Signaling in Legume–Rhizobia Symbiosis
Abstract
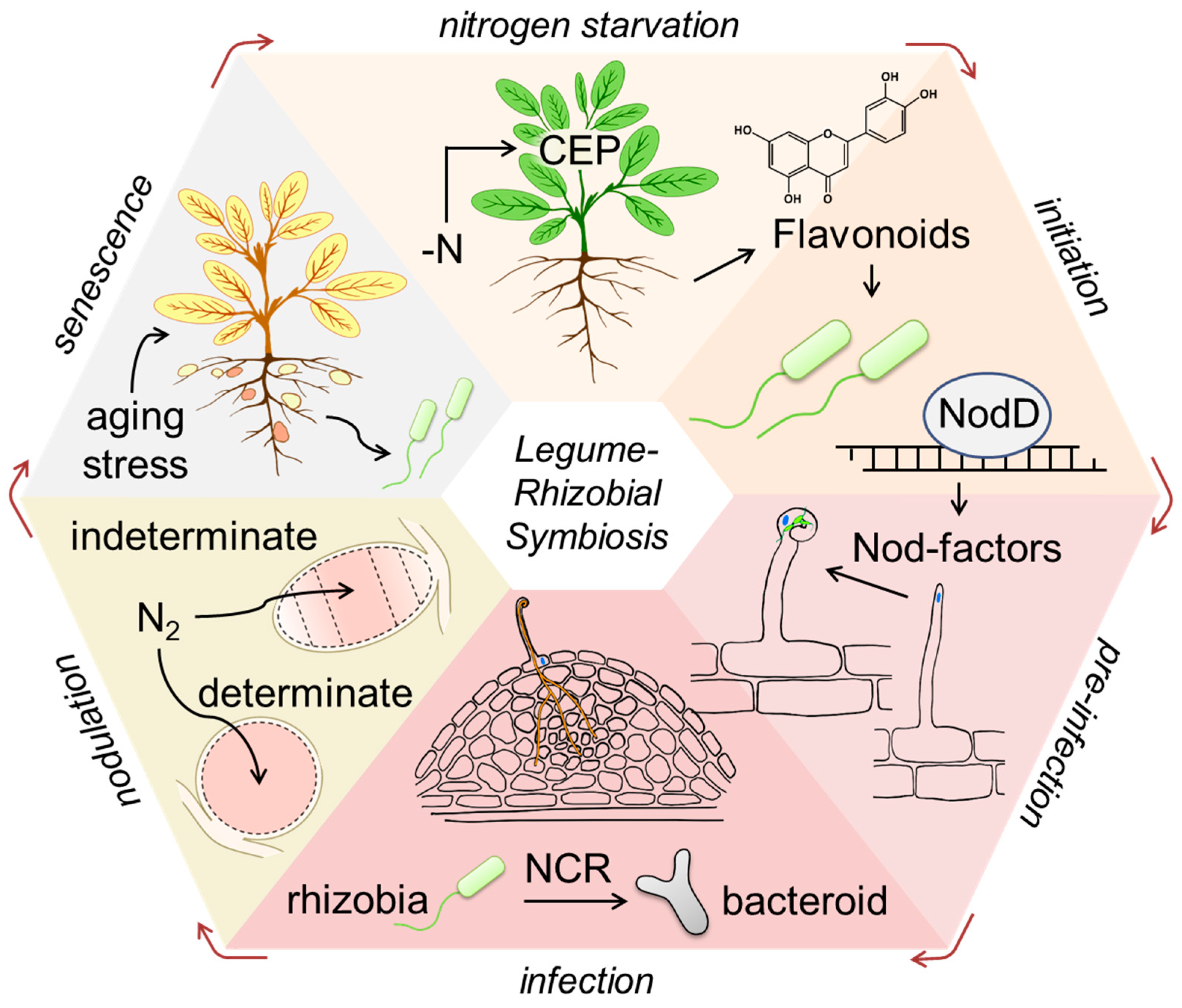
:1. Introduction
2. Mutual Recognition: Flavonoids as the Attractants for Rhizobia
3. Mutual Recognition: Nodulation Protein D (NodD) and Its Activation
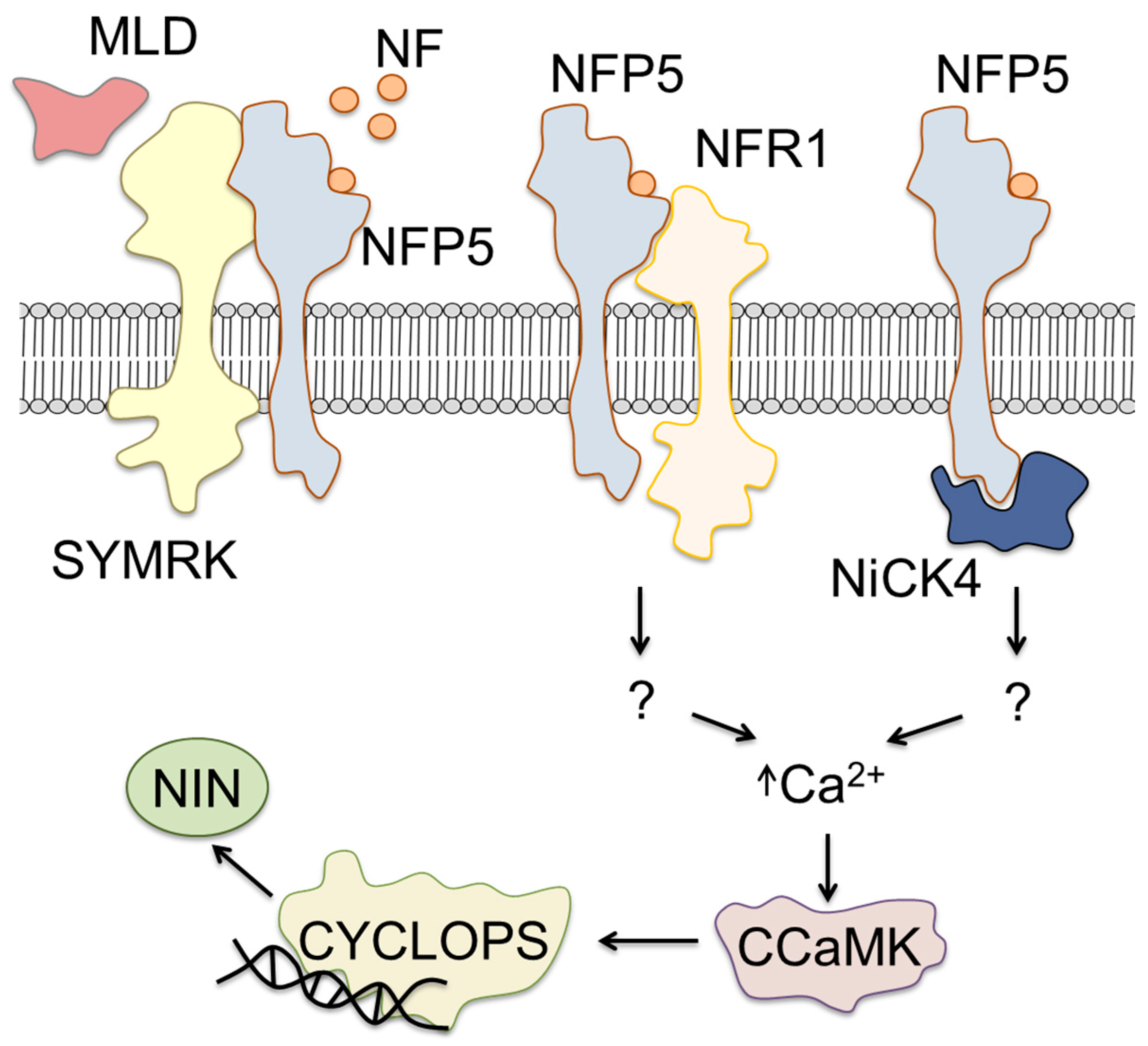
4. Initiation of Legume–Rhizobia Symbiosis
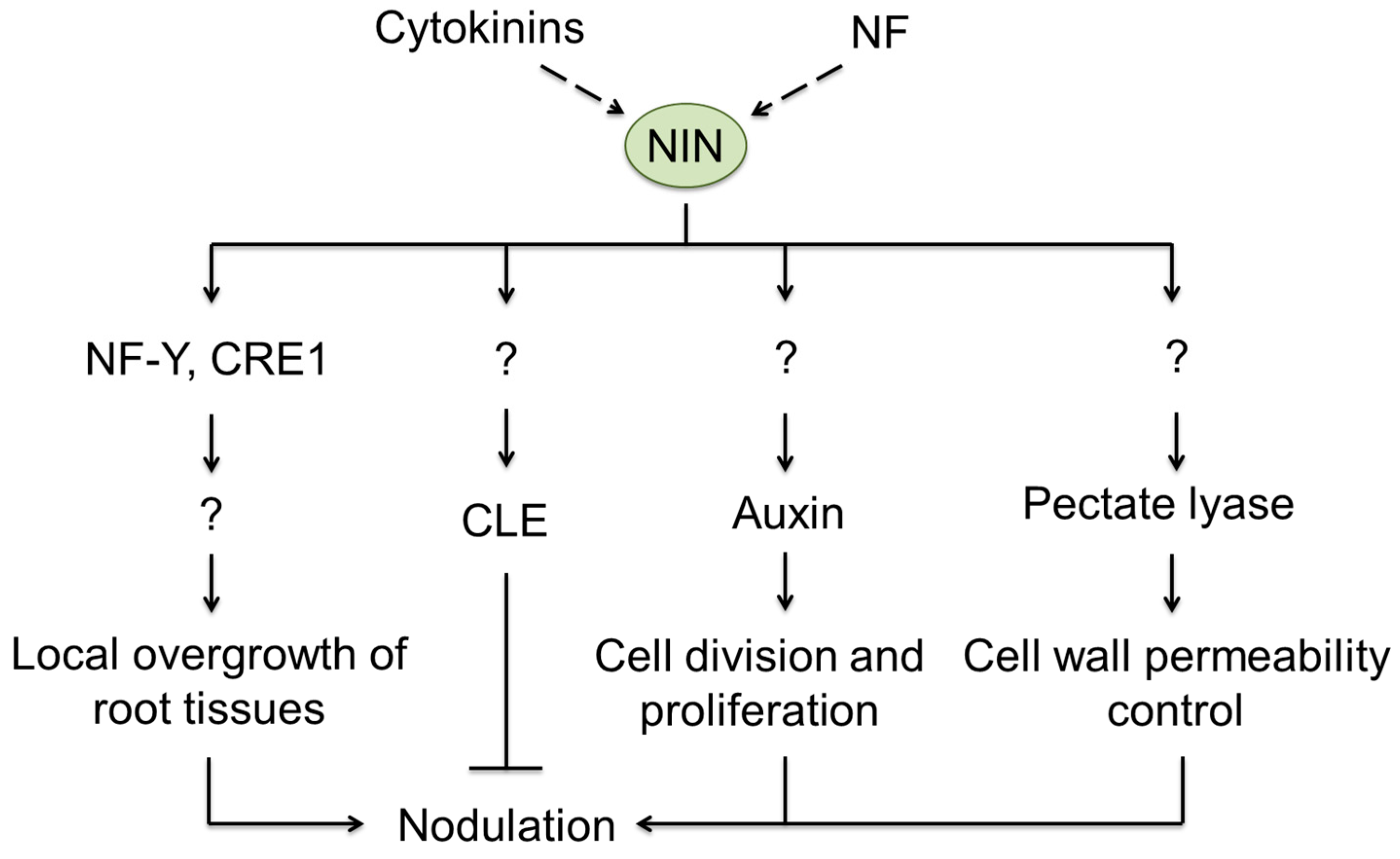
5. Signaling Regulating Rhizobial Infection and Organogenesis
6. Terminal Bacteroid Differentiation Governed by NCR Peptides
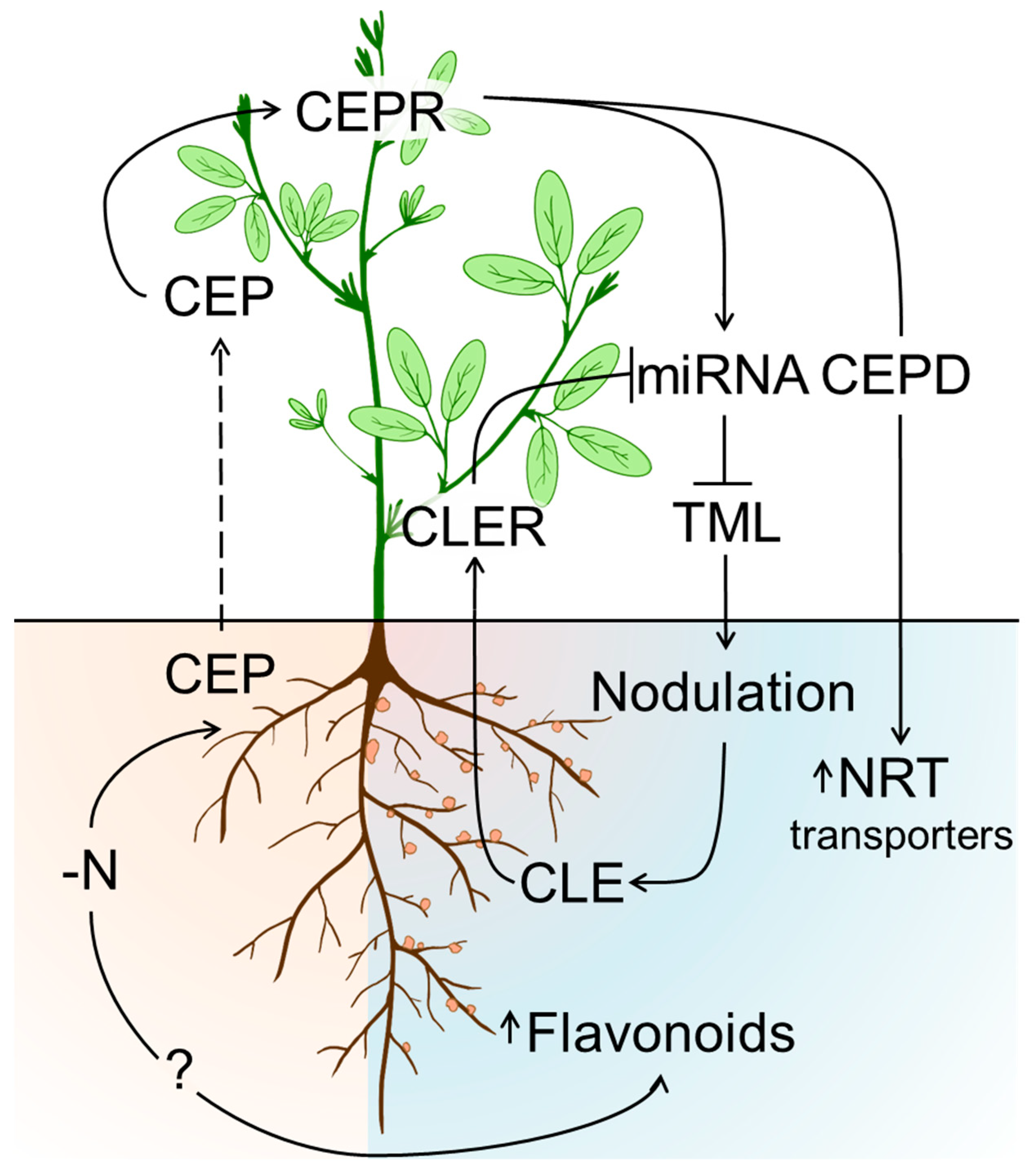
7. Autoregulation of Nodulation
8. Nodule Senescence
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | 1-aminocyclopropane-1-carboxylic acid |
| ADP | Adenosine diphosphate |
| ATP | Adenosine triphosphate |
| CBS1 | Cystathionine-β-synthase-like 1 |
| CCaMK | Calcium/calmodulin-dependent serine/threonine-protein kinase |
| CEP | C-terminally encoded peptide |
| CEPD | C-terminally encoded peptide downstream |
| CEPR | C-terminally encoded peptide receptor |
| CEPs | C-terminally encoded peptides |
| CLEs | CLAVATA3/Embryo-surrounding region-related peptides |
| CRE1 | Cytokinin receptor 1 |
| FA | Fatty acid |
| GSH | Reduced glutathione |
| GSSG | Oxidized form of glutathione |
| IPD3 | Interacting Protein of DMI3 of Medicago truncatula (homolog of the protein CYCLOPS of Lotus japonicus) |
| IT | Infection thread |
| MLD | Malectin-like domain |
| NCR | Nodule-specific cysteine-rich peptide |
| NFR1 | Nod Factor Receptor 1 kinase |
| NFR5 | Nod Factor Receptor 5 kinase |
| NFs | Nod factors |
| NF-Y | Nuclear factor Y |
| NiCK 4 | NFR5-interacting cytoplasmic kinase 4 |
| NIN | Nodulation inception transcription factor |
| NO | Nitric oxide |
| NodA | Acyl transferase |
| NodB | Deacetylase |
| NodC | N-acetyl glucosamine transferase |
| NodD | Nodulation protein D |
| NodM | Glucosamine synthase |
| Nop | Nodulation outer proteins |
| NPL | Nodulation pectate lyase |
| NTR | Nitrogen root transporters |
| ROS | Reactive oxygen species |
| RPG | Rhizobium-directed polar growth protein |
| SAR | Structure activity relationships |
| SYMRK | LRR-containing receptor kinase |
| T3SS | Type III secretory system |
| T4SS | Type IV secretory system |
| VPY | Vapyrin |
References
- Graham, P.H.; Vance, C.P. Nitrogen Fixation in Perspective: An Overview of Research and Extension Needs. Field Crops Res. 2000, 65, 93–106. [Google Scholar] [CrossRef]
- Bhat, T.; Ahmad, D.; Ganai, M.; Khan, O. Nitrogen Fixing Biofertilizers; Mechanism and Growth Promotion: A Review. J. Pure Appl. Microbiol. 2015, 9, 1675–1690. [Google Scholar]
- Compton, K.K.; Scharf, B.E. Rhizobial Chemoattractants, the Taste and Preferences of Legume Symbionts. Front. Plant Sci. 2021, 12, 686465. [Google Scholar] [CrossRef] [PubMed]
- Rainwater, R.; Mukherjee, A. The Legume-Rhizobia Symbiosis Can Be Supported on Mars Soil Simulants. PLoS ONE 2021, 16, e0259957. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, X.; Qin, Q.; Dinkins, R.D.; Zhu, H. The Impacts of Domestication and Breeding on Nitrogen Fixation Symbiosis in Legumes. Front. Genet. 2020, 11, 973. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yang, S.; Tang, F.; Zhu, H. Symbiosis Specificity in the Legume—Rhizobial Mutualism. Cell. Microbiol. 2012, 14, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Desbrosses, G.J.; Stougaard, J. Root Nodulation: A Paradigm for How Plant-Microbe Symbiosis Influences Host Developmental Pathways. Cell Host Microbe 2011, 10, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Zhukov, V.A.; Shtark, O.Y.; Borisov, A.Y.; Tikhonovich, I.A. Breeding to Improve Symbiotic Effectiveness of Legumes. In Plant Breeding from Laboratories to Fields; IntechOpen: Rijeka, Croatia, 2013; ISBN 978-953-51-1090-3. [Google Scholar]
- Shtark, O.Y.; Borisov, A.Y.; Zhukov, V.A.; Provorov, N.A.; Tikhonovich, I.A. Intimate Associations of Beneficial Soil Microbes with Host Plants. In Soil Microbiology and Sustainable Crop Production; Dixon, G.R., Tilston, E.L., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 119–196. ISBN 978-90-481-9479-7. [Google Scholar]
- Sulima, A.S.; Zhukov, V.A.; Shtark, O.Y.; Borisov, A.Y.; Tikhonovich, I.A. Nod-Factor Signaling in Legume-Rhizobial Symbiosis; IntechOpen: Rijeka, Croatia, 2015; ISBN 978-953-51-2185-5. [Google Scholar]
- Glyan’ko, A.K.; Vasil’eva, G.G. Reactive Oxygen and Nitrogen Species in Legume-Rhizobial Symbiosis: A Review. Appl. Biochem. Microbiol. 2010, 46, 15–22. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, C.; Yang, J.; Yu, N.; Wang, E. Hormone Modulation of Legume-Rhizobial Symbiosis. J. Integr. Plant Biol. 2018, 60, 632–648. [Google Scholar] [CrossRef]
- Velandia, K.; Reid, J.B.; Foo, E. Right Time, Right Place: The Dynamic Role of Hormones in Rhizobial Infection and Nodulation of Legumes. Plant Commun. 2022, 3, 100327. [Google Scholar] [CrossRef]
- Tsyganova, A.; Tsyganov, V. Plant Cell Wall in Symbiotic Interactions. Pectins. Sel’skokhozyaistvennaya Biol. 2019, 54, 446–457. [Google Scholar] [CrossRef]
- Oldroyd, G.E.D.; Murray, J.D.; Poole, P.S.; Downie, J.A. The Rules of Engagement in the Legume-Rhizobial Symbiosis. Annu. Rev. Genet. 2011, 45, 119–144. [Google Scholar] [CrossRef] [PubMed]
- Ovtsyna, A.O.; Dolgikh, E.A.; Kilanova, A.S.; Tsyganov, V.E.; Borisov, A.Y.; Tikhonovich, I.A.; Staehelin, C. Nod Factors Induce Nod Factor Cleaving Enzymes in Pea Roots. Genetic and Pharmacological Approaches Indicate Different Activation Mechanisms. Plant Physiol. 2005, 139, 1051–1064. [Google Scholar] [CrossRef] [PubMed]
- Dénarié, J.; Debellé, F.; Promé, J.C. Rhizobium Lipo-Chitooligosaccharide Nodulation Factors: Signaling Molecules Mediating Recognition and Morphogenesis. Annu. Rev. Biochem. 1996, 65, 503–535. [Google Scholar] [CrossRef] [PubMed]
- D’Haeze, W.; Holsters, M. Nod Factor Structures, Responses, and Perception during Initiation of Nodule Development. Glycobiology 2002, 12, 79R–105R. [Google Scholar] [CrossRef]
- Jones, K.M.; Kobayashi, H.; Davies, B.W.; Taga, M.E.; Walker, G.C. How Rhizobial Symbionts Invade Plants: The Sinorhizobium–Medicago Model. Nat. Rev. Microbiol. 2007, 5, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, F.; Wall, L.; Fabra, A. Starting Points in Plant-Bacteria Nitrogen-Fixing Symbioses: Intercellular Invasion of the Roots. J. Exp. Bot. 2017, 68, 1905–1918. [Google Scholar] [CrossRef]
- Perret, X.; Staehelin, C.; Broughton, W.J. Molecular Basis of Symbiotic Promiscuity. Microbiol. Mol. Biol. Rev. 2000, 64, 180–201. [Google Scholar] [CrossRef]
- Kelly, S.; Sullivan, J.T.; Kawaharada, Y.; Radutoiu, S.; Ronson, C.W.; Stougaard, J. Regulation of Nod Factor Biosynthesis by Alternative NodD Proteins at Distinct Stages of Symbiosis Provides Additional Compatibility Scrutiny. Environ. Microbiol. 2018, 20, 97–110. [Google Scholar] [CrossRef]
- Yang, J.; Lan, L.; Jin, Y.; Yu, N.; Wang, D.; Wang, E. Mechanisms Underlying Legume–Rhizobium Symbioses. J. Integr. Plant Biol. 2022, 64, 244–267. [Google Scholar] [CrossRef]
- Minguillón, S.; Matamoros, M.A.; Duanmu, D.; Becana, M. Signaling by Reactive Molecules and Antioxidants in Legume Nodules. New Phytol. 2022, 236, 815–832. [Google Scholar] [CrossRef]
- Berillo, D.; Kozhahmetova, M.; Lebedeva, L. Overview of the Biological Activity of Anthraquinons and Flavanoids of the Plant Rumex Species. Molecules 2022, 27, 1204. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.E. Multiple Responses of Rhizobia to Flavonoids During Legume Root Infection. In Advances in Botanical Research; Incorporating Advances in Plant Pathology; Academic Press: Cambridge, MA, USA, 2004; Volume 41, pp. 1–62. [Google Scholar]
- Webb, B.A.; Compton, K.K.; del Campo, J.S.M.; Taylor, D.; Sobrado, P.; Scharf, B.E. Sinorhizobium Meliloti Chemotaxis to Multiple Amino Acids Is Mediated by the Chemoreceptor McpU. Mol. Plant-Microbe Interact. 2017, 30, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Moe, L.A. Amino Acids in the Rhizosphere: From Plants to Microbes. Am. J. Bot. 2013, 100, 1692–1705. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga, A.M.; Mena-García, A.; Soria Monzón, A.C.; Rada-Mendoza, M.; Chito, D.M.; Ruiz-Matute, A.I.; Sanz, M.L. Microwave Assisted Extraction of Inositols for the Valorization of Legume By-Products. LWT 2020, 133, 109971. [Google Scholar] [CrossRef]
- Webb, B.A.; Hildreth, S.; Helm, R.F.; Scharf, B.E. Sinorhizobium Meliloti Chemoreceptor McpU Mediates Chemotaxis toward Host Plant Exudates through Direct Proline Sensing. Appl. Environ. Microbiol. 2014, 80, 3404–3415. [Google Scholar] [CrossRef] [PubMed]
- Peters, N.K.; Frost, J.W.; Long, S.R. A Plant Flavone, Luteolin, Induces Expression of Rhizobium Meliloti Nodulation Genes. Science 1986, 233, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Redmond, J.W.; Batley, M.; Djordjevic, M.A.; Innes, R.W.; Kuempel, P.L.; Rolfe, B.G. Flavones Induce Expression of Nodulation Genes in Rhizobium. Nature 1986, 323, 632–635. [Google Scholar] [CrossRef]
- Furuya, M.; Galston, A.W. Flavonoid Complexes in Pisum Sativum L.—I: Nature and Distribution of the Major Components. Phytochemistry 1965, 4, 285–296. [Google Scholar] [CrossRef]
- Ferrer, J.-L.; Austin, M.B.; Stewart, C.; Noel, J.P. Structure and Function of Enzymes Involved in the Biosynthesis of Phenylpropanoids. Plant Physiol. Biochem. 2008, 46, 356–370. [Google Scholar] [CrossRef]
- Cesco, S.; Neumann, G.; Tomasi, N.; Pinton, R.; Weisskopf, L. Release of Plant-Borne Flavonoids into the Rhizosphere and Their Role in Plant Nutrition. Plant Soil 2010, 329, 1–25. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as Antioxidants in Plants: Location and Functional Significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.J.; Morris, P.; Hooker, J.E. Perception and Modification of Plant Flavonoid Signals by Rhizosphere Microorganisms. Env. Microbiol 2006, 8, 1867–1880. [Google Scholar] [CrossRef] [PubMed]
- Biała-Leonhard, W.; Zanin, L.; Gottardi, S.; de Brito Francisco, R.; Venuti, S.; Valentinuzzi, F.; Mimmo, T.; Cesco, S.; Bassin, B.; Martinoia, E.; et al. Identification of an Isoflavonoid Transporter Required for the Nodule Establishment of the Rhizobium-Fabaceae Symbiotic Interaction. Front. Plant Sci. 2021, 12, 758213. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, A.; Shitan, N.; Yazaki, K. Involvement of a Soybean ATP-Binding Cassette-Type Transporter in the Secretion of Genistein, a Signal Flavonoid in Legume-Rhizobium Symbiosis. Plant Physiol. 2007, 144, 2000–2008. [Google Scholar] [CrossRef] [PubMed]
- León-Barrios, M.; Dakora, F.D.; Joseph, C.M.; Phillips, D.A. Isolation of Rhizobium Meliloti Nod Gene Inducers from Alfalfa Rhizosphere Soil. Appl. Environ. Microbiol. 1993, 59, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Nouwen, N.; Fardoux, J.; Giraud, E. NodD1 and NodD2 Are Not Required for the Symbiotic Interaction of Bradyrhizobium ORS285 with Nod-Factor-Independent Aeschynomene Legumes. PLoS ONE 2016, 11, e0157888. [Google Scholar] [CrossRef]
- Hungria, M.; Joseph, C.M.; Phillips, D.A. Rhizobium Nod Gene Inducers Exuded Naturally from Roots of Common Bean (Phaseolus Vulgaris L.). Plant Physiol. 1991, 97, 759–764. [Google Scholar] [CrossRef]
- Deng, B.; Li, Y.; Xu, D.; Ye, Q.; Liu, G. Nitrogen Availability Alters Flavonoid Accumulation in Cyclocarya Paliurus via the Effects on the Internal Carbon/Nitrogen Balance. Sci. Rep. 2019, 9, 2370. [Google Scholar] [CrossRef]
- Coronado, C.; Zuanazzi, J.A.S.; Sallaud, C.; Quirion, J.C.; Esnault, R.; Husson, H.P.; Kondorosi, A.; Ratet, P. Alfalfa Root Flavonoid Production Is Nitrogen Regulated. Plant Physiol. 1995, 108, 533–542. [Google Scholar] [CrossRef]
- Bag, S.; Mondal, A.; Majumder, A.; Mondal, S.K.; Banik, A. Flavonoid Mediated Selective Cross-Talk between Plants and Beneficial Soil Microbiome. Phytochem. Rev. 2022, 21, 1739–1760. [Google Scholar] [CrossRef]
- Sharma, K.; Chaturvedi, U.; Sharma, S.; Vaishnav, A.; Singh, S. Fenugreek-Rhizobium Symbiosis and Flavonoids Under Stress Condition. In Antioxidants in Plant-Microbe Interaction; Springer: Berlin/Heidelberg, Germany, 2021; pp. 449–459. [Google Scholar]
- Huang, X.-F.; Chaparro, J.M.; Reardon, K.F.; Zhang, R.; Shen, Q.; Vivanco, J.M. Rhizosphere Interactions: Root Exudates, Microbes, and Microbial Communities. Botany 2014, 92, 267–275. [Google Scholar] [CrossRef]
- Abdel-Lateif, K.; Bogusz, D.; Hocher, V. The Role of Flavonoids in the Establishment of Plant Roots Endosymbioses with Arbuscular Mycorrhiza Fungi, Rhizobia and Frankia Bacteria. Plant Signal. Behav. 2012, 7, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, M.A.; Mathesius, U.; Arioli, T.; Weinman, J.J.; Gärtner, E. Chalcone Synthase Gene Expression in Transgenic Subterranean Clover Correlates with Localised Accumulation of Flavonoids. Funct. Plant Biol. 1997, 24, 119–132. [Google Scholar] [CrossRef]
- Peer, W.A.; Brown, D.E.; Tague, B.W.; Muday, G.K.; Taiz, L.; Murphy, A.S. Flavonoid Accumulation Patterns of Transparent Testa Mutants of Arabidopsis. Plant Physiol. 2001, 126, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Nouwen, N.; Gargani, D.; Giraud, E. The Modification of the Flavonoid Naringenin by Bradyrhizobium Sp. Strain ORS285 Changes the Nod Genes Inducer Function to a Growth Stimulator. Mol. Plant-Microbe Interact. 2019, 32, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.; Lagunas, B.; Gifford, M.L. Determinants of Host Range Specificity in Legume-Rhizobia Symbiosis. Front. Microbiol. 2020, 11, 585749. [Google Scholar] [CrossRef]
- Compton, K.K.; Hildreth, S.B.; Helm, R.F.; Scharf, B.E. An Updated Perspective on Sinorhizobium Meliloti Chemotaxis to Alfalfa Flavonoids. Front. Microbiol. 2020, 11, 581482. [Google Scholar] [CrossRef]
- Nadal, M.; Paszkowski, U. Polyphony in the Rhizosphere: Presymbiotic Communication in Arbuscular Mycorrhizal Symbiosis. Curr. Opin. Plant Biol. 2013, 16, 473–479. [Google Scholar] [CrossRef]
- Singla, P.; Garg, N. Plant Flavonoids: Key Players in Signaling, Establishment, and Regulation of Rhizobial and Mycorrhizal Endosymbioses. In Mycorrhiza—Function, Diversity, State of the Art; Varma, A., Prasad, R., Tuteja, N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 133–176. ISBN 978-3-319-53064-2. [Google Scholar]
- Shang, J.Y.; Zhang, P.; Jia, Y.W.; Lu, Y.N.; Wu, Y.; Ji, S.; Chen, L.; Wang, E.T.; Chen, W.X.; Sui, X.H. Coordinated Regulation of Symbiotic Adaptation by NodD Proteins and NolA in the Type I Peanut Bradyrhizobial Strain Bradyrhizobium Zhanjiangense CCBAU51778. Microbiol. Res. 2022, 265, 127188. [Google Scholar] [CrossRef]
- Peck, M.C.; Fisher, R.F.; Long, S.R. Diverse Flavonoids Stimulate NodD1 Binding to Nod Gene Promoters in Sinorhizobium Meliloti. J. Bacteriol. 2006, 188, 5417–5427. [Google Scholar] [CrossRef]
- Györgypal, Z.; Iyer, N.; Kondorosi, A. Three Regulatory NodD Alleles of Diverged Flavonoid-Specificity Are Involved in Host-Dependent Nodulation by Rhizobium Meliloti. Molec. Gen. Genet. 1988, 212, 85–92. [Google Scholar] [CrossRef]
- Wassem, R.; Marin, A.M.; Daddaoua, A.; Monteiro, R.A.; Chubatsu, L.S.; Ramos, J.L.; Deakin, W.J.; Broughton, W.J.; Pedrosa, F.O.; Souza, E.M. A NodD-like Protein Activates Transcription of Genes Involved with Naringenin Degradation in a Flavonoid-Dependent Manner in Herbaspirillum Seropedicae. Environ. Microbiol. 2017, 19, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, J.; Long, S.R. The Rhizobium Meliloti GroELc Locus Is Required for Regulation of Early Nod Genes by the Transcription Activator NodD. Genes Dev. 1995, 9, 714–729. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, M.; Kumaki, T.; Hashimoto, S.; Saeki, K.; Ayabe, S.; Higashitani, A.; Akashi, T.; Sato, S.; Aoki, T. Phenolic Acids Induce Nod Factor Production in Lotus japonicus–Mesorhizobium Symbiosis. Microbes Environ. 2022, 37, ME21094. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.W.B.; Beynon, J.L.; Buchanan-Wollaston, A.V.; Setchell, S.M.; Hirsch, P.R.; Beringer, J.E. High Frequency Transfer of Nodulating Ability between Strains and Species of Rhizobium. Nature 1978, 276, 634–636. [Google Scholar] [CrossRef]
- Djordjevic, M.A.; Zurkowski, W.; Shine, J.; Rolfe, B.G. Sym Plasmid Transfer to Various Symbiotic Mutants of Rhizobium Trifolii, R. Leguminosarum, and R. Meliloti. J. Bacteriol. 1983, 156, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Carlson, R.W.; Sanjuan, J.; Bhat, U.R.; Glushka, J.; Spaink, H.P.; Wijfjes, A.H.; van Brussel, A.A.; Stokkermans, T.J.; Peters, N.K.; Stacey, G. The Structures and Biological Activities of the Lipo-Oligosaccharide Nodulation Signals Produced by Type I and II Strains of Bradyrhizobium Japonicum. J. Biol. Chem. 1993, 268, 18372–18381. [Google Scholar] [CrossRef] [PubMed]
- Sanjuan, J.; Carlson, R.W.; Spaink, H.P.; Bhat, U.R.; Barbour, W.M.; Glushka, J.; Stacey, G. A 2-O-Methylfucose Moiety Is Present in the Lipo-Oligosaccharide Nodulation Signal of Bradyrhizobium Japonicum. Proc. Natl. Acad. Sci. USA 1992, 89, 8789–8793. [Google Scholar] [CrossRef]
- Spaink, H.P.; Lugtenberg, B.J. Role of Rhizobial Lipo-Chitin Oligosaccharide Signal Molecules in Root Nodule Organogenesis. Plant Mol. Biol. 1994, 26, 1413–1422. [Google Scholar] [CrossRef]
- Spaink, H.P. Rhizobial Lipo-Oligosaccharides: Answers and Questions. Plant Mol. Biol. 1992, 20, 977–986. [Google Scholar] [CrossRef]
- Göttfert, M. Regulation and Function of Rhizobial Nodulation Genes. FEMS Microbiol. Lett. 1993, 104, 39–63. [Google Scholar] [CrossRef]
- Gladchuk, A.; Shumilina, J.; Kusnetsova, A.; Bureiko, K.; Billig, S.; Tsarev, A.; Alexandrova, I.; Leonova, L.; Zhukov, V.A.; Tikhonovich, I.A.; et al. High-Throughput Fingerprinting of Rhizobial Free Fatty Acids by Chemical Thin-Film Deposition and Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry. Methods Protoc. 2020, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Parniske, M. Arbuscular Mycorrhiza: The Mother of Plant Root Endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Maillet, F.; Poinsot, V.; André, O.; Puech-Pagès, V.; Haouy, A.; Gueunier, M.; Cromer, L.; Giraudet, D.; Formey, D.; Niebel, A.; et al. Fungal Lipochitooligosaccharide Symbiotic Signals in Arbuscular Mycorrhiza. Nature 2011, 469, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Antolín-Llovera, M.; Ried, M.K.; Parniske, M. Cleavage of the SYMBIOSIS RECEPTOR-LIKE KINASE Ectodomain Promotes Complex Formation with Nod Factor Receptor 5. Curr. Biol. 2014, 24, 422–427. [Google Scholar] [CrossRef]
- Bhuvaneswari, T.V.; Turgeon, B.G.; Bauer, W.D. Early Events in the Infection of Soybean (Glycine Max L. Merr) by Rhizobium Japonicum. Plant Physiol. 1980, 66, 1027–1031. [Google Scholar] [CrossRef]
- Radutoiu, S.; Madsen, L.H.; Madsen, E.B.; Felle, H.H.; Umehara, Y.; Grønlund, M.; Sato, S.; Nakamura, Y.; Tabata, S.; Sandal, N.; et al. Plant Recognition of Symbiotic Bacteria Requires Two LysM Receptor-like Kinases. Nature 2003, 425, 585–592. [Google Scholar] [CrossRef]
- Indrasumunar, A.; Kereszt, A.; Searle, I.; Miyagi, M.; Li, D.; Nguyen, C.D.T.; Men, A.; Carroll, B.J.; Gresshoff, P.M. Inactivation of Duplicated Nod Factor Receptor 5 (NFR5) Genes in Recessive Loss-of-Function Non-Nodulation Mutants of Allotetraploid Soybean (Glycine Max L. Merr.). Plant Cell Physiol. 2010, 51, 201–214. [Google Scholar] [CrossRef]
- Zhukov, V.; Radutoiu, S.; Madsen, L.H.; Rychagova, T.; Ovchinnikova, E.; Borisov, A.; Tikhonovich, I.; Stougaard, J. The Pea Sym37 Receptor Kinase Gene Controls Infection-Thread Initiation and Nodule Development. Mol. Plant-Microbe Interact. 2008, 21, 1600–1608. [Google Scholar] [CrossRef]
- Kirienko, A.N.; Porozov, Y.B.; Malkov, N.V.; Akhtemova, G.A.; Le Signor, C.; Thompson, R.; Saffray, C.; Dalmais, M.; Bendahmane, A.; Tikhonovich, I.A.; et al. Role of a Receptor-like Kinase K1 in Pea Rhizobium Symbiosis Development. Planta 2018, 248, 1101–1120. [Google Scholar] [CrossRef]
- Li, R.; Knox, M.R.; Edwards, A.; Hogg, B.; Ellis, T.H.N.; Wei, G.; Downie, J.A. Natural Variation in Host-Specific Nodulation of Pea Is Associated with a Haplotype of the SYM37 LysM-Type Receptor-Like Kinase. Mol. Plant-Microbe Interact. 2011, 24, 1396–1403. [Google Scholar] [CrossRef]
- Sulima, A.S.; Zhukov, V.A.; Afonin, A.A.; Zhernakov, A.I.; Tikhonovich, I.A.; Lutova, L.A. Selection Signatures in the First Exon of Paralogous Receptor Kinase Genes from the Sym2 Region of the Pisum Sativum L. Genome. Front. Plant Sci. 2017, 8, 1957. [Google Scholar] [CrossRef] [PubMed]
- Murakami, E.; Cheng, J.; Gysel, K.; Bozsoki, Z.; Kawaharada, Y.; Hjuler, C.T.; Sørensen, K.K.; Tao, K.; Kelly, S.; Venice, F.; et al. Epidermal LysM Receptor Ensures Robust Symbiotic Signalling in Lotus japonicus. eLife 2018, 7, e33506. [Google Scholar] [CrossRef]
- Radutoiu, S.; Madsen, L.H.; Madsen, E.B.; Jurkiewicz, A.; Fukai, E.; Quistgaard, E.M.H.; Albrektsen, A.S.; James, E.K.; Thirup, S.; Stougaard, J. LysM Domains Mediate Lipochitin-Oligosaccharide Recognition and Nfr Genes Extend the Symbiotic Host Range. EMBO J. 2007, 26, 3923–3935. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, C.; Oldroyd, G.E.D. Plant Signalling in Symbiosis and Immunity. Nature 2017, 543, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.E.M.M.; Nadzieja, M.; Madsen, L.H.; Bücherl, C.A.; Dam, S.; Sandal, N.N.; Couto, D.; Derbyshire, P.; Uldum-Berentsen, M.; Schroeder, S.; et al. A Lotus japonicus Cytoplasmic Kinase Connects Nod Factor Perception by the NFR5 LysM Receptor to Nodulation. Proc. Natl. Acad. Sci. USA 2019, 116, 14339–14348. [Google Scholar] [CrossRef]
- Singh, S.; Katzer, K.; Lambert, J.; Cerri, M.; Parniske, M. CYCLOPS, a DNA-Binding Transcriptional Activator, Orchestrates Symbiotic Root Nodule Development. Cell Host Microbe 2014, 15, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, S.; Kim, J.; Muñoz, A.; Heckmann, A.B.; Downie, J.A.; Oldroyd, G.E.D. GRAS Proteins Form a DNA Binding Complex to Induce Gene Expression during Nodulation Signaling in Medicago truncatula. Plant Cell 2009, 21, 545–557. [Google Scholar] [CrossRef]
- Eckardt, N.A. Nodulation Signaling in Legumes Depends on an NSP1-NSP2 Complex. Plant Cell 2009, 21, 367. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, R. Nitrogen and Phosphorus Signaling and Transport During Legume–Rhizobium Symbiosis. Front. Plant Sci. 2021, 12, 683601. [Google Scholar] [CrossRef]
- Sulima, A.S.; Zhukov, V.A.; Kulaeva, O.A.; Vasileva, E.N.; Borisov, A.Y.; Tikhonovich, I.A. New Sources of Sym2A Allele in the Pea (Pisum Sativum L.) Carry the Unique Variant of Candidate LysM-RLK Gene LykX. PeerJ 2019, 7, e8070. [Google Scholar] [CrossRef]
- Gage, D.J. Infection and Invasion of Roots by Symbiotic, Nitrogen-Fixing Rhizobia during Nodulation of Temperate Legumes. Microbiol. Mol. Biol. Rev. 2004, 68, 280–300. [Google Scholar] [CrossRef] [PubMed]
- Fournier, J.; Timmers, A.C.J.; Sieberer, B.J.; Jauneau, A.; Chabaud, M.; Barker, D.G. Mechanism of Infection Thread Elongation in Root Hairs of Medicago truncatula and Dynamic Interplay with Associated Rhizobial Colonization. Plant Physiol. 2008, 148, 1985–1995. [Google Scholar] [CrossRef] [PubMed]
- Leigh, J.A.; Coplin, D.L. Exopolysaccharides in Plant-Bacterial Interactions. Annu. Rev. Microbiol. 1992, 46, 307–346. [Google Scholar] [CrossRef] [PubMed]
- van Workum, W.A.T.; Cremers, H.C.J.C.; Wijfjes, A.H.M.; van der Kolk, C.; Wijffelman, C.A.; Kijne, J.W. Cloning and Characterization of Four Genes of Rhizobium Leguminosarum Bv. Trifolii Involved in Exopolysaccharide Production and Nodulation. Mol. Plant-Microbe Interact. 1997, 10, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-P.; Walker, G.C. Succinoglycan Is Required for Initiation and Elongation of Infection Threads during Nodulation of Alfalfa ByRhizobium Meliloti. J. Bacteriol. 1998, 180, 5183–5191. [Google Scholar] [CrossRef] [PubMed]
- Kawaharada, Y.; Kelly, S.; Nielsen, M.W.; Hjuler, C.T.; Gysel, K.; Muszyński, A.; Carlson, R.W.; Thygesen, M.B.; Sandal, N.; Asmussen, M.H.; et al. Receptor-Mediated Exopolysaccharide Perception Controls Bacterial Infection. Nature 2015, 523, 308–312. [Google Scholar] [CrossRef]
- Kawaharada, Y.; Nielsen, M.W.; Kelly, S.; James, E.K.; Andersen, K.R.; Rasmussen, S.R.; Füchtbauer, W.; Madsen, L.H.; Heckmann, A.B.; Radutoiu, S.; et al. Differential Regulation of the Epr3 Receptor Coordinates Membrane-Restricted Rhizobial Colonization of Root Nodule Primordia. Nat. Commun. 2017, 8, 14534. [Google Scholar] [CrossRef]
- Wong, J.E.M.M.; Gysel, K.; Birkefeldt, T.G.; Vinther, M.; Muszyński, A.; Azadi, P.; Laursen, N.S.; Sullivan, J.T.; Ronson, C.W.; Stougaard, J.; et al. Structural Signatures in EPR3 Define a Unique Class of Plant Carbohydrate Receptors. Nat. Commun. 2020, 11, 3797. [Google Scholar] [CrossRef]
- Maillet, F.; Fournier, J.; Mendis, H.C.; Tadege, M.; Wen, J.; Ratet, P.; Mysore, K.S.; Gough, C.; Jones, K.M. Sinorhizobium Meliloti Succinylated High-Molecular-Weight Succinoglycan and the Medicago truncatula LysM Receptor-like Kinase MtLYK10 Participate Independently in Symbiotic Infection. Plant J. 2020, 102, 311–326. [Google Scholar] [CrossRef]
- Okazaki, S.; Kaneko, T.; Sato, S.; Saeki, K. Hijacking of Leguminous Nodulation Signaling by the Rhizobial Type III Secretion System. Proc. Natl. Acad. Sci. USA 2013, 110, 17131–17136. [Google Scholar] [CrossRef]
- Safronova, V.I.; Guro, P.V.; Sazanova, A.L.; Kuznetsova, I.G.; Belimov, A.A.; Yakubov, V.V.; Chirak, E.R.; Afonin, A.M.; Gogolev, Y.V.; Andronov, E.E.; et al. Rhizobial Microsymbionts of Kamchatka Oxytropis Species Possess Genes of the Type III and VI Secretion Systems, Which Can Affect the Development of Symbiosis. Mol. Plant-Microbe Interact. 2020, 33, 1232–1241. [Google Scholar] [CrossRef]
- Okazaki, S.; Tittabutr, P.; Teulet, A.; Thouin, J.; Fardoux, J.; Chaintreuil, C.; Gully, D.; Arrighi, J.-F.; Furuta, N.; Miwa, H.; et al. Rhizobium-Legume Symbiosis in the Absence of Nod Factors: Two Possible Scenarios with or without the T3SS. ISME J. 2016, 10, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Hueck, C.J. Type III Protein Secretion Systems in Bacterial Pathogens of Animals and Plants. Microbiol. Mol. Biol. Rev. 1998, 62, 379–433. [Google Scholar] [CrossRef] [PubMed]
- Viprey, V.; Del Greco, A.; Golinowski, W.; Broughton, W.J.; Perret, X. Symbiotic Implications of Type III Protein Secretion Machinery in Rhizobium. Mol. Microbiol. 1998, 28, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Marie, C.; Deakin, W.J.; Viprey, V.; Kopciñska, J.; Golinowski, W.; Krishnan, H.B.; Perret, X.; Broughton, W.J. Characterization of Nops, Nodulation Outer Proteins, Secreted via the Type III Secretion System of NGR234. Mol. Plant-Microbe Interact. 2003, 16, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Staehelin, C.; Krishnan, H.B. Nodulation Outer Proteins: Double-Edged Swords of Symbiotic Rhizobia. Biochem. J. 2015, 470, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Guerrero, I.; Pérez-Montaño, F.; Medina, C.; Ollero, F.J.; López-Baena, F.J. NopC Is a Rhizobium-Specific Type 3 Secretion System Effector Secreted by Sinorhizobium (Ensifer) Fredii HH103. PLoS ONE 2015, 10, e0142866. [Google Scholar] [CrossRef] [PubMed]
- Deakin, W.J.; Marie, C.; Saad, M.M.; Krishnan, H.B.; Broughton, W.J. NopA Is Associated with Cell Surface Appendages Produced by the Type III Secretion System of Rhizobium Sp. Strain NGR234. Mol. Plant-Microbe Interact. 2005, 18, 499–507. [Google Scholar] [CrossRef]
- Lorio, J.C.; Kim, W.S.; Krishnan, H.B. NopB, a Soybean Cultivar-Specificity Protein from Sinorhizobium Fredii USDA257, Is a Type III Secreted Protein. Mol. Plant-Microbe Interact. 2004, 17, 1259–1268. [Google Scholar] [CrossRef]
- Rodrigues, J.A.; López-Baena, F.J.; Ollero, F.J.; Vinardell, J.M.; Espuny, M.D.R.; Bellogín, R.A.; Ruiz-Sainz, J.E.; Thomas, J.R.; Sumpton, D.; Ault, J.; et al. NopM and NopD Are Rhizobial Nodulation Outer Proteins: Identification Using LC-MALDI and LC-ESI with a Monolithic Capillary Column. J. Proteome Res. 2007, 6, 1029–1037. [Google Scholar] [CrossRef]
- Busset, N.; Gully, D.; Teulet, A.; Fardoux, J.; Camuel, A.; Cornu, D.; Severac, D.; Giraud, E.; Mergaert, P. The Type III Effectome of the Symbiotic Bradyrhizobium Vignae Strain ORS3257. Biomolecules 2021, 11, 1592. [Google Scholar] [CrossRef] [PubMed]
- Safronova, V.; Belimov, A.; Sazanova, A.; Chirak, E.; Kuznetsova, I.; Andronov, E.; Pinaev, A.; Tsyganova, A.; Seliverstova, E.; Kitaeva, A.; et al. Two Broad Host Range Rhizobial Strains Isolated From Relict Legumes Have Various Complementary Effects on Symbiotic Parameters of Co-Inoculated Plants. Front. Microbiol. 2019, 10, 514. [Google Scholar] [CrossRef] [PubMed]
- Safronova, V.; Sazanova, A.; Belimov, A.; Guro, P.; Kuznetsova, I.; Karlov, D.; Chirak, E.; Yuzikhin, O.; Verkhozina, A.; Afonin, A.; et al. Synergy between Rhizobial Co-Microsymbionts Leads to an Increase in the Efficiency of Plant-Microbe Interactions. Microorganisms 2023, 11, 1206. [Google Scholar] [CrossRef] [PubMed]
- Coba de la Peña, T.; Fedorova, E.; Pueyo, J.J.; Lucas, M.M. The Symbiosome: Legume and Rhizobia Co-Evolution toward a Nitrogen-Fixing Organelle? Front. Plant Sci. 2018, 8, 2229. [Google Scholar] [CrossRef] [PubMed]
- Fournier, J.; Teillet, A.; Chabaud, M.; Ivanov, S.; Genre, A.; Limpens, E.; de Carvalho-Niebel, F.; Barker, D.G. Remodeling of the Infection Chamber before Infection Thread Formation Reveals a Two-Step Mechanism for Rhizobial Entry into the Host Legume Root Hair. Plant Physiol. 2015, 167, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Becker, A. Classic Spotlight: Bacteroids—Views of an Enigmatic Bacterial State in Root Nodule Symbiosis through the Centuries. J. Bacteriol. 2017, 199, e00741-16. [Google Scholar] [CrossRef] [PubMed]
- Jordan, D.C.; Grinyer, I.; Coulter, W.H. Electron Microscopy of Infection Threads and Bacteria in Young Root Nodules of Medicago Sativa. J. Bacteriol. 1963, 86, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Dart, P.J.; Mercer, F.V. Fine Structure of Bacteroids in Root Nodules of Vigna Sinensis, Acacia Longifolia, Viminaria Juncea, and Lupinus Angustifolius. J. Bacteriol. 1966, 91, 1314–1319. [Google Scholar] [CrossRef]
- Goodchild, D.J.; Bergersen, F.J. Electron Microscopy of the Infection and Subsequent Development of Soybean Nodule Cells. J. Bacteriol. 1966, 92, 204–213. [Google Scholar] [CrossRef]
- MacKenzie, C.R.; Vail, W.J.; Jordan, D.C. Ultrastructure of Free-Living and Nitrogen-Fixing Forms of Rhizobium Meliloti as Revealed by Freeze-Etching. J. Bacteriol. 1973, 113, 387–393. [Google Scholar] [CrossRef]
- Tsyganova, A.V.; Brewin, N.J.; Tsyganov, V.E. Structure and Development of the Legume-Rhizobial Symbiotic Interface in Infection Threads. Cells 2021, 10, 1050. [Google Scholar] [CrossRef]
- Brewin, N.J. Plant Cell Wall Remodelling in the Rhizobium–Legume Symbiosis. Crit. Rev. Plant Sci. 2004, 23, 293–316. [Google Scholar] [CrossRef]
- Liu, C.-W.; Breakspear, A.; Stacey, N.; Findlay, K.; Nakashima, J.; Ramakrishnan, K.; Liu, M.; Xie, F.; Endre, G.; de Carvalho-Niebel, F.; et al. A Protein Complex Required for Polar Growth of Rhizobial Infection Threads. Nat. Commun. 2019, 10, 2848. [Google Scholar] [CrossRef] [PubMed]
- Arrighi, J.-F.; Godfroy, O.; de Billy, F.; Saurat, O.; Jauneau, A.; Gough, C. The RPG Gene of Medicago truncatula Controls Rhizobium-Directed Polar Growth during Infection. Proc. Natl. Acad. Sci. USA 2008, 105, 9817–9822. [Google Scholar] [CrossRef] [PubMed]
- Yokota, K.; Fukai, E.; Madsen, L.H.; Jurkiewicz, A.; Rueda, P.; Radutoiu, S.; Held, M.; Hossain, M.S.; Szczyglowski, K.; Morieri, G.; et al. Rearrangement of Actin Cytoskeleton Mediates Invasion of Lotus japonicus Roots by Mesorhizobium Loti. Plant Cell 2009, 21, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Liao, J.; James, E.K.; Sato, S.; Tabata, S.; Jurkiewicz, A.; Madsen, L.H.; Stougaard, J.; Ross, L.; Szczyglowski, K. Lotus japonicus ARPC1 Is Required for Rhizobial Infection. Plant Physiol. 2012, 160, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Sinharoy, S.; Liu, C.; Breakspear, A.; Guan, D.; Shailes, S.; Nakashima, J.; Zhang, S.; Wen, J.; Torres-Jerez, I.; Oldroyd, G.; et al. A Medicago truncatula Cystathionine-β-Synthase-like Domain-Containing Protein Is Required for Rhizobial Infection and Symbiotic Nitrogen Fixation. Plant Physiol. 2016, 170, 2204–2217. [Google Scholar] [CrossRef] [PubMed]
- Kiss, E.; Oláh, B.; Kaló, P.; Morales, M.; Heckmann, A.B.; Borbola, A.; Lózsa, A.; Kontár, K.; Middleton, P.; Downie, J.A.; et al. LIN, a Novel Type of U-Box/WD40 Protein, Controls Early Infection by Rhizobia in Legumes. Plant Physiol. 2009, 151, 1239–1249. [Google Scholar] [CrossRef]
- Soyano, T.; Kouchi, H.; Hirota, A.; Hayashi, M. NODULE INCEPTION Directly Targets NF-Y Subunit Genes to Regulate Essential Processes of Root Nodule Development in Lotus japonicus. PLOS Genet. 2013, 9, e1003352. [Google Scholar] [CrossRef]
- Gonzalez-Rizzo, S.; Crespi, M.; Frugier, F. The Medicago truncatula CRE1 Cytokinin Receptor Regulates Lateral Root Development and Early Symbiotic Interaction with Sinorhizobium Meliloti. Plant Cell 2006, 18, 2680–2693. [Google Scholar] [CrossRef]
- Kohlen, W.; Ng, J.L.P.; Deinum, E.E.; Mathesius, U. Auxin Transport, Metabolism, and Signalling during Nodule Initiation: Indeterminate and Determinate Nodules. J. Exp. Bot. 2018, 69, 229–244. [Google Scholar] [CrossRef]
- Shtark, O.Y.; Shishova, M.F.; Povydysh, M.N.; Avdeeva, G.S.; Zhukov, V.A.; Tikhonovich, I.A. Strigolactones as Regulators of Symbiotrophy of Plants and Microorganisms. Russ. J. Plant Physiol. 2018, 65, 151–167. [Google Scholar] [CrossRef]
- Fiorilli, V.; Wang, J.Y.; Bonfante, P.; Lanfranco, L.; Al-Babili, S. Apocarotenoids: Old and New Mediators of the Arbuscular Mycorrhizal Symbiosis. Front. Plant Sci. 2019, 10, 1186. [Google Scholar] [CrossRef] [PubMed]
- Van Zeijl, A.; Liu, W.; Xiao, T.T.; Kohlen, W.; Yang, W.-C.; Bisseling, T.; Geurts, R. The Strigolactone Biosynthesis Gene DWARF27 Is Co-Opted in Rhizobium Symbiosis. BMC Plant Biol. 2015, 15, 260. [Google Scholar] [CrossRef] [PubMed]
- Foo, E.; Davies, N.W. Strigolactones Promote Nodulation in Pea. Planta 2011, 234, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Foo, E.; Yoneyama, K.; Hugill, C.J.; Quittenden, L.J.; Reid, J.B. Strigolactones and the Regulation of Pea Symbioses in Response to Nitrate and Phosphate Deficiency. Mol. Plant 2013, 6, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Van Dingenen, J.; De Keyser, A.; Desmet, S.; Clarysse, A.; Beullens, S.; Michiels, J.; Planque, M.; Goormachtig, S. Strigolactones Repress Nodule Development and Senescence in Pea. Plant J. 2023, 116, 7–22. [Google Scholar] [CrossRef]
- Okamoto, S.; Shinohara, H.; Mori, T.; Matsubayashi, Y.; Kawaguchi, M. Root-Derived CLE Glycopeptides Control Nodulation by Direct Binding to HAR1 Receptor Kinase. Nat. Commun. 2013, 4, 2191. [Google Scholar] [CrossRef] [PubMed]
- Kereszt, A.; Mergaert, P.; Montiel, J.; Endre, G.; Kondorosi, É. Impact of Plant Peptides on Symbiotic Nodule Development and Functioning. Front. Plant Sci. 2018, 9, 1026. [Google Scholar] [CrossRef]
- Xie, F.; Murray, J.D.; Kim, J.; Heckmann, A.B.; Edwards, A.; Oldroyd, G.E.D.; Downie, J.A. Legume Pectate Lyase Required for Root Infection by Rhizobia. Proc. Natl. Acad. Sci. USA 2012, 109, 633–638. [Google Scholar] [CrossRef]
- Mergaert, P. Differentiation of Symbiotic Nodule Cells and Their Rhizobium Endosymbionts. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2020; Volume 94, pp. 149–180. ISBN 978-0-08-102798-1. [Google Scholar]
- Kereszt, A.; Mergaert, P.; Kondorosi, E. Bacteroid Development in Legume Nodules: Evolution of Mutual Benefit or of Sacrificial Victims? Mol. Plant-Microbe Interact. 2011, 24, 1300–1309. [Google Scholar] [CrossRef]
- Ledermann, R.; Schulte, C.C.M.; Poole, P.S. How Rhizobia Adapt to the Nodule Environment. J. Bacteriol. 2021, 203, e00539-20. [Google Scholar] [CrossRef]
- Mergaert, P.; Uchiumi, T.; Alunni, B.; Evanno, G.; Cheron, A.; Catrice, O.; Mausset, A.-E.; Barloy-Hubler, F.; Galibert, F.; Kondorosi, A.; et al. Eukaryotic Control on Bacterial Cell Cycle and Differentiation in the Rhizobium–Legume Symbiosis. Proc. Natl. Acad. Sci. USA 2006, 103, 5230–5235. [Google Scholar] [CrossRef]
- Nagata, M.; Suzuki, A.; Nagata, M.; Suzuki, A. Effects of Phytohormones on Nodulation and Nitrogen Fixation in Leguminous Plants; IntechOpen: Rijeka, Croatia, 2014; ISBN 978-953-51-1216-7. [Google Scholar]
- Kondorosi, E.; Mergaert, P.; Kereszt, A. A Paradigm for Endosymbiotic Life: Cell Differentiation of Rhizobium Bacteria Provoked by Host Plant Factors. Annu. Rev. Microbiol. 2013, 67, 611–628. [Google Scholar] [CrossRef] [PubMed]
- Kitaeva, A.B.; Gorshkov, A.P.; Kusakin, P.G.; Sadovskaya, A.R.; Tsyganova, A.V.; Tsyganov, V.E. Tubulin Cytoskeleton Organization in Cells of Determinate Nodules. Front. Plant Sci. 2022, 13, 823183. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.M. Developmental Biology of Legume Nodulation. New Phytol. 1992, 122, 211–237. [Google Scholar] [CrossRef] [PubMed]
- Montiel, J.; Downie, J.A.; Farkas, A.; Bihari, P.; Herczeg, R.; Bálint, B.; Mergaert, P.; Kereszt, A.; Kondorosi, É. Morphotype of Bacteroids in Different Legumes Correlates with the Number and Type of Symbiotic NCR Peptides. Proc. Natl. Acad. Sci. USA 2017, 114, 5041–5046. [Google Scholar] [CrossRef]
- Downie, J.A.; Kondorosi, E. Why Should Nodule Cysteine-Rich (NCR) Peptides Be Absent From Nodules of Some Groups of Legumes but Essential for Symbiotic N-Fixation in Others? Front. Agron. 2021, 3. [Google Scholar] [CrossRef]
- Ördögh, L.; Vörös, A.; Nagy, I.; Kondorosi, É.; Kereszt, A. Symbiotic Plant Peptides Eliminate Candida Albicans Both In Vitro and in an Epithelial Infection Model and Inhibit the Proliferation of Immortalized Human Cells. BioMed Res. Int. 2014, 2014, e320796. [Google Scholar] [CrossRef] [PubMed]
- Mergaert, P.; Nikovics, K.; Kelemen, Z.; Maunoury, N.; Vaubert, D.; Kondorosi, A.; Kondorosi, E. A Novel Family in Medicago truncatula Consisting of More Than 300 Nodule-Specific Genes Coding for Small, Secreted Polypeptides with Conserved Cysteine Motifs. Plant Physiol. 2003, 132, 161–173. [Google Scholar] [CrossRef]
- Zorin, E.A.; Kliukova, M.S.; Afonin, A.M.; Gribchenko, E.S.; Gordon, M.L.; Sulima, A.S.; Zhernakov, A.I.; Kulaeva, O.A.; Romanyuk, D.A.; Kusakin, P.G.; et al. A Variable Gene Family Encoding Nodule-Specific Cysteine-Rich Peptides in Pea (Pisum Sativum L.). Front. Plant Sci. 2022, 13, 884726. [Google Scholar] [CrossRef] [PubMed]
- Van de Velde, W.; Zehirov, G.; Szatmari, A.; Debreczeny, M.; Ishihara, H.; Kevei, Z.; Farkas, A.; Mikulass, K.; Nagy, A.; Tiricz, H.; et al. Plant Peptides Govern Terminal Differentiation of Bacteria in Symbiosis. Science 2010, 327, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Mergaert, P. Role of Antimicrobial Peptides in Controlling Symbiotic Bacterial Populations. Nat. Prod. Rep. 2018, 35, 336–356. [Google Scholar] [CrossRef] [PubMed]
- Farkas, A.; Maróti, G.; Dürgő, H.; Györgypál, Z.; Lima, R.M.; Medzihradszky, K.F.; Kereszt, A.; Mergaert, P.; Kondorosi, É. Medicago truncatula Symbiotic Peptide NCR247 Contributes to Bacteroid Differentiation through Multiple Mechanisms. Proc. Natl. Acad. Sci. USA 2014, 111, 5183–5188. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Achom, M.; Wilkinson, H.; Lagunas, B.; Gifford, M.L. Symbiotic Outcome Modified by the Diversification from 7 to over 700 Nodule-Specific Cysteine-Rich Peptides. Genes 2020, 11, 348. [Google Scholar] [CrossRef]
- Collier, J. Regulation of Chromosomal Replication in Caulobacter Crescentus. Plasmid 2012, 67, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Penterman, J.; Abo, R.P.; De Nisco, N.J.; Arnold, M.F.F.; Longhi, R.; Zanda, M.; Walker, G.C. Host Plant Peptides Elicit a Transcriptional Response to Control the Sinorhizobium Meliloti Cell Cycle during Symbiosis. Proc. Natl. Acad. Sci. USA 2014, 111, 3561–3566. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, J.; Li, H.; Yang, S.; Körmöczi, P.; Kereszt, A.; Zhu, H. Nodule-Specific Cysteine-Rich Peptides Negatively Regulate Nitrogen-Fixing Symbiosis in a Strain-Specific Manner in Medicago truncatula. Mol. Plant-Microbe Interact. 2018, 31, 240–248. [Google Scholar] [CrossRef]
- Frolov, A.; Mamontova, T.; Ihling, C.; Lukasheva, E.; Bankin, M.; Chantseva, V.; Vikhnina, M.; Soboleva, A.; Shumilina, J.; Mavropolo-Stolyarenko, G.; et al. Mining Seed Proteome: From Protein Dynamics to Modification Profiles. Biol. Commun. 2018, 63, 43–58. [Google Scholar] [CrossRef]
- Miller, J.K.; Herman, E.M.; Jahn, M.; Bradford, K.J. Strategic Research, Education and Policy Goals for Seed Science and Crop Improvement. Plant Sci. 2010, 179, 645–652. [Google Scholar] [CrossRef]
- Mamontova, T.; Lukasheva, E.; Mavropolo-Stolyarenko, G.; Proksch, C.; Bilova, T.; Kim, A.; Babakov, V.; Grishina, T.; Hoehenwarter, W.; Medvedev, S.; et al. Proteome Map of Pea (Pisum Sativum L.) Embryos Containing Different Amounts of Residual Chlorophylls. Int. J. Mol. Sci. 2018, 19, 4066. [Google Scholar] [CrossRef] [PubMed]
- Mounier, E.; Pervent, M.; Ljung, K.; Gojon, A.; Nacry, P. Auxin-Mediated Nitrate Signalling by NRT1.1 Participates in the Adaptive Response of Arabidopsis Root Architecture to the Spatial Heterogeneity of Nitrate Availability. Plant Cell Environ. 2014, 37, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, K.; Ogawa, M.; Matsubayashi, Y. Identification of a Biologically Active, Small, Secreted Peptide in Arabidopsis by in Silico Gene Screening, Followed by LC-MS-Based Structure Analysis. Plant J. 2008, 55, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Roberts, I.; Smith, S.; De Rybel, B.; Van Den Broeke, J.; Smet, W.; De Cokere, S.; Mispelaere, M.; De Smet, I.; Beeckman, T. The CEP Family in Land Plants: Evolutionary Analyses, Expression Studies, and Role in Arabidopsis Shoot Development. J. Exp. Bot. 2013, 64, 5371–5381. [Google Scholar] [CrossRef] [PubMed]
- Tabata, R.; Sumida, K.; Yoshii, T.; Ohyama, K.; Shinohara, H.; Matsubayashi, Y. Perception of Root-Derived Peptides by Shoot LRR-RKs Mediates Systemic N-Demand Signaling. Science 2014, 346, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Imin, N.; Mohd-Radzman, N.A.; Ogilvie, H.A.; Djordjevic, M.A. The Peptide-Encoding CEP1 Gene Modulates Lateral Root and Nodule Numbers in Medicago truncatula. J. Exp. Bot. 2013, 64, 5395–5409. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, Y.; Tanaka, M.; Tabata, R.; Ogawa-Ohnishi, M.; Matsubayashi, Y. Shoot-to-Root Mobile Polypeptides Involved in Systemic Regulation of Nitrogen Acquisition. Nat. Plants 2017, 3, 17029. [Google Scholar] [CrossRef]
- Gautrat, P.; Laffont, C.; Frugier, F. Compact Root Architecture 2 Promotes Root Competence for Nodulation through the MiR2111 Systemic Effector. Curr. Biol. 2020, 30, 1339–1345.e3. [Google Scholar] [CrossRef]
- Mortier, V.; Den Herder, G.; Whitford, R.; Van de Velde, W.; Rombauts, S.; D’haeseleer, K.; Holsters, M.; Goormachtig, S. CLE Peptides Control Medicago truncatula Nodulation Locally and Systemically. Plant Physiol. 2010, 153, 222–237. [Google Scholar] [CrossRef]
- Yamaguchi, Y.L.; Ishida, T.; Sawa, S. CLE Peptides and Their Signaling Pathways in Plant Development. J. Exp. Bot. 2016, 67, 4813–4826. [Google Scholar] [CrossRef]
- Whitford, R.; Fernandez, A.; De Groodt, R.; Ortega, E.; Hilson, P. Plant CLE Peptides from Two Distinct Functional Classes Synergistically Induce Division of Vascular Cells. Proc. Natl. Acad. Sci. USA 2008, 105, 18625–18630. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Ohnishi, E.; Sato, S.; Takahashi, H.; Nakazono, M.; Tabata, S.; Kawaguchi, M. Nod Factor/Nitrate-Induced CLE Genes That Drive HAR1-Mediated Systemic Regulation of Nodulation. Plant Cell Physiol. 2009, 50, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pei, Y.; Shen, Y.; Zhang, R.; Kang, M.; Ma, Y.; Li, D.; Chen, Y. Progress in the Self-Regulation System in Legume Nodule Development-AON (Autoregulation of Nodulation). Int. J. Mol. Sci. 2022, 23, 6676. [Google Scholar] [CrossRef] [PubMed]
- Takahara, M.; Magori, S.; Soyano, T.; Okamoto, S.; Yoshida, C.; Yano, K.; Sato, S.; Tabata, S.; Yamaguchi, K.; Shigenobu, S.; et al. TOO MUCH LOVE, a Novel Kelch Repeat-Containing F-Box Protein, Functions in the Long-Distance Regulation of the Legume–Rhizobium Symbiosis. Plant Cell Physiol. 2013, 54, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Valmas, M.I.; Sexauer, M.; Markmann, K.; Tsikou, D. Plants Recruit Peptides and Micro RNAs to Regulate Nutrient Acquisition from Soil and Symbiosis. Plants 2023, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- Morandi, D.; Sagan, M.; Prado-Vivant, E.; Duc, G. Influence of Genes Determining Supernodulation on Root Colonization by the Mycorrhizal Fungus Glomus Mosseae in Pisum Sativum and Medicago truncatula Mutants. Mycorrhiza 2000, 10, 37–42. [Google Scholar] [CrossRef]
- Zakaria Solaiman, M.; Senoo, K.; Kawaguchi, M.; Imaizumi-Anraku, H.; Akao, S.; Tanaka, A.; Obata, H. Characterization of Mycorrhizas Formed by Glomus Sp. on Roots of Hypernodulating Mutants of Lotus japonicus. J. Plant Res. 2000, 113, 443–448. [Google Scholar] [CrossRef]
- Müller, L.M.; Harrison, M.J. Phytohormones, miRNAs, and Peptide Signals Integrate Plant Phosphorus Status with Arbuscular Mycorrhizal Symbiosis. Curr. Opin. Plant Biol. 2019, 50, 132–139. [Google Scholar] [CrossRef]
- Funayama-Noguchi, S.; Noguchi, K.; Yoshida, C.; Kawaguchi, M. Two CLE Genes Are Induced by Phosphate in Roots of Lotus japonicus. J. Plant Res. 2011, 124, 155–163. [Google Scholar] [CrossRef]
- Wulf, K.; Sun, J.; Wang, C.; Ho-Plagaro, T.; Kwon, C.-T.; Velandia, K.; Correa-Lozano, A.; Tamayo-Navarrete, M.I.; Reid, J.B.; García Garrido, J.M.; et al. The Role of CLE Peptides in the Suppression of Mycorrhizal Colonization of Tomato. Plant Cell Physiol. 2023, pcad124. [Google Scholar] [CrossRef]
- Kazmierczak, T.; Yang, L.; Boncompagni, E.; Meilhoc, E.; Frugier, F.; Frendo, P.; Bruand, C.; Gruber, V.; Brouquisse, R. Legume Nodule Senescence: A Coordinated Death Mechanism between Bacteria and Plant Cells. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2020; Volume 94, pp. 181–212. ISBN 978-0-08-102798-1. [Google Scholar]
- Roponen, I. The Effect of Darkness on the Leghemoglobin Content and Amino Acid Levels in the Root Nodules of Pea Plants. Physiol. Plant. 2006, 23, 452–460. [Google Scholar] [CrossRef]
- Timmers, A.C.; Soupène, E.; Auriac, M.C.; de Billy, F.; Vasse, J.; Boistard, P.; Truchet, G. Saprophytic Intracellular Rhizobia in Alfalfa Nodules. Mol. Plant-Microbe Interact. 2000, 13, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Pladys, D.; Vance, C.P. Proteolysis during Development and Senescence of Effective and Plant Gene-Controlled Ineffective Alfalfa Nodules. Plant Physiol. 1993, 103, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Van de Velde, W.; Guerra, J.C.P.; Keyser, A.D.; De Rycke, R.; Rombauts, S.; Maunoury, N.; Mergaert, P.; Kondorosi, E.; Holsters, M.; Goormachtig, S. Aging in Legume Symbiosis. A Molecular View on Nodule Senescence in Medicago truncatula. Plant Physiol. 2006, 141, 711–720. [Google Scholar] [CrossRef]
- Franck, S.; Strodtman, K.N.; Qiu, J.; Emerich, D.W. Transcriptomic Characterization of Bradyrhizobium Diazoefficiens Bacteroids Reveals a Post-Symbiotic, Hemibiotrophic-Like Lifestyle of the Bacteria within Senescing Soybean Nodules. Int. J. Mol. Sci. 2018, 19, 3918. [Google Scholar] [CrossRef]
- Serova, T.; Tsyganov, V. Symbiotic Nodule Senescence in Legumes: Molecular-Genetic and Cellular Aspects (Review). Agric. Biol. 2014, 5, 3–15. [Google Scholar] [CrossRef]
- Li, X.; Feng, H.; Wen, J.; Dong, J.; Wang, T. MtCAS31 Aids Symbiotic Nitrogen Fixation by Protecting the Leghemoglobin MtLb120-1 Under Drought Stress in Medicago truncatula. Front. Plant Sci. 2018, 9, 633. [Google Scholar] [CrossRef]
- de Zélicourt, A.; Diet, A.; Marion, J.; Laffont, C.; Ariel, F.; Moison, M.; Zahaf, O.; Crespi, M.; Gruber, V.; Frugier, F. Dual Involvement of a Medicago truncatula NAC Transcription Factor in Root Abiotic Stress Response and Symbiotic Nodule Senescence. Plant J. 2012, 70, 220–230. [Google Scholar] [CrossRef]
- Osmolovskaya, N.; Shumilina, J.; Kim, A.; Didio, A.; Grishina, T.; Bilova, T.; Keltsieva, O.A.; Zhukov, V.; Tikhonovich, I.; Tarakhovskaya, E.; et al. Methodology of Drought Stress Research: Experimental Setup and Physiological Characterization. Int. J. Mol. Sci 2018, 19, 4089. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS Regulation of Plant Development and Stress Responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Ivanova, K.A.; Chernova, E.N.; Kulaeva, O.A.; Tsyganova, A.V.; Kusakin, P.G.; Russkikh, I.V.; Tikhonovich, I.A.; Tsyganov, V.E. The Regulation of Pea (Pisum Sativum L.) Symbiotic Nodule Infection and Defense Responses by Glutathione, Homoglutathione, and Their Ratio. Front. Plant Sci. 2022, 13, 843565. [Google Scholar] [CrossRef]
- Fukudome, M.; Watanabe, E.; Osuki, K.-I.; Imaizumi, R.; Aoki, T.; Becana, M.; Uchiumi, T. Stably Transformed Lotus japonicus Plants Overexpressing Phytoglobin LjGlb1-1 Show Decreased Nitric Oxide Levels in Roots and Nodules as Well as Delayed Nodule Senescence. Plant Cell Physiol. 2019, 60, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Puppo, A.; Groten, K.; Bastian, F.; Carzaniga, R.; Soussi, M.; Lucas, M.M.; de Felipe, M.R.; Harrison, J.; Vanacker, H.; Foyer, C.H. Legume Nodule Senescence: Roles for Redox and Hormone Signalling in the Orchestration of the Natural Aging Process. New Phytol. 2005, 165, 683–701. [Google Scholar] [CrossRef]
- Matamoros, M.A.; Kim, A.; Peñuelas, M.; Ihling, C.; Griesser, E.; Hoffmann, R.; Fedorova, M.; Frolov, A.; Becana, M. Protein Carbonylation and Glycation in Legume Nodules. Plant Physiol 2018, 177, 1510–1528. [Google Scholar] [CrossRef] [PubMed]
- Soboleva, A.; Frolova, N.; Bureiko, K.; Shumilina, J.; Balcke, G.U.; Zhukov, V.A.; Tikhonovich, I.A.; Frolov, A. Dynamics of Reactive Carbonyl Species in Pea Root Nodules in Response to Polyethylene Glycol (PEG)-Induced Osmotic Stress. Int. J. Mol. Sci. 2022, 23, 2726. [Google Scholar] [CrossRef] [PubMed]
- Shumilina, J.; Gorbach, D.; Popova, V.; Tsarev, A.; Kuznetsova, A.; Grashina, M.; Dorn, M.; Lukasheva, E.; Osmolovskaya, N.; Romanovskaya, E.; et al. Protein Glycation and Drought Response of Pea (Pisum Sativum L.) Root Nodule Proteome: A Proteomics Approach. Biol. Commun. 2021, 66, 210–224. [Google Scholar] [CrossRef]
- Shumilina, J.; Kusnetsova, A.; Tsarev, A.; Janse van Rensburg, H.C.; Medvedev, S.; Demidchik, V.; Van den Ende, W.; Frolov, A. Glycation of Plant Proteins: Regulatory Roles and Interplay with Sugar Signalling? Int. J. Mol. Sci. 2019, 20, 2366. [Google Scholar] [CrossRef]
- Bilova, T.; Lukasheva, E.; Brauch, D.; Greifenhagen, U.; Paudel, G.; Tarakhovskaya, E.; Frolova, N.; Mittasch, J.; Balcke, G.; Tissier, A.; et al. A Snapshot of the Plant Glycated Proteome. J. Biol. Chem. 2016, 291, 7621–7636. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, C.; Huang, Y.; Chen, H.; Yuan, S.; Zhou, X. Characteristics and Research Progress of Legume Nodule Senescence. Plants 2021, 10, 1103. [Google Scholar] [CrossRef]
- Bennett, E.J.; Roberts, J.A.; Wagstaff, C. Use of Mutants to Dissect the Role of Ethylene Signalling in Organ Senescence and the Regulation of Yield in Arabidopsis Thaliana. J. Plant Growth Regul. 2014, 33, 56–65. [Google Scholar] [CrossRef]
- Morris, K.; Mackerness, S.A.-H.; Page, T.; John, C.F.; Murphy, A.M.; Carr, J.P.; Buchanan-Wollaston, V. Salicylic Acid Has a Role in Regulating Gene Expression during Leaf Senescence. Plant J. 2000, 23, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.C.; Hong, S.W.; Whang, S.S.; Lim, P.O.; Nam, H.G.; Koo, J.C. Age-Dependent Action of an ABA-Inducible Receptor Kinase, RPK1, as a Positive Regulator of Senescence in Arabidopsis Leaves. Plant Cell Physiol. 2011, 52, 651–662. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Fukushige, H.; Hildebrand, D.F.; Gan, S. Evidence Supporting a Role of Jasmonic Acid in Arabidopsis Leaf Senescence. Plant Physiol. 2002, 128, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Serova, T.A.; Tsyganova, A.V.; Tikhonovich, I.A.; Tsyganov, V.E. Gibberellins Inhibit Nodule Senescence and Stimulate Nodule Meristem Bifurcation in Pea (Pisum Sativum L.). Front. Plant Sci. 2019, 10, 285. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shumilina, J.; Soboleva, A.; Abakumov, E.; Shtark, O.Y.; Zhukov, V.A.; Frolov, A. Signaling in Legume–Rhizobia Symbiosis. Int. J. Mol. Sci. 2023, 24, 17397. https://doi.org/10.3390/ijms242417397
Shumilina J, Soboleva A, Abakumov E, Shtark OY, Zhukov VA, Frolov A. Signaling in Legume–Rhizobia Symbiosis. International Journal of Molecular Sciences. 2023; 24(24):17397. https://doi.org/10.3390/ijms242417397
Chicago/Turabian StyleShumilina, Julia, Alena Soboleva, Evgeny Abakumov, Oksana Y. Shtark, Vladimir A. Zhukov, and Andrej Frolov. 2023. "Signaling in Legume–Rhizobia Symbiosis" International Journal of Molecular Sciences 24, no. 24: 17397. https://doi.org/10.3390/ijms242417397
APA StyleShumilina, J., Soboleva, A., Abakumov, E., Shtark, O. Y., Zhukov, V. A., & Frolov, A. (2023). Signaling in Legume–Rhizobia Symbiosis. International Journal of Molecular Sciences, 24(24), 17397. https://doi.org/10.3390/ijms242417397







