Neurodegeneration of White and Gray Matter in the Hippocampus with FXTAS
Abstract
:1. Introduction
2. Results
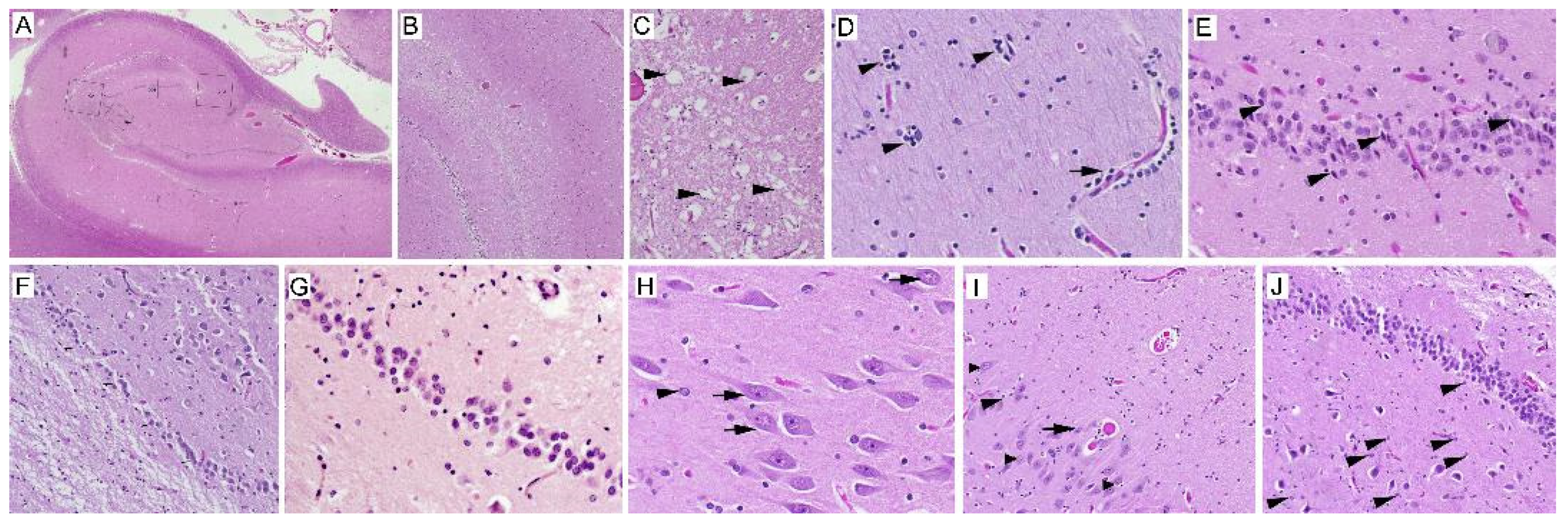
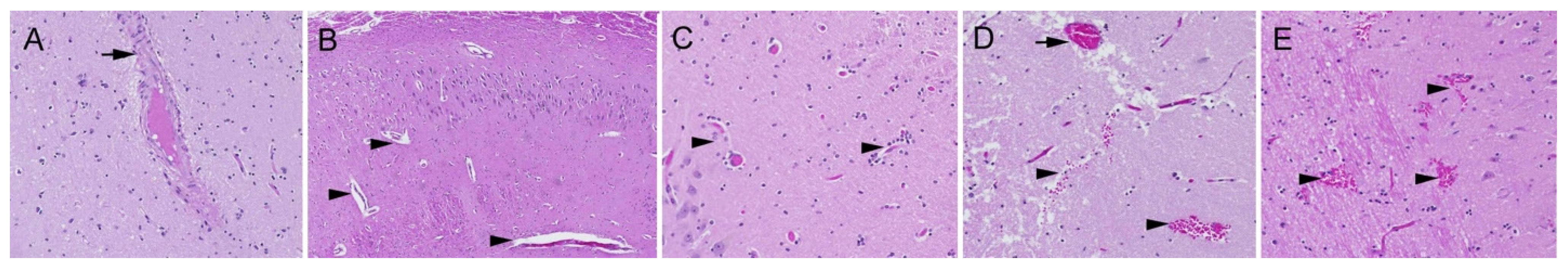
2.1. Neuropathology
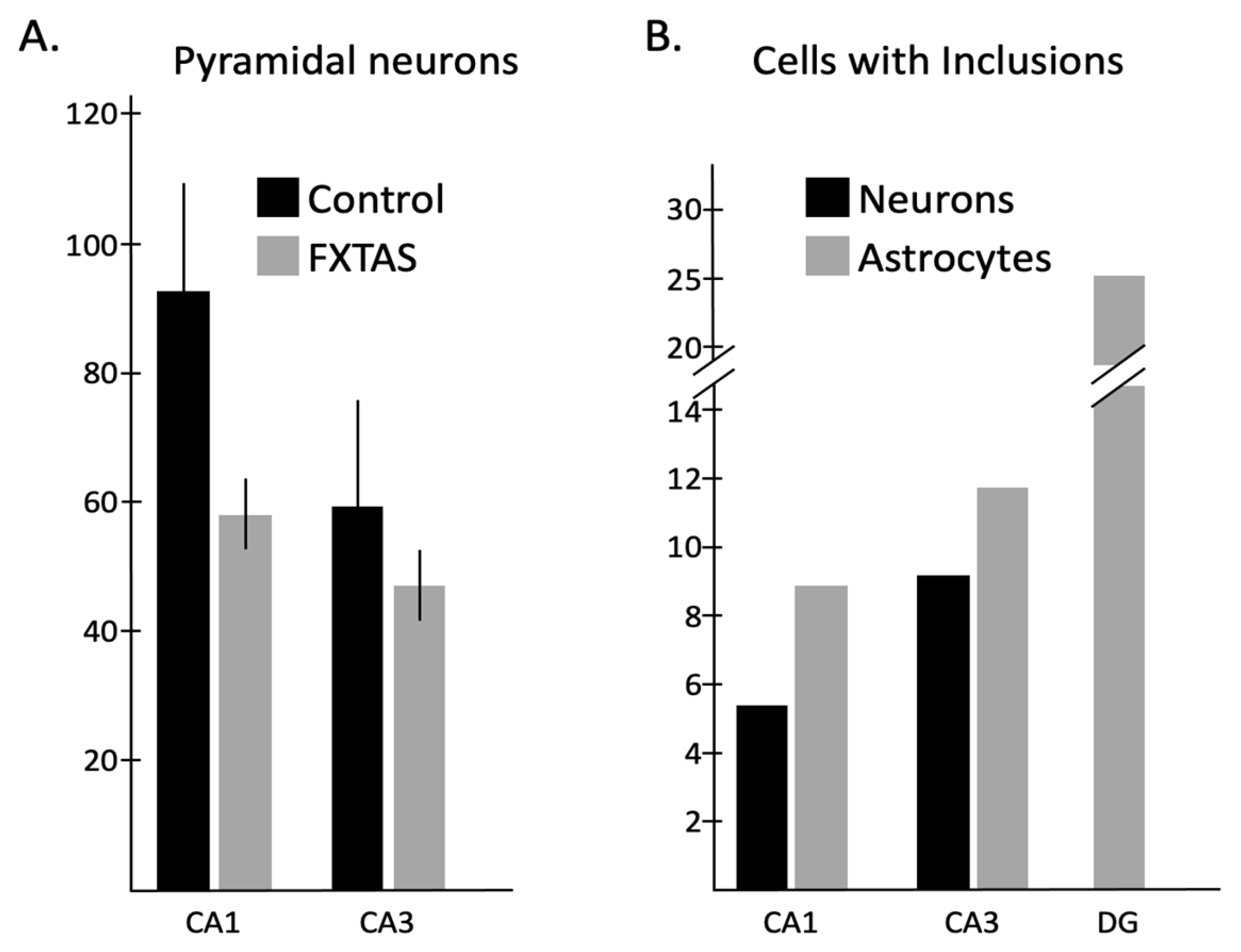
2.2. Quantitative Analysis of the Number of Neurons and Astrocytes
2.3. Quantitative Analysis of the Number of Neurons and Astrocytes with Intranuclear Inclusions
2.4. Correlation Analysis
3. Discussion
3.1. Neuron Loss
3.2. Intranuclear Inclusions
3.3. Correlations
4. Materials and Methods
4.1. Sample Collection
4.2. H&E Staining
4.3. Immunostaining
4.4. FXTAS Neuropathology Evaluation
4.5. Quantification of Cells and Intranuclear Inclusion in the Hippocampus
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cronister, A.; Teicher, J.; Rohlfs, E.M.; Donnenfeld, A.; Hallam, S. Prevalence and instability of fragile X alleles: Implications for offering fragile X prenatal diagnosis. Obstet. Gynecol. 2008, 111, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Oberlé, I.; Rousseau, F.; Heitz, D.; Kretz, C.; Devys, D.; Hanauer, A.; Boué, J.; Bertheas, M.F.; Mandel, J.L. Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science 1991, 252, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Robertson, E.E.; Hall, D.A.; McAsey, A.R.; O’Keefe, J.A. Fragile X-associated Tremor/Ataxia Syndrome: Phenotypic comparisons with other Movement Disorders. Clin. Neuropsychol. 2016, 30, 849–900. [Google Scholar] [CrossRef] [PubMed]
- Berry-Kravis, E.; Ciurlionis, R. Overexpression of fragile X gene (FMR-1) transcripts increases cAMP production in neural cells. J. Neurosci. Res. 1998, 51, 41–48. [Google Scholar] [CrossRef]
- Nolin, S.L.; Brown, W.T.; Glicksman, A.; Houck, G.E., Jr.; Gargano, A.D.; Sullivan, A.; Biancalana, V.; Bröndum-Nielsen, K.; Hjalgrim, H.; Holinski-Feder, E.; et al. Expansion of the fragile X CGG repeat in females with premutation or intermediate alleles. Am. J. Hum. Genet. 2003, 72, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Botta-Orfila, T.; Tartaglia, G.G.; Michalon, A. Molecular Pathophysiology of Fragile X-Associated Tremor/Ataxia Syndrome and Perspectives for Drug Development. Cerebellum 2016, 15, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.A.; Berry-Kravis, E. Chapter 25—Fragile X syndrome and fragile X-associated tremor ataxia syndrome. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 147, pp. 377–391. [Google Scholar]
- Valor, L.M.; Morales, J.C.; Hervás-Corpión, I.; Marín, R. Molecular Pathogenesis and Peripheral Monitoring of Adult Fragile X-Associated Syndromes. Int. J. Mol. Sci. 2021, 22, 8368. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, R.; Levenga, J.; Oostra, B.A. CGG repeat in the FMR1 gene: Size matters. Clin. Genet. 2011, 80, 214–225. [Google Scholar] [CrossRef]
- Brouwer, J.R.; Willemsen, R.; Oostra, B.A. The FMR1 Gene and Fragile X-Associated Tremor/Ataxia Syndrome. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009, 150B, 782–798. [Google Scholar] [CrossRef]
- Todd, P.K.; Oh, S.Y.; Krans, A.; He, F.; Sellier, C.; Frazer, M.; Renoux, A.J.; Chen, K.C.; Scaglione, K.M.; Basrur, V.; et al. CGG repeat-associated translation mediates neurodegeneration in fragile X tremor ataxia syndrome. Neuron 2013, 78, 440–455. [Google Scholar] [CrossRef]
- Hunter, J.; Rivero-Arias, O.; Angelov, A.; Kim, E.; Fotheringham, I.; Leal, J. Epidemiology of fragile X syndrome: A systematic review and meta-analysis. Am. J. Med. Genet. Part A 2014, 164A, 1648–1658. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Revenga, L.; Madrigal, I.; Badenas, C.; Xunclà, M.; Jiménez, L.; Milà, M. Premature ovarian failure and fragile X female premutation carriers: No evidence for a skewed X-chromosome inactivation pattern. Menopause 2009, 16, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Berry-Kravis, E.; Potanos, K.; Weinberg, D.; Zhou, L.; Goetz, C.G. Fragile X-associated tremor/ataxia syndrome in sisters related to X-inactivation. Ann. Neurol. 2005, 57, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Zühlke, C.; Budnik, A.; Gehlken, U.; Dalski, A.; Purmann, S.; Naumann, M.; Schmidt, M.; Bürk, K.; Schwinger, E. FMR1 premutation as a rare cause of late onset ataxia evidence for FXTAS in female carriers. J. Neurol. 2004, 251, 1418–1419. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.A.; O’Keefe, J.A. Fragile X-Associated Tremor Ataxia Syndrome: The Expanding Clinical Picture, Pathophysiology, Epidemiology, and Update on Treatment. Tremor Other Hyperkinet. Mov. 2012, 2, tre-02-56-352-1. [Google Scholar] [CrossRef]
- Hall, D.A.; Robertson-Dick, E.E.; O’Keefe, J.A.; Hadd, A.G.; Zhou, L.; Berry-Kravis, E. X-inactivation in the clinical phenotype of fragile X premutation carrier sisters. Neurol. Genet. 2016, 2, e45. [Google Scholar] [CrossRef] [PubMed]
- Loesch, D.Z.; Litewka, L.; Brotchie, P.; Huggins, R.M.; Tassone, F.; Cook, M. Magnetic resonance imaging study in older fragile X premutation male carriers. Ann. Neurol. 2005, 58, 326–330. [Google Scholar] [CrossRef]
- Louis, E.D.; Vonsattel, J.P.; Honig, L.S.; Lawton, A.; Moskowitz, C.; Ford, B.; Frucht, S. Essential tremor associated with pathologic changes in the cerebellum. Arch. Neurol. 2006, 63, 1189–1193. [Google Scholar] [CrossRef]
- Berry-Kravis, E.; Abrams, L.; Coffey, S.M.; Hall, D.A.; Greco, C.; Gane, L.W.; Grigsby, J.; Bourgeois, J.A.; Finucane, B.; Jacquemont, S.; et al. Fragile X-associated tremor/ataxia syndrome: Clinical features, genetics, and testing guidelines. Mov. Disord. 2007, 22, 2018–2030. [Google Scholar] [CrossRef]
- Bourgeois, J.A. Neuropsychiatry of fragile X-premutation carriers with and without fragile X-associated tremor-ataxia syndrome: Implications for neuropsychology. Clin. Neuropsychol. 2016, 30, 913–928. [Google Scholar] [CrossRef]
- Leehey, M.A. Fragile X-associated Tremor/Ataxia Syndrome (FXTAS): Clinical Phenotype, Diagnosis and Treatment. J. Investig. Med. 2009, 57, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Greco, C.M.; Berman, R.F.; Martin, R.M.; Tassone, F.; Schwartz, P.H.; Chang, A.; Trapp, B.D.; Iwahashi, C.; Brunberg, J.; Grigsby, J.; et al. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS). Brain 2006, 129, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.E.; Adams, J.S.; Nguyen, D.V.; Hessl, D.; Brunberg, J.A.; Tassone, F.; Zhang, W.; Koldewyn, K.; Rivera, S.M.; Grigsby, J.; et al. Psychological symptoms correlate with reduced hippocampal volume in fragile X premutation carriers. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2010, 153B, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Muzar, Z.; Lozano, R. Current research, diagnosis, and treatment of fragile X-associated tremor/ataxia syndrome. Intractable Rare Dis. Res. 2014, 3, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Tassone, F.; Hall, D.A. (Eds.) FXTAS, FXPOI, and Other Premutation Disorders, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Hunsaker, M.R.; Greco, C.M.; Tassone, F.; Berman, R.F.; Willemsen, R.; Hagerman, R.J.; Hagerman, P.J. Rare intranuclear inclusions in the brains of 3 older adult males with fragile x syndrome: Implications for the spectrum of fragile X-associated disorders. J. Neuropathol. Exp. Neurol. 2011, 70, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Tassone, F.; Greco, C.M.; Hunsaker, M.R.; Seritan, A.L.; Berman, R.F.; Gane, L.W.; Jacquemont, S.; Basuta, K.; Jin, L.W.; Hagerman, P.J.; et al. Neuropathological, clinical and molecular pathology in female fragile X premutation carriers with and without FXTAS. Genes Brain Behav. 2012, 11, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Pretto, D.I.; Hunsaker, M.R.; Cunningham, C.L.; Greco, C.M.; Hagerman, R.J.; Noctor, S.C.; Hall, D.A.; Hagerman, P.J.; Tassone, F. Intranuclear inclusions in a fragile X mosaic male. Transl. Neurodegener. 2013, 2, 10. [Google Scholar] [CrossRef]
- Grigsby, J. The fragile X mental retardation 1 gene (FMR1): Historical perspective, phenotypes, mechanism, pathology, and epidemiology. Clin. Neuropsychol. 2016, 30, 815–833. [Google Scholar] [CrossRef]
- Moore, C.J.; Daly, E.M.; Tassone, F.; Tysoe, C.; Schmitz, N.; Ng, V.; Chitnis, X.; McGuire, P.; Suckling, J.; Davies, K.E.; et al. The effect of pre-mutation of X chromosome CGG trinucleotide repeats on brain anatomy. Brain 2004, 127, 2672–2681. [Google Scholar] [CrossRef]
- Jäkälä, P.; Hänninen, T.; Ryynänen, M.; Laakso, M.; Partanen, K.; Mannermaa, A.; Soininen, H. Fragile-X: Neuropsychological test performance, CGG triplet repeat lengths, and hippocampal volumes. J. Clin. Investig. 1997, 100, 331–338. [Google Scholar] [CrossRef]
- Li, Y.; Shen, M.; Stockton, M.E.; Zhao, X. Hippocampal deficits in neurodevelopmental disorders. Neurobiol. Learn. Mem. 2019, 165, 106945. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Koldewyn, K.; Hashimoto, R.; Schneider, A.; Le, L.; Tassone, F.; Cheung, K.; Hagerman, P.; Hessl, D.; Rivera, S.M. Male carriers of the FMR1 premutation show altered hippocampal-prefrontal function during memory encoding. Front. Hum. Neurosci. 2012, 6, 297. [Google Scholar] [CrossRef] [PubMed]
- Capelli, L.P.; Gonçalves, M.R.; Leite, C.C.; Barbosa, E.R.; Nitrini, R.; Vianna-Morgante, A.M. The fragile x-associated tremor and ataxia syndrome (FXTAS). Arq. Neuropsiquiatr. 2010, 68, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Filley, C.M. White matter dementia. Ther. Adv. Neurol. Disord. 2012, 5, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Battistella, G.; Niederhauser, J.; Fornari, E.; Hippolyte, L.; Gronchi Perrin, A.; Lesca, G.; Forzano, F.; Hagmann, P.; Vingerhoets, F.J.; Draganski, B.; et al. Brain structure in asymptomatic FMR1 premutation carriers at risk for fragile X-associated tremor/ataxia syndrome. Neurobiol. Aging 2013, 34, 1700–1707. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.T.; Schmitt, F.A.; Lin, Y.; Abner, E.L.; Jicha, G.A.; Patel, E.; Thomason, P.C.; Neltner, J.H.; Smith, C.D.; Santacruz, K.S.; et al. Hippocampal sclerosis in advanced age: Clinical and pathological features. Brain 2011, 134, 1506–1518. [Google Scholar] [CrossRef]
- Murray, M.E.; Cannon, A.; Graff-Radford, N.R.; Liesinger, A.M.; Rutherford, N.J.; Ross, O.A.; Duara, R.; Carrasquillo, M.M.; Rademakers, R.; Dickson, D.W. Differential clinicopathologic and genetic features of late-onset amnestic dementias. Acta Neuropathol. 2014, 128, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Bachstetter, A.D.; Van Eldik, L.J.; Schmitt, F.A.; Neltner, J.H.; Ighodaro, E.T.; Webster, S.J.; Patel, E.; Abner, E.L.; Kryscio, R.J.; Nelson, P.T. Disease-related microglia heterogeneity in the hippocampus of Alzheimer’s disease, dementia with Lewy bodies, and hippocampal sclerosis of aging. Acta Neuropathol. Commun. 2015, 3, 32. [Google Scholar] [CrossRef]
- Hokkanen, S.R.K.; Hunter, S.; Polvikoski, T.M.; Keage, H.A.D.; Minett, T.; Matthews, F.E.; Brayne, C.; MRC CFAS and CC75C Study Group. Hippocampal sclerosis, hippocampal neuron loss patterns and TDP-43 in the aged population. Brain Pathol. 2018, 28, 548–559. [Google Scholar] [CrossRef]
- Abitbol, M.; Menini, C.; Delezoide, A.L.; Rhyner, T.; Vekemans, M.; Mallet, J. Nucleus basalis magnocellularis and hippocampus are the major sites of FMR-1 expression in the human fetal brain. Nat. Genet. 1993, 4, 147–153. [Google Scholar] [CrossRef]
- Hukema, R.K.; Buijsen, R.A.; Schonewille, M.; Raske, C.; Severijnen, L.A.; Nieuwenhuizen-Bakker, I.; Verhagen, R.F.; van Dessel, L.; Maas, A.; Charlet-Berguerand, N.; et al. Reversibility of neuropathology and motor deficits in an inducible mouse model for FXTAS. Hum. Mol. Genet. 2015, 24, 4948–4957. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Zarnescu, D.C.; Zhang, F.; Pearson, C.E.; Lucchesi, J.C.; Moses, K.; Warren, S.T. RNA-mediated neurodegeneration caused by the fragile X premutation rCGG repeats in Drosophila. Neuron 2003, 39, 739–747. [Google Scholar] [CrossRef]
- Sacino, A.N.; Prokop, S.; Walsh, M.A.; Adamson, J.; Subramony, S.H.; Krans, A.; Todd, P.K.; Giasson, B.I.; Yachnis, A.T. Fragile X-associated tremor ataxia syndrome with co-occurrent progressive supranuclear palsy-like neuropathology. Acta Neuropathol. Commun. 2019, 7, 158. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, R.; Hoogeveen-Westerveld, M.; Reis, S.; Holstege, J.; Severijnen, L.A.; Nieuwenhuizen, I.M.; Schrier, M.; van Unen, L.; Tassone, F.; Hoogeveen, A.T.; et al. The FMR1 CGG repeat mouse displays ubiquitin-positive intranuclear neuronal inclusions; implications for the cerebellar tremor/ataxia syndrome. Hum. Mol. Genet. 2003, 12, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cerdeño, V.; Lechpammer, M.; Hagerman, P.J.; Hagerman, R. Two FMR1 premutation cases without nuclear inclusions. Mov. Disord. 2017, 32, 1328–1329. [Google Scholar] [CrossRef]
| Case Number | Gender | Age | Blood CGG | PMI (h) | Cause of Death |
|---|---|---|---|---|---|
| Control 1 | F | 75 | N/A | 53 | Cardiovascular disease |
| Control 2 | F | 69 | N/A | 60 | Heart failure |
| Control 3 | M | 62 | N/A | 27 | Sudden cardiac death (SCD) |
| Control 4 | F | 63 | N/A | 12 | Hemorrhagic stroke |
| Control 5 | F | 86 | N/A | 61 | Subdural hematoma |
| Control 6 | M | 60 | N/A | 22 | Liver Failure |
| Control 7 | F | 76 | N/A | 5 | Aspergillosis |
| Control 8 | F | 69 | N/A | 57 | Sudden cardia death (SCD) |
| Control 9 | M | 53 | N/A | 45 | Multi-organ dysfunction syndrome (MODS) |
| Case 1 | M | 79 | 88 or 81 | 7.25 | - |
| Case 2 | M | 85 | 89 | - | - |
| Case 3 | F | 66 | 30, 80 | - | - |
| Case 4 | M | 53 | 57 | - | - |
| Case 5 | F | 65 | - | 240 | - |
| Case 6 | M | 69 | 98 | 6.5 | Cardiac failure |
| Case 7 | M | 76 | 87 or 85 | - | - |
| Case 8 | M | - | 89 | 20 | - |
| Case 9 | F | 52 | 36, 75 | - | Multiple Sclerosis |
| Case 10 | F | 86 | 29, 87 | - | - |
| Case 11 | M | 72 | - | 13.5 | Myelodysplasia |
| Case 12 | M | 85 | 66 | 62 | - |
| Case 13 | M | 82 | 67 | - | - |
| Case 14 | M | 82 | 91 | - | - |
| Case 15 | F | 79 | 30, 78 | - | Lung cancer |
| Case 16 | F | 80 | 30, 63 | 5 | Natural causes |
| Case 17 | M | 67 | 112 | - | - |
| Case 18 | M | 66 | 105 | 30 | Cardiorespiratory arrest |
| Case 19 | M | 75 | 89 or 78 | 22 | Lung cancer |
| Case 20 | M | 89 | 75 | - | - |
| Case 21 | M | 77 | 71 or 77 | - | - |
| Case 22 | M | 85 | 86 | 127 | Cardiorespiratory arrest |
| Case 23 | M | 87 | 70 | - | Stroke |
| Case 24 | M | 70 | 62, 75 | 2.5 | - |
| Case 25 | M | 34 | 122 | - | Gunshot |
| Case 26 | - | - | - | - | - |
| Case | CA1 Neuron | CA3 Neuron | DG1 Neuron | DG2 Neuron | CA1 Astrocyte | CA3 Astrocyte | DG1 Astrocyte | DG2 Astrocyte |
|---|---|---|---|---|---|---|---|---|
| Control 1 | 130 | 86 | 82 | 163 | 34 | 39 | 27 | 21 |
| Control 2 | 167 | 73 | 105 | 100 | 28 | 36 | 10 | 10 |
| Control 3 | 96 | 49 | 41 | 82 | 32 | 43 | 22 | 10 |
| Control 4 | 116 | 60 | 82 | 130 | 31 | 56 | 14 | 11 |
| Control 5 | 63 | 47 | 108 | 119 | - | 31 | 8 | - |
| Control 6 | - | 75 | 115 | 117 | - | 28 | 21 | 27 |
| Control 7 | 72 | 53 | 107 | 113 | 61 | 31 | 18 | 12 |
| Control 8 | 45 | 49 | 113 | 163 | 27 | 29 | 12 | 11 |
| Control 9 | 54 | 42 | 123 | - | 15 | 27 | - | - |
| Case Number | CA1 Neurons Inclusion | % CA1 Neurons Inclusion | CA3 Neurons Inclusion | % CA3 Neurons Inclusion | CA1 Astrocyte Inclusion | % CA1 Astrocyte Inclusion | CA3 Astrocyte Inclusion | % CA3 Astrocyte Inclusion | DG1 Astrocyte Inclusion | % DG1 Astrocyte Inclusion | DG2 Astrocyte Inclusion | % DG2 Astrocyte Inclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 4/60 | 6.6 | 8/35 | 22.85 | 2/44 | 4.54 | 3/34 | 8.8 | 10/27 | 37 | 3/23 | 13 |
| Case 2 | 2/36 | 5.5 | 18/75 | 24 | 3/38 | 7.89 | 1/36 | 2.7 | 6/17 | 35.2 | 7/27 | 25.9 |
| Case 3 | 8/94 | 8.5 | 3/40 | 7.5 | 1/10 | 10 | 1/20 | 5 | 0/11 | 0 | 1/5 | 20 |
| Case 4 | 0/57 | 0 | 0/46 | 0 | 0/11 | 0 | 0/64 | 0 | 0/13 | 0 | 0/11 | 0 |
| Case 5 | 4/75 | 5.3 | 4/52 | 7.69 | 0/17 | 0 | 11/20 | 55 | 5/9 | 55.5 | 1/6 | 16.6 |
| Case 6 | 4/50 | 8 | 5/33 | 15.15 | 2/13 | 15.38 | 5/18 | 27.7 | 7/22 | 31.8 | 5/11 | 45.45 |
| Case 7 | 1/68 | 1.47 | 2/60 | 3.33 | 2/28 | 7.1 | 8/53 | 15 | 11/21 | 52.38 | 13/33 | 39.39 |
| Case 8 | 4/55 | 7.27 | 1/34 | 2.94 | 0/39 | 0 | 4/42 | 9.5 | 9/25 | 36 | 2/17 | 11.76 |
| Case 9 | 8/59 | 13.55 | 3/60 | 5 | 1/52 | 1.9 | 1/17 | 5.88 | 0/10 | 0 | 0/7 | 0 |
| Case 10 | 6/50 | 12 | 1/32 | 3.12 | 0/48 | 0 | 0/23 | 0 | 2/12 | 16.6 | 2/10 | 20 |
| Case 11 | 8/60 | 13.33 | 8/28 | 28.5 | 2/14 | 14.28 | 8/29 | 27.58 | 3/7 | 42.85 | 3/6 | 50 |
| Case 12 | 2/90 | 2.22 | 0/33 | 0 | 0/12 | 0 | 0/33 | 0 | 0/17 | 0 | 0/16 | 0 |
| Case 13 | 0/58 | 0 | 0/48 | 0 | 2/26 | 7.69 | 0/28 | 0 | 0/2 | 0 | 0/6 | 0 |
| Case 14 | 4/51 | 7.84 | 12/85 | 14.12 | 0/22 | 0 | 6/49 | 12.24 | 7/15 | 46.6 | 2/9 | 22.2 |
| Case 15 | 4/151 | 2.64 | 2/44 | 4.54 | 5/26 | 19.2 | 6/40 | 15 | 1/14 | 7.1 | 0/12 | 0 |
| Case 16 | 0/85 | 0 | 0/87 | 0 | 0/25 | 0 | 0/42 | 0 | 0/9 | 0 | 0/11 | 0 |
| Case 17 | 4/35 | 11.42 | 4/37 | 10.8 | 6/29 | 20.68 | 9/47 | 19 | 0/6 | 0 | 2/7 | 28.57 |
| Case 18 | 2/47 | 4.25 | - | - | 0/14 | 0 | - | - | 6/11 | 54.5 | 6/19 | 31.57 |
| Case 19 | 0/24 | 0 | 1/39 | 2.56 | 1/16 | 6.25 | 7/33 | 21.2 | 1/10 | 10 | 5/17 | 29.4 |
| Case 20 | 6/22 | 27.27 | 4/59 | 6.78 | 5/12 | 41.6 | 1/35 | 2.85 | 5/19 | 26.3 | 3/10 | 30 |
| Case 21 | 6/85 | 7.06 | 5/47 | 10.64 | 4/28 | 14.28 | 3/36 | 8.3 | 3/16 | 18.75 | 5/12 | 41.66 |
| Case 22 | 1/36 | 2.77 | 4/47 | 8.5 | 7/25 | 28 | 5/35 | 14.2 | 6/11 | 54.5 | 1/11 | 9 |
| Case 23 | 2/35 | 5.71 | 7/46 | 15.2 | 2/6 | 33.3 | 0/18 | 0 | 0/4 | 0 | 2/7 | 28.57 |
| Case 24 | 3/57 | 5.26 | 5/33 | 15.15 | 0/24 | 0 | 5/38 | 13.1 | 1/10 | 10 | 1/12 | 8.3 |
| Case 25 | 4/25 | 16 | 2/31 | 6.45 | 5/23 | 21.73 | 6/30 | 20 | 11/27 | 40.7 | 8/20 | 40 |
| Case 26 | 2/52 | 3.84 | 6/55 | 10.9 | 4/13 | 30.76 | 5/42 | 11.9 | 10/22 | 45.4 | 4/14 | 28.57 |
| CA1 Neurons | CA1 Astroglia | CA3 Neurons | CA3 Astroglia | DG Neurons | DG Astroglia | |
|---|---|---|---|---|---|---|
| Number (+) | 83 of 1517 | 54 of 615 | 105 of 1186 | 95 of 862 | - | 180 of 706 |
| Mean | 5.47 | 8.78 | 8.85 | 11.02 | - | 25.49 |
| Study 1 * | 4.09% | 4.49% | - | - | 2.82% | 6.35% |
| Study 2 * | 10.1% | 10.3% | - | - | 2.1% | 26.6% |
| Study 3 * | 6.59% | 8.84% | - | - | 4.66% | 11.56% |
| Study 4 * | - | - | - | - | 0.68% | 1.34% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kargar, M.; Hagerman, R.J.; Martínez-Cerdeño, V. Neurodegeneration of White and Gray Matter in the Hippocampus with FXTAS. Int. J. Mol. Sci. 2023, 24, 17266. https://doi.org/10.3390/ijms242417266
Kargar M, Hagerman RJ, Martínez-Cerdeño V. Neurodegeneration of White and Gray Matter in the Hippocampus with FXTAS. International Journal of Molecular Sciences. 2023; 24(24):17266. https://doi.org/10.3390/ijms242417266
Chicago/Turabian StyleKargar, Maryam, Randi J. Hagerman, and Verónica Martínez-Cerdeño. 2023. "Neurodegeneration of White and Gray Matter in the Hippocampus with FXTAS" International Journal of Molecular Sciences 24, no. 24: 17266. https://doi.org/10.3390/ijms242417266
APA StyleKargar, M., Hagerman, R. J., & Martínez-Cerdeño, V. (2023). Neurodegeneration of White and Gray Matter in the Hippocampus with FXTAS. International Journal of Molecular Sciences, 24(24), 17266. https://doi.org/10.3390/ijms242417266






