Systematic Review and Meta-Analysis of Dietary Interventions and Microbiome in Phenylketonuria
Abstract
:1. Introduction
2. Materials and Methods
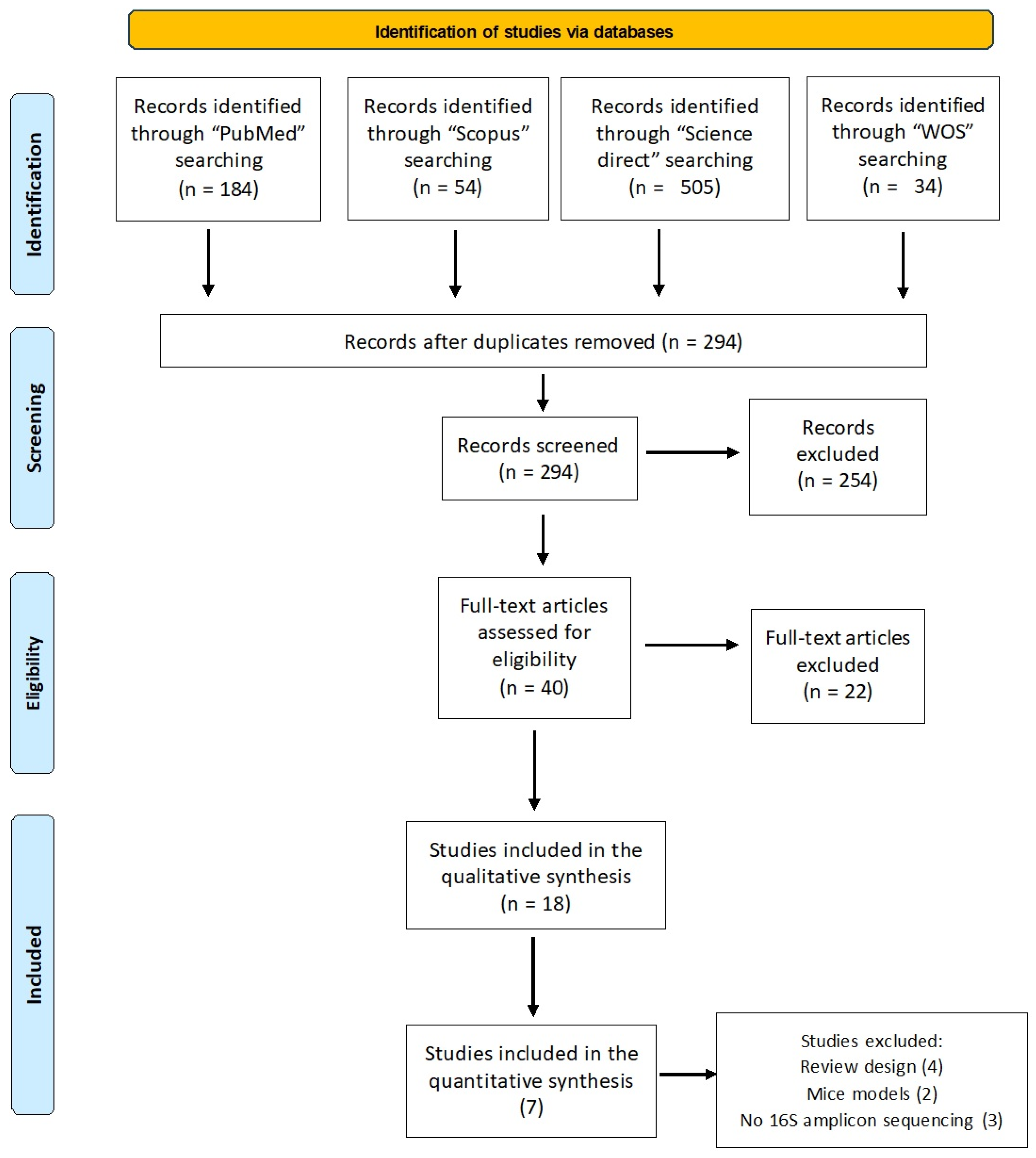
2.1. Selection Protocol and Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction Process and Quality Assessment
2.4. Data Synthesis
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Escribá, R.; Ferrer-Lorente, R. Inborn errors of metabolism: Lessons from iPSC models. Rev. Endocr. Metab. Disord. 2021, 22, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Saudubray, J.M.; Garcia-Cazorla, À. Inborn Errors of Metabolism Overview: Pathophysiology, Manifestations, Evaluation, and Management. Pediatr. Clin. N. Am. 2018, 65, 179–208. [Google Scholar] [CrossRef] [PubMed]
- Colonetti, K.; Roesch, L.F. The microbiome and inborn errors of metabolism: Why we should look carefully at their interplay? Genet. Mol. Biol. 2018, 41, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Elhawary, N.A.; AlJahdali, I.A.; Abumansour, I.S.; Elhawary, E.N.; Gaboon, N.; Dandini, M.; Madkhali, A.; Alosaimi, W.; Alzahrani, A.; Aljohani, F.; et al. Genetic etiology and clinical challenges of phenylketonuria. Hum. Genom. 2022, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Blau, N.; van Spronsen, F.J. Phenylketonuria. Lancet 2010, 376, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Van Spronsen, F.J.; Blau, N. Phenylketonuria. Nat. Rev. Dis. Primers 2021, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Blau, N.; Bélanger-Quintana, A. Management of phenylketonuria in Europe: Survey results from 19 countries. Mol. Genet. Metab. 2010, 99, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Van Wegberg, A.M.J.; MacDonald, A. The complete European guidelines on phenylketonuria: Diagnosis and treatment. Orphanet J. Rare Dis. 2017, 12, 162. [Google Scholar] [CrossRef]
- MacDonald, A.; van Wegberg, A.M.J. PKU dietary handbook to accompany PKU guidelines. Orphanet J. Rare Dis. 2020, 15, 171. [Google Scholar] [CrossRef]
- Macleod, E.L.; Ney, D.M. Nutritional Management of Phenylketonuria. Ann. Nestle Eng. 2010, 68, 58–69. [Google Scholar] [CrossRef]
- Lammi, C.; Bollati, C.; Fiori, L.; Li, J.; Fanzaga, M.; d’Adduzio, L.; Tosi, M.; Burlina, A.; Zuccotti, G.; Verduci, E. Glycomacropeptide (GMP) rescued the oxidative and inflammatory activity of free L-AAs in human Caco-2 cells: New insights that support GMP as a valid and health-promoting product for the dietary management of phenylketonuria (PKU) patients. Food Res. Int. 2023, 173, 113258. [Google Scholar] [CrossRef]
- Pinto, A.; Almeida, M.F.; Ramos, P.C.; Rocha, S.; Guimas, A.; Ribeiro, R.; Martins, E.; Bandeira, A.; MacDonald, A.; Rocha, J.C. Nutritional status in patients with phenylketonuria using glycomacropeptide as their major protein source. Eur. J. Clin. Nutr. 2017, 71, 1230–1234. [Google Scholar] [CrossRef] [PubMed]
- Boyer, S.W.; Barclay, L.J. Inherited Metabolic Disorders: Aspects of Chronic Nutrition Management. Nutr. Clin. Pract. 2015, 30, 502–510. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Filho, J.G.; Carvalho, A.S.E.S.; Alves, J.D.S.; Egea, M.B. Next-generation probiotics as a therapeutic strategy for the treatment of phenylketonuria: A review. Nutr. Rev. 2022, 80, 2100–2112. [Google Scholar] [CrossRef] [PubMed]
- Rizowy, G.M.; Poloni, S. Is the gut microbiota dysbiotic in patients with classical homocystinuria? Biochimie 2020, 173, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Kirby, T.O.; Ochoa-Reparaz, J.; Roullet, J.B.; Gibson, K.M. Dysbiosis of the intestinal microbiome as a component of pathophysiology in the inborn errors of metabolism. Mol. Genet. Metab. 2021, 132, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kirby, T.O.; Brown, M.; Ochoa-Repáraz, J.; Roullet, J.B.; Gibson, K.M. Microbiota Manipulation as a Metagenomic Therapeutic Approach for Rare Inherited Metabolic Disorders. Clin. Pharmacol. Ther. 2019, 106, 505–507. [Google Scholar] [CrossRef]
- Farzi, A.; Fröhlich, E.E.; Holzer, P. Gut Microbiota and the Neuroendocrine System. Neurotherapeutics 2018, 15, 5–22. [Google Scholar] [CrossRef]
- Morban, D.A.H. Phenylketonuria: Central nervous system and microbiome interaction. J. Pediatr. Neonat. Individ. Med. 2017, 6, e060207. [Google Scholar]
- Tagliabue, A.; Ferraris, C.; Uggeri, F.; Trentani, C.; Bertoli, S.; de Giorgis, V.; Veggiotti, P.; Elli, M. Short-term impact of a classical ketogenic diet on gut microbiota in GLUT1 Deficiency Syndrome: A 3-month prospective observational study. Clin. Nutr. ESPEN 2017, 17, 33–37. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Protano, C.; Valeriani, F.; De Giorgi, A.; Marotta, D.; Ubaldi, F.; Napoli, C.; Liguori, G.; Romano Spica, V.; Vitali, M.; Gallè, F. Consumption patterns of energy drinks in university students: A systematic review and meta-analysis. Nutrition 2023, 107, 111904. [Google Scholar] [CrossRef] [PubMed]
- Montanari, C.; Ceccarani, C. Glycomacropeptide Safety and Its Effect on Gut Microbiota in Patients with Phenylketonuria: A Pilot Study. Nutrients 2022, 14, 1883. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thomson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analysis. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Friede, T.; Röver, C.; Wandel, S.; Neuenschwander, B. Meta-analysis of few small studies in orphan diseases. Res. Synth. Methods 2017, 8, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Bender, R.; Friede, T.; Koch, A.; Kuss, O.; Schlattmann, P.; Schwarzer, G.; Skipka, G. Methods for evidence synthesis in the case of very few studies. Res. Synth. Methods 2018, 9, 382–392. [Google Scholar] [CrossRef]
- Santabárbara, J.; Ozamiz-Etxebarria, N.; Idoiaga, N.; Olaya, B.; Bueno-Novitol, J. Meta-Analysis of Prevalence of Depression in Dental Students during COVID-19 Pandemic. Medicina 2021, 57, 1278. [Google Scholar] [CrossRef]
- Faraone, S.V. Interpreting estimates of treatment effects: Implications for managed care. Pharm. Ther. 2008, 33, 700–711. [Google Scholar]
- Al-Zyoud, W.; Nasereddin, A. Culturable gut bacteria lack Escherichia coli in children with phenylketonuria. New Microbes New Infect. 2019, 32, 100616. [Google Scholar] [CrossRef]
- Bassanini, G.; Ceccarani, C. Phenylketonuria Diet Promotes Shifts in Firmicutes Populations. Front. Cell. Infect. Microbiol. 2019, 9, 101. [Google Scholar] [CrossRef]
- MacDonald, A.; Cochrane, B. Specific prebiotics in a formula for infants with Phenylketonuria. Mol. Genet. Metab. 2011, 104, S55–S59. [Google Scholar] [CrossRef] [PubMed]
- Mancilla, V.J.; Mann, A.E.; Zhang, Y.; Allen, M.S. The Adult Phenylketonuria (PKU) Gut Microbiome. Microorganisms 2021, 9, 530. [Google Scholar] [CrossRef] [PubMed]
- McWhorter, N.; Dhillon, J. Preliminary Investigation of Microbiome and Dietary Differences in Patients with Phenylketonuria on Enzyme Substitution Therapy Compared to Traditional Therapies. J. Acad. Nutr. Diet. 2022, 122, 1283–1295. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro de Oliveira, F.; Mendes, R.H. Phenylketonuria and Gut Microbiota: A Controlled Study Based on Next-Generation Sequencing. PLoS ONE 2016, 11, e0157513. [Google Scholar] [CrossRef] [PubMed]
- Sawin, E.A.; De Wolfe, T.J. Glycomacropeptide is a prebiotic that reduces Desulfovibrio bacteria, increases cecal short-chain fatty acids, and is anti-inflammatory in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Shadike, Q. A low abundance of genus Bacteroides in gut microbiota is negatively correlated with blood phenylalanine levels in Uygur patients with phenylketonuria. Transl. Pediatr. 2021, 10, 2521–2532. [Google Scholar] [CrossRef]
- Timmer, C.; Davids, M. Differences in faecal microbiome composition between adult patients with UCD and PKU and healthy control subjects. Mol. Genet. Metab. Rep. 2021, 29, 100794. [Google Scholar] [CrossRef]
- Van der Goot, E.; Vink, S.N. Gut-Microbiome Composition in Response to Phenylketonuria Depends on Dietary Phenylalanine in BTBR Pah_enu2 Mice. Front. Nutr. 2022, 8, 735366. [Google Scholar] [CrossRef]
- Verduci, E.; Moretti, F.; Bassanini, G.; Banderali, G.; Rovelli, V.; Casiraghi, M.C.; Morace, G.; Borgo, F.; Borghi, E. Phenylketonuric diet negatively impacts on butyrate production. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 385–392. [Google Scholar] [CrossRef]
- Verduci, E.; Carbone, M.T.; Borghi, E.; Ottaviano, E.; Burlina, A.; Biasucci, G. Nutrition, Microbiota and role of Gut-Brain Axis in Subjects with Phenylketonuria (PKU): A Review. Nutrients 2020, 12, 3319. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Sigwart, J.D.; Bennett, K.D. Measuring Biodiversity and Extinction-Present and Past. Integr. Comp. Biol. 2018, 58, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Ley, R.E. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Molina, B.; Queipo-Ortuño, M.I. Dietary and Gut Microbiota Polyamines in Obesity- and Age-Related Diseases. Front. Nutr. 2019, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Hasavci, D.; Blank, T. Age-dependent effects of gut microbiota metabolites on brain resident macrophages. Front. Cell. Neurosci. 2022, 16, 944526. [Google Scholar] [CrossRef] [PubMed]
- Huda, M.N.; Salvador, A.C. Gut microbiota and host genetics modulate the effect of diverse diet patterns on metabolic health. Front. Nutr. 2022, 9, 896348. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Tuovinen, E.; Keto, J. Cytokine response of human mononuclear cells induced by intestinal Clostridium species. Anaerobe 2013, 19, 70–76. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Miura, K.; Ohnishi, H. Role of gut microbiota and Toll-like receptors in nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 7381–7391. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, C.; Cavalieri, D. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tian, G. Dietary protein levels and amino acid supplementation patterns alter the composition and functions of colonic microbiota in pigs. Anim. Nutr. 2020, 6, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Radjabzadeh, D.; Boer, C.G. Diversity, compositional and functional differences between gut microbiota of children and adults. Sci. Rep. 2020, 10, 1040. [Google Scholar] [CrossRef] [PubMed]
- Van Spronsen, F.J.; van Wegberg, A.M.; Ahring, K.; Bélanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Giżewska, M.; et al. Key European guidelines for the diagnosis and management of patients with phenylketonuria. Lancet. Diabetes Endocrinol. 2017, 5, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Ilgaz, F.; Ford, S.; O’Driscoll, M.F.; MacDonald, A. Adult PKU Clinics in the UK-Users’ Experiences and Perspectives. Nutrients 2023, 15, 4352. [Google Scholar] [CrossRef] [PubMed]
- Bosch, A.M.; Burlina, A.; Cunningham, A.; Bettiol, E.; Moreau-Stucker, F.; Koledova, E.; Benmedjahed, K.; Regnault, A. Assessment of the impact of phenylketonuria and its treatment on quality of life of patients and parents from seven European countries. Orphanet J. Rare Dis. 2015, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejczyk, A.A.; Zheng, D.; Elinav, E. Diet-microbiota interactions and personalized nutrition. Nat. Rev. Microbiol. 2019, 17, 742–753. [Google Scholar] [CrossRef]
- Hughes, R.L.; Kable, M.E.; Marco, M.; Keim, N.L. The Role of the Gut Microbiome in Predicting Response to Diet and the Development of Precision Nutrition Models. Part II: Results. Adv. Nutr. 2019, 10, 979–998. [Google Scholar] [CrossRef]
- Dong, T.S.; Gupta, A. Influence of Early Life, Diet, and the Environment on the Microbiome. Clin. Gastroenterol. Hepatol. 2019, 17, 231–242. [Google Scholar] [CrossRef]
- Banta, J.E.; Segovia-Siapco, G.; Crocker, C.B.; Montoya, D.; Alhusseini, N. Mental health status and dietary intake among California adults: A population-based survey. Int. J. Food Sci. Nutr. 2019, 70, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef] [PubMed]

| Author, Year, Country, [Ref] | Type of Study | Aim of the Study | Organisms Target | Sample Characteristics | Blood Phe Levels | Treatment/Nutritional Control/Type of Diet | Study Quality * | Findings |
|---|---|---|---|---|---|---|---|---|
| Al-Zyoud et al., 2019, Jordan, [29] | Retrospective study | To compare the microbial profile obtained by traditional bacterial culture of the intestines of PKU patients and controls and to measure the effect of a low protein diet on the microbiota. | Human Childhood | Cases (PKU): 5 subjects Average age: 5 years Gender: 3 M, 2 F Controls: 20 subjects BMI z score: 3 ± 0.3; Average age: 4 years Gender: 11 M, 9 F BMI z score: 2.5 ± 0.3 | No information | Dietary prebiotics: Cases: 5 yes, 0 no Controls: 13 yes, 7 no Probiotics: Cases: 0 yes, 5 no Controls: 8 yes, 12 no Breast milk feeding: Cases: 3 yes, 2 no Controls: 7 yes, 13 no Solid food: Cases: 2 yes and 3 no Controls: 8 yes and 12 no | Poor (3) | A statistically significant (p < 0.01) difference in E. coli presence in the gut flora of PKU subjects vs. control children was reported |
| Bassanini et al., 2019, Italy, [30] | Retrospective study | To study the impact of a Phe-restricted diet on the gut microbiota and possible consequences on the well-being of PKU patients | Human Childhood | PKU group: 21 subjects Average age: 10 ± 3.5 years Gender: 10 M, 11 F BMI z score: 0.2 ± 1; MHP group: 21 subjects Average age: 8 ± 3.4 years Gender: 12 M, 9 F BMI z score: 0.5 ± 1.1 | Blood Phe levels: PKU group: 228 ± 87 μmol/L MHP group: 263 ± 97 μmol/L | Diet: PKU group: Carbs (%) 61.0 ± 7.0; Fibre (grams) 16.0 ± 9.1; Protein (%) 43.2 ± 15.1; Lipids (%) 29.6 ± 6.6; All of the subjects started diet/therapy at diagnosis of the disease, usually within 10 days of birth. MHP group: Carbs (%) 56.0 ± 5.9; Fibre (grams) 8.9 ± 2.6; Protein (%) 52.1 ± 11.1; Lipids (%) 32.6 ± 4.7 | Good (7) | Although there was no significant difference in alpha diversity between the PKU and MHP groups, their gut microbiota analysis showed a marked separation. Notable variations were observed within the Firmicutes phylum. Despite the PKU children consuming more vegetables and fibre, similar to a vegan diet, their gut microbial profile differed from what is commonly observed in individuals following a high-fibre/low-protein diet. The depletion of useful microorganisms, such as F. prausnitzii, was noticed in the gut microbiota of PKU individuals. This suggests that the quality and quantity of carbohydrates consumed play a significant role in determining the observed shifts in Firmicutes within the PKU population. |
| MacDonald et al., 2011, UK, [31] | Exploratory pilot study | To study the influence of adding a specific proprietary blend of prebiotic oligosaccharides to a protein substitute suitable for infants with phenylketonuria | Human Childhood | Subjects: 9 infants with PKU Average age (y): 7.86 weeks (range 7.14–19.43) Gender: 3 M, 6 F BMI: not reported | No information | Infant Phenylalanine-free protein substitute containing prebiotic (scGOS/lcFOS [9:1 ratio]) | Fair (5) | Treated infants showed phenylalanine levels in line with that observed with traditional therapy without prebiotics and higher levels of Bifidobacteria and lower stool pH. |
| Mancilla et al., 2021, USA, [32] | Descriptive study | To characterize the microbiota of PKU patients following a dietary treatment compared to controls | Human Adults | Cases (PKU): 11 subjects Average age: 33 ± 1.98 Gender: 6 M, 4 F (1 not reported by participant) Healthy controls: 21 subjects Average age: 29 ± 3.07 Gender: 11 M, 10 F | No information | No information | Fair (5) | Gut microbiota diversity was lower in PKU individuals (p < 0.001) |
| McWhorter et al., 2022, USA, [33] | Descriptive study | To compare differences in dietary treatment and gut microbiota in adult patients on a PKU liberalized diet and patients on pegvaliase-pqpz therapy | Human Adults | PKU group 1 (Traditional treatment): 6 subjects Average age: 28 (range: 23–40) Gender: 2M, 4F BMI: 30.4 (24.2–35.2) PKU group 2 (pegvaliase-pqpz treatment): 6 subjects Average age: 31 (range: 28–40) Gender: 3 M, 3 F BMI: 29.9 (22.1–39.6) | Mean blood Phe levels (μmol/L): Group 1: 314 (range: 157–468) Group 2: 50 (range: 0–314) | Diet Group 1: Calories (Kcal) 1943 ± 600; Carbs (g) 265.4 ± 103.6; Fibre (g) 16 ± 9.4; Total protein (from natural and medical foods) (g) 88.3 ± 19.6; Protein from foods 24.6 ± 5.0; Lipids (g) 59.9 ± 24.3 Increased intake of many micronutrients, including tyrosine, due to supplementation in medical foods. Group 2: Calories (Kcal) 2220 ± 556; Carbs (g) 290 ± 48.1; Fibre (g) 15.8 ± 6.8; Total protein 78.0 ± 24.9; Protein only from natural foods 78.0 ± 24.9; Lipids 85.6 ± 32.8 | Good (7) | Individuals with PKU who receive treatment using pegvaliase -pqpz and follow a more flexible diet display noticeable variations in their dietary makeup compared to those who receive conventional Phe-restricted diets. Those in the traditional group exhibited a higher level of Verrucomicrobia phylum and Lachnobacterium genus, whilst the pegvaliase-pqpz group showed a greater presence of the Prevotella genus (p < 0.05). |
| Montanari, et al. 2022, Italy, [23] | Prospective study | To evaluate the effect of 6 months GMP supplementation on the intestinal microbiota of PKU patients by comparing their bacterial composition and clinical parameters before and after the intervention | Human Childhood and Adults | Cases (PKU): 9 patients (4 adults, 5 children) Classic PKU: 5 subjects Mild PKU: 4 subjects Average age: 20 years (range 7–38) Gender: 7 M, 2 F Average BMI (Kg/m2): 18.1 ± 1.5 Average BMI z score in paediatric populations: −0.2 ± 1.7 | Mean Phe levels within the range [i.e., 120–360 μmol/L in childhood (<12 years) and 120–600 μmol/L in adolescence and adult age (>12 years)] | Restriction of natural proteins, use of amino acid supplements without Phe, low protein foods in order to reduce Phe intake according to their tolerance. No subject had ever used BH4 tetrahydrobiopterin | Good (8) | GMP seems to be safe from both the microbiological and the clinical point of view. A prebiotic effect of Agathobacter spp. and Subdoligranulum, the butyrate-producer, was observed. Phenylalanine values were kept below the age target and nutritional parameters. |
| Pinheiro De Oliveira et al., 2016, Brazil, [34] | Descriptive study | To characterize the microbiota of PKU patients following a dietary treatment compared to controls | Human Childhood | Cases (PKU): 8 subjects Average age: 4.24 ± 1.74 Gender: 6 M, 2 F BMI: 18.48 ± 1.30 Healthy controls: 10 subjects Average age: 6.06 ± 1.78 Gender: 4 M, 6 F BMI: 16.87 ± 1.55 | Phe blood levels: Cases: PKU 307 μmol/L | Diet: Cases: Calories (Kcal) 1227.92 ± 187.91; Carbs (g) 215.08 ± 39.65; Fibre (g) 17.44 ± 3.42; Protein (g) 79.12 ± 15.41; Lipids (g) 16.12 ± 3.35 Phe-restricted diet supplemented with a prebiotic-free metabolic formula (protein substitute). Controls: Calories (Kcal) 1277.56 ± 78.43; Carbs (g)159.11 ± 9.44; Fibre (g) 12.37± 0.97; Protein (g) 72.82 ± 15.21; Lipids (g) 37.75 ± 4.45 | Good (7) | Distinct taxonomic groups are present within the gut microbiome of PKU patients, which may be modulated by their plasma Phe concentration but it is not clear if these findings are a result of the disease itself or the modified diet. |
| Sawin et al., 2015, USA, [35] | Prospective study | To determine the prebiotic properties of GMP by characterizing caecal and faecal microbiota and caecal concentrations of SCFAs from PKU and wild-type (WT) mice fed diets containing GMP as the primary protein source | Mice | Experiment 1: Wild-type mice (WT): n = 45 Pahenu2 mice (PKU): n = 70 Divided into 3 groups: mice fed high-Phe casein, mice fed low-Phe AA, and mice fed low-Phe GMP Experiments 2 and 3: PKU mice fed AA diet (n = 6) PKU mice fed GMP diet (n = 8) | No information | High Phe level: casein administration (20% casein + 0.3% L-cysteine); low Phe level: supplementation with AA (17.5% free AA); GMP treated (20%) | Poor (4) | Functional foods may be beneficial in the management of PKU, as well as obesity and IBD. |
| Su et al., 2021, China, [36] | Retrospective study | To determine the characteristics of the microbiota in Ugur patients with PKU; to examine the correlation between clinical PKU phenotypes; to compare the microbial composition of PKU and healthy subjects | Human Childhood | Cases (PKU): 11 cases Mean age (y): 4.1 ± 0.7 (range: 0.8–8.25) Gender: 4 M, 7 F Average BMI (kg/m2): 16.36 ± 0.35 Controls: 11 sex-, nation-, and age-matched subjects | Mean blood Phe level (µmol/L): Cases: 481 ± 123 [pre-treatment: 986 ± 168] | Diet: Average daily carbohydrates: 192.36 ± 16.37; Average daily protein 33.46 ± 3.26 Cases: All patients followed a Phe-restricted diet supplemented with a metabolic formula (protein replacement) without prebiotics, and none received tetrahydrobiopterin (BH4), LNAA, or GMP treatment. Recommended daily intake of Phe was 135–330 mg according to guidelines for Chinese populations | Fair (5) | The PKU group showed very low levels of the Bacteroides genus, which was negatively associated with the blood Phe level. The authors propose that the reduced ability to degrade glycans in the PKU group may be due to the capacity of Bacteroides to break down complex and resistant glycans. |
| Timmer et al., 2021, Holland, [37] | Retrospective study | To evaluate and compare the faecal microbiota composition of patients with PKU and healthy controls; to study the influence of protein restriction and AA supplementation on the microbiome composition by comparing results between controls and PKU | Human Adults | PKU: 10 subjects Mean age (y): 35.5 (range: 19–50) Average BMI (kg/m2): 24.3 Controls: 10 subjects Mean age (y): 35.5 (range: 20–58) Average BMI (kg/m2): 23.9 | No information | Diet: Group 1: Calories (Kcal) 1987, Phe intake (g) 31, Carbohydrates (g) 328, Lipids (g) 48, Protein from daily supplemental amino acids (g) 11 Group 2: Calories (Kcal) 1820, Phe intake (g) 19, Carbohydrates (g) 219, Lipids (g) 54, Protein from daily supplemental amino acids (g) 60 Group 3: Calories (Kcal) 1985, Phe intake (g) 83, Carbohydrates (g) 198, Lipids (g) 79.5, Protein from daily supplemental amino acids (g) 0 | Fair (5) | The gut microbiome of PKU patients following a low-protein diet with added amino acids displayed lower diversity when compared to healthy adults not following a specific diet. |
| Van der Goot et al., 2022, Holland, [38] | Prospective study | To study the microbiota of mice with PKU fed diets with different protein contents | Mice | Wild type: n = 14; 7 M, 7 F Pah_enu2 (PKU model mice): n = 42 divided into 3 groups Pah_enu2 group 1—normal diet (high levels of Phe); n = 14; 7 M, 7 F Pah_enu2 group 2—Phe-liberalized diet (33% natural protein restriction); n = 14; 7 M, 7 F Pah_enu2 group 3—Phe-restriction (75% natural protein restriction); n = 14; 7 M, 7 F | No information | Wild-type and Pah_enu2 group 1 mice: baseline diet including 124 g/kg of dietary protein Pah_enu2 group 2: casein reduced by 33% and compensated by a blend of synthetic amino acids (Phe-free) * Pah_enu2 group 3: casein reduced by 75% and compensated by a blend of synthetic amino acids (Phe-free) * * Due to the reduced absorption of synthetic amino acids compared to protein, a putative protein conversion factor was taken into account, and an extra 20% amino acid blend was added | Good (7) | PKU leads to an altered gut microbiome composition in mice, which is least severe on a liberalized Phe-restricted diet |
| Verduci et al., 2018, Italy, [39] | Retrospective study | To compare dietary intake, gut microbiota diversity, and the production of short-chain fatty acids in children with PKU following a low-phenylalanine (Phe) diet and in children with mild hyperphenylalaninemia (MHP) following an unrestricted diet. | Human Adults | PKU group: 21 subjects Gender: 10 M, 11 F MHP group (control): 21 subjects Gender: 10 M, 11 F Average age (y): 8.69 (±3.57) | PKU cases: Mean Phe levels at baseline (µmol/L): 263 ± 97 | PKU cases: Total protein (g) 43.2 (±15.1); Total formula Protein (g) 28.7 (±13.0); Carbs (g) 254.2 (±72.7); Fibre (g) 16.0 (±9.1); Lipids (g) 54.9 (±16.0) 13 different Phe-free, amino acid substitute medical foods (AA-MFs): most AA-MFs contained carbs (11 of 13), vitamins (10 of 13), minerals (10 of 13), and lipids (7 of 13); 3 of 13 supplemented with fibre. None contained probiotics. None of the subjects participating in the study were taking GMP and no patients were on tetrahydrobiopterin (BH4) or LNAA therapy | Poor (4) | The low-Phe diet, characterized by a higher carbohydrate intake, increases glycaemic load, resulting in a different quality of substrates for microbial fermentation. Further analyses, thoroughly evaluating microbial species altered by PKU diet are needed to better investigate gut microbiota in PKU children and supplements. |
| Author, Year, Country, [Ref] | Sequencing Platform | Sequencing Region | Primer Used | Number of Average Reads Obtained per Sample | Passing Filter |
|---|---|---|---|---|---|
| Bassanini et al., 2019, Italy, [30] | MiSeq platform Illumina (2 × 251 base paired-end reads) | Region V3–V4 of the 16S rRNA gene | Illumina 16S rRNA custom primers | 159,914 | 99% |
| Mancilla et al., 2021, USA, [32] | MiSeq platform Illumina (2 × 251 base paired-end reads) | Region V4 of the 16S rRNA gene | Primer pair 515F F-806R 16S rRNA | Not available | Not available |
| McWhorter et al., 2022, USA, [33] | Micro MiSeq platform Illumina (151 × 12 × 151 bp) | Region V4 of the 16S rRNA gene | Primers 515F/806R that directly amplify Bacteria/Archaea | 179,009 | 99% |
| Montanari et al., 2022, Italy, [23] | MiSeq platform Illumina (2 × 251 base paired-end reads) | Region V3–V4 of the 16S rRNA gene | Illumina 16S rRNA custom primers | 15,200 | 99% |
| Pinheiro de Oliveira et al., 2016, Brazil, [34] | Ion Torrent | Region V4 of the 16S rRNA gene | Primers 515F/806R that directly amplify Bacteria/Archaea | 5890 | 99% |
| Su et al., 2021, China, [36] | MiSeq PE300 or NOVAseq PE250 platform Illumina | Region V3–V4 of the 16S rRNA genes | Primer pair 338F-806R 16S rRNA | 16,186 | 99% |
| Timmer et al., 2021, Netherlands, [37] | MiSeq platform Illumina (2 × 251 base paired-end reads) | Region V3–V4 of the 16S rRNA genes | Illumina 16S rRNA custom primers | 25,000 | 99% |
| Author, Year, Country, [Ref] | Biodiversity Indicators Alpha Diversity | Biodiversity Indicators Beta Diversity | F/B | Bacillota | Bacteroidota | Pseudomonadota | Actinomycetota | Verrucomicrobia |
|---|---|---|---|---|---|---|---|---|
| Bassanini et al. 2019, Italy, [30] | ↔ Shannon, OTUs, Chao1 | Microbial communities are statistically different | ↑ | ↑* ↑* Clostridium ↑* Blautia ↑* Ruminococcus ↑* Lachnospiraceae (other) ↓* Faecalibacterium ↓* Veillonellaceae | ↓* | ↑* | ↑* | ↓* |
| Mancilla et al., 2021 [32] | ↓ Shannon, OTUs | Microbial communities are different | ↓ | ↓ ↓ Lactobacillaceae ↓ Blautia | ↑ ↑ Bacteroides ↑ Alistipes | ↑ | ↓ | ↔ |
| McWhorter et al., 2022, USA, [33] | ↑* Shannon, Chao1, Simpson | Microbial communities are similar | ↑* | ↑* ↑Clostridium ↑* Blautia ↑* Coprococcus ↑* Faecalibacterium | ↓* ↓* Alistipes ↓* Prevotella | ↑* | ↑ | ↑* ↑* Akkermansia |
| Montanari et al., 2022, Italy, [23] | ↓ Shannon, Chao1 ↓ OTUs | Microbial communities are similar | ↓* | ↔ ↓* Faecalibacterium | ↓* ↓* Prevotella | ↑* | ↑* ↑* Bifidobacterium | Not available |
| Pinheiro de Oliveira et al., 2016, Brazil, [34] | ↓* Shannon, OTUs, Chao1 | Microbial communities are statistically different | ↓* | ↓ * ↓* Clostridium ↓* Coprococcus ↓* Ruminococcus ↓* Lachnospiraceae ↓* Veillonella ↓ Erysipelotricaceae | ↑* ↑* Prevotella | ↓* | ND | ↑* ↑* Akkermansia |
| Su et al., 2021, China, [36] | ↓* Shannon, Chao1 | Microbial communities are statistically different | ↑* | ↑ ↓* Faecalibacterium | ↓* ↔ Prevotella | ↑* | ↑* ↑* Bifidobacterium ↑* Collinsella | Not available |
| Timmer et al., 2021, Netherlands, [37] | ↓* Shannon, OTUs | Microbial communities are statistically different | ↑* | ↑* ↑* Clostridium ↑* Ruminococcus ↓* Veillonellaceae | ↓* | ↑* | ↑* | ↓* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ubaldi, F.; Frangella, C.; Volpini, V.; Fortugno, P.; Valeriani, F.; Romano Spica, V. Systematic Review and Meta-Analysis of Dietary Interventions and Microbiome in Phenylketonuria. Int. J. Mol. Sci. 2023, 24, 17428. https://doi.org/10.3390/ijms242417428
Ubaldi F, Frangella C, Volpini V, Fortugno P, Valeriani F, Romano Spica V. Systematic Review and Meta-Analysis of Dietary Interventions and Microbiome in Phenylketonuria. International Journal of Molecular Sciences. 2023; 24(24):17428. https://doi.org/10.3390/ijms242417428
Chicago/Turabian StyleUbaldi, Francesca, Claudia Frangella, Veronica Volpini, Paola Fortugno, Federica Valeriani, and Vincenzo Romano Spica. 2023. "Systematic Review and Meta-Analysis of Dietary Interventions and Microbiome in Phenylketonuria" International Journal of Molecular Sciences 24, no. 24: 17428. https://doi.org/10.3390/ijms242417428
APA StyleUbaldi, F., Frangella, C., Volpini, V., Fortugno, P., Valeriani, F., & Romano Spica, V. (2023). Systematic Review and Meta-Analysis of Dietary Interventions and Microbiome in Phenylketonuria. International Journal of Molecular Sciences, 24(24), 17428. https://doi.org/10.3390/ijms242417428








