Cardioprotective Effects of the GRK2 Inhibitor Paroxetine on Isoproterenol-Induced Cardiac Remodeling by Modulating NF-κB Mediated Prohypertrophic and Profibrotic Gene Expression
Abstract
1. Introduction
2. Results
2.1. Effects of Paroxetine on ISO-Induced Cardiac Injury, Hypertrophic, and Fibrotic Markers
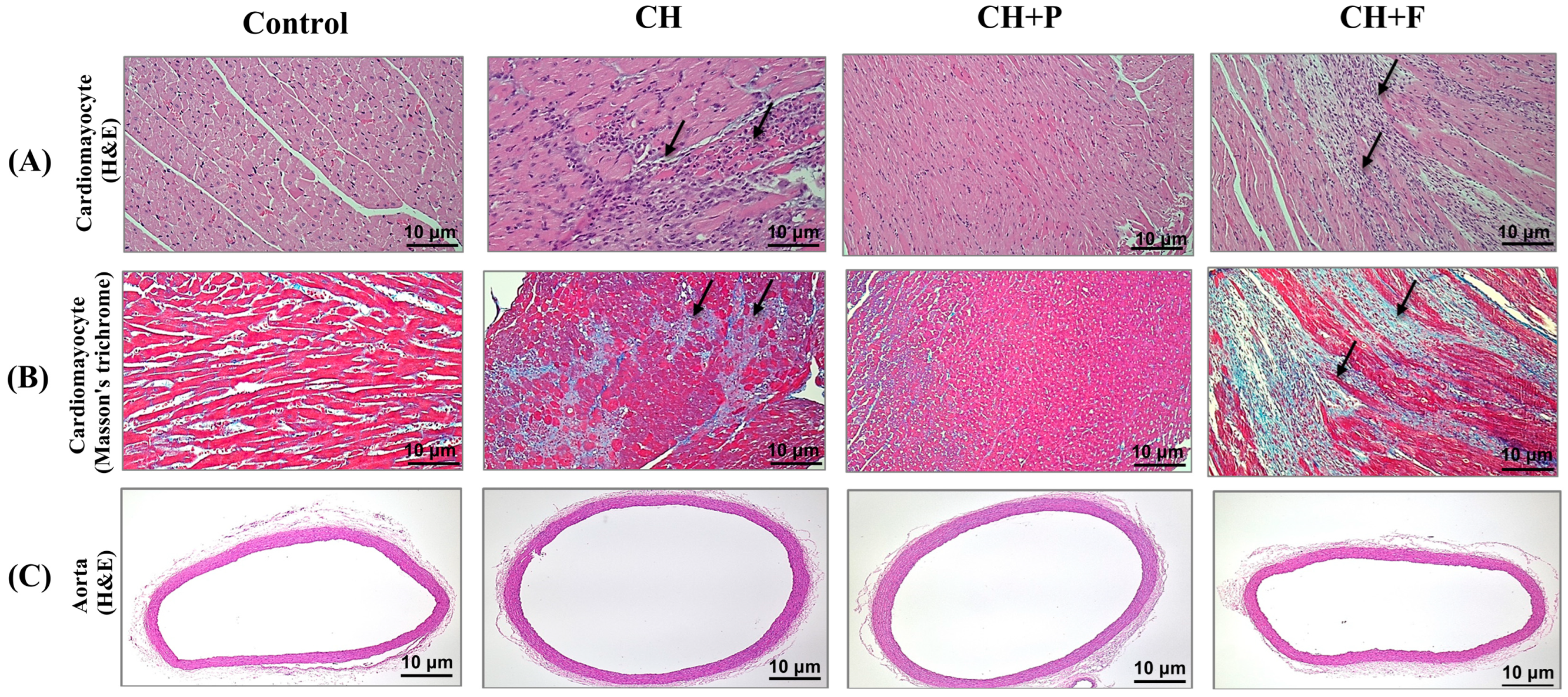
2.2. Effect of Paroxetine on Cardiac Myocyte Morphology and Fibrosis Development in Hypertrophic Heart
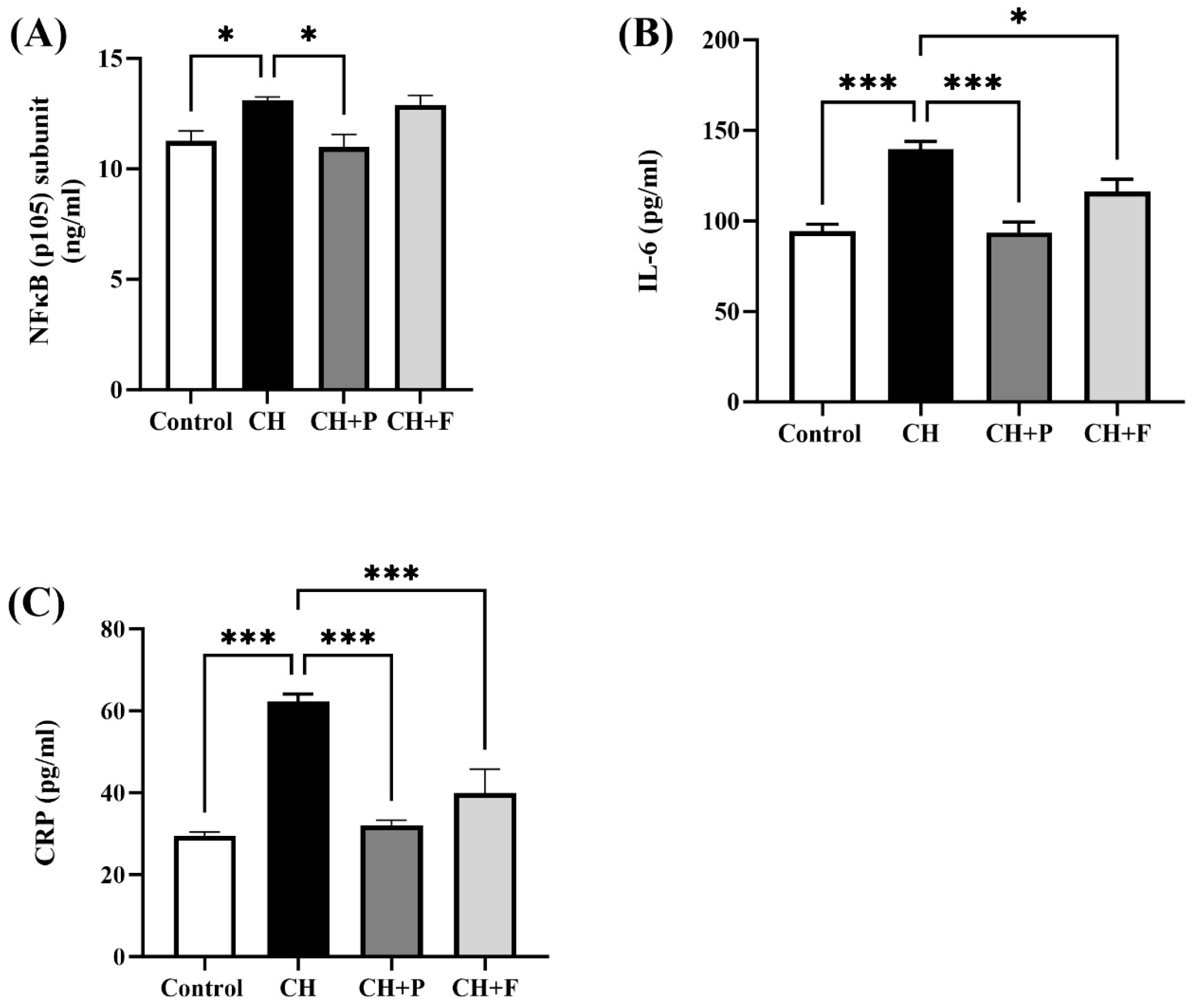
2.3. Effect of Paroxetine on NF-κB and Inflammatory Biomarkers
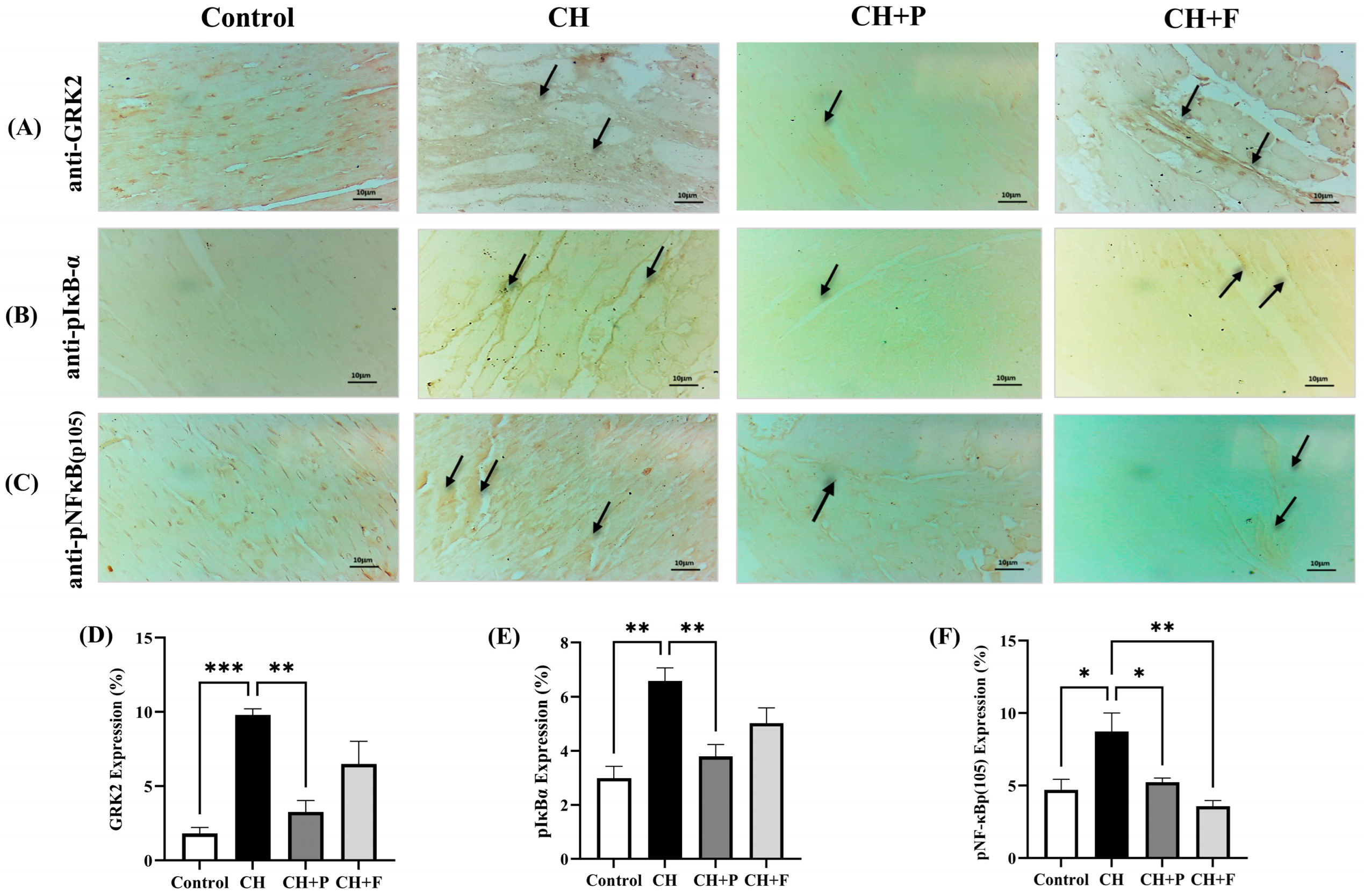
2.4. Effect of Paroxetine on GRK2, pIκBα, and pNF-κB(p105) Expression
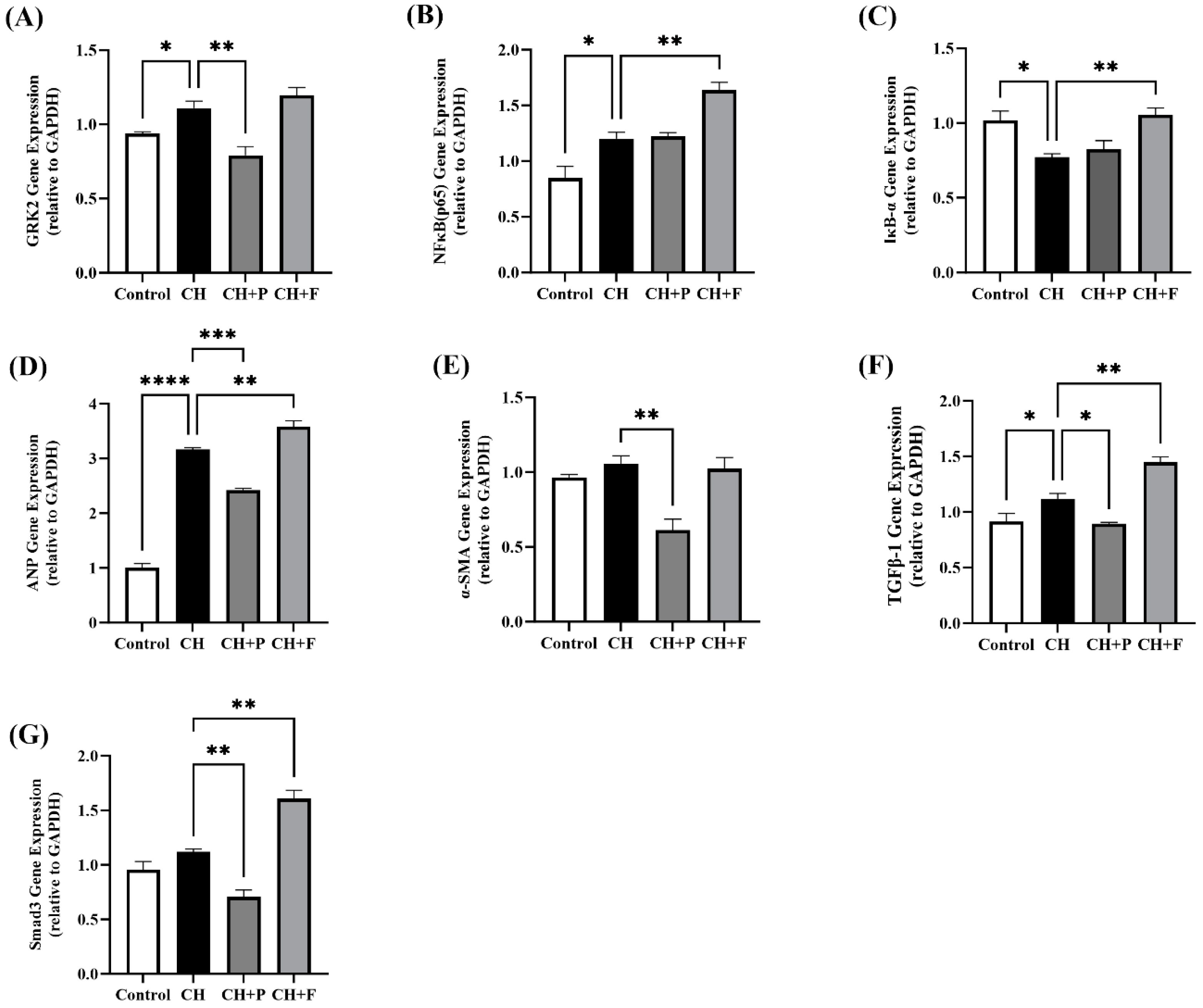
2.5. Effect of Paroxetine on Prohypertrophic and Profibrotic Gene Expression
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. Animals
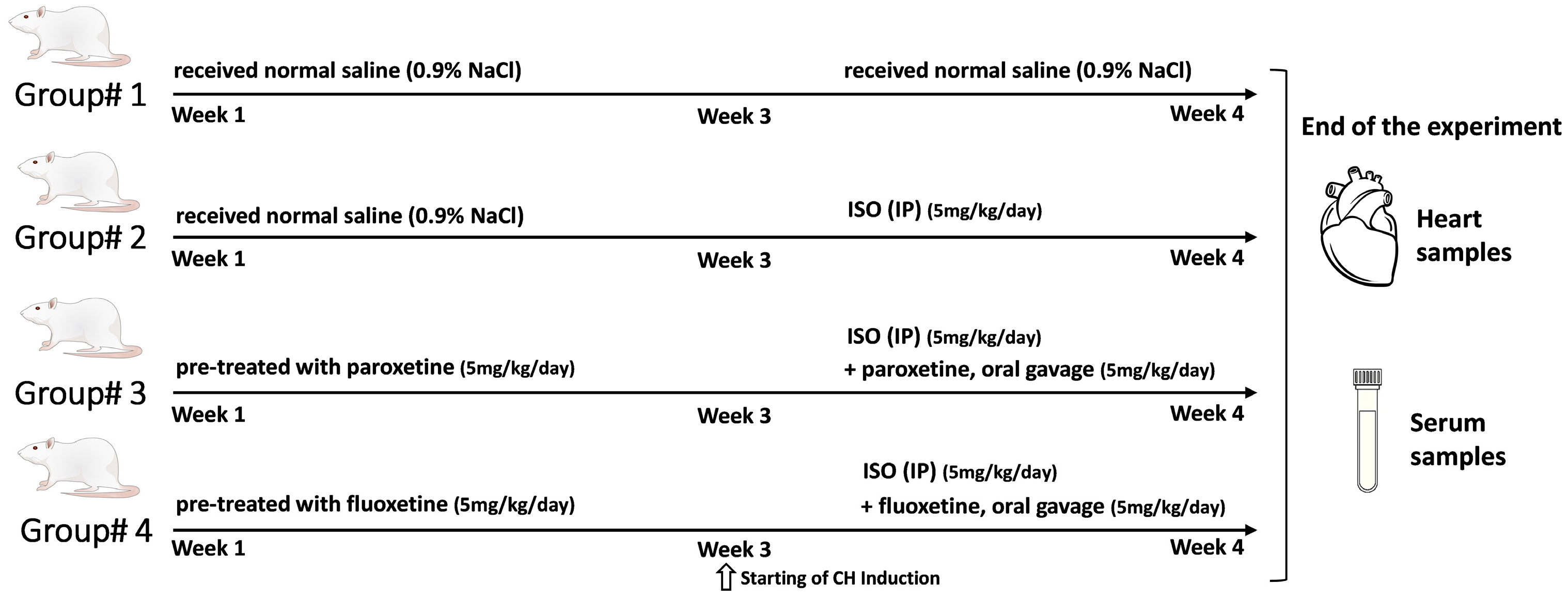
4.3. Experimental Animal Design
4.4. Evaluating of Heart Weight/Body Weight Ratio (HW/BW)
4.5. Examination of Cardiac Injury, Hypertrophic, and Fibrotic Markers
4.6. Evaluation of NF-κB and Inflammatory Biomarkers
4.7. Histopathology of Heart Tissues
4.8. Immunohistochemistry of Heart Tissues
4.9. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANP | Atrial natriuretic peptide |
| α-SMA | α-smooth muscle actin |
| BNP | Brain natriuretic peptide |
| β-MHC | β-myosin heavy chain |
| CH | Cardiac hypertrophy |
| CRP | C-reactive protein |
| CK-MB | Creatine kinase MB |
| ECM | Extracellular matrix |
| ELISA | enzyme-linked immunosorbent assay |
| GPCR | G-protein-coupled receptor |
| GRK | G protein-coupled receptor kinase |
| GRK2 | G protein-coupled receptor kinase 2 |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| HYP | Hydroxyproline |
| IκBα | Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha |
| IL1 | Interleukin-1 |
| IL6 | Interleukin-6 |
| ISO | Isoproterenol |
| IP | intraperitoneal |
| NF-κB | Nuclear factor-kappa-light-chain-enhancer of activated B-cells |
| Smad3 | Mothers against decapentaplegic homolog 3 |
| Tn-I | Troponin 1 |
| TGF-β | Transforming growth factor-β |
| TNFα | Tumor necrosis factor-α |
| SSRI | Selective Serotonin Reuptake Inhibitor |
References
- WHO. Cardiovascular Diseases; WHO. June 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 29 October 2023).
- Roth, G.A.; Mensah, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risks: A Compass for Global Action. J. Am. Coll. Cardiol. 2020, 76, 2980–2981. [Google Scholar] [CrossRef]
- Wu, Q.Q.; Xiao, Y.; Yuan, Y.; Ma, Z.G.; Liao, H.H.; Liu, C.; Zhu, J.X.; Yang, Z.; Deng, W.; Tang, Q.Z. Mechanisms contributing to cardiac remodelling. Clin. Sci. 2017, 131, 2319–2345. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.P.; Zhang, Y.S.; Xu, X.; Zhou, Q.; Li, J.D.; Yan, C. Vinpocetine Attenuates Pathological Cardiac Remodeling by Inhibiting Cardiac Hypertrophy and Fibrosis. Cardiovasc. Drugs Ther. 2017, 31, 157–166. [Google Scholar] [CrossRef]
- Frey, N.; Katus, H.A.; Olson, E.N.; Hill, J.A. Hypertrophy of the Heart. Circulation 2004, 109, 1580–1589. [Google Scholar] [CrossRef]
- Zhu, L.; Li, C.; Liu, Q.; Xu, W.; Zhou, X. Molecular biomarkers in cardiac hypertrophy. J. Cell. Mol. Med. 2019, 23, 1671–1677. [Google Scholar] [CrossRef]
- Samak, M.; Fatullayev, J.; Sabashnikov, A.; Zeriouh, M.; Schmack, B.; Farag, M.; Popov, A.F.; Dohmen, P.M.; Choi, Y.H.; Wahlers, T.; et al. Cardiac Hypertrophy: An Introduction to Molecular and Cellular Basis. Med. Sci. Monit. Basic Res. 2016, 22, 75–79. [Google Scholar] [CrossRef]
- Shimizu, I.; Minamino, T. Physiological and pathological cardiac hypertrophy. J. Mol. Cell. Cardiol. 2016, 97, 245–262. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Nishikimi, T.; Kuwahara, K. Atrial and brain natriuretic peptides: Hormones secreted from the heart. Peptides 2019, 111, 18–25. [Google Scholar] [CrossRef]
- McMullen, J.R.; Jennings, G.L. Differences between Pathological and Physiological Cardiac Hypertrophy: Novel Therapeutic Strategies to Treat Heart Failure. Clin. Exp. Pharmacol. Physiol. 2007, 34, 255–262. [Google Scholar] [CrossRef]
- Shinde, A.V.; Humeres, C.; Frangogiannis, N.G. The role of α-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 298–309. [Google Scholar] [CrossRef]
- Kong, P.; Christia, P.; Frangogiannis, N.G. The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. 2014, 71, 549–574. [Google Scholar] [CrossRef]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac Fibrosis: The Fibroblast Awakens. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Cardiac fibrosis. Cardiovasc. Res. 2021, 117, 1450–1488. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Yong, Q.C.; Thomas, C.M. Do multiple nuclear factor kappa B activation mechanisms explain its varied effects in the heart? Ochsner J. 2013, 13, 157–165. [Google Scholar]
- Murga, C.; Arcones, A.C.; Cruces-Sande, M.; Briones, A.M.; Salaices, M.; Mayor, F., Jr. G Protein-Coupled Receptor Kinase 2 (GRK2) as a Potential Therapeutic Target in Cardiovascular and Metabolic Diseases. Front. Pharmacol. 2019, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Mayor, F., Jr.; Murga, C. G Protein-Coupled Receptor Kinases Take Central Stage. Cells 2022, 12, 23. [Google Scholar] [CrossRef]
- Sato, P.Y.; Chuprun, J.K.; Schwartz, M.; Koch, W.J. The Evolving Impact of G Protein-Coupled Receptor Kinases in Cardiac Health and Disease. Physiol. Rev. 2015, 95, 377–404. [Google Scholar] [CrossRef] [PubMed]
- Bencivenga, L.; Palaia, M.E.; Sepe, I.; Gambino, G.; Komici, K.; Cannavo, A.; Femminella, G.D.; Rengo, G. Why Do We Not Assess Sympathetic Nervous System Activity in Heart Failure Management: Might GRK2 Serve as a New Biomarker? Cells 2021, 10, 457. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, P.; Reinkober, J.; Meinhardt, E.; Tscheschner, H.; Gao, E.; Schumacher, S.M.; Yuan, A.; Backs, J.; Most, P.; Wieland, T.; et al. G protein-coupled receptor kinase 2 promotes cardiac hypertrophy. PLoS ONE 2017, 12, e0182110. [Google Scholar] [CrossRef]
- Umbarkar, P.; Ejantkar, S.; Tousif, S.; Lal, H. Mechanisms of Fibroblast Activation and Myocardial Fibrosis: Lessons Learned from FB-Specific Conditional Mouse Models. Cells 2021, 10, 2412. [Google Scholar] [CrossRef]
- Kowalska, M.; Nowaczyk, J.; Fijałkowski, Ł.; Nowaczyk, A. Paroxetine—Overview of the Molecular Mechanisms of Action. Int. J. Mol. Sci. 2021, 22, 1662. [Google Scholar] [CrossRef]
- Kosić, M.; Nešić, Z.; Glumac, S.; Vasić, M.; Pajović, V.; Savić, B.; Japundžić-Žigon, N. Paroxetine mitigates cardiac remodelling by doxorubicin and increases survival. Biomed. Pharmacother. 2022, 145, 112411. [Google Scholar] [CrossRef]
- Thal, D.M.; Homan, K.T.; Chen, J.; Wu, E.K.; Hinkle, P.M.; Huang, Z.M.; Chuprun, J.K.; Song, J.; Gao, E.; Cheung, J.Y.; et al. Paroxetine is a direct inhibitor of g protein-coupled receptor kinase 2 and increases myocardial contractility. ACS Chem. Biol. 2012, 7, 1830–1839. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wang, Q.; Guo, R.; Xu, L.; Chen, Q.M.; Hou, Y. Effects of paroxetine-mediated inhibition of GRK2 expression on depression and cardiovascular function in patients with myocardial infarction. Neuropsychiatr. Dis. Treat. 2016, 12, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- Kristoff, T.H.; Emily, W.; Michael, W.W.; Puja, S.; Scott, D.L.; John, J.G.T. Structural and Functional Analysis of G Protein–Coupled Receptor Kinase Inhibition by Paroxetine and a Rationally Designed Analog. Mol. Pharmacol. 2014, 85, 237. [Google Scholar] [CrossRef]
- Waldschmidt, H.V.; Homan, K.T.; Cato, M.C.; Cruz-Rodríguez, O.; Cannavo, A.; Wilson, M.W.; Song, J.; Cheung, J.Y.; Koch, W.J.; Tesmer, J.J.G.; et al. Structure-Based Design of Highly Selective and Potent G Protein-Coupled Receptor Kinase 2 Inhibitors Based on Paroxetine. J. Med. Chem. 2017, 60, 3052–3069. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhou, M.; Wen, G.; Huang, Y.; Wu, J.; Peng, L.; Jiang, W.; Yuan, H.; Lu, Y.; Cai, J. Paroxetine Attenuates Cardiac Hypertrophy Via Blocking GRK2 and ADRB1 Interaction in Hypertension. J. Am. Heart Assoc. 2021, 10, e016364. [Google Scholar] [CrossRef]
- Schumacher, S.M.; Gao, E.; Zhu, W.; Chen, X.; Chuprun, J.K.; Feldman, A.M.; Tesmer, J.J.G.; Koch, W.J. Paroxetine-Mediated GRK2 Inhibition Reverses Cardiac Dysfunction and Remodeling After Myocardial Infarction. J. Card. Fail. 2015, 21, S109. [Google Scholar] [CrossRef][Green Version]
- Najafi, A.; Sequeira, V.; Kuster, D.W.; van der Velden, J. β-adrenergic receptor signalling and its functional consequences in the diseased heart. Eur. J. Clin. Investig. 2016, 46, 362–374. [Google Scholar] [CrossRef]
- Leenen, F.H.H.; White, R.; Yuan, B. Isoproterenol-induced cardiac hypertrophy: Role of circulatory versus cardiac renin-angiotensin system. Am. J. Physiol.-Heart Circ. Physiol. 2001, 281, H2410–H2416. [Google Scholar] [CrossRef]
- Kumar, S.; Seth, S.; Jaiswal, A.; Enjamoori, R.; Dinda, A.K.; Ray, R.; Maulik, S.K. Chronic β-adrenergic activation-induced left ventricular systolic dysfunction is associated with systemic release of TNF-α and IL-1-β in rats. Pharmacol. Rep. 2009, 61, 870–876. [Google Scholar] [CrossRef]
- Bin-Dayel, A.F.; Abdel Baky, N.A.; Fadda, L.; Mohammad, R.A.; Al-Mohanna, F. Effect of aliskiren and carvedilol on expression of Ca2+/calmodulin-dependent protein kinase II δ-subunit isoforms in cardiac hypertrophy rat model. Toxicol. Mech. Methods 2016, 26, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Vidal, M.; Wieland, T.; Lohse, M.J.; Lorenz, K. β-Adrenergic receptor stimulation causes cardiac hypertrophy via a Gβγ/Erk-dependent pathway. Cardiovasc. Res. 2012, 96, 255–264. [Google Scholar] [CrossRef]
- Guo, B.Y.; Li, Y.J.; Han, R.; Yang, S.L.; Shi, Y.H.; Han, D.R.; Zhou, H.; Wang, M. Telmisartan attenuates isoproterenol-induced cardiac remodeling in rats via regulation of cardiac adiponectin expression. Acta Pharmacol. Sin. 2011, 32, 449–455. [Google Scholar] [CrossRef]
- Bourdier, G.; Robelet, S. Isoproterenol-induced heart failure in rat heart: Evaluation and cardioprotective strategy. Arch. Cardiovasc. Dis. Suppl. 2019, 11, 230. [Google Scholar] [CrossRef]
- Li, N.; Shan, S.; Li, X.-Q.; Chen, T.-T.; Qi, M.; Zhang, S.-N.; Wang, Z.-Y.; Zhang, L.-L.; Wei, W.; Sun, W.-Y. G Protein-coupled receptor kinase 2 as novel therapeutic target in fibrotic diseases. Front. Immunol. 2022, 12, 822345. [Google Scholar] [CrossRef]
- Sorriento, D.; Santulli, G.; Franco, A.; Cipolletta, E.; Napolitano, L.; Gambardella, J.; Gomez-Monterrey, I.; Campiglia, P.; Trimarco, B.; Iaccarino, G. Integrating GRK2 and NFkappaB in the pathophysiology of cardiac hypertrophy. J. Cardiovasc. Transl. Res. 2015, 8, 493–502. [Google Scholar] [CrossRef]
- Travers, J.G.; Kamal, F.A.; Valiente-Alandi, I.; Nieman, M.L.; Sargent, M.A.; Lorenz, J.N.; Molkentin, J.D.; Blaxall, B.C. Pharmacological and activated fibroblast targeting of Gβγ-GRK2 after myocardial ischemia attenuates heart failure progression. J. Am. Coll. Cardiol. 2017, 70, 958–971. [Google Scholar] [CrossRef]
- Lieu, M.; Koch, W.J. GRK2 and GRK5 as therapeutic targets and their role in maladaptive and pathological cardiac hypertrophy. Expert Opin. Ther. Targets 2019, 23, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Steury, M.D.; McCabe, L.R.; Parameswaran, N. G protein-coupled receptor kinases in the inflammatory response and signaling. Adv. Immunol. 2017, 136, 227–277. [Google Scholar] [PubMed]
- Patial, S.; Luo, J.; Porter, K.J.; Benovic, J.L.; Parameswaran, N. G-Protein coupled receptor kinases mediate TNF alpha-induced NF kappaB signaling via direct interaction with and phosphorylation of I kappa B alpha (38.15). J. Immunol. 2009, 182, 38.15. [Google Scholar] [CrossRef]
- Xu, F.; Sun, S.; Wang, X.; Ni, E.; Zhao, L.; Zhu, W. GRK2 mediates arginine vasopressin-induced interleukin-6 production via nuclear factor-κB signaling neonatal rat cardiac fibroblast. Mol. Pharmacol. 2017, 92, 278–284. [Google Scholar] [CrossRef]
- Woodall, M.C.; Woodall, B.P.; Gao, E.; Yuan, A.; Koch, W.J. Cardiac fibroblast GRK2 deletion enhances contractility and remodeling following ischemia/reperfusion injury. Circ. Res. 2016, 119, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, F.; Zhang, L.; Wang, X.; Wang, Y.; Woo, A.Y.H.; Zhu, W. GRK2/β-arrestin mediates arginine vasopressin-induced cardiac fibroblast proliferation. Clin. Exp. Pharmacol. Physiol. 2017, 44, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, W.-W.; Sun, J.-H.; Yang, H.-X.; Xu, G.-R.; Zhang, Y.; Song, Q.-H.; Zhang, C.; Liu, W.-Z.; Liu, X.-C.; et al. Modified citrus pectin prevents isoproterenol-induced cardiac hypertrophy associated with p38 signalling and TLR4/JAK/STAT3 pathway. Biomed. Pharmacother. 2021, 143, 112178. [Google Scholar] [CrossRef]
- Goncalves, G.K.; Caldeira de Oliveira, T.H.; de Oliveira Belo, N. Cardiac Hypertrophy and Brain Natriuretic Peptide Levels in an Ovariectomized Rat Model Fed a High-Fat Diet. Med. Sci. Monit. Basic Res. 2017, 23, 380–391. [Google Scholar] [CrossRef]
- Saleem, N.; Prasad, A.; Goswami, S.K. Apocynin prevents isoproterenol-induced cardiac hypertrophy in rat. Mol. Cell. Biochem. 2018, 445, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Al-Rasheed, N.M.; Al-Rasheed, N.M.; Hasan, I.H.; Al-Amin, M.A.; Al-Ajmi, H.N.; Mahmoud, A.M. Sitagliptin attenuates cardiomyopathy by modulating the JAK/STAT signaling pathway in experimental diabetic rats. Drug Des. Dev. Ther. 2016, 10, 2095–2107. [Google Scholar] [CrossRef]
- Crowe, A.R.; Yue, W. Semi-quantitative Determination of Protein Expression using Immunohistochemistry Staining and Analysis: An Integrated Protocol. Bio Protoc. 2019, 9, e3465. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Feng, Y.; Bao, Y.; Ding, J.; Li, H.; Liu, W.; Wang, X.; Guan, H.; Chen, Z. MicroRNA-130a attenuates cardiac fibrosis after myocardial infarction through TGF-β/Smad signaling by directly targeting TGF-β receptor 1. Bioengineered 2022, 13, 5779–5791. [Google Scholar] [CrossRef]
- Miri, S.; Rasooli, A.; Brar, S.K. Data on changes of NF-κB gene expression in liver and lungs as a biomarker and hepatic injury in CLP-induced septic rats. Data Brief 2019, 25, 104117. [Google Scholar] [CrossRef] [PubMed]
- Peinnequin, A.; Mouret, C.; Birot, O.; Alonso, A.; Mathieu, J.; Clarençon, D.; Agay, D.; Chancerelle, Y.; Multon, E. Rat pro-inflammatory cytokine and cytokine related mRNA quantification by real-time polymerase chain reaction using SYBR green. BMC Immunol. 2004, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.M.; Lam, A.; Abraham, J.A.; Schreiner, G.F.; Joly, A.H. CTGF expression is induced by TGF- beta in cardiac fibroblasts and cardiac myocytes: A potential role in heart fibrosis. J. Mol. Cell. Cardiol. 2000, 32, 1805–1819. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ai, J.; Feng, J.; Zheng, J.; Tang, K.; Shuai, Z.; Yang, J. Effect of paeoniflorin on cardiac remodeling in chronic heart failure rats through the transforming growth factor β1/Smad signaling pathway. Cardiovasc. Diagn. Ther. 2019, 9, 272–280. [Google Scholar] [CrossRef]
- Fu, S.; Li, Y.L.; Wu, Y.T.; Yue, Y.; Qian, Z.Q.; Yang, D.L. Icariside II attenuates myocardial fibrosis by inhibiting nuclear factor-κB and the TGF-β1/Smad2 signalling pathway in spontaneously hypertensive rats. Biomed. Pharmacother. 2018, 100, 64–71. [Google Scholar] [CrossRef]
- Fiordelisi, A.; Iaccarino, G.; Morisco, C.; Coscioni, E.; Sorriento, D. NFkappaB is a Key Player in the Crosstalk between Inflammation and Cardiovascular Diseases. Int. J. Mol. Sci. 2019, 20, 1599. [Google Scholar] [CrossRef]

| Gene | Forward | Reverse |
|---|---|---|
| GRK2 | 5′-TGGCCCCGGAAGTCCTA-3′ | 5′-CCGCAACAACTTGAAGAGCAT-3′ |
| NFκB (p65) | 5′-CGCAAAAGGACCTACGAGAC-3′ | 5′-TGGGGGAAAACTCATCAAAG-3′ |
| IκBα | 5′-TGAGTACCTGGACTTGCAGAACG-3′ | 5′-TGTAGATGCCTCTCCAAGGATGG-3′ |
| α-SMA | 5′-GCGTGGCTATTCCTTCGTGACTAC-3′ | 5′-CGTCAGGCAGTTCGTAGCTCTTC-3′ |
| ANP | 5′-CAGGCCATATTGGAGCAAATC-3′ | 5′-CTCATCTTCTACCGGCATCTT-3′ |
| TGF-β1 | 5′-GCTGCTGACCCCCACTGAT-3′ | 5′-GCCACTGCCGGACAACTC-3′ |
| Smad3 | 5′-GGCAGGATGTTTCCAGCTA-3′ | 5′-GCAGTCCACAGA CCATGTCA-3′ |
| GAPDH | 5′-GACATGCCGCCTGGAGAAAC-3′ | 5′-AGCCCAGGATGCCCTTTAGT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonazi, A.S.; Bin Dayel, A.F.; Albuaijan, D.A.; Bin Osfur, A.S.; Hakami, F.M.; Alzayed, S.S.; Almotairi, A.R.; Khan, M.R.; Alharbi, H.M.; Ali, R.A.; et al. Cardioprotective Effects of the GRK2 Inhibitor Paroxetine on Isoproterenol-Induced Cardiac Remodeling by Modulating NF-κB Mediated Prohypertrophic and Profibrotic Gene Expression. Int. J. Mol. Sci. 2023, 24, 17270. https://doi.org/10.3390/ijms242417270
Alonazi AS, Bin Dayel AF, Albuaijan DA, Bin Osfur AS, Hakami FM, Alzayed SS, Almotairi AR, Khan MR, Alharbi HM, Ali RA, et al. Cardioprotective Effects of the GRK2 Inhibitor Paroxetine on Isoproterenol-Induced Cardiac Remodeling by Modulating NF-κB Mediated Prohypertrophic and Profibrotic Gene Expression. International Journal of Molecular Sciences. 2023; 24(24):17270. https://doi.org/10.3390/ijms242417270
Chicago/Turabian StyleAlonazi, Asma S., Anfal F. Bin Dayel, Danah A. Albuaijan, Alhanouf S. Bin Osfur, Fatemah M. Hakami, Shaden S. Alzayed, Ahmad R. Almotairi, Mohammad R. Khan, Hana M. Alharbi, Rehab A. Ali, and et al. 2023. "Cardioprotective Effects of the GRK2 Inhibitor Paroxetine on Isoproterenol-Induced Cardiac Remodeling by Modulating NF-κB Mediated Prohypertrophic and Profibrotic Gene Expression" International Journal of Molecular Sciences 24, no. 24: 17270. https://doi.org/10.3390/ijms242417270
APA StyleAlonazi, A. S., Bin Dayel, A. F., Albuaijan, D. A., Bin Osfur, A. S., Hakami, F. M., Alzayed, S. S., Almotairi, A. R., Khan, M. R., Alharbi, H. M., Ali, R. A., Alamin, M. A., Alghibiwi, H. K., Alrasheed, N. M., & Alhosaini, K. A. (2023). Cardioprotective Effects of the GRK2 Inhibitor Paroxetine on Isoproterenol-Induced Cardiac Remodeling by Modulating NF-κB Mediated Prohypertrophic and Profibrotic Gene Expression. International Journal of Molecular Sciences, 24(24), 17270. https://doi.org/10.3390/ijms242417270






