Antarctic Krill Oil from Euphausia superba Ameliorates Carrageenan-Induced Thrombosis in a Mouse Model
Abstract
:1. Introduction
2. Results
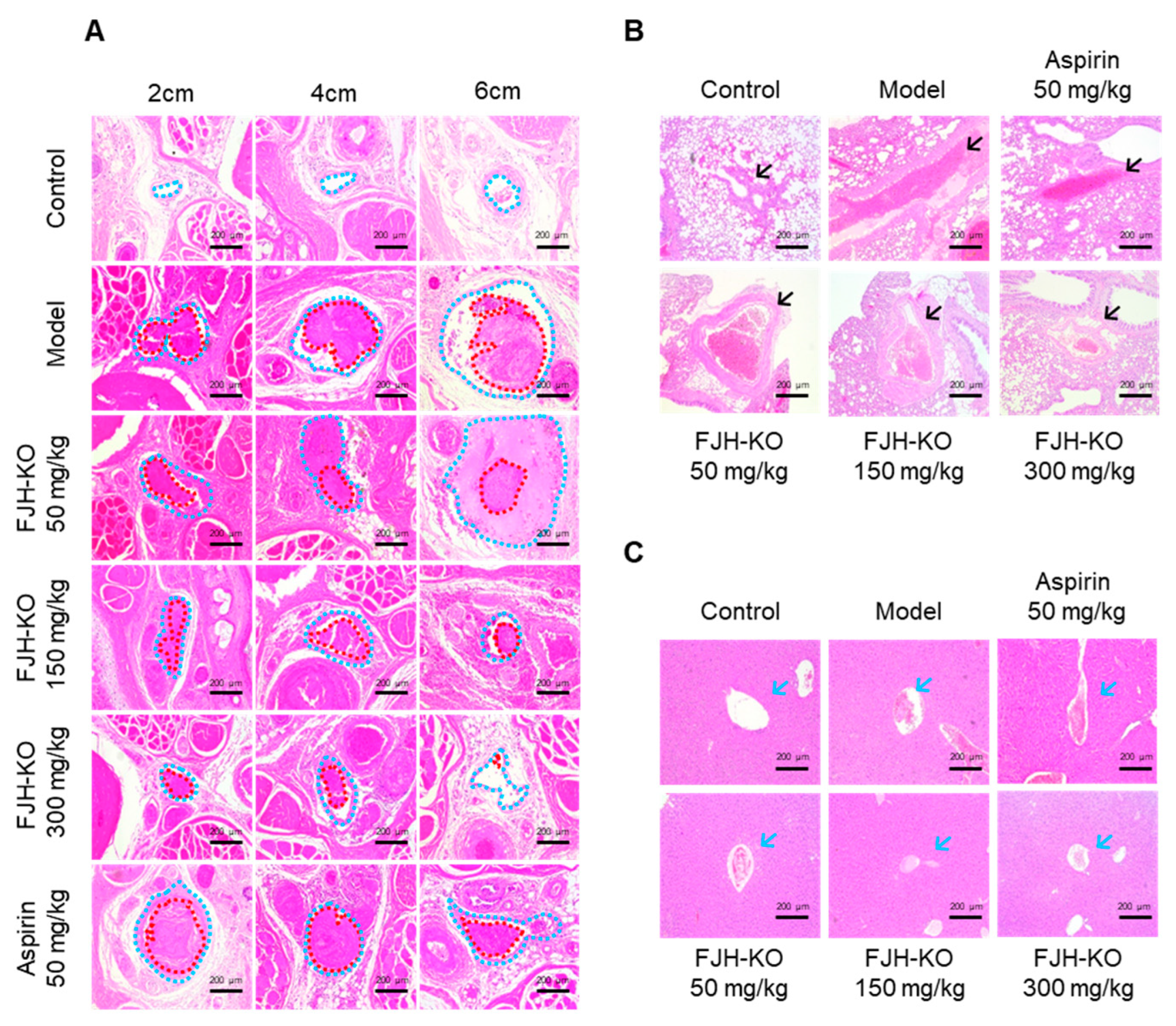
2.1. FJH-KO Attenuates Carrageenan-Induced Thrombosis in Mouse Tail
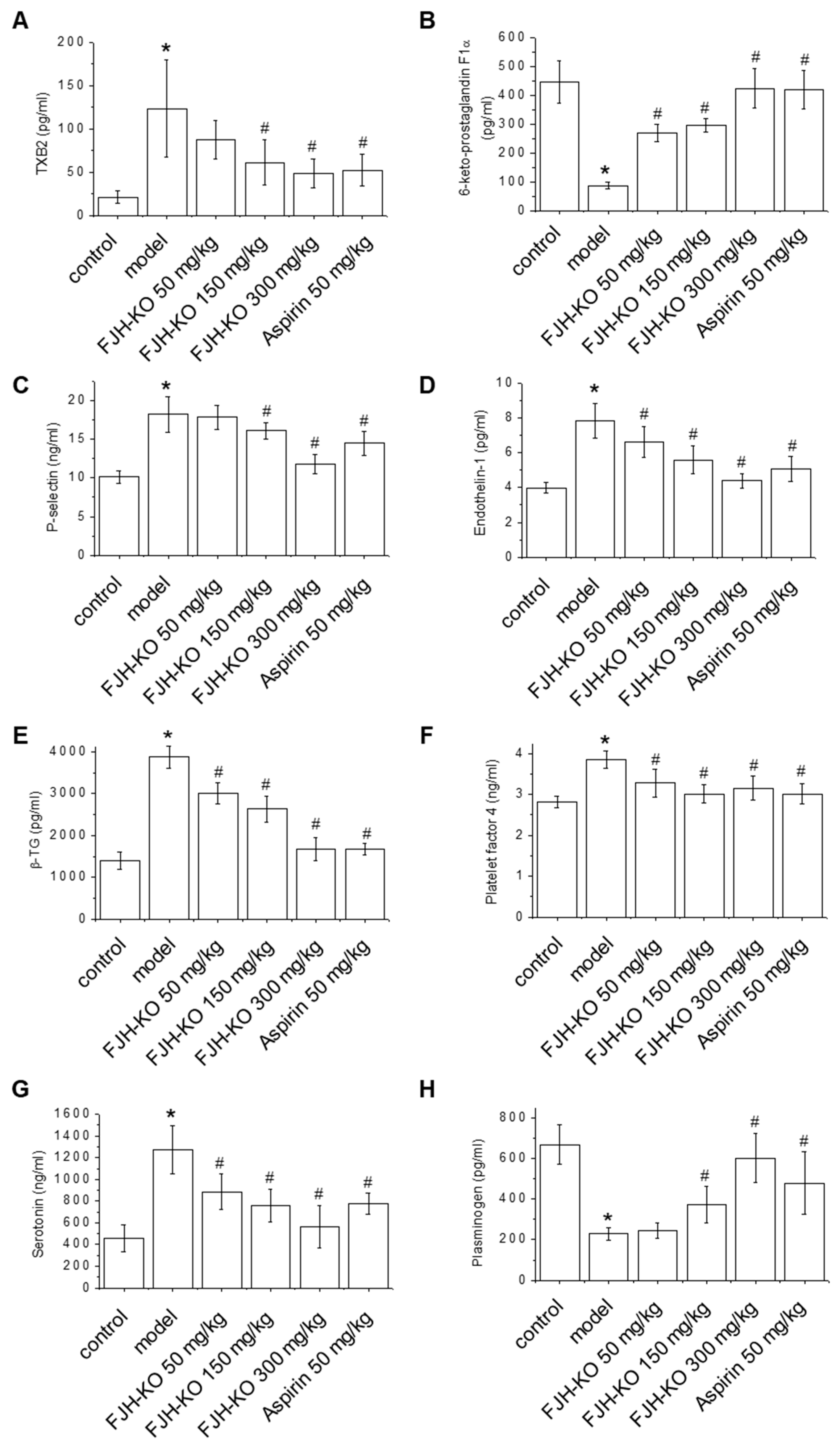
2.2. Effects of FJH-KO on Thrombosis Parameters
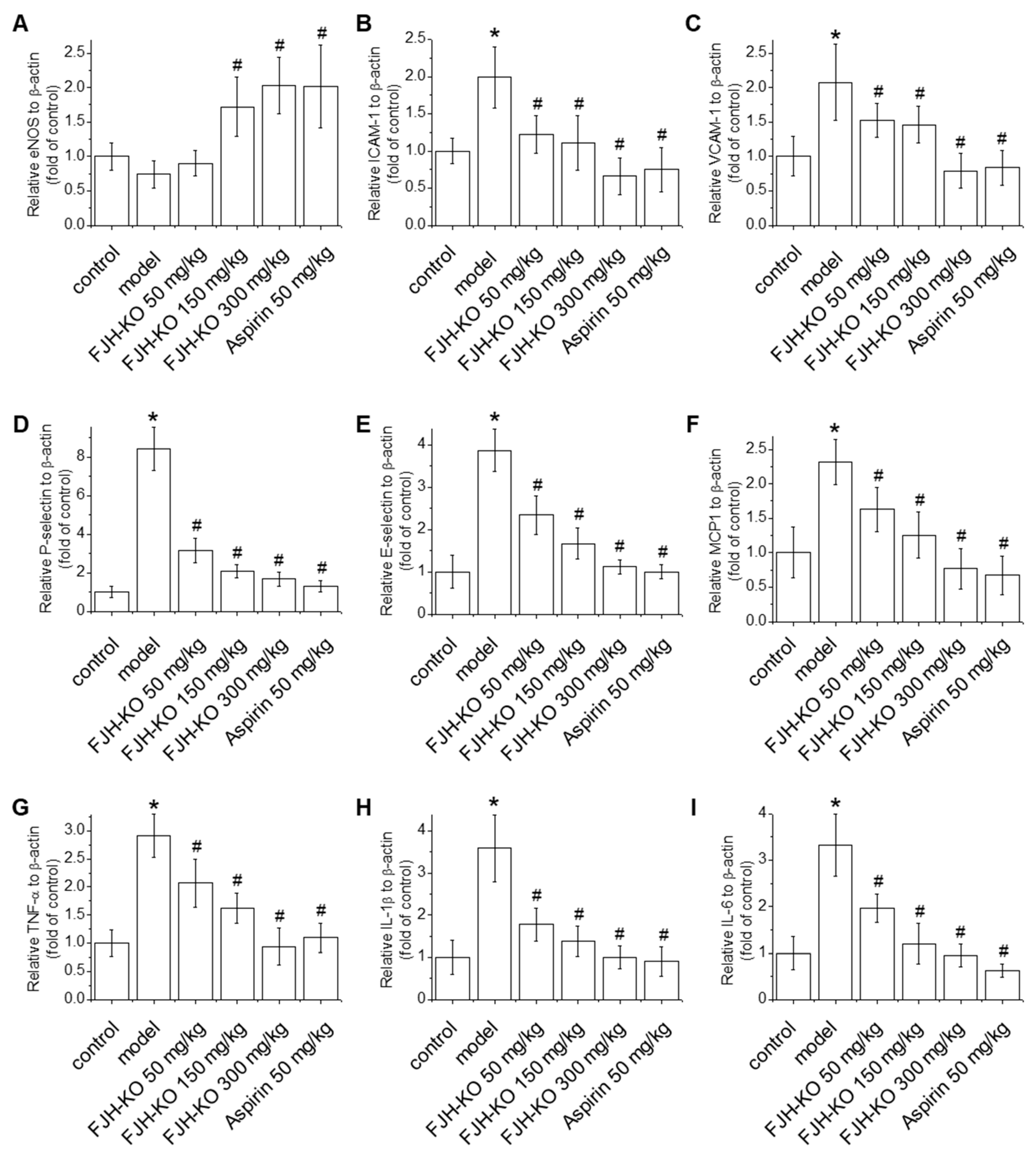
2.3. Effects of FJH-KO on Proinflammatory Cytokine Levels in Mouse Plasma
2.4. Effects of FJH-KO on Vascular Endothelial Dysfunction and Inflammation in Mouse Blood Vessels
2.5. Effects of FJH-KO on Carrageenan-Induced Tissue Thrombosis in Mice
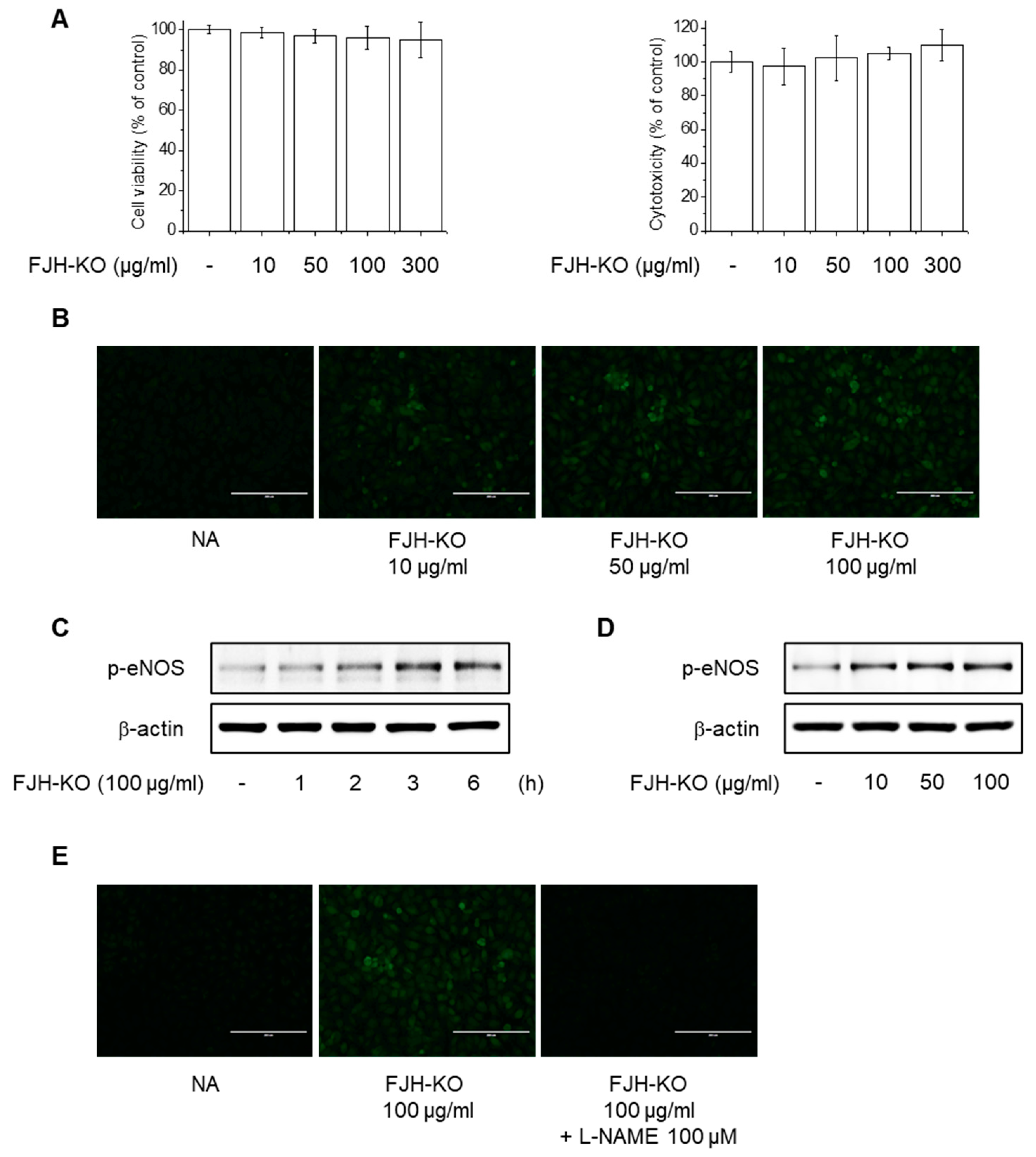
2.6. Effects of FJH-KO on NO Production and eNOS Phosphorylation in Endothelial Cells
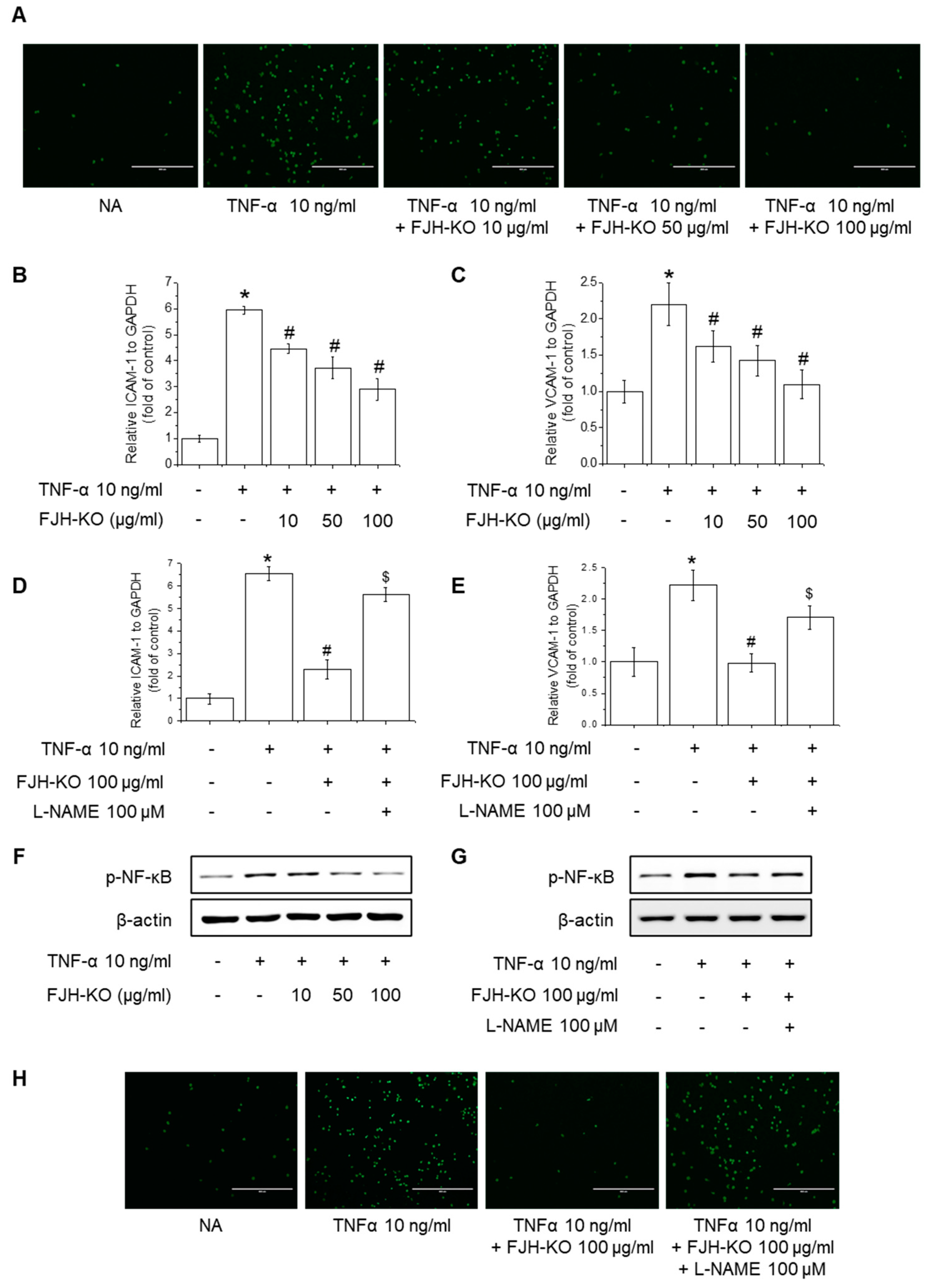
2.7. Effects of FJH-KO on TNF-α-Induced Monocyte Adhesion and Expression of Adhesion Molecules in Endothelial Cells
3. Discussion
4. Materials and Methods
4.1. Preparation of the Extract
4.2. Chemicals and Reagents
4.3. Carrageenan-Induced Thrombosis Mouse Model
4.4. Tail Bleeding Assay
4.5. Thrombus Length Measurement
4.6. Cell Culture and Treatment
4.7. Cell Viability and Cytotoxicity Assay
4.8. Real-Time PCR
4.9. Western Blotting
4.10. Hematoxylin and Eosin (H&E) Staining
4.11. ELISA
4.12. Measurement of NO Production
4.13. Monocyte Adhesion
4.14. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Thomas, H.; Diamond, J.; Vieco, A.; Chaudhuri, S.; Shinnar, E.; Cromer, S.; Perel, P.; Mensah, G.A.; Narula, J.; Johnson, C.O.; et al. Global Atlas of Cardiovascular Disease 2000–2016: The Path to Prevention and Control. Glob. Heart 2018, 13, 143–163. [Google Scholar] [CrossRef] [PubMed]
- Caron, F.; Anand, S.S. Antithrombotic therapy in aortic diseases: A narrative review. Vasc. Med. 2017, 22, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, L. The role of platelets in the initial stages of atherosclerosis. J. Thromb. Haemost. 2006, 4, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Asada, Y. Underlying mechanisms of thrombus formation/growth in atherothrombosis and deep vein thrombosis. Pathol. Int. 2023, 73, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.; Leopold, J.A.; Loscalzo, J. Vasoactive substances: Nitric oxide and endothelial dysfunction in atherosclerosis. Vasc. Pharmacol. 2002, 38, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Gkaliagkousi, E.; Ritter, J.; Ferro, A. Platelet-derived nitric oxide signaling and regulation. Circ. Res. 2007, 101, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xue, Y.; Ding, T.; Sun, J. Enhancement of proinflammatory and procoagulant responses to silica particles by monocyte-endothelial cell interactions. Part. Fibre Toxicol. 2012, 9, 36. [Google Scholar] [CrossRef]
- Watanabe-Kusunoki, K.; Nakazawa, D.; Ishizu, A.; Atsumi, T. Thrombomodulin as a Physiological Modulator of Intravascular Injury. Front. Immunol. 2020, 11, 575890. [Google Scholar] [CrossRef]
- Yau, J.W.; Teoh, H.; Verma, S. Endothelial cell control of thrombosis. BMC Cardiovasc. Disord. 2015, 15, 130. [Google Scholar] [CrossRef]
- Branchford, B.R.; Carpenter, S.L. The Role of Inflammation in Venous Thromboembolism. Front. Pediatr. 2018, 6, 142. [Google Scholar] [CrossRef]
- Aksu, K.; Donmez, A.; Keser, G. Inflammation-induced thrombosis: Mechanisms, disease associations and management. Curr. Pharm. Des. 2012, 18, 1478–1493. [Google Scholar]
- Ghoshal, K.; Bhattacharyya, M. Overview of platelet physiology: Its hemostatic and nonhemostatic role in disease pathogenesis. Sci. World J. 2014, 2014, 781857. [Google Scholar] [CrossRef]
- Patrono, C.; Morais, J.; Baigent, C.; Collet, J.P.; Fitzgerald, D.; Halvorsen, S.; Rocca, B.; Siegbahn, A.; Storey, R.F.; Vilahur, G. Antiplatelet Agents for the Treatment and Prevention of Coronary Atherothrombosis. J. Am. Coll. Cardiol. 2017, 70, 1760–1776. [Google Scholar] [CrossRef] [PubMed]
- Kunamneni, A.; Abdelghani, T.T.; Ellaiah, P. Streptokinase—The drug of choice for thrombolytic therapy. J. Thromb. Thrombolysis 2007, 23, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Krill oil Monograph. Altern. Med. Rev. 2010, 15, 84–86.
- Parolini, C.; Bjorndal, B.; Busnelli, M.; Manzini, S.; Ganzetti, G.S.; Dellera, F.; Ramsvik, M.; Bruheim, I.; Berge, R.K.; Chiesa, G. Effect of Dietary Components from Antarctic Krill on Atherosclerosis in apoE-Deficient Mice. Mol. Nutr. Food Res. 2017, 61, 1700098. [Google Scholar] [CrossRef] [PubMed]
- Adili, R.; Voigt, E.M.; Bormann, J.L.; Foss, K.N.; Hurley, L.J.; Meyer, E.S.; Veldman, A.J.; Mast, K.A.; West, J.L.; Whiteheart, S.W.; et al. In vivo modeling of docosahexaenoic acid and eicosapentaenoic acid-mediated inhibition of both platelet function and accumulation in arterial thrombi. Platelets 2019, 30, 271–279. [Google Scholar] [CrossRef]
- Larson, M.K.; Tormoen, G.W.; Weaver, L.J.; Luepke, K.J.; Patel, I.A.; Hjelmen, C.E.; Ensz, N.M.; McComas, L.S.; McCarty, O.J. Exogenous modification of platelet membranes with the omega-3 fatty acids EPA and DHA reduces platelet procoagulant activity and thrombus formation. Am. J. Physiol. Cell Physiol. 2013, 304, C273–C279. [Google Scholar] [CrossRef]
- Lee, M.; Kim, D.; Park, S.J.; Yun, J.M.; Oh, D.H.; Lee, J. Antarctic Krill Oil Ameliorates Monosodium Iodoacetate-Induced Irregularities in Articular Cartilage and Inflammatory Response in the Rat Models of Osteoarthritis. Nutrients 2020, 12, 3550. [Google Scholar] [CrossRef]
- Kim, O.K.; Kim, D.; Lee, M.; Park, S.H.; Jung, J.; Lee, J. Krill Oil Attenuates Inflammation in Monosodium Iodoacetate-Induced Osteoarthritic Rats, SW982 Synovial Cell Line, and Primary Chondrocytes. J. Med. Food 2022, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Bian, C.; Ji, L.; Qu, H.; Wang, Z. A Novel Polysaccharide from AuriculariaAuricula Alleviates Thrombosis Induced by Carrageenan in Mice. Molecules 2022, 27, 4831. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, K.L.; Owen, J. Plasma levels of beta-thromboglobulin and platelet factor 4 as indices of platelet activation in vivo. Blood 1981, 57, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, H.; Yang, J.; Mu, J.; Wang, R.; Zhao, X. Effect of soybean milk fermented with Lactobacillus plantarum HFY01 isolated from yak yogurt on weight loss and lipid reduction in mice with obesity induced by a high-fat diet. RSC Adv. 2020, 10, 34276–34289. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Wang, W.; Yin, X.; Wang, L.; Gong, L.; Liao, F.; Liang, R. The Joint Effect of a Combination of Components from the Fruit of Crataegus pinnatifida Bge. Var. major N.E. Br. and the Root of Salvia miltiorrhiza Bge. With Exercises on Swimming in Focal Cerebral Infraction in Rat. Front. Physiol. 2020, 11, 574535. [Google Scholar] [CrossRef]
- Merten, M.; Thiagarajan, P. P-selectin in arterial thrombosis. Z. Kardiol. 2004, 93, 855–863. [Google Scholar] [CrossRef]
- Bohm, F.; Pernow, J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc. Res. 2007, 76, 8–18. [Google Scholar] [CrossRef]
- Chen, N.; Chen, L.; Jiang, S.; Wang, Z.; Liu, T. Predictive value of P-selectin and endothelin-1 for vascular restenosis after interventional procedures for peripheral artery disease. Exp. Ther. Med. 2019, 17, 3907–3912. [Google Scholar] [CrossRef]
- Wu, J.F.; Yang, Y.H.; Pang, B.S. Platelet factor 4 and beta-thromboglobulin in blood plasma of patients with acute exacerbation of chronic obstructive pulmonary disease. Zhonghua Yi Xue Za Zhi 2013, 93, 1378–1382. [Google Scholar]
- Su, Y.; Chen, Y.; Zhang, W.; Liu, L.; Cao, X.; Wu, J. Platelet factor 4 and beta-thromboglobulin mRNAs in circulating microparticles of trauma patients as diagnostic markers for deep vein thrombosis. J. Thromb. Thrombolysis 2020, 50, 525–532. [Google Scholar] [CrossRef]
- Rieder, M.; Gauchel, N.; Bode, C.; Duerschmied, D. Serotonin: A platelet hormone modulating cardiovascular disease. J. Thromb. Thrombolysis 2021, 52, 42–47. [Google Scholar] [CrossRef]
- Madoiwa, S. Recent advances in disseminated intravascular coagulation: Endothelial cells and fibrinolysis in sepsis-induced DIC. J. Intensive Care 2015, 3, 8. [Google Scholar] [CrossRef]
- Poredos, P.; Jezovnik, M.K. The role of inflammation in venous thromboembolism and the link between arterial and venous thrombosis. Int. Angiol. 2007, 26, 306–311. [Google Scholar] [PubMed]
- Moore, C.; Tymvios, C.; Emerson, M. Functional regulation of vascular and platelet activity during thrombosis by nitric oxide and endothelial nitric oxide synthase. Thromb. Haemost. 2010, 104, 342–349. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, Q.; Xia, X.; Yang, L. Lagopsis supina ameliorates myocardial ischemia injury by regulating angiogenesis, thrombosis, inflammation, and energy metabolism through VEGF, ROS and HMGB1 signaling pathways in rats. Phytomedicine 2023, 120, 155050. [Google Scholar] [CrossRef] [PubMed]
- Katneni, U.K.; Alexaki, A.; Hunt, R.C.; Schiller, T.; DiCuccio, M.; Buehler, P.W.; Ibla, J.C.; Kimchi-Sarfaty, C. Coagulopathy and Thrombosis as a Result of Severe COVID-19 Infection: A Microvascular Focus. Thromb. Haemost. 2020, 120, 1668–1679. [Google Scholar] [CrossRef]
- Zeng, S.; Yi, R.; Tan, F.; Sun, P.; Cheng, Q.; Zhao, X. Lactobacillus plantarum HFY05 Attenuates Carrageenan-Induced Thrombosis in Mice by Regulating NF-kappaB Pathway-Associated Inflammatory Responses. Front. Nutr. 2022, 9, 813899. [Google Scholar] [CrossRef]
- Gan, X.; Zhao, H.; Wei, Y.; Jiang, Q.; Wen, C.; Ying, Y. Role of miR-92a-3p, oxidative stress, and p38MAPK/NF-kappaB pathway in rats with central venous catheter related thrombosis. BMC Cardiovasc. Disord. 2020, 20, 150. [Google Scholar] [CrossRef]
- Zgheel, F.; Alhosin, M.; Rashid, S.; Burban, M.; Auger, C.; Schini-Kerth, V.B. Redox-sensitive induction of Src/PI3-kinase/Akt and MAPKs pathways activate eNOS in response to EPA:DHA 6:1. PLoS ONE 2014, 9, e105102. [Google Scholar] [CrossRef]
- Matsumoto, T.; Nakayama, N.; Ishida, K.; Kobayashi, T.; Kamata, K. Eicosapentaenoic acid improves imbalance between vasodilator and vasoconstrictor actions of endothelium-derived factors in mesenteric arteries from rats at chronic stage of type 2 diabetes. J. Pharmacol. Exp. Ther. 2009, 329, 324–334. [Google Scholar] [CrossRef]
- Lenox, C.E.; Bauer, J.E. Potential adverse effects of omega-3 Fatty acids in dogs and cats. J. Vet. Intern. Med. 2013, 27, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Lin, M.; Piao, L.; Li, X.; Yang, F.; Zhang, J.; Xiao, B.; Zhang, Q.; Song, W.L.; Yin, H.; et al. Aspirin enhances protective effect of fish oil against thrombosis and injury-induced vascular remodelling. Br. J. Pharmacol. 2015, 172, 5647–5660. [Google Scholar] [CrossRef] [PubMed]




| Gene | Sequences | |
|---|---|---|
| Mouse β-actin | F | AGCCATGTACGTAGCCATCC |
| R | CTCTCAGCTGTGGTGGTGAA | |
| Mouse eNOS | F | CGCAAGAGGAAGGAGTCTAGCA |
| R | TCGAGCAAAGGCACAGAAGTGG | |
| Mouse ICAM-1 | F | AAACCAGACCCTGGAACTGCAC |
| R | GCCTGGCATTTCAGAGTCTGCT | |
| Mouse VCAM-1 | F | GCTATGAGGATGGAAGACTCTGG |
| R | ACTTGTGCAGCCACCTGAGATC | |
| Mouse P-Selectin | F | AAGATGCCTGGCTACTGGACAC |
| R | CAAGAGGCTGAACGCAGGTCAT | |
| Mouse E-Selectin | F | GGACACCACAAATCCCAGTCTG |
| R | TCGCAGGAGAACTCACAACTGG | |
| Mouse MCP1 | F | GCTACAAGAGGATCACCAGCAG |
| R | GTCTGGACCCATTCCTTCTTGG | |
| Mouse TNF-α | F | GGTGCCTATGTCTCAGCCTCTT |
| R | GCCATAGAACTGATGAGAGGGAG | |
| Mouse IL-1β | F | TGGACCTTCCAGGATGAGGACA |
| R | GTTCATCTCGGAGCCTGTAGTG | |
| Mouse IL-6 | F | TACCACTTCACAAGTCGGAGGC |
| R | CTGCAAGTGCATCATCGTTGTTC | |
| Human GAPDH | F | GGGGAGCCAAAAGGGTCATC |
| R | TGGTTCACACCCATGACGAA | |
| Human ICAM-1 | F | GGCAGCGTAGGGTAAGGTT |
| R | CCGGAAGGTGTATGAACTGA | |
| Human VCAM-1 | F | CAGGCTGTGACTCCCCATTT |
| R | CCCTCATTCGTCACCTTCCC | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.H.; Lee, S.Y.; Chae, J.Y.; Kim, J.W.; Kim, J.-H.; Jeong, H.G. Antarctic Krill Oil from Euphausia superba Ameliorates Carrageenan-Induced Thrombosis in a Mouse Model. Int. J. Mol. Sci. 2023, 24, 17440. https://doi.org/10.3390/ijms242417440
Lee GH, Lee SY, Chae JY, Kim JW, Kim J-H, Jeong HG. Antarctic Krill Oil from Euphausia superba Ameliorates Carrageenan-Induced Thrombosis in a Mouse Model. International Journal of Molecular Sciences. 2023; 24(24):17440. https://doi.org/10.3390/ijms242417440
Chicago/Turabian StyleLee, Gi Ho, Seung Yeon Lee, Ju Yeon Chae, Jae Won Kim, Jin-Hee Kim, and Hye Gwang Jeong. 2023. "Antarctic Krill Oil from Euphausia superba Ameliorates Carrageenan-Induced Thrombosis in a Mouse Model" International Journal of Molecular Sciences 24, no. 24: 17440. https://doi.org/10.3390/ijms242417440
APA StyleLee, G. H., Lee, S. Y., Chae, J. Y., Kim, J. W., Kim, J.-H., & Jeong, H. G. (2023). Antarctic Krill Oil from Euphausia superba Ameliorates Carrageenan-Induced Thrombosis in a Mouse Model. International Journal of Molecular Sciences, 24(24), 17440. https://doi.org/10.3390/ijms242417440





