Comparison of Steroidogenic and Ovulation-Inducing Effects of Orthosteric and Allosteric Agonists of Luteinizing Hormone/Chorionic Gonadotropin Receptor in Immature Female Rats
Abstract
1. Introduction
2. Results
2.1. Induction of Ovulation in Immature Female Rats, the Ovarian Weight and Body Weight/Ovarian Weight Ratio in Animals and the Effect of Treatment with TP4/2 and hCG
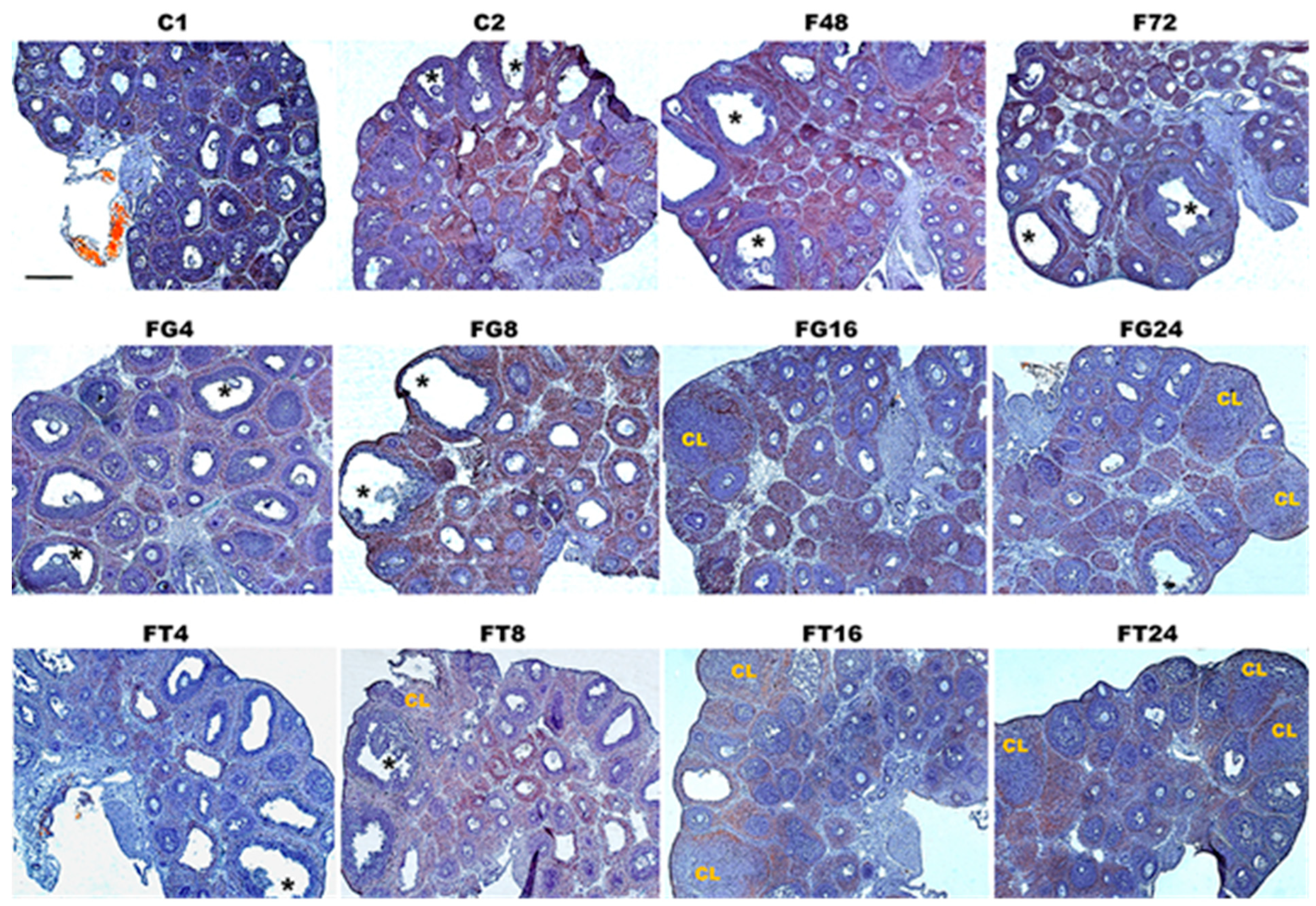
2.2. The Number of Tertiary and Preovulatory Follicles and Corpus Luteum in Immature Female Rats, and the Effect of Treatment with TP4/2 and hCG
2.3. Plasma Levels of Estradiol, Progesterone, and Luteinizing Hormone in Immature Female Rats, and the Effect of Treatment with TP4/2 and hCG
2.4. The Expression of Steroidogenic Genes and the LH/hCG Receptor Gene in the Ovaries of Immature Female Rats, and the Effect of Treatment with TP4/2 and hCG
2.5. The Expression of the Genes Encoding the Isoforms A and B of Vascular Endothelial Growth Factor in the Ovaries of Immature Female Rats, and the Effect of TP4/2 and hCG Treatment
2.6. The Expression of Genes Involved in Late Stages of Folliculogenesis and Ovulation in the Ovaries of Immature Female Rats and the Effect of TP4/2 and hCG Treatment
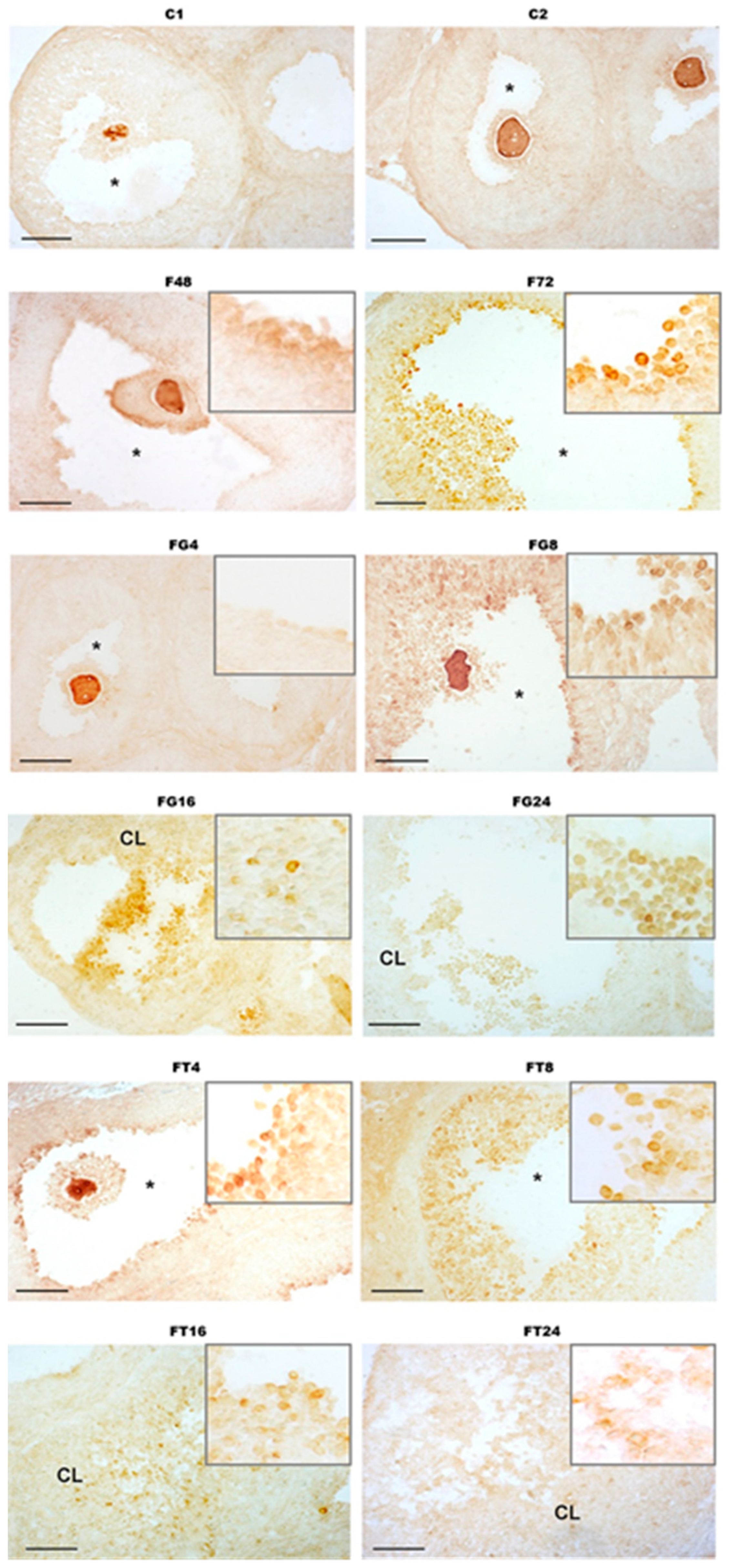
2.7. Immunohistochemical Analysis of the Distribution of Metalloproteinase ADAMTS-1 in the Ovaries of Immature Female Rats and the Effect of Treatment with TP4/2 and hCG
3. Discussion
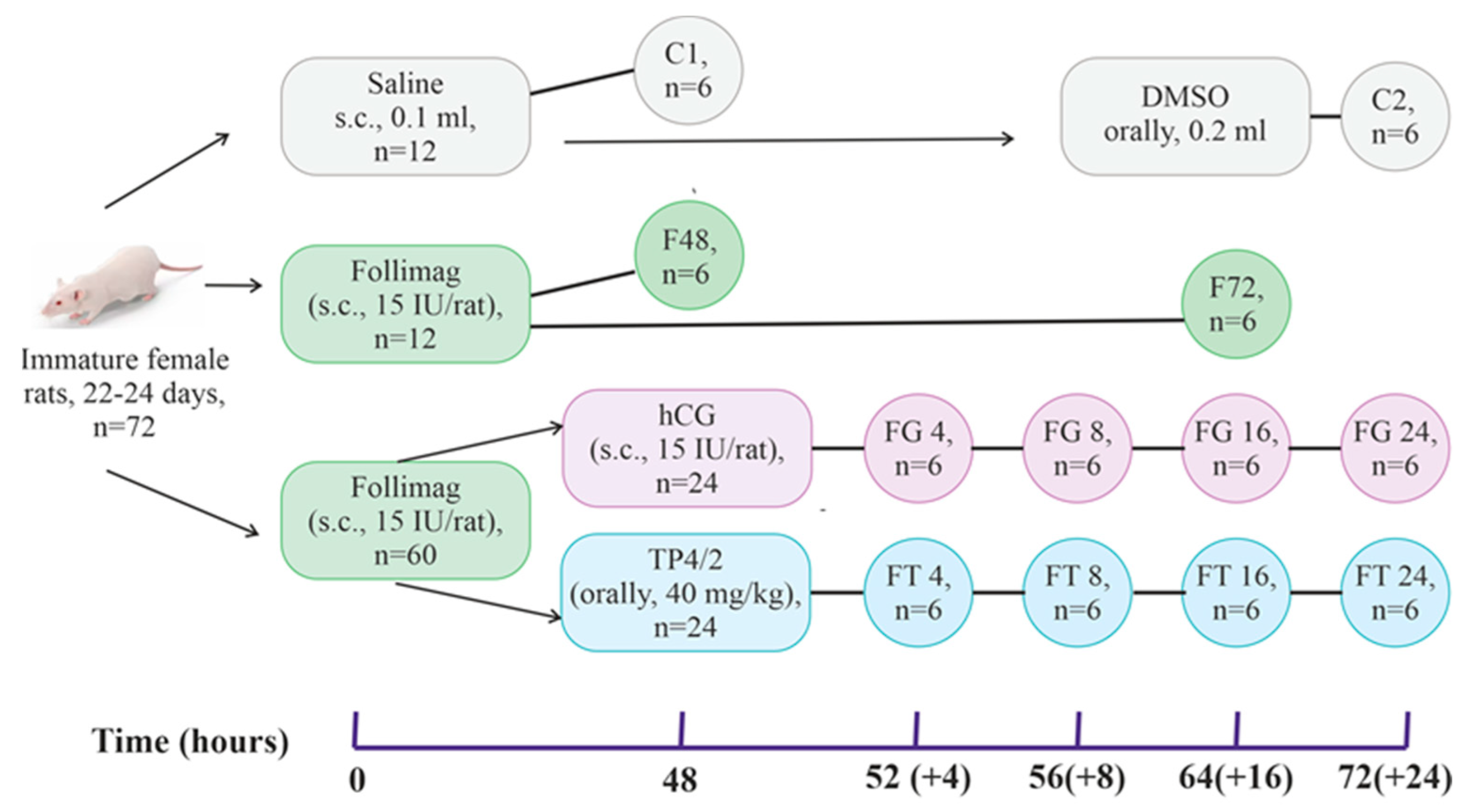
4. Materials and Methods
4.1. Experimental Animals and Ethical Standards for Working with Animals
4.2. The Drugs and Biochemical Reagents
4.3. Procedure for Ovulation Induction in Immature Female Wistar Rats
4.4. Morphological Analysis of the Ovaries
4.5. Immunohistochemical Analysis of the Distribution of Metalloproteinase ADAMTS-1
4.6. Determination of Plasma Levels of Hormones
4.7. Assessment of Ovarian Gene Expression
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADAMTS-1 (gene Adamts-1) | matrix metalloproteinase with thrombospondin motifs 1 (A disintegrin and metalloproteinase with thrombospondin motif) |
| ART | assisted reproductive technology |
| CL | corpus luteum |
| COX-2 (gene Cox-2) | cyclooxygenase-2 |
| CYP11A1 (gene Cyp11a1) | cytochrome P450scc |
| CYP17A1 (gene Cyp17a1) | cytochrome P450 17A1/steroid 17α-monooxygenase |
| CYP19A1 (gene Cyp19a1) | cytochrome P450A1 (aromatase) |
| EGR-1 (gene Egr-1) | early growth response protein-1 |
| FSH | follicle-stimulating hormone |
| GnRH | gonadotropin-releasing hormone |
| hCG | human chorionic gonadotropin |
| LH | luteinizing hormone |
| LH/hCG receptor | luteinizing hormone/human chorionic gonadotropin receptor |
| MT-1 (gene Mt-1) | metallothionein-1 |
| OHSS | ovarian hyperstimulation syndrome |
| StAR (gene Star) | cholesterol-transporting protein (steroidogenic acute regulatory) |
| TP4/2 | 5-amino-N-(tert-butyl)-4-(3-(1-methyl-1H-pyrazole-4-carboxamido)phenyl)-2-(methylthio)thieno[2,3-d]pyrimidine-6-carboxamide |
| VEGF | vascular endothelial growth factor, including the isoforms A (VEGF-A) and B (VEGF-B) |
References
- Martinez, F.; Racca, A.; Rodríguez, I.; Polyzos, N.P. Ovarian stimulation for oocyte donation: A systematic review and meta-analysis. Hum. Reprod. Update 2021, 27, 673–696. [Google Scholar] [CrossRef] [PubMed]
- Segaloff, D.L.; Wang, H.Y.; Richards, J.S. Hormonal regulation of luteinizing hormone/chorionic gonadotropin receptor mRNA in rat ovarian cells during follicular development and luteinization. Mol. Endocrinol. 1990, 4, 1856–1865. [Google Scholar] [CrossRef] [PubMed]
- Menon, B.; Sinden, J.; Franzo-Romain, M.; Botta, R.B.; Menon, K.M. Regulation of LH receptor mRNA binding protein by miR-122 in rat ovaries. Endocrinology 2013, 154, 4826–4834. [Google Scholar] [CrossRef] [PubMed]
- Menon, B.; Gulappa, T.; Menon, K.M. miR-122 Regulates LH Receptor Expression by Activating Sterol Response Element Binding Protein in Rat Ovaries. Endocrinology 2015, 156, 3370–3380. [Google Scholar] [CrossRef]
- Veldhuis, J.D.; Liu, P.Y.; Takahashi, P.Y.; Keenan, D.M. Dynamic testosterone responses to near-physiological LH pulses are determined by the time pattern of prior intravenous LH infusion. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E720–E728. [Google Scholar] [CrossRef]
- Jiang, X.; Dias, J.A.; He, X. Structural biology of glycoprotein hormones and their receptors: Insights to signaling. Mol. Cell Endocrinol. 2014, 382, 424–451. [Google Scholar] [CrossRef]
- Casarini, L.; Simoni, M. Recent advances in understanding gonadotropin signaling. Fac. Rev. 2021, 10, 41. [Google Scholar] [CrossRef]
- Riccetti, L.; Yvinec, R.; Klett, D.; Gallay, N.; Combarnous, Y.; Reiter, E.; Simoni, M.; Casarini, L.; Ayoub, M.A. Human Luteinizing Hormone and Chorionic Gonadotropin Display Biased Agonism at the LH and LH/CG Receptors. Sci. Rep. 2017, 7, 940. [Google Scholar] [CrossRef]
- Banker, M.; Garcia-Velasco, J.A. Revisiting ovarian hyper stimulation syndrome: Towards OHSS free clinic. J. Hum. Reprod. Sci. 2015, 8, 13–17. [Google Scholar] [CrossRef]
- Namavar Jahromi, B.; Parsanezhad, M.E.; Shomali, Z.; Bakhshai, P.; Alborzi, M.; Moin Vaziri, N.; Anvar, Z. Ovarian Hyperstimulation Syndrome: A Narrative Review of Its Pathophysiology, Risk Factors, Prevention, Classification, and Management. Iran. J. Med. Sci. 2018, 43, 248–260. [Google Scholar]
- Cerrillo, M.; Rodríguez, S.; Mayoral, M.; Pacheco, A.; Martínez-Salazar, J.; Garcia-Velasco, J.A. Differential regulation of VEGF after final oocyte maturation with GnRH agonist versus hCG: A rationale for OHSS reduction. Fertil. Steril. 2009, 91 (Suppl. S4), 1526–1528. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.; Chuderland, D.; Ron-El, R.; Shalgi, R.; Ben-Ami, I. GnRH Agonist Triggering Modulates PEDF to VEGF Ratio Inversely to hCG in Granulosa Cells. J. Clin. Endocrinol. Metab. 2015, 100, E1428–E1436. [Google Scholar] [CrossRef]
- Engmann, L.L.; Maslow, B.S.; Kaye, L.A.; Griffin, D.W.; DiLuigi, A.J.; Schmidt, D.W.; Grow, D.R.; Nulsen, J.C.; Benadiva, C.A. Low dose human chorionic gonadotropin administration at the time of gonadotropin releasing-hormone agonist trigger versus 35 h later in women at high risk of developing ovarian hyperstimulation syndrome—A prospective randomized double-blind clinical trial. J. Ovarian Res. 2019, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Heitman, L.H.; Oosterom, J.; Bonger, K.M.; Timmers, C.M.; Wiegerinck, P.H.; Ijzerman, A.P. [3H]-Org 43553, the first low-molecular-weight agonistic and allosteric radioligand for the human luteinizing hormone receptor. Mol. Pharmacol. 2008, 73, 518–524. [Google Scholar] [CrossRef] [PubMed]
- van Koppen, C.J.; Zaman, G.J.; Timmers, C.M.; Kelder, J.; Mosselman, S.; van de Lagemaat, R.; Smit, M.J.; Hanssen, R.G. A signaling-selective, nanomolar potent allosteric low molecular weight agonist for the human luteinizing hormone receptor. Naunyn Schmiedebergs Arch. Pharmacol. 2008, 378, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Nataraja, S.G.; Yu, H.N.; Palmer, S.S. Discovery and Development of Small Molecule Allosteric Modulators of Glycoprotein Hormone Receptors. Front. Endocrinol. 2015, 6, 142. [Google Scholar] [CrossRef]
- Shpakov, A.O. Allosteric Regulation of G-Protein-Coupled Receptors: From Diversity of Molecular Mechanisms to Multiple Allosteric Sites and Their Ligands. Int. J. Mol. Sci. 2023, 24, 6187. [Google Scholar] [CrossRef]
- van Straten, N.C.; Schoonus-Gerritsma, G.G.; van Someren, R.G.; Draaijer, J.; Adang, A.E.; Timmers, C.M.; Hanssen, R.G.; van Boeckel, C.A. The first orally active low molecular weight agonists for the LH receptor: Thienopyr(im)idines with therapeutic potential for ovulation induction. Chembiochem 2002, 3, 1023–1026. [Google Scholar] [CrossRef]
- van de Lagemaat, R.; Timmers, C.M.; Kelder, J.; van Koppen, C.; Mosselman, S.; Hanssen, R.G. Induction of ovulation by a potent, orally active, low molecular weight agonist (Org 43553) of the luteinizing hormone receptor. Hum. Reprod. 2009, 24, 640–648. [Google Scholar] [CrossRef]
- van de Lagemaat, R.; Raafs, B.C.; van Koppen, C.; Timmers, C.M.; Mulders, S.M.; Hanssen, R.G. Prevention of the onset of ovarian hyperstimulation syndrome (OHSS) in the rat after ovulation induction with a low molecular weight agonist of the LH receptor compared with hCG and rec-LH. Endocrinology 2011, 152, 4350–4357. [Google Scholar] [CrossRef]
- Gerrits, M.; Mannaerts, B.; Kramer, H.; Addo, S.; Hanssen, R. First evidence of ovulation induced by oral LH agonists in healthy female volunteers of reproductive age. J. Clin. Endocrinol. Metab. 2013, 98, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Shpakov, A.O.; Dar’in, D.V.; Derkach, K.V.; Lobanov, P.S. The stimulating influence of thienopyrimidine compounds on the adenylyl cyclase signaling systems in the rat testes. Dokl. Biochem. Biophys. 2014, 456, 104–107. [Google Scholar] [CrossRef]
- Derkach, K.V.; Dar’in, D.V.; Bakhtyukov, A.A.; Lobanov, P.S.; Shpakov, A.O. In vitro and in vivo studies of functional activity of new low molecular weight agonists of the luteinizing hormone receptor. Biochem. Mosc. Suppl. Ser. B 2016, 10, 294–300. [Google Scholar] [CrossRef]
- Bakhtyukov, A.A.; Derkach, K.V.; Gureev, M.A.; Dar’in, D.V.; Sorokoumov, V.N.; Romanova, I.V.; Morina, I.Y.; Stepochkina, A.M.; Shpakov, A.O. Comparative Study of the Steroidogenic Effects of Human Chorionic Gonadotropin and Thieno[2,3-D]pyrimidine-Based Allosteric Agonist of Luteinizing Hormone Receptor in Young Adult, Aging and Diabetic Male Rats. Int. J. Mol. Sci. 2020, 21, 7493. [Google Scholar] [CrossRef]
- Bakhtyukov, A.A.; Derkach, K.V.; Sorokoumov, V.N.; Stepochkina, A.M.; Romanova, I.V.; Morina, I.Y.; Zakharova, I.O.; Bayunova, L.V.; Shpakov, A.O. The Effects of Separate and Combined Treatment of Male Rats with Type 2 Diabetes with Metformin and Orthosteric and Allosteric Agonists of Luteinizing Hormone Receptor on Steroidogenesis and Spermatogenesis. Int. J. Mol. Sci. 2021, 23, 198. [Google Scholar] [CrossRef]
- Fokina, E.A.; Derkach, K.V.; Bakhtyukov, A.A.; Sorokoumov, V.N.; Lebedev, I.A.; Morina, I.Y.; Shpakov, A.O. Stimulation of ovulation in immature female rats using orthosteric and allosteric luteinizing hormone receptor agonists. Dokl. Biochem. Biophys. 2022, 507, 345–349. [Google Scholar] [CrossRef]
- Bakhtyukov, A.A.; Derkach, K.V.; Fokina, E.A.; Lebedev, I.A.; Sorokoumov, V.N.; Bayunova, L.V.; Shpakov, A.O. Effect of Different Luteinizing Hormone Receptor Agonists on Ovarian Steroidogenesis in Mature Female Rats. J. Evol. Biochem. Physiol. 2023, 59, 57–68. [Google Scholar] [CrossRef]
- Derkach, K.V.; Dar’in, D.V.; Lobanov, P.S.; Shpakov, A.O. Intratesticular, intraperitoneal, and oral administration of thienopyrimidine derivatives increases the testosterone level in male rats. Dokl. Biol. Sci. 2014, 459, 326–329. [Google Scholar] [CrossRef]
- Miller, W.L.; Auchus, R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.S.; Ascoli, M. Endocrine, Paracrine, and Autocrine Signaling Pathways That Regulate Ovulation. Trends Endocrinol. Metab. 2018, 29, 313–325. [Google Scholar] [CrossRef]
- Soares, S.R. Etiology of OHSS and use of dopamine agonists. Fertil. Steril. 2012, 97, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fang, L.; Zhang, R.; Wang, S.; Li, Y.; Yan, Y.; Yu, Y.; Cheng, J.C.; Sun, Y.P. Melatonin stimulates VEGF expression in human granulosa-lutein cells: A potential mechanism for the pathogenesis of ovarian hyperstimulation syndrome. Mol. Cell Endocrinol. 2020, 518, 110981. [Google Scholar] [CrossRef] [PubMed]
- Espey, L.L.; Richards, J.S. Temporal and spatial patterns of ovarian gene transcription following an ovulatory dose of gonadotropin in the rat. Biol. Reprod. 2002, 67, 1662–1670. [Google Scholar] [CrossRef] [PubMed]
- Mantri, M.; Zhang, H.H.; Spanos, E.; Ren, Y.A.; De Vlaminck, I. A Spatiotemporal Molecular Atlas of the Ovulating Mouse Ovary. bioRxiv 2023. [Google Scholar] [CrossRef]
- Yung, Y.; Maman, E.; Konopnicki, S.; Cohen, B.; Brengauz, M.; Lojkin, I.; Dal Canto, M.; Fadini, R.; Dor, J.; Hourvitz, A. ADAMTS-1: A new human ovulatory gene and a cumulus marker for fertilization capacity. Mol. Cell Endocrinol. 2010, 328, 104–108. [Google Scholar] [CrossRef]
- Ma, Y.; Jin, J.; Tong, X.; Yang, W.; Ren, P.; Dai, Y.; Pan, Y.; Zhang, Y.; Zhang, S. ADAMTS1 and HSPG2 mRNA levels in cumulus cells are related to human oocyte quality and controlled ovarian hyperstimulation outcomes. J. Assist. Reprod. Genet. 2020, 37, 657–667. [Google Scholar] [CrossRef]
- Yang, G.; Yao, G.; Xu, Z.; Fan, H.; Liu, X.; He, J.; Kong, Y.; Kong, D.; Bai, Y.; He, Q.; et al. Expression Level of ADAMTS1 in Granulosa Cells of PCOS Patients Is Related to Granulosa Cell Function, Oocyte Quality, and Embryo Development. Front. Cell Dev. Biol. 2021, 9, 647522. [Google Scholar] [CrossRef]
- Jiang, Y.; He, Y.; Pan, X.; Wang, P.; Yuan, X.; Ma, B. Advances in Oocyte Maturation In Vivo and In Vitro in Mammals. Int. J. Mol. Sci. 2023, 24, 9059. [Google Scholar] [CrossRef]
- Palermo, R. Differential actions of FSH and LH during folliculogenesis. Reprod. Biomed. Online 2007, 15, 326–337. [Google Scholar] [CrossRef]
- Conforti, A.; Vaiarelli, A.; Cimadomo, D.; Bagnulo, F.; Peluso, S.; Carbone, L.; Di Rella, F.; De Placido, G.; Ubaldi, F.M.; Huhtaniemi, I.; et al. Pharmacogenetics of FSH Action in the Female. Front. Endocrinol. 2019, 10, 398. [Google Scholar] [CrossRef]
- Fitzpatrick, S.L.; Carlone, D.L.; Robker, R.L.; Richards, J.S. Expression of aromatase in the ovary: Down-regulation of mRNA by the ovulatory luteinizing hormone surge. Steroids 1997, 62, 197–206. [Google Scholar] [CrossRef]
- Hsueh, A.J.; Adashi, E.Y.; Jones, P.B.; Welsh, T.H., Jr. Hormonal regulation of the differentiation of cultured ovarian granulosa cells. Endocr. Rev. 1984, 5, 76–127. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, X.; Segaloff, D.L. A novel cyclic adenosine 3′,5′-monophosphate-responsive element involved in the transcriptional regulation of the lutropin receptor gene in granulosa cells. Mol. Endocrinol. 2000, 14, 1498–1508. [Google Scholar] [CrossRef][Green Version]
- Yoshino, M.; Mizutani, T.; Yamada, K.; Tsuchiya, M.; Minegishi, T.; Yazawa, T.; Kawata, H.; Sekiguchi, T.; Kajitani, T.; Miyamoto, K. Early growth response gene-1 regulates the expression of the rat luteinizing hormone receptor gene. Biol. Reprod. 2002, 66, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Imai, F.; Kishi, H.; Nakao, K.; Nishimura, T.; Minegishi, T. IL-6 up-regulates the expression of rat LH receptors during granulosa cell differentiation. Endocrinology 2014, 155, 1436–1444. [Google Scholar] [CrossRef] [PubMed]
- Kishi, H.; Kitahara, Y.; Imai, F.; Nakao, K.; Suwa, H. Expression of the gonadotropin receptors during follicular development. Reprod. Med. Biol. 2017, 17, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Khalil, E.; Metwally, M.; Bahgaat, H.; Kassab, A.; El-Shafey, A. Differential expression of FSHR and LHR genes and proteins during development of rabbit ovarian follicles. Zygote 2022, 30, 577–583. [Google Scholar] [CrossRef]
- Singh, P.; Krishna, A. Effects of GnRH agonist treatment on steroidogenesis and folliculogenesis in the ovary of cyclic mice. J. Ovarian Res. 2010, 3, 26. [Google Scholar] [CrossRef]
- Bakhtyukov, A.A.; Derkach, K.V.; Romanova, I.V.; Sorokoumov, V.N.; Sokolova, T.V.; Govdi, A.I.; Morina, I.Y.; Perminova, A.A.; Shpakov, A.O. Effect of low-molecular-weight allosteric agonists of the luteinizing hormone receptor on its expression and distribution in rat testes. J. Evol. Biochem. Physiol. 2021, 57, 208–220. [Google Scholar] [CrossRef]
- Minegishi, T.; Tano, M.; Abe, Y.; Nakamura, K.; Ibuki, Y.; Miyamoto, K. Expression of luteinizing hormone/human chorionic gonadotrophin (LH/HCG) receptor mRNA in the human ovary. Mol. Hum. Reprod. 1997, 3, 101–107. [Google Scholar] [CrossRef]
- Yung, Y.; Aviel-Ronen, S.; Maman, E.; Rubinstein, N.; Avivi, C.; Orvieto, R.; Hourvitz, A. Localization of luteinizing hormone receptor protein in the human ovary. Mol. Hum. Reprod. 2014, 20, 844–849. [Google Scholar] [CrossRef]
- Yung, Y.; Maman, E.; Ophir, L.; Rubinstein, N.; Barzilay, E.; Yerushalmi, G.M.; Hourvitz, A. Progesterone antagonist, RU486, represses LHCGR expression and LH/hCG signaling in cultured luteinized human mural granulosa cells. Gynecol. Endocrinol. 2014, 30, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, J.V.; Kristensen, S.G.; Nielsen, M.E.; Humaidan, P.; Dal Canto, M.; Fadini, R.; Schmidt, K.T.; Ernst, E.; Yding Andersen, C. LH-receptor gene expression in human granulosa and cumulus cells from antral and preovulatory follicles. J. Clin. Endocrinol. Metab. 2012, 97, E1524–E1531. [Google Scholar] [CrossRef] [PubMed]
- LaPolt, P.S.; Oikawa, M.; Jia, X.C.; Dargan, C.; Hsueh, A.J. Gonadotropin-induced up- and down-regulation of rat ovarian LH receptor message levels during follicular growth, ovulation and luteinization. Endocrinology 1990, 126, 3277–3279. [Google Scholar] [CrossRef]
- Nakamura, K.; Minegishi, T.; Takakura, Y.; Miyamoto, K.; Hasegawa, Y.; Ibuki, Y.; Igarashi, M. Hormonal regulation of gonadotropin receptor mRNA in rat ovary during follicular growth and luteinization. Mol. Cell Endocrinol. 1991, 82, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, E.G.; Tournaye, H.; Verpoest, W.; Camus, M.; Vernaeve, V.; Van Steirteghem, A.; Devroey, P. Early and late ovarian hyperstimulation syndrome: Early pregnancy outcome and profile. Hum. Reprod. 2005, 20, 636–641. [Google Scholar] [CrossRef]
- Bodri, D.; Guillén, J.J.; Galindo, A.; Mataró, D.; Pujol, A.; Coll, O. Triggering with human chorionic gonadotropin or a gonadotropin-releasing hormone agonist in gonadotropin-releasing hormone antagonist-treated oocyte donor cycles: Findings of a large retrospective cohort study. Fertil. Steril. 2009, 91, 365–371. [Google Scholar] [CrossRef]
- Kasap, E.; Turan, G.A.; Eskicioğlu, F.; Cengiz, H.; Gur, E.B.; Sivrikoz, O.N.; Genc, M.; Yılmaz, O. Comparison between resveratrol and cabergoline in preventing ovarian hyperstimulation syndrome in a rat model. Gynecol. Endocrinol. 2016, 32, 634–640. [Google Scholar] [CrossRef]
- Hortu, I.; Karadadas, E.; Ozceltik, G.; Tavmergen, E.; Tavmergen Goker, E.N.; Yigitturk, G.; Erbas, O. Oxytocin and cabergoline alleviate ovarian hyperstimulation syndrome (OHSS) by suppressing vascular endothelial growth factor (VEGF) in an experimental model. Arch. Gynecol. Obstet. 2021, 303, 1099–1108. [Google Scholar] [CrossRef]
- Darabi, Z.; Basir, Z.; Tabandeh, M.R.; Ghotbeddin, Z. Coenzyme Q10 improves ovarian histology and attenuates the expression of angiogenesis-associated proteins in the ovary of rats with experimental hyperstimulation syndrome. Iran. J. Basic. Med. Sci. 2022, 25, 989–996. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, J.; He, X.; Li, N.; Miao, Y.; Li, B.; Shao, X.; Wang, N. Ginkgo biloba extract 761 reduces vascular permeability of the ovary and improves the symptom of ovarian hyperstimulation syndrome in a rat model. Gynecol. Endocrinol. 2022, 38, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Harada, M.; Hirota, Y.; Zhao, L.; Yoshino, O.; Urata, Y.; Izumi, G.; Takamura, M.; Hirata, T.; Koga, K.; et al. A potential role of endoplasmic reticulum stress in development of ovarian hyperstimulation syndrome. Mol. Cell Endocrinol. 2016, 428, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Martazanova, B.; Mishieva, N.; Vtorushina, V.; Vedikhina, I.; Levkov, L.; Korneeva, I.; Kirillova, A.; Krechetova, L.; Abubakirov, A.; Sukhikh, G.T. Angiogenic cytokine and interleukin 8 levels in early luteal phase after triggering ovulation with gonadotropin-releasing hormone agonist in high-responder patients. Am. J. Reprod. Immunol. 2021, 85, e13381. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, R.; Luo, X.; Xu, F.; Yang, M.; Zheng, L.; Wu, Q.; Jiang, W.; Li, Y. Vascular endothelial growth factor B regulates insulin secretion in β cells of type 2 diabetes mellitus mice via PLCγ and the IP3R-evoked Ca2+/CaMK2 signaling pathway. Mol. Med. Rep. 2023, 28, 197. [Google Scholar] [CrossRef] [PubMed]
- Arjunan, P.; Lin, X.; Tang, Z.; Du, Y.; Kumar, A.; Liu, L.; Yin, X.; Huang, L.; Chen, W.; Chen, Q.; et al. VEGF-B is a potent antioxidant. Proc. Natl. Acad. Sci. USA 2018, 115, 10351–10356. [Google Scholar] [CrossRef]
- Albrecht, I.; Kopfstein, L.; Strittmatter, K.; Schomber, T.; Falkevall, A.; Hagberg, C.E.; Lorentz, P.; Jeltsch, M.; Alitalo, K.; Eriksson, U.; et al. Suppressive effects of vascular endothelial growth factor-B on tumor growth in a mouse model of pancreatic neuroendocrine tumorigenesis. PLoS ONE 2010, 5, e14109. [Google Scholar] [CrossRef]
- Sanmartín, E.; Sirera, R.; Usó, M.; Blasco, A.; Gallach, S.; Figueroa, S.; Martínez, N.; Hernando, C.; Honguero, A.; Martorell, M.; et al. A gene signature combining the tissue expression of three angiogenic factors is a prognostic marker in early-stage non-small cell lung cancer. Ann. Surg. Oncol. 2014, 21, 612–620. [Google Scholar] [CrossRef]
- Lee, C.; Chen, R.; Sun, G.; Liu, X.; Lin, X.; He, C.; Xing, L.; Liu, L.; Jensen, L.D.; Kumar, A.; et al. VEGF-B prevents excessive angiogenesis by inhibiting FGF2/FGFR1 pathway. Signal Transduct. Target. Ther. 2023, 8, 305. [Google Scholar] [CrossRef]
- Olofsson, B.; Korpelainen, E.; Pepper, M.S.; Mandriota, S.J.; Aase, K.; Kumar, V.; Gunji, Y.; Jeltsch, M.M.; Shibuya, M.; Alitalo, K.; et al. Vascular endothelial growth factor B (VEGF-B) binds to VEGF receptor-1 and regulates plasminogen activator activity in endothelial cells. Proc. Natl. Acad. Sci. USA 1998, 95, 11709–11714. [Google Scholar] [CrossRef]
- Ho, V.C.; Duan, L.J.; Cronin, C.; Liang, B.T.; Fong, G.H. Elevated vascular endothelial growth factor receptor-2 abundance contributes to increased angiogenesis in vascular endothelial growth factor receptor-1-deficient mice. Circulation 2012, 126, 741–752. [Google Scholar] [CrossRef]
- Karaman, S.; Leppänen, V.M.; Alitalo, K. Vascular endothelial growth factor signaling in development and disease. Development 2018, 145, dev151019. [Google Scholar] [CrossRef] [PubMed]
- Christenson, L.K.; Gunewardena, S.; Hong, X.; Spitschak, M.; Baufeld, A.; Vanselow, J. Research resource: Preovulatory LH surge effects on follicular theca and granulosa transcriptomes. Mol. Endocrinol. 2013, 27, 1153–1171. [Google Scholar] [CrossRef] [PubMed]
- Lussier, J.G.; Diouf, M.N.; Lévesque, V.; Sirois, J.; Ndiaye, K. Gene expression profiling of upregulated mRNAs in granulosa cells of bovine ovulatory follicles following stimulation with hCG. Reprod. Biol. Endocrinol. 2017, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Guerrero-Netro, H.; Estienne, A.; Cao, B.; Price, C.A. Regulation and action of early growth response 1 in bovine granulosa cells. Reproduction 2017, 154, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Sayasith, K.; Brown, K.A.; Lussier, J.G.; Doré, M.; Sirois, J. Characterization of bovine early growth response factor-1 and its gonadotropin-dependent regulation in ovarian follicles prior to ovulation. J. Mol. Endocrinol. 2006, 37, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.L.; Sadovsky, Y.; Swirnoff, A.H.; Polish, J.A.; Goda, P.; Gavrilina, G.; Milbrandt, J. Luteinizing hormone deficiency and female infertility in mice lacking the transcription factor NGFI-A (Egr-1). Science 1996, 273, 1219–1221. [Google Scholar] [CrossRef]
- Topilko, P.; Schneider-Maunoury, S.; Levi, G.; Trembleau, A.; Gourdji, D.; Driancourt, M.A.; Rao, C.V.; Charnay, P. Multiple pituitary and ovarian defects in Krox-24 (NGFI-A, Egr-1)-targeted mice. Mol. Endocrinol. 1998, 12, 107–122. [Google Scholar] [CrossRef]
- Carletti, M.Z.; Christenson, L.K. Rapid effects of LH on gene expression in the mural granulosa cells of mouse periovulatory follicles. Reproduction 2009, 137, 843–855. [Google Scholar] [CrossRef]
- Punetha, M.; Chouhan, V.S.; Sonwane, A.; Singh, G.; Bag, S.; Green, J.A.; Whitworth, K.; Sarkar, M. Early growth response gene mediates in VEGF and FGF signaling as dissected by CRISPR in corpus luteum of water buffalo. Sci. Rep. 2020, 10, 6849. [Google Scholar] [CrossRef]
- Berisha, B.; Rodler, D.; Schams, D.; Sinowatz, F.; Pfaffl, M.W. Prostaglandins in Superovulation Induced Bovine Follicles During the Preovulatory Period and Early Corpus Luteum. Front. Endocrinol. 2019, 10, 467. [Google Scholar] [CrossRef]
- Geng, T.; Sun, Y.; Cheng, L.; Cao, Y.; Zhang, M.; Hong, Z.; Ma, L.; Zhang, Y. Downregulation of LHCGR Attenuates COX-2 Expression and Induces Luteinized Unruptured Follicle Syndrome in Endometriosis. Front. Endocrinol. 2022, 13, 853563. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.J.; Lennard, D.E.; Lee, C.A.; Tiano, H.F.; Morham, S.G.; Wetsel, W.C.; Langenbach, R. Anovulation in cyclooxygenase-2-deficient mice is restored by prostaglandin E2 and interleukin-1beta. Endocrinology 1999, 140, 2685–2695. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.M.; Stouffer, R.L. Follicular administration of a cyclooxygenase inhibitor can prevent oocyte release without alteration of normal luteal function in rhesus monkeys. Hum. Reprod. 2002, 17, 2825–2831. [Google Scholar] [CrossRef] [PubMed]
- Niringiyumukiza, J.D.; Cai, H.; Xiang, W. Prostaglandin E2 involvement in mammalian female fertility: Ovulation, fertilization, embryo development and early implantation. Reprod. Biol. Endocrinol. 2018, 16, 43. [Google Scholar] [CrossRef]
- Russell, D.L.; Doyle, K.M.; Ochsner, S.A.; Sandy, J.D.; Richards, J.S. Processing and localization of ADAMTS-1 and proteolytic cleavage of versican during cumulus matrix expansion and ovulation. J. Biol. Chem. 2003, 278, 42330–42339. [Google Scholar] [CrossRef]
- Hernández-Delgado, P.; Felix-Portillo, M.; Martínez-Quintana, J.A. ADAMTS Proteases: Importance in Animal Reproduction. Genes 2023, 14, 1181. [Google Scholar] [CrossRef]
- Xiao, S.; Li, Y.; Li, T.; Chen, M.; Xu, Y.; Wen, Y.; Zhou, C. Evidence for decreased expression of ADAMTS-1 associated with impaired oocyte quality in PCOS patients. J. Clin. Endocrinol. Metab. 2014, 99, E1015–E1021. [Google Scholar] [CrossRef]
- GohariTaban, S.; Amiri, I.; Soleimani Asl, S.; Saidijam, M.; Yavangi, M.; Khanlarzadeh, E.; Mohammadpour, N.; Shabab, N.; Artimani, T. Abnormal expressions of ADAMTS-1, ADAMTS-9 and progesterone receptors are associated with lower oocyte maturation in women with polycystic ovary syndrome. Arch. Gynecol. Obstet. 2019, 299, 277–286. [Google Scholar] [CrossRef]
- Xie, Y.; Guo, W.; Shen, X.; Yu, W.; Kuang, Y.; Chen, Q.; Long, H.; Lyu, Q.; Wang, L. A delayed ovulation of progestin-primed ovarian stimulation (PPOS) by downregulating the LHCGR/PGR pathway. iScience 2023, 26, 107357. [Google Scholar] [CrossRef]
- Rosewell, K.L.; Al-Alem, L.; Zakerkish, F.; McCord, L.; Akin, J.W.; Chaffin, C.L.; Brännström, M.; Curry, T.E., Jr. Induction of proteinases in the human preovulatory follicle of the menstrual cycle by human chorionic gonadotropin. Fertil. Steril. 2015, 103, 826–833. [Google Scholar] [CrossRef]
- Zhu, Y. Metalloproteases in gonad formation and ovulation. Gen. Comp. Endocrinol. 2021, 314, 113924. [Google Scholar] [CrossRef] [PubMed]
- Henríquez, S.; Kohen, P.; Muñoz, A.; Godoy, A.; Orge, F.; Strauss, J.F., 3rd; Devoto, L. In-vitro study of gonadotrophin signaling pathways in human granulosa cells in relation to progesterone receptor expression. Reprod. Biomed. Online 2017, 35, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Doyle, K.M.; Russell, D.L.; Sriraman, V.; Richards, J.S. Coordinate transcription of the ADAMTS-1 gene by luteinizing hormone and progesterone receptor. Mol. Endocrinol. 2004, 18, 2463–2478. [Google Scholar] [CrossRef]
- Espey, L.L.; Ujioka, T.; Okamura, H.; Richards, J.S. Metallothionein-1 messenger RNA transcription in steroid-secreting cells of the rat ovary during the periovulatory period. Biol. Reprod. 2003, 68, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, R.G.J.M.; Timmers, C.M. Thieno[2,3-d]pyrimidines with Combined LH and FSH Agonistic Activity. Patent WO2003020726, 13 March 2003. [Google Scholar]
- Pedersen, T.; Peters, H. Proposal for a classification of oocytes and follicles in the mouse ovary. J. Reprod. Fertil. 1968, 17, 555–557. [Google Scholar] [CrossRef]
- Hirshfield, A.N. Development of follicles in the mammalian ovary. Int. Rev. Cytol. 1991, 124, 43–101. [Google Scholar] [CrossRef]
- Brown, H.M.; Dunning, K.R.; Robker, R.L.; Pritchard, M.; Russell, D.L. Requirement for ADAMTS-1 in extracellular matrix remodeling during ovarian folliculogenesis and lymphangiogenesis. Dev. Biol. 2006, 300, 699–709. [Google Scholar] [CrossRef]
- Carter, L.E.; Cook, D.P.; Collins, O.; Gamwell, L.F.; Dempster, H.A.; Wong, H.W.; McCloskey, C.W.; Garson, K.; Vuong, N.H.; Vanderhyden, B.C. COX2 is induced in the ovarian epithelium during ovulatory wound repair and promotes cell survival. Biol. Reprod. 2019, 101, 961–974. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
| Rat Group | Body Weight, g | Weight of the Right and Left Ovaries, mg | Ratio of Ovary Weight/Body Weight, % |
|---|---|---|---|
| C1 | 53.9 ± 1.6 | 11.5 ± 1.0 | 0.021 ± 0.002 |
| C2 | 62.3 ± 1.9 | 15.0 ± 1.1 a | 0.024 ± 0.001 |
| F48 | 55.4 ± 1.7 | 26.4 ± 0.9 a | 0.048 ± 0.002 a |
| F72 | 63.7 ± 2.0 a | 27.0 ± 1.4 a | 0.043 ± 0.002 a |
| FG4 | 57.1 ± 2.1 | 36.5 ± 2.2 a,b,c | 0.064 ± 0.003 a,b,c |
| FG8 | 56.1 ± 1.8 | 39.5 ± 2.3 a,b,c | 0.071 ± 0.004 a,b,c |
| FG16 | 61.7 ± 1.5 | 47.3 ± 2.5 a,b,c,d | 0.077 ± 0.004 a,b,c,d |
| FG24 | 62.1 ± 2.8 | 43.4 ± 1.5 a,b,c | 0.070 ± 0.004 a,b,c |
| FT4 | 56.6 ± 1.8 | 28.8 ± 1.8 a | 0.051 ± 0.003 a |
| FT8 | 58.4 ± 1.8 | 31.9 ± 2.0 a | 0.055 ± 0.003 a,c |
| FT16 | 59.0 ± 2.0 | 37.4 ± 1.7 a,b,c,d | 0.064 ± 0.003 a,b,c,d |
| FT24 | 58.6 ± 2.2 | 39.9 ± 1.2 a,b,c,d,e | 0.069 ± 0.003 a,b,c,d,e |
| Rat Group | Tertiary (Antral) Follicles, Units | Preovulatory Follicles, Units | CL, Units |
|---|---|---|---|
| C1 | 54.5 (48.0; 61.8) | 2.0 (0.8; 4.3) | ND |
| C2 | 51.5 (41.3; 55.0) | 2.0 (2.0; 3.5) | ND |
| F48 | 52.5 (47.8; 58.0) | 4.5 (4.0; 5.5) | ND |
| F72 | 52.5 (45.5; 57.8) | 6.5 (5.8; 8.5) | ND |
| FG4 | 64.0 (53.0; 73.0) | 12.5 (9.0; 18.8) a,b | ND |
| FG8 | 50.0 (45.3; 51.3) | 4.0 (2.8; 5.3) d | 0.3 ± 0.2 (0, 0, 1, 0, 0, 1) |
| FG16 | 41.0 (39.3; 45.0) | 1.5 (1.0: 4.0) c,d | 7.8 ± 1.0 (9, 4, 11, 6, 9, 8) |
| FG24 | 32.0 (28.5; 37.5) a,b,c,d,e,f | 1.0 (0.0; 2.0) b,c,d | 3.5 ± 0.3 (3, 3, 4, 5, 3, 3) |
| FT4 | 59.5 (52.5; 69.0) | 14.0 (9.8; 19.5) a,b | ND |
| FT8 | 46.5 (43.5; 54.0) | 4.5 (3.5; 6.3) d | 0.2 ± 0.2 (0, 0, 0, 1, 0, 0) |
| FT16 | 47.0 (44.5; 52.0) | 2.0 (1.0; 3.3) c,d | 2.5 ± 0.4 (2, 4, 1, 3, 3, 2) # |
| FT24 | 41.5 (35.8; 46.3) | 0.0 (0.0; 1.0) b,c,d,e | 7.2 ± 0.8 (5, 6, 9, 6, 7, 10) # |
| Rat Group | Estradiol, nmol/L | Progesterone, nmol/L | LH, ng/mL |
|---|---|---|---|
| C1 | 0.73 (0.66; 0.76) | 20.84 (14.69; 23.32) | 0.293 (0.249; 0.305) |
| C2 | 0.64 (0.43; 0.73) | 21.91 (15.71; 27.41) | 0.267 (0.248; 0.347) |
| F48 | 1.41 (1.10; 1.77) a | 19.69 (18.86; 27.50) | 0.080 (0.055; 0.087) a |
| F72 | 0. 58 (0.47; 0.71) | 23.87 (19.15; 29.80) | 0.199 (0.114; 0.256) b |
| FG4 | 5.01 (2.80; 6.06) a,b,c | 18.71 (15.52; 22.39) | 0.206 (0.137; 0.362) b |
| FG8 | 1.12 (1.00; 1.24) a,d | 35.95 (28.97; 38.01) a | 0.064 (0.036; 0.102) a,d |
| FG16 | 0.75 (0.73; 0.77) b,d,e | 62.02 (49.17; 78.90) a,b,c,d,e | 0.164 (0.117; 0.205) a,b |
| FG24 | 1.01 (0.94; 1,10) a,d,f | 12.75 (9.70; 14.29) c,f,e | 0.166 (0.134; 0.256) b,e |
| FT4 | 4.40 (2.83; 5.30) a,b,c | 19.01 (14.82; 20.78) | 0.258 (0.228; 0.318) b |
| FT8 | 1.65 (1.54; 1.69) a,c,d,# | 25.21 (19.69; 27.86) # | 0.167 (0.156; 0.277) b,# |
| FT16 | 1.15 (1.01; 1.27) a,d,# | 40.69 (35.14; 46.76) a,b,c,d,e,# | 0.240 (0.183; 0.275) b |
| FT24 | 1.22 (0.96; 1.35) a,d | 14.09 (13.12; 19.07) f | 0.223 (0.190; 0.282) b |
| Rat Group | Lhcgr | Star | Cyp11a1 | Cyp17a1 | Cyp19a1 |
|---|---|---|---|---|---|
| C1 | 0.98 (0.82; 1.19) | 1.17 (1.15; 1.21) | 0.94 (0.87; 1.30) | 0.96 (0.83; 1.32) | 0.99 (0.73; 1.29) |
| C2 | 1.06 (1.00; 1.08) | 1.15 (0.77; 1.40) | 0.89 (0.85; ±0.96) | 1.07 (1.02; 1.67) | 1.04 (0.93; 1.31) |
| F48 | 6.97 (6.18; 8.95) a | 1.16 (1.05; 1.65) | 4.47 (4.11; 5.14) a | 1.07 (0.72; 1.16) | 1.31 (0.93; 1.62) |
| F72 | 5.99 (4.02; 7.77) a | 4.41 (2.41; 5.04) a,b | 4.82 (2.50; 6.51) a | 1.63 (1.17; 1.94) | 0.75 (0.60; 0.88) |
| FG4 | 8.64 (6.57; 10.45) a | 14.53 (12.62; 21.11) a,b,c | 14.61 (13.59; 16.60) a,b,c | 3.14 (2.11; 3.68) a,b | 2.48 (2.27; 3.00) a,b,c |
| FG8 | 0.71 (0.45; 1.00) b,c,d | 15.92 (14.09; 18.17) a,b,c | 7.52 (6.93; 9.31) a,b,d | 6.10 (4.81; 7.84) a,b,c | 0.13 (0.08; 0.16) a,b,c,d |
| FG16 | 0.52 (0.36; 0.76) b,c,d | 18.90 (13.20; 20.98) a,b,c | 14.46 (11.78; 16.91) a,b,c,e | 5.09 (4.29; 6.22) a,b,c | 0.13 (0.08; 0.17) a,b,c,d |
| FG24 | 0.65 (0.56; 0.73) b,c,d | 12.74 (10.14; 12.89) a,b,c | 14.02 (12.00; 14.77) a,b,c,e | 2.50 (2.15; 2.89) a,b,e,f | 0.14 (0.11; 0.23) a,b,c,d |
| FT4 | 8.88 (7.79; 9.90) a | 15.69 (13.64; 19.81) a,b,c | 13.91 (11.67; 15.41) a,b,c | 2.43 (1.99; 3.35) a,b | 1.73 (1.58; 3.14) |
| FT8 | 8.79 (7.94; 11.35) a,# | 6.73 (5.02; 9.22) a,b,d,# | 9.06 (7.17; 10.23) a,b,c | 4.93 (4.37; 7.10) a,b,c,d | 0.73 (0.67; 0.85) d,# |
| FT16 | 7.84 (6.90; 11.20) a,# | 7.42 (6.17; 8.57) a,b,d,# | 9.28 (9.06; 9.44) a,b,c,d,# | 4.63 (4.30; 5.25) a,b,c | 0.93 (0.83; 1.02) # |
| FT24 | 5.97 (4.88; 7.12) a,# | 3.73 (3.12; 4.34) a,b,d,# | 7.69 (7.43; 8.76) a,b,c,d,# | 2.84 (1.90; 3.08) a,b,e,f | 0.91 (0.66; 1.01) d,# |
| Rat Group | Vegf-a | Vegf-b |
|---|---|---|
| C1 | 1.04 (0.86; 1.31) | 0.93 (0.59; 1.34) |
| C2 | 1.21 (0.96; 1.34) | 0.99 (0.85; 1.20) |
| F48 | 0.72 (0.69; 0.86) | 0.08 (0.05; 0.17) a |
| F72 | 1.38 (0.97; 1.93) | 0.07 (0.04; 0,08) a |
| FG4 | 3.34 (2.84; 3.60) a,b | 0.12 (0.06; 0.22) a |
| FG8 | 2.24 (2.09; 2.51) a,b | 0.15 (0.08; 0.17) a |
| FG16 | 2.70 (2.05; 3.36) a,b | 0.10 (0.09, 0.12) a |
| FG24 | 1.87 (1.62; 2,12) b,d | 0.17 (0.11; 0.25) a |
| FT4 | 2.98 (1.91; 3.45) b | 0.07 (0.05; 0.08) a |
| FT8 | 1.05 (0.95; 1.09) d,# | 0.07 (0.06; 0.09) a |
| FT16 | 1.16 (0.87; 1.25) # | 0.05 (0.04; 0.06) a,# |
| FT24 | 1.09 (0.73; 1.18) d,# | 0.04 (0.03; 0.05) a,e,# |
| Rat Group | Cox-2 | Mt-1 | Adamts-1 | Egr-1 |
|---|---|---|---|---|
| C1 | 0.98 (0.52; 1.51) | 1.10 (0.90; 1.19) | 1.07 (0.73; 1.26) | 1.07 (0.86; 1.39) |
| C2 | 0.96 (0.61; 1.48) | 0.96 (0.83; 1.34) | 1.00 (0.73; 1.21) | 1.15 (0.84; 1.42) |
| F48 | 0.28 (0.22; 0.48) | 1.06 (0.96; 1.21) | 1.29 (1.06; 1.54) | 1.68 (1.61; 2.18) |
| F72 | 1.32 (0.66; 1.89) | 1.48 (1.21; 1.51) | 2.37 (2.22; 3.06) a,b | 4.13 (2.30; 5.95) a |
| FG4 | 35.90 (26.66; 46.92) a,b,c | 1.30 (1.06; 1.63) | 2.09 (1.89; 2.29) a,b | 44.52 (26.24; 51.72) a,b,c |
| FG8 | 1.96 (1.19; 3.88) b,d | 2.25 (1.40; 2.51) | 5.18 (4.93; 6.43) a,b | 2.24 (1.89; 2.42) d |
| FG16 | 0.59 (0.54; 0.84) d | 5.22 (3.52; 6.32) a,b,c,d,e | 2.54 (2.33; 3.19) a,b,c,d | 1.79 (1.61; 2.81) d |
| FG24 | 0.64 (0.49; 0,95) d | 11.16 (10.17; 15.26) a,b,c,d,e,f | 1.87 (1.39; 2.71) a,b | 2.34 (2.02; 2.42) d |
| FT4 | 29.75 (22.45; 35.83) a,b,c | 1.99 (1.62; 2.54) | 2.73 (2.38; 4.23) a,b | 42.49 (29.87; 53.28) a,b,c |
| FT8 | 2.51 (1.24; 4.11) b,d | 3.09 (2.14; 3.35) | 4.92 (3.27; 5.14) a,b | 2.58 (1.96; 3.28) a,d |
| FT16 | 0.53 (0.47; 0.59) d | 3.28 (2.86; 3.35) a,b,c | 3.01 (2.70; 3.35) a,b | 2.47 (2.22; 2.69) d |
| FT24 | 0.35 (0.31; 0.48) d,e | 4.54 (3.91; 5.77) a,b,c,d,# | 1.81 (1.73; 2.01) a,b,e,f | 1.04 (0.94; 1.29) c,d,e,# |
| Genes | Forward/Reverse Sequence | Product Size (bp) | Annealing Temperature (°C) | Genbank |
|---|---|---|---|---|
| StAR | (For) AAGGCTGGAAGAAGGAAAGC (Rev) CACCTGGCACCACCTTACTT | 66 | 55 | NM_031558.3 |
| Cyp11a1 | (For) TATTCCGCTTTGCCTTTGAG (Rev) CACGATCTCCTCCAACATCC | 74 | 55 | NM_017286.3 |
| Cyp17a1 | (For) CATCCCCCACAAGGCTAAC (Rev) TGTGTCCTTGGGGACAGTAAA | 61 | 55 | XM_006231435.3 |
| Cyp19a1 | (For) GGTATCAGCCTGTCGTGGAC (Rev) AGCCTGTGCATTCTTCCGAT | 118 | 56 | NM_017085.2 |
| Lhcgr | (For) CTGCGCTGTCCTGGCC (Rev) CGACCTCATTAAGTCCCCTGAA | 103 | 55 | NM_012978.1 |
| Vegf-a | (For) CACTGGACCCTGGCTTTACT (Rev) GACGTCCATGAACTTCACCA | 62 | 55 | NM_001287114.1 |
| Vegf-b | (For) GCACAAATCAGATGGTGAGAGA (Rev) CAGGAGATGGTTGATGGCTTAG | 101 | 55 | XM_006230911.4 |
| Cox-2 | (For) ATCAAAGCCTTCGCCACTCA (Rev) ACGGGGCCTTCAAAATGTCT | 79 | 55 | NM_017232.4 |
| Egr-1 | (For) CGTAATCCAAGGGGGTCCAG (Rev) GTGTAAGCTCATCCGAGCGA | 196 | 55 | NM_012551.3 |
| Adamts-1 | (For) CTGCTGCCCTCAGGTGTAAA (Rev) TGAGTGGACTAAAGCTGCGG | 187 | 55 | NM_024400.2 |
| Mt-1 | (For) CTGGCACCACACCTTCTACA (Rev) ATGCTCGGTAGAAAACGGGG | 94 | 55 | NM_138826.4 |
| Actb | (For) CTGGCACCACACCTTCTACA (Rev) AGGTCTCAAACATGATCTGGGT | 125 | 55 | NM_031144.3 |
| 18S rRNA | (For) GGACACGGACAGGATTGACA (Rev) ACCCACGGAATCGAGAAAGA | 50 | 56 | XM_039106097.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derkach, K.V.; Lebedev, I.A.; Morina, I.Y.; Bakhtyukov, A.A.; Pechalnova, A.S.; Sorokoumov, V.N.; Kuznetsova, V.S.; Romanova, I.V.; Shpakov, A.O. Comparison of Steroidogenic and Ovulation-Inducing Effects of Orthosteric and Allosteric Agonists of Luteinizing Hormone/Chorionic Gonadotropin Receptor in Immature Female Rats. Int. J. Mol. Sci. 2023, 24, 16618. https://doi.org/10.3390/ijms242316618
Derkach KV, Lebedev IA, Morina IY, Bakhtyukov AA, Pechalnova AS, Sorokoumov VN, Kuznetsova VS, Romanova IV, Shpakov AO. Comparison of Steroidogenic and Ovulation-Inducing Effects of Orthosteric and Allosteric Agonists of Luteinizing Hormone/Chorionic Gonadotropin Receptor in Immature Female Rats. International Journal of Molecular Sciences. 2023; 24(23):16618. https://doi.org/10.3390/ijms242316618
Chicago/Turabian StyleDerkach, Kira V., Ivan A. Lebedev, Irina Yu. Morina, Andrey A. Bakhtyukov, Alena S. Pechalnova, Viktor N. Sorokoumov, Veronica S. Kuznetsova, Irina V. Romanova, and Alexander O. Shpakov. 2023. "Comparison of Steroidogenic and Ovulation-Inducing Effects of Orthosteric and Allosteric Agonists of Luteinizing Hormone/Chorionic Gonadotropin Receptor in Immature Female Rats" International Journal of Molecular Sciences 24, no. 23: 16618. https://doi.org/10.3390/ijms242316618
APA StyleDerkach, K. V., Lebedev, I. A., Morina, I. Y., Bakhtyukov, A. A., Pechalnova, A. S., Sorokoumov, V. N., Kuznetsova, V. S., Romanova, I. V., & Shpakov, A. O. (2023). Comparison of Steroidogenic and Ovulation-Inducing Effects of Orthosteric and Allosteric Agonists of Luteinizing Hormone/Chorionic Gonadotropin Receptor in Immature Female Rats. International Journal of Molecular Sciences, 24(23), 16618. https://doi.org/10.3390/ijms242316618





