The Alga Uronema belkae Has Two Structural Types of [FeFe]-Hydrogenases with Different Biochemical Properties
Abstract
1. Introduction
2. Results
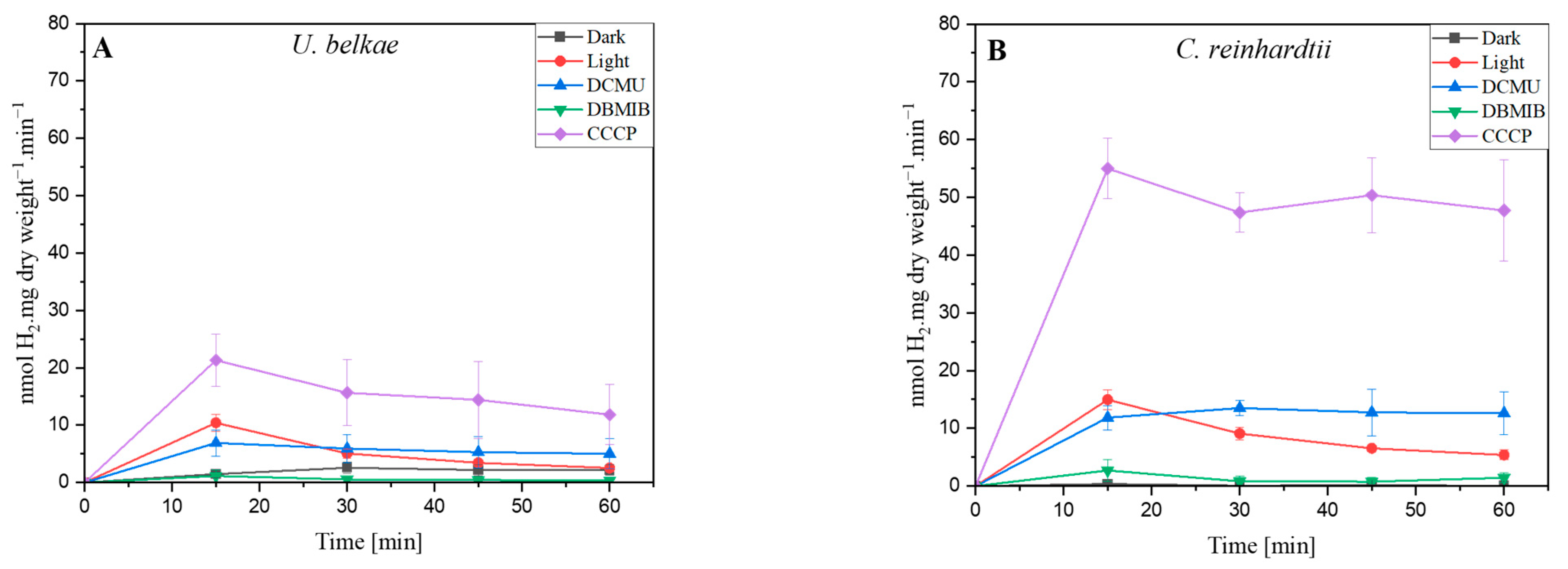
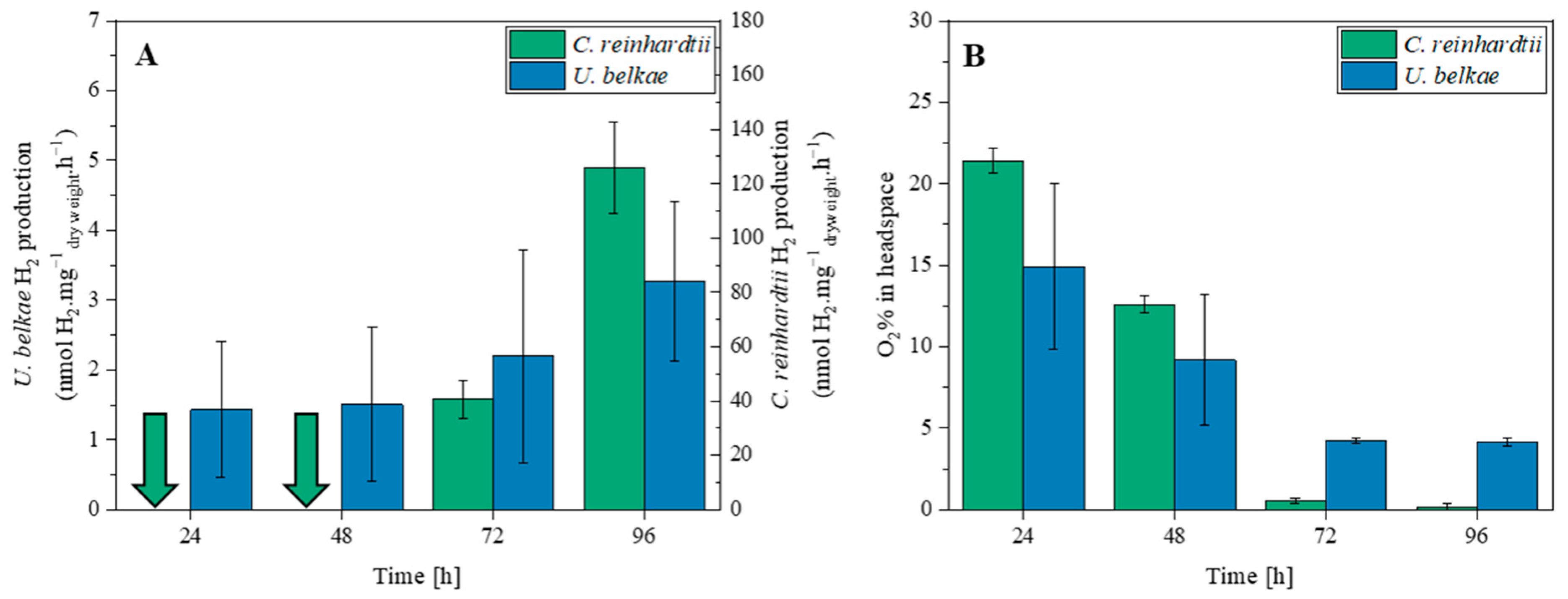
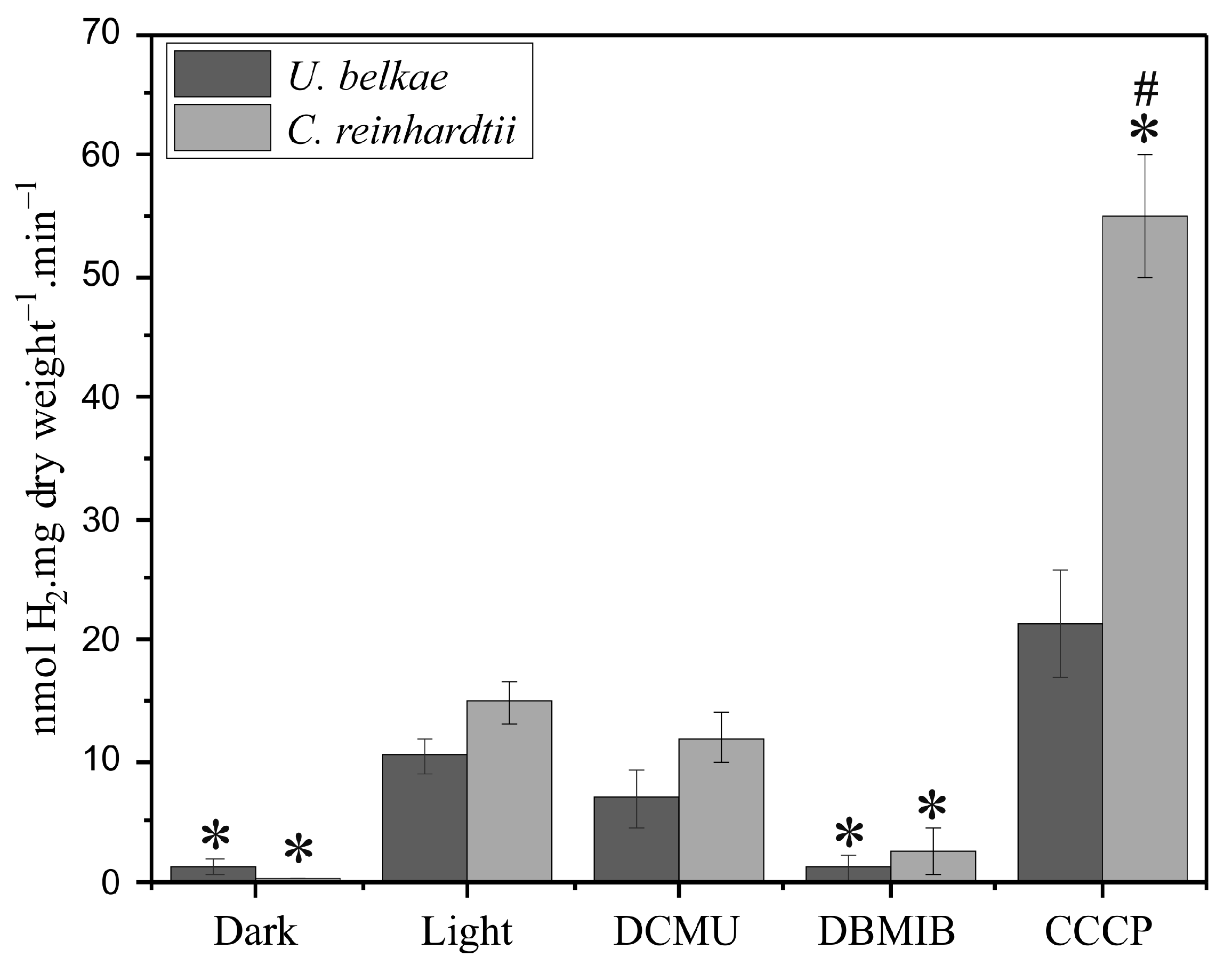
2.1. H2 Production of U. belkae Is Connected to Photosynthesis
2.2. Recombinant UbHydA1 and UbHydA2 Are Active [FeFe]-Hydrogenases
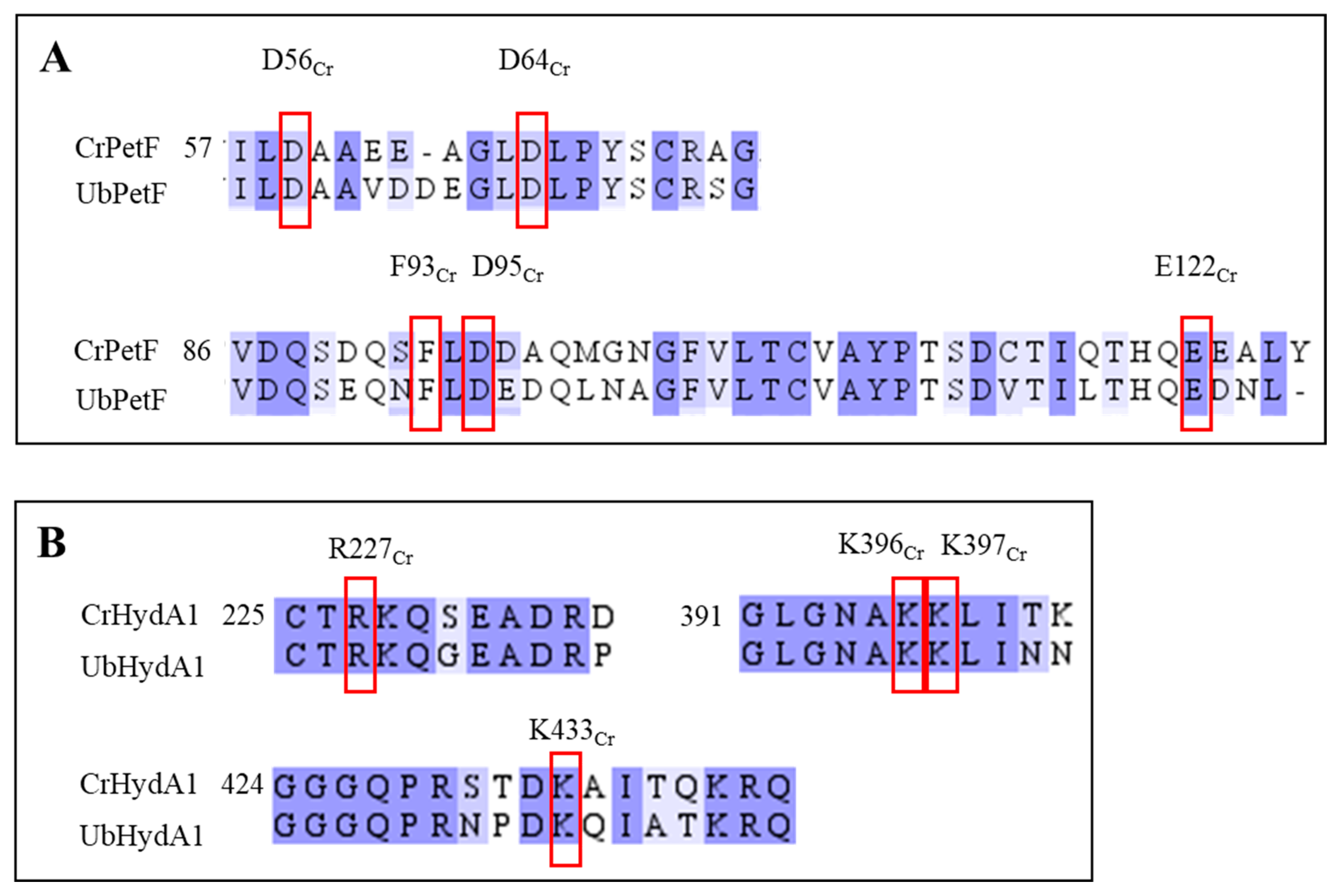
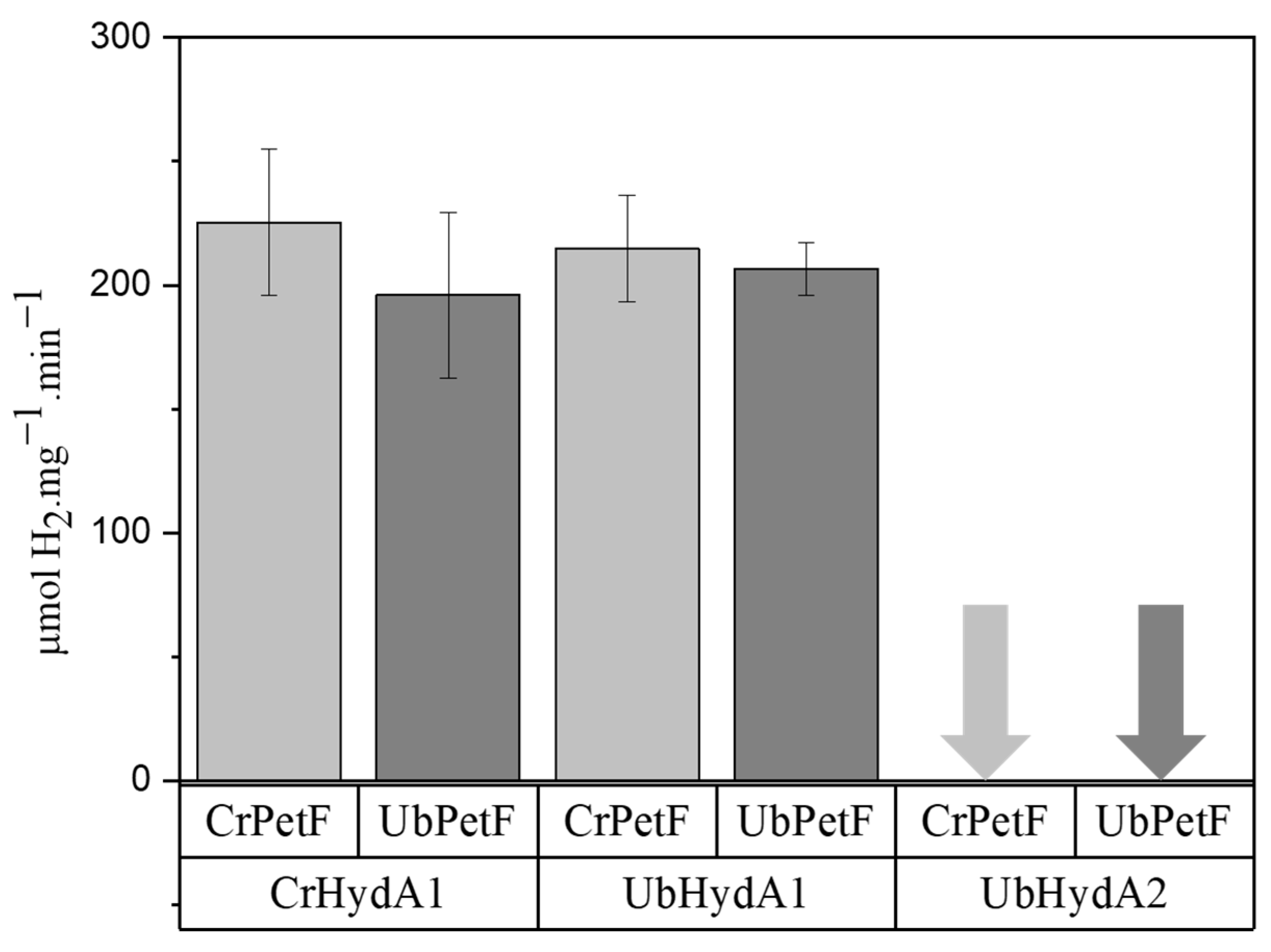
2.3. UbHydA1, but Not UbHydA2, Interacts with Photosynthetic Ferredoxin
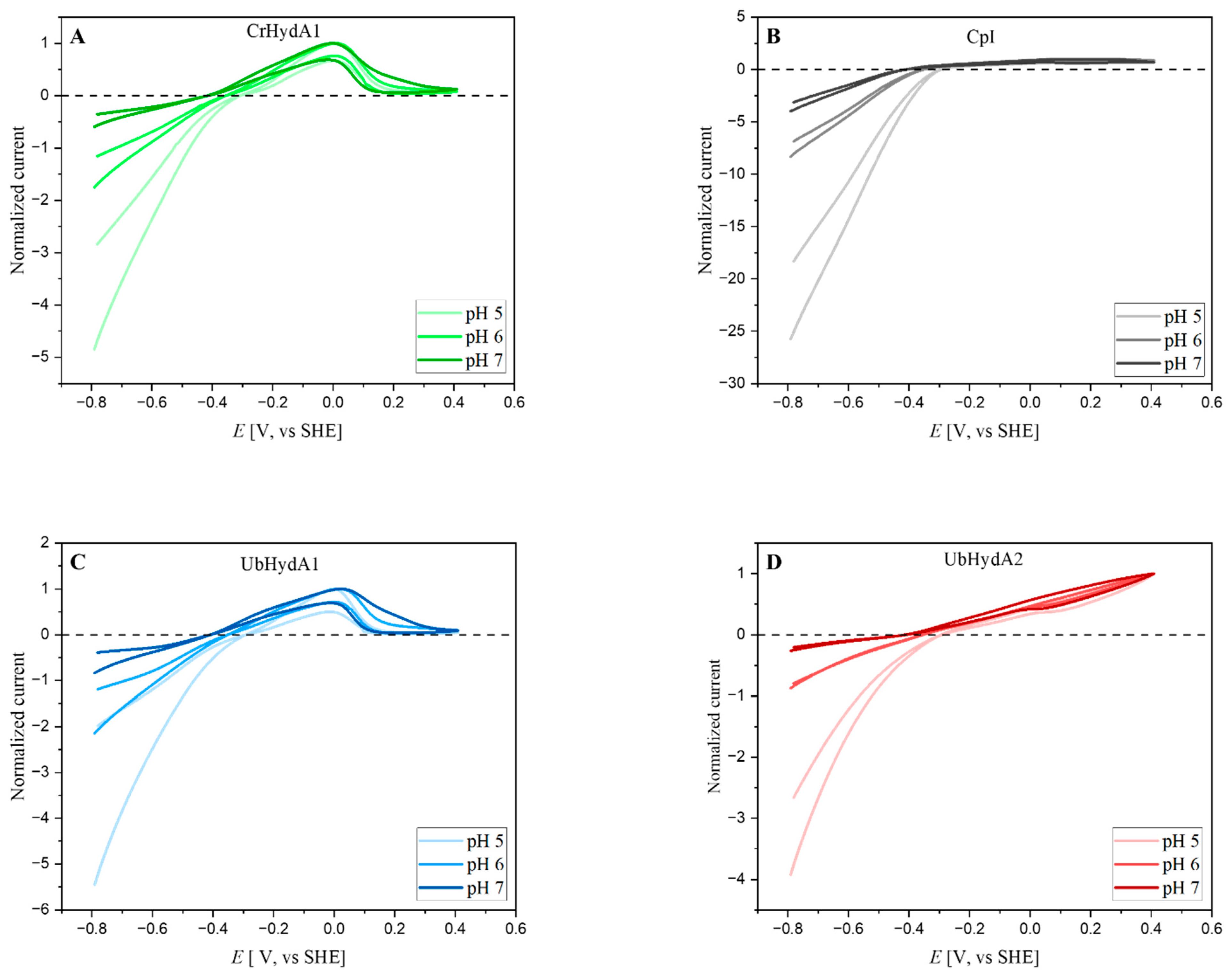
2.4. Electrochemical Characterization of UbHydA1 and UbHydA2
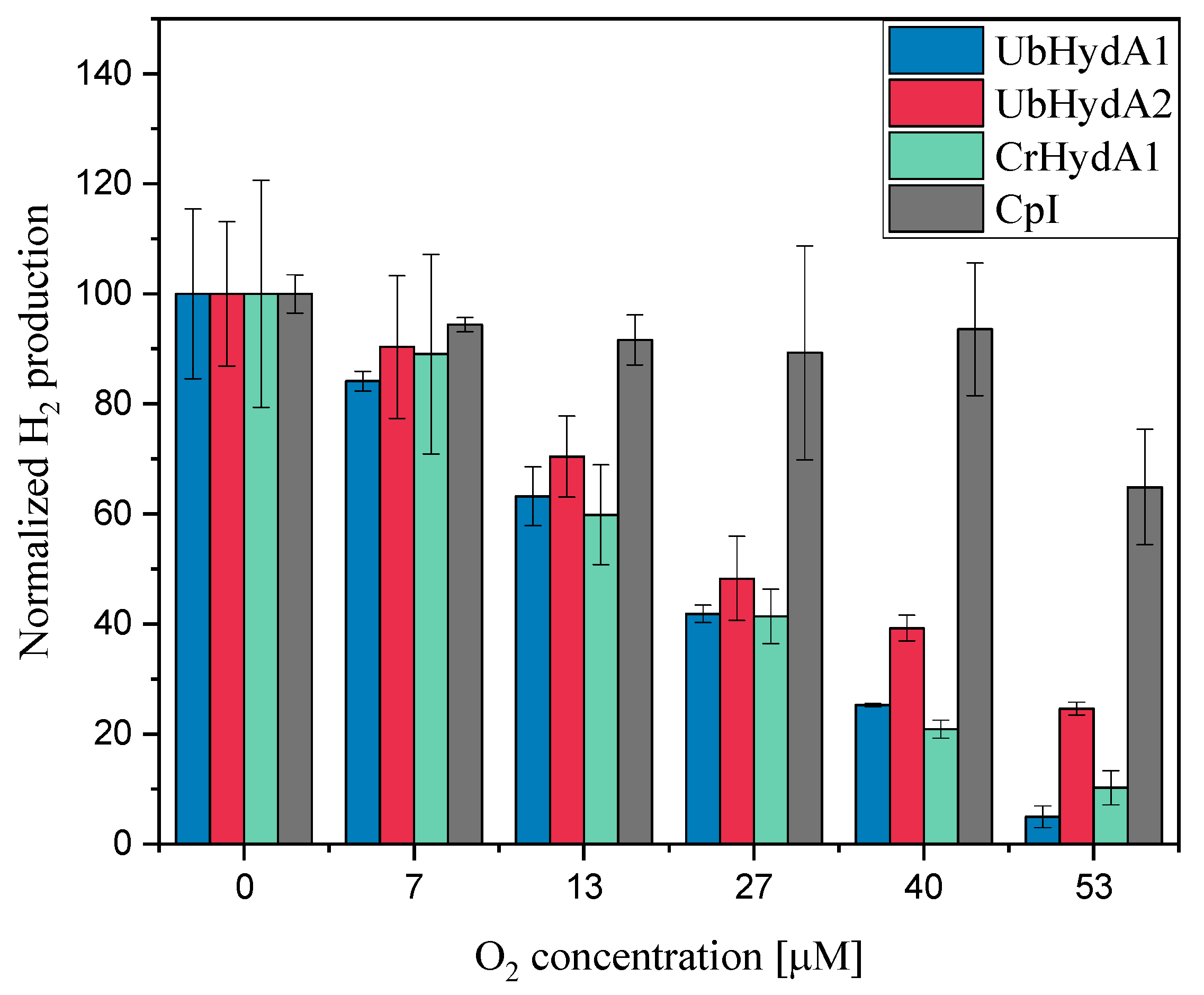
2.5. Oxygen Sensitivity of UbHydA1 and UbHydA2
3. Discussion
4. Material and Methods
4.1. Identification of [FeFe]-Hydrogenase- and PetF-Encoding Genes in Sequence Databases
4.2. Algal Strains, Growth Conditions and Induction of H2 Production
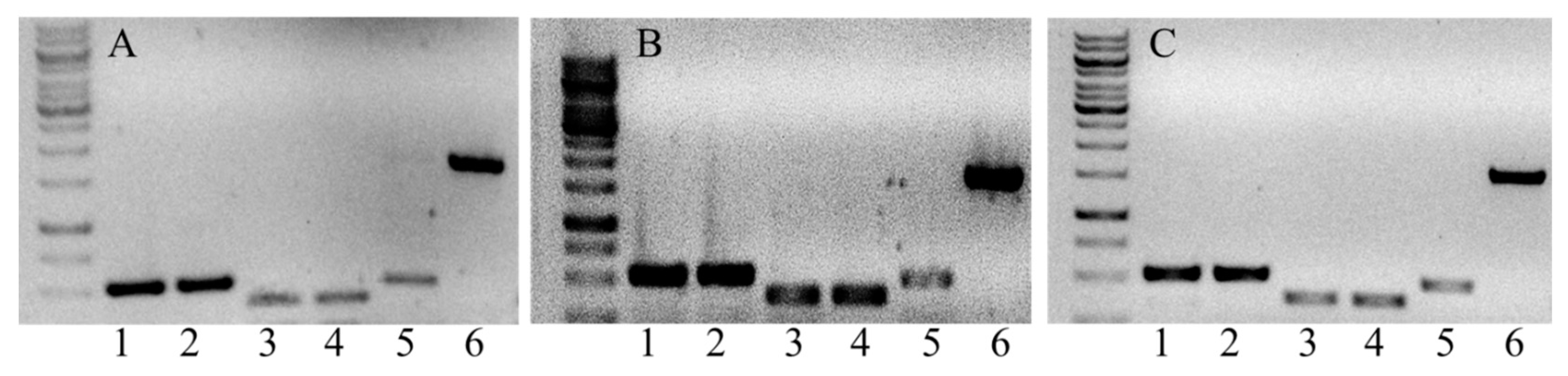
4.3. DNA and RNA Analysis
4.4. Recombinant Protein Production and Purification
4.5. Determination of H2 Production Activity of Recombinant Hydrogenases
4.6. Protein Film Electrochemistry
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Sequences A1: Sequences of U. belkae Hydrogenases and PetF
- >UbHydA1
- MAVAEPKADCDCGPKAAGPHWQQALDLLDAKDKSKLFVVQIAPAVRVAISEPFGLPSGTITIGQIVTGLRQLGFDVVFDTLFGADLTIMEEGTELLHRLKDHLEGNPKNEEPLPMFTSCCPGWVELVEKSYPDMIPYLSSCKSPQMMLGAIIKNYFADVAGYAPQDVISCSVMPCTRKQGEADRPAGATTGLARDVDHVITTAELAKIFQDRGIDLPNLPESPLDNPIGEGSGAGQLFGTTGGVMEAAVRTVYELVTGQPMERINLTEVRGLDGIKEATLVLKPAPDSILGKWSGEGEGLPIRIAVANGLGNAKKLINNIKDGSAKYDFVEVMACPGGCISGGGQPRNPDKQIATKRQQSMYTIDERMTLRRSHDNVFIQQLYAKFLGKPGSHKAHDLLHTPYIPGGPAKQ
- >UbHydA2
- MSDKAAAQPPNQRRSLHNLHVPQNSTPRTRAAAHLLIPFRKQHAATTPRQHNIAEAQPHACVTINGRSVPFSPGQTILQAAANAGVQIPSICYHPRLPKSPGTCRVCLVSVDGRMRPSCVTEAQAGQVVETDSEEVREHVRGQLALLRTNHPDDCMTCDVNGRCEFQRLITQYQVPLMPKTLRIQNHTHAQEIKGLEDFDASAKAAPLGLDEPPSNASSPPPTTPESCASITAAAAASANTTTLRHHETPGLVSHGVAISVDQDKCIKCGRCVTMCQEVQQMNVLGWVGRGQEAHVGVVDDAELQQSRCIECGQCVSICPVGALTEHSEWRQVMQLLRSKRKIMVVQTAPAVRVAIGEEVGLAPGAISTGQLVTALRQLGFDYVFDTNFSADLTIMEEGSELLQRLQHHWQHPDSPAHTPPHEDPRFKGAGSAGAPKAGHHTPGPLPMFTSCCPAWINEVERDRPELIPHLSTCKSPQGMMGAVVKSIWAKKMGLNPEDVVLVSVMPCTAKKHEARREELLPEQPQGDALTPADTTTSVYTSTSGDGITGGDRVVCQEPARDVDFVLTTRELGTMLRQMGIPLASLEAGDYDHPLAGGTGAAVLFGATGGVMEAALRTVAAVVGGAPLPRLEMEAIRGMAGVKEATLQLEGPGLPGGAKEVRVAVASGIGAAQQLLDKVQSGQVNYDFIEVMSCPGGCIGGGGQPKTKDPTAILKRMDATYTIDQDSTLRQSHENPDVQQLYKDALEAPGSHDAHKLLHTHYTDRSSEVKS
- >UbPetF
- MAYKVTLKTPDGDKTFECADDKYILDAAVDDEGLDLPYSCRSGGCCTCTGKIVSGTVDQSEQNFLDEDQLNAGFVLTCVAYPTSDVTILTHQEDNL
Appendix A.2. Sequences A2: Codon-Optimized Sequences Encoding Hydrogenases and PetF for Expression in E. coli
- >UbHydA1_Codon_Optimized
- ATGGCAGTTGCAGAACCGAAAGCAGATTGTGATTGTGGTCCGAAAGCCGCAGGTCCGCATTGGCAGCAGGCACTGGATCTGCTGGATGCAAAAGATAAAAGCAAACTGTTTGTGGTTCAGATTGCACCGGCAGTTCGTGTTGCAATTAGCGAACCGTTTGGTCTGCCGAGCGGCACCATTACCATTGGTCAGATTGTTACCGGTCTGCGTCAGCTGGGTTTTGATGTTGTTTTTGATACCCTGTTTGGTGCCGATCTGACCATTATGGAAGAAGGCACCGAACTGCTGCATCGTCTGAAAGATCATCTGGAAGGTAATCCGAAAAATGAAGAACCGCTGCCGATGTTTACCAGCTGTTGTCCTGGTTGGGTTGAACTGGTTGAAAAAAGCTATCCTGATATGATTCCGTATCTGAGCAGCTGTAAAAGTCCGCAGATGATGCTGGGTGCAATCATCAAAAACTATTTTGCAGATGTTGCAGGTTATGCACCGCAGGATGTTATTAGCTGTAGCGTTATGCCGTGCACACGTAAACAGGGTGAAGCAGATCGTCCGGCAGGCGCAACCACCGGTCTGGCACGTGATGTTGATCATGTTATTACCACCGCAGAACTGGCAAAAATCTTTCAGGATCGTGGTATTGATCTGCCGAATCTGCCGGAAAGTCCGCTGGATAATCCGATTGGTGAAGGTAGCGGTGCAGGTCAGCTGTTTGGTACAACCGGTGGTGTTATGGAAGCAGCCGTTCGTACCGTTTATGAACTGGTGACGGGTCAGCCGATGGAACGTATTAATCTGACCGAAGTTCGTGGTCTGGATGGTATTAAAGAAGCAACCCTGGTTCTGAAACCGGCACCGGATAGCATTCTTGGTAAATGGTCAGGTGAAGGTGAAGGCCTGCCGATTCGTATTGCAGTTGCAAATGGTCTGGGTAATGCCAAAAAACTGATCAACAACATTAAAGACGGCAGCGCCAAATATGATTTTGTTGAAGTTATGGCATGTCCCGGTGGCTGTATTAGCGGTGGTGGTCAGCCTCGTAATCCGGATAAACAAATTGCAACCAAACGTCAGCAGAGCATGTATACCATTGATGAACGTATGACCCTGCGTCGTAGCCATGATAATGTTTTTATTCAGCAACTGTACGCCAAGTTTCTGGGTAAACCGGGTAGCCATAAAGCACATGATCTGCTGCATACCCCGTATATTCCAGGTGGTCCGGCAAAACAG
- >UbHydA2_Codon_Optimized
- ATGAGCGATAAAGCAGCAGCACAGCCTCCGAATCAGCGTCGTAGCCTGCATAATCTGCATGTTCCGCAGAATAGCACACCGCGTACACGTGCAGCAGCCCATCTGCTGATTCCGTTTCGTAAACAGCATGCAGCAACAACACCGCGTCAGCATAATATTGCCGAAGCACAGCCGCATGCATGTGTTACAATTAATGGTCGTAGCGTTCCGTTTAGTCCGGGTCAGACCATTCTGCAGGCAGCAGCAAATGCCGGTGTTCAGATTCCGAGCATTTGTTATCATCCGCGTCTGCCGAAAAGTCCGGGTACATGTCGTGTTTGTCTGGTTAGCGTTGATGGTCGCATGCGTCCGAGCTGTGTTACCGAAGCGCAGGCAGGTCAGGTTGTTGAAACCGATAGCGAAGAGGTTCGTGAACATGTGCGTGGTCAGCTGGCACTGCTGCGTACCAATCATCCTGATGACTGTATGACCTGTGATGTGAATGGTCGTTGTGAATTTCAGCGTCTGATTACCCAGTATCAGGTTCCGCTGATGCCGAAAACACTGCGTATTCAGAATCATACCCATGCGCAAGAAATTAAAGGCCTGGAAGATTTTGATGCAAGCGCAAAAGCAGCACCGCTGGGTTTAGATGAACCGCCTAGCAATGCAAGCAGTCCGCCTCCGACCACACCGGAAAGCTGTGCAAGCATTACCGCAGCAGCAGCCGCAAGCGCCAATACCACCACACTGCGTCATCATGAAACCCCTGGTCTGGTGAGCCATGGTGTTGCAATTAGCGTGGATCAGGATAAATGTATTAAATGCGGTCGTTGCGTTACCATGTGTCAAGAGGTGCAGCAGATGAATGTTTTAGGTTGGGTTGGTCGTGGTCAAGAGGCACATGTTGGTGTTGTGGATGATGCAGAACTGCAGCAGAGCCGTTGTATTGAATGTGGTCAGTGTGTTAGCATTTGTCCGGTTGGTGCACTGACCGAACATTCAGAATGGCGTCAGGTTATGCAGCTGCTGCGTTCAAAACGCAAAATTATGGTTGTTCAGACCGCACCGGCAGTTCGTGTTGCCATTGGTGAAGAAGTTGGTCTGGCACCGGGTGCGATTAGCACAGGCCAGCTGGTTACCGCACTGCGCCAGCTGGGTTTTGATTATGTTTTTGATACCAACTTCAGCGCAGATCTGACCATTATGGAAGAAGGTAGCGAACTGCTGCAGCGTTTACAGCATCATTGGCAGCATCCGGATAGTCCGGCACATACCCCTCCGCATGAAGATCCGCGTTTTAAAGGTGCAGGTAGCGCAGGCGCACCGAAAGCAGGTCATCATACACCGGGTCCGCTGCCGATGTTTACCAGCTGTTGTCCGGCATGGATTAATGAAGTTGAACGTGATCGTCCGGAACTGATTCCGCATCTGAGCACCTGTAAAAGTCCGCAGGGCATGATGGGTGCAGTTGTTAAAAGCATTTGGGCCAAAAAAATGGGTCTGAATCCGGAAGATGTTGTGCTGGTGAGCGTTATGCCGTGTACCGCAAAAAAACATGAAGCACGTCGTGAAGAACTGTTACCGGAACAGCCGCAGGGTGATGCACTGACACCGGCAGATACCACAACCAGCGTTTATACCAGCACCTCTGGTGATGGTATTACCGGTGGTGATCGTGTTGTTTGTCAAGAACCGGCACGTGATGTTGATTTTGTTCTGACCACACGTGAACTGGGCACCATGCTGCGTCAGATGGGTATTCCGCTGGCAAGCCTGGAAGCCGGTGATTATGATCATCCGTTAGCCGGTGGTACAGGTGCAGCCGTTCTGTTTGGTGCAACCGGTGGCGTTATGGAAGCAGCACTGCGTACCGTTGCCGCAGTTGTTGGTGGTGCACCGCTGCCTCGTCTGGAAATGGAAGCAATTCGTGGTATGGCAGGCGTTAAAGAAGCAACCCTGCAGCTGGAAGGTCCGGGTCTGCCTGGTGGTGCCAAAGAAGTTCGCGTTGCAGTTGCAAGCGGTATTGGTGCAGCCCAGCAACTGCTGGATAAAGTTCAGAGCGGTCAGGTGAACTATGATTTTATTGAAGTTATGAGCTGTCCCGGTGGTTGTATTGGTGGTGGTGGCCAGCCGAAAACCAAAGATCCGACCGCAATTCTGAAACGTATGGATGCAACCTATACCATCGATCAGGATTCAACCCTGCGTCAGTCACATGAAAATCCGGATGTACAGCAACTGTATAAAGATGCACTGGAAGCACCGGGTAGCCATGATGCACATAAACTGTTACATACCCATTACACCGATCGTAGCAGCGAAGTGAAAAGC
- >UbPetF_Codon_Optimized
- ATGGCATATAAAGTGACCCTGAAAACACCGGATGGTGATAAAACCTTTGAATGTGCCGATGACAAATATATCCTGGATGCAGCAGTTGATGATGAAGGTCTGGATCTGCCGTATAGCTGTCGTAGCGGTGGTTGTTGTACCTGTACCGGTAAAATTGTTAGCGGCACCGTTGATCAGAGCGAACAGAATTTTCTGGATGAAGATCAGCTGAATGCAGGTTTTGTTCTGACCTGTGTTGCATATCCGACCAGTGATGTTACCATTCTGACCCATCAAGAAGATAACCTG
Appendix A.3
References
- Lubitz, W.; Ogata, H.; Rudiger, O.; Reijerse, E. Hydrogenases. Chem. Rev. 2014, 114, 4081–4148. [Google Scholar] [CrossRef] [PubMed]
- Frey, M. Hydrogenases: Hydrogen-activating enzymes. ChemBioChem 2002, 3, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Wittkamp, F.; Senger, M.; Stripp, S.T.; Apfel, U.P. [FeFe]-Hydrogenases: Recent developments and future perspectives. Chem. Commun. 2018, 54, 5934–5942. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.W.; Lanzilotta, W.N.; Lemon, B.J.; Seefeldt, L.C. X-ray crystal structure of the Fe-only hydrogenase (CpI) from Clostridium pasteurianum to 1.8 angstrom resolution. Science 1998, 282, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Britt, R.D.; Rao, G.; Tao, L. Biosynthesis of the catalytic H-cluster of [FeFe] hydrogenase: The roles of the Fe–S maturase proteins HydE, HydF, and HydG. Chem. Sci. 2020, 11, 10313–10323. [Google Scholar] [CrossRef] [PubMed]
- Sommer, C.; Rumpel, S.; Roy, S.; Farès, C.; Artero, V.; Fontecave, M.; Reijerse, E.; Lubitz, W. Spectroscopic investigations of a semi-synthetic [FeFe] hydrogenase with propane di-selenol as bridging ligand in the binuclear subsite: Comparison to the wild type and propane di-thiol variants. JBIC J. Biol. Inorg. Chem. 2018, 23, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Birrell, J.A.; Rodríguez-Maciá, P.; Reijerse, E.J.; Martini, M.A.; Lubitz, W. The catalytic cycle of [FeFe] hydrogenase: A tale of two sites. Coord. Chem. Rev. 2021, 449, 214191. [Google Scholar] [CrossRef]
- Sommer, C.; Adamska-Venkatesh, A.; Pawlak, K.; Birrell, J.A.; Rüdiger, O.; Reijerse, E.J.; Lubitz, W. Proton coupled electronic rearrangement within the H-cluster as an essential step in the catalytic cycle of [FeFe] hydrogenases. J. Am. Chem. Soc. 2017, 139, 1440–1443. [Google Scholar] [CrossRef]
- Ratzloff, M.W.; Artz, J.H.; Mulder, D.W.; Collins, R.T.; Furtak, T.E.; King, P.W. CO-bridged H-cluster intermediates in the catalytic mechanism of [FeFe]-hydrogenase CaI. J. Am. Chem. Soc. 2018, 140, 7623–7628. [Google Scholar] [CrossRef]
- Meyer, J. [FeFe] hydrogenases and their evolution: A genomic perspective. Cell Mol. Life Sci. 2007, 64, 1063–1084. [Google Scholar] [CrossRef]
- Mulder, D.W.; Shepard, E.M.; Meuser, J.E.; Joshi, N.; King, P.W.; Posewitz, M.C.; Broderick, J.B.; Peters, J.W. Insights into [FeFe]-hydrogenase structure, mechanism, and maturation. Structure 2011, 19, 1038–1052. [Google Scholar] [CrossRef] [PubMed]
- Happe, T.; Naber, J.D. Isolation, characterization and N-terminal amino acid sequence of hydrogenase from the green alga Chlamydomonas reinhardtii. Eur. J. Biochem. 1993, 214, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Florin, L.; Tsokoglou, A.; Happe, T. A novel type of iron hydrogenase in the green Alga Scenedesmus obliquus is linked to the photosynthetic electron transport chain. J. Biol. Chem. 2001, 276, 6125–6132. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.W. Structure and mechanism of iron-only hydrogenases. Curr. Opin. Struct. Biol. 1999, 9, 670–676. [Google Scholar] [CrossRef]
- Kümmerle, R.; Atta, M.; Scuiller, J.; Gaillard, J.; Meyer, J. Structural similarities between the N-terminal domain of Clostridium pasteurianum hydrogenase and plant-type ferredoxins. Biochemistry 1999, 38, 1938–1943. [Google Scholar] [CrossRef] [PubMed]
- Artz, J.H.; Mulder, D.W.; Ratzloff, M.W.; Lubner, C.E.; Zadvornyy, O.A.; LeVan, A.X.; Williams, S.G.; Adams, M.W.; Jones, A.K.; King, P.W.; et al. Reduction potentials of [FeFe]-hydrogenase accessory iron–sulfur clusters provide insights into the energetics of proton reduction catalysis. J. Am. Chem. Soc. 2017, 139, 9544–9550. [Google Scholar] [CrossRef] [PubMed]
- Gauquelin, C.; Baffert, C.; Richaud, P.; Kamionka, E.; Etienne, E.; Guieysse, D.; Girbal, L.; Fourmond, V.; André, I.; Guigliarelli, B.; et al. Roles of the F-domain in [FeFe] hydrogenase. Biochim. Biophys. Acta (BBA)-Bioenerg. 2018, 1859, 69–77. [Google Scholar] [CrossRef]
- Vignais, P.M. Hydrogenases and H+-reduction in primary energy conservation. Bioenerg. Energy Conserv. Convers. 2007, 45, 223–252. [Google Scholar]
- Gaffron, H. Reduction of carbon dioxide with molecular hydrogen in green algae. Nature 1939, 143, 204–205. [Google Scholar] [CrossRef]
- Gaffron, H.; Rubin, J. Fermentative and photochemical production of hydrogen in algae. J. Gen. Physiol. 1942, 26, 219–240. [Google Scholar] [CrossRef]
- Healey, F.P. Hydrogen evolution by several algae. Planta 1970, 91, 220–226. [Google Scholar] [CrossRef]
- Atteia, A.; van Lis, R.; Tielens, A.G.M.; Martin, W.F. Anaerobic energy metabolism in unicellular photosynthetic eukaryotes. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2013, 1827, 210–223. [Google Scholar] [CrossRef]
- Catalanotti, C.; Yang, W.; Posewitz, M.C.; Grossman, A.R. Fermentation metabolism and its evolution in algae. Front. Plant Sci. 2013, 4, 150. [Google Scholar] [CrossRef] [PubMed]
- Posewitz, M.C. Metabolism and genetics of algal hydrogen production. In Microalgal Hydrogen Production: Achievements and Perspectives; Royal Society of Chemistry: London, UK, 2018; pp. 167–188. [Google Scholar]
- Hemschemeier, A.; Posewitz, M.C.; Happe, T. Hydrogenases and hydrogen production. In The Chlamydomonas Sourcebook; Elsevier: Amsterdam, The Netherlands, 2023; pp. 343–367. [Google Scholar]
- Gaffron, H. Carbon dioxide reduction with molecular hydrogen in green algae. Am. J. Bot. 1940, 27, 273–283. [Google Scholar] [CrossRef]
- Gaffron, H. The effect of specific poisons upon the photo-reduction with hydrogen in green algae. J. Gen. Physiol. 1942, 26, 195. [Google Scholar] [CrossRef][Green Version]
- Roessler, P.G.; Lien, S. Purification of hydrogenase from Chlamydomonas reinhardtii. Plant Physiol. 1984, 75, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Happe, T.; Kaminski, A. Differential regulation of the Fe-hydrogenase during anaerobic adaptation in the green alga Chlamydomonas reinhardtii. Eur. J. Biochem. 2002, 269, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.W.; Schut, G.J.; Boyd, E.S.; Mulder, D.W.; Shepard, E.M.; Broderick, J.B.; King, P.W.; Adams, M.W. [FeFe]-and [NiFe]-hydrogenase diversity, mechanism, and maturation. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2015, 1853, 1350–1369. [Google Scholar] [CrossRef] [PubMed]
- Stripp, S.T.; Goldet, G.; Brandmayr, C.; Sanganas, O.; Vincent, K.A.; Haumann, M.; Armstrong, F.A.; Happe, T. How oxygen attacks [FeFe] hydrogenases from photosynthetic organisms. Proc. Natl. Acad. Sci. USA 2009, 106, 17331–17336. [Google Scholar] [CrossRef]
- Ueno, Y.; Kurano, N.; Miyachi, S. Purification and characterization of hydrogenase from the marine green alga, Chlorococcum littorale. FEBS Lett. 1999, 443, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.; Kuhlgert, S.; Hippler, M.; Happe, T. Characterization of the key step for light-driven hydrogen evolution in green algae. J. Biol. Chem. 2009, 284, 36620–36627. [Google Scholar] [CrossRef] [PubMed]
- Meuser, J.E.; Boyd, E.S.; Ananyev, G.; Karns, D.; Radakovits, R.; Narayana Murthy, U.M.; Ghirardi, M.L.; Dismukes, G.C.; Peters, J.W.; Posewitz, M.C. Evolutionary significance of an algal gene encoding an [FeFe]-hydrogenase with F-domain homology and hydrogenase activity in Chlorella variabilis NC64A. Planta 2011, 234, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Price, D.C.; Chan, C.X.; Yoon, H.S.; Yang, E.C.; Qiu, H.; Weber, A.P.M.; Schwacke, R.; Gross, J.; Blouin, N.A.; Lane, C.; et al. Cyanophora paradoxa genome elucidates origin of photosynthesis in algae and plants. Science 2012, 335, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Smith, P.R.; Mehta, K.; Swartz, J.R. Development of a synthetic pathway to convert glucose to hydrogen using cell free extracts. Int. J. Hydrogen Energy 2015, 40, 9113–9124. [Google Scholar] [CrossRef]
- Engelbrecht, V.; Rodríguez-Maciá, P.; Esselborn, J.; Sawyer, A.; Hemschemeier, A.; Rüdiger, O.; Lubitz, W.; Winkler, M.; Happe, T. The structurally unique photosynthetic Chlorella variabilis NC64A hydrogenase does not interact with plant-type ferredoxins. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2017, 1858, 771–778. [Google Scholar] [CrossRef]
- One Thousand Plant Transcriptomes Initiative. One thousand plant transcriptomes and the phylogenomics of green plants. Nature 2019, 574, 679–685. [Google Scholar] [CrossRef]
- Carpenter, E.J.; Matasci, N.; Ayyampalayam, S.; Wu, S.; Sun, J.; Yu, J.; Jimenez Vieira, F.R.; Bowler, C.; Dorrell, R.G.; Gitzendanner, M.A.; et al. Access to RNA-sequencing data from 1,173 plant species: The 1000 Plant transcriptomes initiative (1KP). GigaScience 2019, 8, giz126. [Google Scholar] [CrossRef]
- Melis, A.; Zhang, L.; Forestier, M.; Ghirardi, M.L.; Seibert, M. Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green alga Chlamydomonas reinhardtii. Plant Physiol. 2000, 122, 127–136. [Google Scholar] [CrossRef]
- Stuart, T.S.; Gaffron, H. The mechanism of hydrogen photoproduction by several algae: I. The effect of inhibitors of photophosphorylation. Planta 1972, 106, 91–100. [Google Scholar] [CrossRef]
- Gibbs, M.; Gfeller, R.P.; Chen, C. Fermentative metabolism of Chlamydomonas reinhardii: III. Photoassimilation of acetate. Plant Physiol. 1986, 82, 160–166. [Google Scholar] [CrossRef]
- Redding, K.; Cournac, L.; Vassiliev, I.R.; Golbeck, J.H.; Peltier, G.; Rochaix, J.-D. Photosystem I Is Indispensable for Photoautotrophic Growth, CO2 Fixation, and H2 Photoproduction in Chlamydomonas reinhardtii. J. Biol. Chem. 1999, 274, 10466–10473. [Google Scholar] [CrossRef]
- Engelbrecht, V.; Liedtke, K.; Rutz, A.; Yadav, S.; Günzel, A.; Happe, T. One isoform for one task? The second hydrogenase of Chlamydomonas reinhardtii prefers hydrogen uptake. Int. J. Hydrogen Energy 2021, 46, 7165–7175. [Google Scholar] [CrossRef]
- Armstrong, F.A.; Evans, R.M.; Megarity, C.F. Protein film electrochemistry of iron–sulfur enzymes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 387–407. [Google Scholar]
- Vincent, K.A.; Parkin, A.; Armstrong, F.A. Investigating and exploiting the electrocatalytic properties of hydrogenases. Chem. Rev. 2007, 107, 4366–4413. [Google Scholar] [CrossRef]
- Pandey, K.; Islam, S.T.A.; Happe, T.; Armstrong, F.A. Frequency and potential dependence of reversible electrocatalytic hydrogen interconversion by [FeFe]-hydrogenases. Proc. Natl. Acad. Sci. USA 2017, 114, 3843–3848. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, F.A.; Evans, R.M.; Hexter, S.V.; Murphy, B.J.; Roessler, M.M.; Wulff, P. Guiding principles of hydrogenase catalysis instigated and clarified by protein film electrochemistry. Acc. Chem. Res. 2016, 49, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Vincent, K.A.; Parkin, A.; Lenz, O.; Albracht, S.P.; Fontecilla-Camps, J.C.; Cammack, R.; Friedrich, B.; Armstrong, F.A. Electrochemical definitions of O2 sensitivity and oxidative inactivation in hydrogenases. J. Am. Chem. Soc. 2005, 127, 18179–18189. [Google Scholar] [CrossRef] [PubMed]
- Goldet, G.; Brandmayr, C.; Stripp, S.T.; Happe, T.; Cavazza, C.; Fontecilla-Camps, J.C.; Armstrong, F.A. Electrochemical kinetic investigations of the reactions of [FeFe]-hydrogenases with carbon monoxide and oxygen: Comparing the importance of gas tunnels and active-site electronic/redox effects. J. Am. Chem. Soc. 2009, 131, 14979–14989. [Google Scholar] [CrossRef] [PubMed]
- Artz, J.H.; Zadvornyy, O.A.; Mulder, D.W.; Keable, S.M.; Cohen, A.E.; Ratzloff, M.W.; Williams, S.G.; Ginovska, B.; Kumar, N.; Song, J.; et al. Tuning catalytic bias of hydrogen gas producing hydrogenases. J. Am. Chem. Soc. 2019, 142, 1227–1235. [Google Scholar] [CrossRef]
- Kubas, A.; Orain, C.; De Sancho, D.; Saujet, L.; Sensi, M.; Gauquelin, C.; Meynial-Salles, I.; Soucaille, P.; Bottin, H.; Baffert, C.; et al. Mechanism of O2 diffusion and reduction in FeFe hydrogenases. Nat. Chem. 2017, 9, 88–95. [Google Scholar] [CrossRef]
- Cohen, J.; Kim, K.; King, P.; Seibert, M.; Schulten, K. Finding gas diffusion pathways in proteins: Application to O2 and H2 transport in CpI [FeFe]-hydrogenase and the role of packing defects. Structure 2005, 13, 1321–1329. [Google Scholar] [CrossRef]
- Cohen, J.; Kim, K.; Posewitz, M.; Ghirardi, M.L.; Schulten, K.; Seibert, M.; King, P. Molecular dynamics and experimental investigation of H2 and O2 diffusion in [Fe]-hydrogenase. Biochem. Soc. Trans. 2005, 33, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Bruska, M.K.; Stiebritz, M.T.; Reiher, M. Regioselectivity of H cluster oxidation. J. Am. Chem. Soc. 2011, 133, 20588–20603. [Google Scholar] [CrossRef] [PubMed]
- Mebs, S.; Kositzki, R.; Duan, J.; Kertess, L.; Senger, M.; Wittkamp, F.; Apfel, U.P.; Happe, T.; Stripp, S.T.; Winkler, M.; et al. Hydrogen and oxygen trapping at the H-cluster of [FeFe]-hydrogenase revealed by site-selective spectroscopy and QM/MM calculations. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2018, 1859, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Morra, S. Fantastic [FeFe]-hydrogenases and where to find them. Front. Microbiol. 2022, 13, 853626. [Google Scholar] [CrossRef]
- Morra, S.; Arizzi, M.; Valetti, F.; Gilardi, G. Oxygen stability in the new [FeFe]-hydrogenase from Clostridium beijerinckii SM10 (CbA5H). Biochemistry 2016, 55, 5897–5900. [Google Scholar] [CrossRef]
- Kosourov, S.; Nagy, V.; Shevela, D.; Jokel, M.; Messinger, J.; Allahverdiyeva, Y. Water oxidation by photosystem II is the primary source of electrons for sustained H2 photoproduction in nutrient-replete green algae. Proc. Natl. Acad. Sci. USA 2020, 117, 29629–29636. [Google Scholar] [CrossRef]
- Milrad, Y.; Schweitzer, S.; Feldman, Y.; Yacoby, I. Green algal hydrogenase activity is outcompeted by carbon fixation before inactivation by oxygen takes place. Plant Physiol. 2018, 177, 918–926. [Google Scholar] [CrossRef]
- Gfeller, R.P.; Gibbs, M. Fermentative metabolism of Chlamydomonas reinhardtii: II. Role of plastoquinone. Plant Physiol. 1985, 77, 509–511. [Google Scholar] [CrossRef]
- Stuart, T.S.; Gaffron, H. The mechanism of hydrogen photoproduction by several algae: II. The contribution of photosystem II. Planta 1972, 106, 101–112. [Google Scholar] [CrossRef]
- Benemann, J. Hydrogen biotechnology: Progress and prospects. Nat. Biotechnol. 1996, 14, 1101–1103. [Google Scholar] [CrossRef]
- Baltz, A.; Dang, K.V.; Beyly, A.; Auroy, P.; Richaud, P.; Cournac, L.; Peltier, G. Plastidial expression of type II NAD(P)H dehydrogenase increases the reducing state of plastoquinones and hydrogen photoproduction rate by the indirect pathway in Chlamydomonas reinhardtii. Plant Physiol. 2014, 165, 1344–1352. [Google Scholar] [CrossRef]
- Winkler, M.; Hemschemeier, A.; Gotor, C.; Melis, A.; Happe, T. [Fe]-hydrogenases in green algae: Photo-fermentation and hydrogen evolution under sulfur deprivation. Int. J. Hydrogen Energy 2002, 27, 1431–1439. [Google Scholar] [CrossRef]
- Yang, W.; Wittkopp, T.M.; Li, X.; Warakanont, J.; Dubini, A.; Catalanotti, C.; Kim, R.G.; Nowack, E.C.; Mackinder, L.C.; Aksoy, M.; et al. Critical role of Chlamydomonas reinhardtii ferredoxin-5 in maintaining membrane structure and dark metabolism. Proc. Natl. Acad. Sci. USA 2015, 112, 14978–14983. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, A.; Winkler, M. Evolution of Chlamydomonas reinhardtii ferredoxins and their interactions with [FeFe]-hydrogenases. Photosynth. Res. 2017, 134, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Peden, E.A.; Boehm, M.; Mulder, D.W.; Davis, R.; Old, W.M.; King, P.W.; Ghirardi, M.L.; Dubini, A. Identification of global ferredoxin interaction networks in Chlamydomonas reinhardtii. J. Biol. Chem. 2013, 288, 35192–35209. [Google Scholar] [CrossRef] [PubMed]
- Günzel, A.; Engelbrecht, V.; Happe, T. Changing the tracks: Screening for electron transfer proteins to support hydrogen production. JBIC J. Biol. Inorg. Chem. 2022, 27, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Boehm, M.; Alahuhta, M.; Mulder, D.W.; Peden, E.A.; Long, H.; Brunecky, R.; Lunin, V.V.; King, P.W.; Ghirardi, M.L.; Dubini, A. Crystal structure and biochemical characterization of Chlamydomonas FDX2 reveal two residues that, when mutated, partially confer FDX2 the redox potential and catalytic properties of FDX1. Photosynth. Res. 2016, 128, 45–57. [Google Scholar] [CrossRef]
- Winkler, M.; Esselborn, J.; Happe, T. Molecular basis of [FeFe]-hydrogenase function: An insight into the complex interplay between protein and catalytic cofactor. Biochim. Biophys. Acta (BBA)-Bioenerg. 2013, 1827, 974–985. [Google Scholar] [CrossRef]
- Sybirna, K.; Ezanno, P.; Baffert, C.; Léger, C.; Bottin, H. Arginine171 of Chlamydomonas reinhardtii [Fe–Fe] hydrogenase HydA1 plays a crucial role in electron transfer to its catalytic center. Int. J. Hydrogen Energy 2013, 38, 2998–3002. [Google Scholar] [CrossRef]
- Harris, E.H. Chlamydomonas Sourcebook; Academic Press: San Diego, CA, USA, 1989. [Google Scholar]
- Hemschemeier, A.; Melis, A.; Happe, T. Analytical approaches to photobiological hydrogen production in unicellular green algae. Photosynth. Res. 2009, 102, 523–540. [Google Scholar] [CrossRef] [PubMed]
- Mus, F.; Dubini, A.; Seibert, M.; Posewitz, M.C.; Grossman, A.R. Anaerobic acclimation in Chlamydomonas reinhardtii: Anoxic gene expression, hydrogenase induction, and metabolic pathways. J. Biol. Chem. 2007, 282, 25475–25486. [Google Scholar] [CrossRef]
- Akhtar, M.K.; Jones, P.R. Deletion of iscR stimulates recombinant clostridial Fe–Fe hydrogenase activity and H2-accumulation in Escherichia coli BL21 (DE3). Appl. Microbiol. Biotechnol. 2008, 78, 853–862. [Google Scholar] [CrossRef]
- Kuchenreuther, J.M.; Grady-Smith, C.S.; Bingham, A.S.; George, S.J.; Cramer, S.P.; Swartz, J.R. High-yield expression of heterologous [FeFe] hydrogenases in Escherichia coli. PLoS ONE 2010, 5, e15491. [Google Scholar] [CrossRef] [PubMed]
- Berggren, G.; Adamska, A.; Lambertz, C.; Simmons, T.R.; Esselborn, J.; Atta, M.; Gambarelli, S.; Mouesca, J.M.; Reijerse, E.; Lubitz, W.; et al. Biomimetic assembly and activation of [FeFe]-hydrogenases. Nature 2013, 499, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Esselborn, J.; Lambertz, C.; Adamska-Venkatesh, A.; Simmons, T.; Berggren, G.; Noth, J.; Siebel, J.; Hemschemeier, A.; Artero, V.; Reijerse, E.; et al. Spontaneous activation of [FeFe]-hydrogenases by an inorganic [2Fe] active site mimic. Nat. Chem. Biol. 2013, 9, 607–609. [Google Scholar] [CrossRef] [PubMed]

| Enzyme | pH 5 | pH 6 | pH 7 |
|---|---|---|---|
| UbHydA1 | 0.47 ± 0.01 | 0.58 ± 0.04 | 0.92 ± 0.02 |
| CrHydA1 | 0.46 ± 0.02 | 0.7 ± 0.01 | 1.36 ± 0.06 |
| UbHydA2 | 0.31 ± 0.01 | 0.72 ± 0.001 | 1.74 ± 0.05 |
| CpI | 0.11 ± 0.03 | 0.19 ± 0.01 | 0.28 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alavi, G.; Engelbrecht, V.; Hemschemeier, A.; Happe, T. The Alga Uronema belkae Has Two Structural Types of [FeFe]-Hydrogenases with Different Biochemical Properties. Int. J. Mol. Sci. 2023, 24, 17311. https://doi.org/10.3390/ijms242417311
Alavi G, Engelbrecht V, Hemschemeier A, Happe T. The Alga Uronema belkae Has Two Structural Types of [FeFe]-Hydrogenases with Different Biochemical Properties. International Journal of Molecular Sciences. 2023; 24(24):17311. https://doi.org/10.3390/ijms242417311
Chicago/Turabian StyleAlavi, Ghazal, Vera Engelbrecht, Anja Hemschemeier, and Thomas Happe. 2023. "The Alga Uronema belkae Has Two Structural Types of [FeFe]-Hydrogenases with Different Biochemical Properties" International Journal of Molecular Sciences 24, no. 24: 17311. https://doi.org/10.3390/ijms242417311
APA StyleAlavi, G., Engelbrecht, V., Hemschemeier, A., & Happe, T. (2023). The Alga Uronema belkae Has Two Structural Types of [FeFe]-Hydrogenases with Different Biochemical Properties. International Journal of Molecular Sciences, 24(24), 17311. https://doi.org/10.3390/ijms242417311






