Childhood Obesity: Insight into Kidney Involvement
Abstract
1. Epidemiology
2. Childhood Obesity Definition and BMI Related CKD Progression
3. Impact of Obesity on Kidney Outcomes in Children
4. Hereditary Factors of Obesity
- I.
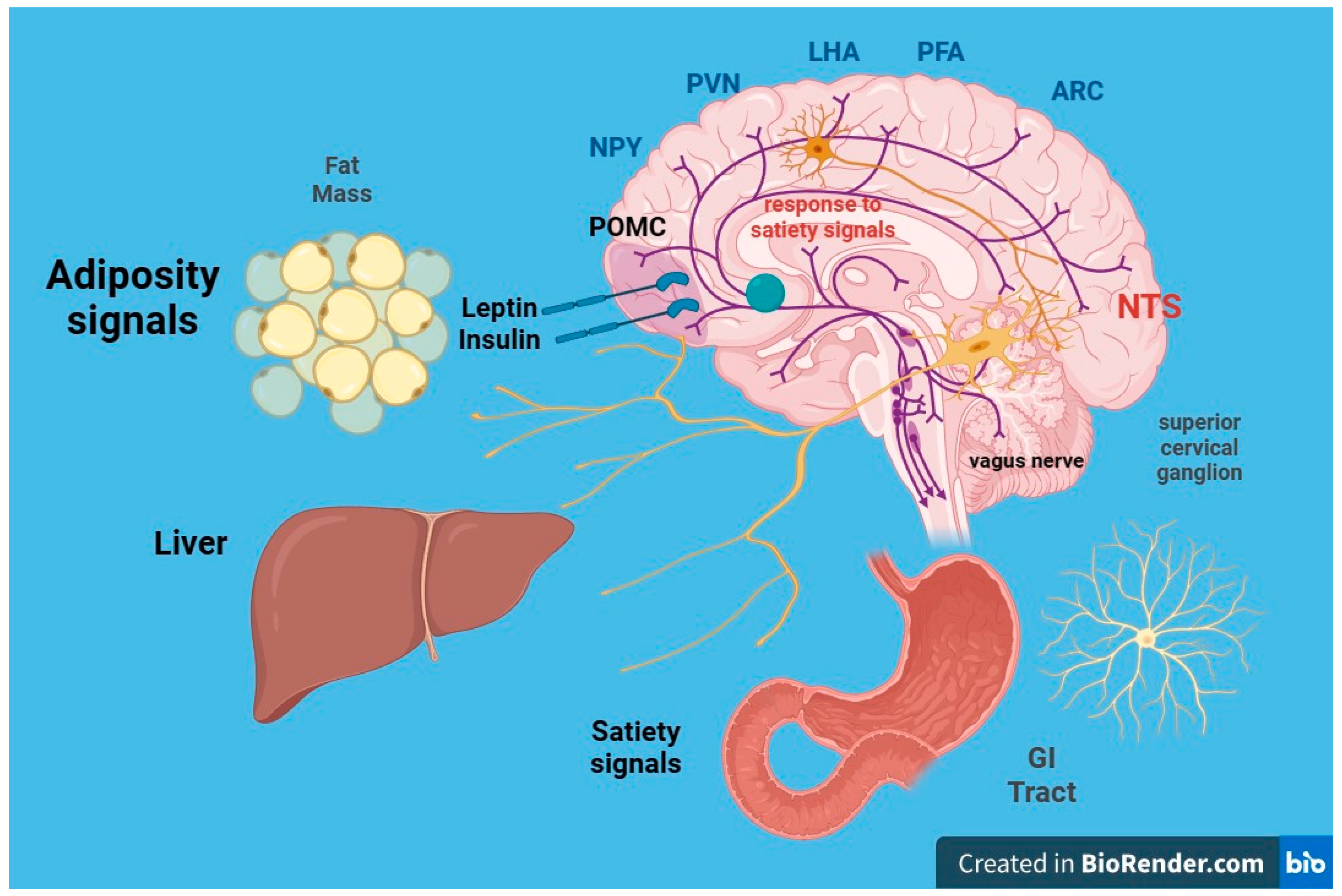
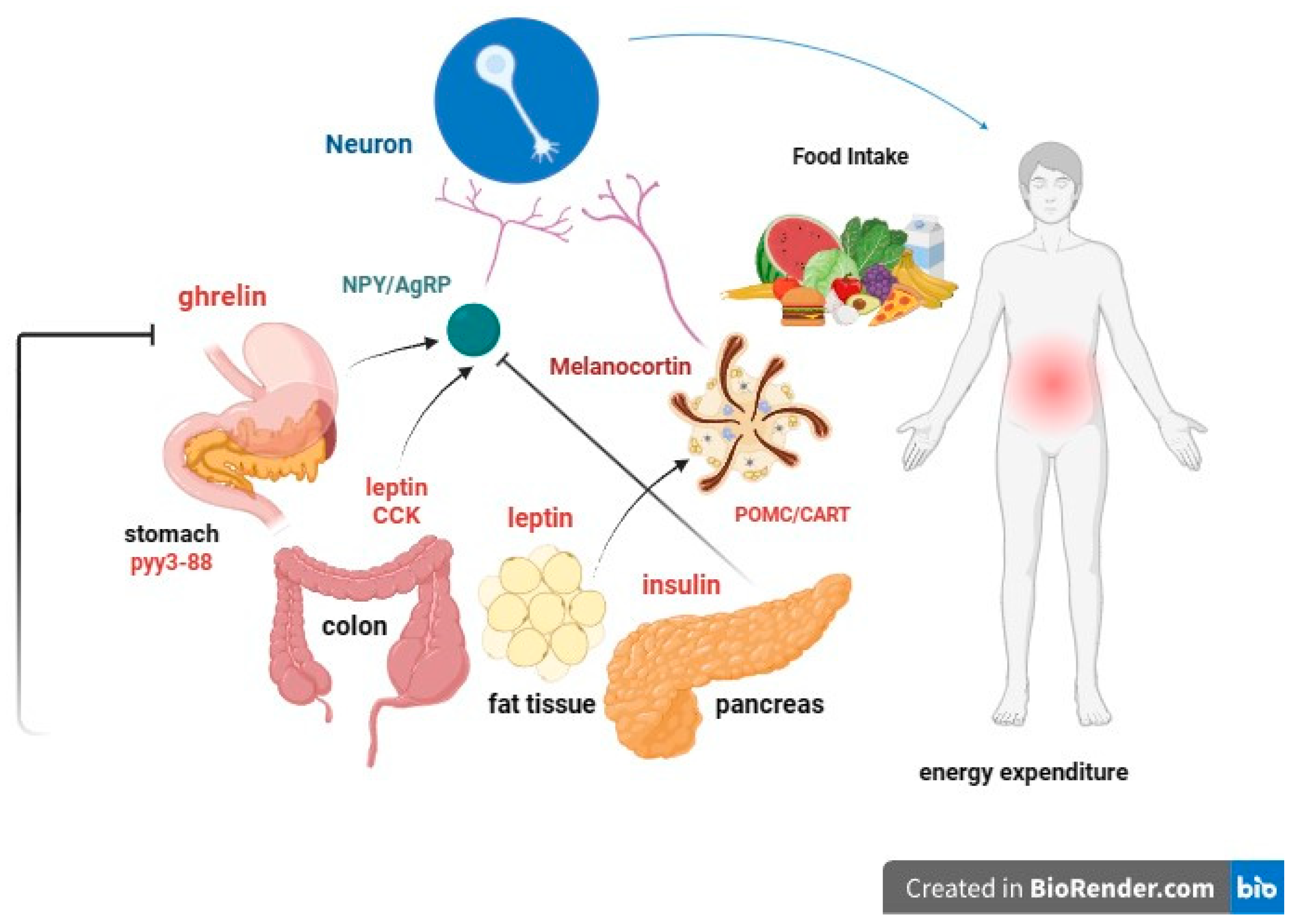
- leptin: Produced by adipose cells in the placenta and, to a lesser extent, in the intestine. The Ob (Obese) or Lep (Leptin) gene codes for leptin, which signals to the brain, regarding the levels of stored fat in the body. Leptin-deficient mice (Ob mice) show hyperphagia, insulin resistance, hyperinsulinemia, and infertility. By increasing adiposity, resistance to the action of leptin occurs. Although obesity due to leptin deficiency has been studied, most people with obesity do not have any abnormalities in the leptin gene. This suggests that obesity may be caused by either leptin deficiency or genetic defects in the leptin receptor itself [49,50].
- II.
- Prohormone convertase 1/3 (PC1/3): a congenital deficiency of the PCSK1 gene, responsible for proprotein convertase 1/3, can lead to a severe multihormonal disorder characterized by early-onset obesity [51].
- III.
- IV.
- Proopiomelanocortin (POMC) or melanocyte-stimulating hormone (MSH): It transmits the appetite-suppressing effect of leptin through MC4R. Mutations in POMC gene can also lead to early-onset obesity due to severe hyperphagia. ACTH (AdrenoCorticoTropic Hormone) is produced by POMC in the hypothalamus, as is alpha-MSH, a key factor in reducing food intake [55,56].
- V.
- Guanine nucleotide-binding protein G, alpha stimulant (GNAS) gene mutations: these are associated with Albright’s hereditary osteodystrophy (i.e., type 1 pseudohypoparathyroidism), and characterized by early-onset obesity, along with other features such as developmental delay, short stature, brachydactyly, subcutaneous ossifications, pseudohypoparathyroidism (hypocalcemia, resistance to parathormone), and resistance to thyrotropin (elevated thyroid stimulating hormone with normal or low-free thyroxine) [57].
5. Correlation between Low Birth Weight, Obesity, and CKD
6. Neurohormonal, Metabolic and Immunological Effects of Obesity on Kidney Function
6.1. Insulin Resistance
6.2. Leptin
6.3. Adiponectin
6.4. Other Adipokines
7. Hypertension and Obesity in Children
8. Renal Biomarkers in Obese Children
| Proximal Tubular Injury Markers | Distal Tubular Injury Markers | Glomerular Injury Markers |
|---|---|---|
| Cystatin C | N-acetyl-beta-D-glucosaminadase (NAG) | Cystatin C |
| N-acetyl-beta-D-glucosaminadase (NAG) | Neutrophil gelatinase-associated lipocalin (NGAL) | Podocin |
| Neutrophil gelatinase-associated lipocalin (NGAL) | Alpha-1-acid glycoprotein (AGP) | Nephrin |
| Alpha-1-acid glycoprotein (AGP) | Podocalyxin (PCX) | |
| Alpha-1-acid glycoprotein (AGP) |
9. Proteomic and Metabolomic Approaches to the Discovery of Novel Biomarkers of Kidney Injury
10. Obesity: Mechanisms of Kidney Damage
11. Cancer, Stones, and Gout: Impact of Obesity on Kidneys
12. Obesity-Related Glomerulopathy: An Overview
13. Obesity as a Risk Factor in Children with Kidney Transplants
14. Prevention and Management
15. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lobstein, T.; Baur, L.; Uauy, R. Obesity in children and young people: A crisis in public health. Obes. Rev. 2004, 5 (Suppl. 1), 4–104. [Google Scholar] [CrossRef] [PubMed]
- WHO. Obesity and Overweight. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 2 November 2023).
- Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [CrossRef] [PubMed]
- Gao, L.; Peng, W.; Xue, H.; Wu, Y.; Zhou, H.; Jia, P.; Wang, Y. Spatial-temporal trends in global childhood overweight and obesity from 1975 to 2030: A weight mean center and projection analysis of 191 countries. Glob. Health 2023, 19, 53. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Seeman, T.E.; Nianogo, R.; Okubo, Y. The effect of poverty on the relationship between household education levels and obesity in U.S. children and adolescents: An observational study. Lancet Reg. Health Am. 2023, 25, 100565. [Google Scholar] [CrossRef]
- Câmara, N.O.; Iseki, K.; Kramer, H.; Liu, Z.H.; Sharma, K. Kidney disease and obesity: Epidemiology, mechanisms and treatment. Nat. Rev. Nephrol. 2017, 13, 181–190. [Google Scholar] [CrossRef]
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Russo, D.; Del Prete, M.; Battaglia, Y.; Russo, L. Risk for chronic kidney disease in high school students: Italian report for World Kidney Day 2008–2009. J. Nephrol. 2011, 24, 250–253. [Google Scholar] [CrossRef]
- Battaglia, Y.; Russo, L.; Spadola, R.; Russo, D. Awareness of kidney diseases in general population and in high school students. Italian report for World Kidney Days 2010–2011. J. Nephrol. 2012, 25, 843–846. [Google Scholar] [CrossRef]
- Chong, L.S.H.; Sautenet, B.; Tong, A.; Hanson, C.S.; Samuel, S.; Zappitelli, M.; Dart, A.; Furth, S.; Eddy, A.A.; Groothoff, J.; et al. Range and Heterogeneity of Outcomes in Randomized Trials of Pediatric Chronic Kidney Disease. J. Pediatr. 2017, 186, 110–117.e11. [Google Scholar] [CrossRef]
- Chou, H.H.; Chiou, Y.Y.; Chiou, Y.H.; Tain, Y.L.; Wang, H.H.; Yu, M.C.; Hsu, C.C.; Lin, C.Y. Mortality Risks among Various Primary Renal Diseases in Children and Adolescents on Chronic Dialysis. J. Clin. Med. 2018, 7, 414. [Google Scholar] [CrossRef]
- Assadi, F. The Growing Epidemic of Chronic Kidney Disease: Preventive Strategies to Delay the Risk for Progression to ESRD. In Primordial Prevention of Non Communicable Disease; Kelishadi, R., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 57–59. [Google Scholar]
- Vivante, A.; Golan, E.; Tzur, D.; Leiba, A.; Tirosh, A.; Skorecki, K.; Calderon-Margalit, R. Body Mass Index in 1.2 Million Adolescents and Risk for End-Stage Renal Disease. Arch. Intern. Med. 2012, 172, 1644–1650. [Google Scholar] [CrossRef] [PubMed]
- Bonthuis, M.; van Stralen, K.J.; Verrina, E.; Groothoff, J.W.; Alonso Melgar, Á.; Edefonti, A.; Fischbach, M.; Mendes, P.; Molchanova, E.A.; Paripović, D.; et al. Underweight, overweight and obesity in paediatric dialysis and renal transplant patients. Nephrol. Dial. Transplant. 2013, 28, iv195–iv204. [Google Scholar] [CrossRef] [PubMed]
- Warady, B.A.; Chadha, V. Chronic kidney disease in children: The global perspective. Pediatr. Nephrol. 2007, 22, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Harambat, J.; van Stralen, K.J.; Kim, J.J.; Tizard, E.J. Epidemiology of chronic kidney disease in children. Pediatr. Nephrol. 2012, 27, 363–373. [Google Scholar] [CrossRef]
- Kuczmarski, R.J.; Ogden, C.L.; Guo, S.S.; Grummer-Strawn, L.M.; Flegal, K.M.; Mei, Z.; Wei, R.; Curtin, L.R.; Roche, A.F.; Johnson, C.L. 2000 CDC Growth Charts for the United States: Methods and development. Vital Health Stat. 2002, 11, 1–190. [Google Scholar]
- Kelly, A.S.; Barlow, S.E.; Rao, G.; Inge, T.H.; Hayman, L.L.; Steinberger, J.; Urbina, E.M.; Ewing, L.J.; Daniels, S.R. Severe obesity in children and adolescents: Identification, associated health risks, and treatment approaches: A scientific statement from the American Heart Association. Circulation 2013, 128, 1689–1712. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; Song, Y.; Caballero, B.; Cheskin, L.J. Association between obesity and kidney disease: A systematic review and meta-analysis. Kidney Int. 2008, 73, 19–33. [Google Scholar] [CrossRef]
- Fox, C.S.; Larson, M.G.; Leip, E.P.; Culleton, B.; Wilson, P.W.; Levy, D. Predictors of new-onset kidney disease in a community-based population. JAMA 2004, 291, 844–850. [Google Scholar] [CrossRef]
- Russo, D.; Morrone, L.F.; Errichiello, C.; De Gregorio, M.G.; Imbriaco, M.; Battaglia, Y.; Russo, L.; Andreucci, M.; Di Iorio, B.R. Impact of BMI on cardiovascular events, renal function, and coronary artery calcification. Blood Purif. 2014, 38, 1–6. [Google Scholar] [CrossRef]
- Garofalo, C.; Borrelli, S.; Minutolo, R.; Chiodini, P.; De Nicola, L.; Conte, G. A systematic review and meta-analysis suggests obesity predicts onset of chronic kidney disease in the general population. Kidney Int. 2017, 91, 1224–1235. [Google Scholar] [CrossRef]
- Postorino, M.; Marino, C.; Tripepi, G.; Zoccali, C. Abdominal obesity and all-cause and cardiovascular mortality in end-stage renal disease. J. Am. Coll. Cardiol. 2009, 53, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; McCulloch, C.E.; Iribarren, C.; Darbinian, J.; Go, A.S. Body mass index and risk for end-stage renal disease. Ann. Intern. Med. 2006, 144, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Maring, B.; Greenspan, L.C.; Chandra, M.; Daniels, S.R.; Sinaiko, A.; Prineas, R.J.; Parker, E.D.; Adams, K.F.; Daley, M.F.; Sherwood, N.E.; et al. Comparing US paediatric and adult weight classification at the transition from late teenage to young adulthood. Pediatr. Obes. 2015, 10, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Umer, A.; Kelley, G.A.; Cottrell, L.E.; Giacobbi, P., Jr.; Innes, K.E.; Lilly, C.L. Childhood obesity and adult cardiovascular disease risk factors: A systematic review with meta-analysis. BMC Public Health 2017, 17, 683. [Google Scholar] [CrossRef] [PubMed]
- Gepstein, V.; Weiss, R. Obesity as the Main Risk Factor for Metabolic Syndrome in Children. Front. Endocrinol. 2019, 10, 568. [Google Scholar] [CrossRef] [PubMed]
- Stern-Zimmer, M.; Calderon-Margalit, R.; Skorecki, K.; Vivante, A. Childhood risk factors for adulthood chronic kidney disease. Pediatr. Nephrol. 2021, 36, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, G.T.; Nasri, H. World Kidney Day 2014: Increasing awareness of chronic kidney disease and aging. J. Ren. Inj. Prev. 2014, 3, 3–4. [Google Scholar] [CrossRef]
- Wahba, I.M.; Mak, R.H. Obesity and Obesity-Initiated Metabolic Syndrome: Mechanistic Links to Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2007, 2, 550–562. [Google Scholar] [CrossRef]
- Batsis, J.A.; Romero-Corral, A.; Collazo-Clavell, M.L.; Sarr, M.G.; Somers, V.K.; Lopez-Jimenez, F. Effect of bariatric surgery on the metabolic syndrome: A population-based, long-term controlled study. Mayo Clin. Proc. 2008, 83, 897–907. [Google Scholar] [CrossRef]
- Sun, J.; Wang, C.; Zhao, M.; Lee, P.M.Y.; Xi, B.; Yu, Y.; Li, J. Childhood diabetes mellitus and early-onset kidney diseases later in life: A nationwide population-based matched cohort study. BMC Med. 2022, 20, 428. [Google Scholar] [CrossRef]
- Jadresic, L.; Silverwood, R.J.; Kinra, S.; Nitsch, D. Can childhood obesity influence later chronic kidney disease? Pediatr. Nephrol. 2019, 34, 2457–2477. [Google Scholar] [CrossRef] [PubMed]
- Pourghazi, F.; Mohammadi, S.; Eslami, M.; Zoshk, M.Y.; Asadi, S.; Ejtahed, H.S.; Qorbani, M. Association between Childhood Obesity and Later Life Kidney Disorders: A Systematic Review. J. Ren. Nutr. 2023, 33, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Lalan, S.; Jiang, S.; Ng, D.K.; Kupferman, F.; Warady, B.A.; Furth, S.; Mitsnefes, M.M. Cardiometabolic Risk Factors, Metabolic Syndrome, and Chronic Kidney Disease Progression in Children. J. Pediatr. 2018, 202, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Correia-Costa, L.; Azevedo, A.; Caldas Afonso, A. Childhood Obesity and Impact on the Kidney. Nephron 2019, 143, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Silverwood, R.J.; Pierce, M.; Hardy, R.; Thomas, C.; Ferro, C.; Savage, C.; Sattar, N.; Kuh, D.; Nitsch, D. Early-life overweight trajectory and CKD in the 1946 British birth cohort study. Am. J. Kidney Dis. 2013, 62, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Silverwood, R.J.; Pierce, M.; Thomas, C.; Hardy, R.; Ferro, C.; Sattar, N.; Whincup, P.; Savage, C.; Kuh, D.; Nitsch, D. Association between younger age when first overweight and increased risk for CKD. J. Am. Soc. Nephrol. 2013, 24, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Filler, G.; Reimão, S.M.; Kathiravelu, A.; Grimmer, J.; Feber, J.; Drukker, A. Pediatric nephrology patients are overweight: 20 years’ experience in a single Canadian tertiary pediatric nephrology clinic. Int. Urol. Nephrol. 2007, 39, 1235–1240. [Google Scholar] [CrossRef]
- Wong, C.S.; Gipson, D.S.; Gillen, D.L.; Emerson, S.; Koepsell, T.; Sherrard, D.J.; Watkins, S.L.; Stehman-Breen, C. Anthropometric measures and risk of death in children with end-stage renal disease. Am. J. Kidney Dis. 2000, 36, 811–819. [Google Scholar] [CrossRef]
- Berthoud, H.R.; Morrison, C. The brain, appetite, and obesity. Annu. Rev. Psychol. 2008, 59, 55–92. [Google Scholar] [CrossRef]
- Loos, R.J. Genetic determinants of common obesity and their value in prediction. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 211–226. [Google Scholar] [CrossRef]
- Aucella, F.; Gesuete, A.; Battaglia, Y. A “nephrological” approach to physical activity. Kidney Blood Press. Res. 2014, 39, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Frayling, T.M.; Timpson, N.J.; Weedon, M.N.; Zeggini, E.; Freathy, R.M.; Lindgren, C.M.; Perry, J.R.; Elliott, K.S.; Lango, H.; Rayner, N.W.; et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007, 316, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Dina, C.; Meyre, D.; Gallina, S.; Durand, E.; Körner, A.; Jacobson, P.; Carlsson, L.M.; Kiess, W.; Vatin, V.; Lecoeur, C.; et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat. Genet. 2007, 39, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Claussnitzer, M.; Dankel, S.N.; Kim, K.H.; Quon, G.; Meuleman, W.; Haugen, C.; Glunk, V.; Sousa, I.S.; Beaudry, J.L.; Puviindran, V.; et al. FTO Obesity Variant Circuitry and Adipocyte Browning in Humans. N. Engl. J. Med. 2015, 373, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Czajkowski, P.; Adamska-Patruno, E.; Bauer, W.; Fiedorczuk, J.; Krasowska, U.; Moroz, M.; Gorska, M.; Kretowski, A. The Impact of FTO Genetic Variants on Obesity and Its Metabolic Consequences is Dependent on Daily Macronutrient Intake. Nutrients 2020, 12, 3255. [Google Scholar] [CrossRef] [PubMed]
- Gaulton, K.J. Mechanisms of Type 2 Diabetes Risk Loci. Curr. Diabetes Rep. 2017, 17, 72. [Google Scholar] [CrossRef] [PubMed]
- Trasande, L.; Cronk, C.; Durkin, M.; Weiss, M.; Schoeller, D.; Gall, E.; Hewitt, J.; Carrel, A.; Landrigan, P.; Gillman, M. Environment and obesity in the National Children’s Study. Cienc. Saude Coletiva 2010, 15, 195–210. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dardeno, T.A.; Chou, S.H.; Moon, H.S.; Chamberland, J.P.; Fiorenza, C.G.; Mantzoros, C.S. Leptin in human physiology and therapeutics. Front. Neuroendocrinol. 2010, 31, 377–393. [Google Scholar] [CrossRef]
- Stijnen, P.; Tuand, K.; Varga, T.V.; Franks, P.W.; Aertgeerts, B.; Creemers, J.W. The association of common variants in PCSK1 with obesity: A HuGE review and meta-analysis. Am. J. Epidemiol. 2014, 180, 1051–1065. [Google Scholar] [CrossRef]
- Lubrano-Berthelier, C.; Le Stunff, C.; Bougnères, P.; Vaisse, C. A homozygous null mutation delineates the role of the melanocortin-4 receptor in humans. J. Clin. Endocrinol. Metab. 2004, 89, 2028–2032. [Google Scholar] [CrossRef]
- Savastano, D.M.; Tanofsky-Kraff, M.; Han, J.C.; Ning, C.; Sorg, R.A.; Roza, C.A.; Wolkoff, L.E.; Anandalingam, K.; Jefferson-George, K.S.; Figueroa, R.E.; et al. Energy intake and energy expenditure among children with polymorphisms of the melanocortin-3 receptor. Am. J. Clin. Nutr. 2009, 90, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, I.S.; Yeo, G.S.; Keogh, J.M.; Aminian, S.; Jebb, S.A.; Butler, G.; Cheetham, T.; O’Rahilly, S. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J. Clin. Investig. 2000, 106, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Krude, H.; Biebermann, H.; Schnabel, D.; Tansek, M.Z.; Theunissen, P.; Mullis, P.E.; Grüters, A. Obesity due to proopiomelanocortin deficiency: Three new cases and treatment trials with thyroid hormone and ACTH4-10. J. Clin. Endocrinol. Metab. 2003, 88, 4633–4640. [Google Scholar] [CrossRef] [PubMed]
- Cignarelli, M.; Lamacchia, O. Obesity and kidney disease. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Mendes de Oliveira, E.; Keogh, J.M.; Talbot, F.; Henning, E.; Ahmed, R.; Perdikari, A.; Bounds, R.; Wasiluk, N.; Ayinampudi, V.; Barroso, I.; et al. Obesity-Associated GNAS Mutations and the Melanocortin Pathway. N. Engl. J. Med. 2021, 385, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Hoy, W.E.; Rees, M.; Kile, E.; Mathews, J.D.; Wang, Z. A new dimension to the Barker hypothesis: Low birthweight and susceptibility to renal disease. Kidney Int. 1999, 56, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.I.; Jones, A.; Goulden, P.A. Birth weight, stress, and the metabolic syndrome in adult life. Ann. N. Y. Acad. Sci. 2006, 1083, 28–36. [Google Scholar] [CrossRef]
- Abitbol, C.L.; Chandar, J.; Rodríguez, M.M.; Berho, M.; Seeherunvong, W.; Freundlich, M.; Zilleruelo, G. Obesity and preterm birth: Additive risks in the progression of kidney disease in children. Pediatr. Nephrol. 2009, 24, 1363–1370. [Google Scholar] [CrossRef]
- Greenbaum, L.A.; Muñoz, A.; Schneider, M.F.; Kaskel, F.J.; Askenazi, D.J.; Jenkins, R.; Hotchkiss, H.; Moxey-Mims, M.; Furth, S.L.; Warady, B.A. The association between abnormal birth history and growth in children with CKD. Clin. J. Am. Soc. Nephrol. 2011, 6, 14–21. [Google Scholar] [CrossRef]
- Grillo, M.A.; Mariani, G.; Ferraris, J.R. Prematurity and Low Birth Weight in Neonates as a Risk Factor for Obesity, Hypertension, and Chronic Kidney Disease in Pediatric and Adult Age. Front. Med. 2021, 8, 769734. [Google Scholar] [CrossRef]
- Chen, J.; Muntner, P.; Hamm, L.L.; Jones, D.W.; Batuman, V.; Fonseca, V.; Whelton, P.K.; He, J. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann. Intern. Med. 2004, 140, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. The distinction of metabolically ‘healthy’ from ‘unhealthy’ obese individuals. Curr. Opin. Lipidol. 2010, 21, 38–43. [Google Scholar] [CrossRef] [PubMed]
- González, E.; Gutiérrez, E.; Morales, E.; Hernández, E.; Andres, A.; Bello, I.; Díaz-González, R.; Leiva, O.; Praga, M. Factors influencing the progression of renal damage in patients with unilateral renal agenesis and remnant kidney. Kidney Int. 2005, 68, 263–270. [Google Scholar] [CrossRef][Green Version]
- Marcantoni, C.; Ma, L.J.; Federspiel, C.; Fogo, A.B. Hypertensive nephrosclerosis in African Americans versus Caucasians. Kidney Int. 2002, 62, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.L.; Sui, Y.; Guan, J.; He, L.; Zhu, X.; Fan, R.R.; Xu, G.; Kong, A.P.; Ho, C.S.; Lai, F.M.; et al. Fat redistribution and adipocyte transformation in uninephrectomized rats. Kidney Int. 2008, 74, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Roubicek, T.; Bartlova, M.; Krajickova, J.; Haluzikova, D.; Mraz, M.; Lacinova, Z.; Kudla, M.; Teplan, V.; Haluzik, M. Increased production of proinflammatory cytokines in adipose tissue of patients with end-stage renal disease. Nutrition 2009, 25, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Martinez Cantarin, M.P.; Whitaker-Menezes, D.; Lin, Z.; Falkner, B. Uremia induces adipose tissue inflammation and muscle mitochondrial dysfunction. Nephrol. Dial. Transplant. 2017, 32, 943–951. [Google Scholar] [CrossRef]
- Ambarkar, M.; Pemmaraju, S.V.; Gouroju, S.; Manohar, S.M.; Bitla, A.R.; Yajamanam, N.; Vishnubhotla, S. Adipokines and their Relation to Endothelial Dysfunction in Patients with Chronic Kidney Disease. J. Clin. Diagn. Res. 2016, 10, BC04–BC08. [Google Scholar] [CrossRef]
- Xiang, D.M.; Song, X.Z.; Zhou, Z.M.; Liu, Y.; Dai, X.Y.; Huang, X.L.; Hou, F.F.; Zhou, Q.G. Chronic kidney disease promotes chronic inflammation in visceral white adipose tissue. Am. J. Physiol. Ren. Physiol. 2017, 312, F689–F701. [Google Scholar] [CrossRef]
- D’Apolito, M.; Du, X.; Zong, H.; Catucci, A.; Maiuri, L.; Trivisano, T.; Pettoello-Mantovani, M.; Campanozzi, A.; Raia, V.; Pessin, J.E.; et al. Urea-induced ROS generation causes insulin resistance in mice with chronic renal failure. J. Clin. Investig. 2010, 120, 203–213. [Google Scholar] [CrossRef]
- Martos-Rus, C.; Katz-Greenberg, G.; Lin, Z.; Serrano, E.; Whitaker-Menezes, D.; Domingo-Vidal, M.; Roche, M.; Ramaswamy, K.; Hooper, D.C.; Falkner, B.; et al. Macrophage and adipocyte interaction as a source of inflammation in kidney disease. Sci. Rep. 2021, 11, 2974. [Google Scholar] [CrossRef]
- Sun, K.; Tordjman, J.; Clément, K.; Scherer, P.E. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013, 18, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Spencer, M.; Unal, R.; Zhu, B.; Rasouli, N.; McGehee, R.E., Jr.; Peterson, C.A.; Kern, P.A. Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2011, 96, E1990–E1998. [Google Scholar] [CrossRef] [PubMed]
- Chun, T.H.; Hotary, K.B.; Sabeh, F.; Saltiel, A.R.; Allen, E.D.; Weiss, S.J. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell 2006, 125, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, D.; Cline, M.A.; Gilbert, E.R. Chronic stress, epigenetics, and adipose tissue metabolism in the obese state. Nutr. Metab. 2020, 17, 88. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Llorens, S.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Talebi, M.; Shakibaei, M.; Samarghandian, S. An Overview of the Role of Adipokines in Cardiometabolic Diseases. Molecules 2020, 25, 5218. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Syamala, S.; Xiao, J.; Muntner, P. Relationship between Plasma Leptin Level and Chronic Kidney Disease. Int. J. Nephrol. 2012, 2012, 269532. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Adipokines—Removing road blocks to obesity and diabetes therapy. Mol. Metab. 2014, 3, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Fasshauer, M.; Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef]
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef]
- Lee, B.T.; Ahmed, F.A.; Hamm, L.L.; Teran, F.J.; Chen, C.-S.; Liu, Y.; Shah, K.; Rifai, N.; Batuman, V.; Simon, E.E.; et al. Association of C-reactive protein, tumor necrosis factor-alpha, and interleukin-6 with chronic kidney disease. BMC Nephrol. 2015, 16, 77. [Google Scholar] [CrossRef] [PubMed]
- Tesauro, M.; Mascali, A.; Franzese, O.; Cipriani, S.; Cardillo, C.; Di Daniele, N. Chronic kidney disease, obesity, and hypertension: The role of leptin and adiponectin. Int. J. Hypertens. 2012, 2012, 943605. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; McCulloch, C.; Brakeman, P.; Portale, A.; Hsu, C.Y. Being overweight modifies the association between cardiovascular risk factors and microalbuminuria in adolescents. Pediatrics 2008, 121, 37–45. [Google Scholar] [CrossRef]
- Nielsen, R.; Christensen, E.I.; Birn, H. Megalin and cubilin in proximal tubule protein reabsorption: From experimental models to human disease. Kidney Int. 2016, 89, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, A.; Sattar, A.; Hashim, R.; Khan, S.P.; Younus, M.; Khan, F.A. Serum leptin values in the healthy obese and non-obese subjects of Rawalpindi. J. Pak. Med. Assoc. 2013, 63, 245–248. [Google Scholar] [PubMed]
- Mao, S.; Fang, L.; Liu, F.; Jiang, S.; Wu, L.; Zhang, J. Leptin and chronic kidney diseases. J. Recept. Signal Transduct. Res. 2018, 38, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.; Ziyadeh, F.N. Leptin and renal fibrosis. Contrib. Nephrol. 2006, 151, 175–183. [Google Scholar] [CrossRef]
- Lee, M.P.; Orlov, D.; Sweeney, G. Leptin induces rat glomerular mesangial cell hypertrophy, but does not regulate hyperplasia or apoptosis. Int. J. Obes. 2005, 29, 1395–1401. [Google Scholar] [CrossRef]
- Briffa, J.F.; McAinch, A.J.; Poronnik, P.; Hryciw, D.H. Adipokines as a link between obesity and chronic kidney disease. Am. J. Physiol. Physiol. 2013, 305, F1629–F1636. [Google Scholar] [CrossRef]
- Noor, S.; Alam, F.; Fatima, S.S.; Khan, M.; Rehman, R. Role of Leptin and dyslipidemia in chronic kidney disease. Pak. J. Pharm. Sci. 2018, 31, 893–897. [Google Scholar]
- Liu, B.; Qiao, J.; Hu, J.; Fan, M.; Zhao, Y.; Su, H.; Wang, Z.; Yu, Q.; Ma, Q.; Li, Y.; et al. Leptin promotes endothelial dysfunction in chronic kidney disease by modulating the MTA1-mediated WNT/β-catenin pathway. Mol. Cell. Biochem. 2020, 473, 155–166. [Google Scholar] [CrossRef]
- Alix, P.M.; Guebre-Egziabher, F.; Soulage, C.O. Leptin as an uremic toxin: Deleterious role of leptin in chronic kidney disease. Biochimie 2014, 105, 12–21. [Google Scholar] [CrossRef]
- Esfahani, M.; Movahedian, A.; Baranchi, M.; Goodarzi, M.T. Adiponectin: An adipokine with protective features against metabolic syndrome. Iran. J. Basic Med. Sci. 2015, 18, 430–442. [Google Scholar]
- Zhu, Q.; Scherer, P.E. Immunologic and endocrine functions of adipose tissue: Implications for kidney disease. Nat. Rev. Nephrol. 2018, 14, 105–120. [Google Scholar] [CrossRef]
- Ohashi, K.; Iwatani, H.; Kihara, S.; Nakagawa, Y.; Komura, N.; Fujita, K.; Maeda, N.; Nishida, M.; Katsube, F.; Shimomura, I.; et al. Exacerbation of Albuminuria and Renal Fibrosis in Subtotal Renal Ablation Model of Adiponectin-Knockout Mice. Arter. Thromb. Vasc. Biol. 2007, 27, 1910–1917. [Google Scholar] [CrossRef]
- Sawaguchi, T.; Nakajima, T.; Haruyama, A.; Hasegawa, T.; Shibasaki, I.; Nakajima, T.; Kaneda, H.; Arikawa, T.; Obi, S.; Sakuma, M.; et al. Association of serum leptin and adiponectin concentrations with echocardiographic parameters and pathophysiological states in patients with cardiovascular disease receiving cardiovascular surgery. PLoS ONE 2019, 14, e0225008. [Google Scholar] [CrossRef]
- Jing, Y.; Jin, S.; Qiao, R.; Fang, J. Association of adipocytokines with obesity and insulin resistance in Korean-Chinese and Han nationality pupils of Yanbian area. Wei Sheng Yan Jiu 2015, 44, 581–585. [Google Scholar]
- Song, S.H.; Oh, T.R.; Choi, H.S.; Kim, C.S.; Ma, S.K.; Oh, K.H.; Ahn, C.; Kim, S.W.; Bae, E.H. High serum adiponectin as a biomarker of renal dysfunction: Results from the KNOW-CKD study. Sci. Rep. 2020, 10, 5598. [Google Scholar] [CrossRef]
- Kuo, I.C.; Wu, P.H.; Lin, H.Y.; Niu, S.W.; Huang, J.C.; Hung, C.C.; Chiu, Y.W.; Chen, H.C. The association of adiponectin with metabolic syndrome and clinical outcome in patients with non-diabetic chronic kidney disease. PLoS ONE 2019, 14, e0220158. [Google Scholar] [CrossRef] [PubMed]
- Pabalan, N.; Tiongco, R.E.; Pandac, J.K.; Paragas, N.A.; Lasta, S.L.; Gallego, N.; Jarjanazi, H.; Pineda-Cortel, M.R. Association and biomarker potential of elevated serum adiponectin with nephropathy among type 1 and type 2 diabetics: A meta-analysis. PLoS ONE 2018, 13, e0208905. [Google Scholar] [CrossRef] [PubMed]
- Briones, A.M.; Cat, A.N.D.; Callera, G.E.; Yogi, A.; Burger, D.; He, Y.; Corrêa, J.W.; Gagnon, A.M.; Gomez-Sanchez, C.E.; Gomez-Sanchez, E.P.; et al. Adipocytes Produce Aldosterone through Calcineurin-Dependent Signaling Pathways. Hypertension 2012, 59, 1069–1078. [Google Scholar] [CrossRef]
- D’Agati, V.D.; Chagnac, A.; de Vries, A.P.J.; Levi, M.; Porrini, E.; Herman-Edelstein, M.; Praga, M. Obesity-related glomerulopathy: Clinical and pathologic characteristics and pathogenesis. Nat. Rev. Nephrol. 2016, 12, 453–471. [Google Scholar] [CrossRef]
- Kotchen, T.A. Obesity-Related Hypertension: Epidemiology, Pathophysiology, and Clinical Management. Am. J. Hypertens. 2010, 23, 1170–1178. [Google Scholar] [CrossRef]
- Falkner, B.; Gidding, S. Childhood Obesity and Blood Pressure. Hypertension 2011, 58, 754–755. [Google Scholar] [CrossRef]
- Battaglia, Y.; Esposito, P.; Corrao, S.; Russo, L.; Balducci, A.; Storari, A.; Russo, D. Evaluation of Hypertension, Proteinuria, and Abnormalities of Body Weight in Italian Adolescents Participating in the World Kidney Days. Kidney Blood Press. Res. 2020, 45, 286–296. [Google Scholar] [CrossRef]
- Bakris, G.L.; Ritz, E. The message for World Kidney Day 2009: Hypertension and kidney disease: A marriage that should be prevented. Clin. J. Am. Soc. Nephrol. 2009, 4, 517–519. [Google Scholar] [CrossRef]
- El-Atat, F.A.; Stas, S.N.; McFarlane, S.I.; Sowers, J.R. The Relationship between Hyperinsulinemia, Hypertension and Progressive Renal Disease. J. Am. Soc. Nephrol. 2004, 15, 2816–2827. [Google Scholar] [CrossRef] [PubMed]
- Staples, A.O.; Greenbaum, L.A.; Smith, J.M.; Gipson, D.S.; Filler, G.; Warady, B.A.; Martz, K.; Wong, C.S. Association between clinical risk factors and progression of chronic kidney disease in children. Clin. J. Am. Soc. Nephrol. 2010, 5, 2172–2179. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Eckert, G.J.; DiMeglio, L.A.; Yu, Z.; Jung, J.; Pratt, J.H. Intensified effect of adiposity on blood pressure in overweight and obese children. Hypertension 2011, 58, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Sorof, J.M.; Lai, D.; Turner, J.; Poffenbarger, T.; Portman, R.J. Overweight, ethnicity, and the prevalence of hypertension in school-aged children. Pediatrics 2004, 113 (3 Pt 1), 475–482. [Google Scholar] [CrossRef]
- Lubrano, R.; Travasso, E.; Raggi, C.; Guido, G.; Masciangelo, R.; Elli, M. Blood pressure load, proteinuria and renal function in pre-hypertensive children. Pediatr. Nephrol. 2009, 24, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Mitsnefes, M.; Flynn, J.; Cohn, S.; Samuels, J.; Blydt-Hansen, T.; Saland, J.; Kimball, T.; Furth, S.; Warady, B. Masked hypertension associates with left ventricular hypertrophy in children with CKD. J. Am. Soc. Nephrol. 2010, 21, 137–144. [Google Scholar] [CrossRef]
- Shatat, I.F.; Flynn, J.T. Relationships between renin, aldosterone, and 24-hour ambulatory blood pressure in obese adolescents. Pediatr. Res. 2011, 69, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J. The changing face of pediatric hypertension in the era of the childhood obesity epidemic. Pediatr. Nephrol. 2013, 28, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Bucher, B.S.; Ferrarini, A.; Weber, N.; Bullo, M.; Bianchetti, M.G.; Simonetti, G.D. Primary hypertension in childhood. Curr. Hypertens. Rep. 2013, 15, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Archbold, K.H.; Vasquez, M.M.; Goodwin, J.L.; Quan, S.F. Effects of sleep patterns and obesity on increases in blood pressure in a 5-year period: Report from the Tucson Children’s Assessment of Sleep Apnea Study. J. Pediatr. 2012, 161, 26–30. [Google Scholar] [CrossRef]
- Pacifico, L.; Anania, C.; Osborn, J.F.; Ferraro, F.; Bonci, E.; Olivero, E.; Chiesa, C. Low 25(OH)D3 levels are associated with total adiposity, metabolic syndrome, and hypertension in Caucasian children and adolescents. Eur. J. Endocrinol. 2011, 165, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Nakagawa, T.; Sanchez-Lozada, L.G.; Shafiu, M.; Sundaram, S.; Le, M.; Ishimoto, T.; Sautin, Y.Y.; Lanaspa, M.A. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes 2013, 62, 3307–3315. [Google Scholar] [CrossRef]
- Yanik, M.; Feig, D.I. Serum urate: A biomarker or treatment target in pediatric hypertension? Curr. Opin. Cardiol. 2013, 28, 433–438. [Google Scholar] [CrossRef]
- van Dam, M.; Pottel, H.; Vreugdenhil, A.C.E. Creatinine-based GFR-estimating equations in children with overweight and obesity. Pediatr. Nephrol. 2022, 37, 2393–2403. [Google Scholar] [CrossRef]
- Kim, S.S.; Song, S.H.; Kim, I.J.; Jeon, Y.K.; Kim, B.H.; Kwak, I.S.; Lee, E.K.; Kim, Y.K. Urinary Cystatin C and Tubular Proteinuria Predict Progression of Diabetic Nephropathy. Diabetes Care 2013, 36, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Mak, R.H. Early markers of obesity-related renal injury in childhood. Pediatr. Nephrol. 2015, 30, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.E.; Reinhard, H.; Zdunek, D.; Hess, G.; Gutiérrez, O.M.; Wolf, M.; Parving, H.H.; Jacobsen, P.K.; Rossing, P. Tubular markers are associated with decline in kidney function in proteinuric type 2 diabetic patients. Diabetes Res. Clin. Pract. 2012, 97, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Goknar, N.; Oktem, F.; Ozgen, I.T.; Torun, E.; Kuçukkoc, M.; Demir, A.D.; Cesur, Y. Determination of early urinary renal injury markers in obese children. Pediatr. Nephrol. 2015, 30, 139–144. [Google Scholar] [CrossRef]
- Valerio, G.; Maffeis, C.; Saggese, G.; Ambruzzi, M.A.; Balsamo, A.; Bellone, S.; Bergamini, M.; Bernasconi, S.; Bona, G.; Calcaterra, V.; et al. Diagnosis, treatment and prevention of pediatric obesity: Consensus position statement of the Italian Society for Pediatric Endocrinology and Diabetology and the Italian Society of Pediatrics. Ital. J. Pediatr. 2018, 44, 88. [Google Scholar] [CrossRef]
- Filler, G.; Bökenkamp, A.; Hofmann, W.; Le Bricon, T.; Martínez-Brú, C.; Grubb, A. Cystatin C as a marker of GFR--history, indications, and future research. Clin. Biochem. 2005, 38, 1–8. [Google Scholar] [CrossRef]
- Bacchetta, J.; Cochat, P.; Rognant, N.; Ranchin, B.; Hadj-Aissa, A.; Dubourg, L. Which creatinine and cystatin C equations can be reliably used in children? Clin. J. Am. Soc. Nephrol. 2011, 6, 552–560. [Google Scholar] [CrossRef]
- Miliku, K.; Bakker, H.; Dorresteijn, E.M.; Cransberg, K.; Franco, O.H.; Felix, J.F.; Jaddoe, V.W. Childhood Estimates of Glomerular Filtration Rate Based on Creatinine and Cystatin C: Importance of Body Composition. Am. J. Nephrol. 2017, 45, 320–326. [Google Scholar] [CrossRef]
- van Dam, M.; Pottel, H.; Vreugdenhil, A.C.E. Relation between obesity-related comorbidities and kidney function estimation in children. Pediatr. Nephrol. 2023, 38, 1867–1876. [Google Scholar] [CrossRef]
- Foster, M.C.; Hwang, S.J.; Larson, M.G.; Lichtman, J.H.; Parikh, N.I.; Vasan, R.S.; Levy, D.; Fox, C.S. Overweight, obesity, and the development of stage 3 CKD: The Framingham Heart Study. Am. J. Kidney Dis. 2008, 52, 39–48. [Google Scholar] [CrossRef]
- Praga, M.; Hernández, E.; Herrero, J.C.; Morales, E.; Revilla, Y.; Díaz-González, R.; Rodicio, J.L. Influence of obesity on the appearance of proteinuria and renal insufficiency after unilateral nephrectomy. Kidney Int. 2000, 58, 2111–2118. [Google Scholar] [CrossRef]
- Csernus, K.; Lanyi, E.; Erhardt, E.; Molnar, D. Effect of childhood obesity and obesity-related cardiovascular risk factors on glomerular and tubular protein excretion. Eur. J. Pediatr. 2005, 164, 44–49. [Google Scholar] [CrossRef]
- Ferris, M.; Hogan, S.L.; Chin, H.; Shoham, D.A.; Gipson, D.S.; Gibson, K.; Yilmaz, S.; Falk, R.J.; Jennette, J.C. Obesity, albuminuria, and urinalysis findings in US young adults from the Add Health Wave III study. Clin. J. Am. Soc. Nephrol. 2007, 2, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Medyńska, A.; Chrzanowska, J.; Kościelska-Kasprzak, K.; Bartoszek, D.; Żabińska, M.; Zwolińska, D. Alpha-1 Acid Glycoprotein and Podocin mRNA as Novel Biomarkers for Early Glomerular Injury in Obese Children. J. Clin. Med. 2021, 10, 4129. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.; Ma, Q.; Prada, A.; Mitsnefes, M.; Zahedi, K.; Yang, J.; Barasch, J.; Devarajan, P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J. Am. Soc. Nephrol. 2003, 14, 2534–2543. [Google Scholar] [CrossRef]
- Bolignano, D.; Lacquaniti, A.; Coppolino, G.; Donato, V.; Campo, S.; Fazio, M.R.; Nicocia, G.; Buemi, M. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2009, 4, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Mackowiak-Lewandowicz, K.; Ostalska-Nowicka, D.; Zaorska, K.; Kaczmarek, E.; Zachwieja, J.; Witt, M.; Nowicki, M. Chronic kidney disease predictors in obese adolescents. Pediatr. Nephrol. 2022, 37, 2479–2488. [Google Scholar] [CrossRef] [PubMed]
- Bolignano, D.; Lacquaniti, A.; Coppolino, G.; Donato, V.; Fazio, M.R.; Nicocia, G.; Buemi, M. Neutrophil gelatinase-associated lipocalin as an early biomarker of nephropathy in diabetic patients. Kidney Blood Press. Res. 2009, 32, 91–98. [Google Scholar] [CrossRef]
- Şen, S.; Özalp Kızılay, D.; Taneli, F.; Özen, Ç.; Ertan, P.; Özunan, İ.; Yıldız, R.; Ersoy, B. Urinary NGAL is a Potential Biomarker for Early Renal Injury in Insulin Resistant Obese Non-diabetic Children. J. Clin. Res. Pediatr. Endocrinol. 2021, 13, 400–407. [Google Scholar] [CrossRef]
- Han, W.K.; Bailly, V.; Abichandani, R.; Thadhani, R.; Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002, 62, 237–244. [Google Scholar] [CrossRef]
- Han, W.K.; Waikar, S.S.; Johnson, A.; Betensky, R.A.; Dent, C.L.; Devarajan, P.; Bonventre, J.V. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 2008, 73, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Suwanpen, C.; Nouanthong, P.; Jaruvongvanich, V.; Pongpirul, K.; Pongpirul, W.A.; Leelahavanichkul, A.; Kanjanabuch, T. Urinary podocalyxin, the novel biomarker for detecting early renal change in obesity. J. Nephrol. 2016, 29, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Musa, N.; Ramzy, T.; Hamdy, A.; Arafa, N.; Hassan, M. Assessment of urinary podocalyxin as a marker of glomerular injury in obesity-related kidney disease in children and adolescents with obesity compared to urinary albumin creatinine ratio. Clin. Obes. 2021, 11, e12452. [Google Scholar] [CrossRef] [PubMed]
- Ostalska-Nowicka, D.; Mackowiak-Lewandowicz, K.; Perek, B.; Zaorska, K.; Zachwieja, J.; Nowicki, M. Megalin—A facultative marker of obesity-related glomerulopathy in children. J. Biol. Regul. Homeost. Agents 2019, 33, 415–420. [Google Scholar] [PubMed]
- Fukuda, A.; Wickman, L.T.; Venkatareddy, M.P.; Wang, S.Q.; Chowdhury, M.A.; Wiggins, J.E.; Shedden, K.A.; Wiggins, R.C. Urine podocin:nephrin mRNA ratio (PNR) as a podocyte stress biomarker. Nephrol. Dial. Transplant. 2012, 27, 4079–4087. [Google Scholar] [CrossRef]
- Pereira, S.V.; Dos Santos, M.; Rodrigues, P.G.; do Nascimento, J.F.; Timm, J.R.; Zancan, R.; Friedman, R.; Veronese, F.V. Increased urine podocyte-associated messenger RNAs in severe obesity are evidence of podocyte injury. Obesity 2015, 23, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Minakawa, A.; Fukuda, A.; Sato, Y.; Kikuchi, M.; Kitamura, K.; Wiggins, R.C.; Fujimoto, S. Podocyte hypertrophic stress and detachment precedes hyperglycemia or albuminuria in a rat model of obesity and type2 diabetes-associated nephropathy. Sci. Rep. 2019, 9, 18485. [Google Scholar] [CrossRef]
- Mangat, G.; Nair, N.; Barat, O.; Abboud, B.; Pais, P.; Bagga, S.; Raina, R. Obesity-related glomerulopathy in children: Connecting pathophysiology to clinical care. Clin. Kidney J. 2023, 16, 611–618. [Google Scholar] [CrossRef]
- Cisek, K.; Krochmal, M.; Klein, J.; Mischak, H. The application of multi-omics and systems biology to identify therapeutic targets in chronic kidney disease. Nephrol. Dial. Transplant. 2016, 31, 2003–2011. [Google Scholar] [CrossRef]
- Benito, S.; Sánchez, A.; Unceta, N.; Andrade, F.; Aldámiz-Echevarria, L.; Goicolea, M.A.; Barrio, R.J. LC-QTOF-MS-based targeted metabolomics of arginine-creatine metabolic pathway-related compounds in plasma: Application to identify potential biomarkers in pediatric chronic kidney disease. Anal. Bioanal. Chem. 2016, 408, 747–760. [Google Scholar] [CrossRef]
- Brooks, E.R.; Lin, D.C.; Langman, C.B.; Thompson, J.W.; St John-Williams, L.; Furth, S.L.; Warady, B.; Haymond, S. Metabolomic Patterns in Adolescents with Mild to Moderate CKD. Kidney Int. Rep. 2019, 4, 720–723. [Google Scholar] [CrossRef] [PubMed]
- Benito, S.; Sánchez-Ortega, A.; Unceta, N.; Andrade, F.; Aldámiz-Echevarria, L.; Goicolea, M.A.; Barrio, R.J. Untargeted metabolomics for plasma biomarker discovery for early chronic kidney disease diagnosis in pediatric patients using LC-QTOF-MS. Analyst 2018, 143, 4448–4458. [Google Scholar] [CrossRef]
- Decramer, S.; Wittke, S.; Mischak, H.; Zürbig, P.; Walden, M.; Bouissou, F.; Bascands, J.L.; Schanstra, J.P. Predicting the clinical outcome of congenital unilateral ureteropelvic junction obstruction in newborn by urinary proteome analysis. Nat. Med. 2006, 12, 398–400. [Google Scholar] [CrossRef]
- Sharma, K.; Karl, B.; Mathew, A.V.; Gangoiti, J.A.; Wassel, C.L.; Saito, R.; Pu, M.; Sharma, S.; You, Y.H.; Wang, L.; et al. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J. Am. Soc. Nephrol. 2013, 24, 1901–1912. [Google Scholar] [CrossRef] [PubMed]
- Sood, M.M.; Murphy, M.S.Q.; Hawken, S.; Wong, C.A.; Potter, B.K.; Burns, K.D.; Tsampalieros, A.; Atkinson, K.M.; Chakraborty, P.; Wilson, K. Association Between Newborn Metabolic Profiles and Pediatric Kidney Disease. Kidney Int. Rep. 2018, 3, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, U.T.; Sekula, P. The Promise of Metabolomics in Decelerating CKD Progression in Children. Clin. J. Am. Soc. Nephrol. 2021, 16, 1152–1154. [Google Scholar] [CrossRef]
- Denburg, M.R.; Xu, Y.; Abraham, A.G.; Coresh, J.; Chen, J.; Grams, M.E.; Feldman, H.I.; Kimmel, P.L.; Rebholz, C.M.; Rhee, E.P.; et al. Metabolite Biomarkers of CKD Progression in Children. Clin. J. Am. Soc. Nephrol. 2021, 16, 1178–1189. [Google Scholar] [CrossRef] [PubMed]
- Wendt, R.; He, T.; Latosinska, A.; Siwy, J.; Mischak, H.; Beige, J. Proteomic characterization of obesity-related nephropathy. Clin. Kidney J. 2020, 13, 684–692. [Google Scholar] [CrossRef]
- Sawyer, A.; Zeitler, E.; Trachtman, H.; Bjornstad, P. Kidney Considerations in Pediatric Obesity. Curr. Obes. Rep. 2023, 12, 332–344. [Google Scholar] [CrossRef]
- Chagnac, A.; Weinstein, T.; Korzets, A.; Ramadan, E.; Hirsch, J.; Gafter, U. Glomerular hemodynamics in severe obesity. Am. J. Physiol. Ren. Physiol. 2000, 278, F817–F822. [Google Scholar] [CrossRef]
- Mathew, A.V.; Okada, S.; Sharma, K. Obesity related kidney disease. Curr. Diabetes Rev. 2011, 7, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Yim, H.E.; Yoo, K.H. Obesity and chronic kidney disease: Prevalence, mechanism, and management. Clin. Exp. Pediatr. 2021, 64, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Kwakernaak, A.J.; Toering, T.J.; Navis, G. Body mass index and body fat distribution as renal risk factors: A focus on the role of renal haemodynamics. Nephrol. Dial. Transplant. 2013, 28 (Suppl. 4), iv42–iv49. [Google Scholar] [CrossRef] [PubMed]
- Helal, I.; Fick-Brosnahan, G.M.; Reed-Gitomer, B.; Schrier, R.W. Glomerular hyperfiltration: Definitions, mechanisms and clinical implications. Nat. Rev. Nephrol. 2012, 8, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Chagnac, A.; Zingerman, B.; Rozen-Zvi, B.; Herman-Edelstein, M. Consequences of Glomerular Hyperfiltration: The Role of Physical Forces in the Pathogenesis of Chronic Kidney Disease in Diabetes and Obesity. Nephron 2019, 143, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Hallow, K.M.; Gebremichael, Y.; Helmlinger, G.; Vallon, V. Primary proximal tubule hyperreabsorption and impaired tubular transport counterregulation determine glomerular hyperfiltration in diabetes: A modeling analysis. Am. J. Physiol. Ren. Physiol. 2017, 312, F819–F835. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Thomson, S.C. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat. Rev. Nephrol. 2020, 16, 317–336. [Google Scholar] [CrossRef]
- Shah, S.; Hussain, T. Enhanced angiotensin II-induced activation of Na+, K+-ATPase in the proximal tubules of obese Zucker rats. Clin. Exp. Hypertens. 2006, 28, 29–40. [Google Scholar] [CrossRef]
- Gai, Z.; Wang, T.; Visentin, M.; Kullak-Ublick, G.A.; Fu, X.; Wang, Z. Lipid Accumulation and Chronic Kidney Disease. Nutrients 2019, 11, 722. [Google Scholar] [CrossRef]
- Ayina, C.N.; Noubiap, J.J.; Etoundi Ngoa, L.S.; Boudou, P.; Gautier, J.F.; Mengnjo, M.K.; Mbanya, J.C.; Sobngwi, E. Association of serum leptin and adiponectin with anthropomorphic indices of obesity, blood lipids and insulin resistance in a Sub-Saharan African population. Lipids Health Dis. 2016, 15, 96. [Google Scholar] [CrossRef]
- Choi, S.R.; Lim, J.H.; Kim, M.Y.; Kim, E.N.; Kim, Y.; Choi, B.S.; Kim, Y.S.; Kim, H.W.; Lim, K.M.; Kim, M.J.; et al. Adiponectin receptor agonist AdipoRon decreased ceramide, and lipotoxicity, and ameliorated diabetic nephropathy. Metab. Clin. Exp. 2018, 85, 348–360. [Google Scholar] [CrossRef] [PubMed]
- de Vries, A.P.; Ruggenenti, P.; Ruan, X.Z.; Praga, M.; Cruzado, J.M.; Bajema, I.M.; D’Agati, V.D.; Lamb, H.J.; Pongrac Barlovic, D.; Hojs, R.; et al. Fatty kidney: Emerging role of ectopic lipid in obesity-related renal disease. Lancet. Diabetes Endocrinol. 2014, 2, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Woollard, J.R.; Wang, S.; Korsmo, M.J.; Ebrahimi, B.; Grande, J.P.; Textor, S.C.; Lerman, A.; Lerman, L.O. Increased glomerular filtration rate in early metabolic syndrome is associated with renal adiposity and microvascular proliferation. Am. J. Physiol. Ren. Physiol. 2011, 301, F1078–F1087. [Google Scholar] [CrossRef] [PubMed]
- Verani, R.R. Obesity-associated focal segmental glomerulosclerosis: Pathological features of the lesion and relationship with cardiomegaly and hyperlipidemia. Am. J. Kidney Dis. 1992, 20, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Adeosun, S.O.; Gordon, D.M.; Weeks, M.F.; Moore, K.H.; Hall, J.E.; Hinds, T.D., Jr.; Stec, D.E. Loss of biliverdin reductase—A promotes lipid accumulation and lipotoxicity in mouse proximal tubule cells. Am. J. Physiol. Ren. Physiol. 2018, 315, F323–F331. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, S.; Wiebe, N.; Fried, L.F.; Tonelli, M. Statins for improving renal outcomes: A meta-analysis. J. Am. Soc. Nephrol. 2006, 17, 2006–2016. [Google Scholar] [CrossRef] [PubMed]
- Butani, L. Prospective monitoring of lipid profiles in children receiving pravastatin preemptively after renal transplantation. Pediatr. Transplant. 2005, 9, 746–753. [Google Scholar] [CrossRef]
- Gunta, S.S.; Mak, R.H. Is obesity a risk factor for chronic kidney disease in children? Pediatr. Nephrol. 2013, 28, 1949–1956. [Google Scholar] [CrossRef]
- Weinberg, J.M. Lipotoxicity. Kidney Int. 2006, 70, 1560–1566. [Google Scholar] [CrossRef]
- Thomas, M.E.; Harris, K.P.; Walls, J.; Furness, P.N.; Brunskill, N.J. Fatty acids exacerbate tubulointerstitial injury in protein-overload proteinuria. Am. J. Physiol. Ren. Physiol. 2002, 283, F640–F647. [Google Scholar] [CrossRef]
- Moorhead, J.F.; Chan, M.K.; El-Nahas, M.; Varghese, Z. Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet 1982, 2, 1309–1311. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, C.; Izquierdo-Lahuerta, A.; Vivas, Y.; Velasco, I.; Yeo, T.K.; Chen, S.; Medina-Gomez, G. Renal Lipotoxicity-Associated Inflammation and Insulin Resistance Affects Actin Cytoskeleton Organization in Podocytes. PLoS ONE 2015, 10, e0142291. [Google Scholar] [CrossRef] [PubMed]
- Beddhu, S.; Pappas, L.M.; Ramkumar, N.; Samore, M. Effects of body size and body composition on survival in hemodialysis patients. J. Am. Soc. Nephrol. 2003, 14, 2366–2372. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.H. Massive obesity and the kidney. A morphologic and statistical study. Am. J. Pathol. 1975, 81, 117–130. [Google Scholar] [PubMed]
- Kambham, N.; Markowitz, G.S.; Valeri, A.M.; Lin, J.; D’Agati, V.D. Obesity-related glomerulopathy: An emerging epidemic. Kidney Int. 2001, 59, 1498–1509. [Google Scholar] [CrossRef] [PubMed]
- Kramer, H. Obesity and chronic kidney disease. Contrib. Nephrol. 2006, 151, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Praga, M.; Morales, E.; Herrero, J.C.; Pérez Campos, A.; Domínguez-Gil, B.; Alegre, R.; Vara, J.; Martínez, M.A. Absence of hypoalbuminemia despite massive proteinuria in focal segmental glomerulosclerosis secondary to hyperfiltration. Am. J. Kidney Dis. 1999, 33, 52–58. [Google Scholar] [CrossRef]
- Tran, H.A. Obesity-Related Glomerulopathy. J. Clin. Endocrinol. Metab. 2004, 89, 6358–6359. [Google Scholar] [CrossRef][Green Version]
- Serra, A.; Romero, R.; Lopez, D.; Navarro, M.; Esteve, A.; Perez, N.; Alastrue, A.; Ariza, A. Renal injury in the extremely obese patients with normal renal function. Kidney Int. 2008, 73, 947–955. [Google Scholar] [CrossRef]
- Chen, H.M.; Li, S.J.; Chen, H.P.; Wang, Q.W.; Li, L.S.; Liu, Z.H. Obesity-related glomerulopathy in China: A case series of 90 patients. Am. J. Kidney Dis. 2008, 52, 58–65. [Google Scholar] [CrossRef]
- Ku, E.; Glidden, D.V.; Hsu, C.Y.; Portale, A.A.; Grimes, B.; Johansen, K.L. Association of Body Mass Index with Patient-Centered Outcomes in Children with ESRD. J. Am. Soc. Nephrol. 2016, 27, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Wall, A.; Lee, G.H.; Maldonado, J.; Magnus, D. Medical Contraindications to Transplant Listing in the USA: A Survey of Adult and Pediatric Heart, Kidney, Liver, and Lung Programs. World J. Surg. 2019, 43, 2300–2308. [Google Scholar] [CrossRef] [PubMed]
- el-Agroudy, A.E.; Wafa, E.W.; Gheith, O.E.; Shehab el-Dein, A.B.; Ghoneim, M.A. Weight gain after renal transplantation is a risk factor for patient and graft outcome. Transplantation 2004, 77, 1381–1385. [Google Scholar] [CrossRef]
- Abdelrahman, S.M.; Samir, B.; Alazem, E.A.A.; Musa, N. Effect of pre and post-transplant body mass index on pediatric kidney transplant outcomes. BMC Pediatr. 2022, 22, 299. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.W.; Isbel, N.M.; Brown, A.M.; Kay, T.D.; Franzen, K.; Hawley, C.M.; Campbell, S.B.; Wall, D.; Griffin, A.; Nicol, D.L. The effect of obesity on renal transplant outcomes. Transplantation 2002, 74, 675–681. [Google Scholar] [CrossRef]
- Scheuermann, U.; Babel, J.; Pietsch, U.-C.; Weimann, A.; Lyros, O.; Semmling, K.; Hau, H.-M.; Seehofer, D.; Rademacher, S.; Sucher, R. Recipient obesity as a risk factor in kidney transplantation. BMC Nephrol. 2022, 23, 37. [Google Scholar] [CrossRef]
- Meier-Kriesche, H.U.; Vaghela, M.; Thambuganipalle, R.; Friedman, G.; Jacobs, M.; Kaplan, B. The effect of body mass index on long-term renal allograft survival. Transplantation 1999, 68, 1294–1297. [Google Scholar] [CrossRef]
- Yaseri, M.; Alipoor, E.; Seifollahi, A.; Rouhifard, M.; Salehi, S.; Hosseinzadeh-Attar, M.J. Association of obesity with mortality and clinical outcomes in children and adolescents with transplantation: A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2021, 22, 847–858. [Google Scholar] [CrossRef]
- Hegyi, P.J.; Soós, A.; Hegyi, P.; Szakács, Z.; Hanák, L.; Váncsa, S.; Ocskay, K.; Pétervári, E.; Balaskó, M.; Eröss, B.; et al. Pre-transplant Sarcopenic Obesity Worsens the Survival after Liver Transplantation: A Meta-Analysis and a Systematic Review. Front. Med. 2020, 7, 599434. [Google Scholar] [CrossRef]
- Ryan, T.D.; Zafar, F.; Siegel, R.M.; Villa, C.R.; Bryant III, R.; Chin, C.J.P.T. Obesity class does not further stratify outcome in overweight and obese pediatric patients after heart transplantation. Pediatr. Transplant. 2018, 22, e13161. [Google Scholar] [CrossRef]
- Davies, R.R.; Haldeman, S.; McCulloch, M.A.; Gidding, S.S.; Pizarro, C. Low body mass index is associated with increased waitlist mortality among children listed for heart transplant. J. Heart Lung Transplant. 2015, 34, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Barker, C.C.; Agovi, M.A.; Logan, B.; Lazarus, H.M.; Ballen, K.K.; Gupta, V.; Hale, G.A.; Frangoul, H.; Ho, V.; Rizzo, J.D.; et al. Childhood obesity and outcomes after bone marrow transplantation for patients with severe aplastic anemia. Biol. Blood Marrow Transplant. 2011, 17, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Weissenbacher, A.; Jara, M.; Ulmer, H.; Biebl, M.; Bösmüller, C.; Schneeberger, S.; Mayer, G.; Pratschke, J.; Öllinger, R. Recipient and donor body mass index as important risk factors for delayed kidney graft function. Transplantation 2012, 93, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Berkman, E.R.; Richardson, K.L.; Clark, J.D.; Dick, A.A.S.; Lewis-Newby, M.; Diekema, D.S.; Wightman, A.G. An ethical analysis of obesity as a contraindication of pediatric kidney transplant candidacy. Pediatr. Nephrol. 2023, 38, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, R.; Gracida, C.; Cancino, J.; Ibarra, A. Effect of obese living donors on the outcome and metabolic features in recipients of kidney transplantation. Transplant. Proc. 2006, 38, 888–889. [Google Scholar] [CrossRef] [PubMed]
- Delmonico, F. A Report of the Amsterdam Forum On the Care of the Live Kidney Donor: Data and Medical Guidelines. Transplantation 2005, 79 (Suppl. 6), S53–S66. [Google Scholar] [PubMed]
- Gracida, C.; Espinoza, R.; Cancino, J. Can a living kidney donor become a kidney recipient? Transplant. Proc. 2004, 36, 1630–1631. [Google Scholar] [CrossRef]
- Pesavento, T.E.; Henry, M.L.; Falkenhain, M.E.; Cosio, F.G.; Bumgardner, G.L.; Elkhammas, E.A.; Pelletier, R.P.; Ferguson, R.M. Obese living kidney donors: Short-term results and possible implications1. Transplantation 1999, 68, 1491–1496. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Taler, S.J.; Prieto, M.; Cosio, F.G.; Textor, S.C.; Kudva, Y.C.; Chow, G.K.; Ishitani, M.B.; Larson, T.S.; Stegall, M.D. Obesity in living kidney donors: Clinical characteristics and outcomes in the era of laparoscopic donor nephrectomy. Am. J. Transplant. 2005, 5, 1057–1064. [Google Scholar] [CrossRef]
- Locke, J.E.; Reed, R.D.; Massie, A.; MacLennan, P.A.; Sawinski, D.; Kumar, V.; Mehta, S.; Mannon, R.B.; Gaston, R.; Lewis, C.E.; et al. Obesity increases the risk of end-stage renal disease among living kidney donors. Kidney Int. 2017, 91, 699–703. [Google Scholar] [CrossRef]
- Shen, W.W.; Chen, H.M.; Chen, H.; Xu, F.; Li, L.S.; Liu, Z.H. Obesity-related glomerulopathy: Body mass index and proteinuria. Clin. J. Am. Soc. Nephrol. 2010, 5, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Morales, E.; Valero, M.A.; León, M.; Hernández, E.; Praga, M. Beneficial effects of weight loss in overweight patients with chronic proteinuric nephropathies. Am. J. Kidney Dis. 2003, 41, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, S.D.; Yehnert, H.; Moustarah, F.; Schreiber, M.J.; Schauer, P.R.; Beddhu, S. Weight loss interventions in chronic kidney disease: A systematic review and meta-analysis. Clin. J. Am. Soc. Nephrol. 2009, 4, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Stabouli, S.; Polderman, N.; Nelms, C.L.; Paglialonga, F.; Oosterveld, M.J.S.; Greenbaum, L.A.; Warady, B.A.; Anderson, C.; Haffner, D.; Desloovere, A.; et al. Assessment and management of obesity and metabolic syndrome in children with CKD stages 2–5 on dialysis and after kidney transplantation-clinical practice recommendations from the Pediatric Renal Nutrition Taskforce. Pediatr. Nephrol. 2022, 37, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Bensignor, M.O.; Kelly, A.S.; Arslanian, S. Anti-obesity pharmacotherapy for treatment of pediatric type 2 diabetes: Review of the literature and lessons learned from adults. Front. Endocrinol. 2022, 13, 1043650. [Google Scholar] [CrossRef] [PubMed]
- Jerome, G.J.; Fink, T.; Brady, T.; Young, D.R.; Dickerson, F.B.; Goldsholl, S.; Findling, R.L.; Stepanova, E.A.; Scheimann, A.; Dalcin, A.T.; et al. Physical Activity Levels and Screen Time among Youth with Overweight/Obesity Using Mental Health Services. Int. J. Environ. Res. Public Health 2022, 19, 2261. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; He, Y.; Venn, A.J.; Jose, M.D.; Tian, J. Childhood modifiable risk factors and later life chronic kidney disease: A systematic review. BMC Nephrol. 2023, 24, 184. [Google Scholar] [CrossRef]
- Martínez-Montoro, J.I.; Morales, E.; Cornejo-Pareja, I.; Tinahones, F.J.; Fernández-García, J.C. Obesity-related glomerulopathy: Current approaches and future perspectives. Obes. Rev. 2022, 23, e13450. [Google Scholar] [CrossRef]
- Cirillo, L.; Ravaglia, F.; Errichiello, C.; Anders, H.J.; Romagnani, P.; Becherucci, F. Expectations in children with glomerular diseases from SGLT2 inhibitors. Pediatr. Nephrol. 2022, 37, 2997–3008. [Google Scholar] [CrossRef]

| Factor | Obesity Kidney-Related Damage |
|---|---|
| Hemodynamic factors | Glomerular hyperfiltration [165,166,167] Increased podocyte injury [104] Glomerulomegaly and glomerulosclerosis [104] Excessive tubular sodium reabsorption [168,169,170] Increased sympathetic activity [104] Increased renin–angiotensin–aldosterone system (RAAS) activity [170] |
| Metabolic effects | Abnormal lipid metabolism [64,77,171] Adipokine dysregulation [78,82,86,87,88,89,90,92,93,95,100,172,173] Increased insulin resistance [99,172] Increased inflammation [99,172] Increased oxidative stress [171,174,175] |
| Lipid nephrotoxicity | Excessive renal fat accumulation [174,175,176] Glomerular and tubular cell injuries [171,175,176] Mitochondrial dysfunction, oxidative stress and inflammation [171,175,177] Increased free fatty acid toxicity to proximal tubular cells [171,177] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carullo, N.; Zicarelli, M.; Michael, A.; Faga, T.; Battaglia, Y.; Pisani, A.; Perticone, M.; Costa, D.; Ielapi, N.; Coppolino, G.; et al. Childhood Obesity: Insight into Kidney Involvement. Int. J. Mol. Sci. 2023, 24, 17400. https://doi.org/10.3390/ijms242417400
Carullo N, Zicarelli M, Michael A, Faga T, Battaglia Y, Pisani A, Perticone M, Costa D, Ielapi N, Coppolino G, et al. Childhood Obesity: Insight into Kidney Involvement. International Journal of Molecular Sciences. 2023; 24(24):17400. https://doi.org/10.3390/ijms242417400
Chicago/Turabian StyleCarullo, Nazareno, Mariateresa Zicarelli, Ashour Michael, Teresa Faga, Yuri Battaglia, Antonio Pisani, Maria Perticone, Davide Costa, Nicola Ielapi, Giuseppe Coppolino, and et al. 2023. "Childhood Obesity: Insight into Kidney Involvement" International Journal of Molecular Sciences 24, no. 24: 17400. https://doi.org/10.3390/ijms242417400
APA StyleCarullo, N., Zicarelli, M., Michael, A., Faga, T., Battaglia, Y., Pisani, A., Perticone, M., Costa, D., Ielapi, N., Coppolino, G., Bolignano, D., Serra, R., & Andreucci, M. (2023). Childhood Obesity: Insight into Kidney Involvement. International Journal of Molecular Sciences, 24(24), 17400. https://doi.org/10.3390/ijms242417400










