Polymer Biocompositions and Nanobiocomposites Based on P3HB with Polyurethane and Montmorillonite
Abstract
:1. Introduction
2. Results
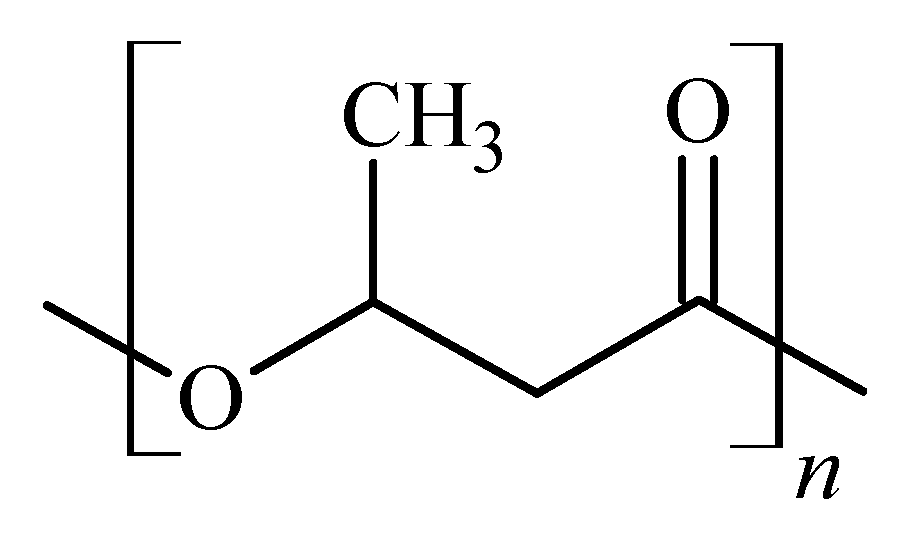
2.1. Polymer Blends of Poly(3-hydroxybutyrate)—Polyurethane
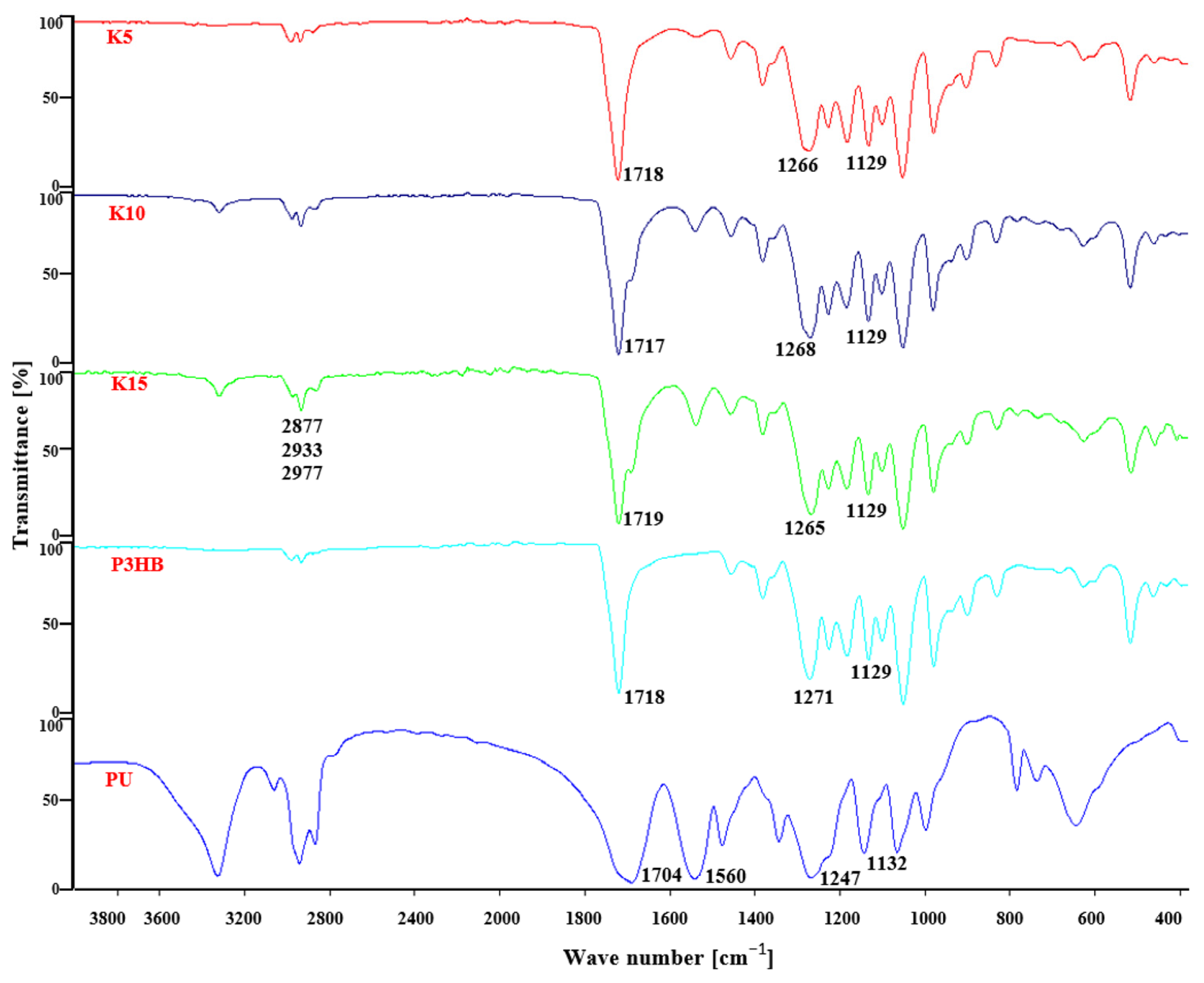
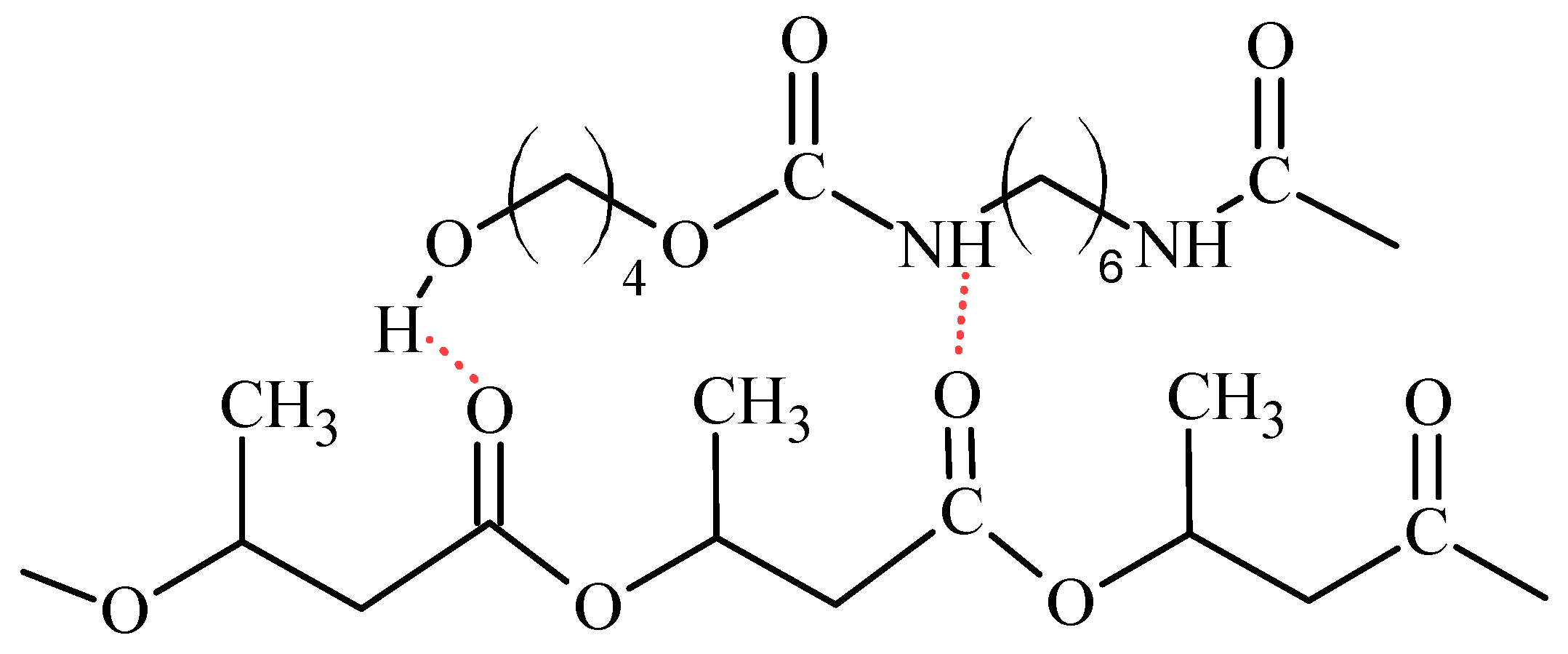
2.2. Spectral Analysis of the Produced Polymer Compositions
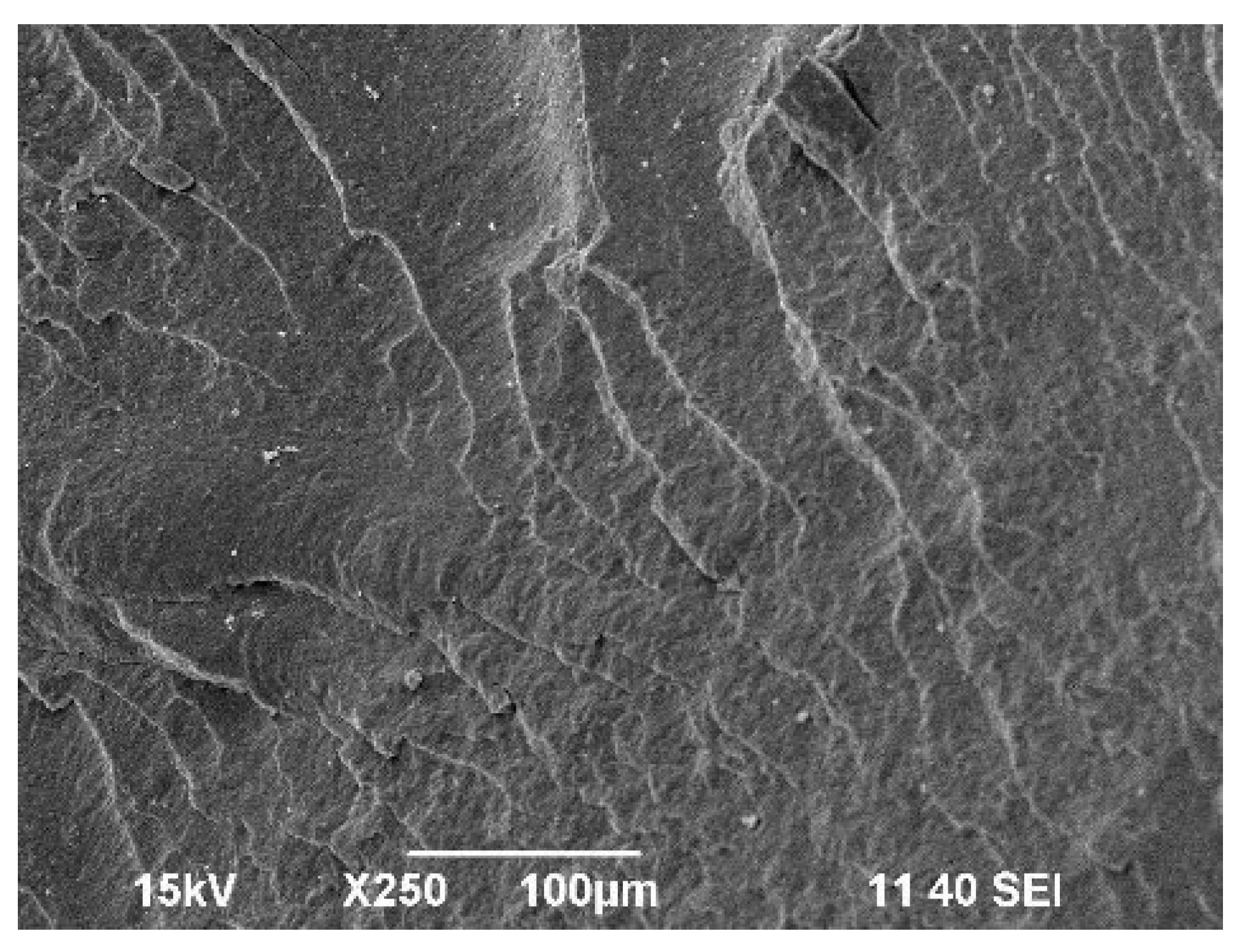
2.3. Morphology Analysis of the Obtained Polymer Compositions
2.4. Mechanical Properties of the Prepared P3HB–PU Polymer Blends
2.5. Thermal Stability of the Produced P3HB Polymer Blends
2.6. Thermal Properties of P3HB–PU Polymer Blends
2.7. Production of Hybrid Nanobiocomposites
2.8. FTIR Analysis of Hybrid Nanobiocomposites
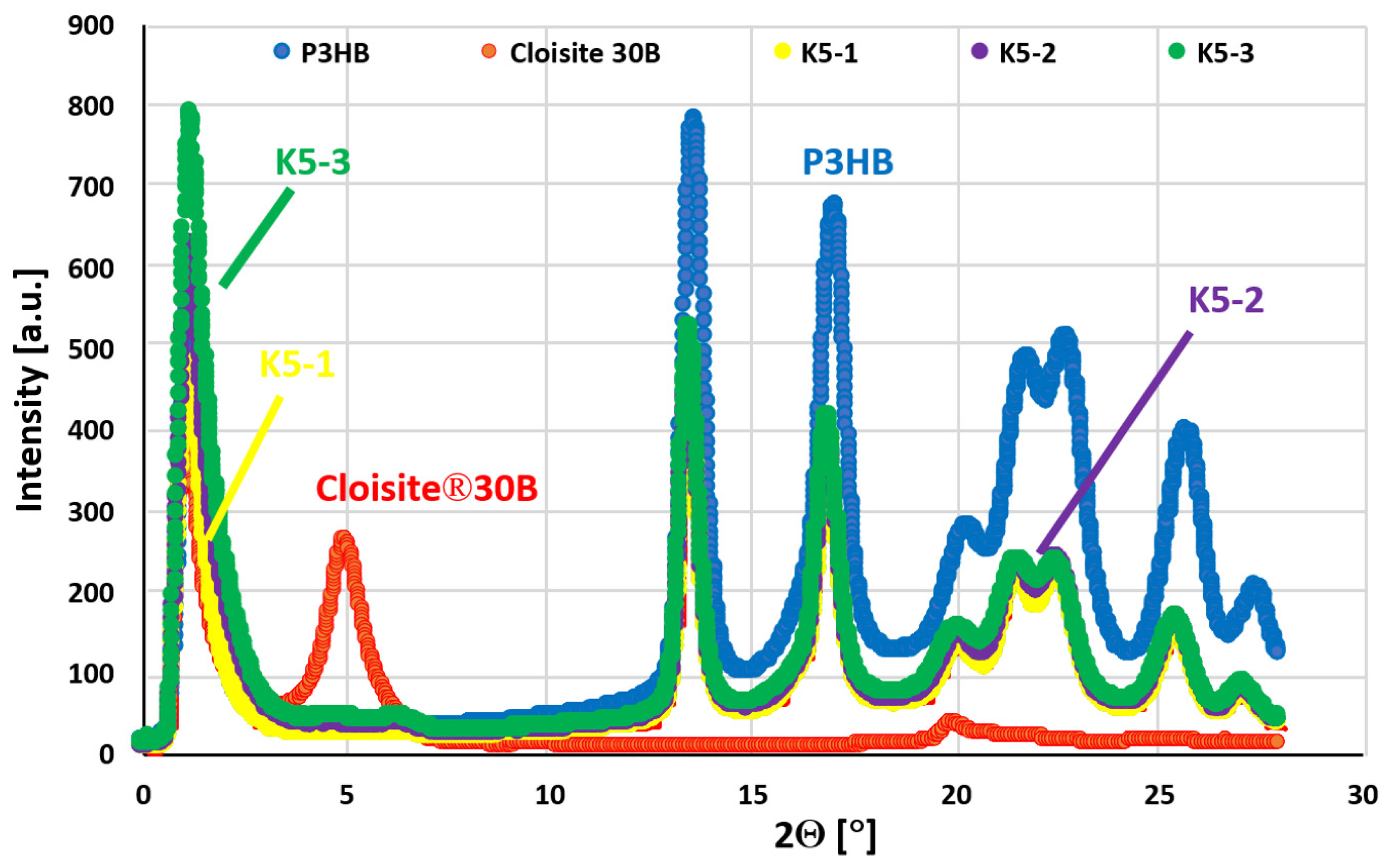
2.9. Structure Analysis of Hybrid P3HB–PU–Cloisite®30B Nanobiocomposites
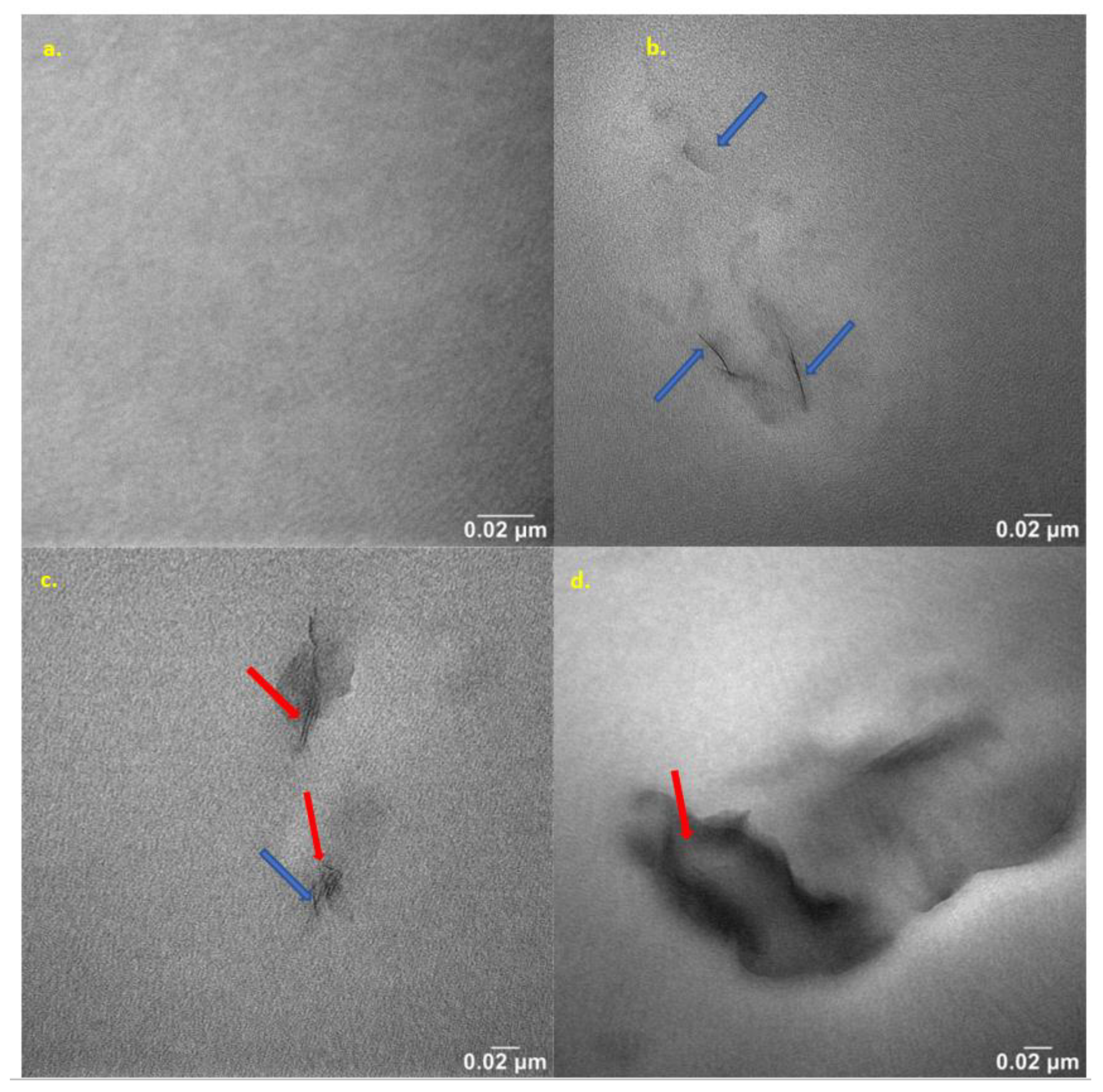
2.10. Nanostructure Analysis of Produced Hybrid Nanobiocomposites
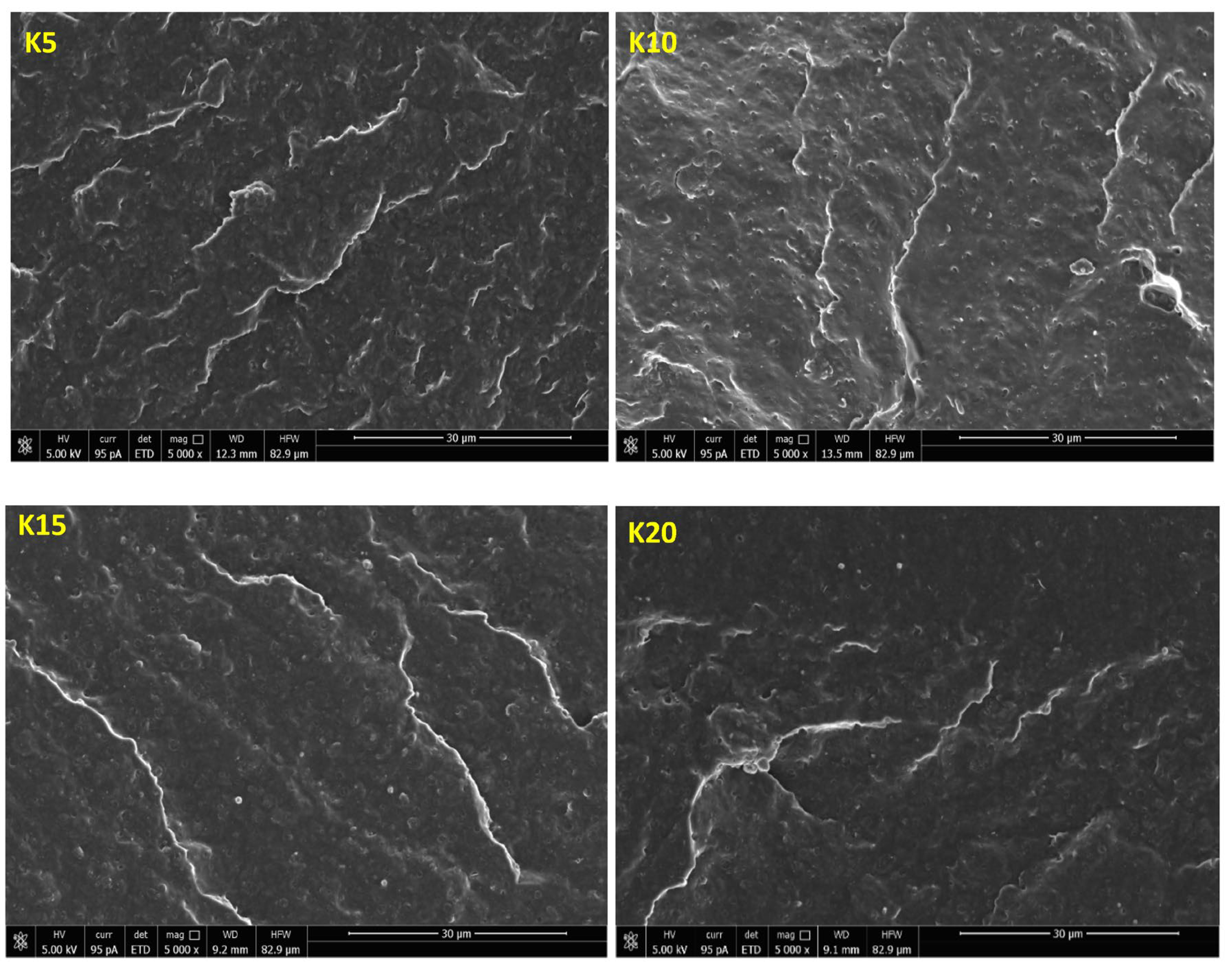
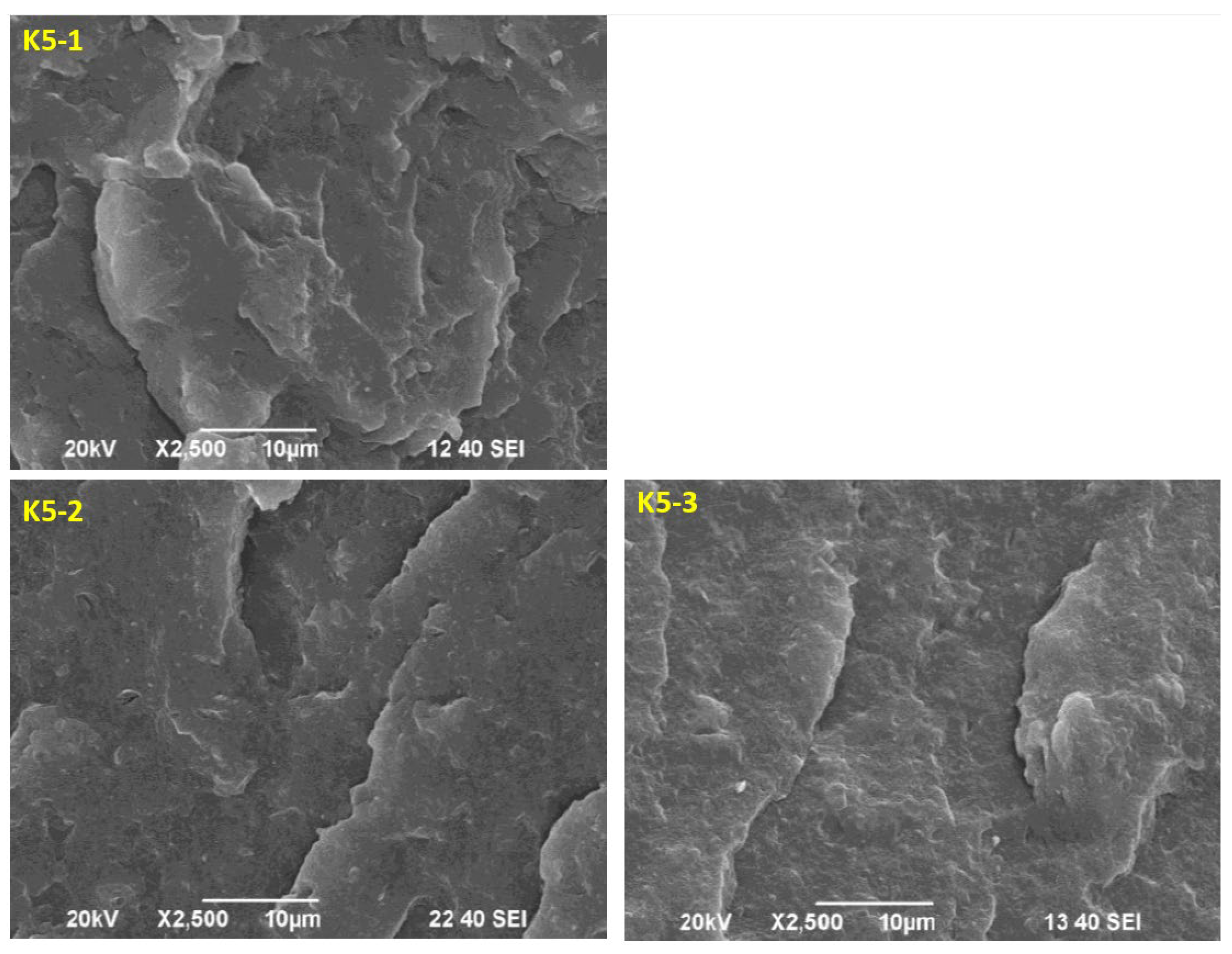
2.11. Morphology Analysis of the Obtained Hybrid Nanobiocomposites
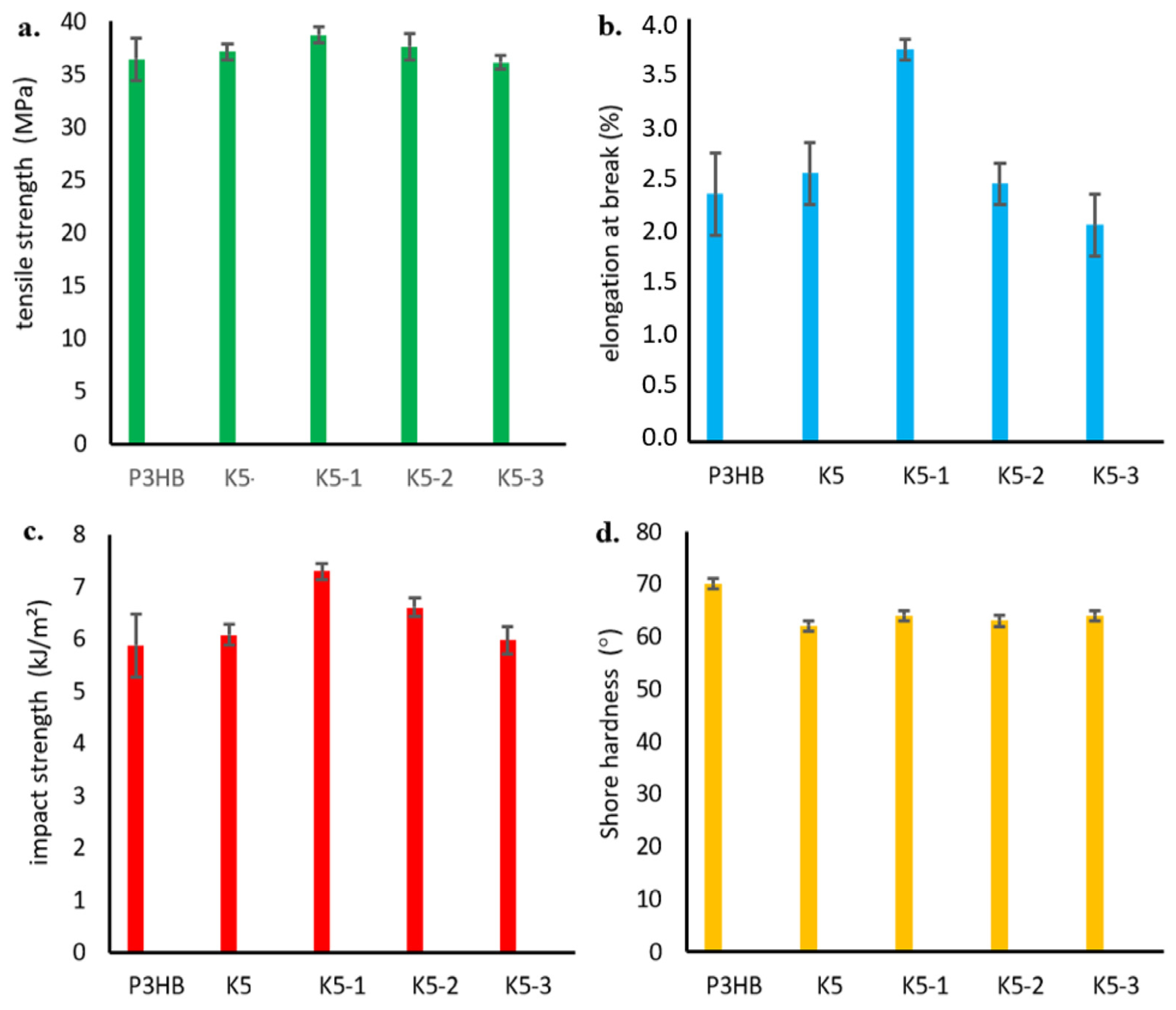
2.12. Mechanical Properties of the Obtained Nanobiocomposites Hybrids
2.13. Thermogravimetric Analysis of Nanobiocomposites
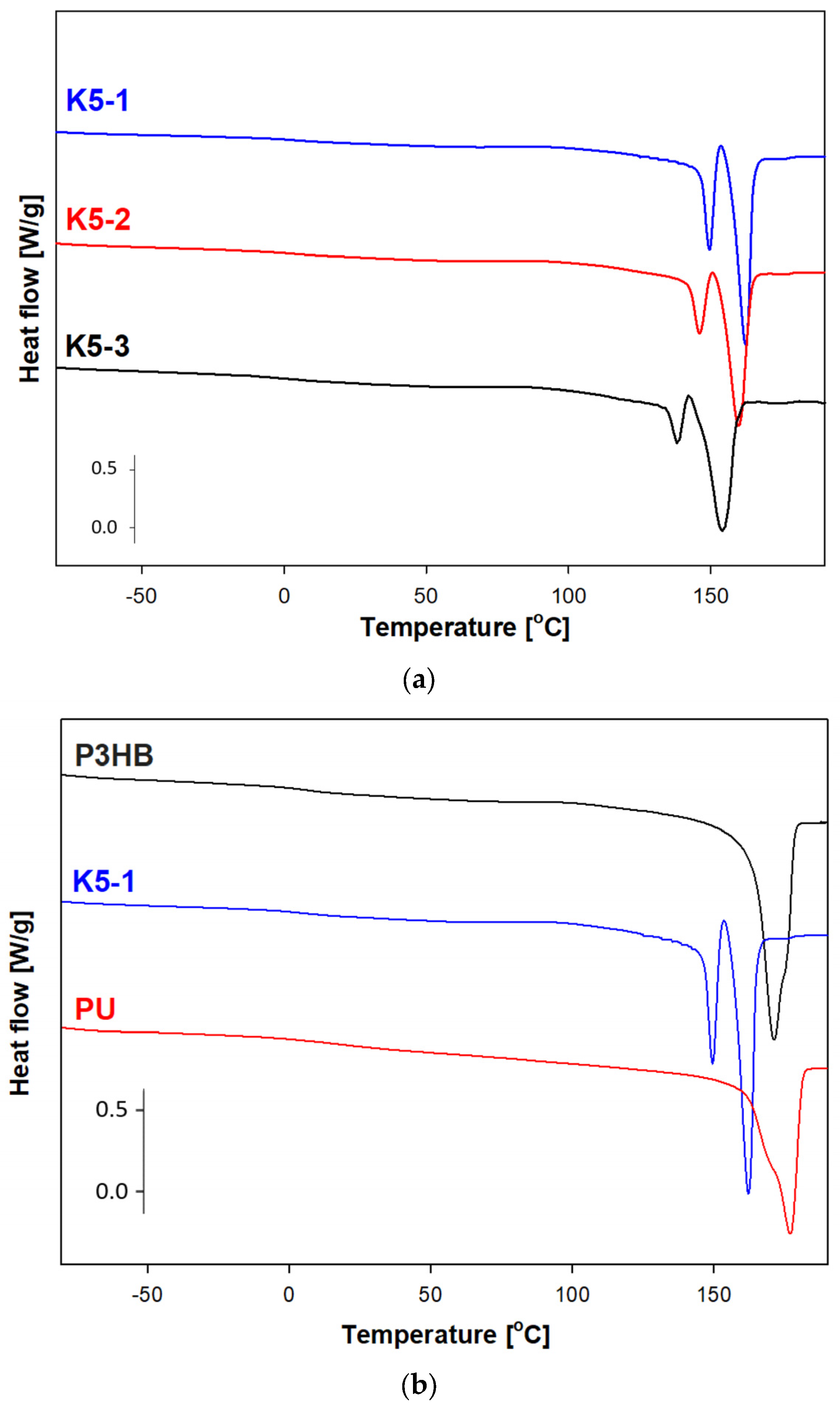
2.14. Thermal Analysis Based on the Differential Scanning Calorimetry Measurements
3. Discussion
4. Materials and Methods
4.1. Materials
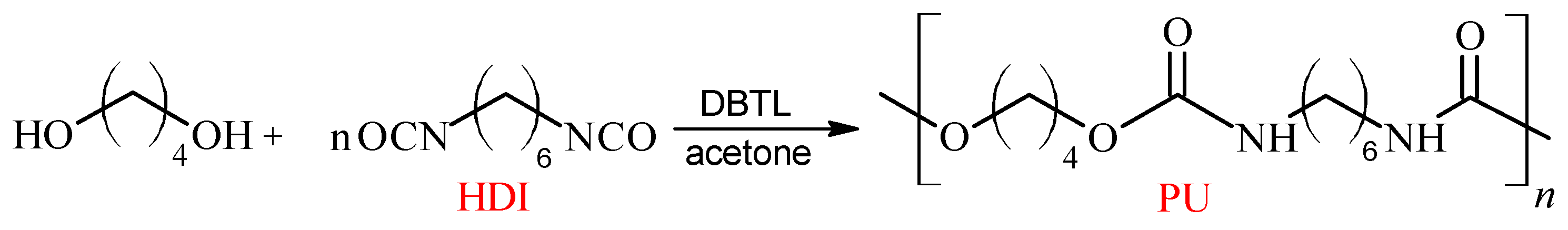
4.1.1. Synthesis of Linear Polyurethane
4.1.2. Preparation of Polymer Blends
4.1.3. Preparation of Polymer Nanobiocompositions
4.2. Analytical Methods
4.2.1. Small-Angle X-ray Scattering
4.2.2. Transmission Electron Microscopy
4.2.3. Scanning Electron Microscopy
4.2.4. FTIR Spectroscopy
4.2.5. Mechanical Properties
4.2.6. Thermogravimetric Analysis
4.2.7. DSC Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalia, V.C.; Patel, S.K.S.; Shanmugam, R.; Lee, J.-K. Polyhydroxyalkanoates: Trends and advances toward biotechnological applications. Bioresour. Technol. 2021, 326, 124737. [Google Scholar] [CrossRef] [PubMed]
- Leja, K.; Lewandowicz, G. Polymer biodegradation and biodegradable polymers—A review. Polish J. Environ. Stud. 2010, 19, 255–266. [Google Scholar]
- Hablot, E.; Bordes, P.; Pollet, E.; Averous, L. Thermal and thermo-mechanical degradation of poly(3-hydroxybutyrate)-based multiphase systems. Polym. Degrad. Stab. 2008, 93, 413–421. [Google Scholar] [CrossRef]
- Chen, G.Q.; Wu, Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 2005, 26, 6565–6578. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Sami, N.; Yasin, D.; Ahmad, N.; Fatma, T. Biomedical applications of environmentally friendly polyhydroxyalkanoates. Int. J. Biol. Macromol. 2020, 183, 549–563. [Google Scholar] [CrossRef] [PubMed]
- Tarrahi, R.; Fathi, Z.; Özgür, M.; Seydibeyoğlu, K.; Doustkhah, E.; Khataee, A. Polyhydroxyalkanoates (PHA): From production to nanoarchitecture. Int. J. Biol. Macromol. 2020, 146, 596–619. [Google Scholar] [CrossRef] [PubMed]
- Dias, Y.J.; Robles, J.R.; Sinha-Ray, S.; Abiade, J.; Pourdeyhimi, B.; Niemczyk-Soczynska, B.; Kolbuk, D.; Sajkiewicz, P.; Yarin, A.L. Solution-Blown Poly(hydroxybutyrate) and ε-Poly-L-lysine Submicro- and Microfiber-Based Sustainable Nonwovens with Antimicrobial Activity for Single-Use Applications. ACS Biomater. Sci. Eng. 2021, 7, 3980–3992. [Google Scholar] [CrossRef]
- Poli, A.; Di Donato, P.; Abbamondi, G.R.; Nicolaus, B. Synthesis, Production, and Biotechnological Applications of Exopolysaccharides and Polyhydroxyalkanoates. Archaea 2011, 2011, 693253. [Google Scholar] [CrossRef]
- Sudesh, K.; Abe, H.; Doi, H. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Fernandes, E.G.; Pietrini, M.; Chiellini, E. Thermo-mechanical and morphological characterization of plasticized poly[(R)-3-hydroxybutyric acid]. Macrom. Symp. 2004, 218, 157–164. [Google Scholar] [CrossRef]
- El-Taweel, S.H.; Höhne, G.W.H.; Mansour, A.A.; Stoll, B. Glass transition and the rigid amorphous phase in semicrystalline blends of bacterial polyhydroxybutyrate PHB with low molecular mass atactic R, S-PHB-diol. Polymer 2004, 45, 983–992. [Google Scholar] [CrossRef]
- Pan, Y.; Inoue, Y. Polymorphism and isomorphism in biodegradable polyesters. Prog. Polym. Sci. 2009, 34, 605–640. [Google Scholar] [CrossRef]
- Yokouchi, M.; Chatani, Y.; Tadokoro, H.; Teranishi, K.; Tani, H. Structural studies of polyesters: Molecular and crystal structures of optically active and racemic poly (β-hydroxybutyrate). Polymer 1973, 14, 267–272. [Google Scholar] [CrossRef]
- Lenz, R.W.; Marchessault, R.H. Bacterial Polyesters: Biosynthesis, Biodegradable Plastics and Biotechnology. Biomacromolecules 2005, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.N.; Lee, M.Y.; Park, W.H. Thermal stabilization of poly(3-hydroxybutyrate) by poly(glycidyl methacrylate). J. Appl. Polym. Sci. 2022, 83, 2945–2952. [Google Scholar] [CrossRef]
- Khanna, S.; Srivastava, A.K. Recent advances in microbial polyhydroxyalkanoates. Process. Biochem. 2005, 40, 607–619. [Google Scholar] [CrossRef]
- Palmeiro-Sánchez, T.; O’Flaherty, V.; Lens, P.N. Polyhydroxyalkanoate bio-production and its rise as biomaterial of the future. J. Biotechnol. 2022, 348, 10–25. [Google Scholar] [CrossRef]
- Ribeiro, P.L.L.; Figueiredo, T.V.B.; Moura, L.E.; Druzian, J.I. Chemical modification of cellulose nanocrystals and their application in thermoplastic starch (TPS) and poly(3-hydroxybutyrate) (P3HB) nanocomposites. Polym. Adv. Technol. 2019, 30, 573–583. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Then, Y.Y.; Loo, Y.Y. Epoxidized Vegetable oils plasticized poly(lactic acid) biocomposites: Mechanical, thermal and morphology properties. Molecules 2014, 19, 16024–16038. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Ferri, J.M.; Montanes, N.; Lopez-Martinez, J.; Balart, R. Plasticization effects of epoxidized vegetable oils on mechanical properties of poly(3-hydroxybutyrate). Polym. Int. 2016, 65, 1157–1164. [Google Scholar] [CrossRef]
- Alakrach, A.M.; Zulkepli, N.N.; Dahham, O.S.; AL-Rashdi, A.A.; Johari, I.; Razlan, Z.M.; Ibrahim, Z.; Khairunizam, W. Physical properties of plasticized PLA/HNTs bionanocomposites: Effects of plasticizer type and content. IOP Conf. Ser. Mater. Sci. Eng. 2019, 557, 012067. [Google Scholar] [CrossRef]
- Zhao, Y.; Qu, J.; Feng, Y.; Wu, Z.; Chen, F.; Tang, H. Mechanical and thermal properties of epoxidized soybean oil plasticized polybutylene succinate blends. Polym. Adv. Technol. 2012, 23, 632–638. [Google Scholar] [CrossRef]
- Seydibeyoglu, M.O.; Misra, M.; Mohanty, A. Progress in bio-based plastics and plasticizing modifications. Int. J. Plast. Technol. 2010, 14, 1–16. [Google Scholar]
- Ferri, J.M.; Samper, M.; Garcia-Sanoguera, D.; Reig, M.; Fenollar, O.; Balart, R. Plasticizing effect of biobased epoxidized fatty acid esters on mechanical and thermal properties of poly(lactic acid). J. Mater. Sci. 2016, 51, 5356–5366. [Google Scholar] [CrossRef]
- Jost, V.; Kopitzky, R. Blending of Polyhydroxybutyrate-co-valerate with Polylactic Acid for Packaging Applications—Reflections on Miscibility and Effects on the Mechanical and Barrier Properties. Chem. Biochem. Eng. Q. 2015, 29, 221–246. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, X.; Wang, X.; Chen, X.; Chen, G.Q.; Xu, K. Processability modifications of poly (3-hydroxybutyrate) by plasticizing, blending, and stabilizing. J. Appl. Polym.Sci. 2008, 107, 166–173. [Google Scholar] [CrossRef]
- Ali, F.; Chang, Y.-W.; Kang, S.C.; Yoon, J.Y. Thermal, mechanical and rheological properties of poly (lactic acid)/epoxidized soybean oil blends. Polym. Bull. 2009, 62, 91–98. [Google Scholar] [CrossRef]
- Silverajah, V.S.G.; Ibrahimm, N.A.; Zainuddin, N.; Yunus, W.M.Z.W.; Abu, H. Mechanical, thermal and morphological properties of poly(lactic acid)/epoxidized palm olein blends. Molecules 2012, 17, 11729–11747. [Google Scholar] [CrossRef]
- Al-Mulla, E.A.J.; Yunus, W.M.Z.W.; Ibrahim, N.A.B.; Ab Rahman, M.Z. Properties of epoxidized palm oil plasticized poly(lactic acid). J. Mater. Sci. 2010, 45, 1942–1946. [Google Scholar] [CrossRef]
- Silverajah, V.S.G.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Abu, H.; Woei, C.B. Thermal and Morphological Characterization of Poly(lactic acid)/Epoxidized Palm Oil Blend. Int. J. Mol. Sci. 2012, 13, 5878–5898. [Google Scholar] [CrossRef]
- Prempeh, N.; Li, J.; Liu, D.; Das, K.; Maiti, S.; Zhang, Y. Plasticizing effects of epoxidized sun flower oil on biodegradable polylactide films: A comparative study. Polym. Sci. Ser. A 2014, 56, 856–863. [Google Scholar] [CrossRef]
- Fernández-Ronco, M.P.; Gradzik, B.; Gooneie, A.; Hufenus, R.; El Fray, M. Tuning poly(3 hydroxybutyrate) (P3HB) properties by tailored segmented bio copolymers. ACS Sustain. Chem. Eng. 2017, 5, 11060–11068. [Google Scholar] [CrossRef]
- Kozlowska, A.; Gromadzki, D.; El Fray, M.; Štěpánek, P. Morphology evaluation of biodegradable copolyesters based on dimerized fatty acid studied by DSC, SAXS and WAXS. Fibres Text. East Eur. 2008, 16, 85. [Google Scholar]
- Mirmusavi, M.H. Assessing the physical and mechanical properties of poly 3-hydroxybutyrate-chitosan-multi-walled carbon nanotube/silk nano–micro composite scaffold for long-term healing tissue engineering applications. Micro. Nano. Lett. 2018, 13, 829–834. [Google Scholar] [CrossRef]
- Zarei, M.; Karbasi, S. Evaluation of the effects of multiwalled carbon nanotubes on electrospun poly (3-hydroxybutirate) scaffold for tissue engineering applications. J. Porous Mat. 2018, 25, 259–272. [Google Scholar] [CrossRef]
- Barud, H.S.; Souza, J.L.; Santos, D.B.; Crespi, M.S.; Ribeiro, C.A.; Messaddeq, Y.; Ribeiro, S.J.L. Bacterial cellulose/poly(3-hydroxybutyrate) composite membranes. Carbohydr. Polym. 2011, 83, 1279–1284. [Google Scholar] [CrossRef]
- Thiré, R.M.S.M.; Ribeiro, T.A.A.; Andrade, C.T. Effect of starch addition on compression-molded poly(3-hydroxybutyrate)/starch blends. J. Appl. Polym. Sci. 2006, 100, 4338–4347. [Google Scholar] [CrossRef]
- Dos Santos, B.R.; Bacalhau, F.B.; Pereira, T.D.S. Chitosan-montmorillonite microspheres: A sustainable fertilizer delivery system. Carbohydr. Polym. 2015, 127, 340–346. [Google Scholar] [CrossRef]
- Souza, J.L.; de Campos, A.; França, D.; Faez, R. PHB and montmorillonite clay composites as KNO3 and NPK support for a controlled release. J. Polym. Environ. 2019, 27, 2089–2097. [Google Scholar] [CrossRef]
- Isa, M.R.M.; Hassan, A.; Nordin, N.A.; Thirmizir, M.Z.A.; Ishak, Z.A.M. Mechanical, rheological and thermal properties of montmorillonite-modified polyhydroxybutyrate composites. High Perform. Polym. 2020, 32, 192–200. [Google Scholar] [CrossRef]
- D’Amico, D.A.; Manfredi, L.B.; Cyras, V.P. Relationship between thermal properties, morphology, and crystallinity of nanocomposites based on polyhydroxybutyrate. J. Appl. Polym. Sci. 2012, 123, 200–208. [Google Scholar] [CrossRef]
- Joshi, S. Can nanotechnology improve the sustainability of biobased products? The case of layered silicate biopolymer nanocomposites. J. Ind. Ecol. 2014, 12, 474–489. [Google Scholar] [CrossRef]
- Yan, X.U.; Zhou, W.; Ma, X.; Sun, B. Fabrication and characterization of poly(3-hydroxybutyrate-co3-hydroxyhexanoate) modified with nano-montmorillonite biocomposite. e-Polymers 2021, 21, 38–46. [Google Scholar] [CrossRef]
- Hussain, F.; Hojjati, M.; Okamoto, M. Polymer-matrix nanocomposites, processing, manufacturing, and application. J. Compos. Mater. 2006, 40, 1511–1575. [Google Scholar] [CrossRef]
- Li, D.N.; Fu, J.R.; Ma, X.J. Improvement in thermal, mechanical, and barrier properties of biocomposite of poly(3-hydroxybutyrateco-3-hydroxyhexanoate)/modified nano-SiO2. Polym Compos. 2020, 41, 381–390. [Google Scholar] [CrossRef]
- Risyon, N.P.; Othman, S.H.; Basha, R.K.; Talib, R.A. Effect of halloysite nanoclay concentration and addition of glycerol on mechanical properties of bionanocomposite films. Polym. Polym. Compos. 2016, 24, 795–801. [Google Scholar] [CrossRef]
- Souza, A.C.; Goto, G.E.O.; Mainardi, J.A.; Coelho, A.C.V.; Tadini, C.C. Cassava starch composite films incorporated with cinnamon essential oil: Antimicrobial activity, microstructure, mechanical and barrier properties. LWT-Food Sci. Technol. 2013, 54, 346–352. [Google Scholar] [CrossRef]
- Jandas, P.J.; Prabakaran, K.; Kumar, R.; Mohanty, S.; Nayak, S.K. Eco-friendly poly (hydroxybutyrate) nanocomposites: Preparation and characterization. J. Polym. Res. 2021, 28, 285. [Google Scholar] [CrossRef]
- Botana, A.; Mollo, M.; Eisenberg, P.; Torres Sanchez, R.M. Effect of modified montmorillonite on biodegradable PHB nanocomposites. Appl. Clay Sci. 2010, 47, 263–270. [Google Scholar] [CrossRef]
- PN-EN 1242:2006; Kleje—Oznaczanie zawartości grup izocyjanianowych. Polski Komitet Normalizacyjny: Warsaw, Poland, 2006.
- PN-C-89052-03:1985; Polietery do poliuretanów—Metody badań—Oznaczanie liczby hydroksylowej. Polski Komitet Normalizacyjny: Warsaw, Poland, 1985.
- PN-EN ISO 527-2:2012; Tworzywa sztuczne—Oznaczanie właściwości mechanicznych przy statycznym rozciąganiu—Część 2: Warunki badań tworzyw sztucznych przeznaczonych do różnych technik formowania. Polski Komitet Normalizacyjny: Warsaw, Poland, 2012.
- PN-EN ISO 179-1:2010; Plastics—Determination of Charpy Impact Properties—Part 1: Non-Instrumented Impact Test. Polish Committee for Standardization: Warsaw, Poland, 2010.
- PN-EN ISO 868:2004; Tworzywa sztuczne i ebonit—Oznaczanie twardości przy wciskaniu z zastosowaniem twardościomierza (twardość Shore’a). Polski Komitet Normalizacyjny: Warsaw, Poland, 2004.




| Content (m/m%) | Sample Designation | ||
|---|---|---|---|
| P3HB | PU | Cloisite® | |
| 95 | 5 | 0 | K5 |
| 90 | 10 | 0 | K10 |
| 85 | 15 | 0 | K15 |
| 80 | 20 | 0 | K20 |
| 94 | 5 | 1 | K5-1 |
| 93 | 5 | 2 | K5-2 |
| 92 | 5 | 3 | K5-3 |
| Sample | Ton (°C) | T50% (°C) | Tmax1 (°C) | Residue 600 °C (m/m%) |
|---|---|---|---|---|
| P3HB | 221 | 246 | 278 | 1.01 |
| K5 | 234 | 268 | 278 | 0.94 |
| K10 | 266 | 277 | 278 | 0.75 |
| K15 | 268 | 279 | 280 | 0.86 |
| K20 | 268 | 279 | 279 | 0.64 |
| K5-1 | 245 | 279 | 279 | 1.58 |
| K5-2 | 245 | 279 | 278 | 5.64 |
| K5-3 | 241 | 278 | 276 | 3.65 |
| Sample | Tg (°C) | ΔCp (J·g−1·°C−1) | Tm (onset) (°C) | Tm1 (°C) | Tm2 (°C) | ΔHf (J·g−1) | Tc (°C) | ΔHc (J·g−1) |
|---|---|---|---|---|---|---|---|---|
| P3HB | 7.70 | 0.1620 | 159.73 | 165.75 | - | 91.93 | 90.50 | 88.79 |
| K5 | 4.45 | 0.2257 | 149.92 | 153.30 | 165.30 | 92.98 | 88.00 | 79.06 |
| K10 | 6.02 | 0.0680 | 145.03 | 150.95 | 163.50 | 88.14 | 85.60 | 68.98 |
| K15 | 2.40 | 0.2294 | 140.81 | 144.55 | 158.10 | 94.55 | 80.80 | 67.04 |
| K20 | 2.30 | 0.1875 | 135.53 | 140.12 | 153.20 | 92.67 | 76.40 | 66.00 |
| Sample | Tg (°C) | ΔCp (J·g−1·°C−1) | Tm (onset) (°C) | Tm1 (°C) | Tm2 (°C) | ΔHf (J·g−1) | Tc (°C) | ΔHc (J·g−1) |
|---|---|---|---|---|---|---|---|---|
| K5-1 | 4.50 | 0.2059 | 146.70 | 149.50 | 162.20 | 90.75 | 86.70 | 68.34 |
| K5-2 | 4.40 | 0.2328 | 142.00 | 146.00 | 159.80 | 89.78 | 82.87 | 68.97 |
| K5-3 | 5.25 | 0.3752 | 134.60 | 138.20 | 153.80 | 85.70 | 77.65 | 63.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzykowska, B.; Czerniecka-Kubicka, A.; Białkowska, A.; Bakar, M.; Hęclik, K.; Dobrowolski, L.; Longosz, M.; Zarzyka, I. Polymer Biocompositions and Nanobiocomposites Based on P3HB with Polyurethane and Montmorillonite. Int. J. Mol. Sci. 2023, 24, 17405. https://doi.org/10.3390/ijms242417405
Krzykowska B, Czerniecka-Kubicka A, Białkowska A, Bakar M, Hęclik K, Dobrowolski L, Longosz M, Zarzyka I. Polymer Biocompositions and Nanobiocomposites Based on P3HB with Polyurethane and Montmorillonite. International Journal of Molecular Sciences. 2023; 24(24):17405. https://doi.org/10.3390/ijms242417405
Chicago/Turabian StyleKrzykowska, Beata, Anna Czerniecka-Kubicka, Anita Białkowska, Mohamed Bakar, Karol Hęclik, Lucjan Dobrowolski, Michał Longosz, and Iwona Zarzyka. 2023. "Polymer Biocompositions and Nanobiocomposites Based on P3HB with Polyurethane and Montmorillonite" International Journal of Molecular Sciences 24, no. 24: 17405. https://doi.org/10.3390/ijms242417405
APA StyleKrzykowska, B., Czerniecka-Kubicka, A., Białkowska, A., Bakar, M., Hęclik, K., Dobrowolski, L., Longosz, M., & Zarzyka, I. (2023). Polymer Biocompositions and Nanobiocomposites Based on P3HB with Polyurethane and Montmorillonite. International Journal of Molecular Sciences, 24(24), 17405. https://doi.org/10.3390/ijms242417405







