Glycyrol Prevents the Progression of Psoriasis-like Skin Inflammation via Immunosuppressive and Anti-Inflammatory Actions
Abstract
:1. Introduction
2. Results
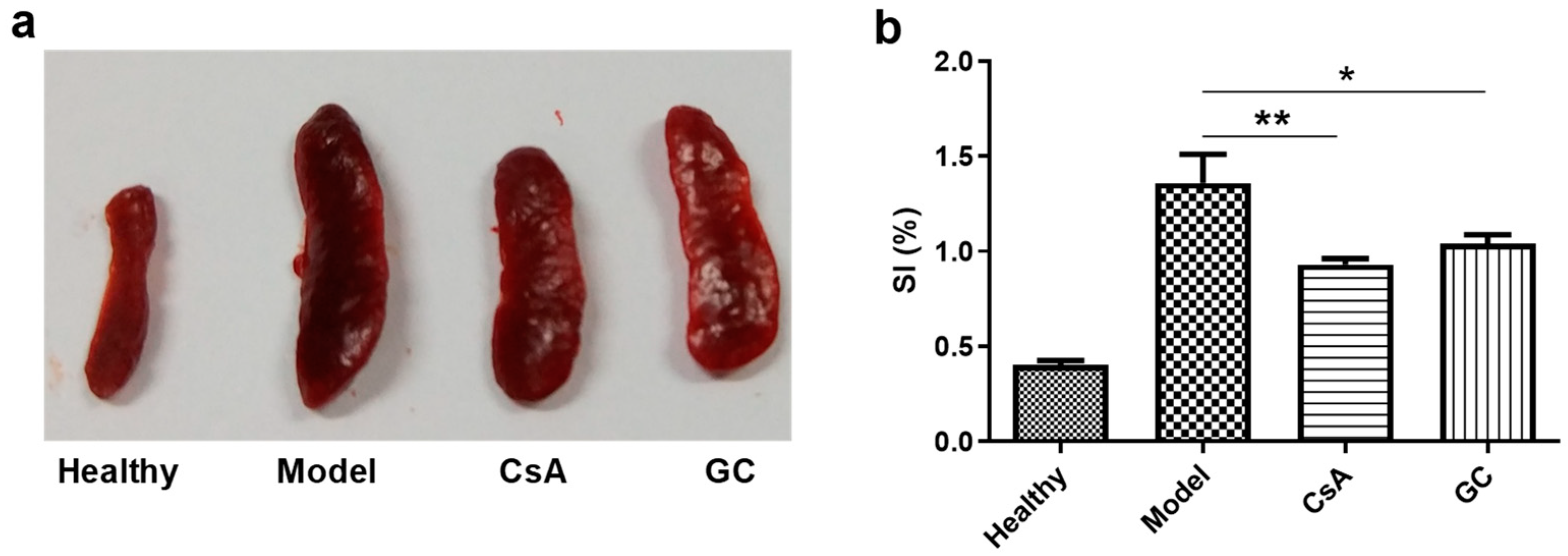
2.1. GC Alleviates Psoriatic Dermatitis
2.2. GC Shows No Detectible Effects on the Functions of the Kidney and Liver
2.3. GC Attenuates IMQ-Induced Immune Stimulation
2.4. GC Alleviates the Inflammatory Response of Lipopolysaccharide-Induced HaCaT Cells
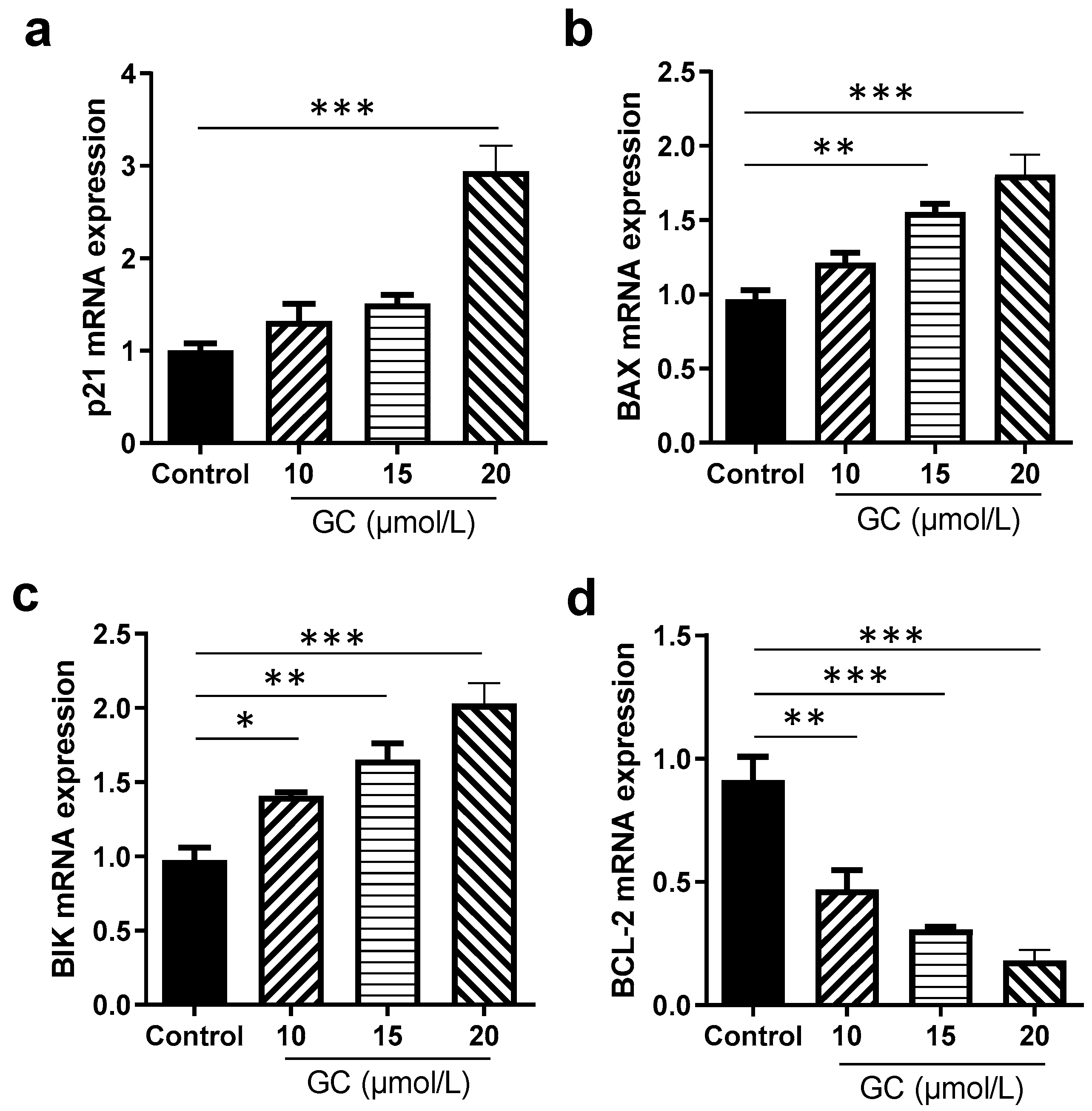
2.5. GC Induces the Apoptosis of Keratinocytes
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Culture and Treatment of Cells
4.3. Animals
4.4. Establishment and Treatment of the Psoriasis-like Mouse Model
4.5. Evaluation of the Severity of Psoriasis
4.6. Evaluation of the Splenic Index
4.7. Pathology
4.8. Peripheral Whole Blood Count Test
4.9. Quantitative Real-Time PCR
4.10. Apoptosis Assay of HaCaT Cells
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, W.; Li, H.; Zhang, M.; Wang, M.; Zhong, Y.; Wu, H.; Yang, Y.; Morel, L.; Wei, Q. Quercitrin ameliorates the development of systemic lupus erythematosus-like disease in a chronic graft-versus-host murine model. Am. J. Phys.-Ren. Phys. 2016, 311, F217–F226. [Google Scholar] [CrossRef]
- Lowes, M.A.; Bowcock, A.M.; Krueger, J.G. Pathogenesis and therapy of psoriasis. Nature 2007, 445, 866–873. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, J.; Huang, D.; Zhong, X.; Kong, L.; Ding, Y.; Bi, X.; Deng, H.; Chen, J.; Gu, J.; et al. Lesion area reduction and the amelioration of anxiety and depression states in psoriasis patients: A prospective clinical study. J. Affect. Disord. 2024, 344, 335–338. [Google Scholar] [CrossRef]
- Yadav, S.; Pawar, G.; Kulkarni, P.; Ferris, C.; Amiji, M. CNS delivery and anti-Inflammatory effects of intranasally administered cyclosporine-A in cationic nanoformulations. J. Pharmacol. Exp. Ther. 2019, 370, 843–854. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.; Huo, R.; Zhai, T.; Li, H.; Wu, P.; Zhu, X.; Zhou, Z.; Shen, B.; Li, N. Paeoniflorin inhibits skin lesions in imiquimod-induced psoriasis-like mice by downregulating inflammation. Int. Immunopharmacol. 2015, 24, 392–399. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X.; Lu, Y.; Sun, X.-Y.; Ze, K.; Zhou, Y.-Q.; Lie, W.; Li, X.; Li, H.-J.; Li, B. Celastrol gel ameliorates imiquimod-induced psoriasis-like dermatitis in mice by targeting Langerhans cells. Biomed. Pharmacother. 2022, 147, 112644. [Google Scholar] [CrossRef]
- Lowes, M.A.; Suárez-Fariñas, M.; Krueger, J.G. Immunology of psoriasis. Ann. Rev. Immunol. 2014, 32, 227–255. [Google Scholar] [CrossRef] [PubMed]
- Balak, D.M.W.; Gerdes, S.; Parodi, A.; Salgado-Boquete, L. Long-term safety of oral systemic therapies for psoriasis: A comprehensive review of the literature. Dermatol. Ther. 2020, 10, 589–613. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Wang, Y.; Xu, J.; Zhou, T.; Chen, Z.; Yang, H.; Di, T.; Li, P. Protective effect of Yangxue Jiedu Soup against psoriasis-like lesions by regulating TLR4/NF-κB signaling pathway mediated by secretion of exosome HSP70. Biomed. Pharmacother. 2022, 147, 112604. [Google Scholar] [CrossRef] [PubMed]
- Di, T.; Zhao, J.; Wang, Y.; Han, L.; Guo, X.; Han, X.; Chen, Z.; Li, P.; Lu, C. Tuhuaiyin alleviates imiquimod-induced psoriasis via inhibiting the properties of IL-17-producing cells and remodels the gut microbiota. Biomed. Pharmacother. 2021, 141, 111884. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhang, S.; Zhou, Y.; Lai, S.; Chen, Y.; Geng, Y.; Wang, J. Curcumin alleviates imiquimod-induced psoriasis in progranulin-knockout mice. Eur. J. Pharmacol. 2021, 909, 174431. [Google Scholar] [CrossRef]
- Chan, T.C.; Lee, M.-S.; Huang, W.-C.; Chang, W.-Y.; Krueger, J.G.; Tsai, T.-F. Capsaicin attenuates imiquimod-induced epidermal hyperplasia and cutaneous inflammation in a murine model of psoriasis. Biomed. Pharmacother. 2021, 141, 111950. [Google Scholar] [CrossRef]
- Zhou, W.; Hu, M.; Zang, X.; Liu, Q.; Du, J.; Hu, J.; Zhang, L.; Du, Z.; Xiang, Z. Luteolin attenuates imiquimod–induced psoriasis-like skin lesions in BALB/c mice via suppression of inflammation response. Biomed. Pharmacother. 2020, 131, 110696. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Su, D.; Huang, R.; Shu, F.; Cheng, F.; Zheng, G. Cellular nanovesicles with bioorthogonal targeting enhance photodynamic/photothermal therapy in psoriasis. Acta Biomater. 2021, 134, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tang, Y.; Wang, M.; Wang, Y.; Wang, W.; Pang, M.; Xu, Y. Co-delivery of methotrexate and nicotinamide by cerosomes for topical psoriasis treatment with enhanced efficacy. Int. J. Pharm. 2021, 605, 120826. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; El-Sherbeeny, A.M.; Al-Harbi, M.M.; Almukhlafi, T.S. GPR43 activation enhances psoriasis-like inflammation through epidermal upregulation of IL-6 and dual oxidase 2 signaling in a murine model. Cell. Signal. 2017, 33, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; El-Sherbeeny, A.M.; Alasmari, A.F.; Alanazi, W.A.; Alasmari, F.; Ibrahim, K.E.; Al-Harbi, M.M.; Bakheet, S.A.; et al. Bruton’s tyrosine kinase inhibitor suppresses imiquimod-induced psoriasis-like inflammation in mice through regulation of IL-23/IL-17A in innate immune cells. Int. Immunopharmacol. 2020, 80, 106215. [Google Scholar] [CrossRef]
- Al-Harbi, N.O.; Nadeem, A.; Ahmad, S.F.; Bakheet, S.A.; El-Sherbeeny, A.M.; Ibrahim, K.E.; Alzahrani, K.S.; Al-Harbi, M.M.; Mahmood, H.M.; Alqahtani, F.; et al. Therapeutic treatment with Ibrutinib attenuates imiquimod-induced psoriasis-like inflammation in mice through downregulation of oxidative and inflammatory mediators in neutrophils and dendritic cells. Eur. J. Pharmacol. 2020, 877, 173088. [Google Scholar] [CrossRef]
- Nadeem, A.; Al-Harbi, N.O.; Al-Harbi, M.M.; El-Sherbeeny, A.M.; Ahmad, S.F.; Siddiqui, N.; Ansari, M.A.; Zoheir, K.M.A.; Attia, S.M.; Al-Hosaini, K.A.; et al. Imiquimod-induced psoriasis-like skin inflammation is suppressed by BET bromodomain inhibitor in mice through RORC/IL-17A pathway modulation. Pharmacol. Res. 2015, 99, 248–257. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; Ibrahim, K.E.; Alqahtani, F.; Sobeai, H.M.A.; Alotaibi, M.R. Inhibition of interleukin-2-inducible T-cell kinase causes reduction in imiquimod-induced psoriasiform inflammation through reduction of Th17 cells and enhancement of Treg cells in mice. Biochimie 2020, 179, 146–156. [Google Scholar] [CrossRef]
- Fu, Y.; Zhou, H.; Wang, S.; Wei, Q. Glycyrol suppresses collagen-induced arthritis by regulating autoimmune and inflammatory responses. PLoS ONE 2014, 9, e98137. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xie, Y.; Zhong, Y.; Cen, J.; Wang, L.; Liu, Y.; Zhu, Y.; Tong, L.; Wei, Q. Amelioration of experimental autoimmune encephalomyelitis by isogarcinol extracted from Garcinia mangostana L. Mangosteen. J. Agric. Food Chem. 2016, 64, 9012–9021. [Google Scholar] [CrossRef]
- Schrem, H.; Lück, R.; Becker, T.; Nashan, B.; Klempnauer, J. Update on liver transplantation using cyclosporine. Transpl. Proc. 2004, 36, 2525–2531. [Google Scholar] [CrossRef] [PubMed]
- Berth-Jones, J. The use of ciclosporin in psoriasis. J. Dermatol. Treat. 2005, 16, 258–277. [Google Scholar] [CrossRef]
- van der Fits, L.; Mourits, S.; Voerman, J.S.A.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.-M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009, 182, 5836. [Google Scholar] [CrossRef]
- Baek, J.-O.; Byamba, D.; Wu, W.H.; Kim, T.-G.; Lee, M.-G. Assessment of an imiquimod-induced psoriatic mouse model in relation to oxidative stress. Arch. Dermatol. Res. 2012, 304, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Flutter, B.; Nestle, F.O. TLRs to cytokines: Mechanistic insights from the imiquimod mouse model of psoriasis. Eur. J. Immunol. 2013, 43, 3138–3146. [Google Scholar] [CrossRef]
- Adianti, M.; Aoki, C.; Komoto, M.; Deng, L.; Shoji, I.; Wahyuni, T.S.; Lusida, M.I.; Soetjipto; Fuchino, H.; Kawahara, N.; et al. Anti-hepatitis C virus compounds obtained from Glycyrrhiza uralensis and other Glycyrrhiza species. Microbiol. Immunol. 2014, 58, 180–187. [Google Scholar] [CrossRef]
- Wang, M.-W.; Hao, X.; Chen, K. Biological screening of natural products and drug innovation in China. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 1093–1105. [Google Scholar] [CrossRef]
- Yan, B.; Hou, J.; Li, W.; Luo, L.; Ye, M.; Zhao, Z.; Wang, W. A review on the plant resources of important medicinal licorice. J. Ethnopharmacol. 2023, 301, 115823. [Google Scholar] [CrossRef]
- Chen, F.; Li, N.; Xiu, L.; Liu, H.; Chen, S.; He, C.; Fan, A.; Yu, X.; Wang, X.; Ge, C.; et al. Comparative efficacy of Haizao Yuhu Decoction composed of different varieties of Glycyrrhiza in goiter rats. Evid.-Based Complement. Altern. Med. Ecam 2021, 2021, 4343239. [Google Scholar] [CrossRef]
- Shin, E.M.; Zhou, H.Y.; Guo, L.Y.; Kim, J.A.; Lee, S.H.; Merfort, I.; Kang, S.S.; Kim, H.S.; Kim, S.; Kim, Y.S. Anti-inflammatory effects of glycyrol isolated from Glycyrrhiza uralensis in LPS-stimulated RAW264.7 macrophages. Int. Immunopharmacol. 2008, 8, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tu, Y.; Tong, L.; Zhang, W.; Zheng, J.; Wei, Q. Immunosuppressive activity on the murine immune responses of glycyrol from Glycyrrhiza uralensis via inhibition of calcineurin activity. Pharm. Biol. 2010, 48, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Mabuchi, T.; Chang, T.W.; Quinter, S.; Hwang, S.T. Chemokine receptors in the pathogenesis and therapy of psoriasis. J. Dermatol. Sci. 2012, 65, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Liu, C.; Liu, S.; Zhou, X.; Lu, J.; Li, M.; Zhu, L. Inhibition of JAK1/STAT3 pathway by 2-methoxyestradiol ameliorates psoriatic features in vitro and in an imiquimod-induced psoriasis-like mouse model. Eur. J. Pharmacol. 2022, 933, 175276. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Kang, S.; Chen, H.; Bahetjan, Y.; Zhang, J.; Lu, R.; Zheng, N.; Yang, G.; Yang, X. A composition of ursolic acid derivatives from Ludwigia hyssopifolia induces apoptosis in throat cancer cells via the Akt/mTOR and mitochondrial signaling pathways and by modulating endoplasmic reticulum stress. J. Ethnopharmacol. 2023, 319, 117351. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.-H.; Hsu, C.-T.; Niu, H.-S.; Niu, C.-S.; Cheng, J.-T.; Chen, Z.-C. Cryptotanshinone inhibits STAT3 signaling to alleviate cardiac fibrosis in Type 1-like diabetic rats. Phytother. Res. 2017, 31, 638–646. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.; Wang, X.; Zhou, J.; Wang, M.; Ma, H.; Xiao, S. Increased βTrCP are associated with imiquimod-induced psoriasis-like skin inflammation in mice via NF-κB signaling pathway. Gene 2016, 592, 164–171. [Google Scholar] [CrossRef]
- Kobayashi, K.; Chikazawa, S.; Chen, Y.; Suzuki, S.; Ichimasu, N.; Katagiri, K. Oestrogen inhibits psoriasis-like dermatitis induced by imiquimod in mice in relation to increased IL-10 producing cells despite elevated expression of IL-22, IL-23, IL-17 mRNA. Exp. Dermatol. 2023, 32, 203–209. [Google Scholar] [CrossRef]
- Nakajima, K.; Kanda, T.; Takaishi, M.; Shiga, T.; Miyoshi, K.; Nakajima, H.; Kamijima, R.; Tarutani, M.; Benson, J.M.; Elloso, M.M.; et al. Distinct roles of IL-23 and IL-17 in the development of psoriasis-like lesions in a mouse model. J. Immunol. 2011, 186, 4481–4489. [Google Scholar] [CrossRef]
- Owczarczyk-Saczonek, A.; Czerwinska, J.; Wygonowska, E.; Kasprowicz-Furmanczyk, M.; Placek, W. D-chiro-inositol as a treatment in plaque psoriasis: A randomized placebo-controlled clinical trial. Dermatol. Ther. 2021, 34, e14538. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.; Kaddour-Djebbar, I.; Custer, V.E.; Uaratanawong, R.; Chen, X.; Cohen, E.; Yang, R.; Ajebo, E.; Hossack, S.; Bollag, W.B. Glycerol Improves Skin Lesion Development in the Imiquimod Mouse Model of Psoriasis: Experimental Confirmation of Anecdotal Reports from Patients with Psoriasis. Int. J. Mol. Sci. 2021, 22, 8749. [Google Scholar] [CrossRef] [PubMed]
- Moghadamnia, A.A.; Motallebnejad, M.; Khanian, M. The efficacy of the bioadhesive patches containing licorice extract in the management of recurrent aphthous stomatitis. Phytother. Res. 2009, 23, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, C.V.; Deepak, H.B.; Thiyagarajan, P.; Kathiresan, S.; Sangli, G.K.; Deepak, M.; Agarwal, A. Dual inhibitory effect of Glycyrrhiza glabra (GutGard™) on COX and LOX products. Phytomed 2011, 18, 278–284. [Google Scholar] [CrossRef]
- Lin, P.; Shi, H.-Y.; Lu, Y.-Y.; Lin, J. Centella asiatica alleviates psoriasis through JAK/STAT3-mediated inflammation: An in vitro and in vivo study. J. Ethnopharmacol. 2023, 317, 116746. [Google Scholar] [CrossRef]
- Li, Y.; Guo, D.; Wang, Q.; Li, A.; Yin, S.; Li, S.; Li, Y.; Wang, B.; Guo, T.; Feng, S. Benzoylaconitine alleviates progression of psoriasis via suppressing STAT3 phosphorylation in keratinocytes. Molecules 2023, 28, 4473. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yao, Y.; Li, Y.; Guo, S.; Li, Y.; Zhang, G. Experimental study on the effect of luteolin on the proliferation, apoptosis and expression of inflammation-related mediators in lipopolysaccharide-induced keratinocytes. Int. J. Immunopathol. Pharmacol. 2023, 37, 3946320231169175. [Google Scholar] [CrossRef]
- Wilson, V.G. Growth and differentiation of HaCaT keratinocytes. Methods Mol. Biol. 2014, 1195, 33–41. [Google Scholar]
- Micallef, L.; Belaubre, F.; Pinon, A.; Jayat-Vignoles, C.; Delage, C.; Charveron, M.; Simon, A. Effects of extracellular calcium on the growth-differentiation switch in immortalized keratinocyte HaCaT cells compared with normal human keratinocytes. Exp. Dermatol. 2009, 18, 143–151. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, T.; Tang, M.; Yang, Z.; Pei, H.; Ye, H.; Tang, Y.; Cheng, Z.; Lin, P.; Chen, L. Studies on the anti-psoriasis effects and its mechanism of a dual JAK2/FLT3 inhibitor flonoltinib maleate. Biomed. Pharmacother. 2021, 137, 111373. [Google Scholar] [CrossRef]
- Lee, S.Y.; Nam, S.; Hong, I.K.; Kim, H.; Yang, H.; Cho, H.-J. Antiproliferation of keratinocytes and alleviation of psoriasis by the ethanol extract of Artemisia capillaris. Phytother. Res. 2018, 32, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Moudgil, K.D.; Venkatesha, S.H. The anti-inflammatory and immunomodulatory activities of natural products to control autoimmune inflammation. Int. J. Mol. Sci. 2022, 24, 95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, J.; Liu, L.; Sun, X.; Zhou, Y.; Chen, S.; Lu, Y.; Cai, X.; Hu, M.; Yan, G.; et al. Efficacy and safety of curcumin in psoriasis: Preclinical and clinical evidence and possible mechanisms. Front. Pharmacol. 2022, 13, 903160. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wang, W.; Zhang, Y.; Zeng, Y. Curcumin alleviates imiquimod-induced psoriasis-like inflammation and regulates gut microbiota of mice. Immun. Inflamm. Dis. 2023, 11, e967. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.; Wozel, G. The psoriasis area and severity index is the adequate criterion to define severity in chronic plaque-type psoriasis. Dermatology 2005, 210, 194–199. [Google Scholar] [CrossRef]
- de Porto, A.P.; Lammers, A.J.; Bennink, R.J.; ten Berge, I.J.; Speelman, P.; Hoekstra, J.B. Assessment of splenic function. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1465–1473. [Google Scholar] [CrossRef]
- Cesta, M.F. Normal structure, function, and histology of the spleen. Toxicol. Pathol. 2006, 34, 409–424. [Google Scholar] [CrossRef]





| Primer | Base Sequence (5′ to 3′) |
|---|---|
| β-actin (Forward primer) | AGGGAAATCGTGCGTGACAT |
| β-actin (Reverse primer) | TCCTGCTTGCTGATCCACAT |
| CXCL-1 (Forward primer) | CCCCAAGA ACATCCAAAGTG |
| CXCL-1 (Reverse primer) | GATGCAGGATTGAGGCAAG |
| CXCL-2 (Forward primer) | CCCATGGTTAAGAAAATCATCG |
| CXCL-2 (Reverse primer) | CTTCAGGAACAGCCACCAAT |
| IL-6 (Forward primer) | AGAGTAGTGAGGAACAAGCC |
| IL-6 (Reverse primer) | TACATTTGCCGAAGAGCCCT |
| Primer | Base Sequence (5′ to 3′) |
|---|---|
| GAPDH (Forward primer) | TGTGTCCGTCGTGGATCTGA |
| GAPDH (Reverse primer) | TTGCTGTTGAAGTCGCAGGAG |
| IL-6 (Forward primer) | CAACGATGATGCACTTGCAGA |
| IL-6 (Reverse primer) | CTCCAGGTAGCTATGGTACTCCAGA |
| IL-23p19 (Forward primer) | CTTTGAAGATGTCAGAGTCAAGCAG |
| IL-23p19 (Reverse primer) | ACATGCACCAGCGGGACATA |
| CXCL-3 (Forward primer) | CCCCAGGCTTCAGATAATCA |
| CXCL-3 (Reverse primer) | TCTGATTTAGAATGCAGGTCCTT |
| Primer | Base Sequence (5′ to 3′) |
|---|---|
| β-actin (Forward primer) | AGGGAAATCGTGCGTGACAT |
| β-actin (Reverse primer) | TCCTGCTTGCTGATCCACAT |
| p21 (Forward primer) | CGCTGCAGGACACATGGGGAGCCGAGCAGCG |
| p21 (Reverse primer) | TCGGCTCCCCATGTGTCCT |
| BAX (Forward primer) | CCAAGAAGCTGAGCGAGTGTC |
| BAX (Reverse primer) | TGAGGACTCCAGCACAAAGA |
| BIK (Forward primer) | TAGGATCCATGTCTGAAGTAAGACCCCTC |
| BIK (Reverse primer) | ACTCTCGAGTCACTTGAGCAGCAGGTG |
| BCL-2 (Forward primer) | CTGGGGGAGGATTGTGGCTTCT |
| BCL-2 (Reverse primer) | CTCCAACCCCCGCATCTCGGAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Fu, Y.; Zhu, Z.; Chen, S.; Tong, L.; Wei, Q. Glycyrol Prevents the Progression of Psoriasis-like Skin Inflammation via Immunosuppressive and Anti-Inflammatory Actions. Int. J. Mol. Sci. 2023, 24, 17335. https://doi.org/10.3390/ijms242417335
Liu Y, Fu Y, Zhu Z, Chen S, Tong L, Wei Q. Glycyrol Prevents the Progression of Psoriasis-like Skin Inflammation via Immunosuppressive and Anti-Inflammatory Actions. International Journal of Molecular Sciences. 2023; 24(24):17335. https://doi.org/10.3390/ijms242417335
Chicago/Turabian StyleLiu, Yuanyuan, Yanxia Fu, Ziwei Zhu, Shanzao Chen, Li Tong, and Qun Wei. 2023. "Glycyrol Prevents the Progression of Psoriasis-like Skin Inflammation via Immunosuppressive and Anti-Inflammatory Actions" International Journal of Molecular Sciences 24, no. 24: 17335. https://doi.org/10.3390/ijms242417335
APA StyleLiu, Y., Fu, Y., Zhu, Z., Chen, S., Tong, L., & Wei, Q. (2023). Glycyrol Prevents the Progression of Psoriasis-like Skin Inflammation via Immunosuppressive and Anti-Inflammatory Actions. International Journal of Molecular Sciences, 24(24), 17335. https://doi.org/10.3390/ijms242417335







