4,8,8-tetramethyl-4,8-diazaundecamethylene) bis [4-[[3-methylbenz-1,3-oxazol-2-yl] methylidine]-1,4-dihydroquinolinium] tetraiodide fluorescence. Biochemistry 2002, 41, 15277–15287. [Google Scholar] [CrossRef]
- Krishnamoorthy, G.; Roques, B.; Darlix, J.L.; Mély, Y. DNA condensation by the nucleocapsid protein of HIV-1: A mechanism ensuring DNA protection. Nucleic Acids Res. 2003, 31, 5425–5432. [Google Scholar] [CrossRef]
- Kombrabail, M.H.; Krishnamoorthy, G. Fluorescence dynamics of DNA condensed by the molecular crowding agent poly (ethylene glycol). J. Fluoresc. 2005, 15, 741–747. [Google Scholar] [CrossRef]
- Lerman, L. A transition to a compact form of DNA in polymer solutions. Proc. Natl. Acad. Sci. USA 1971, 68, 1886–1890. [Google Scholar] [CrossRef]
- Obermeier, B.; Wurm, F.; Mangold, C.; Frey, H. Multifunctional Poly (ethylene glycol) s. Angew. Chem. Int. Ed. 2011, 50, 7988–7997. [Google Scholar] [CrossRef] [PubMed]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Lasic, D.D. Liposomes in Gene Delivery; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Bloomfield, V.A. DNA condensation. Curr. Opin. Struct. Biol. 1996, 6, 334–341. [Google Scholar] [CrossRef] [PubMed]
- de Vries, R. DNA condensation in bacteria: Interplay between macromolecular crowding and nucleoid proteins. Biochimie 2010, 92, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Schlick, T.; Li, B.; Olson, W.K. The influence of salt on the structure and energetics of supercoiled DNA. Biophys. J. 1994, 67, 2146–2166. [Google Scholar] [CrossRef]
- Rybenkov, V.V.; Vologodskii, A.V.; Cozzarelli, N.R. The effect of ionic conditions on DNA helical repeat, effective diameter and free energy of supercoiling. Nucleic Acids Res. 1997, 25, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Halperin, A.; Zhulina, E. On the deformation behaviour of collapsed polymers. EPL Europhys. Lett. 1991, 15, 417. [Google Scholar] [CrossRef]
- Craig, A.; Terentjev, E. Stretching globular polymers. I. Single chains. J. Chem. Phys. 2005, 122, 194901. [Google Scholar] [CrossRef]
- Polotsky, A.A.; Smolyakova, E.E.; Birshtein, T.M. Theory of mechanical unfolding of homopolymer globule: All-or-none transition in force-clamp mode vs phase coexistence in position-clamp mode. Macromolecules 2011, 44, 8270–8283. [Google Scholar] [CrossRef][Green Version]
- Neely, W.B. Dextran: Structure and synthesis. In Advances in Carbohydrate Chemistry; Elsevier: Amsterdam, The Netherlands, 1961; Volume 15, pp. 341–369. [Google Scholar]
- Sidebotham, R.L. Dextrans. Adv. Carbohydr. Chem. Biochem. 1974, 30, 371–444. [Google Scholar]
- Díaz-Montes, E. Dextran: Sources, structures, and properties. Polysaccharides 2021, 2, 554–565. [Google Scholar] [CrossRef]
- Zhang, C.; Shao, P.G.; van Kan, J.A.; van der Maarel, J.R. Macromolecular crowding induced elongation and compaction of single DNA molecules confined in a nanochannel. Proc. Natl. Acad. Sci. USA 2009, 106, 16651–16656. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, S.B.; Minton, A.P. Macromolecular crowding: Biochemical, biophysical, and physiological consequences. Annu. Rev. Biophys. Biomol. Struct. 1993, 22, 27–65. [Google Scholar] [CrossRef] [PubMed]
- Farwell, A.P.; Dubord-Tomasetti, S.A. Thyroid hormone regulates the expression of laminin in the developing rat cerebellum. Endocrinology 1999, 140, 4221–4227. [Google Scholar] [CrossRef] [PubMed]
- Peters, T., Jr. Serum albumin. Adv. Protein Chem. 1985, 37, 161–245. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J.K. Meyler’s Side Effects of Drugs: The International Encyclopedia of Adverse Drug Reactions and Interactions; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Tabaka, M.; Kalwarczyk, T.; Szymanski, J.; Hou, S.; Holyst, R. The effect of macromolecular crowding on mobility of biomolecules, association kinetics, and gene expression in living cells. Front. Phys. 2014, 2, 54. [Google Scholar] [CrossRef]
- Ellis, R.J. Macromolecular crowding: Obvious but underappreciated. Trends Biochem. Sci. 2001, 26, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, I.M.; Turoverov, K.K.; Uversky, V.N. What macromolecular crowding can do to a protein. Int. J. Mol. Sci. 2014, 15, 23090–23140. [Google Scholar] [CrossRef]
- Minton, A.P. Macromolecular crowding. Curr. Biol. 2006, 16, R269–R271. [Google Scholar] [CrossRef]
- Hagen, S.J. Solvent viscosity and friction in protein folding dynamics. Curr. Protein Pept. Sci. 2010, 11, 385–395. [Google Scholar] [CrossRef]
- De Sancho, D.; Sirur, A.; Best, R.B. Molecular origins of internal friction effects on protein-folding rates. Nat. Commun. 2014, 5, 4307. [Google Scholar] [CrossRef]
- Bonnet, G.; Krichevsky, O.; Libchaber, A. Kinetics of conformational fluctuations in DNA hairpin-loops. Proc. Natl. Acad. Sci. USA 1998, 95, 8602–8606. [Google Scholar] [CrossRef]
- Wallace, M.I.; Ying, L.; Balasubramanian, S.; Klenerman, D. Non-Arrhenius kinetics for the loop closure of a DNA hairpin. Proc. Natl. Acad. Sci. USA 2001, 98, 5584–5589. [Google Scholar] [CrossRef]
- Busby, S.; Kolb, A.; Buc, H.; Buc, H.; Strick, T. Where it all begins: An overview of promoter recognition and open complex formation. In RNA Polymerases as Molecular Motors (Rsc Biomolecular Sciences); Royal Society of Chemistry: London, UK, 2009. [Google Scholar]
- Losick, R. In vitro transcription. Annu. Rev. Biochem. 1972, 41, 409–446. [Google Scholar] [CrossRef]
- Dangkulwanich, M.; Ishibashi, T.; Bintu, L.; Bustamante, C. Molecular mechanisms of transcription through single-molecule experiments. Chem. Rev. 2014, 114, 3203–3223. [Google Scholar] [CrossRef]
- Hansen, M.M.; Meijer, L.H.; Spruijt, E.; Maas, R.J.; Rosquelles, M.V.; Groen, J.; Heus, H.A.; Huck, W.T. Macromolecular crowding creates heterogeneous environments of gene expression in picolitre droplets. Nat. Nanotechnol. 2016, 11, 191–197. [Google Scholar] [CrossRef]
- Lerner, E.; Chung, S.; Allen, B.L.; Wang, S.; Lee, J.; Lu, S.W.; Grimaud, L.W.; Ingargiola, A.; Michalet, X.; Alhadid, Y.; et al. Backtracked and paused transcription initiation intermediate of Escherichia coli RNA polymerase. Proc. Natl. Acad. Sci. USA 2016, 113, E6562–E6571. [Google Scholar] [CrossRef] [PubMed]
- Rashid, R.; Chee, S.M.L.; Raghunath, M.; Wohland, T. Macromolecular crowding gives rise to microviscosity, anomalous diffusion and accelerated actin polymerization. Phys. Biol. 2015, 12, 034001. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Trochimczyk, P.; Sun, L.; Wisniewska, A.; Kalwarczyk, T.; Zhang, X.; Wielgus-Kutrowska, B.; Bzowska, A.; Holyst, R. How can macromolecular crowding inhibit biological reactions? The enhanced formation of DNA nanoparticles. Sci. Rep. 2016, 6, 22033. [Google Scholar] [CrossRef] [PubMed]
- Kalwarczyk, T.; Ziebacz, N.; Bielejewska, A.; Zaboklicka, E.; Koynov, K.; Szymanski, J.; Wilk, A.; Patkowski, A.; Gapinski, J.; Butt, H.J.; et al. Comparative analysis of viscosity of complex liquids and cytoplasm of mammalian cells at the nanoscale. Nano Lett. 2011, 11, 2157–2163. [Google Scholar] [CrossRef]
- Plaxco, K.W.; Baker, D. Limited internal friction in the rate-limiting step of a two-state protein folding reaction. Proc. Natl. Acad. Sci. USA 1998, 95, 13591–13596. [Google Scholar] [CrossRef]
- Jas, G.S.; Eaton, W.A.; Hofrichter, J. Effect of viscosity on the kinetics of α-helix and β-hairpin formation. J. Phys. Chem. B 2001, 105, 261–272. [Google Scholar] [CrossRef]
- Pabit, S.A.; Roder, H.; Hagen, S.J. Internal friction controls the speed of protein folding from a compact configuration. Biochemistry 2004, 43, 12532–12538. [Google Scholar] [CrossRef]
- Kramers, H.A. Brownian motion in a field of force and the diffusion model of chemical reactions. Physica 1940, 7, 284–304. [Google Scholar] [CrossRef]
- Shin, J.; Cherstvy, A.G.; Metzler, R. Kinetics of polymer looping with macromolecular crowding: Effects of volume fraction and crowder size. Soft Matter 2015, 11, 472–488. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, A.; Duxbury, P.M.; Mackay, M.E. Multifunctional Nanocomposites with Reduced Viscosity. Macromolecules 2007, 40, 9427–9434. [Google Scholar] [CrossRef]
- Wyart, F.B.; de Gennes, P.G. Viscosity at small scales in polymer melts. Eur. Phys. J. E 2000, 1, 93–97. [Google Scholar] [CrossRef]
- Dormann, D.; Haass, C. Fused in sarcoma (FUS): An oncogene goes awry in neurodegeneration. Mol. Cell. Neurosci. 2013, 56, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Shakya, A.; Park, S.; Rana, N.; King, J.T. Liquid-liquid phase separation of histone proteins in cells: Role in chromatin organization. Biophys. J. 2020, 118, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kutateladze, T.G. Liquid–liquid phase separation is an intrinsic physicochemical property of chromatin. Nat. Struct. Mol. Biol. 2019, 26, 1085–1086. [Google Scholar] [CrossRef] [PubMed]
- Booij, H.; Bungenberg de Jong, H. Colloid Systems. In Biocolloids and Their Interactions; Springer: Berlin/Heidelberg, Germany, 1956; pp. 8–14. [Google Scholar]
- Oparin, A.I.; Synge, A. The Origin of Life on the Earth, 3rd. Rev.; Academic Press: New York, NY, USA, 1957. [Google Scholar]
- Stewart, R.J.; Weaver, J.C.; Morse, D.E.; Waite, J.H. The tube cement of Phragmatopoma californica: A solid foam. J. Exp. Biol. 2004, 207, 4727–4734. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, C.; Stewart, R.J.; Waite, J.H. Cement proteins of the tube-building polychaete Phragmatopoma californica. J. Biol. Chem. 2005, 280, 42938–42944. [Google Scholar] [CrossRef] [PubMed]
- Brangwynne, C.P.; Eckmann, C.R.; Courson, D.S.; Rybarska, A.; Hoege, C.; Gharakhani, J.; Jülicher, F.; Hyman, A.A. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 2009, 324, 1729–1732. [Google Scholar] [CrossRef] [PubMed]
- Bungenberg de Jong, H.G. Crystallisation-coacervation-flocculation. Colloid Sci. 1949, 2, 232–258. [Google Scholar]
- Marianelli, A.M.; Miller, B.M.; Keating, C.D. Impact of macromolecular crowding on RNA/spermine complex coacervation and oligonucleotide compartmentalization. Soft Matter 2018, 14, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Minton, A.P. The effect of volume occupancy upon the thermodynamic activity of proteins: Some biochemical consequences. Mol. Cell. Biochem. 1983, 55, 119–140. [Google Scholar] [CrossRef] [PubMed]
- Winzor, D.J.; Wills, P.R. Thermodynamic nonideality of enzyme solutions supplemented with inert solutes: Yeast hexokinase revisited. Biophys. Chem. 1995, 57, 103–110. [Google Scholar] [CrossRef]
- Minton, A.P. [7] Molecular crowding: Analysis of effects of high concentrations of inert cosolutes on biochemical equilibria and rates in terms of volume exclusion. Methods Enzymol. 1998, 295, 127–149. [Google Scholar]
- Madden, T.L.; Herzfeld, J. Crowding-induced organization of cytoskeletal elements: I. Spontaneous demixing of cytosolic proteins and model filaments to form filament bundles. Biophys. J. 1993, 65, 1147–1154. [Google Scholar] [CrossRef]
- Banani, S.F.; Lee, H.O.; Hyman, A.A.; Rosen, M.K. Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18, 285–298. [Google Scholar] [CrossRef]
- Alberti, S. The wisdom of crowds: Regulating cell function through condensed states of living matter. J. Cell Sci. 2017, 130, 2789–2796. [Google Scholar] [CrossRef]
- Fox, A.H.; Lam, Y.W.; Leung, A.K.; Lyon, C.E.; Andersen, J.; Mann, M.; Lamond, A.I. Paraspeckles: A novel nuclear domain. Curr. Biol. 2002, 12, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Lamond, A.I.; Sleeman, J.E. Nuclear substructure and dynamics. Curr. Biol. 2003, 13, R825–R828. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cioce, M.; Lamond, A.I. Cajal bodies: A long history of discovery. Annu. Rev. Cell Dev. Biol. 2005, 21, 105–131. [Google Scholar] [CrossRef]
- Pederson, T. The nucleus introduced. Cold Spring Harb. Perspect. Biol. 2011, 3, a000521. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.; Kedersha, N.; Ivanov, P. Stress granules, P-bodies and cancer. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2015, 1849, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Banani, S.F.; Rice, A.M.; Peeples, W.B.; Lin, Y.; Jain, S.; Parker, R.; Rosen, M.K. Compositional control of phase-separated cellular bodies. Cell 2016, 166, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Buchan, J.R. mRNP granules: Assembly, function, and connections with disease. RNA Biol. 2014, 11, 1019–1030. [Google Scholar] [CrossRef]
- Elbaum-Garfinkle, S.; Kim, Y.; Szczepaniak, K.; Chen, C.C.H.; Eckmann, C.R.; Myong, S.; Brangwynne, C.P. The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. Proc. Natl. Acad. Sci. USA 2015, 112, 7189–7194. [Google Scholar] [CrossRef]
- Schuster, B.S.; Reed, E.H.; Parthasarathy, R.; Jahnke, C.N.; Caldwell, R.M.; Bermudez, J.G.; Ramage, H.; Good, M.C.; Hammer, D.A. Controllable protein phase separation and modular recruitment to form responsive membraneless organelles. Nat. Commun. 2018, 9, 2985. [Google Scholar] [CrossRef]
- Hernandez-Verdun, D. Assembly and disassembly of the nucleolus during the cell cycle. Nucleus 2011, 2, 189–194. [Google Scholar] [CrossRef]
- Fox, A.H.; Lamond, A.I. Paraspeckles. Cold Spring Harb. Perspect. Biol. 2010, 2, a000687. [Google Scholar] [CrossRef] [PubMed]
- Lallemand-Breitenbach, V.; de Thé, H. PML nuclear bodies. Cold Spring Harb. Perspect. Biol. 2010, 2, a000661. [Google Scholar] [CrossRef]
- Odijk, T. Osmotic compaction of supercoiled DNA into a bacterial nucleoid. Biophys. Chem. 1998, 73, 23–29. [Google Scholar] [CrossRef]
- Zimmerman, S.B. Studies on the compaction of isolated nucleoids from Escherichia coli. J. Struct. Biol. 2004, 147, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, S.B. Shape and compaction of Escherichia coli nucleoids. J. Struct. Biol. 2006, 156, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, S.B. Cooperative transitions of isolated Escherichia coli nucleoids: Implications for the nucleoid as a cellular phase. J. Struct. Biol. 2006, 153, 160–175. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.C.; Spakowitz, A.J.; Theriot, J.A. Bacterial chromosomal loci move subdiffusively through a viscoelastic cytoplasm. Phys. Rev. Lett. 2010, 104, 238102. [Google Scholar] [CrossRef]
- Javer, A.; Long, Z.; Nugent, E.; Grisi, M.; Siriwatwetchakul, K.; Dorfman, K.D.; Cicuta, P.; Cosentino Lagomarsino, M. Short-time movement of E. coli chromosomal loci depends on coordinate and subcellular localization. Nat. Commun. 2013, 4, 3003. [Google Scholar] [CrossRef]
- Parry, B.R.; Surovtsev, I.V.; Cabeen, M.T.; O’Hern, C.S.; Dufresne, E.R.; Jacobs-Wagner, C. The bacterial cytoplasm has glass-like properties and is fluidized by metabolic activity. Cell 2014, 156, 183–194. [Google Scholar] [CrossRef]
- Polovnikov, K.; Gherardi, M.; Cosentino-Lagomarsino, M.; Tamm, M. Fractal folding and medium viscoelasticity contribute jointly to chromosome dynamics. Phys. Rev. Lett. 2018, 120, 088101. [Google Scholar] [CrossRef] [PubMed]
- Dame, R.T.; Tark-Dame, M. Bacterial chromatin: Converging views at different scales. Curr. Opin. Cell Biol. 2016, 40, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Benza, V.G.; Bassetti, B.; Dorfman, K.D.; Scolari, V.F.; Bromek, K.; Cicuta, P.; Lagomarsino, M.C. Physical descriptions of the bacterial nucleoid at large scales, and their biological implications. Rep. Prog. Phys. 2012, 75, 076602. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, M.; Scolari, V.; Dame, R.T.; Lagomarsino, M.C. Chromosome Structure and Dynamics in Bacteria: Theory and Experiments. In Modeling the 3D Conformation of Genomes; CRC Press: Boca Raton, FL, USA, 2019; pp. 207–230. [Google Scholar]
- Dame, R.T.; Wuite, G.J. On the Role of H-NS in the Organization of Bacterial Chromatin: From Bulk to Single Molecules and Back. Biophys. J. 2003, 85, 4146–4148. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cunha, S.; Odijk, T.; Süleymanoglu, E.; Woldringh, C.L. Isolation of the Escherichia coli nucleoid. Biochimie 2001, 83, 149–154. [Google Scholar] [CrossRef]
- Pelletier, J.; Halvorsen, K.; Ha, B.Y.; Paparcone, R.; Sandler, S.J.; Woldringh, C.L.; Wong, W.P.; Jun, S. Physical manipulation of the Escherichia coli chromosome reveals its soft nature. Proc. Natl. Acad. Sci. USA 2012, 109, E2649–E2656. [Google Scholar] [CrossRef]
- Wegner, A.S.; Alexeeva, S.; Odijk, T.; Woldringh, C.L. Characterization of Escherichia coli nucleoids released by osmotic shock. J. Struct. Biol. 2012, 178, 260–269. [Google Scholar] [CrossRef]
- Thacker, V.V.; Bromek, K.; Meijer, B.; Kotar, J.; Sclavi, B.; Lagomarsino, M.C.; Keyser, U.F.; Cicuta, P. Bacterial nucleoid structure probed by active drag and resistive pulse sensing. Integr. Biol. 2014, 6, 184–191. [Google Scholar] [CrossRef]
- Winardhi, R.S.; Yan, J.; Kenney, L.J. H-NS regulates gene expression and compacts the nucleoid: Insights from single-molecule experiments. Biophys. J. 2015, 109, 1321–1329. [Google Scholar] [CrossRef]
- Grainger, D.C. Structure and function of bacterial H-NS protein. Biochem. Soc. Trans. 2016, 44, 1561–1569. [Google Scholar] [CrossRef]
- Gao, Y.; Foo, Y.H.; Winardhi, R.S.; Tang, Q.; Yan, J.; Kenney, L.J. Charged residues in the H-NS linker drive DNA binding and gene silencing in single cells. Proc. Natl. Acad. Sci. USA 2017, 114, 12560–12565. [Google Scholar] [CrossRef]
- Yamanaka, Y.; Winardhi, R.S.; Yamauchi, E.; Nishiyama, S.i.; Sowa, Y.; Yan, J.; Kawagishi, I.; Ishihama, A.; Yamamoto, K. Dimerization site 2 of the bacterial DNA-binding protein H-NS is required for gene silencing and stiffened nucleoprotein filament formation. J. Biol. Chem. 2018, 293, 9496–9505. [Google Scholar] [CrossRef] [PubMed]
- Lang, B.; Blot, N.; Bouffartigues, E.; Buckle, M.; Geertz, M.; Gualerzi, C.O.; Mavathur, R.; Muskhelishvili, G.; Pon, C.L.; Rimsky, S.; et al. High-affinity DNA binding sites for H-NS provide a molecular basis for selective silencing within proteobacterial genomes. Nucleic Acids Res. 2007, 35, 6330–6337. [Google Scholar] [CrossRef] [PubMed]
- Kahramanoglou, C.; Seshasayee, A.S.; Prieto, A.I.; Ibberson, D.; Schmidt, S.; Zimmermann, J.; Benes, V.; Fraser, G.M.; Luscombe, N.M. Direct and indirect effects of H-NS and Fis on global gene expression control in Escherichia coli. Nucleic Acids Res. 2011, 39, 2073–2091. [Google Scholar] [CrossRef] [PubMed]
- Navarre, W.W.; Porwollik, S.; Wang, Y.; McClelland, M.; Rosen, H.; Libby, S.J.; Fang, F.C. Selective silencing of foreign DNA with low GC content by the H-NS protein in Salmonella. Science 2006, 313, 236–238. [Google Scholar] [CrossRef] [PubMed]
- Gordon, B.R.; Li, Y.; Cote, A.; Weirauch, M.T.; Ding, P.; Hughes, T.R.; Navarre, W.W.; Xia, B.; Liu, J. Structural basis for recognition of AT-rich DNA by unrelated xenogeneic silencing proteins. Proc. Natl. Acad. Sci. USA 2011, 108, 10690–10695. [Google Scholar] [CrossRef] [PubMed]
- Rouviere-Yaniv, J.; Gros, F. Characterization of a novel, low-molecular-weight DNA-binding protein from Escherichia coli. Proc. Natl. Acad. Sci. USA 1975, 72, 3428–3432. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.; Brodkorb, A. The casein micelle: Historical aspects, current concepts and significance. Int. Dairy J. 2008, 18, 677–684. [Google Scholar] [CrossRef]
- Devanand, K.; Selser, J. Asymptotic behavior and long-range interactions in aqueous solutions of poly (ethylene oxide). Macromolecules 1991, 24, 5943–5947. [Google Scholar] [CrossRef]
- Yang, W.; Lee, J.Y.; Nowotny, M. Making and breaking nucleic acids: Two-Mg2+-ion catalysis and substrate specificity. Mol. Cell 2006, 22, 5–13. [Google Scholar] [CrossRef]
- Coppins, R.L.; Hall, K.B.; Groisman, E.A. The intricate world of riboswitches. Curr. Opin. Microbiol. 2007, 10, 176–181. [Google Scholar] [CrossRef]
- Sissi, C.; Palumbo, M. Effects of magnesium and related divalent metal ions in topoisomerase structure and function. Nucleic Acids Res. 2009, 37, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Vashishtha, A.K.; Wang, J.; Konigsberg, W.H. Different divalent cations alter the kinetics and fidelity of DNA polymerases. J. Biol. Chem. 2016, 291, 20869–20875. [Google Scholar] [CrossRef] [PubMed]
- Bloom, A.J. Metal regulation of metabolism. Curr. Opin. Chem. Biol. 2019, 49, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Zhang, B.; Zhao, N. Crowding-activity coupling effect on conformational change of a semi-flexible polymer. Polymers 2019, 11, 1021. [Google Scholar] [CrossRef] [PubMed]
- Sukenik, S.; Sapir, L.; Harries, D. Balance of enthalpy and entropy in depletion forces. Curr. Opin. Colloid Interface Sci. 2013, 18, 495–501. [Google Scholar] [CrossRef]
- Sapir, L.; Harries, D. Macromolecular stabilization by excluded cosolutes: Mean field theory of crowded solutions. J. Chem. Theory Comput. 2015, 11, 3478–3490. [Google Scholar] [CrossRef]
- Sapir, L.; Harries, D. Macromolecular compaction by mixed solutions: Bridging versus depletion attraction. Curr. Opin. Colloid Interface Sci. 2016, 22, 80–87. [Google Scholar] [CrossRef]
- Sapir, L.; Harries, D. Is the depletion force entropic? Molecular crowding beyond steric interactions. Curr. Opin. Colloid Interface Sci. 2015, 20, 3–10. [Google Scholar] [CrossRef]
- Asakura, S.; Oosawa, F. On interaction between two bodies immersed in a solution of macromolecules. J. Chem. Phys. 1954, 22, 1255–1256. [Google Scholar] [CrossRef]
- Asakura, S.; Oosawa, F. Interaction between particles suspended in solutions of macromolecules. J. Polym. Sci. 1958, 33, 183–192. [Google Scholar] [CrossRef]

| Technique | Description |
|---|---|
| Fluorescence Spectroscopy | Fluorescent probes or fluorescently labeled macromolecules are used to monitor changes in the partitioning of proteins [13,14,15,16] or nucleic acids [17,18,19,20,21,22,23] in crowded environments. |
| Dynamic Light Scattering (DLS) | Fluctuations in the Rayleigh scattering of light due to diffusion and interference between particles in a solution can be used to assess changes in particle size and proximity in crowded conditions [24,25,26,27,28]. |
| Small-Angle X-ray Scattering (SAXS) | The scattering of X-rays can be used on non-crystalline samples to determine the average size and shape of monodisperse macromolecules in crowded solutions [26,27,29]. |
| Nuclear Magnetic Resonance (NMR) Spectroscopy | NMR can be used to monitor how crowding changes the electronic environment of nuclear spins in biomolecules and alters spin coupling [30,31,32]. |
| Electron Microscopy | Electrons, which have very short wavelength with respect to photons, can be used to visualize, in vacuum conditions, metal-stained macromolecular structures prepared from dilute to crowded conditions with nanometer-scale resolution [8]. |
| Cryo-Electron Microscopy (Cryo-EM) | Cryo-EM can be used to determine the structure of unstained macromolecular complexes held in tiny droplets of ice in crowded conditions at nanometer-scale resolution [33]. |
| Analytical Ultracentrifugation | Optical detection of the dynamics and extent of migration of macromolecules through solutions of density gradients can be used to determine their sizes and reveal condensation in crowded environments [34,35]. |
| Steady-State and Time-Resolved Fluorescence Resonance Energy Transfer (FRET) | The transfer of energy between natural or exogenous fluorphores in macromolecules can be used to measure distances between them and/or their labeled macromolecules in crowded environments [36,37]. |
| Gel Electrophoresis | Electrophoretic migration of macromolecules through gel meshworks in dilute to crowded solutions can be used to reveal sizes and macromolecular associations [38,39]. |
| Single particle tracking, Tethered particle microscopy, Optical and Magnetic Tweezers | Single particle tracking [40], Tethered particle motion (TPM) [17,41] or force spectroscopy with optical or magnetic tweezing (OT or MT) [17,42,43,44] can be used to reveal the dynamics of conformational changes in crowded environments. |
| Atomic Force Microscopy (AFM) | AFM imaging can be used to observe condensates resulting from the presence of crowders [45]. |
| Isothermal calorimetry | ITC titrations are used to determine association constants, enthalpy and entropy of macromolecular interactions influenced by crowding [46]. |
| Circular Dichroism (CD) | Circular dichroism spectroscopy can be used to study the conformation and association of biomolecules in crowded environments by analyzing their differential absorption of left- and right-circularly polarized light [27,46,47,48]. |
| Convex Lens-Induced Confinement | Pressure from a convex lens can be used to isolate one or a few macromolecules in dilute to crowded solutions to study the conformation of and association between macromolecules [3]. |
| Molecular Dynamics Simulations | Computer simulations can be used to model the behavior of macromolecules in crowded conditions and provide insights into their interactions and dynamics [31]. |
| Effect of Crowding on DNA | Description/Effects |
|---|---|
| DNA Extension/Compaction | DNA can compact or extend depending on the conditions [47,49,50,51,52,53,54,55,56,57,58,59,60]. |
| Branched or rigid crowders may lead to greater compaction due to steric hindrance. | |
| Linear and flexible crowders may induce milder compaction. [2] | |
| Thermal Stability | Macromolecular crowding has been shown to increase the thermal stability of DNA [46,61]. |
| Crowding agents can stabilize DNA structures and reduce denaturation [62,63]. | |
| DNA Configuration (Right/Left-Handed) | Specific conditions and crowders may favor transitions between right- and left-handed DNA helices [64]. |
| Opening of ssDNA Hairpins | Crowding can impact the stability and kinetics of DNA secondary structures like hairpins, which are stabilized by crowding and open more slowly [22,23]. |
| Protein Binding (Nucleoid-Associated) | Crowding can enhance the binding of proteins, such as nucleoid-associated proteins (NAPs), to DNA [41,49,51]. |
| Transcription | Increased crowding has been shown to increase the efficiency of transcription initiation [37] and enhance the transcription rate [65]. |
| Liquid–Liquid Phase Separation | Crowding can contribute to the phase separation of biomolecules, including DNA, leading to the formation of liquid condensates [14,15,43]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collette, D.; Dunlap, D.; Finzi, L. Macromolecular Crowding and DNA: Bridging the Gap between In Vitro and In Vivo. Int. J. Mol. Sci. 2023, 24, 17502. https://doi.org/10.3390/ijms242417502
Collette D, Dunlap D, Finzi L. Macromolecular Crowding and DNA: Bridging the Gap between In Vitro and In Vivo. International Journal of Molecular Sciences. 2023; 24(24):17502. https://doi.org/10.3390/ijms242417502
Chicago/Turabian StyleCollette, Dylan, David Dunlap, and Laura Finzi. 2023. "Macromolecular Crowding and DNA: Bridging the Gap between In Vitro and In Vivo" International Journal of Molecular Sciences 24, no. 24: 17502. https://doi.org/10.3390/ijms242417502
APA StyleCollette, D., Dunlap, D., & Finzi, L. (2023). Macromolecular Crowding and DNA: Bridging the Gap between In Vitro and In Vivo. International Journal of Molecular Sciences, 24(24), 17502. https://doi.org/10.3390/ijms242417502





