A Comparative Study of Different Protocols for Isolation of Murine Neutrophils from Bone Marrow and Spleen
Abstract
1. Introduction
2. Results
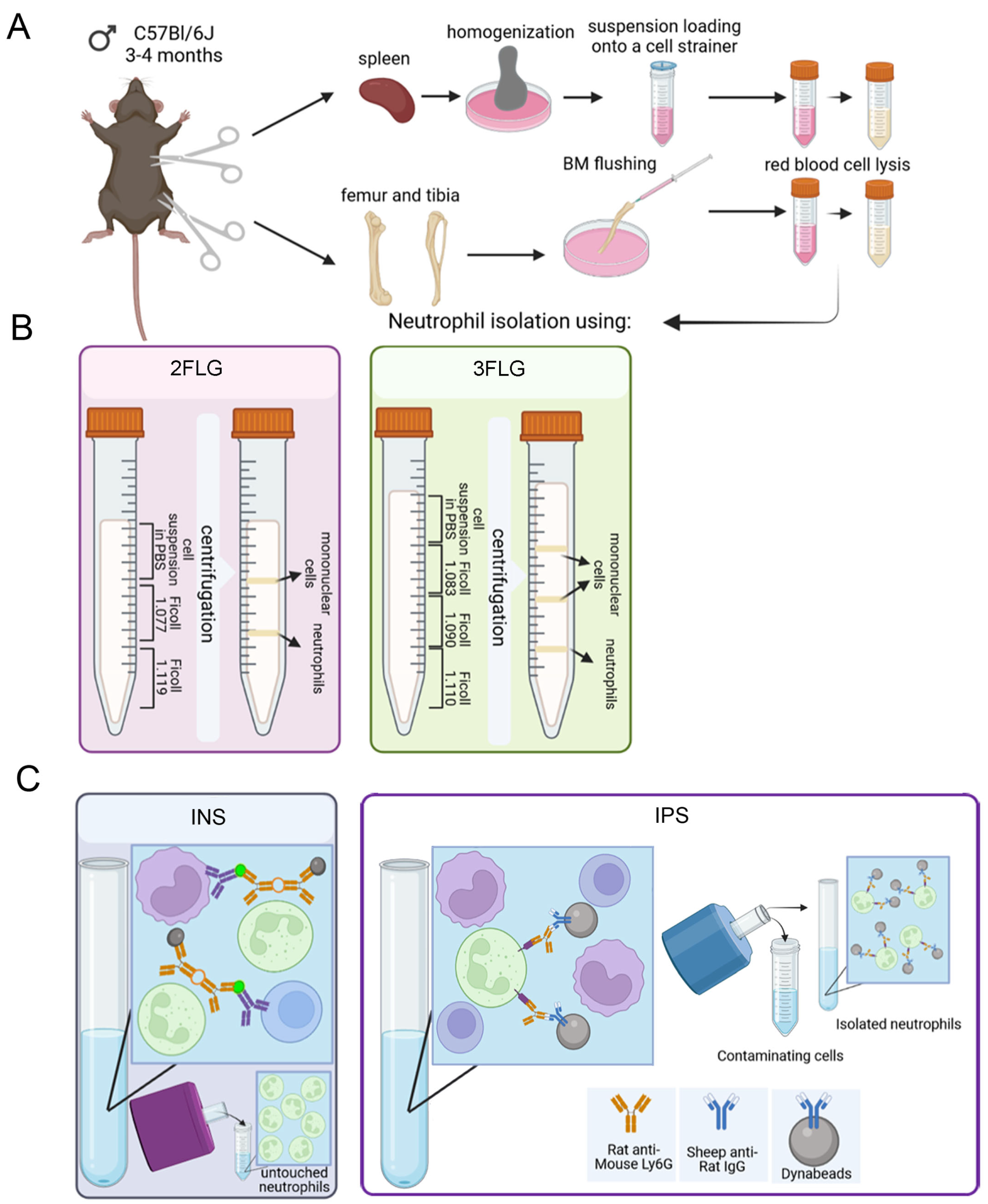
2.1. Protocol Selection for Neutrophil Isolation
2.2. Characterization of the Samples Isolated from Bone Marrow and Spleen
2.3. Comparison of Purity, Viability, and Yield of Neutrophil Samples Isolated from Bone Marrow and Spleen Using Different Protocols
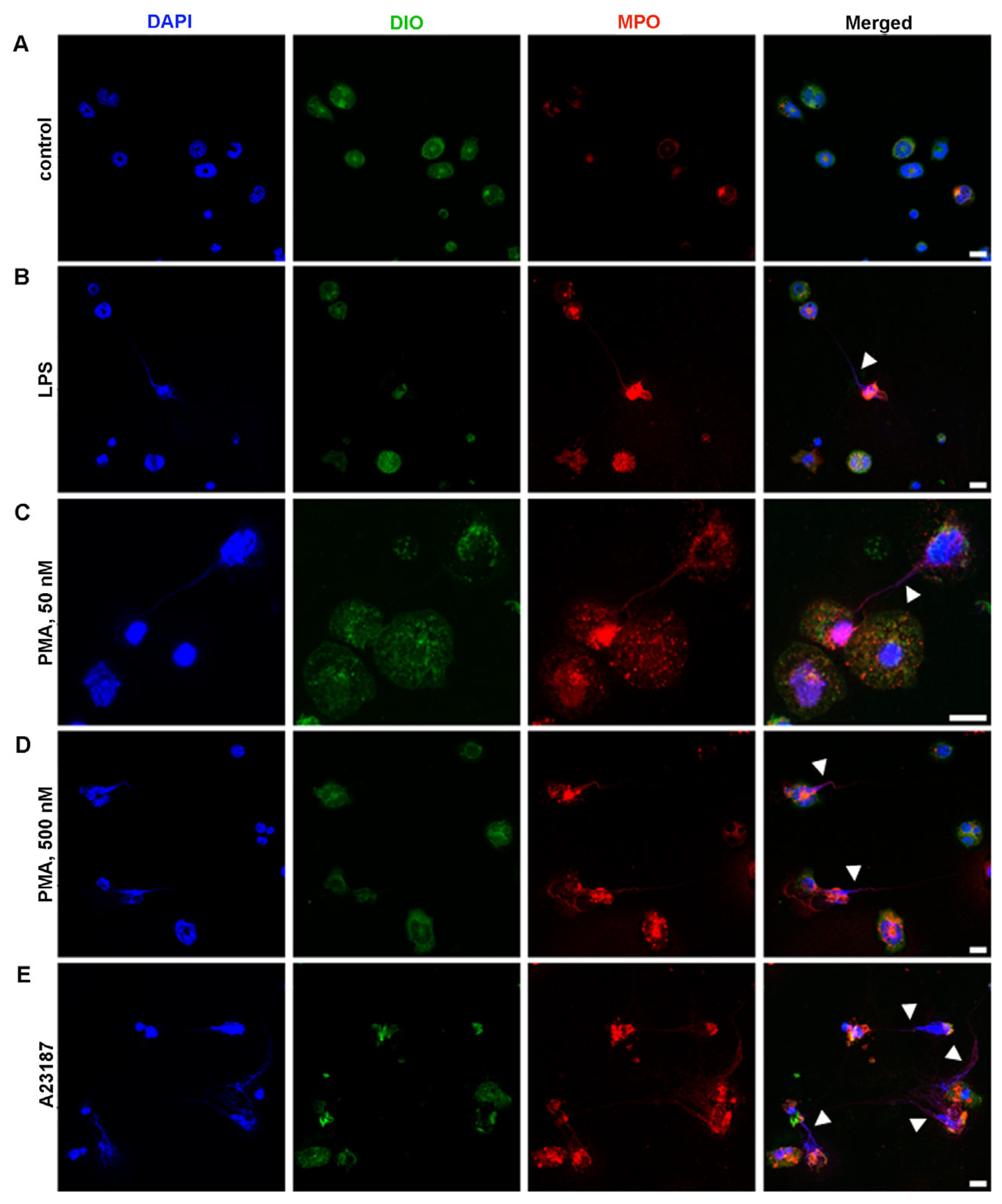
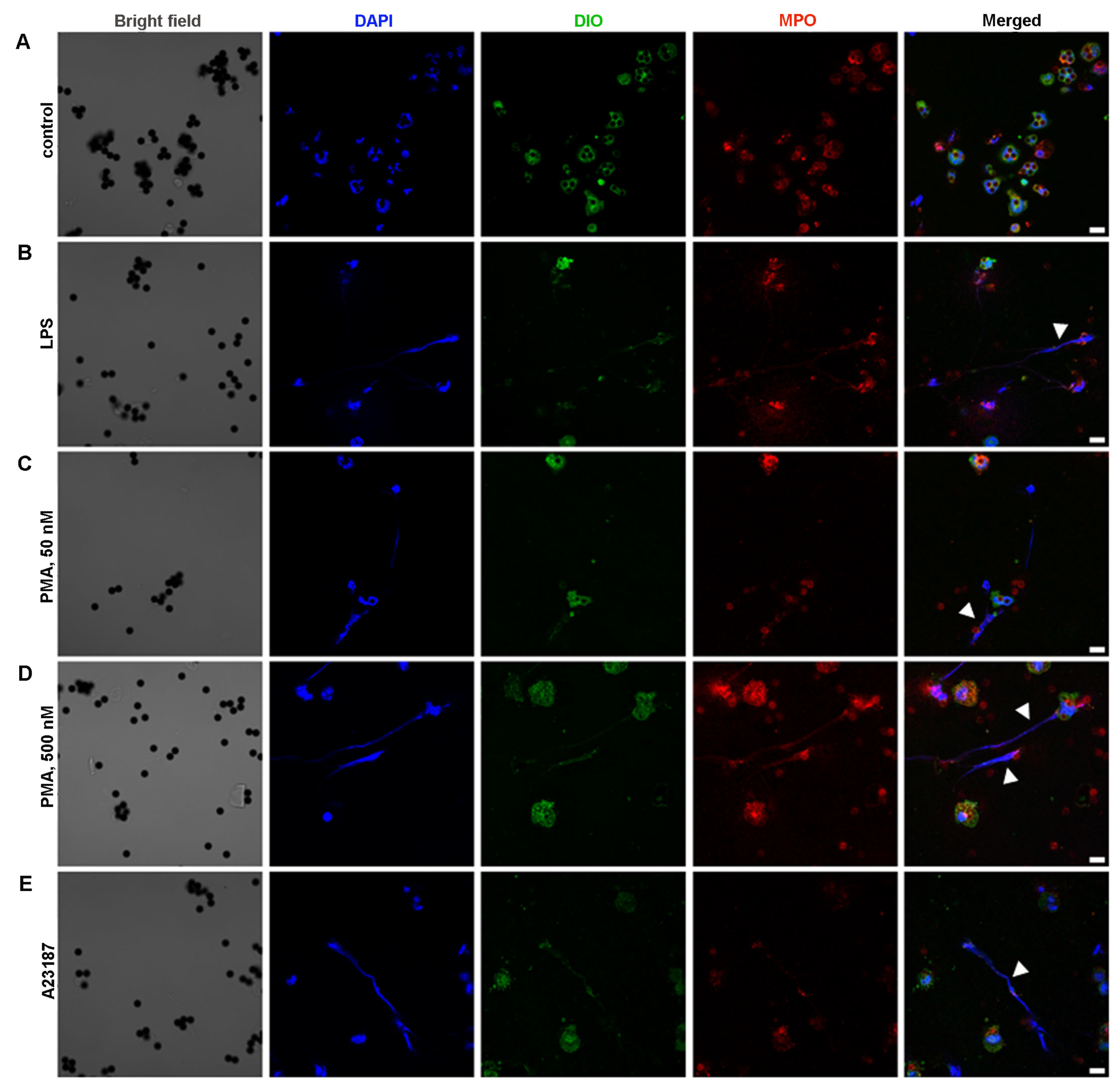
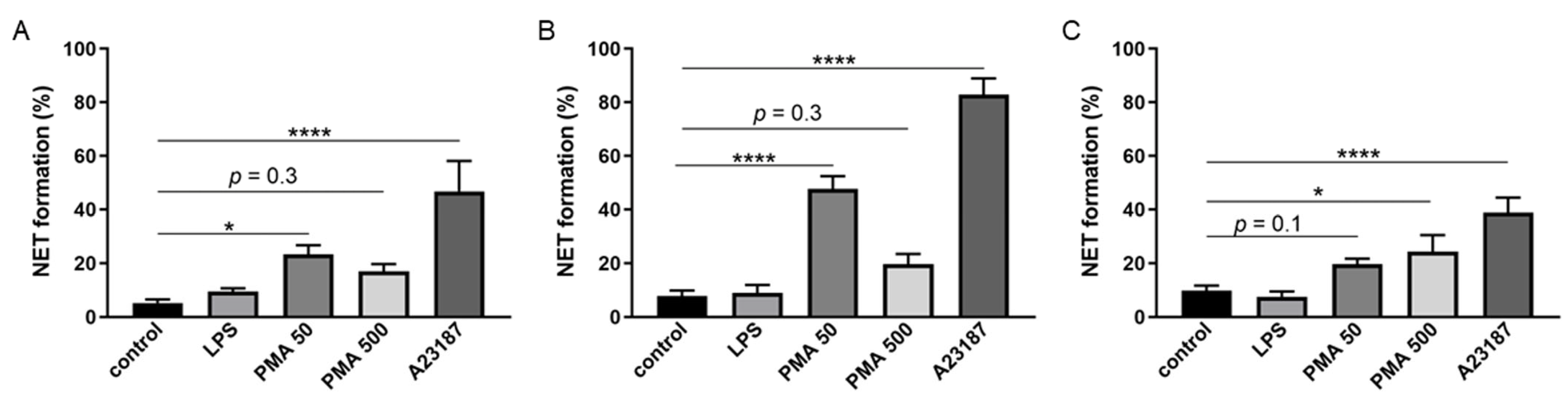
2.4. Ability of BM-Derived Neutrophils Isolated by the 2FLG Protocol and Splenic Neutrophils Isolated Using the IPS Protocol to Produce NETs
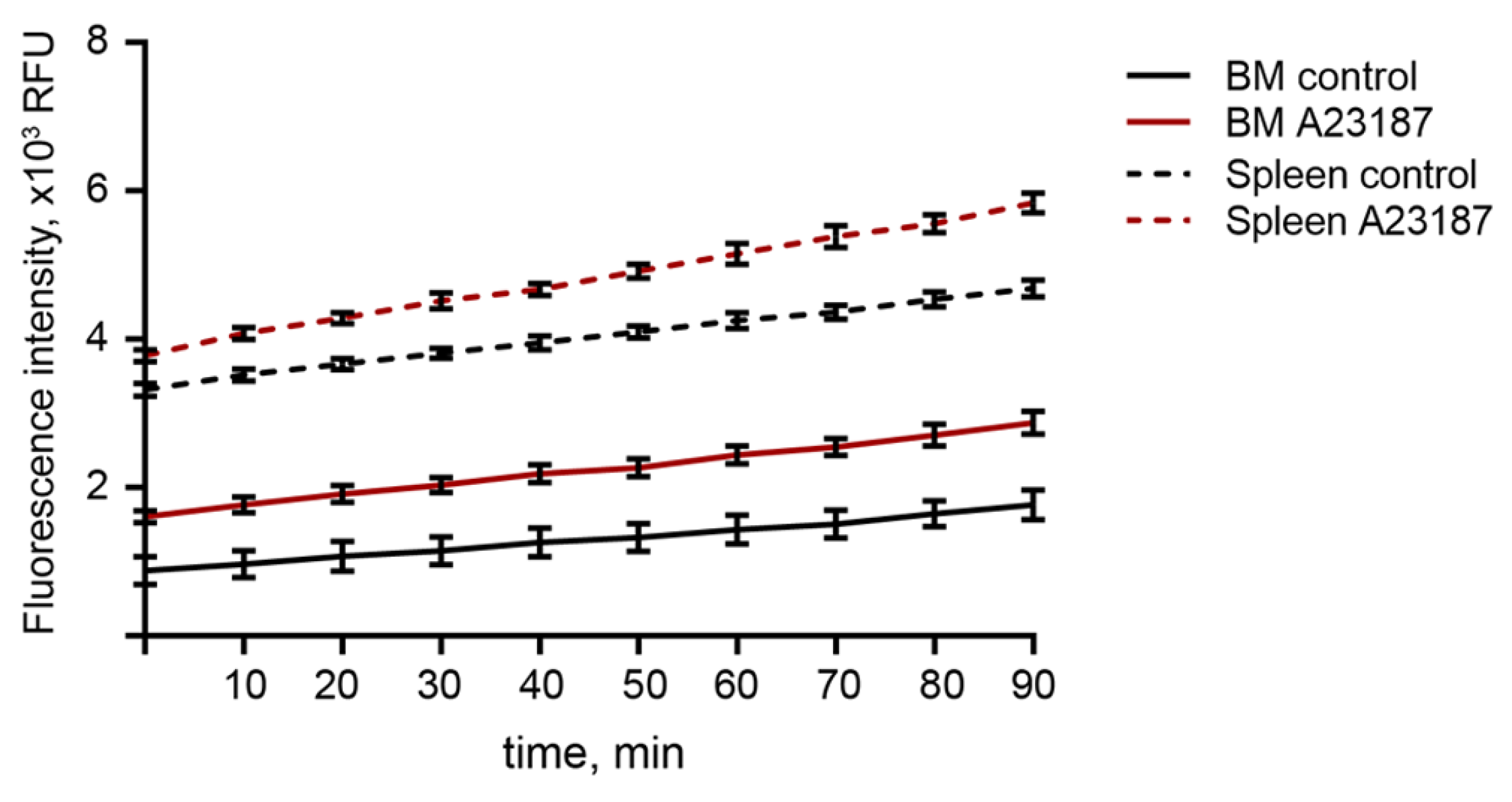
2.5. ROS Production in BM-Derived Neutrophils Isolated Using the 2FLG Protocol and Splenic Neutrophils Isolated Using Dynabeads in Response to Stimulation
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Bone Marrow Cell (BMC) Isolation
4.3. Splenocyte Suspension Preparation
4.4. Preparation of Ficoll Solutions of Different Densities
4.5. Neutrophil Isolation
4.5.1. Ficoll 1.077/1.119 g/mL Density Gradient (Two Layer Density Gradient Protocol, 2FLG Protocol)
4.5.2. Ficoll 1.083/1.090/1.110 g/mL Density Gradient (Three Layer Density Gradient Protocol, 3FLG Protocol)
4.5.3. Immunomagnetic Methods
Immunomagnetic Negative Selection (INS Protocol)
Immunomagnetic Positive Selection (IPS Protocol)
4.6. Neutrophil Characterization
4.6.1. Sample Purity
4.6.2. Cell Viability
4.7. Neutrophil Extracellular Trap Visualization
4.8. Neutrophil Oxidative Burst Test
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russo, M.; Nastasi, C. Targeting the Tumor Microenvironment: A Close Up of Tumor-Associated Macrophages and Neutrophils. Front. Oncol. 2022, 12, 871513. [Google Scholar] [CrossRef]
- Ley, K.; Hoffman, H.M.; Kubes, P.; Cassatella, M.A.; Zychlinsky, A.; Hedrick, C.C.; Catz, S.D. Neutrophils: New Insights and Open Questions. Sci. Immunol. 2018, 3, eaat4579. [Google Scholar] [CrossRef]
- Sounbuli, K.; Mironova, N.; Alekseeva, L. Diverse Neutrophil Functions in Cancer and Promising Neutrophil-Based Cancer Therapies. Int. J. Mol. Sci. 2022, 23, 15827. [Google Scholar] [CrossRef]
- Yang, P.; Li, Y.; Xie, Y.; Liu, Y. Different Faces for Different Places: Heterogeneity of Neutrophil Phenotype and Function. J. Immunol. Res. 2019, 2019, 8016254. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Morales-Primo, A.U.; Becker, I.; Zamora-Chimal, J. Neutrophil Extracellular Trap-Associated Molecules: A Review on Their Immunophysiological and Inflammatory Roles. Int. Rev. Immunol. 2022, 41, 253–274. [Google Scholar] [CrossRef]
- Alekseeva, L.; Mironova, N. Role of Cell-Free DNA and Deoxyribonucleases in Tumor Progression. Int. J. Mol. Sci. 2021, 22, 12246. [Google Scholar] [CrossRef]
- Kaltenmeier, C.; Simmons, R.L.; Tohme, S.; Yazdani, H.O. Neutrophil Extracellular Traps (NETs) in Cancer Metastasis. Cancers 2021, 13, 6131. [Google Scholar] [CrossRef]
- Blanter, M.; Gouwy, M.; Struyf, S. Studying Neutrophil Function in Vitro: Cell Models and Environmental Factors. J. Inflamm. Res. 2021, 14, 141. [Google Scholar] [CrossRef]
- Blanter, M.; Cambier, S.; De Bondt, M.; Vanbrabant, L.; Pörtner, N.; Abouelasrar Salama, S.; Metzemaekers, M.; Marques, P.E.; Struyf, S.; Proost, P.; et al. Method Matters: Effect of Purification Technology on Neutrophil Phenotype and Function. Front. Immunol. 2022, 13, 820058. [Google Scholar] [CrossRef]
- Bonilla, M.C.; Fingerhut, L.; Alfonso-Castro, A.; Elmontaser Mergani, A.; Schwennen, C.; Von Köckritz-Blickwede, M.; de Buhr, N. How Long Does a Neutrophil Live?—The Effect of 24 h Whole Blood Storage on Neutrophil Functions in Pigs. Biomedicines 2020, 8, 278. [Google Scholar] [CrossRef]
- Marchi, L.F.; Sesti-Costa, R.; Chedraoui-Silva, S.; Mantovani, B. Comparison of Four Methods for the Isolation of Murine Blood Neutrophils with Respect to the Release of Reactive Oxygen and Nitrogen Species and the Expression of Immunological Receptors. Comp. Clin. Path. 2014, 23, 1469–1476. [Google Scholar] [CrossRef]
- Swamydas, M.; Lionakis, M.S. Isolation, Purification and Labeling of Mouse Bone Marrow Neutrophils for Functional Studies and Adoptive Transfer Experiments. J. Vis. Exp. 2013, 77, e50586. [Google Scholar] [CrossRef]
- Ubags, N.D.J.; Suratt, B.T. Isolation and Characterization of Mouse Neutrophils. Methods Mol. Biol. 2018, 1809, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Hasenberg, M.; Köhler, A.; Bonifatius, S.; Borucki, K.; Riek-Burchardt, M.; Achilles, J.; Männ, L.; Baumgart, K.; Schraven, B.; Gunzer, M. Rapid Immunomagnetic Negative Enrichment of Neutrophil Granulocytes from Murine Bone Marrow for Functional Studies in Vitro and in Vivo. PLoS ONE 2011, 6, e17314. [Google Scholar] [CrossRef][Green Version]
- Frenea-Robin, M.; Marchalot, J. Basic Principles and Recent Advances in Magnetic Cell Separation. Magnetochemistry 2022, 8, 11. [Google Scholar] [CrossRef]
- Hundhammer, T.; Gruber, M.; Wittmann, S. Paralytic Impact of Centrifugation on Human Neutrophils. Biomedicines 2022, 10, 2896. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, K.; Rankin, S.M. The Secretive Life of Neutrophils Revealed by Intravital Microscopy. Front. Cell Dev. Biol. 2020, 8, 1236. [Google Scholar] [CrossRef] [PubMed]
- Rankin, S.M. The Bone Marrow: A Site of Neutrophil Clearance. J. Leukoc. Biol. 2010, 88, 241–251. [Google Scholar] [CrossRef]
- Scapini, P.; Cassatella, M.A. Location in the Spleen Dictates the Function of Murine Neutrophils. J. Exp. Med. 2017, 214, 1207–1209. [Google Scholar] [CrossRef]
- Deniset, J.F.; Surewaard, B.G.; Lee, W.Y.; Kubes, P. Splenic Ly6Ghigh Mature and Ly6Gint Immature Neutrophils Contribute to Eradication of S. Pneumoniae. J. Exp. Med. 2017, 214, 1333–1350. [Google Scholar] [CrossRef] [PubMed]
- Boxio, R.; Bossenmeyer-Pourié, C.; Steinckwich, N.; Dournon, C.; Nüße, O. Mouse Bone Marrow Contains Large Numbers of Functionally Competent Neutrophils. J. Leukoc. Biol. 2004, 75, 604–611. [Google Scholar] [CrossRef]
- Rahmanian, N.; Bozorgmehr, M.; Torabi, M.; Akbari, A.; Zarnani, A.H. Cell Separation: Potentials and Pitfalls. Prep. Biochem. Biotechnol. 2017, 47, 38–51. [Google Scholar] [CrossRef]
- Coquery, C.M.; Loo, W.; Buszko, M.; Lannigan, J.; Erickson, L.D. Optimized Protocol for the Isolation of Spleen-Resident Murine Neutrophils. Cytom. A 2012, 81, 806. [Google Scholar] [CrossRef]
- Bucher, K.; Schmitt, F.; Mothes, B.; Blumendeller, C.; Schäll, D.; Piekorz, R.; Hirsch, E.; Nürnberg, B.; Beer-Hammer, S. Deficiency of PI3-Kinase Catalytic Isoforms P110γ and P110δ in Mice Enhances the IL-17/G-CSF Axis and Induces Neutrophilia. Cell Commun. Signal. 2017, 15, 28. [Google Scholar] [CrossRef]
- Jia, X.; Zhai, T.; Qu, C.; Ye, J.; Zhao, J.; Liu, X.; Zhang, J.A.; Qian, Q. Metformin Reverses Hashimoto’s Thyroiditis by Regulating Key Immune Events. Front. Cell Dev. Biol. 2021, 9, 685522. [Google Scholar] [CrossRef]
- Hoult, J.R.S.; Nourshargh, S. Phorbol Myristate Acetate Enhances Human Polymorphonuclear Neutrophil Release of Granular Enzymes but Inhibits Chemokinesis. Br. J. Pharmacol. 1985, 86, 533–537. [Google Scholar] [CrossRef]
- Inozemtsev, V.; Sergunova, V.; Vorobjeva, N.; Kozlova, E.; Sherstyukova, E.; Lyapunova, S.; Chernysh, A. Stages of NETosis Development upon Stimulation of Neutrophils with Activators of Different Types. Int. J. Mol. Sci. 2023, 24, 12355. [Google Scholar] [CrossRef]
- Pieterse, E.; Rother, N.; Yanginlar, C.; Hilbrands, L.B.; van der Vlag, J. Neutrophils Discriminate between Lipopolysaccharides of Different Bacterial Sources and Selectively Release Neutrophil Extracellular Traps. Front. Immunol. 2016, 7, 484. [Google Scholar] [CrossRef]
- Marin Oyarzún, C.P.; Carestia, A.; Lev, P.R.; Glembotsky, A.C.; Castro Ríos, M.A.; Moiraghi, B.; Molinas, F.C.; Marta, R.F.; Schattner, M.; Heller, P.G. Neutrophil Extracellular Trap Formation and Circulating Nucleosomes in Patients with Chronic Myeloproliferative Neoplasms. Sci. Rep. 2016, 6, 38738. [Google Scholar] [CrossRef]
- Kleinholz, C.L.; Riek-Burchardt, M.; Seiß, E.A.; Amore, J.; Gintschel, P.; Philipsen, L.; Bousso, P.; Relja, B.; Schraven, B.; Handschuh, J.; et al. Ly6G Deficiency Alters the Dynamics of Neutrophil Recruitment and Pathogen Capture during Leishmania Major Skin Infection. Sci. Rep. 2021, 11, 15071. [Google Scholar] [CrossRef]
- O’Connell, K.E.; Mikkola, A.M.; Stepanek, A.M.; Vernet, A.; Hall, C.D.; Sun, C.C.; Yildirim, E.; Staropoli, J.F.; Lee, J.T.; Brown, D.E. Practical Murine Hematopathology: A Comparative Review and Implications for Research. Comp. Med. 2015, 65, 96. [Google Scholar] [PubMed]
- Hidalgo, A.; Chilvers, E.R.; Summers, C.; Koenderman, L. The Neutrophil Life Cycle. Trends Immunol. 2019, 40, 584–597. [Google Scholar] [CrossRef]
- Heib, T.; Gross, C.; Müller, M.L.; Stegner, D.; Pleines, I. Isolation of Murine Bone Marrow by Centrifugation or Flushing for the Analysis of Hematopoietic Cells—A Comparative Study. Platelets 2021, 32, 601–607. [Google Scholar] [CrossRef]
- Thiama, H.R.; Wong, S.L.; Qiu, R.; Kittisopikul, M.; Vahabikashi, A.; Goldman, A.E.; Goldman, R.D.; Wagner, D.D.; Waterman, C.M. NETosis Proceeds by Cytoskeleton and Endomembrane Disassembly and PAD4-Mediated Chromatin Decondensation and Nuclear Envelope Rupture. Proc. Natl. Acad. Sci. USA 2020, 117, 7326–7337. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.E.; Hallett, M.B. Neutrophil Cell Shape Change: Mechanism and Signalling during Cell Spreading and Phagocytosis. Int. J. Mol. Sci. 2019, 20, 1383. [Google Scholar] [CrossRef]
- Sergunova, V.; Inozemtsev, V.; Vorobjeva, N.; Kozlova, E.; Sherstyukova, E.; Lyapunova, S.; Chernysh, A. Morphology of Neutrophils during Their Activation and NETosis: Atomic Force Microscopy Study. Cells 2023, 12, 2199. [Google Scholar] [CrossRef] [PubMed]
- Takei, H.; Araki, A.; Watanabe, H.; Ichinose, A.; Sendo, F. Rapid Killing of Human Neutrophils by the Potent Activator Phorbol 12-Myristate 13-Acetate (PMA) Accompanied by Changes Different from Typical Apoptosis or Necrosis. J. Leukoc. Biol. 1996, 59, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Damascena, H.L.; Silveira, W.A.A.; Castro, M.S.; Fontes, W. Neutrophil Activated by the Famous and Potent PMA (Phorbol Myristate Acetate). Cells 2022, 11, 2889. [Google Scholar] [CrossRef]
- Yipp, B.G.; Kubes, P. NETosis: How Vital Is It? Blood 2013, 122, 2784–2794. [Google Scholar] [CrossRef]
- Hoppenbrouwers, T.; Autar, A.S.A.; Sultan, A.R.; Abraham, T.E.; Van Cappellen, W.A.; Houtsmuller, A.B.; Van Wamel, W.J.B.; Van Beusekom, H.M.M.; Van Neck, J.W.; De Maat, M.P.M. In Vitro Induction of NETosis: Comprehensive Live Imaging Comparison and Systematic Review. PLoS ONE 2017, 12, e0176472. [Google Scholar] [CrossRef] [PubMed]
- Ståhl, A.L.; Sartz, L.; Nelson, A.; Békássy, Z.D.; Karpman, D. Shiga Toxin and Lipopolysaccharide Induce Platelet-Leukocyte Aggregates and Tissue Factor Release, a Thrombotic Mechanism in Hemolytic Uremic Syndrome. PLoS ONE 2009, 4, e6990. [Google Scholar] [CrossRef] [PubMed]
- Dedkova, E.; Sigova, A.; Zinchenko, V. Mechanism of Action of Calcium Ionophores on Intact Cells: Ionophore-Resistant Cells. Membr. Cell Biol. 2000, 13, 357–368. [Google Scholar] [PubMed]
- Douda, D.N.; Khan, M.A.; Grasemann, H.; Palaniyar, N. SK3 Channel and Mitochondrial ROS Mediate NADPH Oxidase-Independent NETosis Induced by Calcium Influx. Proc. Natl. Acad. Sci. USA 2015, 112, 2817–2822. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Caldwell, C.C.; Nomellini, V. Distinct Neutrophil Populations in the Spleen During PICS. Front. Immunol. 2020, 11, 534199. [Google Scholar] [CrossRef] [PubMed]
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018, 9, 113. [Google Scholar] [CrossRef]
- McGill, C.J.; Lu, R.J.; Benayoun, B.A. Protocol for Analysis of Mouse Neutrophil NETosis by Flow Cytometry. STAR Protoc. 2021, 2, 100948. [Google Scholar] [CrossRef]
- De Meo, M.L.; Shahzad, M.H.; Spicer, J.D. Visualizing NETosis Using a Novel Neutrophil Extracellular Trap-Specific Marker. Methods Mol. Biol. 2023, 2614, 71–80. [Google Scholar] [CrossRef]
- Nadesalingam, A.; Chen, J.H.K.; Farahvash, A.; Khan, M.A. Hypertonic Saline Suppresses NADPH Oxidase-Dependent Neutrophil Extracellular Trap Formation and Promotes Apoptosis. Front. Immunol. 2018, 9, 359. [Google Scholar] [CrossRef]
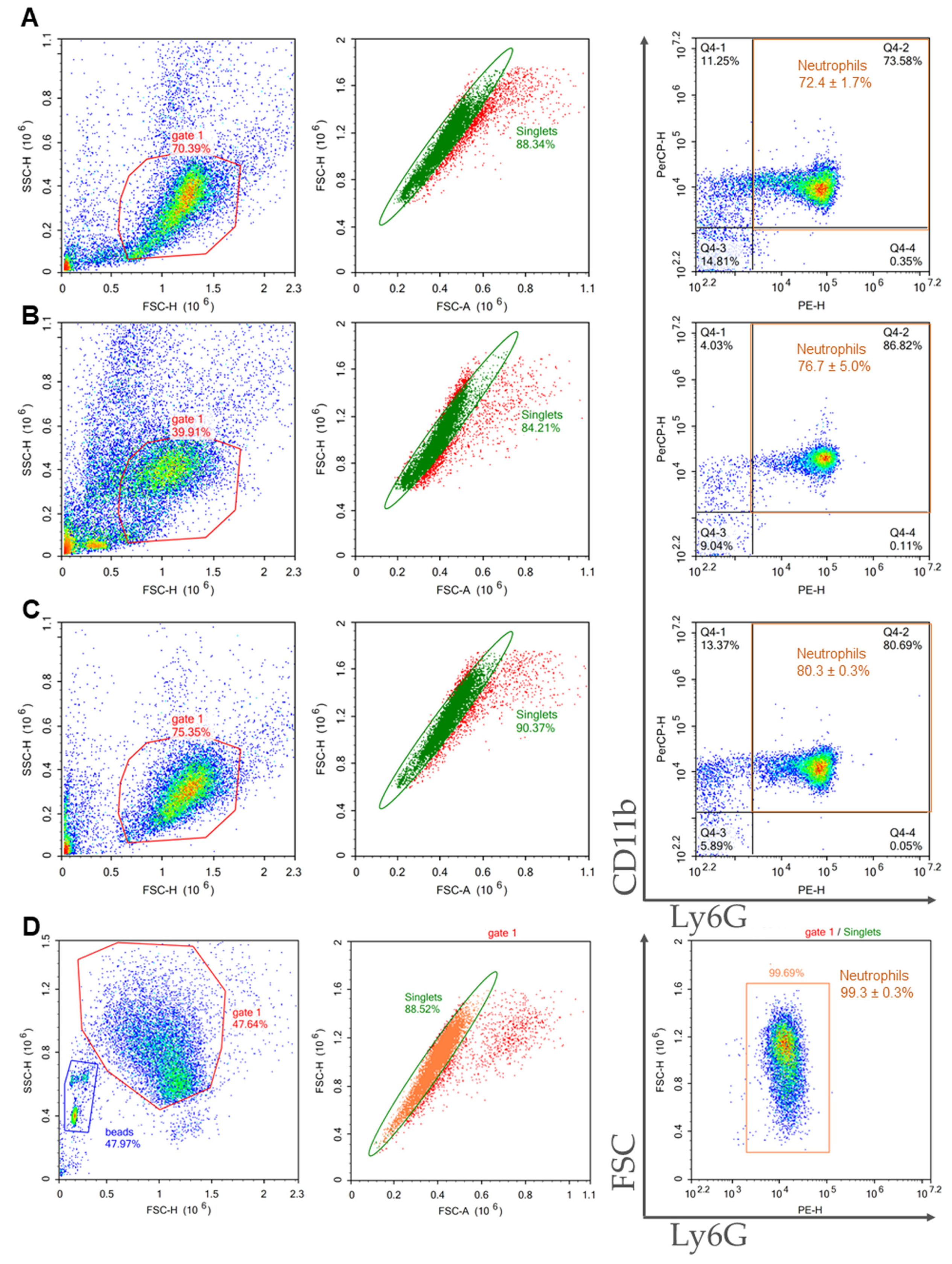
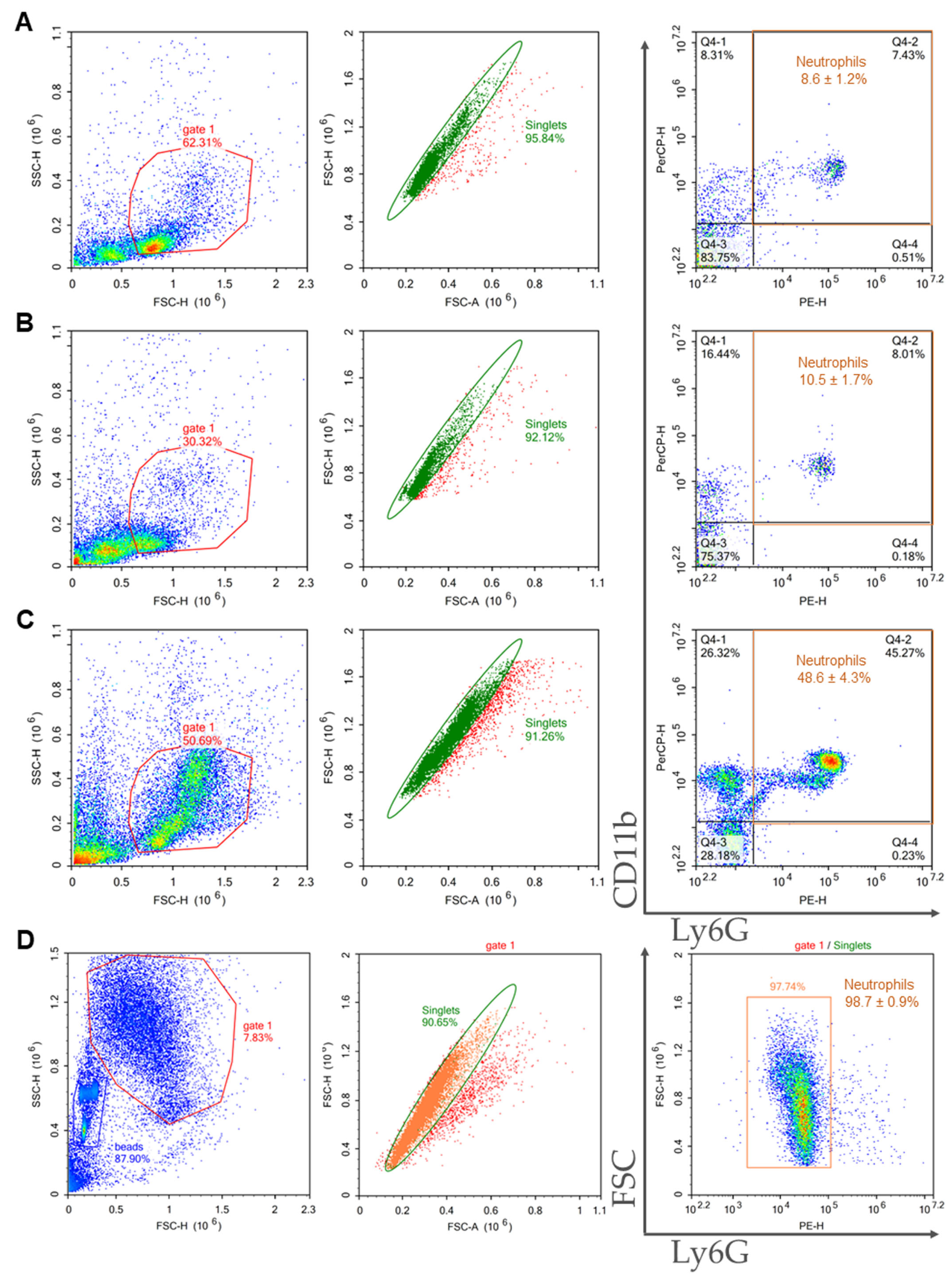
| Method of Isolation | Sample Purity, % | Viability, % | General Yield, ×106 | Neutrophil Yield, ×106 | Cost |
|---|---|---|---|---|---|
| 2FLG | 72.4 ± 1.7 | 89.8 ± 1.4 | 8.6 ± 2.8 | 6.3 ± 2.1 | the lowest |
| 3FLG | 76.7 ± 5.0 | 91.0 ± 2.9 | 0.5 ± 0.1 | 0.4 ± 0.04 | middle |
| INS | 80.3 ± 0.3 | 94.3 ± 0.8 | 10.7 ± 1.1 | 8.6 ± 0.9 | the highest |
| IPS | 99.3 ± 0.3 | 91.6 ± 0.3 | 4.3 ± 1.4 | 4.3 ± 1.4 | high |
| Method of Isolation | Sample Purity, % | Viability, % | General Yield, ×106 | Neutrophil Yield, ×106 | Cost |
|---|---|---|---|---|---|
| 2FLG | 8.6 ± 1.2 | 74.0 ± 7.0 | 18.1 ± 1.9 | 1.5 ± 0.3 | the lowest |
| 3FLG | 10.5 ± 1.7 | 86.0 ± 4.0 | 0.8 ± 0.1 | 0.08 ± 0.02 | middle |
| INS | 48.6 ± 4.3 | 89.6 ± 2.0 | 6.5 ± 2.4 | 3.3 ± 1.5 | the highest |
| IPS | 98.7 ± 0.5 | 88.5 ± 4.5 | 0.7 ± 0.25 | 0.69 ± 0.25 | high |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sounbuli, K.; Alekseeva, L.A.; Markov, O.V.; Mironova, N.L. A Comparative Study of Different Protocols for Isolation of Murine Neutrophils from Bone Marrow and Spleen. Int. J. Mol. Sci. 2023, 24, 17273. https://doi.org/10.3390/ijms242417273
Sounbuli K, Alekseeva LA, Markov OV, Mironova NL. A Comparative Study of Different Protocols for Isolation of Murine Neutrophils from Bone Marrow and Spleen. International Journal of Molecular Sciences. 2023; 24(24):17273. https://doi.org/10.3390/ijms242417273
Chicago/Turabian StyleSounbuli, Khetam, Ludmila A. Alekseeva, Oleg V. Markov, and Nadezhda L. Mironova. 2023. "A Comparative Study of Different Protocols for Isolation of Murine Neutrophils from Bone Marrow and Spleen" International Journal of Molecular Sciences 24, no. 24: 17273. https://doi.org/10.3390/ijms242417273
APA StyleSounbuli, K., Alekseeva, L. A., Markov, O. V., & Mironova, N. L. (2023). A Comparative Study of Different Protocols for Isolation of Murine Neutrophils from Bone Marrow and Spleen. International Journal of Molecular Sciences, 24(24), 17273. https://doi.org/10.3390/ijms242417273







