Thrombocytes and Platelet-Rich Plasma as Modulators of Reproduction and Fertility
Abstract
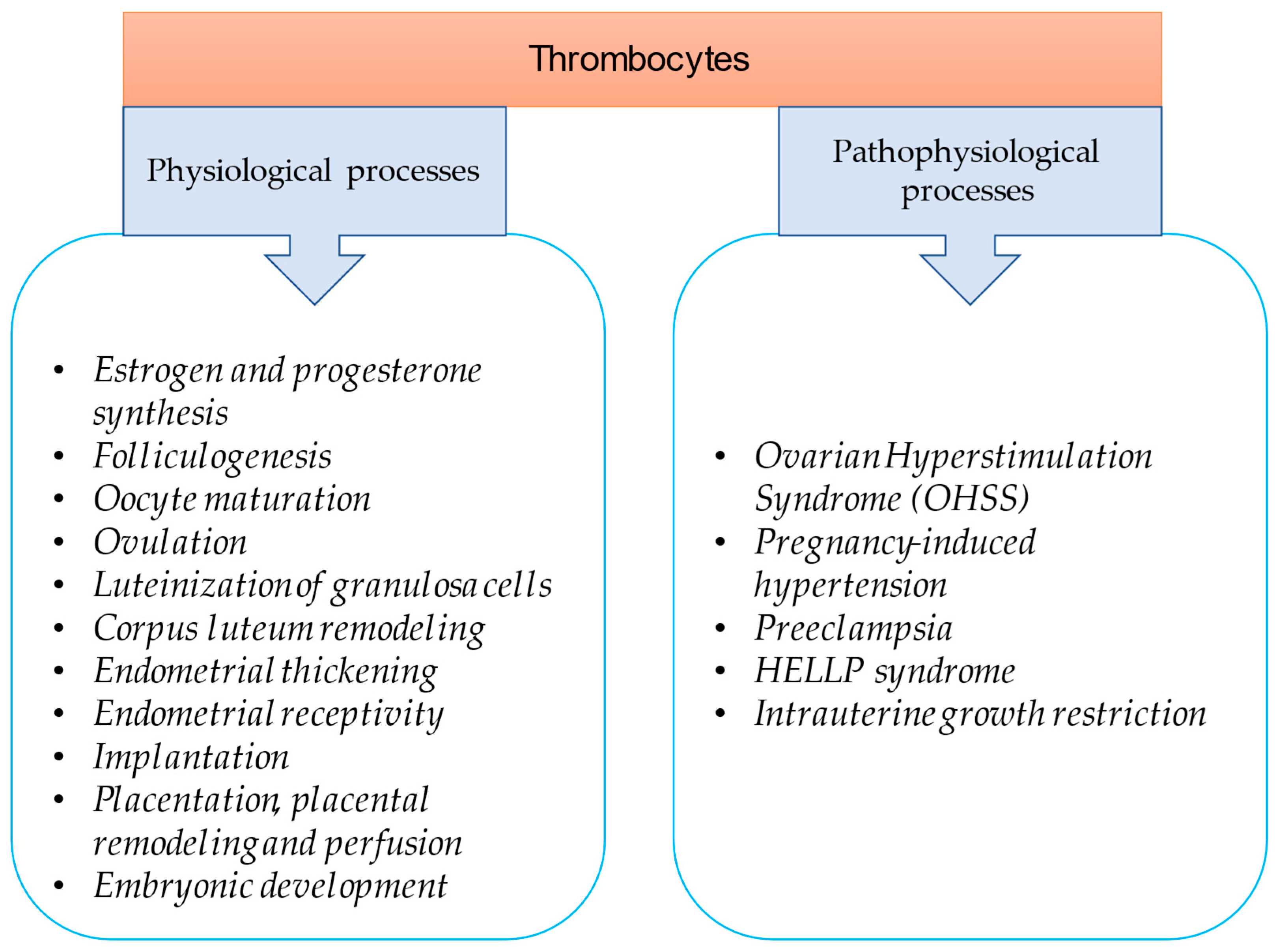
:1. Introduction
2. Ovarian Function Is Controlled by Platelet-Associated Regulatory System (PARS)
3. Thrombocytes in Early Pregnancy
3.1. Thrombocytopenia as an Initial Maternal Response to Fertilization
3.2. Early Pregnancy Factor (EPF) and Lymphocyte–Thrombocyte Adhesion
4. Role of Platelets in Ovarian Hyperstimulation Syndrome
4.1. Platelet Activation in OHSS
4.2. Low-Dose Aspirin Therapy to Prevent OHSS
5. Endometrial Effects of Platelets
6. Placental Effects of Platelets
7. Aspirin Treatment in Placenta-Mediated Obstetrical Complications
8. Platelet-Rich Plasma (PRP) Treatment
8.1. Intraovarian Application of Platelet-Rich Plasma
8.2. Intrauterine Administration of Platelet-Rich Plasma
9. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| OHSS | ovarian hyperstimulation syndrome |
| PRP | platelet-rich plasma |
| PARS | platelet-associated regulatory system |
| IVF | in vitro fertilization |
| PAF | platelet-activating factor |
| TXB2 | thromboxane B2 |
| TXA2 | thromboxane A2 |
| BDNF | brain-derived neurotrophic factor |
| PMSG | pregnant mare serum gonadotropin |
| EPF | early pregnancy factor |
| VEGF | vascular endothelial growth factor |
| PDGF | platelet-derived growth factor |
| hCG | human chorionic gonadotropin |
| PDGF A | platelet-derived growth factor A |
| IGF-1 | insulin-like growth factor |
| PSGL-1 | P-selectin glycoprotein ligand-1 |
| HELLP syndrome | Hemolysis, Elevated Liver enzymes, Low Platelets syndrome |
| ASPRE | Combined Multimarker Screening and Randomized Patient Treatment with Aspirin for Evidence-Based Preeclampsia Prevention study |
| PAPP A | pregnancy-associated plasma protein A |
| AMH | antimüllerian hormone |
| RIF | recurrent implantation failure |
References
- Leslie, M. Cell biology. Beyond clotting: The powers of platelets. Science 2010, 328, 562–564. [Google Scholar] [CrossRef]
- Fitzgerald, J.R.; Loughman, A.; Keane, F.; Brennan, M.; Knobel, M.; Higgins, J.; Visai, L.; Speziale, P.; Cox, D.; Foster, T.J. Fibronectin-binding proteins of Staphylococcus aureus mediate activation of human platelets via fibrinogen and fibronectin bridges to integrin GPIIb/IIIa and IgG binding to the FcgammaRIIa receptor. Mol. Microbiol. 2006, 59, 212–230. [Google Scholar] [CrossRef] [PubMed]
- Bodis, J.; Papp, S.; Vermes, I.; Sulyok, E.; Tamas, P.; Farkas, B.; Zambo, K.; Hatzipetros, I.; Kovacs, G.L. “Platelet-associated regulatory system (PARS)” with particular reference to female reproduction. J. Ovarian Res. 2014, 7, 55. [Google Scholar] [CrossRef]
- Atkinson, L.; Martin, F.; Sturmey, R.G. Intraovarian injection of platelet-rich plasma in assisted reproduction: Too much too soon? Human. Reprod. 2021, 36, 1737–1750. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, Z.S.; Jackson, S.P. The role of platelets in atherothrombosis. Hematol. Am. Soc. Hematol. Educ. Program. 2011, 2011, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Semple, J.W.; Italiano, J.E., Jr.; Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011, 11, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Nagy, B.; Sulyok, E.; Varnagy, A.; Barabas, A.; Kovacs, K.; Bodis, J. The role of platelets in reproduction. Orv. Hetil. 2022, 163, 1254–1260. [Google Scholar] [CrossRef]
- Stiegler, G.; Stohlawetz, P.; Brugger, S.; Jilma, B.; Vierhapper, H.; Hocker, P.; Panzer, S. Elevated numbers of reticulated platelets in hyperthyroidism: Direct evidence for an increase of thrombopoiesis. Br. J. Haematol. 1998, 101, 656–658. [Google Scholar] [CrossRef]
- Erem, C.; Kocak, M.; Hacihasanoglu, A.; Yilmaz, M.; Saglam, F.; Ersoz, H.O. Blood coagulation, fibrinolysis and lipid profile in patients with primary hyperparathyroidism: Increased plasma factor VII and X activities and D-Dimer levels. Exp. Clin. Endocrinol. Diabetes 2008, 116, 619–624. [Google Scholar] [CrossRef]
- Nguyen, T.; Ghebrehiwet, B.; Peerschke, E.I. Staphylococcus aureus protein A recognizes platelet gC1qR/p33: A novel mechanism for staphylococcal interactions with platelets. Infect. Immun. 2000, 68, 2061–2068. [Google Scholar] [CrossRef]
- Peerschke, E.I.; Yin, W.; Ghebrehiwet, B. Platelet mediated complement activation. Adv. Exp. Med. Biol. 2008, 632, 81–91. [Google Scholar]
- Gay, L.J.; Felding-Habermann, B. Contribution of platelets to tumour metastasis. Nat. Rev. Cancer 2011, 11, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Bodis, J.; Torok, A.; Tinneberg, H.R.; Hanf, V.; Hamori, M.; Cledon, P. Influence of serotonin on progesterone and estradiol secretion of cultured human granulosa cells. Fertil. Steril. 1992, 57, 1008–1011. [Google Scholar] [CrossRef] [PubMed]
- Bódis, J.; Sulyok, E.; Várnagy, Á.; Koppán, M.; Kovács, L.G. Investigations of follicular fluid biomarkers in patients undergoing in vitro fertilization. [A tüszőfolyadék biomarkereinek vizsgálata in vitro fertilizációs kezelésben részesült betegekben]. Orv. Hetil. 2021, 162, 523–529. (In Hungarian) [Google Scholar] [CrossRef]
- Bodis, J.; Koppan, M.; Kornya, L.; Tinneberg, H.R.; Torok, A. Influence of melatonin on basal and gonadotropin-stimulated progesterone and estradiol secretion of cultured human granulosa cells and in the superfused granulosa cell system. Gynecol. Obstet. Investig. 2001, 52, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, P.H. The degradation of platelet-activating factor by high-density lipoprotein in rat plasma. Effect of ethynyloestradiol administration. Biochem. J. 1987, 246, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Maki, N.; Hoffman, D.R.; Johnston, J.M. Platelet-activating factor acetylhydrolase activity in maternal, fetal, and newborn rabbit plasma during pregnancy and lactation. Proc. Natl. Acad. Sci. USA 1988, 85, 728–732. [Google Scholar] [CrossRef]
- Furukawa, K.; Fujiwara, H.; Sato, Y.; Zeng, B.X.; Fujii, H.; Yoshioka, S.; Nishi, E.; Nishio, T. Platelets are novel regulators of neovascularization and luteinization during human corpus luteum formation. Endocrinology 2007, 148, 3056–3064. [Google Scholar] [CrossRef]
- Hashimoto, K. Sigma-1 receptor chaperone and brain-derived neurotrophic factor: Emerging links between cardiovascular disease and depression. Prog. Neurobiol. 2013, 100, 15–29. [Google Scholar] [CrossRef]
- O’Neill, C. Thrombocytopenia is an initial maternal response to fertilization in mice. J. Reprod. Fertil. 1985, 73, 559–566. [Google Scholar] [CrossRef]
- Bodis, J.; Torok, A.; Tinneberg, H.R. Hypothesis of preeclampsia requires inclusion of the role of platelets. Am. J. Obstet. Gynecol. 1997, 177, 243–244. [Google Scholar] [PubMed]
- Li, X.M.; Sagawa, N.; Ihara, Y.; Okagaki, A.; Hasegawa, M.; Inamori, K.; Itoh, H.; Mori, T.; Ban, C. The involvement of platelet-activating factor in thrombocytopenia and follicular rupture during gonadotropin-induced superovulation in immature rats. Endocrinology 1991, 129, 3132–3138. [Google Scholar] [CrossRef]
- Check, J.H.; Bollendorf, A.; Askari, H.A. Evidence for a leukocyte adhesion factor produced by the early embryo. Am. J. Reprod. Immunol. 1995, 34, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.G.; Zheng, Z.Q. A study of early pregnancy factor activity in preimplantation. Am. J. Reprod. Immunol. 1997, 37, 359–364. [Google Scholar] [PubMed]
- Morton, H. Early pregnancy factor: An extracellular chaperonin 10 homologue. Immunol. Cell Biol. 1998, 76, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.U.; Chou, C.H.; Lee, H.; Ho, C.H.; Lin, C.W.; Yang, Y.S. Lysophosphatidic acid up-regulates expression of interleukin-8 and -6 in granulosa-lutein cells through its receptors and nuclear factor-kappaB dependent pathways: Implications for angiogenesis of corpus luteum and ovarian hyperstimulation syndrome. J. Clin. Endocrinol. Metab. 2008, 93, 935–943. [Google Scholar] [CrossRef]
- Siess, W. Athero- and thrombogenic actions of lysophosphatidic acid and sphingosine-1-phosphate. Biochim. Biophys. Acta 2002, 1582, 204–215. [Google Scholar] [CrossRef]
- Neulen, J.; Yan, Z.; Raczek, S.; Weindel, K.; Keck, C.; Weich, H.A.; Marme, D.; Breckwoldt, M. Human chorionic gonadotropin-dependent expression of vascular endothelial growth factor/vascular permeability factor in human granulosa cells: Importance in ovarian hyperstimulation syndrome. J. Clin. Endocrinol. Metab. 1995, 80, 1967–1971. [Google Scholar]
- Elchalal, U.; Schenker, J.G. The pathophysiology of ovarian hyperstimulation syndrome—Views and ideas. Human. Reprod. 1997, 12, 1129–1137. [Google Scholar] [CrossRef]
- Vasiljevic, M.P.M.; Tasic, L.; Stanimirovic, B.; Dikic, S.; Mandic, V.; Jeremic, D. The significance of the ovarian arteriolar vasodilatation in pathogenesis of the ovarian hyperstimulation syndrome. Srp. Arh. Celok. Lek. 2006, 134, 344–347. [Google Scholar]
- Varnagy, A.; Bodis, J.; Manfai, Z.; Wilhelm, F.; Busznyak, C.; Koppan, M. Low-dose aspirin therapy to prevent ovarian hyperstimulation syndrome. Fertil. Steril. 2010, 93, 2281–2284. [Google Scholar] [CrossRef]
- Rubinstein, M.; Marazzi, A.; Polak de Fried, E. Low-dose aspirin treatment improves ovarian responsiveness, uterine and ovarian blood flow velocity, implantation, and pregnancy rates in patients undergoing in vitro fertilization: A prospective, randomized, double-blind placebo-controlled assay. Fertil. Steril. 1999, 71, 825–829. [Google Scholar] [CrossRef]
- Khairy, M.; Banerjee, K.; El-Toukhy, T.; Coomarasamy, A.; Khalaf, Y. Aspirin in women undergoing in vitro fertilization treatment: A systematic review and meta-analysis. Fertil. Steril. 2007, 88, 822–831. [Google Scholar] [CrossRef]
- Paule, S.; Nebl, T.; Webb, A.I.; Vollenhoven, B.; Rombauts, L.J.; Nie, G. Proprotein convertase 5/6 cleaves platelet-derived growth factor A in the human endometrium in preparation for embryo implantation. Mol. Hum. Reprod. 2015, 21, 262–270. [Google Scholar] [CrossRef]
- Smith, S.; Francis, R.; Guilbert, L.; Baker, P.N. Growth factor rescue of cytokine mediated trophoblast apoptosis. Placenta 2002, 23, 322–330. [Google Scholar] [CrossRef]
- Shivdasani, R.A.; Rosenblatt, M.F.; Zucker-Franklin, D.; Jackson, C.W.; Hunt, P.; Saris, C.J.; Orkin, S.H. Transcription factor NF-E2 is required for platelet formation independent of the actions of thrombopoietin/MGDF in megakaryocyte development. Cell 1995, 81, 695–704. [Google Scholar] [CrossRef]
- Sato, Y.; Fujiwara, H.; Zeng, B.X.; Higuchi, T.; Yoshioka, S.; Fujii, S. Platelet-derived soluble factors induce human extravillous trophoblast migration and differentiation: Platelets are a possible regulator of trophoblast infiltration into maternal spiral arteries. Blood 2005, 106, 428–435. [Google Scholar] [CrossRef]
- Sato, Y.; Fujiwara, H.; Konishi, I. Role of platelets in placentation. Med. Mol. Morphol. 2010, 43, 129–133. [Google Scholar] [CrossRef]
- de Almeida, L.G.N.; Young, D.; Chow, L.; Nicholas, J.; Lee, A.; Poon, M.C.; Dufour, A.; Agbani, E.O. Proteomics and Metabolomics Profiling of Platelets and Plasma Mediators of Thrombo-Inflammation in Gestational Hypertension and Preeclampsia. Cells 2022, 11, 1256. [Google Scholar] [CrossRef]
- James, A.H.; Brancazio, L.R.; Price, T. Aspirin and reproductive outcomes. Obstet. Gynecol. Surv. 2008, 63, 49–57. [Google Scholar] [CrossRef]
- Roberge, S.; Nicolaides, K.; Demers, S.; Hyett, J.; Chaillet, N.; Bujold, E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: Systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2017, 216, 110–120 e6. [Google Scholar] [CrossRef]
- Rolnik, D.L.; Wright, D.; Poon, L.C.Y.; Syngelaki, A.; O’Gorman, N.; de Paco Matallana, C.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; et al. ASPRE trial: Performance of screening for preterm pre-eclampsia. Ultrasound Obstet. Gynecol. 2017, 50, 492–495. [Google Scholar] [CrossRef]
- Rolnik, D.L.; Wright, D.; Poon, L.C.; O’Gorman, N.; Syngelaki, A.; de Paco Matallana, C.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N. Engl. J. Med. 2017, 377, 613–622. [Google Scholar] [CrossRef]
- Hoffman, M.K.; Goudar, S.S.; Kodkany, B.S.; Metgud, M.; Somannavar, M.; Okitawutshu, J.; Lokangaka, A.; Tshefu, A.; Bose, C.L.; Mwapule, A.; et al. Low-dose aspirin for the prevention of preterm delivery in nulliparous women with a singleton pregnancy (ASPIRIN): A randomised, double-blind, placebo-controlled trial. Lancet 2020, 395, 285–293. [Google Scholar] [CrossRef]
- Csaba, I.F.; Sulyok, E.; Ertl, T. Relationship of maternal treatment with indomethacin to persistence of fetal circulation syndrome. J. Pediatr. 1978, 92, 484. [Google Scholar] [CrossRef]
- Csaba, I.; Sulyok, E.; Ertl, T. A császármetsést követő újszülöttkori respiratorikus distress syndroma pathomechanizmusáról [Pathomechanism of neonatal respiratory distress syndrome following cesarean section]. Orv. Hetil. 1978, 119, 2623–2628. [Google Scholar]
- Lin, Y.; Qi, J.; Sun, Y. Platelet-Rich Plasma as a Potential New Strategy in the Endometrium Treatment in Assisted Reproductive Technology. Front. Endocrinol. 2021, 12, 707584. [Google Scholar] [CrossRef]
- Dhurat, R.; Sukesh, M. Principles and Methods of Preparation of Platelet-Rich Plasma: A Review and Author’s Perspective. J. Cutan. Aesthet. Surg. 2014, 7, 189–197. [Google Scholar] [CrossRef]
- Pavlovic, V.; Ciric, M.; Jovanovic, V.; Stojanovic, P. Platelet Rich Plasma: A short overview of certain bioactive components. Open Med. 2016, 11, 242–247. [Google Scholar] [CrossRef]
- Bors, S.I.; Ibanescu, I.; Bors, A.; Abdoon, A.S.S. Platelet-rich plasma in animal reproductive medicine: Prospective and applications. Reprod. Domest. Anim. 2022, 57, 1287–1294. [Google Scholar] [CrossRef]
- Ghallab, R.S.; El-Beskawy, M.; El-Shereif, A.A.; Rashad, A.M.A.; Elbehiry, M.A. Impact of intrauterine infusion of Platelets-Rich plasma on endometritis and reproductive performance of Arabian mare. Reprod. Domest. Anim. 2023, 58, 622–629. [Google Scholar] [CrossRef]
- Segabinazzi, L.; Canisso, I.F.; Podico, G.; Cunha, L.L.; Novello, G.; Rosser, M.F.; Loux, S.C.; Lima, F.S.; Alvarenga, M.A. Intrauterine Blood Plasma Platelet-Therapy Mitigates Persistent Breeding-Induced Endometritis, Reduces Uterine Infections, and Improves Embryo Recovery in Mares. Antibiotics 2021, 10, 490. [Google Scholar] [CrossRef]
- Petryk, N.; Petryk, M. Ovarian Rejuvenation through Platelet-Rich Autologous Plasma (PRP)—A Chance to Have a Baby without Donor Eggs, Improving the Life Quality of Women Suffering from Early Menopause without Synthetic Hormonal Treatment. Reprod. Sci. 2020, 27, 1975–1982. [Google Scholar] [CrossRef]
- Bodis, J. Role of platelets in female reproduction. Human. Reprod. 2022, 37, 384–385. [Google Scholar] [CrossRef]
- Sharara, F.I.; Lelea, L.L.; Rahman, S.; Klebanoff, J.S.; Moawad, G.N. A narrative review of platelet-rich plasma (PRP) in reproductive medicine. J. Assist. Reprod. Genet. 2021, 38, 1003–1012. [Google Scholar] [CrossRef]
- Barad, D.H.; Albertini, D.F.; Molinari, E.; Gleicher, N. Preliminary report of intraovarian injections of autologous platelet-rich plasma (PRP) in extremely poor prognosis patients with only oocyte donation as alternative: A prospective cohort study. Hum. Reprod. Open 2022, 2022, hoac027. [Google Scholar] [CrossRef]
- Sills, E.S.; Wood, S.H. Autologous activated platelet-rich plasma injection into adult human ovary tissue: Molecular mechanism, analysis, and discussion of reproductive response. Biosci. Rep. 2019, 39, BSR20190805. [Google Scholar] [CrossRef]
- Cakiroglu, Y.; Saltik, A.; Yuceturk, A.; Karaosmanoglu, O.; Kopuk, S.Y.; Scott, R.T.; Tiras, B.; Seli, E. Effects of intraovarian injection of autologous platelet rich plasma on ovarian reserve and IVF outcome parameters in women with primary ovarian insufficiency. Aging 2020, 12, 10211–10222. [Google Scholar] [CrossRef]
- Mouanness, M.; Ali-Bynom, S.; Jackman, J.; Seckin, S.; Merhi, Z. Use of Intra-uterine Injection of Platelet-rich Plasma (PRP) for Endometrial Receptivity and Thickness: A Literature Review of the Mechanisms of Action. Reprod. Sci. 2021, 28, 1659–1670. [Google Scholar] [CrossRef]
- Nazari, L.; Salehpour, S.; Hoseini, S.; Zadehmodarres, S.; Azargashb, E. Effects of autologous platelet-rich plasma on endometrial expansion in patients undergoing frozen-thawed embryo transfer: A double-blind RCT. Int. J. Reprod. Biomed. 2019, 17, 443–448. [Google Scholar] [CrossRef]
- Farimani, M.; Poorolajal, J.; Rabiee, S.; Bahmanzadeh, M. Successful pregnancy and live birth after intrauterine administration of autologous platelet-rich plasma in a woman with recurrent implantation failure: A case report. Int. J. Reprod. Biomed. 2017, 15, 803–806. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagy, B.; Kovács, K.; Sulyok, E.; Várnagy, Á.; Bódis, J. Thrombocytes and Platelet-Rich Plasma as Modulators of Reproduction and Fertility. Int. J. Mol. Sci. 2023, 24, 17336. https://doi.org/10.3390/ijms242417336
Nagy B, Kovács K, Sulyok E, Várnagy Á, Bódis J. Thrombocytes and Platelet-Rich Plasma as Modulators of Reproduction and Fertility. International Journal of Molecular Sciences. 2023; 24(24):17336. https://doi.org/10.3390/ijms242417336
Chicago/Turabian StyleNagy, Bernadett, Kálmán Kovács, Endre Sulyok, Ákos Várnagy, and József Bódis. 2023. "Thrombocytes and Platelet-Rich Plasma as Modulators of Reproduction and Fertility" International Journal of Molecular Sciences 24, no. 24: 17336. https://doi.org/10.3390/ijms242417336
APA StyleNagy, B., Kovács, K., Sulyok, E., Várnagy, Á., & Bódis, J. (2023). Thrombocytes and Platelet-Rich Plasma as Modulators of Reproduction and Fertility. International Journal of Molecular Sciences, 24(24), 17336. https://doi.org/10.3390/ijms242417336






