Endothelial Glycocalyx of Peritubular Capillaries in Experimental Diabetic Nephropathy: A Target of ACE Inhibitor-Induced Kidney Microvascular Protection
Abstract
:1. Introduction
2. Results
2.1. Laboratory, Systemic, and Renal Function Parameters
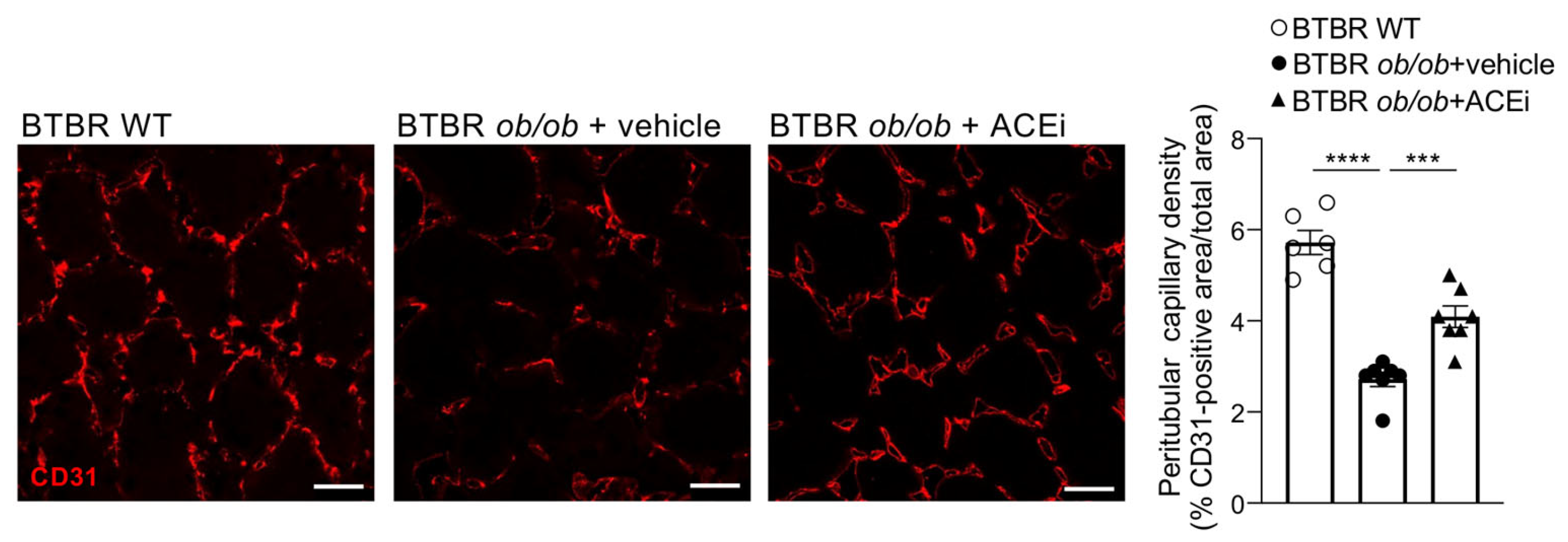
2.2. Kidneys of Diabetic Mice Exhibit Peritubular Capillary Rarefaction, Which Is Limited by ACEi Treatment
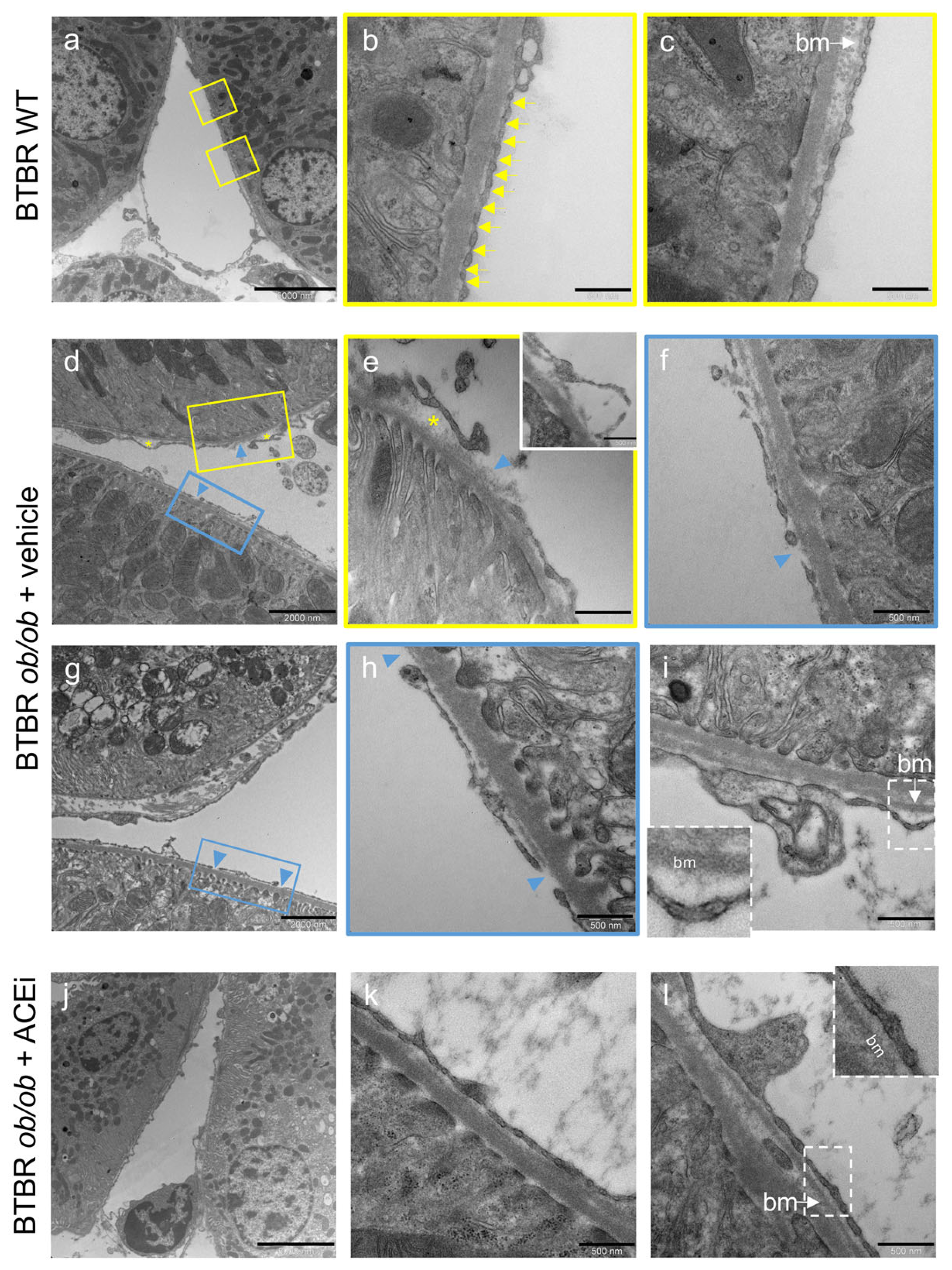
2.3. The Ultrastructure of Peritubular Capillaries Is Altered in Diabetic Mice and Ameliorated by ACEi Treatment
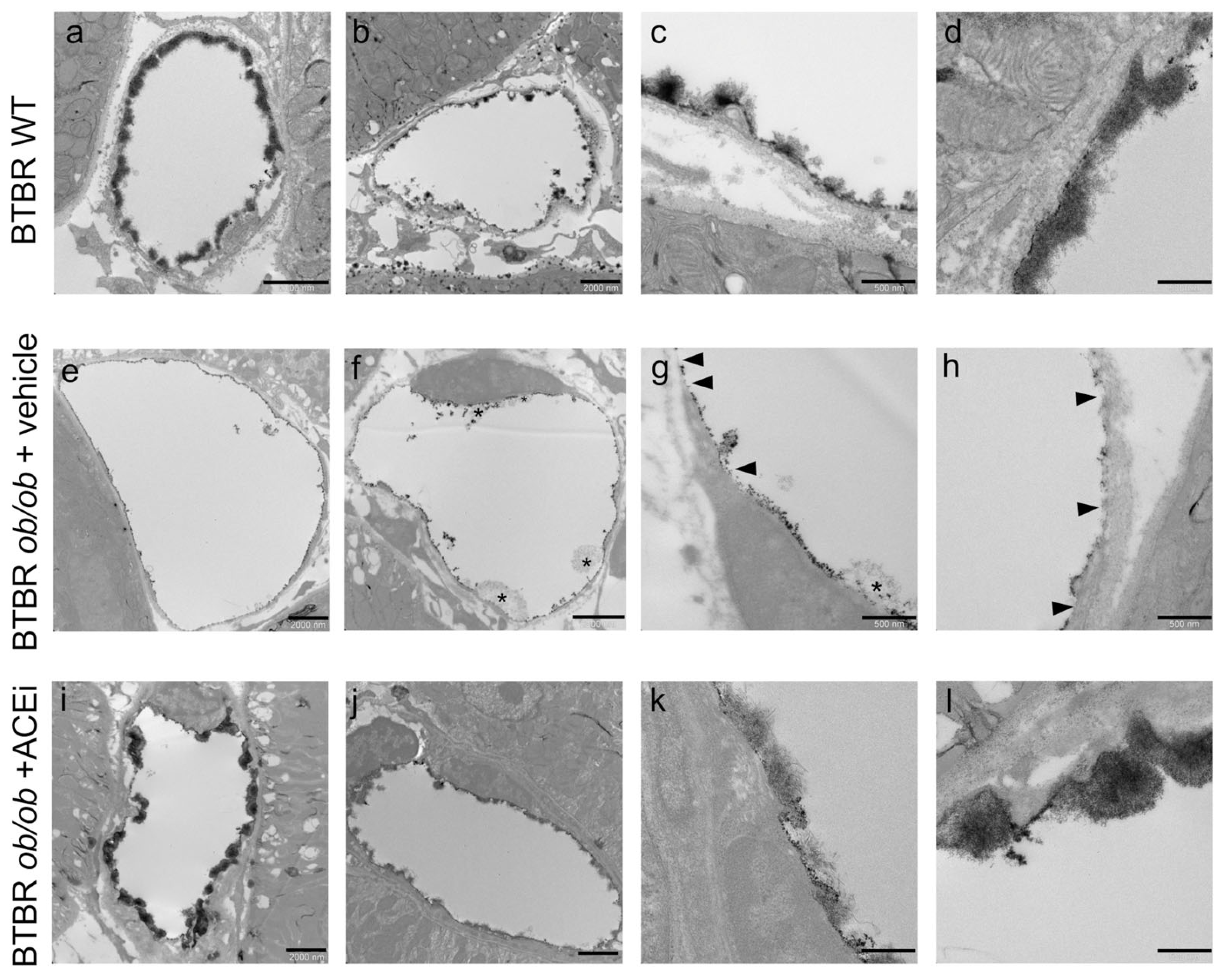
2.4. The Endothelial Glycocalyx of Peritubular Capillaries Is Altered in Diabetic Mice and Preserved by ACEi Treatment
2.5. Staining for Sialic Acid Is Reduced in Peritubular Capillaries of Diabetic Mice and Is Improved by ACEi Treatment
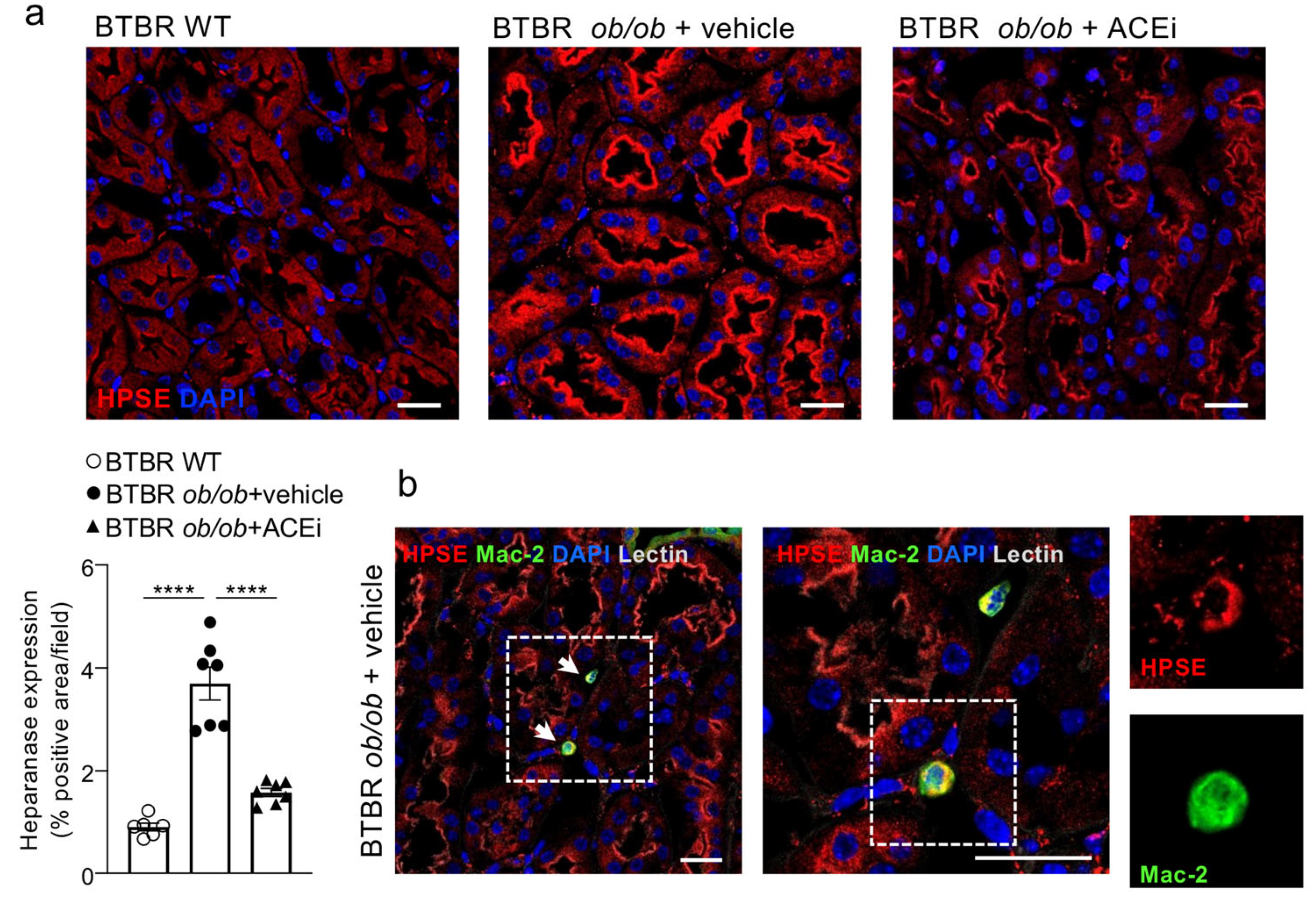
2.6. Interstitial Heparanase 1 Is Overexpressed in Diabetic Mice and Reduced by ACEi Treatment
2.7. ACEi Treatment Attenuates Renal Interstitial Inflammation in Diabetic Mice
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Biochemical and Renal Function Parameters
4.3. Systolic Blood Pressure
4.4. Transmission Electron Microscopy Analysis
4.4.1. Ultrastructure of Peritubular Capillaries
4.4.2. Endothelial Glycocalyx
4.5. Immunohistochemistry
4.5.1. Immunofluorescence Analysis
4.5.2. Immunoperoxidase Analysis
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kida, Y. Peritubular Capillary Rarefaction: An Underappreciated Regulator of CKD Progression. Int. J. Mol. Sci. 2020, 21, 8255. [Google Scholar] [CrossRef] [PubMed]
- Bohle, A.; Mackensen-Haen, S.; Wehrmann, M. Significance of Postglomerular Capillaries in the Pathogenesis of Chronic Renal Failure. Kidney Blood Press. Res. 1996, 19, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Chakraborty, S.; Nguyen, V.; Nguyen, C.; Kim, B.K.; Shim, S.I.; Suki, W.N.; Truong, L.D. Peritubular Capillary Loss Is Associated with Chronic Tubulointerstitial Injury in Human Kidney: Altered Expression of Vascular Endothelial Growth Factor. Hum. Pathol. 2000, 31, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Eardley, K.S.; Kubal, C.; Zehnder, D.; Quinkler, M.; Lepenies, J.; Savage, C.O.; Howie, A.J.; Kaur, K.; Cooper, M.S.; Adu, D.; et al. The Role of Capillary Density, Macrophage Infiltration and Interstitial Scarring in the Pathogenesis of Human Chronic Kidney Disease. Kidney Int. 2008, 74, 495–504. [Google Scholar] [CrossRef]
- Serón, D.; Alexopoulos, E.; Raftery, M.J.; Hartley, B.; Cameron, J.S. Number of Interstitial Capillary Cross-Sections Assessed by Monoclonal Antibodies: Relation to Interstitial Damage. Nephrol. Dial. Transplant. 1990, 5, 889–893. [Google Scholar] [CrossRef]
- Menshikh, A.; Scarfe, L.; Delgado, R.; Finney, C.; Zhu, Y.; Yang, H.; de Caestecker, M.P. Capillary Rarefaction Is More Closely Associated with CKD Progression after Cisplatin, Rhabdomyolysis, and Ischemia-Reperfusion-Induced AKI than Renal Fibrosis. Am. J. Physiol. Renal Physiol. 2019, 317, F1383–F1397. [Google Scholar] [CrossRef]
- Rabelink, T.J.; Wijewickrama, D.C.; de Koning, E.J. Peritubular Endothelium: The Achilles Heel of the Kidney? Kidney Int. 2007, 72, 926–930. [Google Scholar] [CrossRef]
- Bábíčková, J.; Klinkhammer, B.M.; Buhl, E.M.; Djudjaj, S.; Hoss, M.; Heymann, F.; Tacke, F.; Floege, J.; Becker, J.U.; Boor, P. Regardless of Etiology, Progressive Renal Disease Causes Ultrastructural and Functional Alterations of Peritubular Capillaries. Kidney Int. 2017, 91, 70–85. [Google Scholar] [CrossRef]
- Ermert, K.; Buhl, E.M.; Klinkhammer, B.M.; Floege, J.; Boor, P. Reduction of Endothelial Glycocalyx on Peritubular Capillaries in Chronic Kidney Disease. Am. J. Pathol. 2023, 193, 138–147. [Google Scholar] [CrossRef]
- Reitsma, S.; Slaaf, D.W.; Vink, H.; van Zandvoort, M.A.M.J.; oude Egbrink, M.G.A. The Endothelial Glycocalyx: Composition, Functions, and Visualization. Pflug. Arch. 2007, 454, 345–359. [Google Scholar] [CrossRef]
- Ballermann, B.J.; Nyström, J.; Haraldsson, B. The Glomerular Endothelium Restricts Albumin Filtration. Front. Med. 2021, 8, 766689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, D.; Song, J.W.; Zullo, J.; Lipphardt, M.; Coneh-Gould, L.; Goligorsky, M.S. Endothelial Cell Dysfunction and Glycocalyx—A Vicious Circle. Matrix Biol. J. Int. Soc. Matrix Biol. 2018, 71–72, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Cosgun, Z.C.; Fels, B.; Kusche-Vihrog, K. Nanomechanics of the Endothelial Glycocalyx: From Structure to Function. Am. J. Pathol. 2020, 190, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Maric-Bilkan, C.; Flynn, E.R.; Chade, A.R. Microvascular Disease Precedes the Decline in Renal Function in the Streptozotocin-Induced Diabetic Rat. Am. J. Physiol. Renal Physiol. 2012, 302, F308–F315. [Google Scholar] [CrossRef]
- Cassis, P.; Locatelli, M.; Corna, D.; Villa, S.; Rottoli, D.; Cerullo, D.; Abbate, M.; Remuzzi, G.; Benigni, A.; Zoja, C. Addition of Cyclic Angiotensin-(1-7) to Angiotensin-Converting Enzyme Inhibitor Therapy Has a Positive Add-on Effect in Experimental Diabetic Nephropathy. Kidney Int. 2019, 96, 906–917. [Google Scholar] [CrossRef]
- Lindenmeyer, M.T.; Kretzler, M.; Boucherot, A.; Berra, S.; Yasuda, Y.; Henger, A.; Eichinger, F.; Gaiser, S.; Schmid, H.; Rastaldi, M.P.; et al. Interstitial Vascular Rarefaction and Reduced VEGF-A Expression in Human Diabetic Nephropathy. J. Am. Soc. Nephrol. 2007, 18, 1765–1776. [Google Scholar] [CrossRef]
- Pinter, G.G.; Atkins, J.L. Role of Postglomerular Microvessels in Pathophysiology of Diabetic Nephropathy. Assessment and Hypothesis. Diabetes 1991, 40, 791–795. [Google Scholar] [CrossRef]
- Hudkins, K.L.; Pichaiwong, W.; Wietecha, T.; Kowalewska, J.; Banas, M.C.; Spencer, M.W.; Mühlfeld, A.; Koelling, M.; Pippin, J.W.; Shankland, S.J.; et al. BTBR Ob/Ob Mutant Mice Model Progressive Diabetic Nephropathy. J. Am. Soc. Nephrol. 2010, 21, 1533–1542. [Google Scholar] [CrossRef]
- Locatelli, M.; Zoja, C.; Conti, S.; Cerullo, D.; Corna, D.; Rottoli, D.; Zanchi, C.; Tomasoni, S.; Remuzzi, G.; Benigni, A. Empagliflozin Protects Glomerular Endothelial Cell Architecture in Experimental Diabetes through the VEGF-A/Caveolin-1/PV-1 Signaling Pathway. J. Pathol. 2022, 256, 468–479. [Google Scholar] [CrossRef]
- Robertson, R.T.; Levine, S.T.; Haynes, S.M.; Gutierrez, P.; Baratta, J.L.; Tan, Z.; Longmuir, K.J. Use of Labeled Tomato Lectin for Imaging Vasculature Structures. Histochem. Cell Biol. 2015, 143, 225–234. [Google Scholar] [CrossRef]
- Bojar, D.; Meche, L.; Meng, G.; Eng, W.; Smith, D.F.; Cummings, R.D.; Mahal, L.K. A Useful Guide to Lectin Binding: Machine-Learning Directed Annotation of 57 Unique Lectin Specificities. ACS Chem. Biol. 2022, 17, 2993–3012. [Google Scholar] [CrossRef] [PubMed]
- Koshy, V.; Avasthi, P.S. The Anionic Sites at Luminal Surface of Peritubular Capillaries in Rats. Kidney Int. 1987, 31, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Khanna, M.; Parish, C.R. Heparanase: Historical Aspects and Future Perspectives. Adv. Exp. Med. Biol. 2020, 1221, 71–96. [Google Scholar] [CrossRef] [PubMed]
- Wada, J.; Makino, H. Inflammation and the Pathogenesis of Diabetic Nephropathy. Clin. Sci. Lond. Engl. 1979 2013, 124, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Chow, F.; Ozols, E.; Nikolic-Paterson, D.J.; Atkins, R.C.; Tesch, G.H. Macrophages in Mouse Type 2 Diabetic Nephropathy: Correlation with Diabetic State and Progressive Renal Injury. Kidney Int. 2004, 65, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, R.; Kitamura, H.; Yamanaka, N. Peritubular Capillary Injury during the Progression of Experimental Glomerulonephritis in Rats. J. Am. Soc. Nephrol. 2000, 11, 47–56. [Google Scholar] [CrossRef]
- Kramann, R.; Wongboonsin, J.; Chang-Panesso, M.; Machado, F.G.; Humphreys, B.D. Gli1+ Pericyte Loss Induces Capillary Rarefaction and Proximal Tubular Injury. J. Am. Soc. Nephrol. 2017, 28, 776–784. [Google Scholar] [CrossRef]
- Hvala, A.; Ferluga, D.; Rott, T.; Kobenter, T.; Koselj-Kajtna, M.; Trnacević, S. Peritubular Capillary Changes in Alport Syndrome, Diabetic Glomerulopathy, Balkan Endemic Nephropathy and Hemorrhagic Fever with Renal Syndrome. Ultrastruct. Pathol. 2005, 29, 451–459. [Google Scholar] [CrossRef]
- Rostgaard, J.; Qvortrup, K. Electron Microscopic Demonstrations of Filamentous Molecular Sieve Plugs in Capillary Fenestrae. Microvasc. Res. 1997, 53, 1–13. [Google Scholar] [CrossRef]
- D’Addio, M.; Frey, J.; Otto, V.I. The Manifold Roles of Sialic Acid for the Biological Functions of Endothelial Glycoproteins. Glycobiology 2020, 30, 490–499. [Google Scholar] [CrossRef]
- Privratsky, J.R.; Paddock, C.M.; Florey, O.; Newman, D.K.; Muller, W.A.; Newman, P.J. Relative Contribution of PECAM-1 Adhesion and Signaling to the Maintenance of Vascular Integrity. J. Cell Sci. 2011, 124, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Kitazume, S.; Imamaki, R.; Ogawa, K.; Komi, Y.; Futakawa, S.; Kojima, S.; Hashimoto, Y.; Marth, J.D.; Paulson, J.C.; Taniguchi, N. Alpha2,6-Sialic Acid on Platelet Endothelial Cell Adhesion Molecule (PECAM) Regulates Its Homophilic Interactions and Downstream Antiapoptotic Signaling. J. Biol. Chem. 2010, 285, 6515–6521. [Google Scholar] [CrossRef] [PubMed]
- Kitazume, S.; Imamaki, R.; Ogawa, K.; Taniguchi, N. Sweet Role of Platelet Endothelial Cell Adhesion Molecule in Understanding Angiogenesis. Glycobiology 2014, 24, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Liu, A.; Miranda-Ribera, A.; Hyun, S.W.; Lillehoj, E.P.; Cross, A.S.; Passaniti, A.; Grimm, P.R.; Kim, B.-Y.; Welling, P.A.; et al. NEU1 Sialidase Regulates the Sialylation State of CD31 and Disrupts CD31-Driven Capillary-like Tube Formation in Human Lung Microvascular Endothelia. J. Biol. Chem. 2014, 289, 9121–9135. [Google Scholar] [CrossRef] [PubMed]
- Betteridge, K.B.; Arkill, K.P.; Neal, C.R.; Harper, S.J.; Foster, R.R.; Satchell, S.C.; Bates, D.O.; Salmon, A.H.J. Sialic Acids Regulate Microvessel Permeability, Revealed by Novel in Vivo Studies of Endothelial Glycocalyx Structure and Function. J. Physiol. 2017, 595, 5015–5035. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.M.; Troy, J.L. Postglomerular Vascular Protein Concentration: Evidence for a Causal Role in Governing Fluid Reabsorption and Glomerulotublar Balance by the Renal Proximal Tubule. J. Clin. Investig. 1971, 50, 336–349. [Google Scholar] [CrossRef]
- Garsen, M.; Lenoir, O.; Rops, A.L.W.M.M.; Dijkman, H.B.; Willemsen, B.; van Kuppevelt, T.H.; Rabelink, T.J.; Berden, J.H.M.; Tharaux, P.-L.; van der Vlag, J. Endothelin-1 Induces Proteinuria by Heparanase-Mediated Disruption of the Glomerular Glycocalyx. J. Am. Soc. Nephrol. 2016, 27, 3545–3551. [Google Scholar] [CrossRef]
- Boels, M.G.S.; Avramut, M.C.; Koudijs, A.; Dane, M.J.C.; Lee, D.H.; van der Vlag, J.; Koster, A.J.; van Zonneveld, A.J.; van Faassen, E.; Gröne, H.-J.; et al. Atrasentan Reduces Albuminuria by Restoring the Glomerular Endothelial Glycocalyx Barrier in Diabetic Nephropathy. Diabetes 2016, 65, 2429–2439. [Google Scholar] [CrossRef]
- Van den Hoven, M.J.; Rops, A.L.; Bakker, M.A.; Aten, J.; Rutjes, N.; Roestenberg, P.; Goldschmeding, R.; Zcharia, E.; Vlodavsky, I.; van der Vlag, J.; et al. Increased Expression of Heparanase in Overt Diabetic Nephropathy. Kidney Int. 2006, 70, 2100–2108. [Google Scholar] [CrossRef]
- Maxhimer, J.B.; Somenek, M.; Rao, G.; Pesce, C.E.; Baldwin, D.; Gattuso, P.; Schwartz, M.M.; Lewis, E.J.; Prinz, R.A.; Xu, X. Heparanase-1 Gene Expression and Regulation by High Glucose in Renal Epithelial Cells: A Potential Role in the Pathogenesis of Proteinuria in Diabetic Patients. Diabetes 2005, 54, 2172–2178. [Google Scholar] [CrossRef]
- Masola, V.; Gambaro, G.; Tibaldi, E.; Onisto, M.; Abaterusso, C.; Lupo, A. Regulation of Heparanase by Albumin and Advanced Glycation End Products in Proximal Tubular Cells. Biochim. Biophys. Acta 2011, 1813, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Lerner, I.; Hermano, E.; Zcharia, E.; Rodkin, D.; Bulvik, R.; Doviner, V.; Rubinstein, A.M.; Ishai-Michaeli, R.; Atzmon, R.; Sherman, Y.; et al. Heparanase Powers a Chronic Inflammatory Circuit That Promotes Colitis-Associated Tumorigenesis in Mice. J. Clin. Investig. 2011, 121, 1709–1721. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.; Rubinstein, A.M.; Gil, N.; Hermano, E.; Li, J.-P.; van der Vlag, J.; Atzmon, R.; Meirovitz, A.; Elkin, M. Role of Heparanase-Driven Inflammatory Cascade in Pathogenesis of Diabetic Nephropathy. Diabetes 2014, 63, 4302–4313. [Google Scholar] [CrossRef]
- Qin, Q.; Niu, J.; Wang, Z.; Xu, W.; Qiao, Z.; Gu, Y. Heparanase Induced by Advanced Glycation End Products (AGEs) Promotes Macrophage Migration Involving RAGE and PI3K/AKT Pathway. Cardiovasc. Diabetol. 2013, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Qin, Q.; Yang, M.; Qiao, Z.; Gu, Y.; Niu, J. Heparanase-Driven Inflammation from the AGEs-Stimulated Macrophages Changes the Functions of Glomerular Endothelial Cells. Diabetes Res. Clin. Pract. 2017, 124, 30–40. [Google Scholar] [CrossRef]
- Sun, L.; Kanwar, Y.S. Relevance of TNF-α in the Context of Other Inflammatory Cytokines in the Progression of Diabetic Nephropathy. Kidney Int. 2015, 88, 662–665. [Google Scholar] [CrossRef]
- Cheng, D.; Liang, R.; Huang, B.; Hou, J.; Yin, J.; Zhao, T.; Zhou, L.; Wu, R.; Qian, Y.; Wang, F. Tumor Necrosis Factor-α Blockade Ameliorates Diabetic Nephropathy in Rats. Clin. Kidney J. 2021, 14, 301–308. [Google Scholar] [CrossRef]
- Donate-Correa, J.; Ferri, C.M.; Sánchez-Quintana, F.; Pérez-Castro, A.; González-Luis, A.; Martín-Núñez, E.; Mora-Fernández, C.; Navarro-González, J.F. Inflammatory Cytokines in Diabetic Kidney Disease: Pathophysiologic and Therapeutic Implications. Front. Med. 2020, 7, 628289. [Google Scholar] [CrossRef]
- Ramnath, R.; Foster, R.R.; Qiu, Y.; Cope, G.; Butler, M.J.; Salmon, A.H.; Mathieson, P.W.; Coward, R.J.; Welsh, G.I.; Satchell, S.C. Matrix Metalloproteinase 9-Mediated Shedding of Syndecan 4 in Response to Tumor Necrosis Factor α: A Contributor to Endothelial Cell Glycocalyx Dysfunction. FASEB J. 2014, 28, 4686–4699. [Google Scholar] [CrossRef]
- Chappell, D.; Hofmann-Kiefer, K.; Jacob, M.; Rehm, M.; Briegel, J.; Welsch, U.; Conzen, P.; Becker, B.F. TNF-Alpha Induced Shedding of the Endothelial Glycocalyx Is Prevented by Hydrocortisone and Antithrombin. Basic Res. Cardiol. 2009, 104, 78–89. [Google Scholar] [CrossRef]
- Nieuwdorp, M.; Meuwese, M.C.; Mooij, H.L.; van Lieshout, M.H.P.; Hayden, A.; Levi, M.; Meijers, J.C.M.; Ince, C.; Kastelein, J.J.P.; Vink, H.; et al. Tumor Necrosis Factor-Alpha Inhibition Protects against Endotoxin-Induced Endothelial Glycocalyx Perturbation. Atherosclerosis 2009, 202, 296–303. [Google Scholar] [CrossRef]
- Henry, C.B.; Duling, B.R. TNF-Alpha Increases Entry of Macromolecules into Luminal Endothelial Cell Glycocalyx. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H2815–H2823. [Google Scholar] [CrossRef] [PubMed]
- Kerkar, S.; Williams, M.; Blocksom, J.M.; Wilson, R.F.; Tyburski, J.G.; Steffes, C.P. TNF-Alpha and IL-1beta Increase Pericyte/Endothelial Cell Co-Culture Permeability. J. Surg. Res. 2006, 132, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, A.; Satoh, M.; Tomita, N.; Sasaki, T.; Kashihara, N. Deterioration of Glomerular Endothelial Surface Layer Induced by Oxidative Stress Is Implicated in Altered Permeability of Macromolecules in Zucker Fatty Rats. Diabetologia 2010, 53, 2056–2065. [Google Scholar] [CrossRef]
- Kramer, A.; van den Hoven, M.; Rops, A.; Wijnhoven, T.; van den Heuvel, L.; Lensen, J.; van Kuppevelt, T.; van Goor, H.; van der Vlag, J.; Navis, G.; et al. Induction of Glomerular Heparanase Expression in Rats with Adriamycin Nephropathy Is Regulated by Reactive Oxygen Species and the Renin-Angiotensin System. J. Am. Soc. Nephrol. 2006, 17, 2513–2520. [Google Scholar] [CrossRef]
- Van den Hoven, M.J.; Waanders, F.; Rops, A.L.; Kramer, A.B.; van Goor, H.; Berden, J.H.; Navis, G.; van der Vlag, J. Regulation of Glomerular Heparanase Expression by Aldosterone, Angiotensin II and Reactive Oxygen Species. Nephrol. Dial. Transplant. 2009, 24, 2637–2645. [Google Scholar] [CrossRef] [PubMed]
- Luis-Lima, S.; Rodríguez-Rodríguez, A.E.; Martín-Higueras, C.; Sierra-Ramos, C.; Carrara, F.; Arnau, M.R.; Alvarez de la Rosa, D.; Salido, E.; Gaspari, F.; Porrini, E. Iohexol Plasma Clearance, a Simple and Reliable Method to Measure Renal Function in Conscious Mice. Pflug. Arch. 2016, 468, 1587–1594. [Google Scholar] [CrossRef]
- Okada, H.; Takemura, G.; Suzuki, K.; Oda, K.; Takada, C.; Hotta, Y.; Miyazaki, N.; Tsujimoto, A.; Muraki, I.; Ando, Y.; et al. Three-Dimensional Ultrastructure of Capillary Endothelial Glycocalyx under Normal and Experimental Endotoxemic Conditions. Crit. Care Lond. Engl. 2017, 21, 261. [Google Scholar] [CrossRef]



| Groups | Body Weight | Blood Glucose | SBP | GFR | uACR |
|---|---|---|---|---|---|
| (g) | (mg/dL) | (mmHg) | (μL/min) | (μg/mg) | |
| BTBR WT | 39 ± 2 | 130 ± 7 | 101 ± 2 | 202 ± 39 | 32 ± 3 |
| (n = 6) | (n = 6) | (n = 6) | (n = 5) | (n = 6) | |
| BTBR ob/ob | |||||
| vehicle | 49 ± 2 * | 554 ± 20 ** | 96 ± 4 | 612 ± 73 * | 861 ± 210 ** |
| (n = 7) | (n = 7) | (n = 7) | (n = 6) | (n = 7) | |
| ACEi | 49 ± 2 | 576 ± 16 | 83 ±4° | 505 ±95 | 307 ± 65 ° |
| (n = 7) | (n = 7) | (n = 7) | (n = 6) | (n = 7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Locatelli, M.; Rottoli, D.; Mahmoud, R.; Abbate, M.; Corna, D.; Cerullo, D.; Tomasoni, S.; Remuzzi, G.; Zoja, C.; Benigni, A.; et al. Endothelial Glycocalyx of Peritubular Capillaries in Experimental Diabetic Nephropathy: A Target of ACE Inhibitor-Induced Kidney Microvascular Protection. Int. J. Mol. Sci. 2023, 24, 16543. https://doi.org/10.3390/ijms242216543
Locatelli M, Rottoli D, Mahmoud R, Abbate M, Corna D, Cerullo D, Tomasoni S, Remuzzi G, Zoja C, Benigni A, et al. Endothelial Glycocalyx of Peritubular Capillaries in Experimental Diabetic Nephropathy: A Target of ACE Inhibitor-Induced Kidney Microvascular Protection. International Journal of Molecular Sciences. 2023; 24(22):16543. https://doi.org/10.3390/ijms242216543
Chicago/Turabian StyleLocatelli, Monica, Daniela Rottoli, Rayan Mahmoud, Mauro Abbate, Daniela Corna, Domenico Cerullo, Susanna Tomasoni, Giuseppe Remuzzi, Carlamaria Zoja, Ariela Benigni, and et al. 2023. "Endothelial Glycocalyx of Peritubular Capillaries in Experimental Diabetic Nephropathy: A Target of ACE Inhibitor-Induced Kidney Microvascular Protection" International Journal of Molecular Sciences 24, no. 22: 16543. https://doi.org/10.3390/ijms242216543
APA StyleLocatelli, M., Rottoli, D., Mahmoud, R., Abbate, M., Corna, D., Cerullo, D., Tomasoni, S., Remuzzi, G., Zoja, C., Benigni, A., & Macconi, D. (2023). Endothelial Glycocalyx of Peritubular Capillaries in Experimental Diabetic Nephropathy: A Target of ACE Inhibitor-Induced Kidney Microvascular Protection. International Journal of Molecular Sciences, 24(22), 16543. https://doi.org/10.3390/ijms242216543







