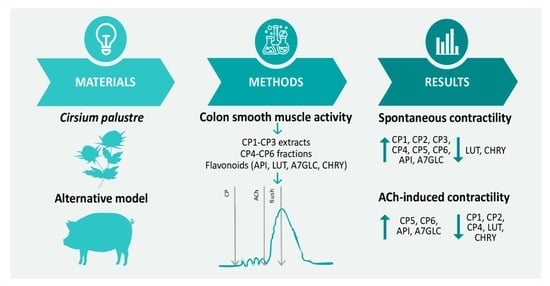
Effects of Cirsium palustre Extracts and Their Main Flavonoids on Colon Motility—An Ex Vivo Study
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Screening of Selected C. palustre Extracts/Fractions
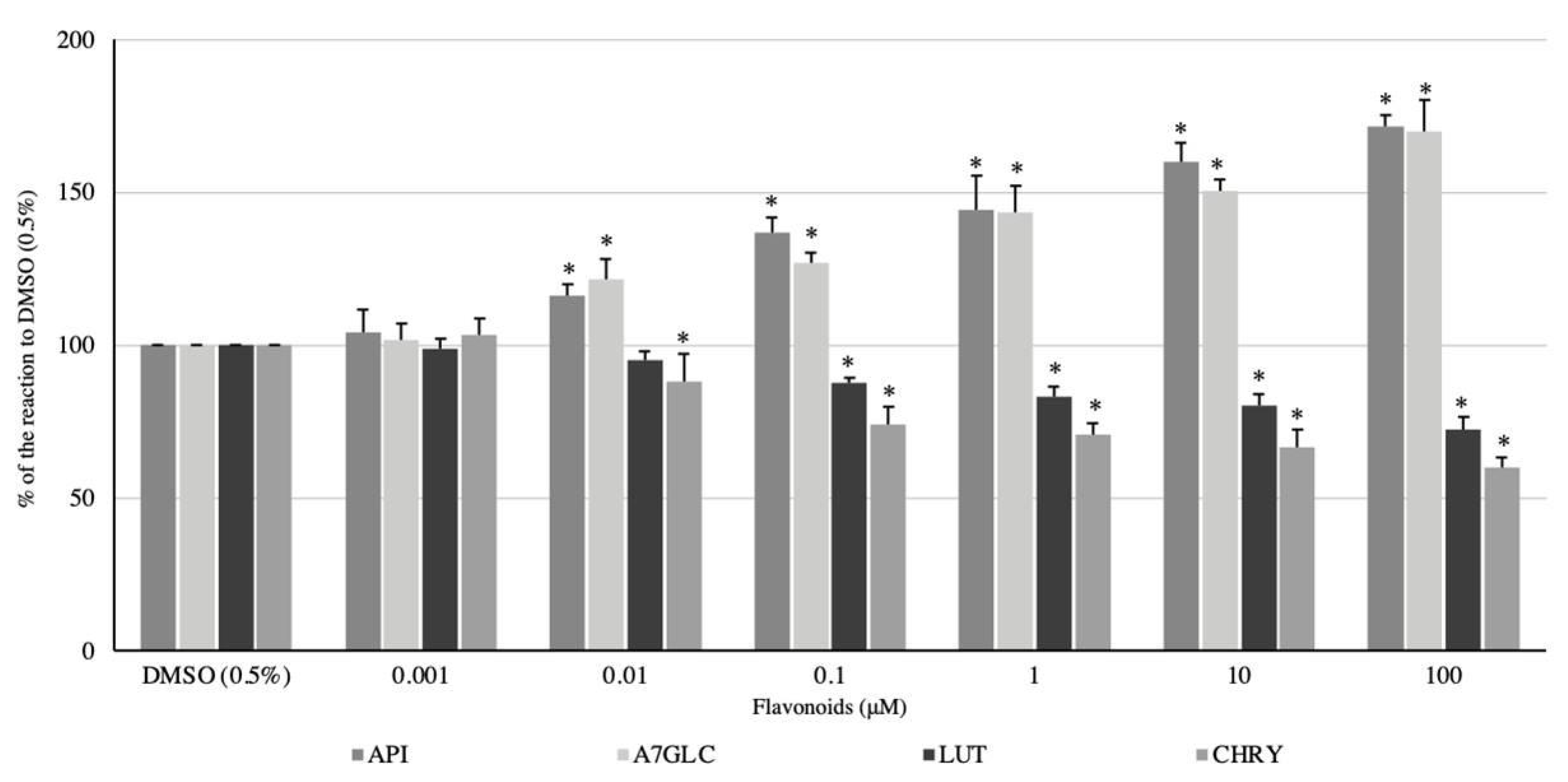
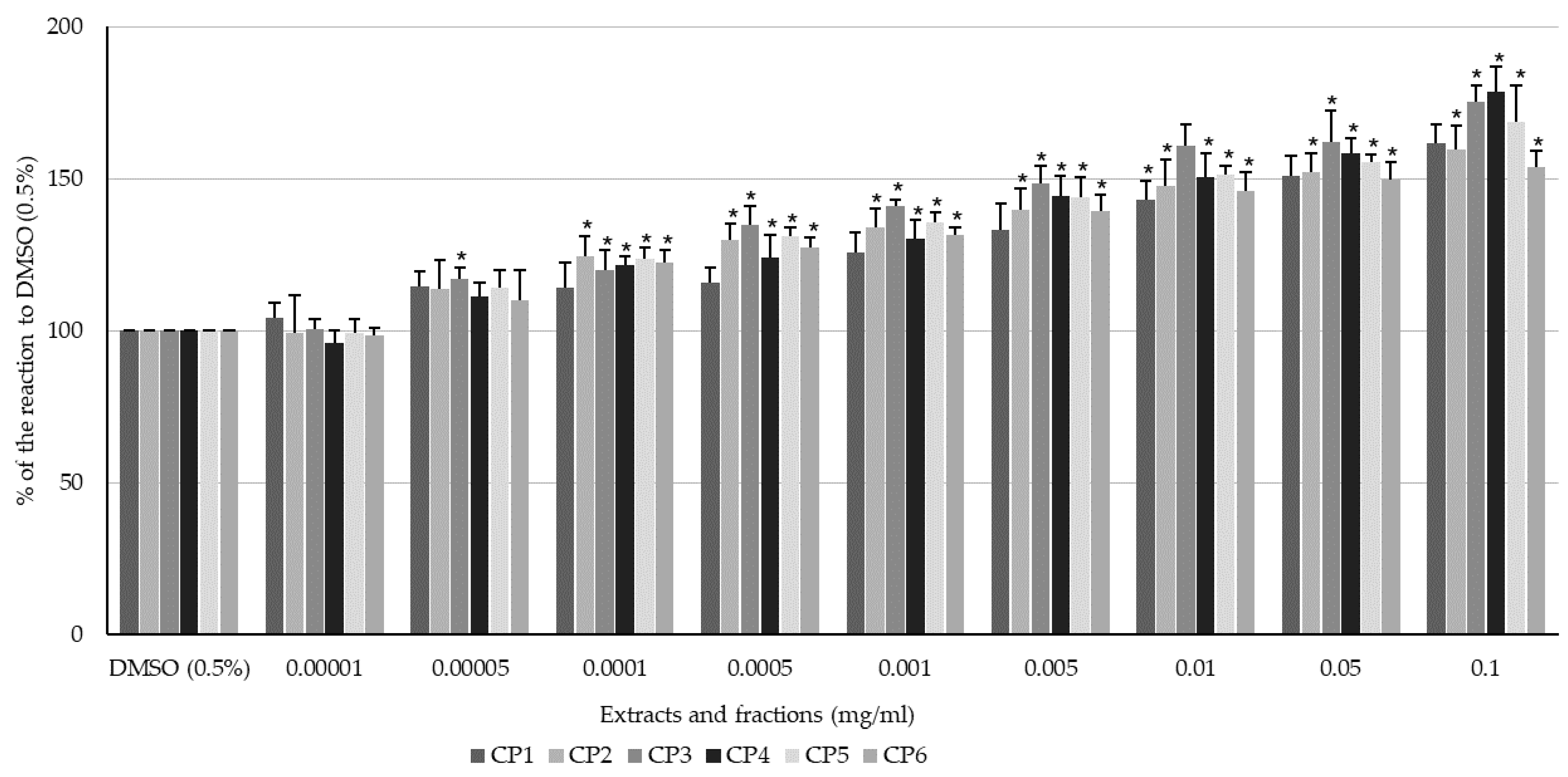
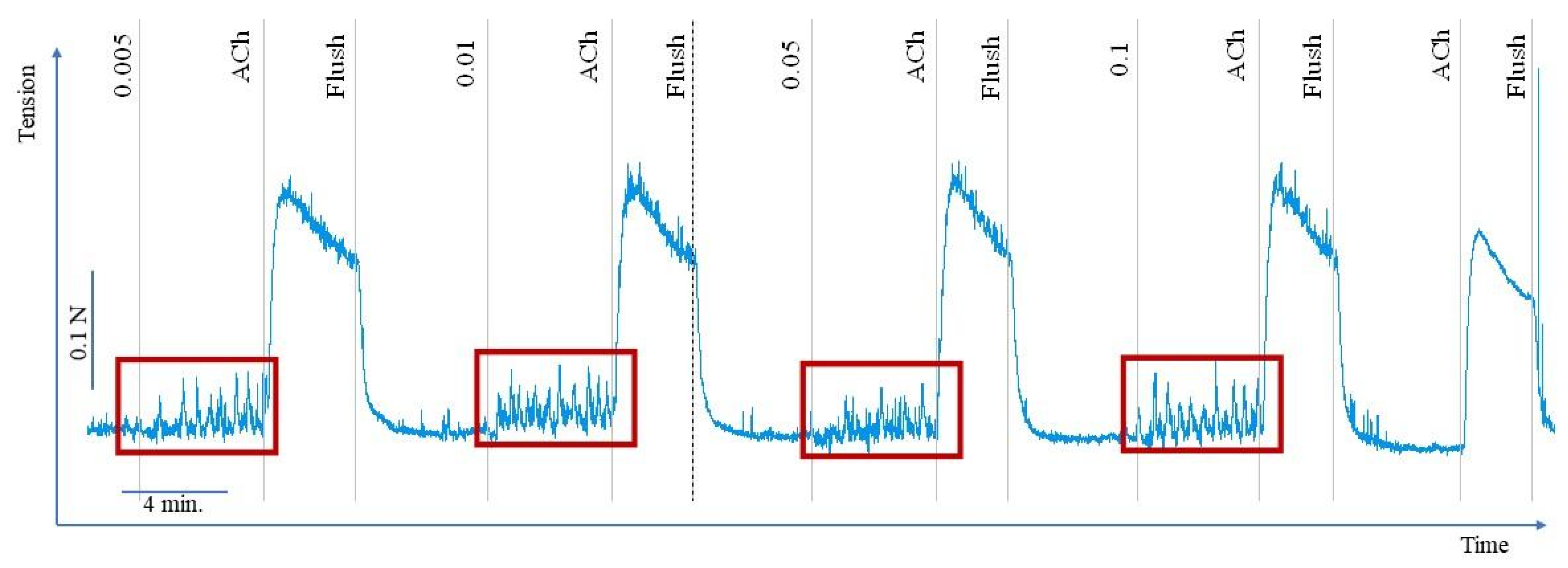
2.2. Effect of Flavonoids and C. palustre Extracts and Fractions on the Spontaneous Contractility of Swine Colonic Smooth Muscle
2.3. Effect of Flavonoids and C. palustre Extracts/Fractions on ACh-Provoked Contractility of Swine Colonic Smooth Muscle
3. Discussion
4. Materials and Methods
4.1. Chemical Solvents, Reagents, and Standards
4.2. Plant Material
4.3. Extraction Procedure for Preparation of Crude CP1-CP3 Extracts and CP4-CP6 Fractions
4.4. Phytochemical Characterization of Extracts and Fractions by LC-PDA-HRMS
4.5. Tissue Collection and Preparation
4.6. Assessment of Smooth Muscle Activity
4.7. Experimental Sequence
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drossman, D.A. Functional gastrointestinal disorders: History, pathophysiology, clinical features, and Rome IV. Gastroenterology 2016, 150, 1262–1279.e2. [Google Scholar] [CrossRef] [PubMed]
- Gubert, C.; Gasparotto, J.; Morais, L.H.; Morais, L.H. Convergent pathways of the gut microbiota-brain axis and neurodegenerative disorders. Gastroenterol. Rep. 2022, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Shivaji, U.N.; Ford, A.C. Prevalence of functional gastrointestinal disorders among consecutive new patient referrals to a gastroenterology clinic. Frontline Gastroenterol. 2014, 5, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, R.; Li, D.; Zhao, L.; Zhu, L. Role of gut microbiota in functional constipation. Gastroenterol. Rep. 2021, 9, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Podolsky, D. Inflammatory bowel disease. Med. Prog. 2002, 347, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Azab, A.; Nassar, A.; Azab, A.N. Anti-inflammatory activity of natural products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef]
- Zardzewiały, W.; Bernacki, R.; Bernacki, P.; Zaremskyi, O.; Dutka, M.; Szawica, D.; Kuźniar, A.; Oleszko, M.; Wąskiewicz, E. Rola mikrobioty jelitowej w zrozumieniu czynnościowych zaburzeń przewodu pokarmowego. J. Educ. Heal. Sport 2023, 30, 96–105. [Google Scholar] [CrossRef]
- Pusceddu, M.M.; Gareau, M.G. Visceral pain: Gut microbiota, a new hope? J. Biomed. Sci. 2018, 25, 1–8. [Google Scholar] [CrossRef]
- Tian, S.; Zhang, H.; Chen, S.; Wu, P.; Chen, M. Global research progress of visceral hypersensitivity and irritable bowel syndrome: Bibliometrics and visualized analysis. Front. Pharmacol. 2023, 14, 1–13. [Google Scholar] [CrossRef]
- Ruiz-Malagón, A.J.; Rodríguez-Sanchez, M.J.; Rodríguez-Sojo, M.J.; Vezza, T.; Pischel, I.; Algieri, F.; Rodríguez-Cabezas, M.E.; Rodríguez-Nogales, A.; Gálvez, J. Intestinal anti-inflammatory and visceral analgesic effects of a Serpylli herba extract in an experimental model of irritable bowel syndrome in rats. Front. Pharmacol. 2022, 13, 1–12. [Google Scholar] [CrossRef]
- Tome, J.; Kamboj, A.K.; Loftus, C.G. Approach to disorders of gut-brain interaction. Mayo Clin. Proc. 2023, 98, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Mearin, F.; Malfertheiner, P. Functional gastrointestinal disorders: Complex treatments for complex pathophysiological mechanisms. Dig. Dis. 2018, 35, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Invasive Species Council of BC. Marsh Plume Thistle Factsheet. Invasive Species Council of British Columbia—ISCBC 2021. Available online: https://bcinvasives.ca/wp-content/uploads/2021/01/Marsh-Plume-Thistle_Factsheet_09_04_2019.pdf (accessed on 17 November 2023).
- Tohver, M. Cirsium palustre: An evasive invasive; University of Michigan Biological Station: Pellston, MI, USA, 1998. [Google Scholar]
- Borawska, M.H.; Czechowska, S.K.; Markiewicz, R.; Socha, K.; Nazaruk, J.; Paka, J.; Isidorov, V.A. Enhancement of antibacterial effects of extracts from Cirsium species using sodium picolinate and estimation of their toxicity. Nat. Prod. Res. 2010, 24, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Kenny, O.; Smyth, T.J.; Walsh, D.; Kelleher, C.T.; Hewage, C.M.; Brunton, N.P. Investigating the potential of under-utilised plants from the Asteraceae family as a source of natural antimicrobial and antioxidant extracts. Food Chem. 2014, 161, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Nazaruk, J. Antioxidant activity and total phenolic content in Cirsium five species from north-east region of Poland. Fitoterapia 2008, 79, 194–196. [Google Scholar] [CrossRef]
- Nazaruk, J.; Czechowska, S.K.; Markiewicz, R.; Borawska, M.H. Polyphenolic compounds and in vitro antimicrobial and antioxidant activity of aqueous extracts from leaves of some Cirsium species. Nat. Prod. Res. 2008, 22, 1583–1588. [Google Scholar] [CrossRef]
- Nazaruk, J.; Wajs-Bonikowska, A.; Bonikowski, R. Components and antioxidant activity of fruits of Cirsium palustre and C. rivulare. Chem. Nat. Compd. 2012, 48, 8–10. [Google Scholar] [CrossRef]
- Nalewajko-Sieliwoniuk, E.; Malejko, J.; Mozolewska, M.; Wołyniec, E.; Nazaruk, J. Determination of polyphenolic compounds in Cirsium palustre (L.) extracts by high performance liquid chromatography with chemiluminescence detection. Talanta 2015, 133, 38–44. [Google Scholar] [CrossRef]
- Nazaruk, J.; Karna, E.; Kalemba, D. The chemical composition of the essential oils of Cirsium palustre and C. rivulare and their antiproliferative effect. Nat. Prod. Commun. 2012, 7, 269–272. [Google Scholar] [CrossRef]
- Chakraborty, T.; Saha, S.; Bisht, N.S. First report on the ethnopharmacological uses of medicinal plants by Monpa tribe from the Zemithang region of Arunachal Pradesh, Eastern Himalayas, India. Plants 2017, 6, 13. [Google Scholar] [CrossRef]
- Aggarwal, G.; Kaur, G.; Bhardwaj, G.; Mutreja, V.; Sohal, H.S.; Nayik, G.A.; Bhardwaj, A.; Sharma, A. Traditional uses, phytochemical composition, pharmacological properties, and the biodiscovery potential of the genus. Cirsium. Chem. 2022, 4, 1161–1192. [Google Scholar] [CrossRef]
- Kolosova, V.B. Name—Text—Ritual: The role of plant characteristics in slavic folk medicine. Folklorica 2010, 10, 44–61. [Google Scholar] [CrossRef]
- Kujawska, M.; Łuczaj, Ł.; Sosnowska, J.; Klepacki, P. Rośliny w Wierzeniach i Zwyczajach Ludowych: Słownik Adama Fischera; Polskie Towarzystwo Ludoznawcze: Wrocław, Poland, 2016. [Google Scholar]
- Szadkowska, D.; Posłuszny, M.; Chłopecka, M.; Strawa, W.J.; Jakimiuk, K.; Tomczyk, M.; Mendel, M. Cirsium palustre extracts as potential modifiers of colon motility in functional gut disorders—An ex vivo study. Planta Med. 2021, 87, 1279. [Google Scholar]
- Szadkowska, D.; Posłuszny, M.; Chłopecka, M.; Strawa, J.W.; Jakimiuk, K.; Augustynowicz, D.; Tomczyk, M.; Mendel, M. Effects of selected Cirsium palustre extracts on intestinal motility—An ex vivo study. Planta Med. 2022, 88, 1486. [Google Scholar]
- Nazaruk, J. Flavonoid compounds from Cirsium palustre (L.) Scop. flower heads. Biochem. Syst. Ecol. 2009, 37, 525–527. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef]
- Black, C.J.; Paine, P.A.; Agrawal, A.; Aziz, I.; Eugenicos, M.P.; Houghton, L.A.; Hungin, P.; Overshott, R.; Vasant, D.H.; Rudd, S.; et al. British Society of Gastroenterology guidelines on the management of functional dyspepsia. Gut 2022, 71, 1697–1723. [Google Scholar] [CrossRef]
- Fukudo, S.; Okumura, T.; Inamori, M.; Okuyama, Y.; Kanazawa, M.; Kamiya, T.; Sato, K.; Shiotani, A.; Naito, Y.; Fujikawa, Y.; et al. Evidence-based clinical practice guidelines for irritable bowel syndrome 2020. J. Gastroenterol. 2021, 56, 193–217. [Google Scholar] [CrossRef]
- Patterson, J.K.; Lei, X.G.; Miller, D.D. The pig as an experimental model for elucidating the mechanisms governing dietary influence on mineral absorption. Exp. Biol. Med. 2008, 233, 651–664. [Google Scholar] [CrossRef]
- Sciascia, Q.; Daş, G.; Metges, C.C. Review: The pig as a model for humans: Effects of nutritional factors on intestinal function and health. J. Anim. Sci. 2016, 94, 441–452. [Google Scholar] [CrossRef]
- Mendel, M.; Chłopecka, M.; Latek, U.; Karlik, W.; Tomczykowa, M.; Strawa, J.; Tomczyk, M. Evaluation of the effects of Bidens tripartita extracts and their main constituents on intestinal motility—An ex vivo study. J. Ethnopharmacol. 2020, 259, 112982. [Google Scholar] [CrossRef] [PubMed]
- Chłopecka, M.; Dziekan, N.; Mendel, M.; Baķała, A.; Małdyk, J.; Wiechetek, M. Evaluation of the time-stability of an alternative research model based on isolated rat gastrointestinal strips. J. Physiol. Pharmacol. 2007, 58, 73–86. [Google Scholar] [PubMed]
- Bahrami, H.R.; Hamedi, S.; Salari, R.; Noras, M. Herbal medicines for the management of irritable bowel syndrome: A systematic review. Electron. Physician 2016, 8, 2719–2725. [Google Scholar] [CrossRef] [PubMed]
- Czigle, S.; Fialová, S.B.; Tóth, J.; Mučaji, P.; Nagy, M. Treatment of gastrointestinal disorders—Plants and potential mechanisms of action of their constituents. Molecules 2022, 27, 2881. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhou, Y.; Wan, L.L.; Ye, J.Z.; Lu, H.L.; Huang, X.; Xu, W.X. Luteolin suppresses colonic smooth muscle motility via inhibiting L-type calcium channel currents in mice. Gen. Physiol. Biophys. 2020, 39, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Gilani, A.H.; Khan, A.U.; Ghayur, M.N.; Ali, S.F.; Herzig, J.W. Antispasmodic effects of rooibos tea (Aspalathus linearis) is mediated predominantly through K+-channel activation. Basic Clin. Pharmacol. Toxicol. 2006, 99, 365–373. [Google Scholar] [CrossRef]
- Lemmens-Gruber, R.; Marchart, E.; Rawnduzi, P.; Engel, N.; Benedek, B.; Kopp, B. Investigation of the spasmolytic activity of the flavonoid fraction of Achillea millefolium on isolated guinea-pig ilea. Arzneimittel-Forschung/Drug Res. 2006, 56, 582–586. [Google Scholar] [CrossRef]
- Sadraei, H.; Ghanadian, M.; Asghari, G.; Sekhavati, N. Antispasmodic activity of apigenin and luteolin, two components of Dracocephalum kotschyi extract, on rat ileum contractions. J. Herbmed. Pharmacol. 2018, 7, 100–105. [Google Scholar] [CrossRef]
- Yu, M.C.; Chen, J.H.; Lai, C.Y.; Han, C.Y.; Ko, W.C. Luteolin, a non-selective competitive inhibitor of phosphodiesterases 1-5, displaced [3H]-rolipram from high-affinity rolipram binding sites and reversed xylazine/ketamine-induced anesthesia. Eur. J. Pharmacol. 2010, 627, 269–275. [Google Scholar] [CrossRef]
- Mendel, M.; Chłopecka, M.; Dziekan, N.; Karlik, W. Modification of abomasum contractility by flavonoids present in ruminants diet: In vitro study. Animal 2016, 10, 1431–1438. [Google Scholar] [CrossRef]
- Gorzalczany, S.; Moscatelli, V.; Acevedo, C.; Ferraro, G. Spasmolytic activity of Artemisia copa aqueous extract and isolated compounds. Nat. Prod. Res. 2013, 27, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, G.; Autore, G.; Izzo, A.A.; Maiolino, P.; Mascolo, N.; Viola, P.; Diurno, M.V.; Capasso, F. Inhibition of intestinal motility and secretion by flavonoids in mice and rats: Structure-activity relationships. J. Pharm. Pharmacol. 1993, 45, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Gharzouli, K.; Holzer, P. Inhibition of guinea pig intestinal peristalsis by the flavonoids quercetin, naringenin, apigenin and genistein. Pharmacology 2004, 70, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, A.; Serio, R.; Mulè, F. Gastric relaxation induced by apigenin and quercetin: Analysis of the mechanism of action. Life Sci. 2009, 85, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Fleer, H.; Verspohl, E.J. Antispasmodic activity of an extract from Plantago lanceolata L. and some isolated compounds. Phytomedicine 2007, 14, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.; Kim, M.-Y.; Cho, J.Y. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef]
- Roudsari, N.M.; Lashgari, N.A.; Momtaz, S.; Farzaei, M.H.; Marques, A.M.; Abdolghaffari, A.H. Natural polyphenols for the prevention of irritable bowel syndrome: Molecular mechanisms and targets; a comprehensive review. J. Pharm. Sci. 2019, 27, 755–780. [Google Scholar] [CrossRef]
- Docsa, T.; Sipos, A.; Cox, C.S.; Uray, K. The role of inflammatory mediators in the development of gastrointestinal motility disorders. Int. J. Mol. Sci. 2022, 23, 6917. [Google Scholar] [CrossRef]
- Fu, R.; Wang, L.; Meng, Y.; Xue, W.; Liang, J.; Peng, Z.; Meng, J.; Zhang, M. Apigenin remodels the gut microbiota to ameliorate ulcerative colitis. Front. Nutr. 2022, 9, 1062961. [Google Scholar] [CrossRef]
- Sun, W.L.; Yang, J.W.; Dou, H.Y.; Li, G.Q.; Li, X.Y.; Shen, L.; Ji, H.F. Anti-inflammatory effect of luteolin is related to the changes in the gut microbiota and contributes to preventing the progression from simple steatosis to nonalcoholic steatohepatitis. Bioorg. Chem. 2021, 112, 104966. [Google Scholar] [CrossRef]
- Xiong, H.H.; Lin, S.Y.; Chen, L.L.; Ouyang, K.H.; Wang, W.J. The interaction between flavonoids and intestinal microbes: A review. Foods 2023, 12, 320. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Zhao, W.; DIng, C.; Tian, H.; Xu, L.; Wang, H.; Ni, L.; Jiang, J.; Gong, J.; Zhu, W.; et al. Potential role of fecal microbiota from patients with slow transit constipation in the regulation of gastrointestinal motility. Sci. Rep. 2017, 7, 441. [Google Scholar] [CrossRef] [PubMed]
- West, C.L.; Stanisz, A.M.; Mao, Y.K.; Champagne-Jorgensen, K.; Bienenstock, J.; Kunze, W.A. Microvesicles from Lactobacillus reuteri (DSM-17938) completely reproduce modulation of gut motility by bacteria in mice. PLoS ONE 2020, 15, e0225481. [Google Scholar] [CrossRef] [PubMed]
- Nazaruk, J.; Galicka, A. The influence of selected flavonoids from the leaves of Cirsium palustre (L.) Scop. on collagen expression in human skin fibroblasts. Phyther. Res. 2014, 28, 1399–1405. [Google Scholar] [CrossRef]
- Strawa, J.; Wajs-Bonikowska, A.; Jakimiuk, K.; Waluk, M.; Poslednik, M.; Nazaruk, J.; Tomczyk, M. Phytochemical examination of Woolly Burdock Arctium tomentosum leaves and flower heads. Chem. Nat. Compd. 2020, 56, 345–347. [Google Scholar] [CrossRef]
- Broda, B.; Mowszowicz, J. Przewodnik do Oznaczania Roślin Leczniczych, Trujących i Użytkowych; Wydawnictwo Lekarskie PZWL: Warsaw, Poland, 1996. [Google Scholar]
- Strawa, J.W.; Jakimiuk, K.; Kita, Z.; Tomczyk, M. In vitro screening for anti-acetylcholinesterase and antioxidant activities of Hottonia palustris L. extracts and their unusual flavonoids. Molecules 2022, 27, 8034. [Google Scholar] [CrossRef]
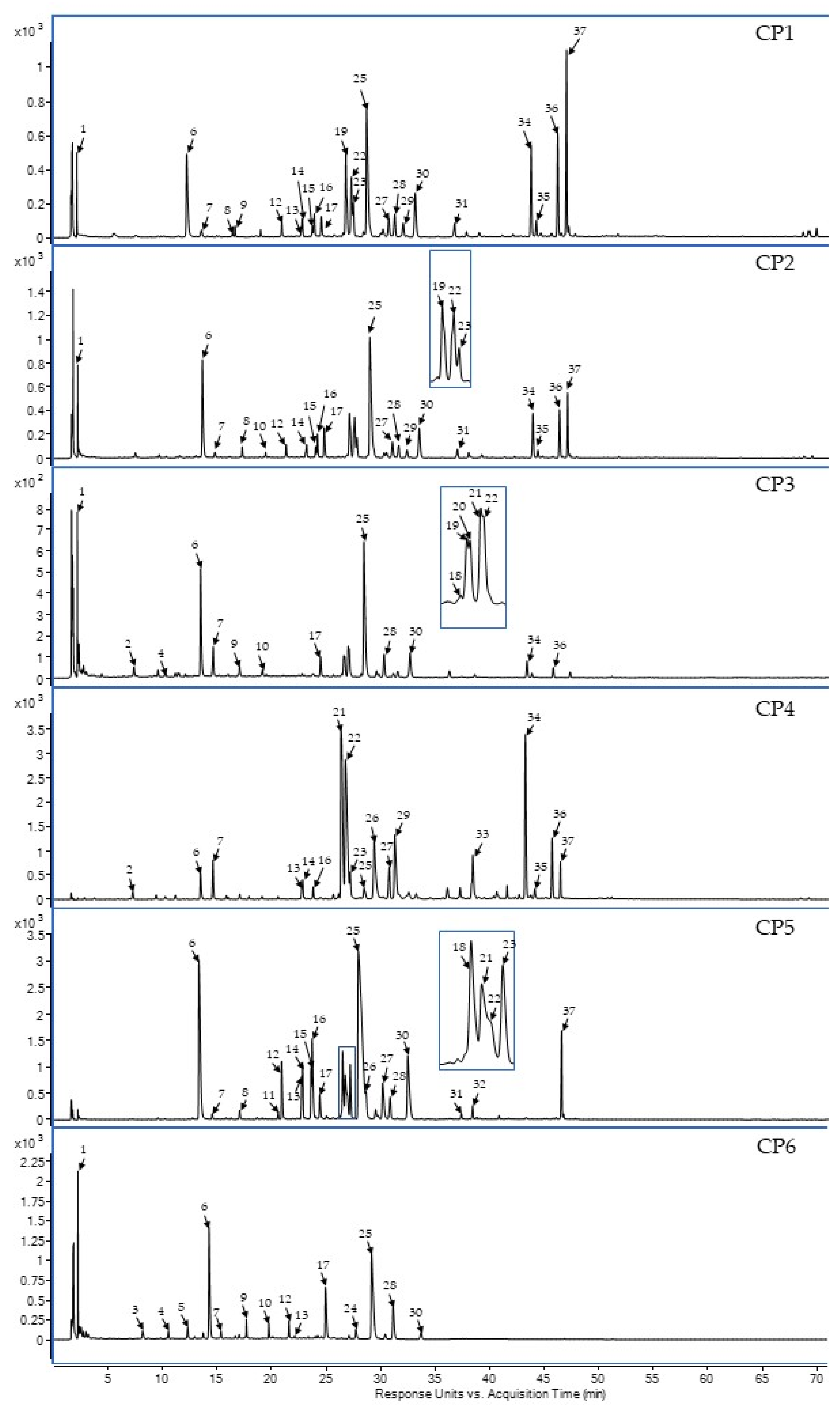



| No. | Rt (min) | UV Spectra (λmax nm) | Observed A | Δ (ppm) B | Formula | Fragmentation C | Compounds | Presence in Extracts/Fraction | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CP1 | CP2 | CP3 | CP4 | CP5 | CP6 | ||||||||
| 1 | 2.29 | 248, 270, 345 | 191.01957 | −0.65 | C6H8O7 | 191 | organic acid derivatives F | x | x | x | x | ||
| 2 | 7.23 | 260, 295 | 153.02000 | 3,66 | C7H6O4 | 153 | phenolic acid derivatives | x | x | ||||
| 3 | 8.13 | 280 | 315.10854 | 1.22 | C14H20O8 | 203, 315 | phenolic acid derivatives | x | |||||
| 4 | 10.5 | 245 sh, 295 sh, 325 | 353.08918 | 3.85 | C16H18O9 | 353, 134 | 3-O-caffeoylquinic acid R | x | x | ||||
| 5 | 12.39 | 270 | 443.19227 | 0.66 | C21H32O10 | 215, 443 | phenolic acid derivatives | x | |||||
| 6 | 14.32 | 245 sh, 295 sh, 325 | 353.08833 | 1.5 | C16H18O9 | 353, 191 | 5-O-caffeoylquinic acid S | x | x | x | x | x | x |
| 7 | 15.32 | 245 sh, 295 sh, 325 | 353.08930 | 4.14 | C16H18O9 | 353, 179 | 4-O-caffeoylquinic acid S,R | x | x | x | x | x | x |
| 8 | 17.04 | 290, 312 | 337.09462 | 3.33 | C16H18O8 | 337, 191 | 5-O-p-coumaroylquinic acid R | x | x | x | |||
| 9 | 17.66 | 265, 345 | 623.12809 | 4.21 | C27H28O17 | 284, 447, 623 | flavone derivative F | x | x | x | |||
| 10 | 19.72 | 264, 343 | 607.13046 | 3.76 | C27H28O16 | 607 | unknown | x | x | x | |||
| 11 | 20.57 | 245 sh, 295 sh, 327 | 367.10349 | 4.62 | C17H19O9 | 367, 179, 135 | 4-O-feruloylquinic acid R | x | |||||
| 12 | 21.55 | 255, 282, 344 | 463.08820 | 3.62 | C21H20O12 | 300, 463 | flavone O-hex F | x | x | x | x | ||
| 13 | 22.74 | 283, 335 | 449.11088 | 4.64 | C21H22O11 | 287, 449 | eriodictyol O-hex isomer F | x | x | x | x | ||
| 14 | 22.82 | 283, 335 | 449.10894 | 4.32 | C21H22O11 | 287, 449 | eriodictyol 7-O-glucoside S | x | x | x | x | ||
| 15 | 22.63 | 255, 267 sh, 348 | 447.09329 | 4.57 | C21H22O11 | 283, 447 | luteolin 7-O-glucoside S | x | x | x | |||
| 16 | 23.67 | 255, 267 sh, 348 | 447.09329 | 4.57 | C21H22O11 | 283, 447 | luteolin O-hex isomer F | x | x | x | x | ||
| 17 | 24.39 | 255, 267 sh, 342 | 461.07255 | 6.55 | C21H18O12 | 285, 461 | flavone O-uronide derivatives F | x | x | x | x | x | |
| 18 | 26.49 | 264, 347 | 491.08311 | 5.73 | C22H20O13 | 315, 447 | cirsimaritin 4’-O-glucoside F | x | x | ||||
| 19 | 26.58 | 246, 296, 327 | 515.12266 | 5.05 | C25H24O12 | 191, 353, 515 | 3,4-O-dicaffeoylquinic acid F | x | x | x | |||
| 20 | 26.67 | 246, 296, 327 | 515.12240 | 6.03 | C25H24O12 | 191, 353, 515 | 3,5-O-dicaffeoylquinic acid S,F | x | |||||
| 21 | 26.72 | 246, 296, 327 | 515.11950 | 5.09 | C25H24O12 | 191, 353, 515 | dicaffeoylquinic acid isomer F | x | x | x | |||
| 22 | 26.92 | 246, 296, 327 | 515.12212 | 5.31 | C25H24O12 | 191, 353, 515 | dicaffeoylquinic acid isomer F | x | x | x | x | x | |
| 23 | 27.18 | 266, 336 | 431.10043 | 4.79 | C21H20O10 | 268, 431 | flavone O-hex isomer F | x | x | x | x | ||
| 24 | 27.68 | 250, 295 sh, 327 | 631.13046 | 3.25 | C29H28O16 | 191, 353, 631 | quinic acid derivatives | x | |||||
| 25 | 29.14 | 266, 336 | 445.07763 | 3.98 | C21H18O11 | 269, 445 | apigenin 7-O-glc (A7GLC) S | x | x | x | x | x | x |
| 26 | 29.79 | 245 sh, 295 sh, 325 | 515.11950 | 4.63 | C25H24O12 | 515 | dicaffeoylquinic acid isomer F | x | x | ||||
| 27 | 30.82 | 266, 350 | 461.10894 | 3.86 | C22H22O11 | 283, 461 | isokaempferide 7-O-glu S | x | x | x | |||
| 28 | 31.12 | 274, 334 | 475.08907 | 2.34 | C22H20O12 | 283, 299, 475 | isokaempferide 7-O-glc F | x | x | x | x | x | |
| 29 | 31.25 | 264, 340 | 431.10021 | 4.52 | C21H20O10 | 284, 431 | flavone derivatives | x | x | x | |||
| 30 | 33.67 | 274, 334 | 475.08820 | 1.84 | C22H20O12 | 255, 299, 475 | flavone O-hex derivatives F | x | x | x | x | x | |
| 31 | 37.36 | 268, 325 | 593.13006 | 4.62 | C30H26O13 | 593 | unknown | x | x | ||||
| 32 | 38.4 | 268, 336 | 459.09329 | 4.8 | C22H20O11 | 269, 459 | apigenin 7-O-(6’’-O-methyl)-glc S | x | |||||
| 33 | 38.48 | 268, 345 | 285.04046 | 3.48 | C15H10O6 | 285 | luteolin (LUT) S | x | |||||
| 34 | 44.24 | 268, 290 sh, 338 | 269.04609 | 2.03 | C15H10O5 | 269 | apigenin (API) S | x | x | x | x | ||
| 35 | 44.67 | 266, 29 sh, 358 | 285.04178 | 4.39 | C15H10O6 | 285 | Kaempferol S | x | x | x | |||
| 36 | 45.69 | 266, 293 sh, 350 | 299.05697 | 2.89 | C16H12O6 | 299 | chrysoeriol (CHRY) S | x | x | x | x | ||
| 37 | 46.56 | 295, 308 | 785.35848 | −1.41 | C38H58O17 | 545, 665, 785 | unknown | x | x | x | x | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szadkowska, D.; Chłopecka, M.; Strawa, J.W.; Jakimiuk, K.; Augustynowicz, D.; Tomczyk, M.; Mendel, M. Effects of Cirsium palustre Extracts and Their Main Flavonoids on Colon Motility—An Ex Vivo Study. Int. J. Mol. Sci. 2023, 24, 17283. https://doi.org/10.3390/ijms242417283
Szadkowska D, Chłopecka M, Strawa JW, Jakimiuk K, Augustynowicz D, Tomczyk M, Mendel M. Effects of Cirsium palustre Extracts and Their Main Flavonoids on Colon Motility—An Ex Vivo Study. International Journal of Molecular Sciences. 2023; 24(24):17283. https://doi.org/10.3390/ijms242417283
Chicago/Turabian StyleSzadkowska, Dominika, Magdalena Chłopecka, Jakub W. Strawa, Katarzyna Jakimiuk, Daniel Augustynowicz, Michał Tomczyk, and Marta Mendel. 2023. "Effects of Cirsium palustre Extracts and Their Main Flavonoids on Colon Motility—An Ex Vivo Study" International Journal of Molecular Sciences 24, no. 24: 17283. https://doi.org/10.3390/ijms242417283
APA StyleSzadkowska, D., Chłopecka, M., Strawa, J. W., Jakimiuk, K., Augustynowicz, D., Tomczyk, M., & Mendel, M. (2023). Effects of Cirsium palustre Extracts and Their Main Flavonoids on Colon Motility—An Ex Vivo Study. International Journal of Molecular Sciences, 24(24), 17283. https://doi.org/10.3390/ijms242417283