Molecular Mechanisms Linking Diabetes with Increased Risk of Thrombosis
Abstract
1. Introduction
2. Mechanisms Influencing Thrombosis
2.1. Endothelial Dysfunction
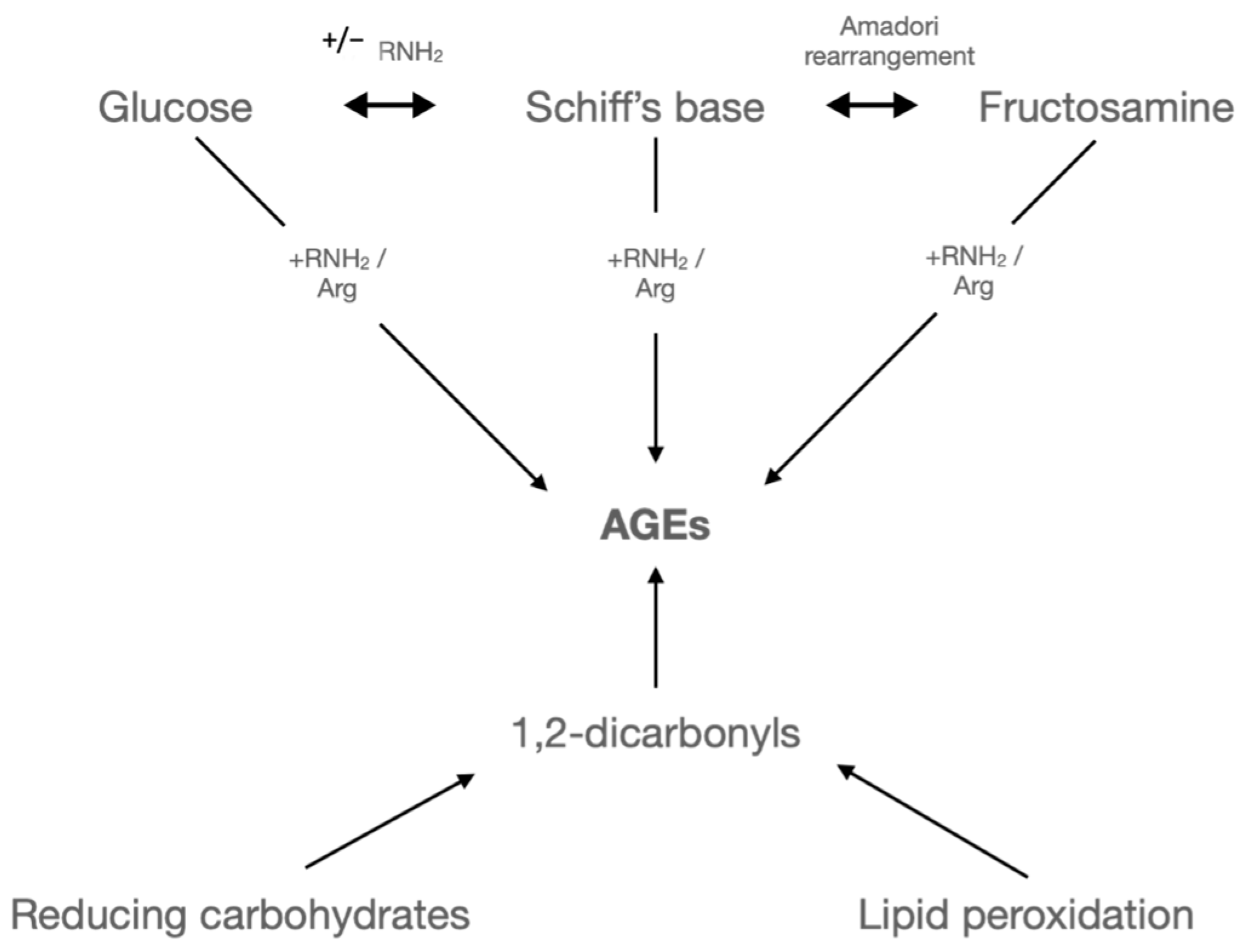
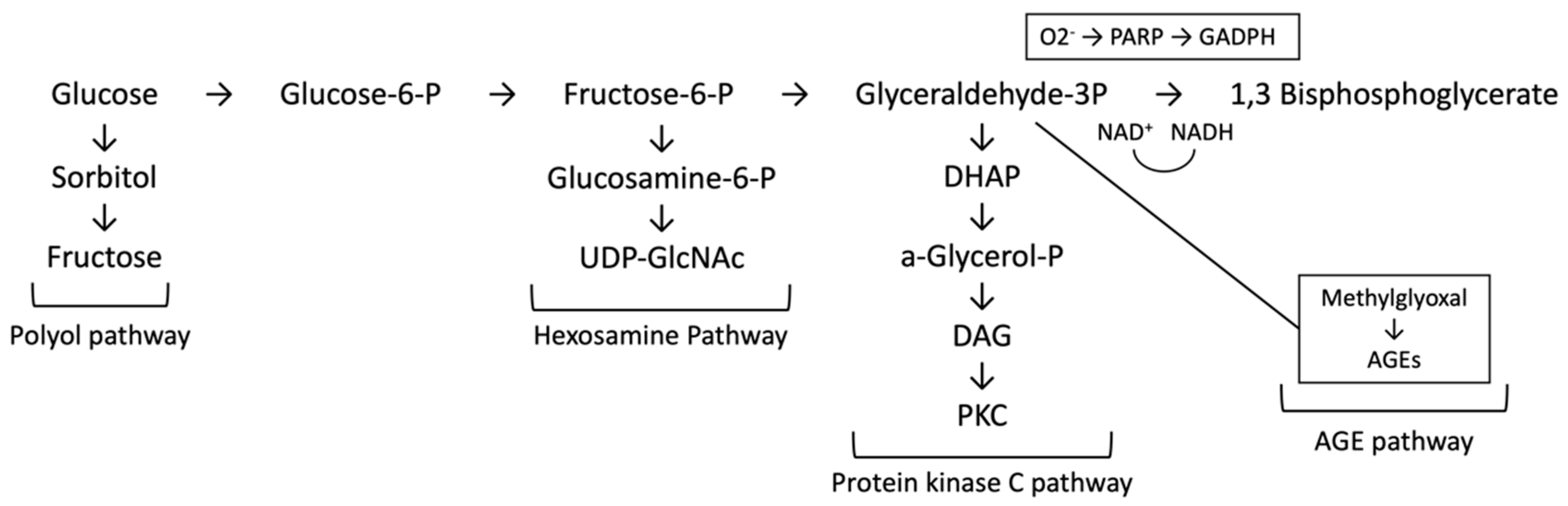
2.2. Oxidative Stress
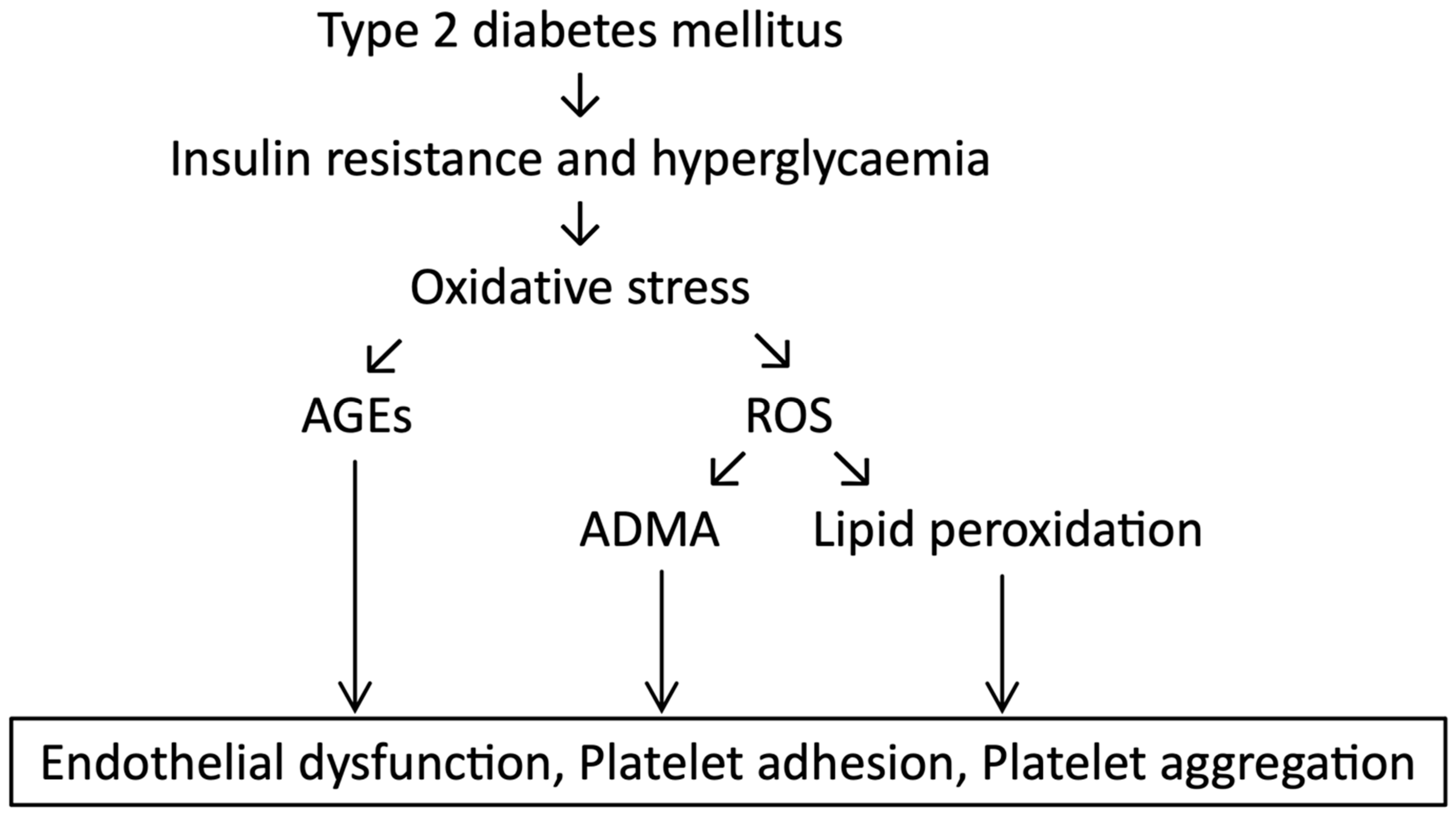
2.3. Platelet Hyperactivity
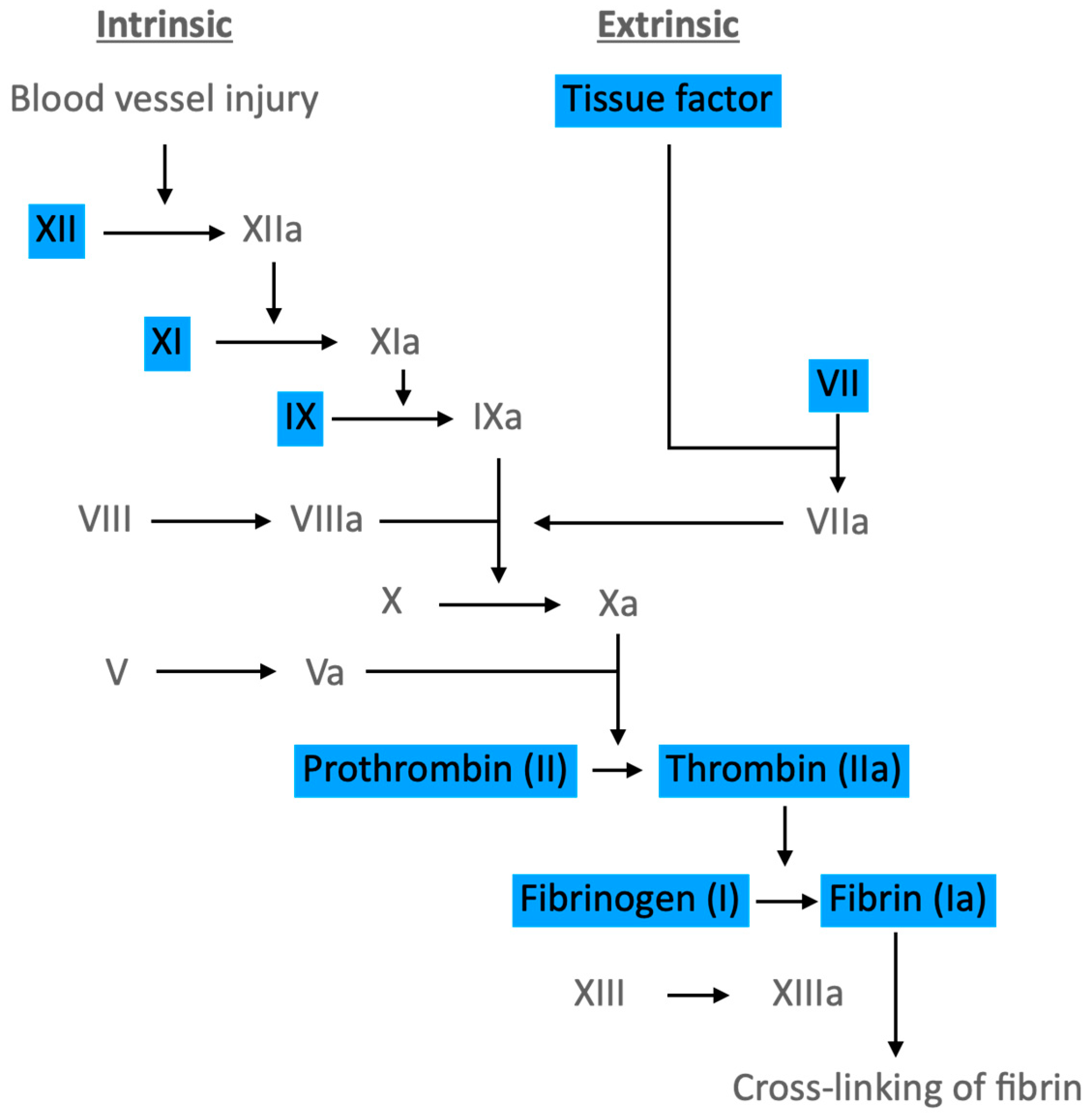
2.4. Coagulation Pathways
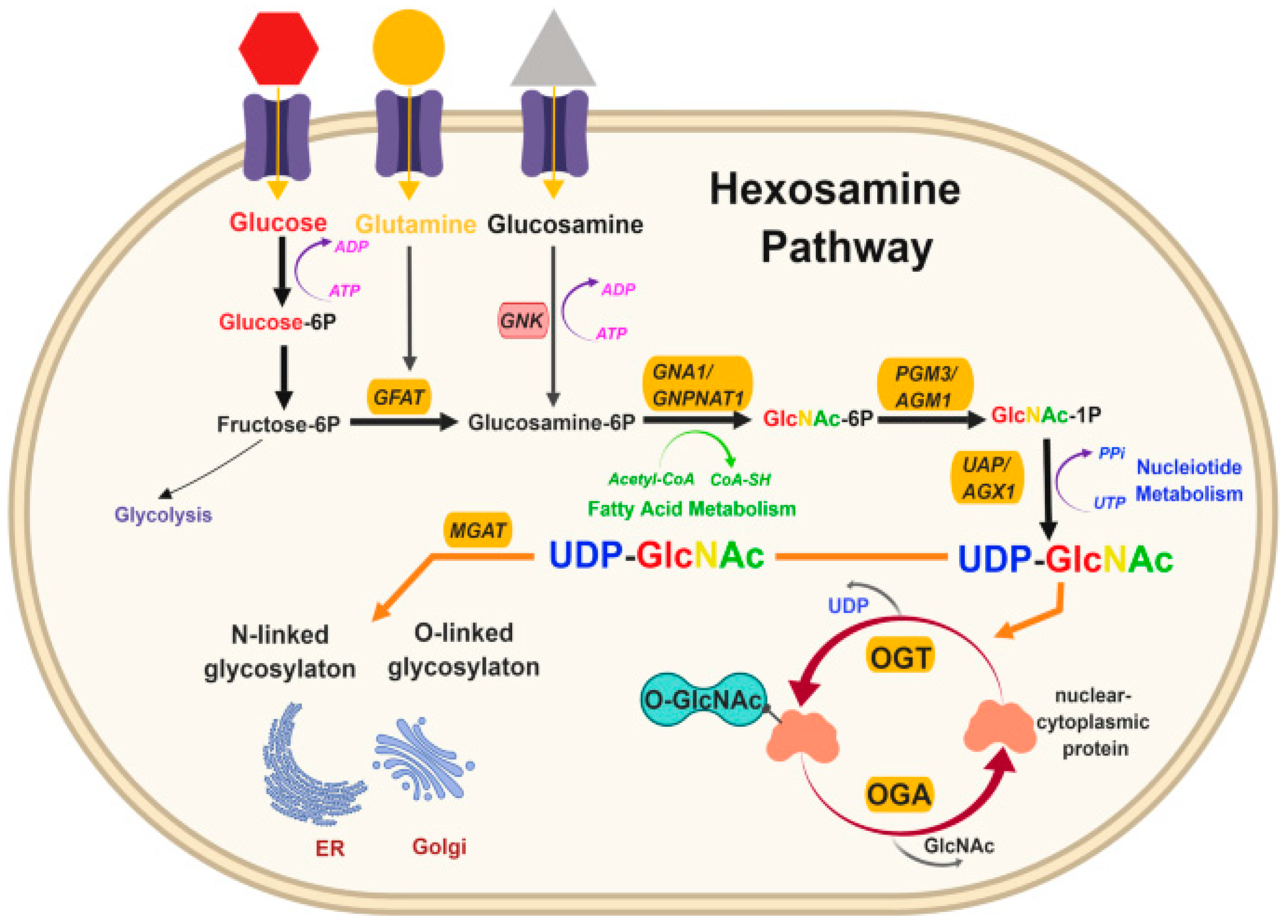
2.5. Post-Translational Modifications
O-GlcNAcylation
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organisation. Global Report on Diabetes; World Health Organisation: Geneva, Switzerland, 2016. [Google Scholar]
- The Cost of Diabetes: Report diabetes.org.uk: Diabetes UK. 2019. Available online: https://www.diabetes.co.uk/cost-of-diabetes.html (accessed on 25 January 2023).
- Nowakowska, M.; Zghebi, S.S.; Ashcroft, D.M.; Buchan, I.; Chew-Graham, C.; Holt, T.; Mallen, C.; Marwijk, H.V.; Peek, N.; Kontopantelis, E.; et al. The comorbidity burden of type 2 diabetes mellitus: Patterns, clusters and predictions from a large English primary care cohort. BMC Med. 2019, 17, 145. [Google Scholar] [CrossRef]
- Byon, C.H.; Kim, S.W. Regulatory Effects of O-GlcNAcylation in Vascular Smooth Muscle Cells on Diabetic Vasculopathy. J. Lipid Atheroscler. 2020, 9, 243–254. [Google Scholar] [CrossRef]
- Landman, G.W.; van Hateren, K.J.; Kleefstra, N.; Groenier, K.H.; Gans, R.O.; Bilo, H.J. The relationship between glycaemic control and mortality in patients with type 2 diabetes in general practice (ZODIAC-11). Br. J. Gen. Pract. 2010, 60, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Pechlivani, N.; Ajjan, R.A. Thrombosis and Vascular Inflammation in Diabetes: Mechanisms and Potential Therapeutic Targets. Front. Cardiovasc. Med. 2018, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; Turgeon, H.; Regan, S. Relationship between glycated haemoglobin levels and mean glucose levels over time. Diabetologia 2007, 50, 2239–2244. [Google Scholar] [CrossRef] [PubMed]
- Crowley, M.J.; Holleman, R.; Klamerus, M.L.; Bosworth, H.B.; Edelman, D.; Heisler, M. Factors associated with persistent poorly controlled diabetes mellitus: Clues to improving management in patients with resistant poor control. Chronic Illn. 2014, 10, 291–302. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Exellence. Type 2 Diabetes in Adults: Management [Guideline]. 2022. Available online: https://www.nice.org.uk/guidance/ng28/chapter/Recommendations#hba1c-measurement-and-targets (accessed on 29 June 2022).
- Ajjan, R.A.; Kietsiriroje, N.; Badimon, L.; Vilahur, G.; Gorog, D.A.; Angiolillo, D.J.; Russell, D.A.; Rocca, B.; Storey, R.F. Antithrombotic therapy in diabetes: Which, when, and for how long? Eur. Heart J. 2021, 42, 2235–2259. [Google Scholar] [CrossRef]
- Holzmann, M.J.; Rathsman, B.; Eliasson, B.; Kuhl, J.; Svensson, A.M.; Nyström, T.; Sartipy, U. Long-Term Prognosis in Patients With Type 1 and 2 Diabetes Mellitus After Coronary Artery Bypass Grafting. J. Am. Coll. Cardiol. 2015, 65, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- Godo, S.; Shimokawa, H. Endothelial Functions. Arterioscler Thromb. Vasc. Biol. 2017, 37, e108–e114. [Google Scholar] [CrossRef]
- Schmidt, A.-M.; Hori, O.; Brett, J.; Yan, S.D.; Wautier, J.-L.; Stern, D. Cellular receptors for advanced glycation end products. Implications for induction of oxidant stress and cellular dysfunction in the pathogenesis of vascular lesions. Arterioscler. Thromb. J. Vasc. Biol. 1994, 14, 1521–1528. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Ott, C.; Jacobs, K.; Haucke, E.; Navarrete Santos, A.; Grune, T.; Simm, A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.-Y.; Cooper, M.E. The Role of Advanced Glycation End Products in Progression and Complications of Diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- McCance, D.R.; Dyer, D.G.; Dunn, J.A.; Bailie, K.E.; Thorpe, S.R.; Baynes, J.W.; Lyons, T.J. Maillard reaction products and their relation to complications in insulin-dependent diabetes mellitus. J. Clin. Investig. 1993, 91, 2470–2478. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, C.; Bucci, I.; Napolitano, G. The Role of the Transcription Factor Nuclear Factor-kappa B in Thyroid Autoimmunity and Cancer. Front. Endocrinol. 2018, 9, 471. [Google Scholar] [CrossRef]
- Wautier, J.L.; Wautier, M.P. Endothelial Cell Participation in Inflammatory Reaction. Int. J. Mol. Sci. 2021, 22, 6341. [Google Scholar] [CrossRef] [PubMed]
- Devangelio, E.; Santilli, F.; Formoso, G.; Ferroni, P.; Bucciarelli, L.; Michetti, N.; Clissa, C.; Ciabattoni, G.; Consoli, A.; Davi, G. Soluble RAGE in type 2 diabetes: Association with oxidative stress. Free Radic. Biol. Med. 2007, 43, 511–518. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Matsui, T.; Takeuchi, M.; Yamagishi, S. Metformin Inhibits Advanced Glycation End Products (AGEs)-induced Renal Tubular Cell Injury by Suppressing Reactive Oxygen Species Generation via Reducing Receptor for AGEs (RAGE) Expression. Horm. Metab. Res. 2012, 44, 891–895. [Google Scholar] [CrossRef]
- Kunt, T.; Forst, T.; Wilhelm, A.; Tritschler, H.; Pfuetzner, A.; Harzer, O.; Engelbach, M.; Zschaebitz, A.; Stofft, E.; Beyer, J. α-lipoic acid reduces expression of vascular cell adhesion molecule-1 and endothelial adhesion of human monocytes after stimulation with advanced glycation end products. Clin. Sci. 1999, 96, 75–82. [Google Scholar] [CrossRef]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef]
- ÖZTÜRK, Z. Diabetes, oxidative stress and endothelial dysfunction. Bezmialem Sci. 2019, 7, 52. [Google Scholar] [CrossRef]
- Elahy, M.; Baindur-Hudson, S.; Cruzat, V.F.; Newsholme, P.; Dass, C.R. Mechanisms of PEDF-mediated protection against reactive oxygen species damage in diabetic retinopathy and neuropathy. J. Endocrinol. 2014, 222, R129–R139. [Google Scholar] [CrossRef] [PubMed]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress-A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Papachristoforou, E.; Lambadiari, V.; Maratou, E.; Makrilakis, K. Association of Glycemic Indices (Hyperglycemia, Glucose Variability, and Hypoglycemia) with Oxidative Stress and Diabetic Complications. J. Diabetes Res. 2020, 2020, 7489795. [Google Scholar] [CrossRef]
- Brownlee, M. The Pathobiology of Diabetic Complications: A Unifying Mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Ceriello, A.; Ihnat, M.A. ‘Glycaemic variability’: A new therapeutic challenge in diabetes and the critical care setting. Diabet. Med. 2010, 27, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Saisho, Y. Glycemic variability and oxidative stress: A link between diabetes and cardiovascular disease? Int. J. Mol. Sci. 2014, 15, 18381–18406. [Google Scholar] [CrossRef]
- de M Bandeira, S.; da Fonseca, L.J.; da S Guedes, G.; Rabelo, L.A.; Goulart, M.O.; Vasconcelos, S.M. Oxidative stress as an underlying contributor in the development of chronic complications in diabetes mellitus. Int. J. Mol. Sci. 2013, 14, 3265–3284. [Google Scholar] [CrossRef]
- Obrosova, I.G. Diabetic painful and insensate neuropathy: Pathogenesis and potential treatments. Neurotherapeutics 2009, 6, 638–647. [Google Scholar] [CrossRef]
- Vazzana, N.; Ranalli, P.; Cuccurullo, C.; Davì, G. Diabetes mellitus and thrombosis. Thromb. Res. 2012, 129, 371–377. [Google Scholar] [CrossRef]
- Gerrard, J.M.; Stuart, M.J.; Rao, G.H.R.; Steffes, M.W.; Mauer, S.M.; Brown, D.M.; White, J.G. Alteration in the balance of prostaglandin and thromboxane synthesis in diabetic rats. J. Lab. Clin. Med. 1980, 95, 950–958. [Google Scholar] [PubMed]
- Chung, A.W.Y.; Jurasz, P.; Hollenberg, M.D.; Radomski, M.W. Mechanisms of action of proteinase-activated receptor agonists on human platelets. Br. J. Pharmacol. 2002, 135, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Colwell, J.A.; Nair, R.M.; Halushka, P.V.; Rogers, C.; Whetsell, A.; Sagel, J. Platelet adhesion and aggregation in diabetes mellitus. Metabolism 1979, 28 (Suppl. 1), 394–400. [Google Scholar] [CrossRef]
- Karpatkin, S. Heterogeneity of human platelets. I. Metabolic and kinetic evidence suggestive of young and old platelets. J. Clin. Investig. 1969, 48, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Santilli, F.; Simeone, P.; Liani, R. 27—The Role of Platelets in Diabetes Mellitus. In Platelets, 4th ed.; Michelson, A.D., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 469–503. [Google Scholar]
- Hekimsoy, Z.; Payzin, B.; Örnek, T.; Kandogan, G. Mean platelet volume in Type 2 diabetic patients. J. Diabetes Its Complicat. 2004, 18, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.L.; Febbraio, M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci. Signal 2009, 2, re3. [Google Scholar] [CrossRef]
- Caligiuri, G. CD31 as a Therapeutic Target in Atherosclerosis. Circ. Res. 2020, 126, 1178–1189. [Google Scholar] [CrossRef]
- Abrams, C.S.; Ellison, N.; Budzynski, A.Z.; Shattil, S.J. Direct detection of activated platelets and platelet-derived microparticles in humans. Blood 1990, 75, 128–138. [Google Scholar] [CrossRef]
- Tschoepe, D.; Spangenberg, P.; Esser, J.; Schwippert, B.; Kehrel, B.; Roesen, P.; Gries, F.A. Flow-cytometric detection of surface membrane alterations and concomitant changes in the cytoskeletal actin status of activated platelets. Cytometry 1990, 11, 652–656. [Google Scholar] [CrossRef]
- Cicmil, M.; Thomas, J.M.; Leduc, M.; Bon, C.; Gibbins, J.M. Platelet endothelial cell adhesion molecule-1 signaling inhibits the activation of human platelets. Blood 2002, 99, 137–144. [Google Scholar] [CrossRef]
- Omoto, S.; Nomura, S.; Shouzu, A.; Hayakawa, T.; Shimizu, H.; Miyake, Y.; Yonemoto, T.; Nishikawa, M.; Fukuhara, S.; Inada, M. Significance of platelet-derived microparticles and activated platelets in diabetic nephropathy. Nephron 1999, 81, 271–277. [Google Scholar] [CrossRef]
- Israels, S.J.; McNicol, A.; Dean, H.J.; Cognasse, F.; Sellers, E.A.C. Markers of Platelet Activation Are Increased in Adolescents With Type 2 Diabetes. Diabetes Care 2014, 37, 2400–2403. [Google Scholar] [CrossRef] [PubMed]
- Eibl, N.; Krugluger, W.; Streit, G.; Schrattbauer, K.; Hopmeier, P.; Schernthaner, G. Improved metabolic control decreases platelet activation markers in patients with type-2 diabetes. Eur. J. Clin. Investig. 2004, 34, 205–209. [Google Scholar] [CrossRef]
- Martin, S. Soluble adhesion molecules in type 1 diabetes mellitus. Horm. Metab. Res. 1997, 29, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Daviet, L.; McGregor, J.L. Vascular biology of CD36: Roles of this new adhesion molecule family in different disease states. Thromb. Haemost. 1997, 78, 65–69. [Google Scholar] [CrossRef]
- Kashyap, S.R.; Ioachimescu, A.G.; Gornik, H.L.; Gopan, T.; Davidson, M.B.; Makdissi, A.; Major, J.; Febbraio, M.; Silverstein, R.L. Lipid-induced insulin resistance is associated with increased monocyte expression of scavenger receptor CD36 and internalization of oxidized LDL. Obesity 2009, 17, 2142–2148. [Google Scholar] [CrossRef] [PubMed]
- Collot-Teixeira, S.; Martin, J.; McDermott-Roe, C.; Poston, R.; McGregor, J.L. CD36 and macrophages in atherosclerosis. Cardiovasc. Res. 2007, 75, 468–477. [Google Scholar] [CrossRef]
- Koonen, D.; Jensen, M.; Handberg, A. Soluble CD36- a marker of the (pathophysiological) role of CD36 in the metabolic syndrome? Arch. Physiol. Biochem. 2011, 117, 57–63. [Google Scholar] [CrossRef]
- Masselli, E.; Pozzi, G.; Vaccarezza, M.; Mirandola, P.; Galli, D.; Vitale, M.; Carubbi, C.; Gobbi, G. ROS in Platelet Biology: Functional Aspects and Methodological Insights. Int. J. Mol. Sci. 2020, 21, 4866. [Google Scholar] [CrossRef]
- Magwenzi, S.; Woodward, C.; Wraith, K.; Aburima, A.; Raslan, Z.; Jones, H.; McNeil, C.; Wheatcroft, S.; Yuldasheva, N.; Naseem, K.M.; et al. Oxidized LDL activates blood platelets through CD36/NOX2–mediated inhibition of the cGMP/protein kinase G signaling cascade. Blood 2015, 125, 2693–2703. [Google Scholar] [CrossRef]
- Mckenzie, S. Macrophage to Foam Cell Differentiation Pathway News-Medical. 2018. Available online: https://www.news-medical.net/life-sciences/Macrophage-to-Foam-Cell-Differentiation-Pathway.aspx (accessed on 17 October 2022).
- Febbraio, M.; Podrez, E.A.; Smith, J.D.; Hajjar, D.P.; Hazen, S.L.; Hoff, H.F.; Sharma, K.; Silverstein, R.L. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J. Clin. Investig. 2000, 105, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M. CD36, a scavenger receptor implicated in atherosclerosis. Exp. Mol. Med. 2014, 46, e99. [Google Scholar] [CrossRef] [PubMed]
- Gresele, P.; Guglielmini, G.; De Angelis, M.; Ciferri, S.; Ciofetta, M.; Falcinelli, E.; Lalli, C.; Ciabattoin, G.; Davì, G.; Bolli, G.B. Acute, short-term hyperglycemia enhances shear stress-induced platelet activation in patients with Type II diabetes mellitus. J. Am. Coll. Cardiol. 2003, 41, 1013–1020. [Google Scholar] [CrossRef]
- Undas, A.; Wiek, I.; Stêpień, E.; Zmudka, K.; Tracz, W. Hyperglycemia Is Associated With Enhanced Thrombin Formation, Platelet Activation, and Fibrin Clot Resistance to Lysis in Patients With Acute Coronary Syndrome. Diabetes Care 2008, 31, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Gaiz, A.; Mosawy, S.; Colson, N.; Singh, I. Thrombotic and cardiovascular risks in type two diabetes; Role of platelet hyperactivity. Biomed. Pharmacother. 2017, 94, 679–686. [Google Scholar] [CrossRef]
- Davidson, K.W.; Barry, M.J.; Mangione, C.M.; US Preventive Services Task Force. Aspirin use to prevent cardiovascular disease: US preventive services task force recommendation statement. JAMA 2022, 327, 1577–1584. [Google Scholar]
- Sierra, C.; Moreno, M.; García-Ruiz, J.C. The physiology of hemostasis. Blood Coagul. Fibrinolysis 2022, 33 (Suppl. S1), S1–S2. [Google Scholar] [CrossRef]
- Gale, A.J. Continuing education course #2: Current understanding of hemostasis. Toxicol. Pathol. 2011, 39, 273–280. [Google Scholar]
- Chaudhry, R.; Usama, S.M.; Babiker, H.M. Physiology, Coagulation Pathways; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Lemkes, B.A.; Hermanides, J.; Devries, J.H.; Holleman, F.; Meijers, J.C.M.; Hoekstra, J.B.L. Hyperglycemia: A prothrombotic factor? J. Thromb. Haemost. 2010, 8, 1663–1669. [Google Scholar] [CrossRef]
- Cefalu, W.T.; Schneider, D.J.; Carlson, H.E.; Migdal, P.; Gan Lim, L.; Izon, M.P.; Kapoor, A.; Bell-Farrow, A.; Terry, J.G.; Sobel, B.E. Effect of combination glipizide GITS/metformin on fibrinolytic and metabolic parameters in poorly controlled type 2 diabetic subjects. Diabetes Care 2002, 25, 2123–2128. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Giugliano, D.; Quatraro, A.; Dello Russo, P.; Torella, R. Blood glucose may condition factor VII levels in diabetic and normal subjects. Diabetologia 1988, 31, 889–891. [Google Scholar] [CrossRef]
- Shrestha, S.K. Simple Coagulation Cascade with Mnemonics Epomedicine. 2017. Available online: https://epomedicine.com/medical-students/simple-coagulation-cascade-mnemonics/ (accessed on 19 October 2022).
- Sambola, A.; Osende, J.; Hathcock, J.; Degen, M.; Nemerson, Y.; Fuster, V.; Crandall, J.; Badimon, J.J. Role of risk factors in the modulation of tissue factor activity and blood thrombogenicity. Circulation 2003, 107, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Boden, G.; Vaidyula, V.R.; Homko, C.; Cheung, P.; Rao, A.K. Circulating tissue factor procoagulant activity and thrombin generation in patients with type 2 diabetes: Effects of insulin and glucose. J. Clin. Endocrinol. Metab. 2007, 92, 4352–4358. [Google Scholar] [CrossRef]
- Hayden, M.R.; Tyagi, S.C.; Kerklo, M.M.; Nicolls, M.R. Type 2 diabetes mellitus as a conformational disease. Jop 2005, 6, 287–302. [Google Scholar]
- Harding, J.J.; Ganea, E. Protection against glycation and similar post-translational modifications of proteins. Biochim. Biophys. Acta 2006, 1764, 1436–1446. [Google Scholar] [CrossRef]
- Chatterjee, M.; Thakur, S.S. Investigation of post-translational modifications in type 2 diabetes. Clin. Proteom. 2018, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Szabat, M.; Lynn, F.C.; Hoffman, B.G.; Kieffer, T.J.; Allan, D.W.; Johnson, J.D. Maintenance of β-cell maturity and plasticity in the adult pancreas: Developmental biology concepts in adult physiology. Diabetes 2012, 61, 1365–1371. [Google Scholar] [CrossRef]
- Gual, P.; Le Marchand-Brustel, Y.; Tanti, J.F. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie 2005, 87, 99–109. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, F.; Bai, M.; Liu, Y.; Zhang, L.; Zhu, Q.; Bi, Y.; Ning, G.; Zhou, L.; Wang, X. The pivotal role of protein acetylation in linking glucose and fatty acid metabolism to β-cell function. Cell Death Disease 2019, 10, 66. [Google Scholar] [CrossRef]
- Choudhary, C.; Kumar, C.; Gnad, F.; Nielsen, M.L.; Rehman, M.; Walther, T.C.; Olsen, J.V.; Mann, M. Lysine Acetylation Targets Protein Complexes and Co-Regulates Major Cellular Functions. Science 2009, 325, 834–840. [Google Scholar] [CrossRef]
- diabetes.co.uk. Diabetes and Heart Disease [Website]. 2019. Available online: https://www.diabetes.co.uk/diabetes-complications/heart-disease.html (accessed on 10 June 2022).
- Paneque, A.; Fortus, H.; Zheng, J.; Werlen, G.; Jacinto, E. The Hexosamine Biosynthesis Pathway: Regulation and Function. Genes 2023, 14, 933. [Google Scholar] [CrossRef] [PubMed]
- Maynard, J.C.; Chalkley, R.J. Methods for Enrichment and Assignment of N-Acetylglucosamine Modification Sites. Mol. Cell. Proteom. 2021, 20, 100031. [Google Scholar] [CrossRef]
- Comer, F.I.; Vosseller, K.; Wells, L.; Accavitti, M.A.; Hart, G.W. Characterization of a Mouse Monoclonal Antibody Specific for O-Linked N-Acetylglucosamine. Anal. Biochem. 2001, 293, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Snow, C.M.; Senior, A.; Gerace, L. Monoclonal antibodies identify a group of nuclear pore complex glycoproteins. J. Cell Biol. 1987, 104, 1143–1156. [Google Scholar] [CrossRef]
- Monsigny, M.; Sene, C.; Obrenovitch, A.; Roche, A.C.; Delmotte, F.; Boschetti, E. Properties of succinylated wheat-germ agglutinin. Eur. J. Biochem. 1979, 98, 39–45. [Google Scholar] [CrossRef]
- Nakanuma, Y.; Sasaki, M.; Kono, N. Succinylated wheat germ agglutinin lectin binding in intrahepatic vessels. A New Histochem. Tool. Arch. Pathol. Lab. Med. 1993, 117, 809–811. [Google Scholar]
- Machon, O.; Baldini, S.F.; Ribeiro, J.P.; Steenackers, A.; Varrot, A.; Lefebvre, T.; Imberty, A. Recombinant fungal lectin as a new tool to investigate O-GlcNAcylation processes. Glycobiology 2017, 27, 123–128. [Google Scholar] [CrossRef]
- Mariappa, D.; Selvan, N.; Borodkin, V.; Alonso, J.; Ferenbach, A.T.; Shepherd, C.; Hopkins-Navratilova, I.; van Aalten, D.M.F. A mutant O-GlcNAcase as a probe to reveal global dynamics of protein O-GlcNAcylation during Drosophila embryonic development. Biochem. J. 2015, 470, 255–262. [Google Scholar] [CrossRef]
- Selvan, N.; Williamson, R.; Mariappa, D.; Campbell, D.G.; Gourlay, R.; Ferenbach, A.T.; Aristotelous, T.; Hopkins-Navratilova, I.; Trost, M.; van Aalten, D.M.F. A mutant O-GlcNAcase enriches Drosophila developmental regulators. Nat. Chem. Biol. 2017, 13, 882–887. [Google Scholar] [CrossRef]
- Zhu, Q.; Yi, W. Chemistry-Assisted Proteomic Profiling of O-GlcNAcylation. Front. Chem. 2021, 9, 702260. [Google Scholar] [CrossRef] [PubMed]
- Prescher, J.A.; Bertozzi, C.R. Chemistry in living systems. Nat. Chem. Biol. 2005, 1, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Hahne, H.; Sobotzki, N.; Nyberg, T.; Helm, D.; Borodkin, V.S.; van Aalten, D.M.; Agnew, B.; Kuster, B. Proteome wide purification and identification of O-GlcNAc-modified proteins using click chemistry and mass spectrometry. J. Proteome Res. 2013, 12, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, J.; Wen, L.; Zhu, H.; Li, S.; Huang, K.; Jiang, K.; Li, X.; Ma, C.; Wang, P.G.; et al. An OGA-Resistant Probe Allows Specific Visualization and Accurate Identification of O-GlcNAc-Modified Proteins in Cells. ACS Chem. Biol. 2016, 11, 3002–3006. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wei, S.; Ji, Y.; Guo, X.; Yang, F. Quantitative proteomics using SILAC: Principles, applications, and developments. Proteomics 2015, 15, 3175–3192. [Google Scholar] [CrossRef] [PubMed]
- Greis, K.D.; Hayes, B.K.; Comer, F.I.; Kirk, M.; Barnes, S.; Lowary, T.L.; Hart, G.W. Selective detection and site-analysis of O-GlcNAc-modified glycopeptides by beta-elimination and tandem electrospray mass spectrometry. Anal. Biochem. 1996, 234, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Whelan, S.A.; Hart, G.W. Identification of O-GlcNAc sites on proteins. Methods Enzymol. 2006, 415, 113–133. [Google Scholar] [PubMed]
- Wells, L.; Vosseller, K.; Cole, R.N.; Cronshaw, J.M.; Matunis, M.J.; Hart, G.W. Mapping sites of O-GlcNAc modification using affinity tags for serine and threonine post-translational modifications. Mol. Cell. Proteom. 2002, 1, 791–804. [Google Scholar] [CrossRef]
- Syka, J.E.; Coon, J.J.; Schroeder, M.J.; Shabanowitz, J.; Hunt, D.F. Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc. Natl. Acad. Sci. USA 2004, 101, 9528–9533. [Google Scholar] [CrossRef]
- Zhao, P.; Viner, R.; Teo, C.F.; Boons, G.J.; Horn, D.; Wells, L. Combining high-energy C-trap dissociation and electron transfer dissociation for protein O-GlcNAc modification site assignment. J. Proteome Res. 2011, 10, 4088–4104. [Google Scholar] [CrossRef]
- Pedowitz, N.J.; Batt, A.R.; Darabedian, N.; Pratt, M.R. MYPT1 O-GlcNAc modification regulates sphingosine-1-phosphate mediated contraction. Nat. Chem. Biol. 2021, 17, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Feghhi, S.; Tooley, W.W.; Sniadecki, N.J. Nonmuscle Myosin IIA Regulates Platelet Contractile Forces Through Rho Kinase and Myosin Light-Chain Kinase. J. Biomech. Eng. 2016, 138, 1045061–1045064. [Google Scholar] [CrossRef] [PubMed]
- George, M.J.; Litvinov, J.; Aroom, K.; Spangler, L.J.; Caplan, H.; Wade, C.E.; Cox, C.S., Jr.; Gill, B.S. Microelectromechanical System Measurement of Platelet Contraction: Direct Interrogation of Myosin Light Chain Phosphorylation. Int. J. Mol. Sci. 2021, 22, 6448. [Google Scholar] [CrossRef] [PubMed]
- Akella, N.M.; Ciraku, L.; Reginato, M.J. Fueling the fire: Emerging role of the hexosamine biosynthetic pathway in cancer. BMC Biol. 2019, 17, 52. [Google Scholar] [CrossRef]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef]
- Muniyappa, R.; Sowers, J.R. Role of insulin resistance in endothelial dysfunction. Rev. Endocr. Metab. Disord. 2013, 14, 5–12. [Google Scholar] [CrossRef]
- Federici, M.; Menghini, R.; Mauriello, A.; Hribal, M.L.; Ferrelli, F.; Lauro, D.; Sbraccia, P.; Spagnoli, L.G.; Sesti, G.; Lauro, R. Insulin-Dependent Activation of Endothelial Nitric Oxide Synthase Is Impaired by O-Linked Glycosylation Modification of Signaling Proteins in Human Coronary Endothelial Cells. Circulation 2002, 106, 466–472. [Google Scholar] [CrossRef]
- Byon, C.H.; Javed, A.; Dai, Q.; Kappes, J.C.; Clemens, T.L.; Darley-Usmar, V.M.; McDonald, J.M.; Chen, Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J. Biol. Chem. 2008, 283, 15319–15327. [Google Scholar] [CrossRef]
- Deng, L.; Huang, L.; Sun, Y.; Heath, J.M.; Wu, H.; Chen, Y. Inhibition of FOXO1/3 promotes vascular calcification. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 175–183. [Google Scholar] [CrossRef]
- Heath, J.M.; Sun, Y.; Yuan, K.; Bradley, W.E.; Litovsky, S.; Dell’Italia, L.G.; Chatham, J.C.; Wu, H.; Chen, Y. Activation of AKT by O-linked N-acetylglucosamine induces vascular calcification in diabetes mellitus. Circ. Res. 2014, 114, 1094–1102. [Google Scholar] [CrossRef]
- Luanpitpong, S.; Poohadsuan, J.; Klaihmon, P.; Kang, X.; Tangkiettrakul, K.; Issaragrisil, S. Metabolic sensor O-GlcNAcylation regulates megakaryopoiesis and thrombopoiesis through c-Myc stabilization and integrin perturbation. Stem Cells 2021, 39, 787–802. [Google Scholar] [CrossRef] [PubMed]
- He, A.; Hu, S.; Pi, Q.; Guo, Y.; Long, Y.; Luo, S.; Xia, Y. Regulation of O-GlcNAcylation on endothelial nitric oxide synthase by glucose deprivation and identification of its O-GlcNAcylation sites. Sci. Rep. 2020, 10, 19364. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.D.; Hart, G.W. AMP-activated protein kinase and p38 MAPK activate O-GlcNAcylation of neuronal proteins during glucose deprivation. J. Biol. Chem. 2008, 283, 13009–13020. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Schimmack, G.; Defronzo, R.A.; Musi, N. AMP-activated protein kinase: Role in metabolism and therapeutic implications. Diabetes Obes. Metab. 2006, 8, 591–602. [Google Scholar] [CrossRef]
- Crawford, G.L.; Hart, G.W.; Whiteheart, S.W. Murine platelets are not regulated by O-linked β-N-acetylglucosamine. Arch. Biochem. Biophys. 2008, 474, 220–224. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batten, L.; Sathyapalan, T.; Palmer, T.M. Molecular Mechanisms Linking Diabetes with Increased Risk of Thrombosis. Int. J. Mol. Sci. 2023, 24, 17465. https://doi.org/10.3390/ijms242417465
Batten L, Sathyapalan T, Palmer TM. Molecular Mechanisms Linking Diabetes with Increased Risk of Thrombosis. International Journal of Molecular Sciences. 2023; 24(24):17465. https://doi.org/10.3390/ijms242417465
Chicago/Turabian StyleBatten, Lucy, Thozhukat Sathyapalan, and Timothy M. Palmer. 2023. "Molecular Mechanisms Linking Diabetes with Increased Risk of Thrombosis" International Journal of Molecular Sciences 24, no. 24: 17465. https://doi.org/10.3390/ijms242417465
APA StyleBatten, L., Sathyapalan, T., & Palmer, T. M. (2023). Molecular Mechanisms Linking Diabetes with Increased Risk of Thrombosis. International Journal of Molecular Sciences, 24(24), 17465. https://doi.org/10.3390/ijms242417465







