Response of Carrot (Daucus carota L.) to Multi-Contaminated Soil from Historic Mining and Smelting Activities
Abstract
:1. Introduction
2. Results
2.1. Cd, Pb and Zn Content in Carrots
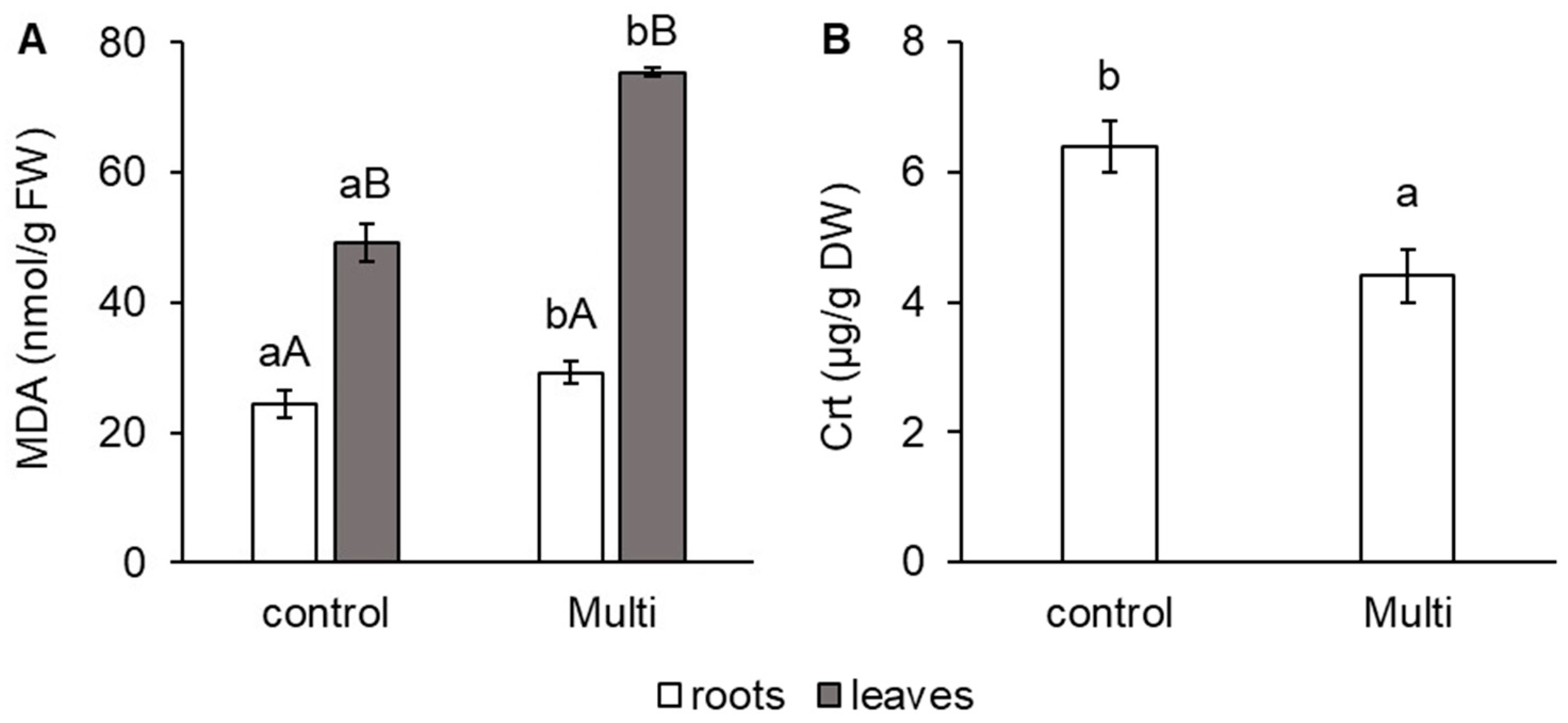
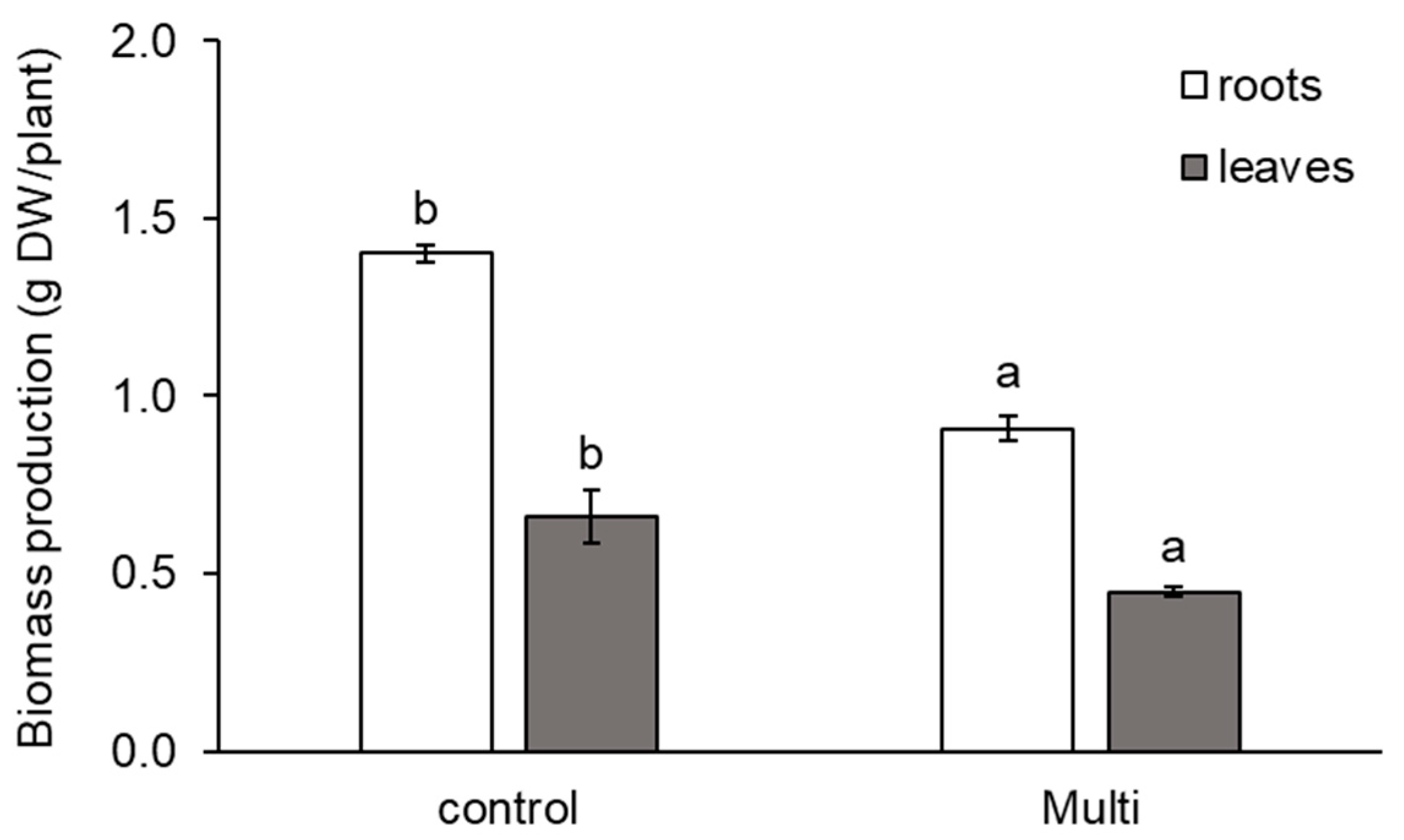
2.2. Malondialdehyde and Carotenoid Content and Biomass Production in Carrots
2.3. Change in Gas-Exchange Parameters, Chlorophyll Fluorescence and Photosynthetic Pigments
2.4. Change in Free Amino Acid Content
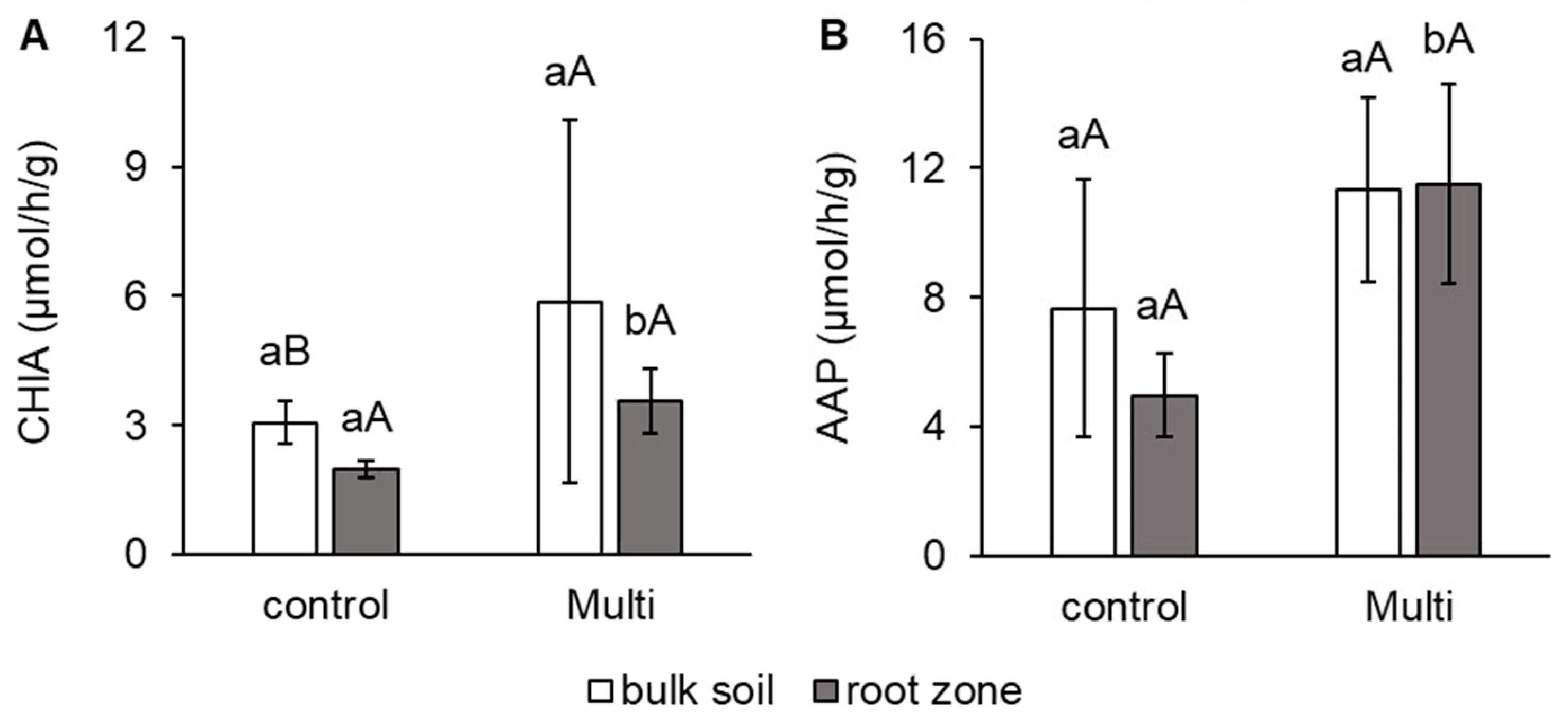
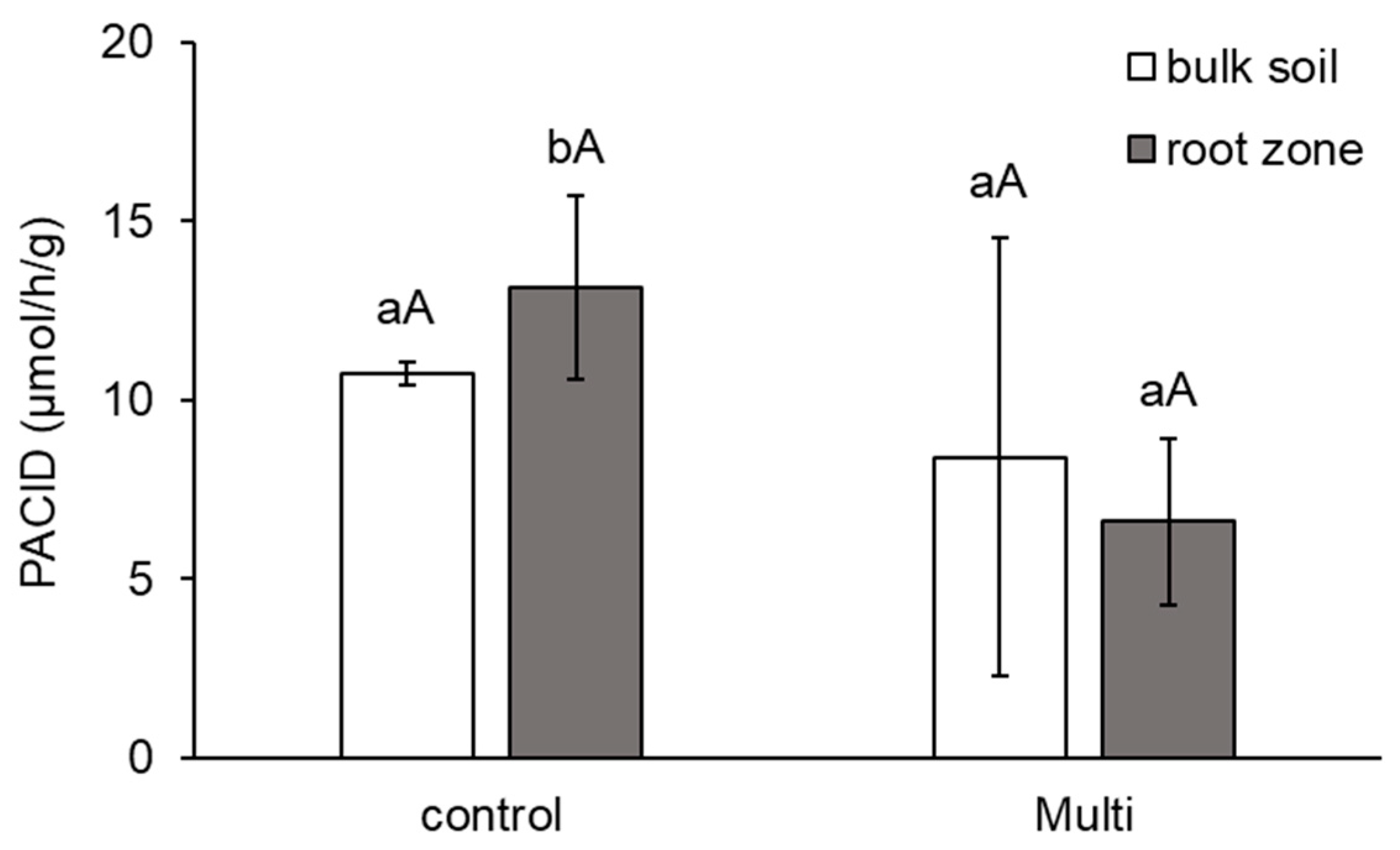
2.5. Morphological and Anatomical Conditions of Carrot Roots and Changes in Enzyme Activities in the Root Zone
3. Discussion
3.1. Accumulation and Translocation of Cd, Pb and Zn in Carrots
3.2. Response of Malondialdehyde and Carotenoids to Cd, Pb and Zn and Biomass Production of Carrot
3.3. Response of Gas-Exchange Parameters and Photosynthetic Pigments to Cd, Pb and Zn
3.4. Response of Free Amino Acids to Cd, Pb and Zn
3.5. Morphological and Anatomical Conditions of Roots and Changes in Enzyme Activities under Cd, Pb and Zn Stress
4. Materials and Methods
4.1. Soil Sampling and Characterisation
4.2. Experimental Design and Plant Material
4.3. Determination of Toxic Elements
4.4. Determination of Malondialdehyde Content
4.5. Determination of Carotenoid and Chlorophyll Content
4.6. Determination of Gas-Exchange Parameters and Chlorophyll Fluorescence
4.7. Determination of Free Amino Acids
4.8. Evaluation of Morphology and Anatomy of Carrot Roots
4.9. Determination of Soil Enzyme Activity
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Özcan, M.M.; Yılmaz, F.G.; Kulluk, D.A. The accumulation of element and heavy metal concentrations in different parts of some carrot and radish types. Environ. Monit. Assess. 2023, 195, 754. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Ansari, T.M. An assessment of toxic heavy metals in soil and plants (Allium cepa and Daucus carota) by GFAAS. Int. J. Environ. Anal. Chem. 2022, 102, 1029–1048. [Google Scholar] [CrossRef]
- Hou, S.; Zheng, N.; Tang, L.; Ji, X. Effects of cadmium and copper mixtures to carrot and pakchoi under greenhouse cultivation condition. Ecotoxicol. Environ. Saf. 2018, 159, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Pietrelli, L.; Menegoni, P.; Papetti, P. Bioaccumulation of heavy metals by herbaceous species grown in urban and rural sites. Ecotoxicol. Environ. Saf. 2022, 233, 141. [Google Scholar] [CrossRef]
- Kebonye, N.M.; Eze, P.N.; John, K.; Agyeman, P.C.; Němeček, K.; Borůvka, L. An in-depth human health risk assessment of potentially toxic elements in highly polluted riverine soils, Příbram (Czech Republic). Environ. Geochem. Health 2021, 44, 369–385. [Google Scholar] [CrossRef]
- Gooday, G.W. Physiology and microbial degradation of chitin and chitosan. In Biochemstry of Microbial Degradation; Ratledge, C., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; pp. 279–312. [Google Scholar]
- Gao, L.; Smith, A.R.; Jones, D.L.; Guo, Y.; Liu, B.; Guo, Z.; Fan, C.H.; Zheng, J.; Cui, X.; Hill, P.W. How do tree species with different successional stages affect soil organic nitrogen transformations? Geoderma 2023, 430, 116319. [Google Scholar] [CrossRef]
- Chowdhury, N.; Rasid, M.M. Heavy metal concentrations and its impact on soil microbial and enzyme activities in agricultural lands around ship yards in Chattogram, Bangladesh. Soil Sci. Annu. 2021, 72, 135994. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, X.; Wang, X.; Zhang, X.; Fang, L. Evaluation methods of heavy metal pollution in soils based on enzyme activities: A review. Soil Ecol. Lett. 2021, 3, 169–177. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Lewu, F.B.; Mulidzi, R.; Ncube, B. The biological activities of β-glucosidase, phosphatase and urease as soil quality indicators: A review. J. Soil Sci. Plant Nutr. 2017, 17, 794–807. [Google Scholar] [CrossRef]
- Gupta, N.; Yadav, K.K.; Kumar, V.; Kumar, S.; Chadd, R.P.; Kumar, A. Trace elements in soil-vegetables interface: Translocation, bioaccumulation, toxicity and amelioration—A review. Sci. Total Environ. 2019, 651, 2927–2942. [Google Scholar] [CrossRef]
- Caracciolo, B.A.; Terenzi, V. Rhizosphere microbial communities and heavy metals. Microorganisms 2021, 9, 1462. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, B.; Bolan, N.S.; Naidu, R. Rhizosphere-induced heavy metal(loid) transformation in relation to bioavailability and remediation. J. Soil Sci. Plant Nutr. 2015, 15, 524–548. [Google Scholar] [CrossRef]
- Bidar, G.; Pelfrêne, A.; Schwartz, C.H.; Waterlot, C.H.; Sahmer, K.; Marot, F.; Douay, F. Urban kitchen gardens: Effect of the soil contamination and parameters on the trace element accumulation in vegetables—A review. Sci. Total Environ. 2020, 738, 139569. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ju, Y.; Mandzhieva, S.; Pinskii, D.; Minkina, T.; Rajput, V.D.; Roane, T.; Huang, S.; Li, Y.; Ma, L.Q.; et al. Sporadic Pb accumulation by plants: Influence of soil biogeochemistry, microbial community and physiological mechanisms. J. Hazard. Mater. 2023, 444, 130391. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S.; Ma, J.F. Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu. Rev. Plant Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef] [PubMed]
- Asati, A.; Pichhode, M.; Nikhil, K. Effect of heavy metals on plants: An overview. Int. J. Appl. Innov. Eng. Manag. 2016, 5, 56–66. [Google Scholar]
- Viehweger, K. How plants cope with heavy metals. Bot. Stud. 2014, 55, 35. [Google Scholar] [CrossRef] [PubMed]
- Pavlíková, D.; Zemanová, V.; Procházková, D.; Pavlík, M.; Száková, J.; Wilhelmová, N. The long-term effect of zinc soil contamination on selected free amino acids playing an important role in plant adaptation to stress and senescence. Ecotoxicol. Environ. Saf. 2014, 100, 166–170. [Google Scholar] [CrossRef]
- Thakur, S.; Singh, L.; Wahid, Z.A.; Siddiqui, M.F.; Atnaw, S.M.; Din, M.F.M. Plant-driven removal of heavy metals from soil: Uptake, translocation, tolerance mechanism, challenges, and future perspectives. Environ. Monit. Assess. 2016, 188, 206. [Google Scholar] [CrossRef]
- Ovečka, M.; Takáč, T. Managing heavy metal toxicity stress in plants: Biological and biotechnological tools. Biotechnol. Adv. 2014, 32, 73–86. [Google Scholar] [CrossRef]
- Gao, Y.; Li, H.; Song, Y.; Zhang, F.; Yang, Z.; Yang, Y.; Grohmann, T. Response of glutathione pools to cadmium stress and the strategy to translocate cadmium from roots to leaves (Daucus carota L.). Sci. Total Environ. 2022, 823, 153575. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Khalid, S.; Abbas, G.; Shahid, N.; Nadeem, M.; Sabir, M.; Aslam, M.; Dumat, C. Heavy metal stress and crop productivity. In Crop Production and Global Environmental Issues; Hakeem, K.R., Ed.; Springer: Cham, Switzerland, 2015; pp. 1–25. [Google Scholar] [CrossRef]
- Ghori, N.-H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Gill, M. Heavy metal stress in plants: A review. Int. J. Adv. Res. 2014, 2, 1043–1055. [Google Scholar]
- Huang, X.; Duan, S.; Wu, Q.; Yu, M.; Shabala, S. Reducing Cadmium accumulation in plants: Structure–function relations and tissue-specific operation of transporters in the spotlight. Plants 2020, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, W.; Wang, M.; Li, Y.; Peng, C. Evaluating the potential health risk of toxic trace elements in vegetables: Accounting for variations in soil factors. Sci. Total Environ. 2017, 584, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, U.; Jiang, W.; Xiukang, W.; Hussain, S.; Ahmad, M.; Maqsood, M.F.; Ali, N.; Ishfaq, M.; Kaleem, M.; Haider, F.U.; et al. Cadmium phytotoxicity, tolerance, and advanced remediation approaches in agricultural soils; A comprehensive review. Front. Plant Sci. 2022, 13, 773815. [Google Scholar] [CrossRef]
- Baruah, N.; Gogoi, N.; Roy, S.; Bora, P.; Chetia, J.; Zahra, N.; Ali, N.; Gogoi, P.; Farooq, M. Phytotoxic responses and plant tolerance mechanisms to cadmium toxicity. J. Soil Sci. Plant Nutr. 2023, 1–22. [Google Scholar] [CrossRef]
- Hamzah Saleem, M.; Usman, K.; Rizwan, M.; Al Jabri, H.; Alsafran, M. Functions and strategies for enhancing zinc availability in plants for sustainable agriculture. Front. Plant Sci. 2022, 13, 1033092. [Google Scholar] [CrossRef]
- Kaur, H.; Garg, N. Zinc toxicity in plants: A review. Planta 2021, 253, 129. [Google Scholar] [CrossRef]
- Natasha, N.; Shahid, M.; Bibi, I.; Iqbal, J.; Khalid, S.; Murtaza, B.; Bakhat, H.F.; Farooq, A.B.U.; Amjad, M.; Hammad, H.M.; et al. Zinc in soil-plant-human system: A data-analysis review. Sci. Total Environ. 2022, 808, 152024. [Google Scholar] [CrossRef]
- Goodarzi, A.; Namdjoyan, S.; Soorki, A.A. Effects of exogenous melatonin and glutathione on zinc toxicity in safflower (Carthamus tinctorius L.) seedlings. Ecotoxicol. Environ. Saf. 2020, 201, 110853. [Google Scholar] [CrossRef]
- Fahr, M.; Laplaze, L.; Bendaou, N.; Hocher, V.; El Mzibri, M.; Bogusz, D.; Smouni, A. Effect of lead on root growth. Front. Plant Sci. 2013, 4, 175. [Google Scholar] [CrossRef]
- Ghani, M.A.; Abbas, M.M.; Ali, B.; Aziz, R.; Qadri, R.W.K.; Noor, A.; Azam, M.; Bahzad, S.; Saleem, M.H.; Abualreesh, M.H.; et al. Alleviating role of gibberellic acid in enhancing plant growth and stimulating phenolic compounds in carrot (Daucus carota L.) under lead stress. Sustainability 2021, 13, 12329. [Google Scholar] [CrossRef]
- Collin, S.; Baskar, A.; Geevarghese, D.M.; Ali, M.N.V.S.; Bahubali, P.; Choudhary, R.; Lvov, V.; Tovar, G.I.; Senatov, F.; Koppala, S.; et al. Bioaccumulation of lead (Pb) and its effects in plants: A review. J. Hazard. Mater. 2022, 3, 100064. [Google Scholar] [CrossRef]
- Dogan, M.; Karatas, M.; Aasim, M. Cadmium and lead bioaccumulation potentials of an aquatic macrophyte Ceratophyllum demersum L.: A laboratory study. Ecotoxicol. Environ. Saf. 2018, 148, 431–440. [Google Scholar] [CrossRef]
- Khan, A.; Khan, S.; Khan, M.A.; Qamar, Z.; Waqas, M. The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: A review. Environ. Sci. Pollut. Res. 2015, 22, 13772–13799. [Google Scholar] [CrossRef]
- Guo, Z.; Gao, Y.; Yuan, X.; Yuan, M.; Huang, L.; Wang, S.; Liu, C.; Duan, C. Effects of heavy metals on stomata in plants: A review. Int. J. Mol. Sci. 2023, 24, 9302. [Google Scholar] [CrossRef]
- Que, F.; Hou, X.-L.; Wang, G.-L.; Xu, Z.-S.; Tan, G.-F.; Li, T.; Wang, Y.-H.; Khadr, A.; Xiong, A.-S. Advances in research on the carrot, an important root vegetable in the Apiaceae family. Hortic. Res. 2019, 6, 69. [Google Scholar] [CrossRef]
- Knez, E.; Kadac-Czapska, K.; Dmochowska-Ślęzak, K.; Grembecka, M. Root vegetables—Composition, health effects, and contaminants. Int. J. Environ. Res. Public Health 2022, 19, 15531. [Google Scholar] [CrossRef]
- do Sousa Lima, F.; do Nascimento, C.W.A.; da Silva Sousa, C. Lead and nutrient allocation in vegetables grown in soil from a battery site. Semin. Cienc. Agrar. 2015, 36, 2483–2491. [Google Scholar] [CrossRef]
- Roy, M.; McDonald, L.M. Metal uptake in plants and health risk assessments in metal-contaminated smelter soils. Land Degrad. Dev. 2015, 26, 785–792. [Google Scholar] [CrossRef]
- Stančić, Z.; Vujević, D.; Gomaz, A.; Bogdan, S.; Vincek, D. Detection of heavy metals in common vegetables at Varaždin city market, Croatia. Arch. Ind. Hyg. Toxicol. 2016, 67, 340–350. [Google Scholar] [CrossRef]
- Andrejić, G.; Gajić, G.; Prica, M.; Dželetović, Ž.; Rakić, T. Zinc accumulation, photosynthetic gas exchange, and chlorophyll a fluorescence in Zn-stressed Miscanthus × giganteus plants. Photosynthetica 2018, 56, 1249–1258. [Google Scholar] [CrossRef]
- Nedelescu, M.; Baconi, D.; Neagoe, A.; Iordache, V.; Stan, M.; Constantinescu, P.; Ciobanu, A.M.; Vardavas, A.I.; Vinceti, M.; Tsatsakis, A.M. Environmental metal contamination and health impact assessment in two industrial regions of Romania. Sci. Total Environ. 2017, 580, 984–995. [Google Scholar] [CrossRef]
- Bakhshayesh, B.E.; Delkash, M.; Scholz, M. Response of vegetables to cadmium-enriched soil. Water 2014, 6, 1246–1256. [Google Scholar] [CrossRef]
- Yang, D.; Guo, Z.; Green, I.D.; Xie, D. Effect of cadmium accumulation on mineral nutrient levels in vegetable crops: Potential implications for human health. Environ. Sci. Pollut. Res. 2016, 23, 19744–19753. [Google Scholar] [CrossRef]
- Basu, A.; Mazumdar, I.; Goswami, K. Concentrations of lead in selected vegetables grown & marketed along major highway in Kolkata (India). IIOAB J. 2013, 4, 32. [Google Scholar]
- Lizarazo, M.F.; Herrera, C.D.; Celis, C.A.; Pombo, L.M.; Teherán, A.A.; Piñeros, L.G.; Rodríguez, O.E. Contamination of staple crops by heavy metals in Sibaté, Colombia. Heliyon 2020, 6, e04212. [Google Scholar] [CrossRef]
- O’Lexy, R.; Kasai, K.; Clark, N.; Fujiwara, T.; Sozzani, R.; Gallagher, K.L. Exposure to heavy metal stress triggers changes in plasmodesmatal permeability via deposition and breakdown of callose. J. Exp. Bot. 2018, 69, 3715–3728. [Google Scholar] [CrossRef]
- Massaccesi, L.; Meneghini, C.; Comaschi, T.; D’Amato, R.; Onofri, A.; Businelli, D. Ligands involved in Pb immobilization and transport in lettuce, radish, tomato and Italian ryegrass. J. Plant Nutr. Soil Sci. 2014, 177, 766–774. [Google Scholar] [CrossRef]
- Orroño, D.I.; Schindler, V.; Lavado, R.S. Heavy metal availability in Pelargonium hortorum rhizosphere: Interactions, uptake and plant accumulation. J. Plant Nutr. 2012, 35, 1374–1386. [Google Scholar] [CrossRef]
- Tran, L.T.T.; Luan, L.V.; Hieu, T.Q.; Van Tan, L. Study on the effect of Cu (II) and Zn (II) on the accumulation of Pb (II) from soil to the biomass of vegetable. Int. J. Agron. 2021, 2021, 6687566. [Google Scholar] [CrossRef]
- Woźniak, A.; Bednarski, W.; Dancewicz, K.; Gabryś, B.; Borowiak-Sobkowiak, B.; Bocianowski, J.; Samardakiewicz, S.; Rucińska-Sobkowiak, R.; Morkunas, I. Oxidative stress links response to lead and Acyrthosiphon pisum in Pisum sativum L. J. Plant Physiol. 2019, 240, 152996. [Google Scholar] [CrossRef]
- Tirani, M.M.; Haghjou, M.M. Reactive oxygen species (ROS), total antioxidant capacity (AOC) and malondialdehyde (MDA) make a triangle in evaluation of zinc stress extension. J. Anim. Plant Sci. 2019, 29, 1100–1111. [Google Scholar]
- Hafizi, Z.; Nasr, N. The effect of zinc oxide nanoparticles on safflower plant growth and physiology. Eng. Technol. Appl. Sci. Res. 2018, 8, 2508–2513. [Google Scholar] [CrossRef]
- Zacchini, M.; Rea, M.; Tullio, M.; de Agazio, M. Increased antioxidative capacity in maize calli during and after oxidative stress induced by a long lead treatment. Plant Physiol. Biochem. 2003, 41, 49–54. [Google Scholar] [CrossRef]
- Reddy, A.M.; Kumar, S.G.; Jyothsnakumari, G.; Thimmanaik, S.; Sudhakar, C. Lead induced changes in antioxidant metabolism of horsegram (Macrotyloma uniflorum (Lam.) Verdc.) and bengalgram (Cicer arietinum L.). Chemosphere 2005, 60, 97–104. [Google Scholar] [CrossRef]
- Bharwana, S.A.; Ali, S.; Farooq, M.A.; Iqbal, N.; Abbas, F.; Ahmad, M.S.A. Alleviation of lead toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes suppressed lead uptake and oxidative stress in cotton. J. Bioremediation Biodegrad. 2013, 4, 187. [Google Scholar] [CrossRef]
- An, Q.; He, X.; Zheng, N.; Hou, S.; Sun, S.; Wang, S.; Penyang, L.; Xiaoqian, L.; Song, X. Physiological and genetic effects of cadmium and copper mixtures on carrot under greenhouse cultivation. Ecotoxicol. Environ. Saf. 2020, 206, 111363. [Google Scholar] [CrossRef]
- Lanier, C.; Bernard, F.; Dumez, S.; Leclercq-Dransart, J.; Lemiere, S.; Vandenbulcke, F.; Nesslany, F.; Platel, A.; Devred, I.; Hayet, A.; et al. Combined toxic effects and DNA damage to two plant species exposed to binary metal mixtures (Cd/Pb). Ecotoxicol. Environ. Saf. 2019, 167, 278–287. [Google Scholar] [CrossRef]
- Rizvi, A.; Khan, M.S. Heavy metal-induced oxidative damage and root morphology alterations of maize (Zea mays L.) plants and stress mitigation by metal tolerant nitrogen-fixing Azotobacter chroococcum. Ecotoxicol. Environ. Saf. 2018, 157, 9–20. [Google Scholar] [CrossRef]
- Rosas-Saavedra, C.; Quiroz, L.F.; Parra, S.; Gonzalez-Calquin, C.; Arias, D.; Ocarez, N.; Lopez, F.; Stange, C. Putative Daucus carota capsanthin-capsorubin synthase (DcCCS) possesses lycopene β-cyclase activity, boosts carotenoid levels, and increases salt tolerance in heterologous plants. Plants 2023, 12, 2788. [Google Scholar] [CrossRef] [PubMed]
- Faiz, S.; Yasin, N.A.; Khan, W.U.; Shah, A.A.; Akram, W.; Ahmad, A.; Ali, A.; Naveed, N.H.; Riaz, L. Role of magnesium oxide nanoparticles in the mitigation of lead-induced stress in Daucus carota: Modulation in polyamines and antioxidant enzymes. Int. J. Phytoremediation 2021, 24, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Agrawal, M.; Agrawal, S.B. Physiological and biochemical responses resulting from cadmium and zinc accumulation in carrot plants. J. Plant Nutr. 2010, 33, 1066–1079. [Google Scholar] [CrossRef]
- Majeed, A.; Muhammad, Z.; Siyar, S. Assessment of heavy metal induced stress responses in pea (Pisum sativum L.). Acta Ecol. Sin. 2019, 39, 284–288. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 2015, 756120. [Google Scholar] [CrossRef]
- Faseela, P.; Sinisha, A.K.; Brestic, M.; Puthur, J.T. Chlorophyll a fluorescence parameters as indicators of a particular abiotic stress in rice. Photosynthetica 2020, 58, 293–300. [Google Scholar] [CrossRef]
- Ou, C.; Cheng, W.; Wang, Z.; Yao, X.; Yang, S. Exogenous melatonin enhances Cd stress tolerance in Platycladus orientalis seedlings by improving mineral nutrient uptake and oxidative stress. Ecotoxicol. Environ. Saf. 2023, 252, 114619. [Google Scholar] [CrossRef]
- Lhotská, M.; Zemanová, V.; Pavlíková, D.; Hnilička, F. Changes in the photosynthetic response of lettuce exposed to toxic element multicontamination under hydroponic conditions. Photosynthetica 2023, 61, 390–397. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, J.; Chen, L. Growth and physiological responses of Pennisetum sp. to cadmium stress under three different soils. Environ. Sci. Pollut. Res. 2021, 28, 14867–14881. [Google Scholar] [CrossRef]
- Shafi, M.; Bakht, J.; Razuddin, H.Y.; Zhang, G.P. Genotypic difference in the inhibition of photosynthesis and chlorophyll fluorescence by salinity and cadmium stresses in wheat. J. Plant Nutr. 2011, 34, 315–323. [Google Scholar] [CrossRef]
- Bernardini, A.; Salvatori, E.; Guerrini, V.; Fusaro, L.; Canepari, S.; Manes, F. Effects of high Zn and Pb concentrations on Phragmites australis (Cav.) Trin. Ex. Steudel: Photosynthetic performance and metal accumulation capacity under controlled conditions. Int. J. Phytoremediation 2016, 18, 16–24. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, L.; Huang, X.; Zhou, Y.; Quan, Q.; Li, Y.; Zhu, X. Response of photosynthesis to different concentrations of heavy metals in Davidia involucrata. PLoS ONE 2020, 15, e0228563. [Google Scholar] [CrossRef]
- He, J.; Ren, Y. Effects of cadmium on seedling growth and photosynthesis characteristics of lettuce (Lactuca sativa L.). Southwest China J. Agric. Sci. 2009, 22, 922–926. [Google Scholar]
- Cheng, S.; Tam, N.F.Y.; Li, R.; Shen, X.; Niu, Z.; Chai, M.; Qiu, G.Y. Temporal variations in physiological responses of Kandelia obovata seedlings exposed to multiple heavy metals. Mar. Pollut. Bull. 2017, 124, 1089–1095. [Google Scholar] [CrossRef]
- Liang, L.; Li, X.; Li, H.; Peng, X.; Zhang, R.; Tang, W.; Dong, Y.; Tang, Y. Intercropping affects the physiology and cadmium absorption of pakchoi, lettuce, and radish seedlings. Environ. Sci. Pollut. Res. 2023, 30, 4744–4753. [Google Scholar] [CrossRef]
- Chandra, R.; Kang, H. Mixed heavy metal stress on photosynthesis, transpiration rate, and chlorophyll content in poplar hybrids. For. Sci. Technol. 2016, 12, 55–61. [Google Scholar] [CrossRef]
- Alamer, K.H.; Galal, T.M. Safety assessment and sustainability of consuming eggplant (Solanum melongena L.) grown in wastewater-contaminated agricultural soils. Sci. Rep. 2022, 12, 9768. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Xu, Z.; Wang, Y.; Teng, Z.; An, M.; Zhang, Y.; Zhu, W.; Xu, N.; Sun, G. Toxic effects of heavy metals Pb and Cd on mulberry (Morus alba L.) seedling leaves: Photosynthetic function and reactive oxygen species (ROS) metabolism responses. Ecotoxicol. Environ. Saf. 2020, 195, 110469. [Google Scholar] [CrossRef]
- Dahlawi, S.; Sadiq, M.; Sabir, M.; Farooqi, Z.U.R.; Saifullah; Qadir, A.A.; Faraj, T.K. Differential response of Brassica cultivars to potentially toxic elements and their distribution in different plant parts irrigated with metal-contaminated water. Sustainability 2023, 15, 1966. [Google Scholar] [CrossRef]
- Zemanová, V.; Pavlík, M.; Pavlíková, D. Cadmium toxicity induced contrasting patterns of concentrations of free sarcosine, specific amino acids and selected microelements in two Noccaea species. PLoS ONE 2017, 12, e0177963. [Google Scholar] [CrossRef]
- Xu, Q.; Qiu, W.; Lin, T.; Yang, Y.; Jiang, Y. Cadmium tolerance in Elodea canadensis Michx: Subcellular distribution and metabolomic analysis. Ecotoxicol. Environ. Saf. 2023, 256, 114905. [Google Scholar] [CrossRef]
- Okunev, R.V. Free amino acid accumulation in soil and tomato plants (Solanum lycopersicum L.) associated with arsenic stress. Water Air Soil Pollut. 2019, 230, 253. [Google Scholar] [CrossRef]
- Pavlíková, D.; Zemanová, V.; Pavlík, M. Health risk and quality assessment of vegetables cultivated on soils from a heavily polluted old mining area. Toxics 2023, 11, 583. [Google Scholar] [CrossRef]
- Zhu, G.; Xiao, H.; Guo, Q.; Zhang, Z.; Zhao, J.; Yang, D. Effects of cadmium stress on growth and amino acid metabolism in two Compositae plants. Ecotoxicol. Environ. Saf. 2018, 158, 300–308. [Google Scholar] [CrossRef]
- Zemanová, V.; Pavlík, M.; Pavlíková, D.; Tlustoš, P. The changes of contents of selected free amino acids associated with cadmium stress in Noccaea caerulescens and Arabidopsis halleri. Plant Soil Environ. 2013, 59, 417–422. [Google Scholar] [CrossRef]
- Wan, S.J.; Si, H.R.; Wang, X.Z.; Chao, L.; Ma, W.; Sun, S.S.; Tang, B.; Tan, X.L.; Wang, S. Regulation of Vicia faba L. response and its effect on Megoura crassicauda reproduction under zinc stress. Int. J. Mol. Sci. 2023, 24, 9659. [Google Scholar] [CrossRef]
- Pavlíková, D.; Zemanová, V.; Pavlík, M. The contents of free amino acids and elements in As-hyperaccumulator Pteris cretica and non-hyperaccumulator Pteris straminea during reversible senescence. Plant Soil Environ. 2017, 63, 455–460. [Google Scholar] [CrossRef]
- Krämer, U.; Cotter-Howells, J.D.; Charnock, J.M.; Baker, A.J.; Smith, J.A.C. Free histidine as a metal chelator in plants that accumulate nickel. Nature 1996, 379, 635–638. [Google Scholar] [CrossRef]
- Pavlíková, D.; Pavlík, M.; Staszková, L.; Motyka, V.; Száková, J.; Tlustoš, P.; Balík, J. Glutamate kinase as a potential biomarker of heavy metal stress in plants. Ecotoxicol. Environ. Saf. 2008, 70, 223–230. [Google Scholar] [CrossRef]
- Majumdar, R.; Barchi, B.; Turlapati, S.A.; Gagne, M.; Minocha, R.; Long, S.; Minocha, S.C. Glutamate, ornithine, arginine, proline, and polyamine metabolic interactions: The pathway is regulated at the post-transcriptional level. Front. Plant Sci. 2016, 7, 78. [Google Scholar] [CrossRef]
- Deng, L.; Yang, X.; Qiu, Y.; Luo, J.; Wu, H.; Liu, X.; Zhao, G.; Gong, H.; Zheng, X.; Li, J. Metabolic and molecular mechanisms underlying the foliar Zn application induced increase of 2-acetyl-1-pyrroline conferring the ‘taro-like’ aroma in pumpkin leaves. Front. Plant Sci. 2023, 14, 1127032. [Google Scholar] [CrossRef]
- Zhang, Y.; He, S.; Zhang, Z.; Xu, H.; Wang, J.; Chen, H.; Liu, Y.; Wang, X.; Li, Y. Glycine transformation induces repartition of cadmium and lead in soil constituents. Environ. Pollut. 2019, 251, 930–937. [Google Scholar] [CrossRef]
- Zemanová, V.; Pavlík, M.; Pavlíková, D.; Tlustoš, P. The significance of methionine, histidine and tryptophan in plant responses and adaptation to cadmium stress. Plant Soil Environ. 2014, 60, 426–432. [Google Scholar] [CrossRef]
- Lux, A.; Lackovič, A.; van Staden, J.; Lišková, D.; Kohanová, J.; Martinka, M. Cadmium translocation by contractile roots differs from that in regular, non-contractile roots. Ann. Bot. 2015, 115, 1149–1154. [Google Scholar] [CrossRef]
- Lentini, M.; De Lillo, A.; Paradisone, V.; Liberti, D.; Landi, S.; Esposito, S. Early responses to cadmium exposure in barley plants: Effects on biometric and physiological parameters. Acta Physiol. Plant. 2018, 40, 178. [Google Scholar] [CrossRef]
- He, S.; Yang, X.; He, Z.; Baligar, V.C. Morphological and physiological responses of plants to cadmium toxicity: A review. Pedosphere 2017, 27, 421–438. [Google Scholar] [CrossRef]
- Kováčik, J.; Babula, P. Fluorescence microscopy as a tool for visualization of metal-induced oxidative stress in plants. Acta Physiol. Plant. 2017, 39, 157. [Google Scholar] [CrossRef]
- Duan, C.; Wang, Y.; Wang, Q.; Ju, W.; Zhang, Z.; Cui, Y.; Yuan, J.; Fan, Q.; Wei, S.; Li, S.; et al. Microbial metabolic limitation of rhizosphere under heavy metal stress: Evidence from soil ecoenzymatic stoichiometry. Environ. Pollut. 2022, 300, 118978. [Google Scholar] [CrossRef]
- Zemanová, V.; Pavlíková, D.; Novák, M.; Dobrev, P.I.; Matoušek, T.; Motyka, V.; Pavlík, M. Arsenic-induced response in roots of arsenic-hyperaccumulator fern and soil enzymatic activity changes. Plant Soil Environ. 2022, 68, 213–222. [Google Scholar] [CrossRef]
- Wahsha, M.; Nadimi-Goki, M.; Fornasier, F.; Al-Jawasehr, R.; Hussein, E.I.; Bini, C. Microbial enzymes as an early warning management tool for monitoring mining site soils. Catena 2017, 148, 40–45. [Google Scholar] [CrossRef]
- Aponte, H.; Meli, P.; Butler, B.; Paolini, J.; Matus, F.; Merino, C.; Cornejo, P.; Kuzyakov, Y. Meta-analysis of heavy metal effects on soil enzyme activities. Sci. Total Environ. 2020, 737, 139744. [Google Scholar] [CrossRef] [PubMed]
- Ciadamidaro, L.; Madejón, P.; Madejón, E. Soil chemical and biochemical properties under Populus alba growing: Three years study in trace element contaminated soils. Appl. Soil Ecol. 2014, 73, 26–33. [Google Scholar] [CrossRef]
- Maurya, S.; Abraham, J.S.; Somasundaram, S.; Toteja, R.; Gupta, R.; Makhija, S. Indicators for assessment of soil quality: A mini-review. Environ. Monit. Assess. 2020, 192, 604. [Google Scholar] [CrossRef] [PubMed]
- Spohn, M.; Kuzyakov, Y. Distribution of microbial- and root-derived phosphatase activities in the rhizosphere depending on P availability and C allocation—Coupling soil zymography with 14C imaging. Soil Biol. Biochem. 2013, 67, 106–113. [Google Scholar] [CrossRef]
- Aponte, H.; Medina, J.; Butler, B.; Meier, S.; Cornejo, P.; Kuzyakov, Y. Soil quality indices for metal(loid) contamination: An enzymatic perspective. Land Degrad. Dev. 2020, 31, 2700–2719. [Google Scholar] [CrossRef]
- Šichorová, K.; Tlustoš, P.; Száková, J.; Kořínek, K.; Balík, J. Horizontal and vertical variability of heavy metals in the soil of a polluted area. Plant Soil Environ. 2004, 50, 525–534. [Google Scholar] [CrossRef]
- Vaněk, A.; Ettler, V.; Grygar, T.; Borůvka, L.; Šebek, O.; Drábek, O. Combined chemical and mineralogical evidence for heavy metal binding in mining-and smelting-affected alluvial soils. Pedosphere 2008, 18, 464–478. [Google Scholar] [CrossRef]
- Czech Ministry of the Environment. Public Notice No. 153/2016 for the Management of Soil Protection; Czech Ministry of the Environment: Prague, Czech Republic, 2016.
- Břendová, K.; Zemanová, V.; Pavlíková, D.; Tlustoš, P. Utilization of biochar and activated carbon to reduce Cd, Pb and Zn phytoavailability and phytotoxicity for plants. J. Environ. Manag. 2016, 181, 637–645. [Google Scholar] [CrossRef]
- Pavlíková, D.; Pavlík, M.; Zemanová, V.; Novák, M.; Doležal, P.; Dobrev, P.I.; Motyka, V.; Kraus, K. Accumulation of toxic arsenic by cherry radish tuber (Raphanus sativus var. sativus Pers.) and its physiological, metabolic and anatomical stress responses. Plants 2023, 12, 1257. [Google Scholar] [CrossRef]
- Lhotská, M.; Zemanová, V.; Pavlík, M.; Pavlíková, D.; Hnilička, F.; Popov, M. Leaf fitness and stress response after the application of contaminated soil dust particulate matter. Sci. Rep. 2022, 12, 10046. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. The spectral determination of chlorophyll a and chlorophyll b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta BBA Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Hanč, A.; Dume, B.; Hrebečková, T. Differences of enzymatic activity during composting and vermicomposting of sewage sludge mixed with straw pellets. Front. Microbiol. 2022, 12, 801107. [Google Scholar] [CrossRef]
- Novák, M.; Zemanová, V.; Pavlík, M.; Procházková, S.; Pavlíková, D. Change in β-glucosidase activity in root zone of ferns under toxic elements soil contamination. Plant Soil Environ. 2023, 69, 124–130. [Google Scholar] [CrossRef]

| Roots | Periderm | Leaves | TF | |||||
|---|---|---|---|---|---|---|---|---|
| Control | Multi | Control | Multi | Control | Multi | Control | Multi | |
| Cd (mg/kg DW) | 0.2 ± 0.01 aA | 4.8 ± 0.5 bA | 0.2 ± 0.04 aAB | 8.4 ± 0.1 bB | 0.3 ± 0.1 aB | 13.8 ± 1.1 bC | 1.5 | 2.9 |
| Pb (mg/kg DW) | <2.0 | 68.8 ± 1.6 C | <2.0 | 16.3 ± 1.0 A | <2.0 | 28.0 ± 1.5 B | - | 0.4 |
| Zn (mg/kg DW) | 10.3 ± 0.5 aA | 25.1 ± 1.0 bA | 17.2 ± 0.6 aB | 24.4 ± 0.6 bA | 16.9 ± 1.4 aB | 64.1 ± 2.3 bB | 1.6 | 2.6 |
| Control | Multi | |
|---|---|---|
| Ci (µmol CO2/mol) | 309.3 ± 4.8 a | 328.8 ± 1.9 b |
| E (mmol H2O/m2/s) | 2.9 ± 0.04 a | 2.9 ± 0.02 a |
| gs (mol H2O/m2/s) | 0.4 ± 0.03 b | 0.3 ± 0.01 a |
| PN (µmol CO2/m2/s) | 12.5 ± 0.2 b | 11.1 ± 0.2 a |
| Fv/Fm (-) | 0.8 ± 0.01 b | 0.7 ± 0.01 a |
| Chl a (mg/kg DW) | 1.8 ± 0.03 b | 1.6 ± 0.06 a |
| Chl b (mg/kg DW) | 0.9 ± 0.04 a | 1.1 ± 0.02 b |
| Control | Multi | ||
|---|---|---|---|
| Ʃ AAs (μmol/kg FW) | roots | 30,600.6 ± 6996.8 a | 23,251.9 ± 2097.9 a |
| leaves | 9871.7 ± 275.5 a | 10,327.5 ± 720.2 a | |
| transport AAs (μmol/kg FW) | roots | 25,547.1 ± 6293.9 a | 19,305.3 ± 1411.6 a |
| leaves | 7136.1 ± 252.8 b | 5661.5 ± 491.2 a | |
| PRO (μmol/kg FW) | roots | 832.8 ± 61.7 b | 449.8 ± 235.6 a |
| leaves | 82.5 ± 0.8 a | 161.3 ± 17.9 b | |
| ORN (μmol/kg FW) | roots | 57.1 ± 8.6 a | 62.8 ± 7.9 a |
| leaves | 56.9 ± 0.2 a | 71.4 ± 1.1 b | |
| GLY (μmol/kg FW) | roots | 86.8 ± 4.5 a | 83.9 ± 6.6 a |
| leaves | 161.7 ± 0.9 a | 182.0 ± 15.7 a | |
| MET (μmol/kg FW) | roots | 71.7 ± 8.9 a | 77.7 ± 3.6 a |
| leaves | 49.0 ± 0.3 a | 64.1 ± 0.2 b | |
| HYP (μmol/kg FW) | roots | nd | nd |
| leaves | 25.5 ± 1.8 a | 27.2 ± 3.5 a | |
| SAR (μmol/kg FW) | roots | nd | nd |
| leaves | nd | 73.7 ± 0.4 |
| Parameters | Control | Multi |
|---|---|---|
| Soil type and subtype | Chernozem Haplic | Cambisol Haplic |
| pHH2O | 7.5 | 6.0 |
| Cation-Exchange Capacity (mmol(+)/kg) | 230.1 ± 5.0 | 165.8 ± 15.1 |
| Total Carbon (%) | 2.0 ± 0.08 | 2.4 ± 0.04 |
| Cdpseudo-total/Cdwater-soluble (mg/kg) | 0.4 ± 0.01/<0.005 | 6.5 ± 1.1/0.01 ± 0.002 |
| Pbpseudo-total/Pbwater-soluble (mg/kg) | 36.6 ± 1.6/<0.1 | 1560.2 ± 167.1/0.4 ± 0.09 |
| Znpseudo-total/Znwater-soluble (mg/kg) | 93.1 ± 1.5/0.03 ± 0.01 | 243.4 ± 8.2/0.1 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novák, M.; Zemanová, V.; Lhotská, M.; Pavlík, M.; Klement, A.; Hnilička, F.; Pavlíková, D. Response of Carrot (Daucus carota L.) to Multi-Contaminated Soil from Historic Mining and Smelting Activities. Int. J. Mol. Sci. 2023, 24, 17345. https://doi.org/10.3390/ijms242417345
Novák M, Zemanová V, Lhotská M, Pavlík M, Klement A, Hnilička F, Pavlíková D. Response of Carrot (Daucus carota L.) to Multi-Contaminated Soil from Historic Mining and Smelting Activities. International Journal of Molecular Sciences. 2023; 24(24):17345. https://doi.org/10.3390/ijms242417345
Chicago/Turabian StyleNovák, Milan, Veronika Zemanová, Marie Lhotská, Milan Pavlík, Aleš Klement, František Hnilička, and Daniela Pavlíková. 2023. "Response of Carrot (Daucus carota L.) to Multi-Contaminated Soil from Historic Mining and Smelting Activities" International Journal of Molecular Sciences 24, no. 24: 17345. https://doi.org/10.3390/ijms242417345
APA StyleNovák, M., Zemanová, V., Lhotská, M., Pavlík, M., Klement, A., Hnilička, F., & Pavlíková, D. (2023). Response of Carrot (Daucus carota L.) to Multi-Contaminated Soil from Historic Mining and Smelting Activities. International Journal of Molecular Sciences, 24(24), 17345. https://doi.org/10.3390/ijms242417345








