Sex-Chromosome-Related Dimorphism in Steroidogenic Enzymes and Androgen Receptor in Response to Testosterone Treatment: An In Vitro Study on Human Primary Skeletal Muscle Cells
Abstract
:1. Introduction
2. Results
2.1. Sex-Chromosome-Related Dimorphism in Steroidogenic Enzyme Gene Expression
2.2. Sex-Chromosome-Related Dimorphism in Androgen Receptor Gene Expression
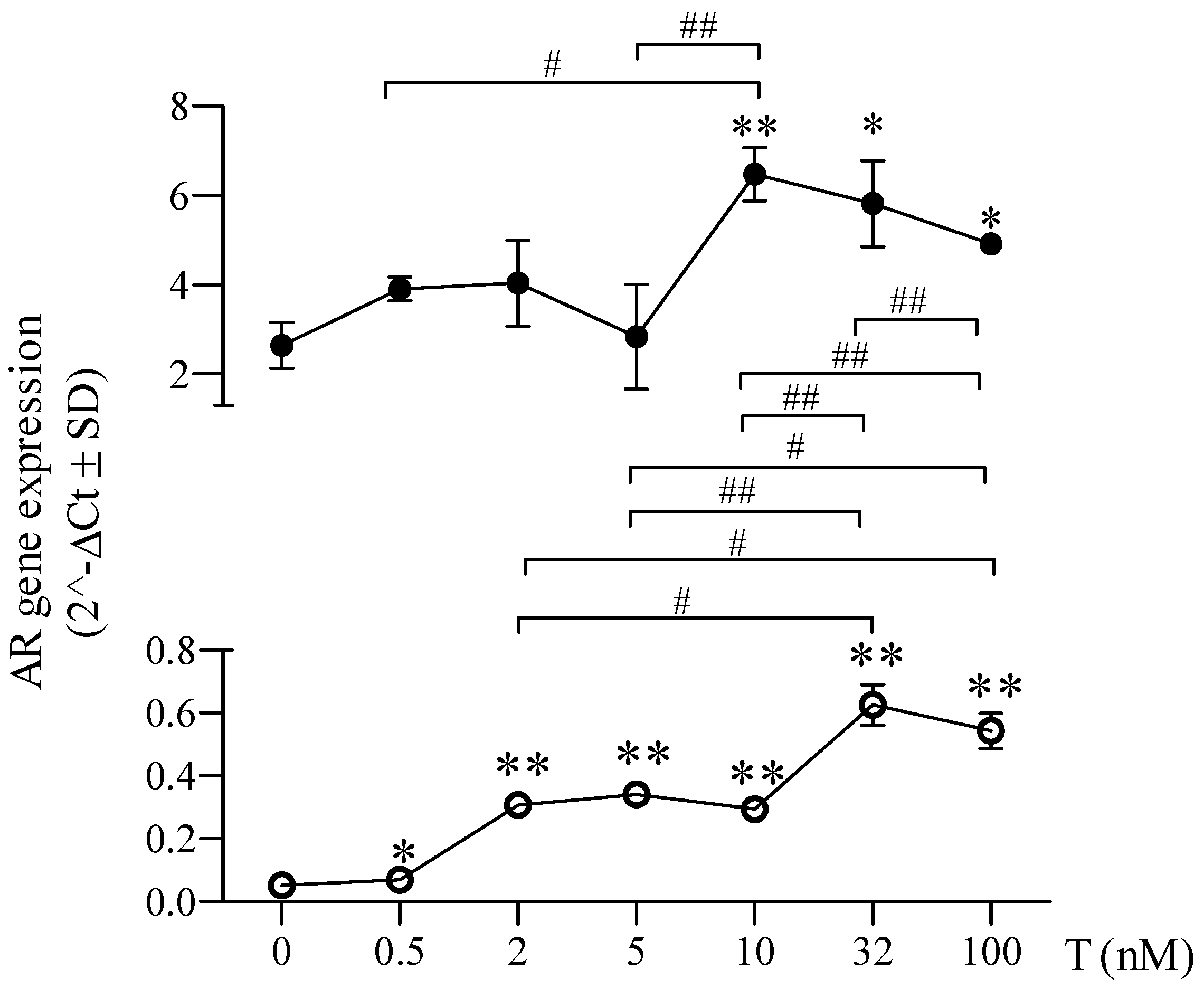
2.3. Sex-Chromosome-Related Dimorphism in Androgen Receptor Expression after Testosterone Treatment
2.4. Sex-Chromosome-Related Dimorphism in Androgen Receptor Cellular Localization after Testosterone Treatment
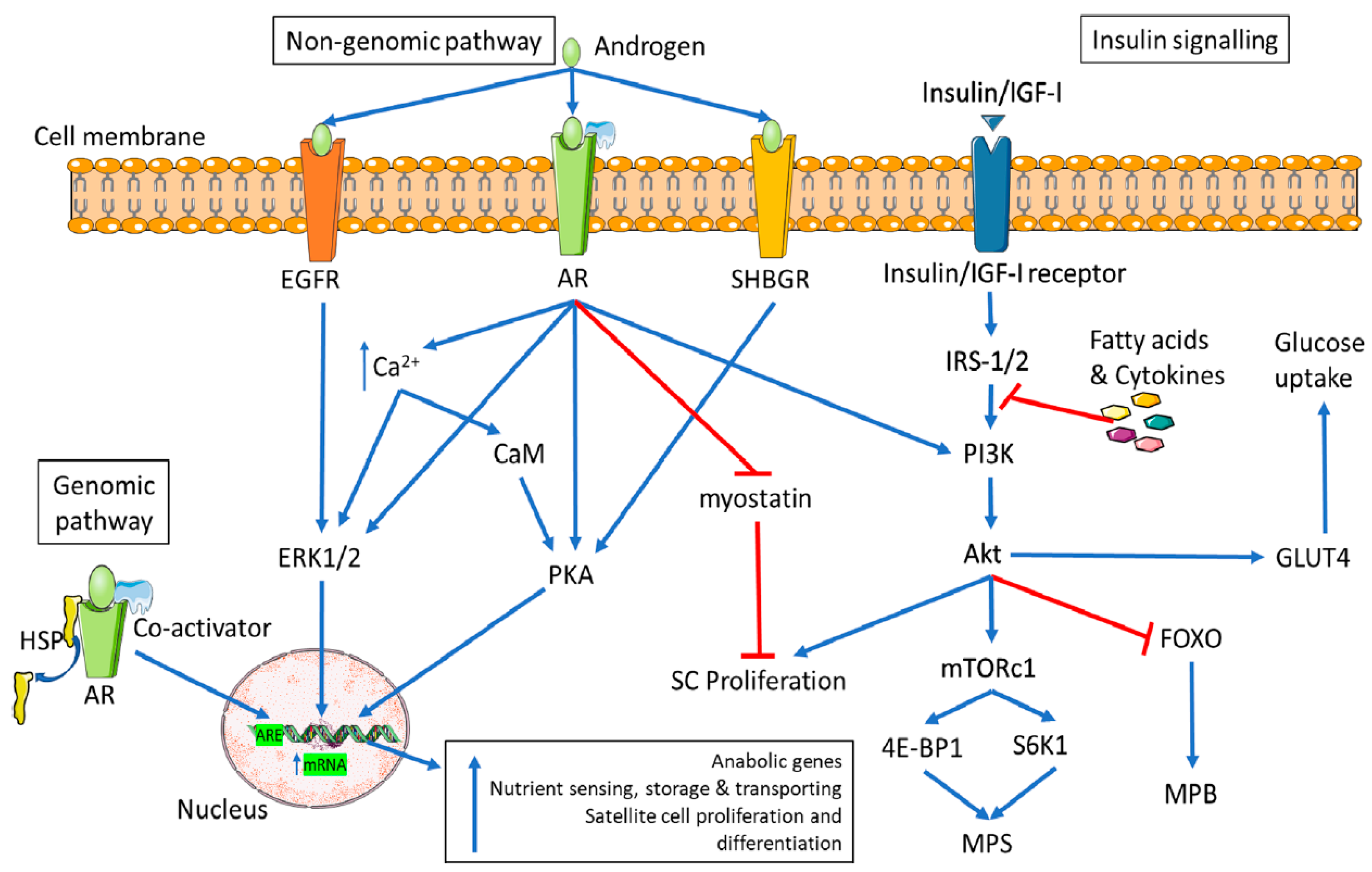
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Cell Treatments
4.3. Immunofluorescence Microscope
4.4. RNA Extraction, Reverse Transcription, and Real-Time Quantitative PCR
4.5. Protein Expression Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nkoke, C.; Jingi, A.M.; Noubiap, J.J.; Teuwafeu, D.; Nkouonlack, C.; Gobina, R.; Djibrilla, S.; Abas, A.; Dzudie, A. Gender differences in cardiovascular risk factors, clinical presentation, and outcome of patients admitted with a hypertensive crisis at the Buea regional hospital, Cameroon. Int. J. Hypertens. 2022, 28, 3062526. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Jiang, X.; Lian, X.; Chen, J.; Song, E.F.; Jin, L.G.; Xia, Z.Y.; Ma, H.C.; Cai, Y. Caloric restriction-mimetics for the reduction of heart failure risk in aging heart: With consideration of gender-related differences. Mil. Med. Res. 2022, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Regitz-Zagrosek, V. Sex and gender differences in health. Sci. Soc. Ser. Sex Sci. EMBO Rep. 2012, 29, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.A.; Arif, M.; Emanuelsson, E.B.; Reitzner, S.M.; Lindholm, M.E.; Mardinoglu, A.; Sundberg, C.J. Skeletal muscle transcriptomic comparison between long-term trained and untrained men and women. Cell Rep. 2020, 31, 154–196. [Google Scholar] [CrossRef] [PubMed]
- Haizlip, K.M.; Harrison, B.C.; Leinwand, L.A. Sex-based differences in skeletal muscle kinetics and fiber-type composition. Physiology 2015, 30, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Welle, S.; Tawil, R.; Thornton, C.A. Sex-related differences in gene expression in human skeletal muscle. PLoS ONE 2008, 2, e1385. [Google Scholar] [CrossRef] [PubMed]
- Antinozzi, C.; Marampon, F.; Corinaldesi, C.; Vicini, E.; Sgrò, P.; Vannelli, G.B.; Lenzi, A.; Crescioli, C.; Di Luigi, L. Testosterone insulin-like effects: An in vitro study on the short-term metabolic effects of testosterone in human skeletal muscle cells. J. Endocrinol. Invest. 2017, 40, 1133–1143. [Google Scholar] [CrossRef]
- Schiffer, L.; Arlt, W.; Storbeck, K.H. Intracrine androgen biosynthesis, metabolism and action revisited. Mol. Cell Endocrinol. 2018, 15, 4–26. [Google Scholar] [CrossRef]
- Lundsgaard, A.M.; Kiens, B. Gender differences in skeletal muscle substrate metabolism—Molecular mechanisms and insulin sensitivity. Front. Endocrinol. 2014, 5, 195. [Google Scholar] [CrossRef]
- Aizawa, K.; Iemitsu, M.; Otsuki, T.; Maeda, S.; Miyauchi, T.; Mesaki, N. Sex differences in steroidogenesis in skeletal muscle following a single bout of exercise in rats. J. Appl. Physiol. 2008, 104, 67–74. [Google Scholar] [CrossRef]
- Handelsman, D.J.; Hirschberg, A.L.; Bermon, S. Circulating testosterone as the hormonal basis of sex differences in athletic performance. Endocr. Rev. 2018, 1, 803–829. [Google Scholar] [CrossRef] [PubMed]
- Piper, T.; Heimbach, S.; Adamczewski, M.; Thevis, M. An in vitro assay approach to investigate the potential impact of different doping agents on the steroid profile. Drug Test. Anal. 2021, 13, 916–928. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.B.; Glintborg, D.; Nielsen, M.F.; Jakobsen, M.A.; Brusgaard, K.; Tan, Q.; Gaster, M. Testosterone treatment increases androgen receptor and aromatase gene expression in myotubes from patients with PCOS and controls but does not induce insulin resistance. Biochem. Biophys. Res. Commun. 2014, 5, 622–626. [Google Scholar] [CrossRef]
- Antinozzi, C.; Duranti, G.; Ceci, R.; Lista, M.; Sabatini, S.; Caporossi, D.; Di Luigi, L.; Sgrò, P.; Dimauro, I. Hydrogen peroxide stimulates dihydrotestosterone release in C2C12 myotubes: A new perspective for exercise-related muscle steroidogenesis? Int. J. Mol. Sci. 2022, 23, 6566. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, C.; Dufau, M.; Catt, K. Gonadotropin binding and stimulation of cyclic adenosine 3′:5′-monophosphate and testosterone production in isolated Leydig cells. J. Biol. Chem. 1975, 25, 8818–8823. [Google Scholar] [CrossRef]
- Sato, K.; Iemitsu, M.; Matsutani, K.; Kurihara, T.; Hamaoka, T.; Fujita, S. Resistance training restores muscle sex steroid hormone steroidogenesis in older men. FASEB J. 2014, 28, 1891–1897. [Google Scholar] [CrossRef]
- McCullough, D.; Webb, R.; Enright, K.J.; Stewart, E.C.; Davies, G.I. How the love of muscle can break a heart: Impact of anabolic androgenic steroids on skeletal muscle hypertrophy, metabolic and cardiovascular health. Rev. Endocr. Metab. Disord. 2021, 22, 389–405. [Google Scholar] [CrossRef]
- Hammes, S.R.; Levin, E.R. Impact of estrogens in males and androgens in females. J. Clin. Investig. 2019, 129, 1818–1826. [Google Scholar] [CrossRef]
- Romero-Moraleda, B.; Coso, J.D.; Gutiérrez-Hellín, J.; Ruiz-Moreno, C.; Grgic, J.; Lara, B. The influence of the menstrual cycle on muscle strength and power performance. J. Hum. Kinet. 2019, 68, 123–133. [Google Scholar] [CrossRef]
- Lynch, N.A.; Metter, E.J.; Lindle, R.S.; Fozard, J.L.; Tobin, J.D.; Roy, T.A.; Fleg, J.L.; Hurley, B.F. Muscle quality. I. Age-associated differences between arm and leg muscle groups. Appl. Physiol. 1999, 86, 188–194. [Google Scholar] [CrossRef]
- Mohamed, M.K.; Abdel-Rahman, A.A. Effect of long-term ovariectomy and estrogen replacement on the expression of estrogen receptor gene in female rats. Eur. J. Endocrinol. 2000, 142, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Vingren, J.L.; Kraemer, W.J.; Hatfield, D.L.; Anderson, J.M.; Volek, J.S.; Ratamess, N.A.; Thomas, G.A.; Ho, J.Y.; Fragala, M.S.; Maresh, C.M. Effect of resistance exercise on muscle steroidogenesis. J. Appl. Physiol. 2008, 105, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Vingren, J.L.; Kraemer, W.J.; Ratamess, N.A.; Anderson, J.M.; Volek, J.S.; Maresh, C.M. Testosterone physiology in resistance exercise and training: The up-stream regulatory elements. Sports Med. 2010, 40, 1037–1053. [Google Scholar] [CrossRef] [PubMed]
- Hackney, A.C. Hypogonadism in exercising males: Dysfunction or adaptive-regulatory adjustment? Front. Endocrinol. 2020, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Dhindsa, S.; Ghanim, H.; Saad, F. Mechanisms underlying the metabolic actions of testosterone in humans: A narrative review. Diabetes Obes. Metab. 2021, 23, 18–28. [Google Scholar] [CrossRef] [PubMed]
- MacLean, H.E.; Chiu, W.S.; Notini, A.J.; Axell, A.M.; Davey, R.A.; McManus, J.F.; Ma, C.; Plant, D.R.; Lynch, G.S.; Zajac, J.D. Impaired skeletal muscle development and function in male, but not female, genomic androgen receptor knockout mice. FASEB J. 2008, 22, 2676–2689. [Google Scholar] [CrossRef]
- Wyce, A.; Bai, Y.; Nagpal, S.; Thompson, C.C. Research Resource: The androgen receptor modulates expression of genes with critical roles in muscle development and function. Mol. Endocrinol. 2010, 24, 1665–1674. [Google Scholar] [CrossRef]
- Di Luigi, L.; Greco, E.A.; Fossati, C.; Aversa, A.; Sgrò, P.; Antinozzi, C. Clinical concerns on sex steroids variability in cisgender and transgender women athletes. Int. J. Sports Med. 2023, 44, 81–94. [Google Scholar] [CrossRef]
- Sikaris, K.; McLachlan, R.I.; Kazlauskas, R.; de Kretser, D.; Holden, C.A.; Handelsman, D.J. Reproductive hormone reference intervals for healthy fertile young men: Evaluation of automated platform assays. J. Clin. Endocrinol. Metab. 2005, 90, 5928–5936. [Google Scholar] [CrossRef]
- Clark, R.V.; Wald, J.A.; Swerdloff, R.S.; Wang, C.; Wu, F.C.W.; Bowers, L.D.; Matsumoto, A.M. Large divergence in testosterone concentrations between men and women: Frame of reference for elite athletes in sex-specific competition in sports, a narrative review. Clin. Endocrinol. 2019, 90, 15–22. [Google Scholar] [CrossRef]
- Davey, R.A.; Grossmann, M. Androgen receptor structure, function and biology: From bench to bedside. Clin. Biochem. 2016, 37, 3–15. [Google Scholar]
- Strauss, R.H.; Liggett, M.T.; Lanese, R.R. Anabolic steroid use and perceived effects in ten weight-trained women athletes. JAMA 1985, 253, 2871–2873. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, V.E.; Bresciani, E.; Meanti, R.; Rizzi, L.; Omeljaniuk, R.J.; Torsello, A. The role of androgens in women’s health and wellbeing. Pharmacol. Res. 2021, 171, 105758. [Google Scholar] [CrossRef] [PubMed]
- Börchers, S.; Krieger, J.P.; Maric, I.; Carl, J.; Abraham, M.; Longo, F.; Asker, M.; Richard, J.E.; Skibicka, K.P. From an empty stomach to anxiolysis: Molecular and behavioral assessment of sex differences in the ghrelin axis of rats. Front. Endocrinol. 2022, 16, 901669. [Google Scholar] [CrossRef] [PubMed]
- Pivtorak, K.; Fedzhaga, I.; Pivtorak, N.; Vozniuk, L.; Klekot, O. Fat and muscle components of body weight and their relationship with the concentration of serum adipokines in patients with nonalcoholic fatty liver disease. Wiad. Lek. 2022, 75, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, Q.; Shi, K.; Lu, Y.; Yu, D.; Shi, X.; Du, W.; Yu, M. Testosterone Promotes the Proliferation of Chicken Embryonic Myoblasts Via Androgen Receptor Mediated PI3K/Akt Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 1152. [Google Scholar] [CrossRef]
- MacLean, H.E.; Moore, A.J.; Sastra, S.A.; Morris, H.A.; Ghasem-Zadeh, A.; Rana, K.; Axell, A.M.; Notini, A.J.; Handelsman, D.J.; Seeman, E.; et al. DNA-binding-dependent androgen receptor signaling contributes to gender differences and has physiological actions in males and females. J. Endocrinol. 2010, 206, 93–103. [Google Scholar] [CrossRef]
- Paronetto, M.P.; Dimauro, I.; Grazioli, E.; Palombo, R.; Guidotti, F.; Fantini, C.; Sgrò, P.; De Francesco, D.; Di Luigi, L.; Capranica, L.; et al. Exercise-mediated downregulation of MALAT1 expression and implications in primary and secondary cancer prevention. Free Radic. Biol. Med. 2020, 160, 28–39. [Google Scholar] [CrossRef]
- Duranti, G.; Maldini, M.; Crognale, D.; Horner, K.; Dimauro, I.; Sabatini, S.; Ceci, R. Moringa Oleifera leaf extract upregulates NRF2/HO-1 expression and ameliorates redox status in C2C12 skeletal muscle cells. Molecules 2021, 26, 5041. [Google Scholar] [CrossRef]





| Gene Name | Forward 5′–3′ | Reverse 5′–3′ |
|---|---|---|
| 5α-R2 | AGTGGAGGGCATGGTGCTAA | TCTCTCACTTAGCACGGGGA |
| 17β-HSD | TTTGCGCTCGAAGGTTTGTG | GCAGTCAAGAAGAGCTCCGT |
| AR | TACCAGCTCACCAAGCTCCT | GATGGGCTTGACTTTCCCAG |
| CYP-19 | ATGTTTCTGGAAATGCTGAAC | CTGTTTCAGATATTTTTCGCTG |
| β-actin | AAC CTGAACCCCAAGGCC | AGCCTGGATAGCAACGTACA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Luigi, L.; Antinozzi, C.; Duranti, G.; Dimauro, I.; Sgrò, P. Sex-Chromosome-Related Dimorphism in Steroidogenic Enzymes and Androgen Receptor in Response to Testosterone Treatment: An In Vitro Study on Human Primary Skeletal Muscle Cells. Int. J. Mol. Sci. 2023, 24, 17382. https://doi.org/10.3390/ijms242417382
Di Luigi L, Antinozzi C, Duranti G, Dimauro I, Sgrò P. Sex-Chromosome-Related Dimorphism in Steroidogenic Enzymes and Androgen Receptor in Response to Testosterone Treatment: An In Vitro Study on Human Primary Skeletal Muscle Cells. International Journal of Molecular Sciences. 2023; 24(24):17382. https://doi.org/10.3390/ijms242417382
Chicago/Turabian StyleDi Luigi, Luigi, Cristina Antinozzi, Guglielmo Duranti, Ivan Dimauro, and Paolo Sgrò. 2023. "Sex-Chromosome-Related Dimorphism in Steroidogenic Enzymes and Androgen Receptor in Response to Testosterone Treatment: An In Vitro Study on Human Primary Skeletal Muscle Cells" International Journal of Molecular Sciences 24, no. 24: 17382. https://doi.org/10.3390/ijms242417382
APA StyleDi Luigi, L., Antinozzi, C., Duranti, G., Dimauro, I., & Sgrò, P. (2023). Sex-Chromosome-Related Dimorphism in Steroidogenic Enzymes and Androgen Receptor in Response to Testosterone Treatment: An In Vitro Study on Human Primary Skeletal Muscle Cells. International Journal of Molecular Sciences, 24(24), 17382. https://doi.org/10.3390/ijms242417382









