CDKN1A/p21 in Breast Cancer: Part of the Problem, or Part of the Solution?
Abstract
:1. Introduction
1.1. Medical Need for Targeted Therapeutics and Biomarkers for the Management of Late-Stage Metastatic Breast Cancer
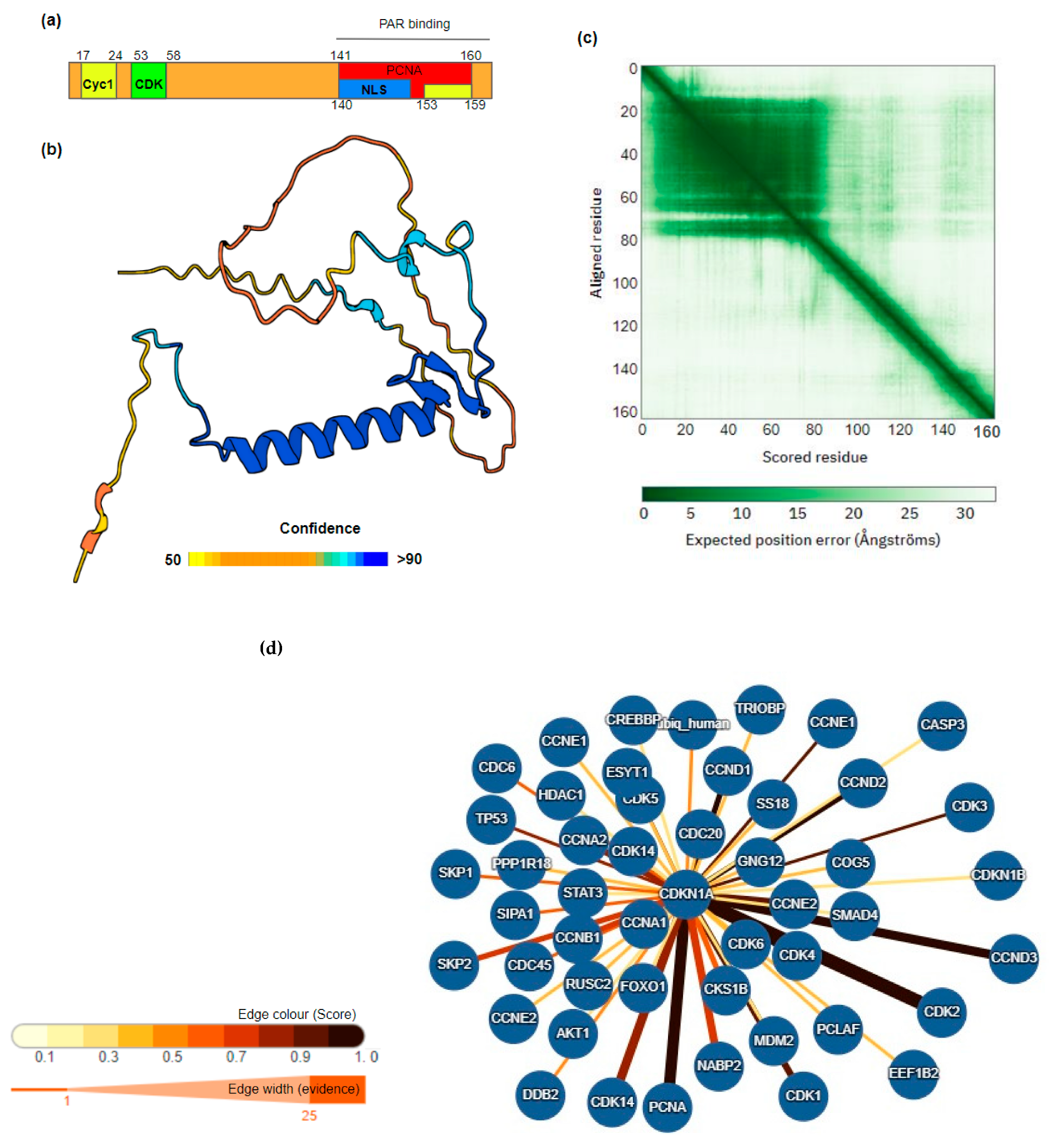
1.2. Cyclin-Dependent Kinase Inhibitor 1A
| Post-Translational Modification | Site | Effector | Observed Effect | Reference |
|---|---|---|---|---|
| Phosphorylation | Thr-55 | MELK (mouse) | Induce interaction CDK2/CDK4 | [16] |
| Thr-57 | MAPK1, MAPK14 and MAPK8 | Cytoplasmic localisation and degradation | [17,18] | |
| Thr-80 | LKB1 | Degradation | [19] | |
| Thr-145 | AKT1, CHEK1, DAPK1, PIM1, PIM2, PKA | Impairs binding to PCNA Enhances protein stability Alters protein localisation | [20,21] | |
| Ser-31 | Unknown | Unknown/identified using mass spectrometry | [22] | |
| Ser-98 | ASK1 | Unknown | [23] | |
| Ser-114 | GSK3-beta | Enhances ubiquitination | [24] | |
| Ser-123 | Unknown | Protein stabilisation | [25] | |
| Ser-130 | CDK6, MAPK, MAPK14, MAPK8 | Impairs stability | [26,27] | |
| Ser 137 | Unknown | [28] | ||
| Ser-153 | DYRK1B, PKCA | Cytoplasmic localisation | [29] | |
| Ser-160 | PKC | Modulates binding to PCNA | [30] | |
| Ser-146 | AKT, CHEK1, DAPK1, LATS2, NUAK1, PKC, PRKCD, STK38 | Impairs binding to PCNA Protein stabilisation | [31,32] | |
| Tyr-151 | Unknown | Unknown | [33] | |
| Ubiquitination | Lys-16 Lys-75 Lys-141 Lys-154 Lys-161 Lys-163 | Breast cancer cell growth-induced | Protein degradation | [34] |
| Acetylation | Lys-141 Lys-154 Lys-161 Lys-163 | HDAC1, TSA induced, cell growth and carcinogenesis inhibited | Protein stabilisation enhanced following acetylation | [35] |
| Methylation | Arg-156 | PRMT6 | Induction of cytoplasmic localisation | [36] |
2. Role of CDKN1A/p21 in Cancer
2.1. Clinical Significance of CDKN1A/p21 Expression Levels
2.1.1. CDKN1A/p21 in Cancer Progression
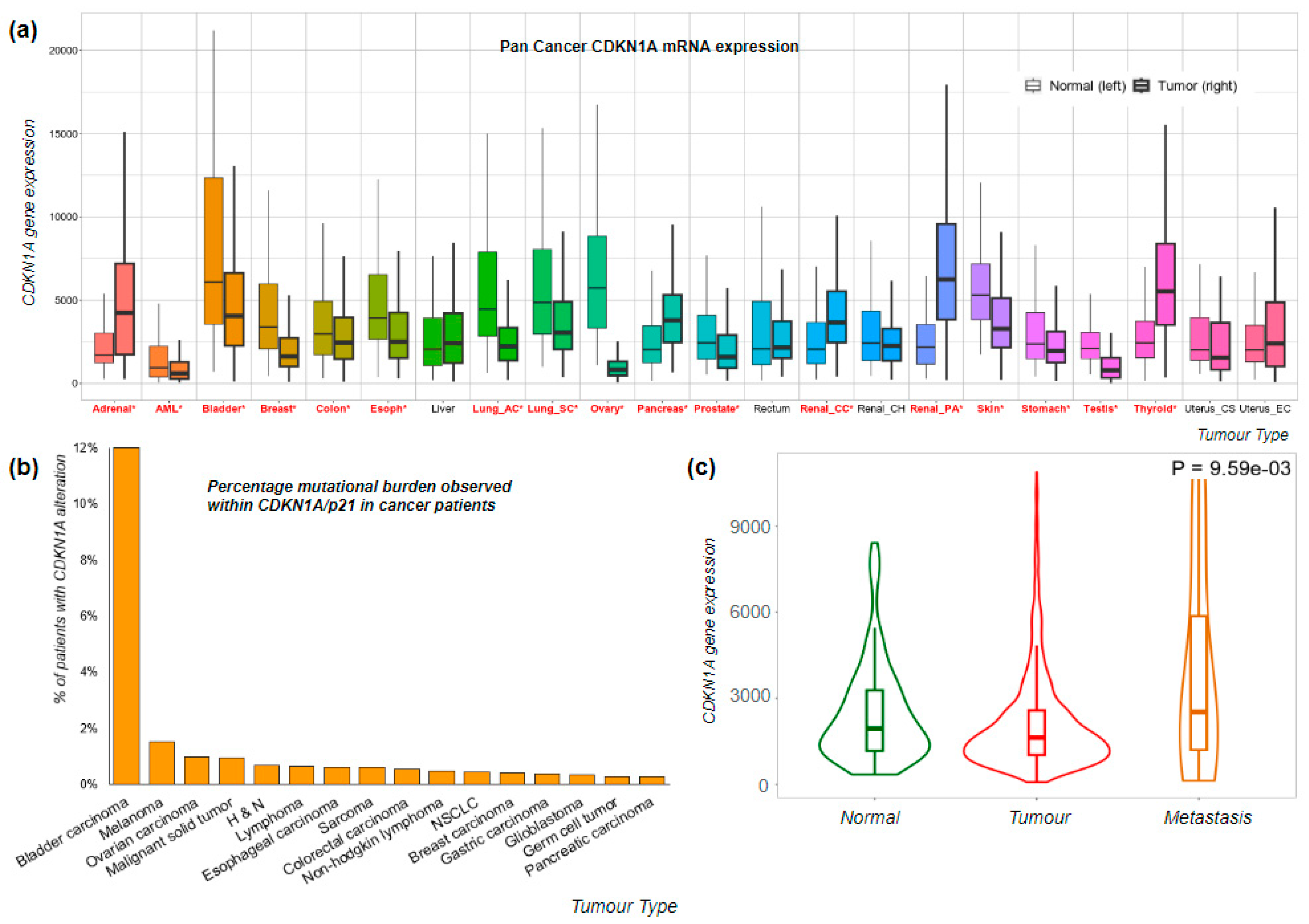
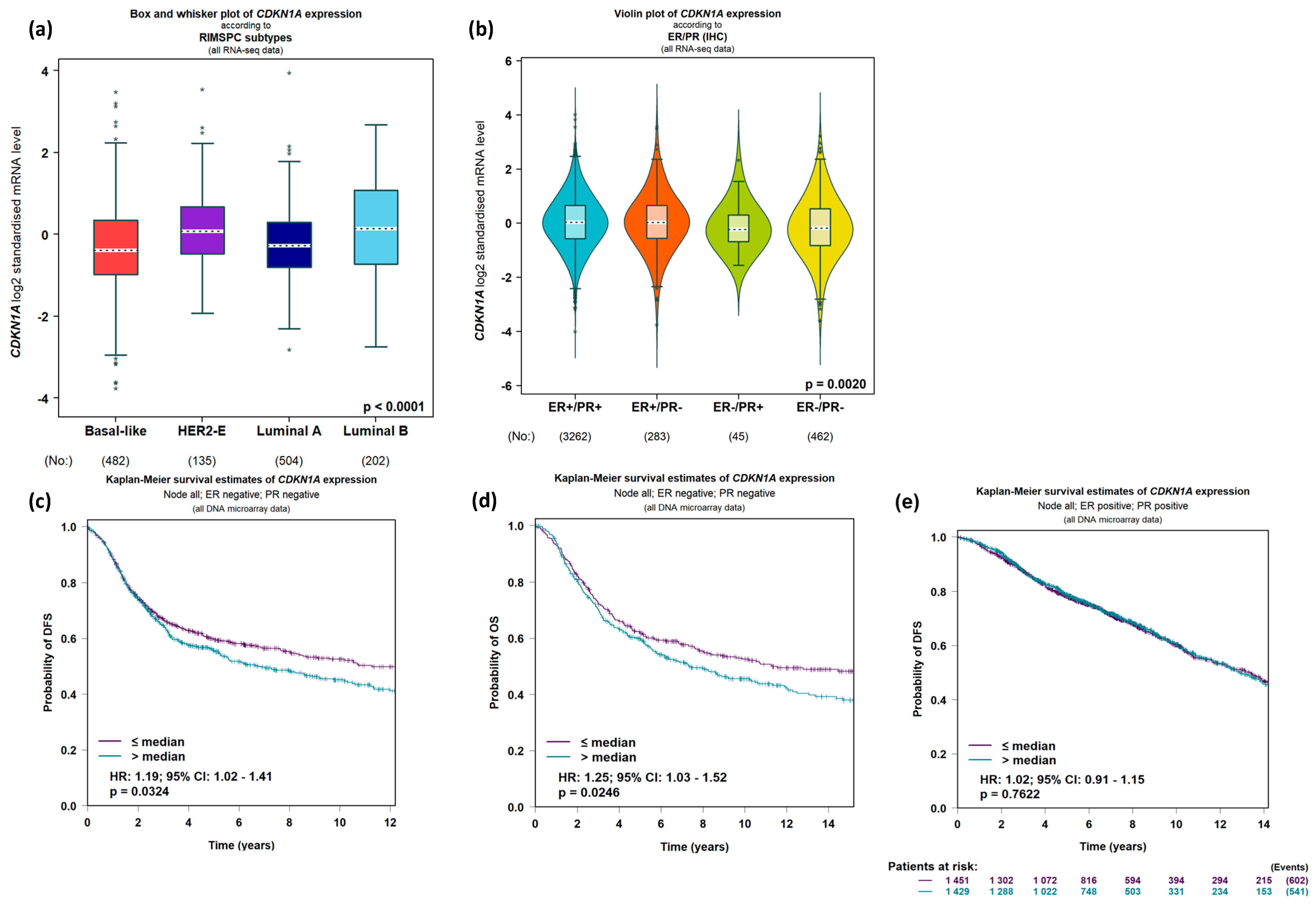
2.1.2. Expression of CDKN1A/p21 Shows Predictive Promise in Breast Cancer
2.1.3. Chemical Modulation of CDKN1A/p21 Expression and Action
2.2. Role of CDKN1A/p21 in Molecular Processes Important to Cancer Progression
2.2.1. Cell Cycle
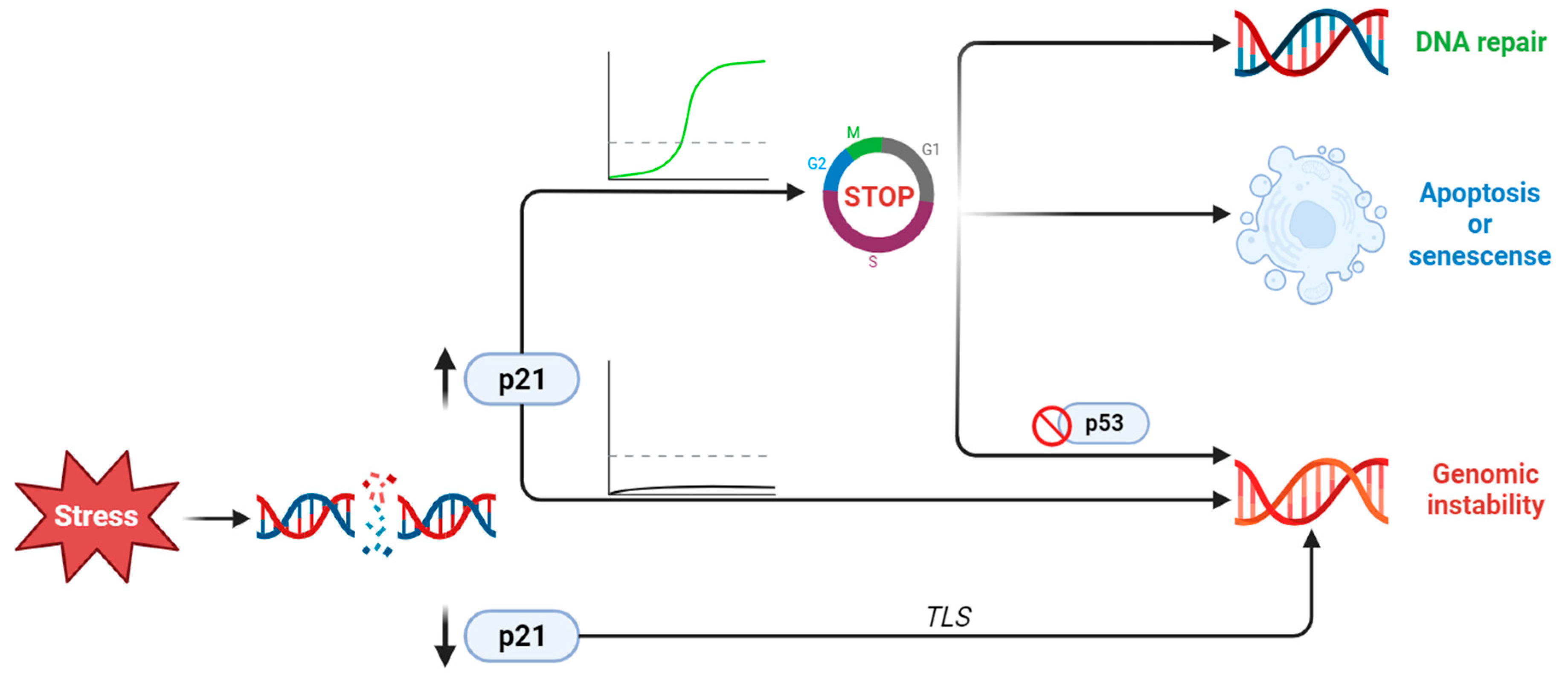
2.2.2. CDKN1A/p21 Is a Key Player in the DNA Damage Response
2.2.3. CDKN1A/p21 Activation or Repression during EMT, Invasion, and Metastatic Transformation
2.2.4. Stem Cells
2.2.5. Apoptosis and Senescence
3. Discussion and Future Directions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT | AKT serine/threonine kinase 1 |
| AML | Acute Myeloid Leukemia |
| ASK1 | Apoptosis signal-regulating kinase 1 |
| BER | Base Excision Repair |
| BCSC | Breast cancer stem cell |
| CDK | Cyclin-dependent kinase |
| CDKN1A | Cyclin-dependent kinase inhibitory protein 1A |
| CHEK1 | Serine/threonine-protein kinase Chk1 |
| CIP | CDK-interacting protein |
| CMTM6 | CKLF-Like MARVEL Transmembrane Domain-Containing Protein 6 |
| CMS4 | Consensus molecular subtype 4 |
| CPEB4 | Cytoplasmic polyadenylation element-binding protein 4 |
| CRC | Colorectal cancer |
| CSC | Cancer stem cell |
| DAPK1 | Death-associated protein kinase 1 |
| DFS | Disease-free survival |
| DNMT1 | DNA Methyltransferase 1 |
| DDR | DNA damage repair |
| DREAM | Dimerisation partner, RB-like, E2F, and multi-vulval class B |
| DYRK1B | Dual-specificity tyrosine-phosphorylation-regulated kinase 1B |
| EMT | Epithelial–mesenchymal transition |
| GSK | Glycogen synthase kinase |
| HCC | Hepatocellular carcinoma |
| HDAC | Histone Deacetylases |
| H and N | Head and Neck |
| HR | Homologous Recombination |
| KIP | Kinase inhibitor protein |
| LATS2 | Serine/threonine-protein kinase LATS2 |
| LEC | Lymphatic endothelial cells |
| LKB1 | Serine/threonine-protein kinase STK11 |
| LMNB2 | Lamin B2 |
| MAPK | MAP kinase-activated protein kinase |
| MELK | Maternal embryonic leucine zipper kinase |
| MPM | Malignant pleural mesotheliomas |
| MMR | Mismatch repair pathway |
| NER | Nucleotide excision repair |
| NHEJ | Non-homologous end joining |
| NSCLC | Non-small-cell lung carcinoma |
| NUAK1 | NUAK family SNF1-like kinase 1 |
| NUDT5 | Nudix hydrolase 5 |
| ODG | Oligodendrogliomas |
| PARP1 | Poly-ADP-ribose polymerase |
| PCNA | Proliferating cell nuclear antigen |
| PDGF | Platelet-Derived Growth Factor |
| PIM1 | Serine/threonine-protein kinase pim1 |
| PKA/B | Protein kinase B (Akt) |
| PRKCD | Protein kinase C delta type |
| PRMT1 | Protein arginine N-methyltransferase 1 |
| PVT1 | Plasmacytoma Variant Translocation 1 |
| RB | Retinoblastoma |
| RCC | Renal cell carcinoma |
| SOX2 | Sex-determining region Y-box 2 |
| STK38 | Serine/threonine-protein kinase 38 |
| STAT1 | Signal transducer and activator of transcription 1 |
| TLS | Translesion DNA synthesis |
| TSA | Trichostatin A |
| ZEB1 | Zinc finger E-box-binding homeobox 1 |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Agostinetto, E.; Gligorov, J.; Piccart, M. Systemic therapy for early-stage breast cancer: Learning from the past to build the future. Nat. Rev. Clin. Oncol. 2022, 19, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA A Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Nardin, S.; Mora, E.; Varughese, F.M.; D’Avanzo, F.; Vachanaram, A.R.; Rossi, V.; Saggia, C.; Rubinelli, S.; Gennari, A. Breast Cancer Survivorship, Quality of Life, and Late Toxicities. Front. Oncol. 2020, 10, 864. [Google Scholar] [CrossRef] [PubMed]
- Riggio, A.I.; Varley, K.E.; Welm, A.L. The lingering mysteries of metastatic recurrence in breast cancer. Br. J. Cancer 2021, 124, 13–26. [Google Scholar] [CrossRef]
- Courtney, D.; Davey, M.G.; Moloney, B.M.; Barry, M.K.; Sweeney, K.; McLaughlin, R.P.; Malone, C.M.; Lowery, A.J.; Kerin, M.J. Breast cancer recurrence: Factors impacting occurrence and survival. Ir. J. Med. Sci. 2022, 191, 2501–2510. [Google Scholar] [CrossRef]
- Zohny, S.F.; Al-Malki, A.L.; Zamzami, M.A.; Choudhry, H. p21Waf1/Cip1: Its paradoxical effect in the regulation of breast cancer. Breast Cancer 2019, 26, 131–137. [Google Scholar] [CrossRef]
- Besson, A.; Dowdy, S.F.; Roberts, J.M. CDK Inhibitors: Cell Cycle Regulators and Beyond. Dev. Cell 2008, 14, 159–169. [Google Scholar] [CrossRef]
- Ullah, Z.; Lee, C.Y.; DePamphilis, M.L. Cip/Kip cyclin-dependent protein kinase inhibitors and the road to polyploidy. Cell Div. 2009, 4, 10. [Google Scholar] [CrossRef]
- Uhlén, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, 2507. [Google Scholar] [CrossRef]
- Asada, M.; Yamada, T.; Ichijo, H.; Delia, D.; Miyazono, K.; Fukumuro, K.; Mizutani, S. Apoptosis inhibitory activity of cytoplasmic p21 Cip1/WAF1 in monocytic differentiation. EMBO J. 1999, 18, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Asada, M.; Ohmi, K.; Delia, D.; Enosawa, S.; Suzuki, S.; Yuo, A.; Suzuki, H.; Mizutani, S. Brap2 Functions as a Cytoplasmic Retention Protein for p21 during Monocyte Differentiation. Mol. Cell. Biol. 2004, 24, 8236–8243. [Google Scholar] [CrossRef]
- Tak, J.; Sabarwal, A.; Shyanti, R.K.; Singh, R.P. Berberine enhances posttranslational protein stability of p21/cip1 in breast cancer cells via down-regulation of Akt. Mol. Cell. Biochem. 2019, 458, 49–59. [Google Scholar] [CrossRef] [PubMed]
- del Toro, N.; Shrivastava, A.; Ragueneau, E.; Meldal, B.; Combe, C.; Barrera, E.; Perfetto, L.; How, K.; Ratan, P.; Shirodkar, G.; et al. The IntAct database: Efficient access to fine-grained molecular interaction data. Nucleic Acids Res. 2022, 50, D648–D653. [Google Scholar] [CrossRef] [PubMed]
- Seong, H.-A.; Ha, H. Thr55 phosphorylation of p21 by MPK38/MELK ameliorates defects in glucose, lipid, and energy metabolism in diet-induced obese mice. Cell Death Dis. 2019, 10, 380. [Google Scholar] [CrossRef]
- Hwang, C.Y.; Lee, C.; Kwon, K.-S. Extracellular Signal-Regulated Kinase 2-Dependent Phosphorylation Induces Cytoplasmic Localization and Degradation of p21Cip1. Mol. Cell. Biol. 2009, 29, 3379–3389. [Google Scholar] [CrossRef]
- Foertsch, F.; Teichmann, N.; Kob, R.; Hentschel, J.; Laubscher, U.; Melle, C. S100A11 is involved in the regulation of the stability of cell cycle regulator p21CIP1/WAF1 in human keratinocyte HaCaT cells. FEBS J. 2013, 280, 3840–3853. [Google Scholar] [CrossRef]
- Esteve-Puig, R.; Gil, R.; González-Sánchez, E.; Bech-Serra, J.J.; Grueso, J.; Hernández-Losa, J.; Moliné, T.; Canals, F.; Ferrer, B.; Cortés, J.; et al. A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21WAF1/CIP1) Degradation. PLOS Genet. 2014, 10, e1004721. [Google Scholar] [CrossRef]
- Li, Y.; Dowbenko, D.; Lasky, L.A. AKT/PKB Phosphorylation of p21Cip/WAF1 Enhances Protein Stability of p21Cip/WAF1 and Promotes Cell Survival. J. Biol. Chem. 2002, 277, 11352–11361. [Google Scholar] [CrossRef]
- Rössig, L.; Jadidi, A.S.; Urbich, C.; Badorff, C.; Zeiher, A.M.; Dimmeler, S. Akt-Dependent Phosphorylation of p21Cip1 Regulates PCNA Binding and Proliferation of Endothelial Cells. Mol. Cell. Biol. 2001, 21, 5644–5657. [Google Scholar] [CrossRef]
- Stuart, S.A.; Houel, S.; Lee, T.; Wang, N.; Old, W.M.; Ahn, N.G. A Phosphoproteomic Comparison of B-RAFV600E and MKK1/2 Inhibitors in Melanoma Cells. Mol. Cell. Proteom. 2015, 14, 1599–1615. [Google Scholar] [CrossRef] [PubMed]
- Colleoni, B.; Paternot, S.; Pita, J.M.; Bisteau, X.; Coulonval, K.; Davis, R.J.; Raspé, E.; Roger, P.P. JNKs function as CDK4-activating kinases by phosphorylating CDK4 and p21. Oncogene 2017, 36, 4349–4361. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Yu, S.J.; Park, Y.G.; Kim, J.; Sohn, J. Glycogen Synthase Kinase 3β Phosphorylates p21WAF1/CIP1 for Proteasomal Degradation after UV Irradiation. Mol. Cell. Biol. 2007, 27, 3187–3198. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, J.; Zhang, M.; Liu, S.; Yan, W.; Jung, J.; Chen, X. Serine 123 Phosphorylation Modulates p21 Protein Stability and Activity by Suppressing Ubiquitin-independent Proteasomal Degradation. J. Biol. Chem. 2012, 287, 34410–34418. [Google Scholar] [CrossRef]
- Yi, T.; Zhai, B.; Yu, Y.; Kiyotsugu, Y.; Raschle, T.; Etzkorn, M.; Seo, H.-C.; Nagiec, M.; Luna, R.E.; Reinherz, E.L.; et al. Quantitative phosphoproteomic analysis reveals system-wide signaling pathways downstream of SDF-1/CXCR4 in breast cancer stem cells. Proc. Natl. Acad. Sci. USA 2014, 111, E2182–E2190. [Google Scholar] [CrossRef]
- Järviluoma, A.; Child, E.S.; Sarek, G.; Sirimongkolkasem, P.; Peters, G.; Ojala, P.M.; Mann, D.J. Phosphorylation of the Cyclin-Dependent Kinase Inhibitor p21Cip1 on Serine 130 Is Essential for Viral Cyclin-Mediated Bypass of a p21Cip1-Imposed G1 Arrest. Mol. Cell. Biol. 2006, 26, 2430–2440. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Nie, L.; Maki, C.G. Cdk2-dependent Inhibition of p21 Stability via a C-terminal Cyclin-binding Motif. J. Biol. Chem. 2005, 280, 29282–29288. [Google Scholar] [CrossRef]
- Hornbeck, P.V.; Zhang, B.; Murray, B.; Kornhauser, J.M.; Latham, V.; Skrzypek, E. PhosphoSitePlus, 2014: Mutations, PTMs and recalibrations. Nucleic Acids Res. 2014, 43, D512–D520. [Google Scholar] [CrossRef]
- Rodríguez-Vilarrupla, A.; Jaumot, M.; Abella, N.; Canela, N.; Brun, S.; Díaz, C.; Estanyol, J.M.; Bachs, O.; Agell, N. Binding of Calmodulin to the Carboxy-Terminal Region of p21 Induces Nuclear Accumulation via Inhibition of Protein Kinase C-Mediated Phosphorylation of Ser153. Mol. Cell. Biol. 2005, 25, 7364–7374. [Google Scholar] [CrossRef]
- Scott, M.T.; Morrice, N.; Ball, K.L. Reversible Phosphorylation at the C-Terminal Regulatory Domain of p21 Waf1/Cip1 Modulates Proliferating Cell Nuclear Antigen Binding. J. Biol. Chem. 2000, 275, 11529–11537. [Google Scholar]
- Wang, Z.; Zhang, Y.; Gu, J.J.; Davitt, C.; Reeves, R.; Magnuson, N.S. Pim-2 phosphorylation of p21Cip1/WAF1 enhances its stability and inhibits cell proliferation in HCT116 cells. Int. J. Biochem. Cell Biol. 2010, 42, 1030–1038. [Google Scholar] [CrossRef]
- Mertins, P.; Yang, F.; Liu, T.; Mani, D.R.; Petyuk, V.A.; Gillette, M.A.; Clauser, K.R.; Qiao, J.W.; Gritsenko, M.A.; Moore, R.J.; et al. Ischemia in Tumors Induces Early and Sustained Phosphorylation Changes in Stress Kinase Pathways but Does Not Affect Global Protein Levels. Mol. Cell. Proteomics 2014, 13, 1690–1704. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Richardson, D.R. Iron chelation and regulation of the cell cycle: 2 mechanisms of posttranscriptional regulation of the universal cyclin-dependent kinase inhibitor p21CIP1/WAF1 by iron depletion. Blood 2007, 110, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Q.; Mao, J.-H.; Weise, A.; Mrasek, K.; Fan, X.; Zhang, X.; Liehr, T.; Lu, K.H.; Balmain, A.; et al. An HDAC1-binding domain within FATS bridges p21 turnover to radiation-induced tumorigenesis. Oncogene 2010, 29, 2659–2671. [Google Scholar] [CrossRef] [PubMed]
- Nakakido, M.; Deng, Z.; Suzuki, T.; Dohmae, N.; Nakamura, Y.; Hamamoto, R. PRMT6 Increases Cytoplasmic Localization of p21CDKN1A in Cancer Cells through Arginine Methylation and Makes More Resistant to Cytotoxic Agents. Oncotarget 2015, 6, 30957–30967. [Google Scholar] [CrossRef] [PubMed]
- Teramen, H.; Tsukuda, K.; Tanaka, N.; Ueno, T.; Kubo, T.; Ando, M.; Soh, J.; Asano, H.; Pass, H.I.; Toyooka, S.; et al. Aberrant Methylation of p21 Gene in Lung Cancer and Malignant Pleural Mesothelioma. Acta Medica Okayama 2011, 65, 179–184. [Google Scholar] [PubMed]
- Bott, S.R.J.; Arya, M.; Kirby, R.S.; Williamson, M. p21WAF1/CIP1 gene is inactivated in metastatic prostatic cancer cell lines by promoter methylation. Prostate Cancer Prostatic Dis. 2005, 8, 321–326. [Google Scholar] [CrossRef]
- Askari, M.; Sobti, R.C.; Nikbakht, M.; Sharma, S.C. Aberrant promoter hypermethylation of p21 (WAF1/CIP1) gene and its impact on expression and role of polymorphism in the risk of breast cancer. Mol. Cell. Biochem. 2013, 382, 19–26. [Google Scholar] [CrossRef]
- Roman-Gomez, J.; Castillejo, J.A.; Jimenez, A.; Gonzalez, M.G.; Moreno, F.; del Carmen Rodriguez, M.; Barrios, M.; Maldonado, J.; Torres, A. 5 CpG Island Hypermethylation Is Associated with Transcriptional Silencing of the p21 CIP1/WAF1/SDI1 Gene and Confers Poor Prognosis in Acute Lymphoblastic Leukemia. 2002. Available online: http://ashpublications.org/blood/article-pdf/99/7/2291/1682050/2291.pdf (accessed on 1 January 2023).
- Xu, M.; Gao, J.; Du, Y.Q.; Gao, D.-J.; Zhang, Y.Q.; Li, Z.S.; Zhang, Y.L.; Li, Y.; Gong, Y.F.; Xu, P. Reduction of pancreatic cancer cell viability and induction of apoptosis mediated by siRNA targeting DNMT1 through suppression of total DNA methyltransferase activity. Mol. Med. Rep. 2010, 3, 699–704. [Google Scholar] [CrossRef]
- Dong, C.-H.; Jiang, T.; Yin, H.; Song, H.; Zhang, Y.; Geng, H.; Shi, P.-C.; Xu, Y.-X.; Gao, H.; Liu, L.-Y.; et al. LMNB2 promotes the progression of colorectal cancer by silencing p21 expression. Cell Death Dis. 2021, 12, 331. [Google Scholar] [CrossRef]
- Yamawaki, K.; Ishiguro, T.; Mori, Y.; Yoshihara, K.; Suda, K.; Tamura, R.; Yamaguchi, M.; Sekine, M.; Kashima, K.; Higuchi, M.; et al. Sox2-dependent inhibition of p21 is associated with poor prognosis of endometrial cancer. Cancer Sci. 2017, 108, 632–640. [Google Scholar] [CrossRef]
- Di, J.; Wang, H.; Zhao, Z.; Zhao, G.; Qin, X.; Han, Z.; Liu, Y. CPEB4 Inhibit Cell Proliferation via Upregulating p21 mRNA Stability in Renal Cell Carcinoma. Front. Cell Dev. Biol. 2021, 9, 687253. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, Y.; Yang, J.; Pan, Q.; Zhao, J.; Song, M.; Yang, C.; Han, Y.; Tang, Y.; Wang, Q.; et al. CMTM6 inhibits tumor growth and reverses chemoresistance by preventing ubiquitination of p21 in hepatocellular carcinoma. Cell Death Dis. 2022, 13, 251. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yeh, N.; Zhu, X.-H.; Leversha, M.; Cordon-Cardo, C.; Ghossein, R.; Singh, B.; Holland, E.; Koff, A. Somatic cell type specific gene transfer reveals a tumor-promoting function for p21Waf1/Cip1. EMBO J. 2007, 26, 4683–4693. [Google Scholar] [CrossRef] [PubMed]
- De la Cueva, E.; García-Cao, I.; Herranz, M.; López, P.; García-Palencia, P.; Flores, J.M.; Serrano, M.; Fernández-Piqueras, J.; Martín-Caballero, J. Tumorigenic activity of p21Waf1/Cip1 in thymic lymphoma. Oncogene 2006, 25, 4128–4132. [Google Scholar] [CrossRef] [PubMed]
- Benson, E.K.; Zhao, B.; Sassoon, D.A.; Lee, S.W.; Aaronson, S.A. Effects of p21 deletion in mouse models of premature aging. Cell Cycle 2009, 8, 2002–2004. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Zhang, P.; Harper, J.W.; Elledge, S.J.; Leder, P.; McLean, M. Mice Lacking p21 c~P7/wAF7 Undergo Normal Development, but Are Defective in Gl Checkpoint Control. Cell 1995, 82, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Groza, T.; Gomez, F.L.; Mashhadi, H.H.; Muñoz-Fuentes, V.; Gunes, O.; Wilson, R.; Cacheiro, P.; Frost, A.; Keskivali-Bond, P.; Vardal, B.; et al. The International Mouse Phenotyping Consortium: Comprehensive knockout phenotyping underpinning the study of human disease. Nucleic Acids Res. 2023, 51, D1038–D1045. [Google Scholar] [CrossRef] [PubMed]
- The AACR Project GENIE Consortium. AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov. 2017, 7, 818–831. [Google Scholar] [CrossRef]
- Zhou, B.P.; Liao, Y.; Xia, W.; Spohn, B.; Lee, M.-H.; Hung, M.-C. Cytoplasmic Localization of p21 Cip1/WAF1 by Akt-Induced Phosphorylation in HER-2/Neu-Overexpressing Cells. 2001. Available online: http://cellbio.nature.com (accessed on 1 January 2023).
- Zhou, Y.; Li, G.; Ji, Y.; Liu, C.; Zhu, J.; Lu, Y. Cytoplasmic p21 induced by p65 prevents doxorubicin-induced cell death in pancreatic carcinoma cell line. J. Biomed. Sci. 2012, 19, 15. [Google Scholar] [CrossRef]
- Vincent, A.J.; Ren, S.; Harris, L.G.; Devine, D.J.; Samant, R.S.; Fodstad, O.; Shevde, L.A. Cytoplasmic translocation of p21 mediates NUPR1-induced chemoresistance: NUPR1 and p21 in chemoresistance. FEBS Lett. 2012, 586, 3429–3434. [Google Scholar] [CrossRef]
- Bartha, Á.; Győrffy, B. TNMplot.com: A Web Tool for the Comparison of Gene Expression in Normal, Tumor and Metastatic Tissues. Int. J. Mol. Sci. 2021, 22, 2622. [Google Scholar] [CrossRef]
- Inoue, H.; Hwang, S.H.; Wecksler, A.T.; Hammock, B.D.; Weiss, R.H. Sorafenib attenuates p21 in kidney cancer cells and augments cell death in combination with DNA-damaging chemotherapy. Cancer Biol. Ther. 2011, 12, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Wettersten, H.I.; Hwang, S.H.; Li, C.; Shiu, E.Y.; Wecksler, A.T.; Hammock, B.D.; Weiss, R.H. A novel p21 attenuator which is structurally related to sorafenib. Cancer Biol. Ther. 2013, 14, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Sax, J.K.; Dash, B.C.; Hong, R.; Dicker, D.T.; El-Deiry, W.S. The Cyclin-Dependent Kinase Inhibitor Butyrolactone Is a Potent Inhibitor of p21WAF1/CIP1 Expression. Cell Cycle 2002, 1, 90–96. [Google Scholar] [CrossRef]
- Park, S.-H.; Wang, X.; Liu, R.; Lam, K.S.; Weiss, R.H. High throughput screening of a small molecule one-bead-one-compound combinatorial library to identify attenuators of p21 as chemotherapy sensitizers. Cancer Biol. Ther. 2008, 7, 2015–2022. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; I Wettersten, H.; Park, S.-H.; Weiss, R.H.; Supuran, C.T.; Winum, J.-Y.; Shull, A.Y.; Farrell, C.L.; Wang, R.; Ma, X.; et al. Small-molecule inhibitors of p21 as novel therapeutics for chemotherapy-resistant kidney cancer. Futur. Med. Chem. 2013, 5, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Seto, E. HDACs and HDAC Inhibitors in Cancer Development and Therapy. Cold Spring Harb. Perspect. Med. 2016, 6, a026831. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Tian, Y.; Zhu, W.-G. The Roles of Histone Deacetylases and Their Inhibitors in Cancer Therapy. Front. Cell Dev. Biol. 2020, 8, 576946. [Google Scholar] [CrossRef] [PubMed]
- Gui, C.-Y.; Ngo, L.; Xu, W.S.; Richon, V.M.; Marks, P.A. Histone Deacetylase (HDAC) Inhibitor Activation of p21 WAF1 Involves Changes in Promoter-Associated Proteins, Including HDAC1. 2004. Available online: https://www.pnas.org (accessed on 1 February 2023).
- Bellucci, L.; Dalvai, M.; Kocanova, S.; Moutahir, F.; Bystricky, K. Activation of p21 by HDAC Inhibitors Requires Acetylation of H2A.Z. PLoS ONE 2013, 8, e54102. [Google Scholar] [CrossRef]
- Huang, B.H.; Laban, M.; Leung, C.H.; Lee, L.; Lee, C.K.; Salto-Tellez, M.; Raju, G.C.; Hooi, S.C. Inhibition of histone deacetylase 2 increases apoptosis and p21Cip1/WAF1 expression, independent of histone deacetylase 1. Cell Death Differ. 2005, 12, 395–404. [Google Scholar] [CrossRef]
- Hrgovic, I.; Doll, M.; Kleemann, J.; Wang, X.-F.; Zoeller, N.; Pinter, A.; Kippenberger, S.; Kaufmann, R.; Meissner, M. The histone deacetylase inhibitor trichostatin a decreases lymphangiogenesis by inducing apoptosis and cell cycle arrest via p21-dependent pathways. BMC Cancer 2016, 16, 763. [Google Scholar] [CrossRef]
- Kakiuchi, A.; Kakuki, T.; Ohwada, K.; Kurose, M.; Kondoh, A.; Obata, K.; Nomura, K.; Miyata, R.; Kaneko, Y.; Konno, T.; et al. HDAC inhibitors suppress the proliferation, migration and invasiveness of human head and neck squamous cell carcinoma cells via p63-mediated tight junction molecules and p21-mediated growth arrest. Oncol. Rep. 2021, 45, 46. [Google Scholar] [CrossRef]
- Kavoosi, F.; Sanaei, M.; Safari, M. Effect of 5′-fluoro-2′-deoxycytidine and sodium butyrate on the gene expression of the intrinsic apoptotic pathway, p21, p27, and p53 genes expression, cell viability, and apoptosis in human hepatocellular carcinoma cell lines. Adv. Biomed. Res. 2023, 12, 24. [Google Scholar] [CrossRef]
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A.M. Cell cycle control in cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Suski, J.M.; Braun, M.; Strmiska, V.; Sicinski, P. Targeting cell-cycle machinery in cancer. Cancer Cell 2021, 39, 759–778. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Hannon, G.J.; Zhang, H.; Casso, D.; Kobayashi, R.; Beach, D. p21 is a universal inhibitor of cyclin kinases. Nature 1993, 366, 701–704. [Google Scholar] [CrossRef]
- Harper, J.W.; Adami, G.R.; Wei, N.; Keyomarsi, K.; Elledge, S.J. The p21 Cdk-Interacting Protein Cipl Is a Potent Inhibitor of Gl Cyclin-Dependent Kinases. Cell 1993, 75, 805–816. [Google Scholar] [CrossRef]
- Barr, A.R.; Cooper, S.; Heldt, F.S.; Butera, F.; Stoy, H.; Mansfeld, J.; Novák, B.; Bakal, C. DNA damage during S-phase mediates the proliferation-quiescence decision in the subsequent G1 via p21 expression. Nat. Commun. 2017, 8, 14728. [Google Scholar] [CrossRef] [PubMed]
- Waga, S.; Hannon, G.J.; Beach, D.; Stillman, B. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature 1994, 369, 574–578. [Google Scholar] [CrossRef]
- Bruning, J.B.; Shamoo, Y. Structural and Thermodynamic Analysis of Human PCNA with Peptides Derived from DNA Polymerase-δ p66 Subunit and Flap Endonuclease-1. Structure 2004, 12, 2209–2219. [Google Scholar] [CrossRef]
- Boehm, E.M.; Gildenberg, M.S.; Washington, M.T. The Many Roles of PCNA in Eukaryotic DNA Replication. Enzymes 2016, 39, 231–254. [Google Scholar] [CrossRef] [PubMed]
- Mansilla, S.F.; De La Vega, M.B.; Calzetta, N.L.; Siri, S.O.; Gottifredi, V. CDK-Independent and PCNA-Dependent Functions of p21 in DNA Replication. Genes 2020, 11, 593. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.D.; Watanabe, K.; Broude, E.V.; Fang, J.; Poole, J.C.; Kalinichenko, T.V.; Roninson, I.B. Effects of p21 Waf1/Cip1/Sdi1 on Cellular Gene Expression: Implications for Carcinogenesis, Senescence, and Age-Related Diseases. 2000. Available online: www.pnas.org (accessed on 1 January 2023).
- Kitaura, H.; Shinshi, M.; Uchikoshi, Y.; Ono, T.; Iguchi-Ariga, S.M.; Ariga, H. Reciprocal Regulation via Protein-Protein Interaction between c-Myc and p21 cip1/waf1/sdi1 in DNA Replication and Transcription. J. Biol. Chem. 2000, 275, 10477–10483. [Google Scholar]
- Engeland, K. Cell cycle arrest through indirect transcriptional repression by p53: I have a DREAM. Cell Death Differ. 2018, 25, 114–132. [Google Scholar] [CrossRef]
- Engeland, K. Cell cycle regulation: p53-p21-RB signaling. Cell Death Differ. 2022, 29, 946–960. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.; Kim, K.S.; Park, J.-K.; Um, H.-D. Involvement of the p53/p21 complex in p53-dependent gene expression. Biochem. Biophys. Res. Commun. 2022, 621, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Ferrándiz, N.; Caraballo, J.M.; García-Gutierrez, L.; Devgan, V.; Rodriguez-Paredes, M.; Lafita, M.C.; Bretones, G.; Quintanilla, A.; Muñoz-Alonso, M.J.; Blanco, R.; et al. p21 as a Transcriptional Co-Repressor of S-Phase and Mitotic Control Genes. PLoS ONE 2012, 7, e37759. [Google Scholar] [CrossRef]
- Marqués-Torrejón, M.; Porlan, E.; Banito, A.; Gómez-Ibarlucea, E.; Lopez-Contreras, A.J.; Fernández-Capetillo, O.; Vidal, A.; Gil, J.; Torres, J.; Fariñas, I. Cyclin-Dependent Kinase Inhibitor p21 Controls Adult Neural Stem Cell Expansion by Regulating Sox2 Gene Expression. Cell Stem Cell 2013, 12, 88–100. [Google Scholar] [CrossRef]
- Hopkins, J.L.; Lan, L.; Zou, L. DNA repair defects in cancer and therapeutic opportunities. Minerva Anestesiol. 2022, 36, 278–293. [Google Scholar] [CrossRef]
- Groelly, F.J.; Fawkes, M.; Dagg, R.A.; Blackford, A.N.; Tarsounas, M. Targeting DNA damage response pathways in cancer. Nat. Rev. Cancer 2023, 23, 78–94. [Google Scholar] [CrossRef]
- He, G.; Siddik, Z.H.; Huang, Z.; Wang, R.; Koomen, J.; Kobayashi, R.; Khokhar, A.R.; Kuang, J. Induction of p21 by p53 following DNA damage inhibits both Cdk4 and Cdk2 activities. Oncogene 2005, 24, 2929–2943. [Google Scholar] [CrossRef]
- Cazzalini, O.; Scovassi, A.I.; Savio, M.; Stivala, L.A.; Prosperi, E. Multiple roles of the cell cycle inhibitor p21CDKN1A in the DNA damage response. Mutat. Res. Mol. Mech. Mutagen. 2010, 704, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Mailand, N.; Gibbs-Seymour, I.; Bekker-Jensen, S. Regulation of PCNA–protein interactions for genome stability. Nat. Rev. Mol. Cell Biol. 2013, 14, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Ticli, G.; Cazzalini, O.; Stivala, L.A.; Prosperi, E. Revisiting the Function of p21CDKN1A in DNA Repair: The Influence of Protein Interactions and Stability. Int. J. Mol. Sci. 2022, 23, 7058. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, A.; Punganuru, S.R.; Madala, H.R.; Srivenugopal, K.S. S-phase Specific Downregulation of Human O6-Methylguanine DNA Methyltransferase (MGMT) and its Serendipitous Interactions with PCNA and p21cip1 Proteins in Glioma Cells. Neoplasia 2018, 20, 305–323. [Google Scholar] [CrossRef] [PubMed]
- Sukhanova, M.V.; Khodyreva, S.N.; Lebedeva, N.A.; Prasad, R.; Wilson, S.H.; Lavrik, O.I. Human base excision repair enzymes apurinic/apyrimidinic endonuclease1 (APE1), DNA polymerase β and poly(ADP-ribose) polymerase 1: Interplay between strand-displacement DNA synthesis and proofreading exonuclease activity. Nucleic Acids Res. 2005, 33, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.; Clemente-Blanco, A. Cell Cycle and DNA Repair Regulation in the Damage Response: Protein Phosphatases Take over the Reins. Int. J. Mol. Sci. 2020, 21, 446. [Google Scholar] [CrossRef]
- Nathans, J.F.; Cornwell, J.A.; Afifi, M.M.; Paul, D.; Cappell, S.D. Cell cycle inertia underlies a bifurcation in cell fates after DNA damage. Sci. Adv. 2021, 7, eabe3882. [Google Scholar] [CrossRef]
- Prives, C.; Gottifredi, V. The p21 and PCNA partnership: A new twist for an old plot. Cell Cycle 2008, 7, 3840–3846. [Google Scholar] [CrossRef]
- Soria, G.; Gottifredi, V. PCNA-coupled p21 degradation after DNA damage: The exception that confirms the rule? DNA Repair 2010, 9, 358–364. [Google Scholar] [CrossRef]
- Sheng, C.; Mendler, I.-H.; Rieke, S.; Snyder, P.; Jentsch, M.; Friedrich, D.; Drossel, B.; Loewer, A. PCNA-Mediated Degradation of p21 Coordinates the DNA Damage Response and Cell Cycle Regulation in Individual Cells. Cell Rep. 2019, 27, 48–58.e7. [Google Scholar] [CrossRef]
- Galanos, P.; Vougas, K.; Walter, D.; Polyzos, A.; Maya-Mendoza, A.; Haagensen, E.J.; Kokkalis, A.; Roumelioti, F.-M.; Gagos, S.; Tzetis, M.; et al. Chronic p53-independent p21 expression causes genomic instability by deregulating replication licensing. Nature 2016, 18, 777–789. [Google Scholar] [CrossRef]
- Sousa, F.G.; Matuo, R.; Soares, D.G.; Escargueil, A.E.; Henriques, J.A.; Larsen, A.K.; Saffi, J. PARPs and the DNA damage response. Carcinogenesis 2012, 33, 1433–1440. [Google Scholar] [CrossRef]
- Chaudhuri, A.R.; Nussenzweig, A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat. Rev. Mol. Cell Biol. 2017, 18, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Cazzalini, O.; Donà, F.; Savio, M.; Tillhon, M.; Maccario, C.; Perucca, P.; Stivala, L.A.; Scovassi, A.I.; Prosperi, E. p21CDKN1A participates in base excision repair by regulating the activity of poly(ADP-ribose) polymerase-1. DNA Repair 2010, 9, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Dutto, I.; Sukhanova, M.; Tillhon, M.; Cazzalini, O.; Stivala, L.A.; Scovassi, A.I.; Lavrik, O.; Prosperi, E. p21CDKN1A Regulates the Binding of Poly(ADP-Ribose) Polymerase-1 to DNA Repair Intermediates. PLoS ONE 2016, 11, e0146031. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, H.; West, M.D.; Allsopp, R.C.; Davison, T.S.; Wu, Y.-S.; Arrowsmith, C.H.; Poirier, G.G.; Benchimol, S. ATM-dependent telomere loss in aging human diploid fibroblasts and DNA damage lead to the post-translational activation of p53 protein involving poly(ADP-ribose) polymerase. EMBO J. 1997, 16, 6018–6033. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowski, J.; Schmid, G.; Węsierska-Gądek, J. Central and carboxy-terminal regions of human p53 protein are essential for interaction and complex formation with PARP-1. J. Cell. Biochem. 2003, 89, 220–232. [Google Scholar] [CrossRef]
- Fischbach, A.; Krüger, A.; Hampp, S.; Assmann, G.; Rank, L.; Hufnagel, M.; Stöckl, M.T.; Fischer, J.M.; Veith, S.; Rossatti, P.; et al. The C-terminal domain of p53 orchestrates the interplay between non-covalent and covalent poly(ADP-ribosyl)ation of p53 by PARP1. Nucleic Acids Res. 2018, 46, 804–822. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Ho, T.L.F.; Hariharan, A.; Goh, H.C.; Wong, Y.L.; Verkaik, N.S.; Lee, M.Y.; Tam, W.L.; van Gent, D.C.; Venkitaraman, A.R.; et al. Rapid recruitment of p53 to DNA damage sites directs DNA repair choice and integrity. Proc. Natl. Acad. Sci. USA 2022, 119, e2113233119. [Google Scholar] [CrossRef]
- Xu, M.; Zhao, X.; Zhao, S.; Yang, Z.; Yuan, W.; Han, H.; Zhang, B.; Zhou, L.; Zheng, S.; Li, M.D. Landscape analysis of lncRNAs shows that DDX11-AS1 promotes cell-cycle progression in liver cancer through the PARP1/p53 axis. Cancer Lett. 2021, 520, 282–294. [Google Scholar] [CrossRef]
- Passos, J.F.; Nelson, G.; Wang, C.; Richter, T.; Simillion, C.; Proctor, C.J.; Miwa, S.; Olijslagers, S.; Hallinan, J.; Wipat, A.; et al. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol. Syst. Biol. 2010, 6, 347. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhang, Z.; Zhou, C.; Liu, L.; Huang, C. Epithelial–mesenchymal transition: The history, regulatory mechanism, and cancer therapeutic opportunities. Medcomm 2022, 3, e144. [Google Scholar] [CrossRef] [PubMed]
- Bracken, C.P.; Goodall, G.J. The many regulators of epithelial−mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2022, 23, 89–90. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, W.; Jung, Y.S.; Chen, X. PUMA Cooperates with p21 to Regulate Mammary Epithelial Morphogenesis and Epithelial-To-Mesenchymal Transition. PLoS ONE 2013, 8, e66464. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Casimiro, M.C.; Wang, C.; Shirley, L.A.; Jiao, X.; Katiyar, S.; Ju, X.; Li, Z.; Yu, Z.; Zhou, J.; et al. p21 CIP1 attenuates Ras- and c-Myc-dependent breast tumor epithelial mesenchymal transition and cancer stem cell-like gene expression in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 19035–19039. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, R.; Ye, Z.; Wang, Y.; Li, X.; Chen, W.; Zhang, M.; Cai, C. PVT1 affects EMT and cell proliferation and migration via regulating p21 in triple-negative breast cancer cells cultured with mature adipogenic medium. Acta Biochim. Biophys. Sin. 2018, 50, 1211–1218. [Google Scholar] [CrossRef]
- Wu, B.-Q.; Jiang, Y.; Zhu, F.; Sun, D.-L.; He, X.-Z. Long Noncoding RNA PVT1 Promotes EMT and Cell Proliferation and Migration through Downregulating p21 in Pancreatic Cancer Cells. Technol. Cancer Res. Treat. 2017, 16, 819–827. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L. MiR-149-3p promotes the cisplatin resistance and EMT in ovarian cancer through downregulating TIMP2 and CDKN1A. J. Ovarian Res. 2021, 14, 165. [Google Scholar] [CrossRef]
- Bueno-Fortes, S.; Muenzner, J.K.; Berral-Gonzalez, A.; Hampel, C.; Lindner, P.; Berninger, A.; Huebner, K.; Kunze, P.; Bäuerle, T.; Erlenbach-Wuensch, K.; et al. A Gene Signature Derived from the Loss of CDKN1A (p21) Is Associated with CMS4 Colorectal Cancer. Cancers 2022, 14, 136. [Google Scholar] [CrossRef]
- Li, X.L.; Hara, T.; Choi, Y.; Subramanian, M.; Francis, P.; Bilke, S.; Walker, R.L.; Pineda, M.; Zhu, Y.; Yang, Y.; et al. A p21-ZEB1 Complex Inhibits Epithelial-Mesenchymal Transition through the MicroRNA 183-96-182 Cluster. Mol. Cell. Biol. 2014, 34, 533–550. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Han, M.; Han, H.; Wang, B.; Li, S.; Zhang, Z.; Zhao, W. Silencing Snail suppresses tumor cell proliferation and invasion by reversing epithelial-to-mesenchymal transition and arresting G2/M phase in non-small cell lung cancer. Int. J. Oncol. 2017, 50, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Wang, J.; Jiang, Y.; Qiu, Y.; Xu, M.; Rong, R.; Zhu, T. Snai1-induced partial epithelial–mesenchymal transition orchestrates p53–p21-mediated G2/M arrest in the progression of renal fibrosis via NF-κB-mediated inflammation. Cell Death Dis. 2021, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Zaremba-Czogalla, M.; Hryniewicz-Jankowska, A.; Tabola, R.; Nienartowicz, M.; Stach, K.; Wierzbicki, J.; Cirocchi, R.; Ziolkowski, P.; Tabaczar, S.; Augoff, K. A novel regulatory function of CDKN1A/p21 in TNFα-induced matrix metalloproteinase 9-dependent migration and invasion of triple-negative breast cancer cells. Cell. Signal. 2018, 47, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Weis, S.M.; Cheresh, D.A. Tumor angiogenesis: Molecular pathways and therapeutic targets. Nat. Med. 2011, 17, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-L.; Chen, H.-H.; Zheng, L.-L.; Sun, L.-P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Kuljaca, S.; Liu, T.; Dwarte, T.; Kavallaris, M.; Haber, M.; Norris, M.D.; Martin-Caballero, J.; Marshall, G.M. The cyclin-dependent kinase inhibitor, p21WAF1, promotes angiogenesis by repressing gene transcription of thioredoxin-binding protein 2 in cancer cells. Carcinogenesis 2009, 30, 1865–1871. [Google Scholar] [CrossRef]
- Mo, Y.; Liang, Z.; Lan, L.; Xiong, X.; Zhang, C.; Liu, W.; Huang, H.; Fan, J.; Yang, L. Extracellular vesicles derived from cervical cancer cells carrying MCM3AP-AS1 promote angiogenesis and tumor growth in cervical cancer via the miR-93/p21 axis. Exp. Cell Res. 2023, 428, 113621. [Google Scholar] [CrossRef]
- Ghahremani, M.F.; Goossens, S.; Nittner, D.; Bisteau, X.; Bartunkova, S.; Zwolinska, A.; Hulpiau, P.; Haigh, K.; Haenebalcke, L.; Drogat, B.; et al. p53 promotes VEGF expression and angiogenesis in the absence of an intact p21-Rb pathway. Cell Death Differ. 2013, 20, 888–897. [Google Scholar] [CrossRef]
- Pontes-Quero, S.; Fernández-Chacón, M.; Luo, W.; Lunella, F.F.; Casquero-Garcia, V.; Garcia-Gonzalez, I.; Hermoso, A.; Rocha, S.F.; Bansal, M.; Benedito, R. High mitogenic stimulation arrests angiogenesis. Nat. Commun. 2019, 10, 2016. [Google Scholar] [CrossRef]
- Batlle, E.; Clevers, H. Cancer stem cells revisited. Nat. Med. 2017, 23, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Kharkar, P.S. Cancer Stem Cell (CSC) Inhibitors in Oncology—A Promise for a Better Therapeutic Outcome: State of the Art and Future Perspectives. J. Med. Chem. 2020, 63, 15279–15307. [Google Scholar] [CrossRef] [PubMed]
- Pickup, K.E.; Pardow, F.; Carbonell-Caballero, J.; Lioutas, A.; Villanueva-Cañas, J.L.; Wright, R.H.G.; Beato, M. Expression of Oncogenic Drivers in 3D Cell Culture Depends on Nuclear ATP Synthesis by NUDT5. Cancers 2019, 11, 1337. [Google Scholar] [CrossRef]
- Zhang, X.; Powell, K.; Li, L. Breast Cancer Stem Cells: Biomarkers, Identification and Isolation Methods, Regulating Mechanisms, Cellular Origin, and Beyond. Cancers 2020, 12, 3765. [Google Scholar] [CrossRef] [PubMed]
- Ruijtenberg, S.; van den Heuvel, S. Coordinating cell proliferation and differentiation: Antagonism between cell cycle regulators and cell type-specific gene expression. Cell Cycle 2016, 15, 196–212. [Google Scholar] [CrossRef]
- Xiao, B.-D.; Zhao, Y.-J.; Jia, X.-Y.; Wu, J.; Wang, Y.-G.; Huang, F. Multifaceted p21 in carcinogenesis, stemness of tumor and tumor therapy. World J. Stem Cells 2020, 12, 481–487. [Google Scholar] [CrossRef]
- Sperka, T.; Wang, J.; Rudolph, K.L. DNA damage checkpoints in stem cells, ageing and cancer. Nat. Rev. Mol. Cell Biol. 2012, 13, 579–590. [Google Scholar] [CrossRef]
- Zhong, G.; Qin, S.; Townsend, D.; Schulte, B.A.; Tew, K.D.; Wang, G.Y. Oxidative stress induces senescence in breast cancer stem cells. Biochem. Biophys. Res. Commun. 2019, 514, 1204–1209. [Google Scholar] [CrossRef]
- Troschel, F.M.; Palenta, H.; Borrmann, K.; Heshe, K.; Hua, S.H.; Yip, G.W.; Kiesel, L.; Eich, H.T.; Götte, M.; Greve, B. Knockdown of the prognostic cancer stem cell marker Musashi-1 decreases radio-resistance while enhancing apoptosis in hormone receptor-positive breast cancer cells via p21WAF1/CIP1. J. Cancer Res. Clin. Oncol. 2021, 147, 3299–3312. [Google Scholar] [CrossRef]
- Insinga, A.; Cicalese, A.; Faretta, M.; Gallo, B.; Albano, L.; Ronzoni, S.; Furia, L.; Viale, A.; Pelicci, P.G. DNA damage in stem cells activates p21, inhibits p53, and induces symmetric self-renewing divisions. Proc. Natl. Acad. Sci. USA 2013, 110, 3931–3936. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.; Rubio, R.; Masip, M.; Catalina, P.; Nieto, A.; de la Cueva, T.; Arriero, M.; Martin, N.S.; de la Cueva, E.; Balomenos, D.; et al. Loss of p53 Induces Tumorigenesis in p21-Deficient Mesenchymal Stem Cells. Neoplasia 2009, 11, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.; Choudhury, A.R.; Rudolph, K.L. A Dual Role of p21 in Stem Cell Aging. Ann. N. Y. Acad. Sci. 2007, 1100, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Oh, M.; Sasaki, J.-I.; Nör, J.E. Inverse and reciprocal regulation of p53/p21 and Bmi-1 modulates vasculogenic differentiation of dental pulp stem cells. Cell Death Dis. 2021, 12, 644. [Google Scholar] [CrossRef] [PubMed]
- Chrysanthou, S.; Flores, J.C.; Dawlaty, M.M. Tet1 Suppresses p21 to Ensure Proper Cell Cycle Progression in Embryonic Stem Cells. Cells 2022, 11, 1366. [Google Scholar] [CrossRef]
- Letai, A. Apoptosis and Cancer. Annu. Rev. Cancer Biol. 2017, 1, 275–294. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Neophytou, C.M.; Trougakos, I.P.; Erin, N.; Papageorgis, P. Apoptosis Deregulation and the Development of Cancer Multi-Drug Resistance. Cancers 2021, 13, 4363. [Google Scholar] [CrossRef]
- Prihantono; Faruk, M. Breast cancer resistance to chemotherapy: When should we suspect it and how can we prevent it? Ann. Med. Surg. 2021, 70, 102793. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, M.; Wang, B.; Zhang, L.; Zhou, F.; Fang, M. Chemoresistance and Metastasis in Breast Cancer Molecular Mechanisms and Novel Clinical Strategies. Front. Oncol. 2021, 11, 658552. [Google Scholar] [CrossRef]
- Gartel, A.L. The conflicting roles of the cdk inhibitor p21(CIP1/WAF1) in apoptosis. Leuk. Res. 2005, 29, 1237–1238. [Google Scholar] [CrossRef] [PubMed]
- Jänicke, R.U.; Sohn, D.; Essmann, F.; Schulze-Osthoff, K. The Multiple Battles Fought by Anti-Apoptotic p21. Cell Cycle 2007, 6, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Kawano, H.; Hayashida, M.; Hayasaki, Y.; Tsutomi, Y.; Akahane, K. Procaspase 3/p21 Complex Formation to Resist Fas-Mediated Cell Death Is Initiated as a Result of the Phosphorylation of p21 by Protein Kinase A. Available online: www.nature.com/cdd (accessed on 1 January 2023).
- Shile, H.; Shu, L.; Dilling, M.B.; Easton, J.; Harwood, F.C.; Ichijo, H.; Houghton, P.J. Sustained Activation of the JNK Cascade and Rapamycin-Induced Apoptosis Are Suppressed by p53/p21 Cip1. Mol. Cell 2003, 11, 1491–1501. [Google Scholar]
- Sohn, D.; Essmann, F.; Schulze-Osthoff, K.; Jänicke, R.U. p21 Blocks Irradiation-Induced Apoptosis Downstream of Mitochondria by Inhibition of Cyclin-Dependent Kinase–Mediated Caspase-9 Activation. Cancer Res. 2006, 66, 11254–11262. [Google Scholar] [CrossRef]
- Megyesi, J.; Tarcsafalvi, A.; Seng, N.; Hodeify, R.; Price, P. Cdk2 phosphorylation of Bcl-xL after stress converts it to a pro-apoptotic protein mimicking Bax/Bak. Cell Death Discov. 2016, 2, 15066. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Ray, R.M.; Johnson, L.R. Cyclin-dependent kinases regulate apoptosis of intestinal epithelial cells. Apoptosis 2014, 19, 451–466. [Google Scholar] [CrossRef]
- Kim, E.M.; Jung, C.-H.; Kim, J.; Hwang, S.-G.; Park, J.K.; Um, H.-D. The p53/p21 Complex Regulates Cancer Cell Invasion and Apoptosis by Targeting Bcl-2 Family Proteins. Cancer Res. 2017, 77, 3092–3100. [Google Scholar] [CrossRef]
- Choi, K.-W.; Suh, H.; Oh, H.L.; Ryou, C.; Lee, C.-H. p21CIP1 Induces Apoptosis via Binding to BCL2 in LNCaP Prostate Cancer Cells Treated with MCS-C3, A Novel Carbocyclic Analog of Pyrrolopyrimidine. Anticancer Res. 2016, 36, 213–220. [Google Scholar]
- Wu, S.; Cetinkaya, C.; Munoz-Alonso, M.J.; von der Lehr, N.; Bahram, F.; Beuger, V.; Eilers, M.; Leon, J.; Larsson, L.-G. Myc represses differentiation-induced p21CIP1 expression via Miz-1-dependent interaction with the p21 core promoter. Oncogene 2003, 22, 351–360. [Google Scholar] [CrossRef]
- Hoffman, B.; A Liebermann, D. Apoptotic signaling by c-MYC. Oncogene 2008, 27, 6462–6472. [Google Scholar] [CrossRef]
- Wyld, L.; Bellantuono, I.; Tchkonia, T.; Morgan, J.; Turner, O.; Foss, F.; George, J.; Danson, S.; Kirkland, J.L. Senescence and Cancer: A Review of Clinical Implications of Senescence and Senotherapies. Cancers 2020, 12, 2134. [Google Scholar] [CrossRef]
- Huang, W.; Hickson, L.J.; Eirin, A.; Kirkland, J.L.; Lerman, L.O. Cellular senescence: The good, the bad and the unknown. Nat. Rev. Nephrol. 2022, 18, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Mylonas, A.; O’loghlen, A. Cellular Senescence and Ageing: Mechanisms and Interventions. Front. Aging 2022, 3, 866718. [Google Scholar] [CrossRef] [PubMed]
- Faggioli, F.; Velarde, M.C.; Wiley, C.D. Cellular Senescence, a Novel Area of Investigation for Metastatic Diseases. Cells 2023, 12, 860. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, K.; Komoda, K.; Mikawa, R.; Asai, A.; Sugimoto, M. Cellular senescence promotes cancer metastasis by enhancing soluble E-cadherin production. iScience 2021, 24, 103022. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.; Gewirtz, D.A. Considering therapy-induced senescence as a mechanism of tumour dormancy contributing to disease recurrence. Br. J. Cancer 2022, 126, 1363–1365. [Google Scholar] [CrossRef] [PubMed]
- Perkins, D.W.; Haider, S.; Robertson, D.; Buus, R.; O’leary, L.; Isacke, C.M. Therapy-induced senescence in normal tissue promotes breast cancer metastasis. Oncogene 2022, 41, 4361–4370. [Google Scholar] [CrossRef]
- Gallanis, G.T.; Sharif, G.M.; Schmidt, M.O.; Friedland, B.N.; Battina, R.; Rahhal, R.; Davis, J.E.; Khan, I.S.; Wellstein, A.; Riegel, A.T. Stromal Senescence following Treatment with the CDK4/6 Inhibitor Palbociclib Alters the Lung Metastatic Niche and Increases Metastasis of Drug-Resistant Mammary Cancer Cells. Cancers 2023, 15, 1908. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, W.; Gao, Y.F.; Qin, X.S.; Zhai, Z.H. Senescence-Like Changes Induced by Expression of p21 Waf1/Cip1 in NIH3T3 Cell Line. 2002. Available online: http://www.cell-research.com (accessed on 1 January 2023).
- Englund, D.A.; Jolliffe, A.; Aversa, Z.; Zhang, X.; Sturmlechner, I.; Sakamoto, A.E.; Zeidler, J.D.; Warner, G.M.; McNinch, C.; White, T.A.; et al. p21 induces a senescence program and skeletal muscle dysfunction. Mol. Metab. 2023, 67, 101652. [Google Scholar] [CrossRef] [PubMed]
- Shtutman, M.; Chang, B.D.; Schools, G.P.; Broude, E.V. Cellular model of p21-induced senescence. Methods Mol. Biol. 2017, 1534, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-H.; Altschuler, S.J.; Wu, L.F. Patterns of Early p21 Dynamics Determine Proliferation-Senescence Cell Fate after Chemotherapy. Cell 2019, 178, 361–373.e12. [Google Scholar] [CrossRef] [PubMed]
- Kartika, I.D.; Kotani, H.; Iida, Y.; Koyanagi, A.; Tanino, R.; Harada, M. Protective role of cytoplasmic p21Cip1/Waf1 in apoptosis of CDK4/6 inhibitor-induced senescence in breast cancer cells. Cancer Med. 2021, 10, 8988–8999. [Google Scholar] [CrossRef]
- Anand, J.; Chiou, L.; Sciandra, C.; Zhang, X.; Hong, J.; Wu, D.; Zhou, P.; Vaziri, C. Roles of trans-lesion synthesis (TLS) DNA polymerases in tumorigenesis and cancer therapy. NAR Cancer 2023, 5, zcad005. [Google Scholar] [CrossRef]
- Stanzione, M.; Zhong, J.; Wong, E.; LaSalle, T.J.; Wise, J.F.; Simoneau, A.; Myers, D.T.; Phat, S.; Sade-Feldman, M.; Lawrence, M.S.; et al. Translesion DNA synthesis mediates acquired resistance to olaparib plus temozolomide in small cell lung cancer. Sci. Adv. 2022, 8, eabn1229. [Google Scholar] [CrossRef]
- Zafar, M.K.; Eoff, R.L. Translesion DNA Synthesis in Cancer: Molecular Mechanisms and Therapeutic Opportunities. Chem. Res. Toxicol. 2017, 30, 1942–1955. [Google Scholar] [CrossRef]
| Cancer Type | Mechanism | Description | Ref |
|---|---|---|---|
| Colorectal | LMNB2 binding to the P3- and P6-binding regions of the CDKN1A promoter | Regulation of CDKN1A promoter by LMNB2 results in silencing of p21 expression and promotes cell proliferation in CRC tissues. | [42] |
| Lung | Suppression of CDKN1A via promoter methylation | Transcriptional inactivation of CDKN1A in lung cancer cell lines and MPM. | [37] |
| Prostate | Methylation within the CDKN1Apromoter, at the 5′ end of a CpG island and STAT1-binding site | Inactivation of CDKN1A expression by methylation promotes cell proliferation and DNA replication in metastatic prostate cancer. | [38] |
| Breast | Aberrant hypermethylation of CDKN1A promoter | Increased risk of breast cancer in hypermethylated CDKN1A promoter reduces CDKN1A mRNA expression in all age groups. Inhibition of G1 arrest. | [39] |
| Lymphoblastic leukaemia | Hypermethylation of CpG islands within the CDKN1A promoter regions led to a decrease in CDKN1A expression | Loss of CDKN1A is associated with disease progression and poorer DFS. Selective growth advantage and accelerated onset of other pathways. | [40] |
| Oligodendrogliomas | In the nucleus, p21 promotes ODG by stabilising cyclin D1–cdk4. Cytosolic p21 binds procaspase 3, desensitising apoptotic stimuli | PDGF signalling produces p21 accumulation in ODG, which associates with cyclinD-cdk4 complexes and increases proliferation. Binding to cytosolic components reduced apoptosis. | [46] |
| Renal | CPEB4 binds to the CDKN1A transcripts and stabilises its mRNA, increasing CDKN1A expression | Increasing CDKN1A expression modulates RCC cell proliferation by inhibiting cell cycle progression due to G1 cell cycle arrest. | [44] |
| Lymphomas | p21 provides survival to TNF- or Fas-triggered apoptosis by binding and inhibiting caspase-3 | Absence of p21 increases lifespan due to reduced incidence of thymic lymphomas. p21-proficient lymphomas have faster growth due to lower apoptotic rate. | [47] |
| Hepatocellular | p21 is stabilised by the repression of ubiquitin-proteasome proteolysis due to CMTM6 binding | Extension of CDKN1A half-life by CMTM6 suppresses HCC cell proliferation, sensitising patients to treatment and increasing survival rates. | [45] |
| Endometrial | p21/CDKN1A gene transcription is repressed via SOX2 binding to the CDKN1A promoter | Inhibition of CDKN1A by SOX2 overexpression correlates with poor prognosis in endometrial cancer due to cell cycle progression. | [43] |
| Pancreatic | DNMT1 silencing results in the upregulation of p21 expression | Upregulation of p21 decreases cell proliferation in pancreatic cancer cell lines. | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manousakis, E.; Miralles, C.M.; Esquerda, M.G.; Wright, R.H.G. CDKN1A/p21 in Breast Cancer: Part of the Problem, or Part of the Solution? Int. J. Mol. Sci. 2023, 24, 17488. https://doi.org/10.3390/ijms242417488
Manousakis E, Miralles CM, Esquerda MG, Wright RHG. CDKN1A/p21 in Breast Cancer: Part of the Problem, or Part of the Solution? International Journal of Molecular Sciences. 2023; 24(24):17488. https://doi.org/10.3390/ijms242417488
Chicago/Turabian StyleManousakis, Evangelos, Clàudia Martinez Miralles, Maria Guimerà Esquerda, and Roni H. G. Wright. 2023. "CDKN1A/p21 in Breast Cancer: Part of the Problem, or Part of the Solution?" International Journal of Molecular Sciences 24, no. 24: 17488. https://doi.org/10.3390/ijms242417488
APA StyleManousakis, E., Miralles, C. M., Esquerda, M. G., & Wright, R. H. G. (2023). CDKN1A/p21 in Breast Cancer: Part of the Problem, or Part of the Solution? International Journal of Molecular Sciences, 24(24), 17488. https://doi.org/10.3390/ijms242417488






